Abstract
Infective endocarditis is a bacterial or fungal infection of the heart valves or endocardial surface, and it frequently forms vegetation and can lead to systemic embolism. Dislodged vegetation rarely results in coronary artery embolism (CAE) and subsequent acute myocardial infarction. A 43-year-old male patient was emergently brought to our hospital for suspected acute myocardial infarction. Coronary angiography was performed and it showed embolism in the left circumflex artery. Thrombus aspiration was performed during coronary angiography. Echocardiography showed formation of vegetation in the posterior leaflet of the mitral valve and multiple blood cultures showed Listeria monocytogenes. Infective endocarditis was diagnosed. Three weeks later, debridement of subacute bacterial endocarditis, mitral valve replacement, and tricuspid valvuloplasty were successfully conducted. Our findings suggest that CAE should be considered in the differential diagnosis of acute myocardial infarction. Aspiration of coronary embolus during coronary angiography followed by surgical intervention of diseased heart valves is a plausible strategy for managing CAE in infective endocarditis.
Keywords: Infective endocarditis, coronary artery embolism, acute myocardial infarction, coronary angiography, Listeria monocytogenes, mitral valve
Introduction
Although the main cause of acute myocardial infarction (AMI) is atherosclerotic coronary artery disease, other causes should also be considered in clinical practice. There are five types of myocardial infarction according to the fourth universal definition of myocardial infarction.1 Identifying the underlying etiology of AMI is important for managing this fatal disease, which affects patients’ prognosis.
Infective endocarditis (IE) is a bacterial or fungal infection of the heart valves or endocardial surface, and it frequently forms vegetation on heart valves and can lead to systemic embolism. Dislodged vegetation rarely results in coronary artery embolism (CAE) and subsequent AMI.
We report a unique case of AMI as the first sign of IE, and the AMI was caused by CAE, which is a rare complication of IE.
Case report
A 43-year-old male patient was brought to the Emergency Department for sudden chest pain with shortness of breath. A physical examination showed a temperature of 38°C, heart rate of 107 beats/minute, respiratory rate of 20 breaths/minute, and blood pressure of 112/69 mmHg. The physical examination also showed a Levine grade II/VI systolic heart murmur at the apex of the heart. His past history was rheumatic heart disease 20 years previously. In the past 6 months, recurrent fever and fatigue occurred. An electrocardiogram (ECG) and measurement of myocardial enzymes, including high-sensitivity cardiac troponin T and creatinine kinase-MB, were performed. The ECG showed tachycardia with 0.15- to 0.20-mm elevation of the ST-segment in leads II, II, and aVF, and ST-segment depression in leads V4 to V5 (Figure 1). The high-sensitivity cardiac troponin T level was 20.11 ng/mL and the creatinine kinase-MB level was 210 U/L. The symptom of chest pain, elevated high-sensitivity cardiac troponin T levels, and ECG findings were consistent with AMI, which has an indication for coronary angiography and primary percutaneous intervention.
Figure 1.
Twelve-lead electrocardiogram showing tachycardia with 0.15 to 0.20-mm elevation of the ST-segment in leads II, II, and aVF, and ST-segment depression in leads V4 to V5.
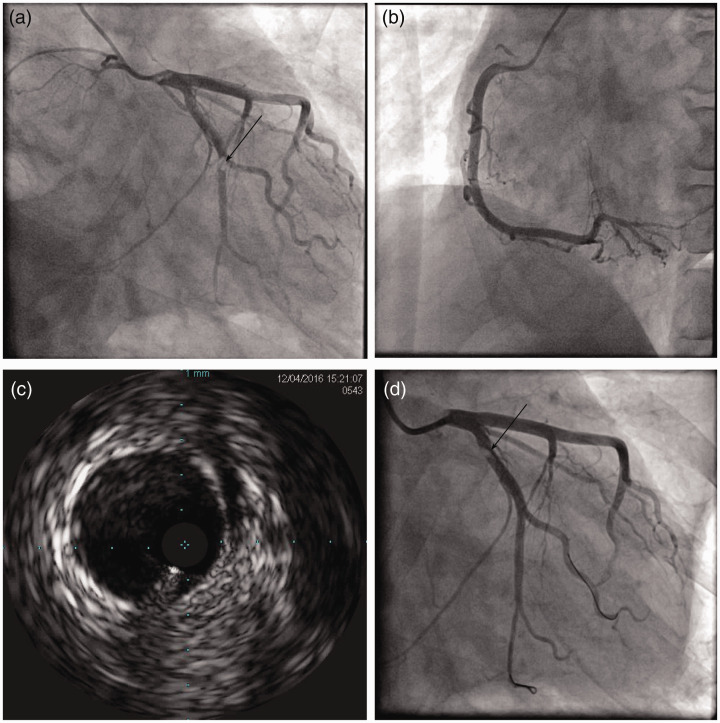
Urgent coronary angiography was performed and it showed normal blood flow in the left main artery, left anterior descending artery, and right coronary artery. The distal left circumflex artery (LCx) had an abrupt occlusion, which resulted in thrombolysis in myocardial infarction flow grade 2 flow (Figure 2a and c). Aspiration of thrombus was conducted and the embolus moved to the proximal LCx (Figure 2d). Antiplatelet and anticoagulant agents were used after coronary angiography.
Figure 2.
Coronary angiography. a: Left coronary angiogram showing an abrupt occlusion in the distal left circumflex artery (black arrow). b: Right coronary angiogram showing a normal artery. c: Intravenous ultrasound showing a thrombus shadow in the distal left circumflex artery without plaques or dissection. d: The embolus moved to the proximal left circumflex artery after thrombus aspiration (black arrow).
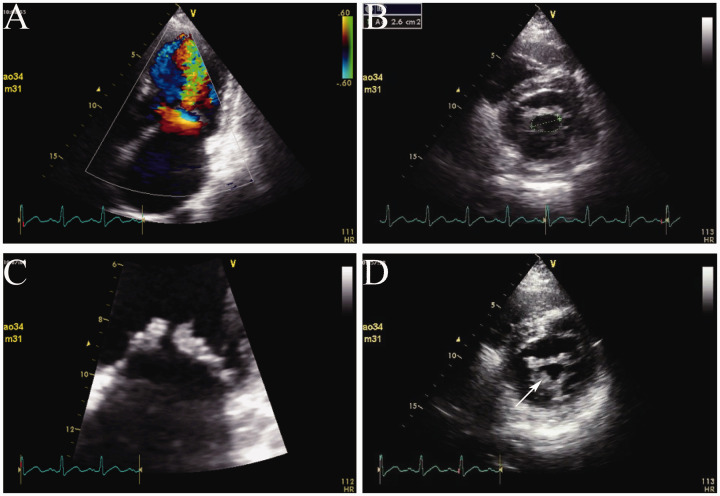
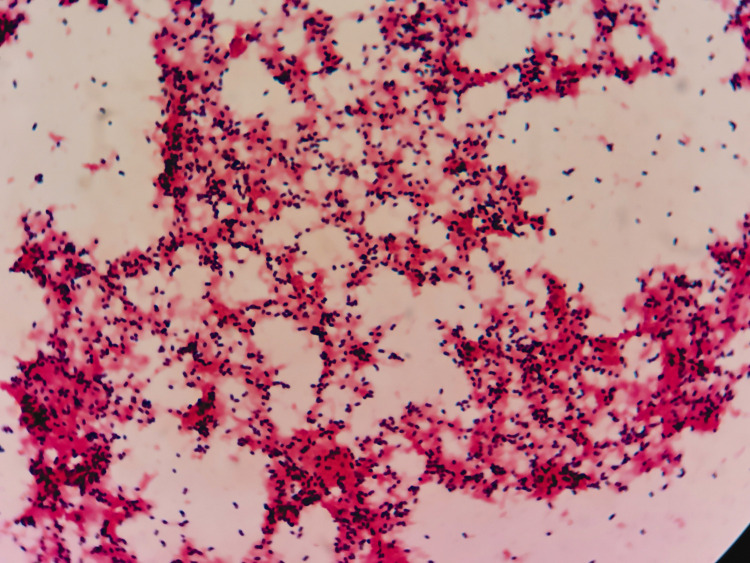
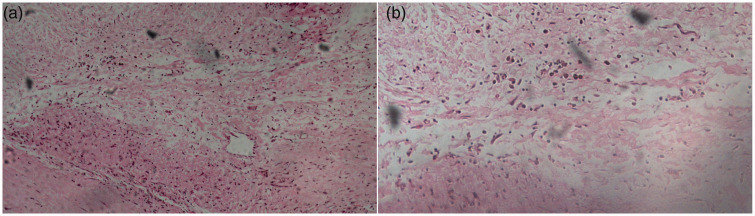
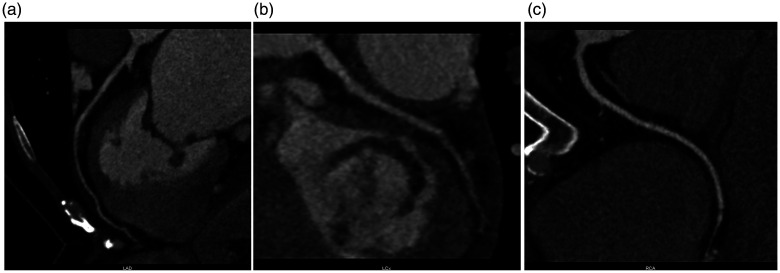
When the patient was transferred to the coronary care unit, he received transthoracic echocardiography. He had mild mitral stenosis with moderate regurgitation and formation of vegetation (12 × 9 mm) in the posterior leaflet of the mitral valve and moderate tricuspid regurgitation (Figure 3). Multiple blood cultures showed Listeria monocytogenes (Figure 4). IE was definitely diagnosed and he was administered teicoplanin and moxifloxacin for anti-infection. His condition became stable 3 weeks later. His infection was controlled and symptoms, such as fever, chest pain, and shortness of breath, were alleviated. Transthoracic echocardiography was repeated and showed vegetation of 8 × 7 mm in the posterior leaflet of the mitral valve, moderate to severe mitral stenosis, severe mitral regurgitation, and moderate tricuspid regurgitation. He was referred to the Department of Cardio-Thoracic Surgery for debridement of subacute bacterial endocarditis, mitral valve replacement (St Jude mechanical valve, St. Jude Medical, Inc., St Paul, MN, USA ), and tricuspid valvuloplasty without major postoperative complications. Postoperative pathological examination of the diseased mitral valve showed infiltration of inflammatory cells and broken collagenous fibers (Figure 5). After surgery, the patient recovered well and returned to work. Coronary computed tomographic angiography showed normal coronary arteries without embolism or stenosis 2 weeks after surgery (Figure 6).
Figure 3.
Transthoracic echocardiography. a: Left atrial enlargement and mitral stenosis. b and c: The anterior and posterior leaflets of the mitral valve (area: 2.6 cm2) are thickened and adhesive. d: Formation of vegetation in the posterior leaflet of the mitral valve (white arrow).
Figure 4.
Blood culture showing Listeria monocytogenes.
Figure 5.
Images of hematoxylin and eosin staining of a diseased mitral valve show infiltration of inflammatory cells and broken collagenous fibers. a: ×100 light microscopy; b: ×200 light microscopy.
Figure 6.
Coronary computed tomographic angiography shows normal coronary arteries without embolism or stenosis. a: Left anterior descending artery; b: left circumflex artery; c: right coronary artery.
Discussion
IE is uncommon, with an annual incidence of approximately 3 to 10 per 100,000 people.2 In developing countries, the most important risk factor for IE is rheumatic heart disease, as described in this case.3,4 A serious complication of IE is an embolic event caused by migration of cardiac vegetation. Dislodged vegetation can cause embolism in systemic blood vessels, leading to ischemic events. Major predictors of embolism in IE are intravenous drug use, Staphylococcus aureus infection, mitral valve vegetation, and vegetation size >10 mm.5
CAE is a rare, but lethal, complication of IE with high morbidity and mortality. Previous observational studies showed that the incidence rate of CAE in IE was approximately 0.5%.6,7 CAE can become an etiology of acute coronary syndrome, including myocardial infarction, but it is not likely to be diagnosed early in clinical practice. Presentation of CAE is difficult to distinguish from atherosclerotic coronary artery disease before angiography because the symptom of chest pain is unspecific. Therefore, initial management of such patients is the same as that for patients with suspected acute coronary syndrome. An ECG, laboratory test, pain relief, and anti-platelet therapy should be performed as preparations for emergent coronary angiography. During coronary angiography, a filling defect suggests CAE.
There are still no guidelines for management of CAE. Thrombus aspiration is a treatment modality for CAE and can also help in assessing the affected coronary artery. If aspiration can restore coronary blood flow with a normal unobstructed coronary artery, CAE is the likely definite diagnosis. Balloon dilation and stent implantation are generally unnecessary. Aspiration may not remove the thrombus entirely. In this situation, antiplatelet and anticoagulant agents should be prescribed. Additionally, patients should be screened for underlying atrial fibrillation and sources of embolic material, including thrombus of the left atrial appendage, left ventricular thrombus, and arteriovenous communications.8
In the current case, the origin of CAE was IE. Therefore, IE should be treated aggressively to prevent future embolic events. In addition to antibiotics, early valve surgery is recommended for patients with IE and valvular dysfunction or recurrent embolic events.9
Conclusions
This report describes the process of diagnosis and treatment of a case of AMI due to CAE, which is a rare complication of IE, thus providing a reference for clinical management. Aspiration of coronary embolus during coronary angiography followed by surgical intervention of diseased heart valves is a plausible strategy for managing CAE in IE.
Acknowledgements
The authors thank Dr Yanda Zhang and Dr Yun Wang for consultation in coronary angiography and coronary computed tomographic angiography.
Ethics statement: The study protocol was approved by the Ethics Committee of Changzheng Hospital (No. CZEC2019-32). Written informed consent was obtained from the patient for publication of the patient’s history and associated images.
Authors’ contributions: JZ, JY, and NL collected the data and wrote the manuscript. ZH performed the coronary angiography. WC, XC, XY, and YL were involved in management of the case. All authors have read and approved the submitted manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by an NSFC grant (81803939) to NL and by the Pyramid Talent Program of Changzheng Hospital (JZ). The funding bodies played no role in design of the study, collection, analysis, and interpretation of the data and in writing the manuscript.
ORCID iD: Jian Zhao https://orcid.org/0000-0002-8567-5925
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018; 138: e618–e651. [DOI] [PubMed] [Google Scholar]
- 2.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387: 882–893. [DOI] [PubMed] [Google Scholar]
- 3.Sunil M, Hieu HQ, Arjan SR, et al. Evolving trends in infective endocarditis in a developing country: a consequence of medical progress? Ann Clin Microbiol Antimicrob 2019; 18: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Chen Y, Xiao T, et al. Epidemiology and risk factors of infective endocarditis in a tertiary hospital in China from 2007 to 2016. BMC Infect Dis 2020; 20: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang A, Tan C, Daneman N, et al. Clinical and echocardiographic predictors of embolism in infective endocarditis: systematic review and meta-analysis. Clin Microbiol Infect 2019; 25: 178–187. [DOI] [PubMed] [Google Scholar]
- 6.Fabri JJ, Issa VS, Pomerantzeff PM, et al. Time-related distribution, risk factors and prognostic influence of embolism in patients with left-sided infective endocarditis. Int J Cardiol 2006; 110: 334–339. [DOI] [PubMed] [Google Scholar]
- 7.Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005; 112: 69–75. [DOI] [PubMed] [Google Scholar]
- 8.Raphael CE, Heit JA, Reeder GS, et al. Coronary Embolus: An Underappreciated Cause of Acute Coronary Syndromes. JACC Cardiovasc Interv 2018; 11: 172–180. [DOI] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]