Key Points
Question
What is the level of air contamination from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in different hospital areas, and what factors are associated with contamination?
Findings
In this systematic review of 24 studies, 17% of air sampled from close patient environments was positive for SARS-CoV-2 RNA, with viability of the virus found in 9% of cultures.
Meaning
In this study, air both close to and distant from patients with coronavirus disease 2019 was frequently contaminated with SARS-CoV-2 RNA; however, few of these samples contained viable viruses.
This systematic review distills the current evidence on air contamination with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital settings and assesses the factors associated with contamination, including viral load and particle size.
Abstract
Importance
Controversy remains regarding the transmission routes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Objective
To review current evidence on air contamination with SARS-CoV-2 in hospital settings and the factors associated with contamination, including viral load and particle size.
Evidence Review
The MEDLINE, Embase, and Web of Science databases were systematically queried for original English-language articles detailing SARS-CoV-2 air contamination in hospital settings between January 1 and October 27, 2020. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. The positivity rate of SARS-CoV-2 viral RNA and culture were described and compared according to the setting, clinical context, air ventilation system, and distance from patients. The SARS-CoV-2 RNA concentrations in copies per meter cubed of air were pooled, and their distribution was described by hospital areas. Particle sizes and SARS-CoV-2 RNA concentrations in copies or median tissue culture infectious dose (TCID50) per meter cubed were analyzed after categorization as less than 1 μm, from 1 to 4 μm, and greater than 4 μm.
Findings
Among 2284 records identified, 24 cross-sectional observational studies were included in the review. Overall, 82 of 471 air samples (17.4%) from close patient environments were positive for SARS-CoV-2 RNA, with a significantly higher positivity rate in intensive care unit settings (intensive care unit, 27 of 107 [25.2%] vs non–intensive care unit, 39 of 364 [10.7%]; P < .001). There was no difference according to the distance from patients (≤1 m, 3 of 118 [2.5%] vs >1-5 m, 13 of 236 [5.5%]; P = .22). The positivity rate was 5 of 21 air samples (23.8%) in toilets, 20 of 242 (8.3%) in clinical areas, 15 of 122 (12.3%) in staff areas, and 14 of 42 (33.3%) in public areas. A total of 81 viral cultures were performed across 5 studies, and 7 (8.6%) from 2 studies were positive, all from close patient environments. The median (interquartile range) SARS-CoV-2 RNA concentrations varied from 1.0 × 103 copies/m3 (0.4 × 103 to 3.1 × 103 copies/m3) in clinical areas to 9.7 × 103 copies/m3 (5.1 × 103 to 14.3 × 103 copies/m3) in the air of toilets or bathrooms. Protective equipment removal and patient rooms had high concentrations per titer of SARS-CoV-2 (varying from 0.9 × 103 to 40 × 103 copies/m3 and 3.8 × 103 to 7.2 × 103 TCID50/m3), with aerosol size distributions that showed peaks in the region of particle size less than 1 μm; staff offices had peaks in the region of particle size greater than 4 μm.
Conclusions and Relevance
In this systematic review, the air close to and distant from patients with coronavirus disease 2019 was frequently contaminated with SARS-CoV-2 RNA; however, few of these samples contained viable viruses. High viral loads found in toilets and bathrooms, staff areas, and public hallways suggest that these areas should be carefully considered.
Introduction
The transmission modes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remain controversial.1 At the emerging stage of the pandemic, many countries implemented high-level precautions, including airborne and contact precautions, to prevent the spread from patients to health care professionals (HCPs).2 An emerging understanding of SARS-CoV-2 epidemiology, which is primarily transmitted from person to person through droplets, led to recommendations for droplet precautions to care for patients hospitalized with coronavirus disease 2019 (COVID-19).3 However, separating transmission dynamics into the dichotomy of droplet vs airborne transmission is probably simplistic. In some circumstances, aerosol particles (<5 μm in diameter) may be produced by individuals with infection and travel more than the 1.50 m commonly used to define transmission routes and contaminate surfaces further away.4
Environmental airflow may ease the spread of large particles.5 The switch from airborne to droplet precautions, combined with a global shortage of face masks and respirators, fed the controversy regarding respiratory protections to prevent transmission of SARS-CoV-2.6,7 This generated a mistrust in personal protective equipment (PPE), particularly regarding surgical masks and their ability to protect HCPs from SARS-CoV-2 transmission. As the World Health Organization recently acknowledged, airborne transmission could occur in crowded and closed environments in the community. This raises the question of whether similar transmission could occur in the hospital.1 Viral contamination of the air surrounding patients with COVID-19 and HCPs in hospitals may have serious implications for outbreak control strategies. We reviewed the current evidence on air contamination with SARS-CoV-2 in hospital settings, the viral load, and associated factors to better assess the risk of cross-transmission of COVID-19 among HCPs and patients.
Methods
Search Strategy
We performed a systematic search of MEDLINE via PubMed, Embase, and Web of Science on October 27, 2020, with terms covering COVID-19 and air contamination in hospital settings in articles published between January 1 and October 27, 2020 (eAppendix in the Supplement). Because of potential delays in indexing of databases, we also searched selected infectious disease journals (eAppendix in the Supplement). We also searched some preprint servers, including BioRxiv and MedRxiv as well as the reference lists of identified articles to find reports of additional studies. We conducted this scoping systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension for scoping reviews (eTable in the Supplement).
Inclusion and Exclusion Criteria
We included all literature related to COVID-19 published in English between January 1, 2020, and October 27, 2020, without restrictions, including original articles, research letters, and comments. We excluded experimental methods and studies performed in dental and primary care settings.
Article Selection and Data Extraction
Two reviewers (G.B. and N.P.S.) screened all titles, abstracts, and full-text articles independently and resolved disagreements by consensus or consultation with a third reviewer (J.C.L.). The following information was then extracted: (1) setting, (2) clinical context, (3) ventilation system, (4) number of air samples performed, (5) sampling method, (6) location of sampler and distance from patients, (7) duration and air volume sampled, (8) method of SARS-CoV-2 search, (9) positivity rate, (10) viral load (SARS-CoV-2 RNA copies per m3), and (11) viral culture results.
Statistical Analysis
We conducted a descriptive analysis of the characteristics of the included literature. We described the setting, patient clinical contexts, ventilation, air sampling and SARS-CoV-2 search methods, and the qualitative and quantitative results according to settings and the hospital area. We categorized the location of air sampling in 5 classes of hospital areas: close patient environments (ie, patient rooms or bays), toilet or bathroom, clinical areas (ie, workstations, anterooms or buffer rooms, corridors, and other spaces in the clinical unit), staff areas (ie, changing rooms, staff rooms including office, meeting rooms, dining rooms, and other staff areas), public areas (hallways and other indoor and outdoor public areas). When possible, we also classified the setting as intensive care unit (ICU) vs non-ICU; the clinical context as severe or critical vs mild, moderate, or asymptomatic; the ventilation system as negative pressure vs natural or mechanical; and the distance from patients as 1 m or less vs greater than 1 to 5 m. The positivity rate of viral RNA and the viral culture were pooled, described, and compared according to categories using a χ2 test. The results of SARS-CoV-2 RNA concentrations in copies per meter cubed of air were pooled, and their distribution was described by hospital areas. The Kruskall-Wallis test was used to compare the nonnormally distributed RNA concentrations across hospital areas. A 2-tailed P < .05 was considered statistically significant. Studies presenting the combined results of particle sizes and SARS-CoV-2 RNA concentrations in copies or median tissue culture infectious dose (TCID50) per meter cubed were analyzed after categorization of sizes as less than 1 μm, 1 to 4 μm, and greater than 4 μm, the thresholds available across the 3 studies.
Results
Search Results
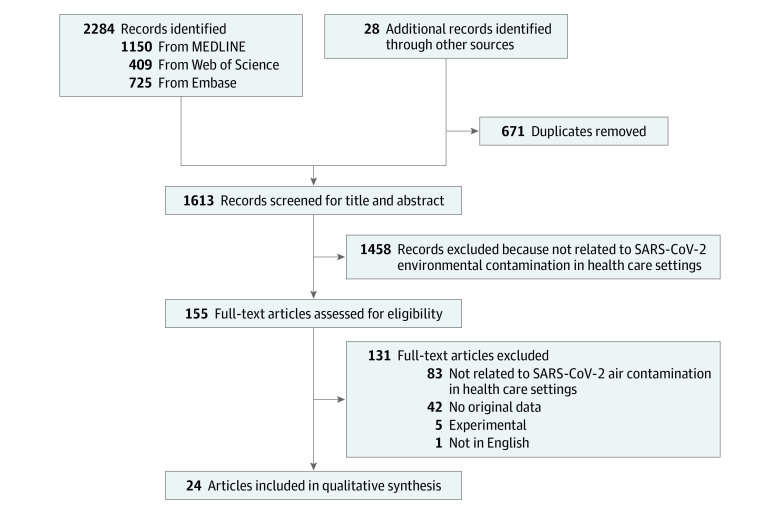
We identified 2284 records, 671 (29.4%) of which were excluded as duplicates. Title and abstract screening were conducted for the remaining 1613 articles, 1458 (90.4%) of which were excluded because they were unrelated to air contamination by SARS-CoV-2 in hospital settings. We retrieved the full text of the 155 remaining articles. After further screening and supplementary searching of articles published or posted between January 1 and October 27, 2020, we identified an additional article, and a total of 24 articles were included in the review (Figure 1).3,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30
Figure 1. Flowchart of the Search Strategy.
Characteristics of Included Articles and Studies
Of the 24 included studies, all were cross-sectional observational studies. Ten studies (41.7%) were from China,9,10,11,15,18,19,20,22,25,29 and the remaining were from the United States (4 [16.7%]),12,13,26,30 Hong Kong (2 [8.3%]),14,21 Korea (2 [8.3%]),24,27 Singapore (2 [8.3%]),3,16 Iran (2 [8.3%]),8,28 the United Kingdom (1 [4.2%]),19 and Italy (1 [4.2%]).23 Of all included articles and studies, 20 (83.3%)3,8,9,10,11,14,15,16,17,20,21,22,23,24,25,26,27,28,29,30 were published in peer-reviewed journals, and 4 (16.7%)12,13,17,18 were posted on preprint servers.
A total of 23 studies (95.8%)3,8,9,10,11,12,13,14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30 sampled the air in the close patient environments, 12 (50.0%)3,9,10,11,12,17,18,19,20,23,24,28 in clinical areas away from patients, 8 (33.3%)9,10,11,12,13,19,23,29 in staff areas, 6 (25.0%)3,9,17,18,19,22 in toilets and/or bathrooms, and 6 (33.3%)9,10,12,17,20,28 in public areas (Table 1). The clinical context of patients hospitalized in the targeted areas was detailed in 18 studies, of which 10 (50.0%)8,9,10,11,22,23,25,27,28,29 were performed in units hospitalizing patients with severe or critical illness, 11 (61.1%)3,11,12,14,15,16,24,25,26,28,30 with patients with mild, moderate, or asymptomatic disease, and 4 studies (22.2%)11,14,25,28 with both categories.
Table 1. Summary of Included Studies Evaluating the Air Contamination With SARS-CoV-2 in the Hospital Environment.
| Source | Setting | Clinical context | Location | Air ventilation | Distance from patients, m | Duration (air volume per sample, L) | Microbiology | SARS-CoV-2 | Positivity, No./total No. (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Viral load, RNA copies/m3 | Viral culture, No./total No. (%) | |||||||||
| ICU patient environments | ||||||||||
| Liu et al,9 2020 | ICU | Severe | IR | Negative pressure | ND | 5 h-7 d (1500-50 400 ) | ddPCR | 31 113 | NA | 2/3 (66) |
| Faridi et al,8 2020 | ICU | Severe | Multiple-bed room | Mechanical or natural | 2-5 | 60 min (90) | RT-PCR; Ct, 38 | NA | NA | 0/9 |
| Guo et al,11 2020 | ICU | Severe | IRs, bay room | 12 Air supplies; 16 air discharges/h | ND | 30 min (300) | RT-qPCR | Mean: 3.8 near air outlet; 1.4 near patients | NA | 13/32 (41); Ct, 35.7 and 44.4 |
| Lei et al,22 2020 | ICU | Critical | Multiple-bed room | ND | 1 | 4 h (1260) | RT-qPCR | NA | NA | 0/1; Ct, 41.5 |
| Li et al,10 2020 | ICU | Severe | Multiple-bed room; 3 beds | 12 Air supplies; 16 air discharges/h | 1-5 | 5 h (21 600) | RT-PCR; Ct, <37 and >40 | NA | NA | 0/9 |
| Zhou et al,19 2020 | ICU | ND | ND | Natural ventilation | 1-5 | 40 min (600-16 000) | RT-qPCR; Ct, 39.5 | NP | NA | 0/5 |
| Razzini et al,23 2020 | ICU | 2 Patients intubated, 1 patient not intubated | Multiple-bed room | Negative pressure | ND | 40 min (2000) | RT-PCR | Mean, 22.7 | NA | 12/12 (100) |
| Ding et al,18 2020 | ICU | Mild | IRs | Negative pressure | 0.5 | 20-30 min (300-10 000) | RT-PCR | NA | NA | 0/26 |
| Ahn et al,27 2020 | ICU | 3 Patients intubated | IRs | Negative pressure and 12 air changes/h | 1 | 20 min (250) | rRT-PCR; Ct, <35; viral culture | NA | NP | 0/3 |
| Kenarhoohi et al,28 2020 | ICU | 6 Critical | Multiple beds; 10 patients | Natural | ≥2 | 180 min (2160) | RT-PCR; Ct, <40 | NA | NA | 0/6 |
| Jin et al,29 2020 | ICU | 1 Critical | IR | Negative pressure | 0.5 | 15 min (6000) | qRT-PCR; Ct, <40 | NA | NA | 1/1 (100) |
| Tan et al,25 2020 | ICU | 9 Severe or critical | Single room | Natural ventilation | 1 | 60 min (300) | RT-PCR | NA | NA | 1/10 (10) |
| Non-ICU patient environments | ||||||||||
| Liu et al,9 2020 | GW | Severe | 4 Single and 1 multiple-bed room; 2 beds | Negative pressure | ND | 5 h (1500) | ddPCR | 0 | NA | 0/2 |
| Faridi et al,8 2020 | GW | Severe | Multiple-bed room; 2-9 beds | Mechanical or natural | 2-5 | 60 min (90) | RT-PCR; Ct, 38 | NA | NA | 0/1 |
| Ong et al,3 2020 | GW | Moderate or mild | IRs | 12 Air exchanges/h | <1 and 2-5 | 4 h (3600) | RT-PCR; Ct, 45 | ND | NA | 0/18 |
| Santarpia et al,12 2020 | IW | Mild | IRs | Negative pressure | Bedside table or desk | 15 min (750) | RT-PCR; Ct, 45; viral culture | NA | 0/10 | 0/18 |
| Guo et al,11 2020 | GW | Mild | Single bay room | 8 Air supplies and 12 air discharges/h | Near patients, near air outlet | 30 min (300) | RT-qPCR | 0.68 near air outlet | NA | 2/16 (12.5) |
| Chia et al,16 2020 | GW | Mild or asymptomatic | IRs | 12 Air changes/h | 1 and 2.1 | 4 h (840) | RT-PCR | Mean (range), 1.3 (0.916-2) | NA | 4/10 (40) |
| Lei et al,22 2020 | IW | Critical | ND | ND | 1 | 4 h (1260) | RT-qPCR | NA | NA | 1/1 (100); Ct, 44.6 |
| Cheng et al,14 2020 | IW | Severe, mild, asymptomatic | IRs | 12 Air changes/h, shelter | 0.1 | 20 min (1000) | RT-PCR | 0 | NA | 0/6 |
| Cheng et al,21 2020 | IW | ND | IRs | Negative pressure | 0.1 | 5 min (1000) | RT-PCR | 0 | NA | 0/8 |
| Zhou et al,17 2020 | IW and GW | ND | ND | ND | ND | ND (1000) | RT-qPCR; Ct, <40.4; viral culture | mean (range), 1.17 (0.16-7.04) | 0/12 | 6/12 (50) |
| Li et al,10 2020 | IW | Severe | Multiple-bed room; 3 beds | 8 Air supplies; 12 air discharges/h | 1-5 | 5 h (21 600) | RT-PCR; Ct, <37 and >40 | NA | NA | 0/18 |
| Wei et al,15 2020 | IW | Mild or asymptomatic | IRs | 12 Air exchanges/h | 0.6 | 15 min (1500) | RT-PCR; Ct, <35 | NA | NA | 0/6 |
| Zhou et al,19 2020 | GW | ND | IRs | Natural | 1-5 | 40 min (600-16 000) | RT-PCR; Ct, <39.5 | NA | NA | 0/16 |
| Santarpia et al,13 2020 | ND | ND | IRs | ND | ND | 30 min (ND) | RT-qPCR; viral culture | 2.41 TCID50/cm3 of air | 3/18 (17) | 18/18 (100) |
| Kim et al,24 2020 | IW and GW | 7 Mild and 1 asymptomatic | IRs; 5-bed room | IR with 15 air changes/h; IR without negative pressure; room without negative air pressure | 2 | 20 min (1000) | rRT-PCR; Ct, <35 | NA | NA | 0/32 |
| Tan et al,25 2020 | IW | 15 Mild | 3 Patients per room | Natural ventilation | 1 | 60 min (300) | RT-PCR | NA | NA | 1/2 (50) |
| Binder et al,26 2020 | IW | 4 Asymptomatic; 16 mild | Single rooms | 14 Air exchange/h | 1, 1.4, 2.2, and 3.2 | 240 min (840) | RT-PCR; viral culture | NA | 0/3 | 3/160 (1.9) |
| Kenarhoohi et al,28 2020 | Laboratory, radiology, internal medicine, emergency | 5 Mild and 1 suspected case | Multiple rooms, 18-30 patients | Natural | ≥2 | 180 min (2160) | RT-PCR; Ct, <40 | NA | NA | 0/6 |
| Lednicky et al,30 2020 | IW | 2 Patients with mild disease | IRs | 6 Air changes/h | 2-4.8 | 180 min (ND) | RT-PCR; viral culture |
Mean (range), 46 (16-94); 2-74 TCID50 U/L of air | 4/4 (100) | 4/4 (100) |
| Toilet or bathroom | ||||||||||
| Liu et al,9 2020 | Non-ICU | NA | Patient mobile toilet room | No ventilation | NA | 20 h (6000) | ddPCR | 1 | NA | 1/1 (100) |
| Ong et al,3 2020 | GW | NA | IRs | ND | NA | 8 h (2400) | RT-PCR; Ct, 45 | NA | NA | 0/6 |
| Lei et al,22 2020 | IW | NA | Patient bathroom | ND | NA | 4 h (1680) | RT-qPCR | NA | NA | 2/2 (100); Ct, 35.6, 35.5 |
| Zhou et al,17 2020 | Cohort ward | NA | Outside patient bay, in the ward | ND | NA | ND (1000) | RT-qPCR; Ct, <40.4; viral culture | 0.464 | 0/2 | 1/2 (50) |
| Zhou et al,19 2020 | Fever clinic | NA | In the ward | Natural | NA | 40 (16 000) | RT-PCR; Ct, <39.5 | NA | NA | 0/3 |
| Ding et al,18 2020 | 4 ICU | NA | Patient bathroom | 4 With negative pressure | NA | 20-30 min (420-10 000) | RT-PCR | NA | NA | 1/7 (14) |
| Clinical areas | ||||||||||
| Liu et al,9 2020 | Non-ICU | ND | Workstation | Natural | ND | 320-1200 min (1600-6000) | ddPCR | 0, 1, 1, 5, 9 | NA | 4/5 (80) |
| Ong et al,3 2020 | ICU and non-ICU | ND | Corridor, anteroom | NA | ND | 15 min (3000); 480 min (2400) | RT-PCR; Ct, 45 | NA | NA | 0/12 |
| Santarpia et al,12 2020 | ND | Mild | Floor adjacent to rooms | NA | >6 ft | 15 min (750) | RT-PCR; Ct, 45; viral culture | 2.58, 3.76 | 0/3 | 2/3 (66) |
| Guo et al,11 2020 | ICU, GW | 1 Severe, ND | Near office, pharmacy, nurse station, corridor, and buffer room | ND | ND | 30 min (27 000-72 000) | RT-qPCR | 0.54 | NA | 1/34 (3) |
| Wu et al,20 2020 | 1 ICU | Ward and various clinical rooms | ND | ND | ND | 30 min (ND) | RT-PCR; Ct, 43 | NA | NA | 0/69 |
| Zhou et al,17 2020 | ICU and non-ICU | ND | Nurse station, ward, ambulatory waiting room, Resus bay, CPAP unit | ND | ND | ND (1000) | RT-qPCR; Ct, <40.4; viral culture | 0.404, 0.035, 1.922, 0.031 | 0/10 | 4/10 (40) |
| Li et al,10 2020 | ICU and non-ICU | Severe | Corridor, clinic, buffer room | 1 With negative pressure | ND | 250 min (21 600) | RT-PCR; Ct, <37 and >40 | NA | NA | 0/45 |
| Zhou et al,19 2020 | ND | ND | Corridor and preroom | Natural | ND | 40 min (16 000) | RT-PCR; Ct, <39.5 | NA | NA | 0/18 |
| Razzini et al,23 2020 | ND | ND | Corridor | ND | ND | 40 min (16 000) | RT-PCR | NA | NA | 8/8 (100); Ct, 31.1 |
| Ding et al,18 2020 | IW | 3 Mild, ND for others | Ward, corridor, nurse station, and storage room | 3 Negative pressure, ND for others | ND | 20-30 (300-10 000) | RT-PCR | NA | NA | 1/12 (8); Ct, 37.8 |
| Kim et al,24 2020 | IW and GW | 7 Mild and 1 asymptomatic | 5 Anterooms, regardless of room type | ND | ND | 20 min (1000) | RT-PCR; Ct, <35 | NA | NA | 0/20 |
| Kenarhoohi et al,28 2020 | ICU entrance | NA | NA | Natural | NA | 180 min (2160) | RT-PCR; Ct, <40 | NA | NA | 0/1 |
| Staff areas | ||||||||||
| Liu et al,9 2020 | ICU | NA | Changing, meeting, and dining rooms, warehouse | Natural and/or mechanical; small air purifier | NA | 300-1200 min (1500-6000) | ddPCR | Mean, 13.8 | NA | 10/13 (77) |
| Santarpia et al,12 2020 | IW | NA | Personal air sample | NA | ND | NA | RT-PCR; Ct, 45 | Mean, 20.037 | NA | 4/4 (100) |
| Guo et al,11 2020 | ICU and GW | NA | Dressing rooms | NA | ND | 30 min (45 000-108 000) | RT-qPCR | NA | NA | 0/36 |
| Santarpia et al,13 2020 | ND | NA | Staff and changing rooms | NA | ND | ND (1000) | RT-qPCR; Ct, <40.4; viral culture | 0.249 | 0/4 | 1/4 (25) |
| Li et al,10 2020 | ICU and IW | NA | Conference room and clean zone | 2/3 With negative pressure | ND | 270-540 min (21 600-43 200) | RT-PCR; Ct, <37 and >40 | NA | NA | 0/45 |
| Zhou et al,19 2020 | ND | NA | Waste storage | Natural | ND | 40 min (16 000) | RT-PCR; Ct, <39.5 | NA | NA | 0/2 |
| Razzini et al,23 2020 | ND | NA | Changing and locker rooms | NA | ND | 40 min (8000-14 000) | RT-PCR | ND | NA | 0/17 |
| Jin et al,29 2020 | ICU | NA | Changing room | Negative pressure | Middle | 15 min (6000) | qRT-PCR; Ct, <40 | NA | NA | 0/1 |
| Public areas | ||||||||||
| Liu et al,9 2020 | NA | NA | Pharmacy, hall, office, store, and supermarket | Mechanical, natural, outdoor | NA | 300-1000 min (1500-5000) | ddPCR | 3, 7, 11, 3 | NA | 4/11 (36) |
| Santarpia et al,12 2020 | NA | NA | Hallway | ND | NA | ND (ND) | RT-PCR; Ct, 45; viral culture | 0.979-8.688 | 0/12 | 8/12 (67) |
| Wu et al,20 2020 | NA | NA | Public area | ND | NA | 30 min (ND) | RT-PCR; Ct, 43 | NA | NA | 0/6 |
| Zhou et al,17 2020 | NA | NA | Main entrance, toilet entrance, and lift area | ND | NA | ND (1000) | RT-qPCR; Ct, <40.4; viral culture | 1.574, 1.545 | 0/3 | 2/3 (67) |
| Li et al,10 2020 | NA | NA | Public area | ND | NA | 270 min (21 600) | RT-PCR; Ct, <37 and >40 | NA | NA | 0/9 |
| Kenarhoohi et al,28 2020 | Hospital entrance | NA | NA | Natural | NA | 180 min (2160) | RT-PCR; Ct, <40 | NA | NA | 0/1 |
Abbreviations: CPAP, continuous positive airway pressure; Ct, cycle threshold; ddPCR, droplet-digital polymerase chain reaction; GW, general ward; ICU, intensive care unit; IR, isolation room; IW, isolation ward; NA, not applicable; ND, not detailed; NP, not performed; RT-PCR, reverse transcription–polymerase chain reaction; RT-qPCR, quantitative reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
A median of 24 air samples were collected per study, varying from 2 to 160 samples. In close patient environments, a median of 10 air samples (range, 1-160) were performed, 2.5 (range, 1-7) in toilets and/or bathrooms, 11 (range, 1-69) in clinical areas, 9 (range, 1-45) in staff areas, and 10 (1-12) in public areas. Overall, 19 studies (79.2%)3,8,9,10,11,12,13,14,15,16,17,19,22,23,25,26,28,30 sampled the air from non-ICU patient rooms, and 12 (50.0%)8,9,10,11,18,19,22,23,25,27,28,29 in ICU rooms. Among the 19 studies3,8,9,10,11,12,14,15,18,19,21,23,24,25,26,27,28,29,30 with the available information, 360 samples were taken in patient rooms with negative pressure and 66 with natural or mechanical ventilation. When pooling the 19 studies3,8,10,11,12,14,15,16,18,19,22,24,25,26,27,28,29,30 detailing the distance from patient, a total of 118 samples were performed 1 m or less from patients and 236 from greater than 1 to 5 m.
All included studies used reverse transcription–polymerase chain reaction (RT-PCR) to identify SARS-CoV-2 RNA, with a quantification of RNA copies per meters cubed or per liter in 8 studies (33.3%). One study9 used a droplet digital RT-PCR method. The viral culture was planned in the methods of 6 studies (20.8%)12,13,17,26,27,30 but performed in 5 (12.5%) of them.12,13,17,26,30 The remaining did not perform viral culture due to negative RT-PCR results. Three studies (12.5%)9,13,16 assessed the particle size in parallel to RNA concentration or viral titer.
RT-PCR and Culture Results by Hospital Areas
A total of 893 air samples were performed across the 24 studies reviewed, including 471 (52.7%) in close patient environments, 237 (26.5%) in clinical areas, 122 (13.7%) in staff areas, 42 (4.7%) in public areas, and 21 (2.4%) in toilets and/or bathrooms (Table 2). Overall, 82 of 471 air samples (17.4%) from close patient environments were positive for SARS-CoV-2 RNA. Among the 107 samples performed in ICU rooms, 27 (25.2%) were positive vs 39 of 364 (10.7%) in non-ICU rooms (P < .001). The air RNA positivity rate was 47 of 360 (13.1%) in rooms with negative pressure and 6 of 66 (9.1%) in rooms with natural or mechanical ventilation. In toilets and/or bathrooms, 5 of 21 samples (23.8%) samples were positive. In clinical areas, the overall positivity rate was 8.4% (20 of 237), varying from 0 of 64 in anterooms or buffer rooms to 6 of 22 (27.2%) at workstations (P < .001). In staff areas, 15 of 122 samples (12.3%) were positive, with 5 of 26 (19.2%) in staff meeting rooms vs 2 of 51 (3.9%) in changing rooms and 8 of 45 (17.8%) in other types of staff rooms (P = .06). Overall, 14 of 42 samples (33.3%) in public areas were positive, with 9 of 16 (56.3%) in hallways, 2 of 18 (11.1%) in other indoor areas, and 3 of 8 (37.5%) in outdoor public areas (P = .01). A total of 81 viral cultures were performed across 3 studies (47 samples [58.0%] from close patient environment, 2 [2.5%] in toilets/bathroom, 13 [16.0%] in clinical areas, 4 [4.9%] in staff areas, and 15 [18.5%] in public areas). Two studies13,30 described positive viral cultures, both from the close patient environment (3 of 39 [7.7%];13 and 4 of 4 [100%]30) in a non-ICU setting.
Table 2. Description of Reverse Transcription–Polymerase Chain Reaction and Culture Results Categorized by Hospital Areas.
| Area | SARS-CoV-2 | ||||
|---|---|---|---|---|---|
| Viral RNA | Viral culture | ||||
| No./total No. | Positivity, % | P value | No./total No. | Positivity, % | |
| Patient environments | |||||
| All | 82/471 | 17.4 | NA | 7/47 | 14.9 |
| Ward | |||||
| ICU | 27/107 | 25.2 | <.001 | NA | NA |
| Non-ICU | 39/364 | 10.7 | 7/47 | 14.9 | |
| Ventilation | |||||
| Negative pressure | 47/360 | 13.1 | .37 | 0/13 | 0 |
| Mechanical or natural | 6/66 | 9.1 | 4/4 | 100 | |
| Distance from patient, m | |||||
| ≤1 | 3/118 | 2.5 | .22 | NA | NA |
| 1-5 | 13/236 | 5.5 | 4/7 | 57.1 | |
| Clinical context | |||||
| Severe or critical | 20/96 | 20.8 | <.001 | NA | NA |
| Mild, moderate, or asymptomatic | 23/303 | 7.6 | 4/17 | 23.5 | |
| Patient toilets or bathrooms | 5/21 | 23.8 | NA | 0/2 | 0 |
| Clinical areas | |||||
| All | 20/237 | 8.4 | NA | 0/13 | 0 |
| Corridor | 9/48 | 18.7 | <.001 | NA | NA |
| Workstation | 6/22 | 27.2 | 0/5 | 0 | |
| Anteroom or buffer room | 0/64 | 0 | NA | NA | |
| Others | 5/103 | 4.8 | 0/8 | 0 | |
| Staff areas | |||||
| All | 15/122 | 12.3 | NA | 0/4 | 0 |
| Changing room | 2/51 | 3.9 | .06 | 0/1 | 0 |
| Meeting or staff room | 5/26 | 19.2 | 0/3 | 0 | |
| Others | 8/45 | 17.8 | NA | NA | |
| Public areas | |||||
| All | 14/42 | 33.3 | NA | 0/15 | 0 |
| Hallways | 9/16 | 56.2 | .01 | 0/14 | 0 |
| Other, indoor | 2/18 | 11.1 | 0/1 | 0 | |
| Outdoor | 3/8 | 37.5 | NA | NA | |
Abbreviations: ICU, intensive care unit; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 RNA Concentrations in Copies per Meters Cubed of Air, According to Hospital Areas
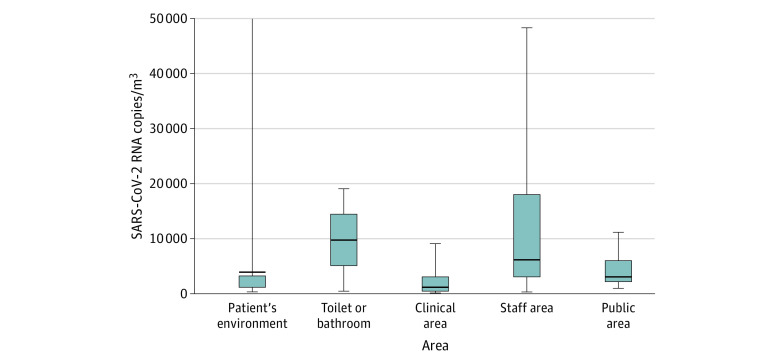
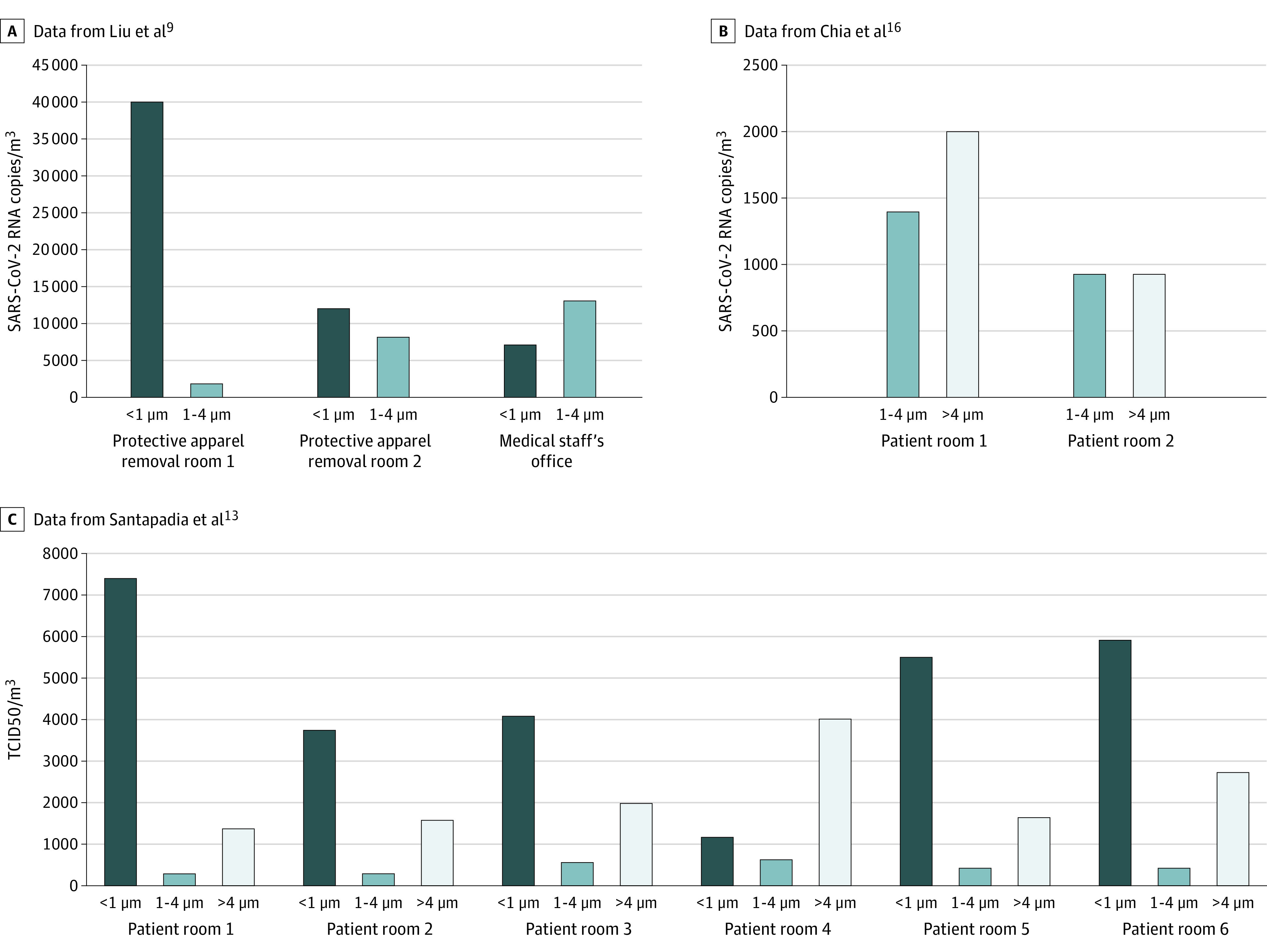
Among studies with SARS-CoV-2 positive air samples11,12,13,16,17,23,30 that performed a quantitative RT-PCR, the median (interquartile range [IQR]) RNA concentrations varied from 1.0 × 103 copies/m3 (0.4 × 103 to 3.1 × 103) in clinical areas to 9.7 × 103 (5.1 × 103 to 14.3 × 103) in the air of toilets and/or bathrooms (Figure 2). The median (IQR) concentration found in close patients environments was 3.8 × 103 (1.2 × 103 to 3.3 × 103) copies/m3 (P < .001). Among the 3 studies9,13,16 that assessed the particle size in air sampled in parallel with the viral load, 1 study16 found an RNA concentration of 2.0 × 103 copies/m3 for particles greater than 4 μm and 1.3 × 103 for particles sized 1 to 4 μm in 1 patient room, and 927 and 916 copies/m3 of those sizes, respectively, in a second room, both at a distance of 1.0 to 2.1 m from patients (Figure 3). A second study9 of 2 PPE removal rooms found 40.0 × 103 and 12.0 × 103 copies/m3 for particles less than 1 μm, and 2.0 × 103 to 8.0 × 103 copies/m3 for particles sized 1 to 4 μm in 2 PPE removal rooms. A concentration of 7.0 × 103 copies/m3 was found for particles less than 1 μm and 13.0 × 103 copies/m3 for particles sized 1 to 4 μm in medical staff offices.31 For the third study that performed viral cultures with air samples from 6 different patients’ room,13 the median (IQR) viral concentration was 4.8 (3.3-5.8) TCID50/m3 for particles less than 1 μm, 4.27 (2.96-5.48) TCID50/m3 for particles sized 1 to 4 μm, and 1.82 (1.6-2.55) TCID50/m3 for particles greater than 4 μm.13
Figure 2. Distribution of Pooled Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA Concentrations in Copies per Meter Cubed of Air, by Hospital Area.
In patient’s environment, there was an outlier, with 1 sample finding 94 000 SARS-CoV-2 RNA copies/m3 in 1 non–intensive care unit room.30
Figure 3. Concentration of Airborne Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Different Aerosol Sizes.
TCID50 indicates median tissue culture infectious dose.
Discussion
This scoping systematic review of the literature suggests that air near and distant from patient environments, including toilets and/or bathrooms, staff areas, and public areas, may carry viral RNA. However, the infectivity of the virus assessed by viral culture was only reported by 2 studies in non-ICU patient rooms. PPE removal and patient rooms had high concentrations per titer of SARS-CoV-2 with aerosol size distributions that showed peaks of particles sized less than 1 μm; for staff offices, the size distribution peaked for particles sized greater than 4 μm.
The results of positivity rate in ICU and non-ICU patient environments were highly heterogeneous and appeared superior in the ICU when pooling the results. In the ICU, 7 of 12 studies did not find SARS-CoV-2 RNA, whereas the remaining did, with 37.5% to 100% positive samples. In non-ICU patient environments, 11 of 19 did not find SARS-CoV-2 RNA, and 8 studies found viral RNA present in from 1.9% to 100% of samples. This heterogeneity may be explained either by a different case mix or by a difference in the methods used for air sampling. The level of severity of patients’ infections was not associated with increased air contamination. Several studies32,33 suggested higher viral loads might be associated with severe clinical outcomes. However, the association between clinical conditions and air contamination may be more complex. The potential opportunistic airborne contamination occurring during aerosol-generating procedures (AGPs) and ventilation at the time of sampling could inform the results. All these factors were poorly detailed in the articles analyzed. The sampling method, including the sampler used; its position in the clinical unit and in relation to patients; the duration of sampling; the volume sampled; and the conditions for transfer to the laboratory were highly variable across studies. The volume of a single room is approximately 40 m3. However, most sample volumes were less than 10 m3, at various airflow rates, for a duration of less than or equal to 1 hour, potentially not reflecting the reality of air contamination. The climatic conditions (eg, temperature and hygrometry) were poorly detailed in studies reviewed, but they may affect the capacity for viral particles to persist in the air.34 The methods for RNA detection varied, especially the cycle threshold (Ct) for PCR positivity, which also varied from 37 to 45. The RT-PCR Ct values are strongly associated with a cultivable virus. The probability of culturing virus declines to 8% in samples with Ct of greater than 35.35 Only 2 studies13,30 described a positive viral culture on samples with SARS-CoV-2 RNA on RT-PCR, suggesting that most samples did not contain enough infectious virus. Most sampling methods affect viral infectivity, which may partly explain these results.36 Future studies should consider these points for better accuracy and comparability of data.
The concentration of SARS-CoV-2 RNA in aerosols detected in isolation wards and in areas where patients were receiving ventilation was very low. However, a higher concentration of viral RNA was found in patient toilets, public areas, and in some medical staff areas. The finding of high concentrations in staff rooms (ie, meeting and dining rooms) is consistent with the possible cross-transmission of COVID-19 among HCPs during breaks. During these periods, face masks are frequently removed in small areas without ventilation. Toilets and staff rooms are often small and poorly ventilated. The presence of SARS-CoV-2 RNA in stool samples has been described in several studies.37,38 Toilet flushing may lead to the aerosolization of RNA in small and nonventilated toilets or bathrooms. In an epidemic setting, public areas are often crowded, with both a high patient flow and high incidence of COVID-19. These factors have to be considered to control the transmission of COVID-19 between nonmasked HCPs in hospitals, especially staff rooms and lockers.
Only 3 studies9,13,16 assessed the size of particles found when searching for SARS-CoV-2. Regarding aerosols of submicrometer size that were observed in PPE removal and patient rooms, the authors of those studies hypothesized the resuspension of virus-laden aerosols from the surfaces of PPE worn by medical staff. The submicrometer virus–laden aerosols may originally come from direct deposition of respiratory droplets or airborne SARS-CoV-2 from a patient to the PPE. On the other hand, floor-deposited SARS-CoV-2 could be the source of virus-laden aerosols greater than 4 μm that were then carried across different areas by medical staff.
The findings of this scoping systematic review are consistent with the accumulated knowledge on other respiratory viruses. SARS-CoV-1 is commonly recognized to be mainly transmitted through large droplets, requiring particular conditions to be airborne transmitted, such as AGPs.39,40 For other respiratory viruses, a 2019 review described the frequent presence of nucleic material (RNA or DNA) in the air around patients with influenza, respiratory syncytial virus, adenovirus, rhinovirus, and other coronaviruses but rarely the presence of viable viruses.41 The current available evidence on hospital air contamination by SARS-CoV-2 leans toward the effectiveness of surgical face masks in most circumstances to prevent cross-transmission of COVID-19 in hospital settings.42 In contrast, AGPs on the respiratory tract require wearing a respirator (N95 or FFP2) to prevent transmission and protect HCPs.5,43 However, the validation of these hypothesis regarding the transmission mode of COVID-19 and the associated efficacy of PPE requires more robust studies. A randomized clinical trial comparing the surgical face mask with respirator may provide important information for recommendations regarding respiratory protection for HCPs in settings in addition to AGPs. Assessing SARS-CoV-2 RNA and viable virus contamination of surgical face masks and respirators worn by HCP according to a panel of procedures with patients with COVID-19 would provide information on exposure in routine practice.
Limitations
This study has limitations. First, the context (ie, location, ventilation, distance, and clinical context) were infrequently detailed in studies. Misclassification may have occurred when variables were categorized without enough detail. Moreover, the sampling and microbiology methods were highly heterogeneous across studies. As explained earlier, these flaws potentially affected the comparability of data and the reliability of pooled data analysis. This issue was avoided by performing categorization only when data were available. Second, for a better clarity of analysis, we did not include surface contamination. However, air and surface contamination are potentially correlated and may ease the understanding of resuspension. Third, we included articles not validated by a peer review process.
Conclusions
In this study, the air around patients hospitalized with COVID-19 was frequently contaminated with SARS-CoV-2 RNA but rarely with viable viruses. The available data suggest that COVID-19 requires particular conditions to be transmitted through the air (such as AGPs), leaning toward the effectiveness of surgical face masks in most circumstances. High viral loads found in toilets and/or bathrooms, staff areas, and public hallways argue for a careful consideration of these areas for the prevention of COVID-19 transmission. However, the presence of viable viruses should be primarily considered, given that it is a required link for the potential of cross-transmission.
eAppendix. Supplementary Methods
eTable. Evaluation of the Quality of Included Studies
References
- 1.World Health Organisation Transmission of SARS-CoV-2: implications for infection prevention precautions. Published online July 9, 2020. Accessed November 23, 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- 2.Birgand G, Mutters NT, Otter JA, et al. . Analysis of national and international guidelines on respiratory protection equipment for COVID-19 in healthcare settings. medRxiv. Preprint published online April 29, 2020. doi: 10.1101/2020.04.23.20077230 [DOI]
- 3.Ong SWX, Tan YK, Chia PY, et al. . Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610-1612. doi: 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. doi: 10.1001/jama.2020.4756 [DOI] [PubMed] [Google Scholar]
- 5.Seto WH. Airborne transmission and precautions: facts and myths. J Hosp Infect. 2015;89(4):225-228. doi: 10.1016/j.jhin.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morawska L, Tang JW, Bahnfleth W, et al. . How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chagla Z, Hota S, Khan S, Mertz D; International Hospital and Community Epidemiology Group . Airborne transmission of COVID-19. Clin Infect Dis. 2020;ciaa1118. doi: 10.1093/cid/ciaa1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faridi S, Niazi S, Sadeghi K, et al. . A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Ning Z, Chen Y, et al. . Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557-560. doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 10.Li YH, Fan YZ, Jiang L, Wang HB. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol Infect. 2020;148:e154. doi: 10.1017/S0950268820001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Z-D, Wang Z-Y, Zhang S-F, et al. . Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1583-1591. doi: 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santarpia JL, Rivera DN, Herrera V, et al. . Aerosol and surface transmission potential of SARS-CoV-2. medRxiv. Preprint published online June 3, 2020. doi: 10.1101/2020.03.23.20039446 [DOI] [Google Scholar]
- 13.Santarpia JL, Herrera VL, Rivera DN, et al. . The infectious nature of patient-generated SARS-CoV-2 aerosol. medRxiv. Preprint published online July 21, 2020. doi: 10.1101/2020.07.13.20041632 [DOI] [Google Scholar]
- 14.Cheng VC-C, Wong S-C, Chan VW-M, et al. . Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2020;41(11):1258-1265. doi: 10.1017/ice.2020.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei L, Lin J, Duan X, et al. . Asymptomatic COVID-19 patients can contaminate their surroundings: an environment sampling study. mSphere. 2020;5(3):e00442-20. doi: 10.1128/mSphere.00442-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chia PY, Coleman KK, Tan YK, et al. . Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications. 2020;11:2800. doi: 10.1038/s41467-020-16670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Otter JA, Price JR, et al. . Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis. 2020;ciaa905. doi: 10.1093/cid/ciaa905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Z, Qian H, Xu B, et al. . Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. medRxiv. Preprint published online April 7, 2020. doi: 10.1101/2020.04.03.20052175 [DOI] [Google Scholar]
- 19.Zhou L, Yao M, Zhang X, et al. . Detection of SARS-CoV-2 in exhaled breath from COVID-19 patients ready for hospital discharge. medRxiv. Preprint published online June 2, 2020. doi: 10.1101/2020.05.31.20115196 [DOI]
- 20.Wu S, Wang Y, Jin X, Tian J, Liu J, Mao Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am J Infect Control. 2020;48(8):910-914. doi: 10.1016/j.ajic.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng VCC, Wong S-C, Chen JHK, et al. . Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41(5):493-498. doi: 10.1017/ice.2020.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei H, Ye F, Liu X, et al. . SARS-CoV-2 environmental contamination associated with persistently infected COVID-19 patients. Influenza Other Respir Viruses. 2020;14(6):688-699. doi: 10.1111/irv.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razzini K, Castrica M, Menchetti L, et al. . SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim UJ, Lee SY, Lee JY, et al. . Air and environmental contamination caused by COVID-19 patients: a multi-center study. J Korean Med Sci. 2020;35(37):e332. doi: 10.3346/jkms.2020.35.e332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan L, Ma B, Lai X, et al. . Air and surface contamination by SARS-CoV-2 virus in a tertiary hospital in Wuhan, China. Int J Infect Dis. 2020;99:3-7. doi: 10.1016/j.ijid.2020.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder RA, Alarja NA, Robie ER, et al. . Environmental and aerosolized severe acute respiratory syndrome coronavirus 2 among hospitalized coronavirus disease 2019 patients. J Infect Dis. 2020;222(11):1798-1806. doi: 10.1093/infdis/jiaa575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn JY, An S, Sohn Y, et al. . Environmental contamination in the isolation rooms of COVID-19 patients with severe pneumonia requiring mechanical ventilation or high-flow oxygen therapy. J Hosp Infect. 2020;106(3):570-576. doi: 10.1016/j.jhin.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenarkoohi A, Noorimotlagh Z, Falahi S, et al. . Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin T, Li J, Yang J, et al. . SARS-CoV-2 presented in the air of an intensive care unit (ICU). Sustain Cities Soc. 2020;102446. doi: 10.1016/j.scs.2020.102446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lednicky JA, Lauzardo M, Fan ZH, et al. . Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476-482. doi: 10.1016/j.ijid.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui RN, Wong SH, Sánchez-Luna SA, et al. . Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35(5):749-759. doi: 10.1111/jgh.15053 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Yan L-M, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656-657. doi: 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469. doi: 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 34.Tang S, Mao Y, Jones RM, et al. . Aerosol transmission of SARS-CoV-2? evidence, prevention and control. Environ Int. 2020;144:106039. doi: 10.1016/j.envint.2020.106039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singanayagam A, Patel M, Charlett A, et al. . Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32). doi: 10.2807/1560-7917.ES.2020.25.32.2001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verreault D, Moineau S, Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev. 2008;72(3):413-444. doi: 10.1128/MMBR.00002-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Xu Y, Gao R, et al. . Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843-1844. doi: 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Guo C, Tang L, et al. . Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434-435. doi: 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy CJ, Milton DK. Airborne transmission of communicable infection—the elusive pathway. N Engl J Med. 2004;350(17):1710-1712. doi: 10.1056/NEJMp048051 [DOI] [PubMed] [Google Scholar]
- 40.Booth TF, Kournikakis B, Bastien N, et al. . Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191(9):1472-1477. doi: 10.1086/429634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32(4):372-379. doi: 10.1097/QCO.0000000000000563 [DOI] [PubMed] [Google Scholar]
- 42.Temkin E; Healthcare Worker COVID-19 Surveillance Working Group . Extremely low prevalence of asymptomatic COVID-19 among healthcare workers caring for COVID-19 patients in Israeli hospitals: a cross-sectional study. Clin Microbiol Infect. 2020;S1198-743X(20)30593-0. doi: 10.1016/j.cmi.2020.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seto WH, Tsang D, Yung RWH, et al. ; Advisors of Expert SARS group of Hospital Authority . Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520. doi: 10.1016/S0140-6736(03)13168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplementary Methods
eTable. Evaluation of the Quality of Included Studies