Abstract
The recurrent emergence of novel, pathogenic coronaviruses (CoVs) severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1; 2002), Middle East respiratory syndrome (MERS)-CoV (2012), and most recently SARS-CoV-2 (2019) has highlighted the need for physiologically informative airway epithelial cell infection models for studying immunity to CoVs and development of antiviral therapies. To address this, we developed an in vitro infection model for two human coronaviruses; alphacoronavirus 229E-CoV (229E) and betacoronavirus OC43-CoV (OC43) in differentiated primary human bronchial epithelial cells (pBECs). Primary BECs from healthy subjects were grown at air-liquid interface (ALI) and infected with 229E or OC43, and replication kinetics and time-course expression of innate immune mediators were assessed. OC43 and 229E-CoVs replicated in differentiated pBECs but displayed distinct replication kinetics: 229E replicated rapidly with viral load peaking at 24 h postinfection, while OC43 replication was slower peaking at 96 h after infection. This was associated with diverse antiviral response profiles defined by increased expression of type I/III interferons and interferon-stimulated genes (ISGs) by 229E compared with no innate immune activation with OC43 infection. Understanding the host-virus interaction for previously established coronaviruses will give insight into pathogenic mechanisms underpinning SARS-CoV-2-induced respiratory disease and other future coronaviruses that may arise from zoonotic sources.
Keywords: coronavirus, epithelial cell, infection, 229E, OC43
INTRODUCTION
In addition to recently emerged zoonotic coronaviruses severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1; 2002), Middle East respiratory syndrome (MERS)-CoV (2012), and SARS-CoV-2 (2019), there are four known endemic human CoVs: NL63, HKU1, OC43-CoV (OC43), and 229E-CoV (229E). 229E and OC43 were identified during the 1960s (19) and since then, they have been implicated in causing mild respiratory disease and, after rhinoviruses, are a leading cause of common colds (10–30%) (14, 23, 26). NL63-CoV (NL63) (27) and HKU1-CoV (HKU1) (32) were later discovered when the SARS-CoV pandemic generated intensified screening for CoVs. CoVs are further divided into four genera based on phylogeny: alpha, beta, gamma, and delta. 229E and NL63 belong to the alpha genera while OC43, HKU1, SARS-CoV-1, SARS-CoV-2, and MERS-CoV are betacoronaviruses (5, 6, 17, 28).
Little is known about replication and innate immune responses to human CoVs in airway epithelial cells. In vitro studies utilizing bronchial epithelial cell (BEC) cultures differentiated at the air-liquid interface (ALI) have reported that 229E host receptor aminopeptidase N is found predominately on nonciliated cells (29). Although the functional cellular receptor for OC43 remains unknown, the OC43 S protein has been shown to use the sugar 5-N-acetyl-9-O-acetylneuraminic acid (Neu5, 9Ac2) as a receptor (15, 21, 22). OC43 has been shown to infect ciliated cells via Neu5, 9Ac2 expression on this cell type (29).
BECs express germ-line encoded pathogen-recognition receptors (PRRs) (24), which upon recognition of a pathogen-derived molecule (viral RNA), leads to the expression of interferons (IFNs), interferon gamma-induced protein (CXCL10) (30), and proinflammatory cytokines such as interleukin-6 (IL-6) (25). Type I interferons (IFN-α/β) and type III IFNs (IFN-λ1, -λ2, -λ3) are particularly important in the innate antiviral response with type III IFNs specifically involved in epithelial innate immune protection to respiratory viruses (9) that involves expression of interferon-stimulated genes (ISGs) such as viperin, 2′,5′-oligoadenylate synthetase (OAS), and protein kinase R (PKR) (7).
In the current study, we developed two human CoV infection models utilizing human primary BECs (pBECs) differentiated at the ALI to investigate the replication kinetics and epithelial innate immune response to OC43- and 229E-CoVs. This study showed that these CoVs have distinct replication kinetics and induce different innate antiviral cytokine profiles. This study supports the use of this in vitro model to investigate interventions targeting viral replication or host antiviral immunity as well as providing insights into mechanisms of coronavirus-induced disease.
MATERIALS AND METHODS
Ethics statements, donor recruitment, and pBEC collection.
pBECs were provided by P. A. B. Wark (The University of Newcastle), obtained from healthy nonsmoking donors during bronchoscopy, with written informed consent. All experiments were conducted with approval from the University of Newcastle Safety Committee (Safety REF# 25/2016 and R5/2017). All subjects underwent fiber-optic bronchoscopy in accordance with standard guidelines (16). pBECs were obtained using a single sheathed nylon cytology brush applied under direct vision. Approximately 4–8 brushings were taken from second to third generation bronchi.
Air-liquid interface culture of pBECs.
pBECs obtained from healthy donors were grown until confluent and differentiated at air liquid-interface (ALI), as previously described (11–13). Briefly, cells were grown in complete BEGM (Lonza) with growth factor supplements in submerged monolayer culture and then seeded at 2 × 105 cells in transwells (cat. no. 3460, Corning) in a 12-well plate with ALI-initial media comprised of 50% BEBM-50% DMEM containing 0.1% hydrocortisone, 0.1% bovine insulin, 0.1% epinephrine, 0.1% transferrin, 0.4% bovine pituitary extract (all from Lonza singlequots, cat. no. CC-3171), and ethanolamine (final concentration 80 μM), MgCl2 (final concentration 0.3 mM), MgSO4 (final concentration 0.4 mM), bovine serum albumin (final concentration 0.5 mg/mL), amphotericin B (final concentration 250 μg/mL), all-trans retinoic acid (30 ng/mL) and 2% penicillin-streptomycin with 10 ng/mL recombinant human epithelial growth factor (rhEGF) until confluent (at least 3 days in both apical and basal compartments). Once confluent, rhEGF concentration was changed to 0.5 ng/ml during the ALI phase for differentiation in the basal compartment (beneath the transwell insert) without apical media. The quality of cultures was confirmed by the presence of ciliated epithelium, mucus-producing cells, pseudostratified structure, and assessment of trans-epithelial electrical resistance. Trans-epithelial electrical resistance (TEER) was measured weekly using an Epithelial Volt/Ohm (TEER) Meter (World Precision Instruments).
Coronavirus propagation.
For OC43 (ATCC number VR-1558) and 229E (ATCC number VR-740), OC43 passage history is unknown, while passage history of 229E shows two passages. American Type Culture Collection (ATCC) indicates that OC43 was propagated on HCT-8 cells and 229E on MRC-5 cells. The ATCC-supplied OC43 titer was 2.8 × 105 median tissue culture infectious dose per milliliter (TCID50/mL) and 229E titer was 2.8 × 104 TCID50/mL. Upon receiving the ATCC stocks both viruses were passaged three times in MRC-5 cells to generate working stocks: OC43 titer was 2 × 108 TCID50/mL and 229E titer was 2 × 106 TCID50/mL and used for studies according to WHO guidelines. MRC-5s were utilized for TCID50 assays using the Karber method.
Coronavirus infection.
Infection time-courses for each donor were conducted independently with one well per treatment using the same stocks of OC43 and 229E for all experiments. pBECs were starved basally with BEBM Minimal media ((Lonza; BEBM + 1% ITS, 0.5% linoleic acid, 2% penicillin-streptomycin and 1% fungizone) for 24 h before infection. ALI cultures were apically infected with OC43 [multiplicity of infection (MOI) 0.1], 229E (MOI 0.1) or mock-infected with starvation media controls for two hours at 33°C (OC43) or 35°C (229E). Transwells were washed with PBS to remove unbound virus and starvation media was apically applied on all wells. pBECs were harvested at 0, 24, 96, or 168 h postinfection. During the time course, basal media was replenished at 48 and 96 h.
Sample collection and analysis from ALI cultures.
Apical media was removed and stored at −80°C for viral infectivity and downstream protein analyses. Cytometric bead array (BD Bioscience) was performed to quantify CXCL10 and IL-6 on a FACS Canto II cytometer (BD BioSciences). Data were analyzed using FCAP Array software. IFN-β (PBL Assay Science) and IL-29/IL-28B (IFN-λ1/3) (R&D Systems) were measured by ELISA using the FLUOstar OPTIMA Multi-Detection Plate Reader (BMG Labtech).
Transwells were collected in RLT buffer (Qiagen) containing 1% 2-mercaptoethanol (2ME) for molecular analyses and in RIPA buffer containing protease inhibitor cocktail (Roche) for protein analyses. RNA extraction was conducted according to manufacturer instructions (Qiagen, RNEasy Mini Kit). Purified RNA was quantitated by spectrophotometry (Nanodrop) and cDNA synthesized using the High Capacity cDNA Reverse Transcription Kit (ABI). For OC43 and 229E nucleocapsid gene PCR detection, standards were produced for quantitative PCR using plasmids. All qPCR analysis was conducted using the ABI 7500 Real Time PCR System. Targets included 18S, IFN-β (Applied Biosystems, Thermofisher Scientific), OC43 (Geneworks), and 229E (Geneworks) N genes (Table 1). All targets were normalized to 18S.
Table 1.
Primers
| Target Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| N-229E | 5′-CAGTCAAATGGGCTGATGCA | 5′-AAAGGGCTATAAAGAGAATAAGGTATTCT | 5′-FAM-CCCTGACGACCACGTTGTGGTTCA-TAMRA |
| N-OC43 | 5′-CGATGAGGCTATTCCGACTAGGT | 5′-CCTTCCTGAGCCTTCAATATAGTAACC | 5′-FAM-TCCGCCTGGCACGGTACTCCCT-TAMRA |
| 18S | 5′-CGCCGCTAGAGGTGAAATTCT | 5′-CATTCTTGGCAAATGCTTTCG | 5′-FAM-ACCGGCGCAAGACGGACCAGA-TAMRA |
| Viperin | CACAAAGAAGTGTCCTGCTTGGT | AAGCGCATATATTCATCCAGAATAAG | FAM-CCTGAATCTAACCAGAAGATGAAAGACTCC-TAMRA |
| OAS | CTGACGCTGACCTGGTTGTCT | CCCCGGCGATTTAACTGAT | FAM-CCTCAGTCCTCTCACCACTTTTCA-TAMRA |
| PKR | AAGGGAACTTTGCGATACATGAG | GCGTAGAGGTCCACTTCCTTTC | FAM-CCAGAACAGATTTCTTCGCAAGACTAT-TAMRA |
Statistical analysis.
All data were analyzed using GraphPad Prism version 8.2.1 software. Log transformed viral load data was compared by two-way ANOVA with Sidak’s multiple comparisons test. Transformed data fitted to a straight line in a QQ plot confirming normal distributions for comparison. Time-course cell mRNA and protein data were compared by two-way ANOVA with Dunnett’s multiple comparisons test. Single time point data were analyzed using nonparametric Friedman test. A P value <0.05 was considered statistically significant.
RESULTS
Donor characteristics.
Differentiated pBECs from healthy donors (n = 8) were grown at the air-liquid interface (ALI) and infected with OC43-CoV or 229E-CoV at a MOI of 0.1. Cells and supernatants were collected at 0, 24, 96, and 168 h after infection. One patient had 0 and 24 h time points only. Patient details are listed in Table 2.
Table 2.
Healthy donor characteristics
| Donor |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Median | IQR | |
| Age, yr | 62 | 78 | 62 | 48 | 74 | 47 | 76 | 53 | 62 | 23.25 |
| Sex | F | F | F | M | M | M | F | F | ||
IQR, interquartile range; F, female; M, male.
Viral replication kinetics of OC43-CoV and 229E-CoV.
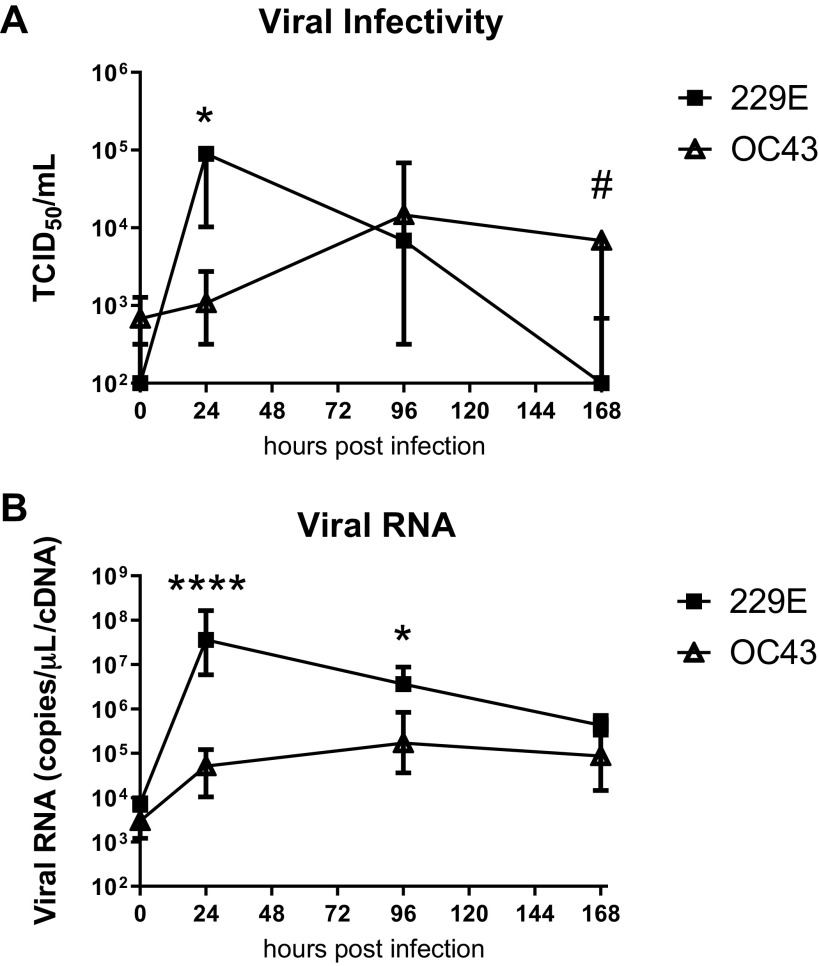
Apical supernatants were measured for 229E and OC43 viral infectivity using the TCID50 assay in MRC-5 cells. Figure 1A shows the viral infectivity for OC43 and 229E over a 7 day (168 h) time course. 229E viral infectivity peaked at 24 h postinfection and was significantly (1,000 fold) greater than OC43 viral load at that time point. 229E virus production decreased after 24 h postinfection and was undetectable by 168 h postinfection. OC43 viral titers increased more slowly than 229E, peaking at 96 h postinfection with sustained production of infectious virus at 168 h postinfection that was significantly higher than 229E at this time. Figure 1B shows the viral RNA expression over the time course. Early (24 h postinfection) expression reflected the viral infectivity results, with 229E RNA synthesis peaking at 24 h, significantly (∼1,000 fold) higher than OC43. OC43 RNA peaked at 96 h with levels remaining significantly lower than 229E. At 168 h postinfection 229E RNA production had reduced and was not significantly different from OC43 by this time.
Fig. 1.
Distinct replication kinetics of OC43 and 229E in differentiated bronchial epithelial cells from healthy donors. Infectivity of released virus collected at apical surface (A) and viral RNA expression (B) at 0, 24, 96, and 168 h postinfection. n = 8 healthy donors, median ± interquartile range (A and B). Log transformed data analyzed by 2-way ANOVA with Sidak’s multiple comparisons test (A and B). *P < 0.05, ****P < 0.0001 increased in 229E vs. OC43, #P < 0.05 increased OC43 vs. 229E.
Innate immune activation during 229E and OC43 infection.
229E infection induced significantly increased IFN-β protein expression at 96 h (∼10-fold mock-infected cells) with levels decreasing by 168 h postinfection but remaining significantly elevated above mock-infected cells. In contrast, OC43 failed to induce IFN-β expression at any time (Fig. 2A). 229E infection also induced type III IFN-λ protein expression –∼1,000-fold over mock-infected cells by 96 h and 168 h postinfection (Fig. 2B). No induction of IFN-λ was detected in OC43-infected BECs. Coinciding with peak expression of IFN-β, expression of ISGs viperin, OAS and PKR were also significantly increased at 96 h postinfection by 229E compared with mock-infected cells. OC43 infection did not induce ISG expression (Fig. 2C).
Fig. 2.
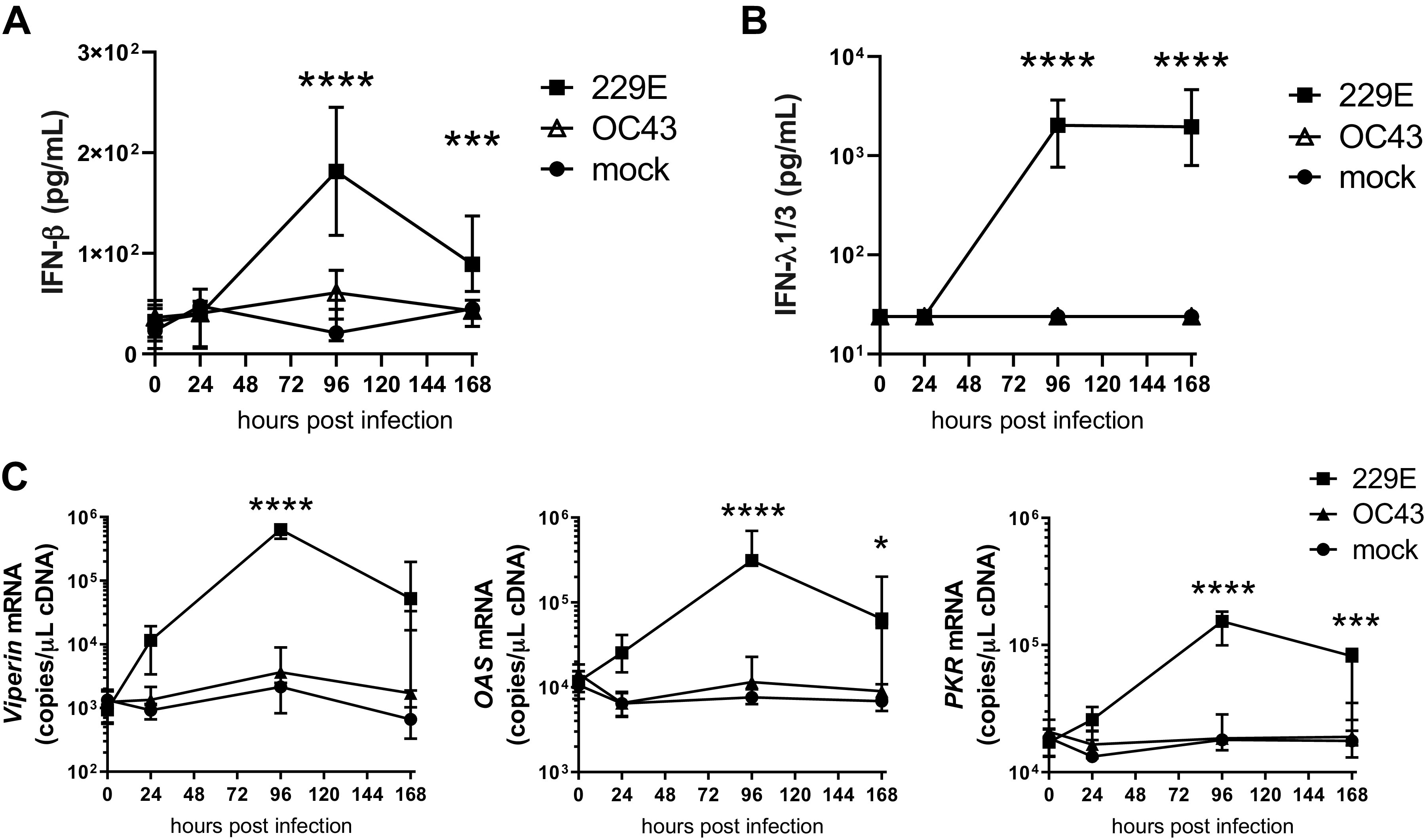
OC43 and 229E coronaviruses induce different type I and III IFN response profiles in primary bronchial epithelial cells. IFN-β protein expression in apical supernatants (A), IFN-λ1/3 protein expression in apical supernatants during 229E- and OC43-coronavirus (CoV) infection at 0, 24, 96, and 168 h postinfection (B), and viperin, 2′,5′-oligoadenylate synthetase (OAS), and protein kinase R (PKR) mRNA expression in whole cell lysates during 229E- and OC43-CoV infection at 0, 24, 96, and 168 h postinfection (C). n = 8 healthy donors, median with interquartile range (A–C). Data analyzed by 2-way ANOVA with Dunnett’s multiple comparison test (A–C). *P < 0.05, ***P < 0.001, ****P < 0.0001 229E compared with mock infected cells.
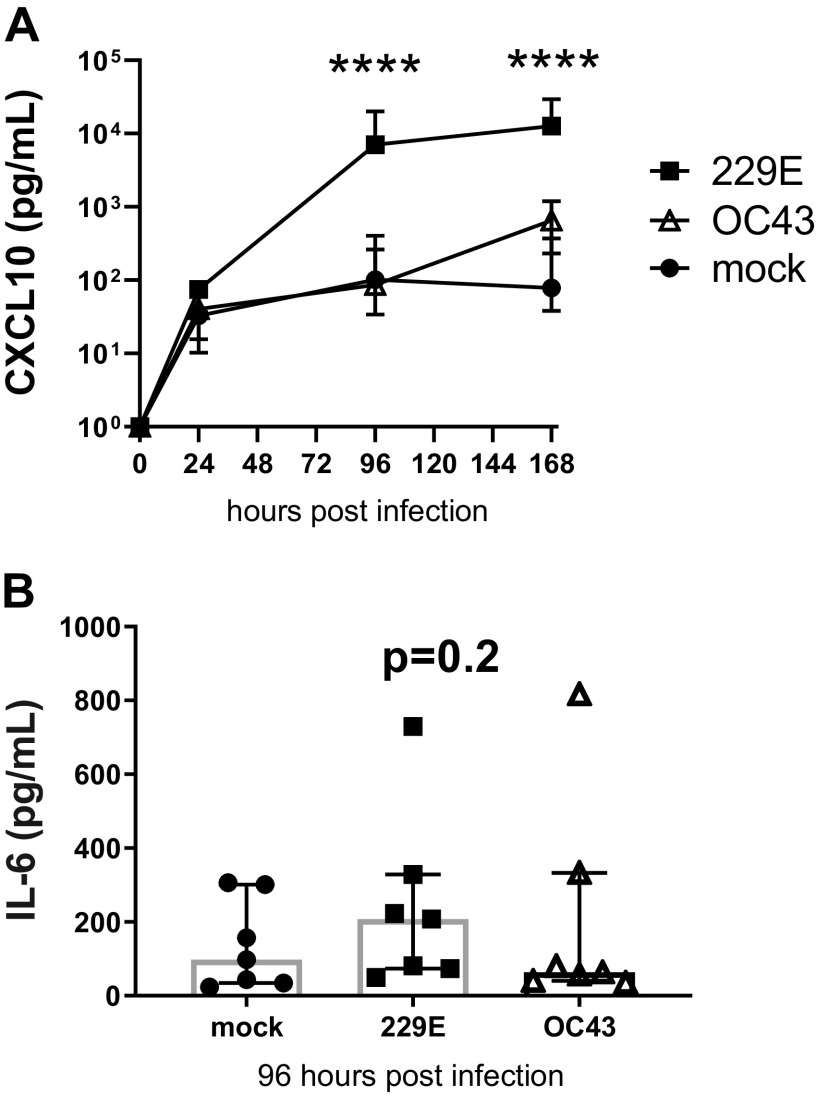
Consistent with type I/III IFNs, 229E infection induced CXCL10 protein expression that was significantly increased (100-fold) over mock-infected cells by 96 to 168 h post infection (Fig. 3A). CXCL10 was also detected in OC43 infection, but this was ∼10-fold less than the magnitude of 229E induction and was not significantly higher than mock-infected cells. Apical supernatants were analyzed for the proinflammatory cytokine interleukin-6 (IL-6) (Fig. 3B). The induction of IL-6 protein during both the OC43 and 229E infection time course was minimal and comparable with mock-infected controls, however assessment at 96 h postinfection indicated a trend (P = 0.2) for induction by 229E.
Fig. 3.
OC43 and 229E coronaviruses induce distinct IFN-associated and inflammatory cytokine profiles in primary bronchial epithelial cells. Apical supernatants were analyzed by cytometric bead array for CXCL10 (A) and IL-6 protein expression (96 h) (B) in differentiated healthy epithelial cultures during 229E- and OC43-coronavirus (CoV) infection. n = 8 healthy donors, median with interquartile range (A and B). Data analyzed by 2-way ANOVA with Dunnett’s multiple comparison test. ****P < 0.0001 compared with mock-infected cells (A). Data analyzed by Friedman test (B).
DISCUSSION
229E and OC43 display distinct replication kinetics.
229E and OC43-CoV infection in differentiated pBECs displayed distinct replication kinetics. OC43 replication steadily increased, with viral infectivity peaking at 96 h postinfection. In contrast, 229E replicated rapidly, peaking at 24 h after which the amount of infectious virus steadily declined reaching the limit of detection by 168 h postinfection. Viral cellular RNA also reduced from this time; however, it remained detectable above (∼1 log) input virus. This persistence of viral RNA, after infectious virus is no longer detectable, has been reported previously with other viruses such as influenza (8, 18). Currently, the diagnosis of coronavirus disease 2019 (COVID-19) relies on the detection of SARS-CoV-2 RNA using PCR, and the persistence of viral RNA has been noted, particularly in the stool (33). However, there are important limitations to molecular methods. Out of 90 RT-PCR SARS-CoV-2-positive samples, only 26 (28.9%) demonstrated viral growth in Vero cells (2). Experimental models such as the one described in this study are important in defining the relationship between duration of viral infectivity and detection of viral RNA.
Pathogenic CoVs have also demonstrated distinct individual replication kinetics. Kindler et al. (20) reported that pBEC-ALI cultures were highly permissive to MERS-CoV infection with peak virus production at 48 h postinfection, while SARS-CoV-1 replicated more slowly in comparison, peaking at 72–96 h post infection (20). More recently, Chu et al. (2020) demonstrated in ex vivo human lung tissue explants that SARS-CoV-2 produced 3.2 fold higher amounts of infectious virus particles within 48 h post-infection and significantly higher viral N antigen expression in comparison to tissues infected with SARS-CoV-1 (4). These findings might explain the high viral load in the respiratory secretions of patients with COVID-19 with mild symptoms or even during the pre-symptom incubation period (34), contributing to its transmissibility and pathogenicity.
229E infection induces higher IFN expression compared with OC43.
A key finding of this study is that 229E infection induced significantly greater expression of IFN-β and IFN-λ1/3 and ISGs compared with OC43. In contrast, there was variable and constitutive expression of IL-6 observed during the time course. IL-6 expression at 96 h postinfection indicated a trend for induction by 229E. This was not apparent for OC43 and presents further evidence of an impaired innate response to this virus, despite its robust replication. Our interpretation of lack of innate immune response to OC43 relates to level of RNA synthesis and threshold for innate immune activation: 229E replicated rapidly to maximize production of infectious virus. High level viral replication demands high level RNA synthesis—the cost of this is activation of innate immunity which limits duration of viral replication. In contrast, OC43 replication built up slowly—this required far less RNA synthesis at any given time which did not trigger innate immunity. This allowed OC43 to replicate productively for an extended time.
Replication without induction of an immune response has also been reported for pathogenic CoVs. This has significant implications for asymptomatic transmission, as immune response and airway inflammation is a primary driver of symptoms. Reduced innate immune response (particularly in the upper respiratory tract) and lack of associated symptoms during the initial phase of infection coupled with high viral shedding differentiates SARS-CoV-2 from SARS-CoV-1 and is almost certainly why COVID-19 has become a pandemic disease (10, 31). SARS-CoV-2 infection induced lower levels of IFNs than SARS-CoV-1 despite significantly higher viral loads in ex vivo cultures of lung biopsies (4). Another study reported that SARS-CoV-2 did not induce IFN expression at all but, in contrast to OC43, elicited a strong chemotactic and inflammatory response (including CCL20, CXCL1, IL-1B, IL-6) in human BECs (1). This lowered antiviral and heightened proinflammatory state is proposed to contribute to COVID-19. This points to OC43 as a useful virus to study treatments that aim to restore an antiviral response to SARS-CoV-2.
This study also highlights the potential for differentiated pBECs to be utilized as a platform for CoV antiviral drug development. Recent studies have focused on cell lines to investigate efficacy of drugs against SARS-CoV-2 replication in vitro (3). Monolayer cultures consist only of basal cells while epithelial cells differentiated at air-liquid interface consist of stratified layers, with tight junctions, ciliated cells, and mucin production. This is particularly important to study OC43 and other CoVs that demonstrate cellular tropism for ciliated cells (29). Additionally, an in vitro infection model may sustain viral replication for many days, with replication kinetics and innate immune activation more representative of that in tissues ex vivo and in vivo. This is particularly important considering that IFN-β, IFN-λ1/3, ISGs, and CXCL10 responses were only detectable towards the latter part of the time course in this study (96–168 h postinfection). Current antiviral drug trials could be transitioned easily from submerged cell lines into differentiated pBECs for high-throughput screening in CoV infection models to expedite identification of efficacious drugs to progress to in vivo testing.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., P.A.B.W., and N.W.B. conceived and designed research; S.L., C.E., and K.S.N. performed experiments; S.L. analyzed data; S.L. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; S.L., P.A.B.W., and N.W.B. edited and revised manuscript; S.L., P.A.B.W., A.C.-Y.H., and N.W.B. approved final version of manuscript.
REFERENCES
- 1.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181: 1036–1045.e9, 2020. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, Boodman C, Bello A, Hedley A, Schiffman Z, Doan K, Bastien N, Li Y, Van Caeseele PG, Poliquin G. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. In press. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178: 104786, 2020. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, Shuai H, Yang D, Hu B, Huang X, Zhang X, Cai JP, Zhou J, Yuan S, Kok KH, To KK, Chan IH, Zhang AJ, Sit KY, Au WK, Yuen KY. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis 71: 1400–1409, 2020. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman VM, Baldwin HJ, Tateno AF, Zerbinati RM, Annan A, Owusu M, Nkrumah EE, Maganga GD, Oppong S, Adu-Sarkodie Y, Vallo P, da Silva Filho LV, Leroy EM, Thiel V, van der Hoek L, Poon LL, Tschapka M, Drosten C, Drexler JF. Evidence for an ancestral association of human coronavirus 229e with bats. J Virol 89: 11858–11870, 2015. doi: 10.1128/JVI.01755-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corman VM, Eckerle I, Memish ZA, Liljander AM, Dijkman R, Jonsdottir H, Juma Ngeiywa KJ, Kamau E, Younan M, Al Masri M, Assiri A, Gluecks I, Musa BE, Meyer B, Müller MA, Hilali M, Bornstein S, Wernery U, Thiel V, Jores J, Drexler JF, Drosten C. Link of a ubiquitous human coronavirus to dromedary camels. Proc Natl Acad Sci USA 113: 9864–9869, 2016. doi: 10.1073/pnas.1604472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosse KM, Monson EA, Beard MR, Helbig KJ. Interferon-stimulated genes as enhancers of antiviral innate immune signaling. J Innate Immun 10: 85–93, 2018. doi: 10.1159/000484258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol 41: 4160–4165, 2003. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 46: 875–890.e6, 2017. doi: 10.1016/j.immuni.2017.04.025.28514692 [DOI] [PubMed] [Google Scholar]
- 10.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the achilles’ heel of current strategies to control Covid-19. N Engl J Med 382: 2158–2160, 2020. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells 26: 2576–2585, 2008. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, Van Eeden S, Bai TR, Dorscheid DR, Knight DA. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol 45: 1090–1100, 2011. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 13.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, Knight DA. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 180: 122–133, 2009. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 14.Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med 121: 190–193, 1966. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 15.Hulswit RJG, Lang Y, Bakkers MJG, Li W, Li Z, Schouten A, Ophorst B, van Kuppeveld FJM, Boons GJ, Bosch BJ, Huizinga EG, de Groot RJ. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci USA 116: 2681–2690, 2019. doi: 10.1073/pnas.1809667116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurd SZ. Workshop summary and guidelines: investigative use of bronchoscopy, lavage, and bronchial biopsies in asthma and other airway diseases. J Allergy Clin Immunol 88: 808–814, 1991. doi: 10.1016/0091-6749(91)90189-U. [DOI] [PubMed] [Google Scholar]
- 17.Huynh J, Li S, Yount B, Smith A, Sturges L, Olsen JC, Nagel J, Johnson JB, Agnihothram S, Gates JE, Frieman MB, Baric RS, Donaldson EF. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol 86: 12816–12825, 2012. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip DKM, Lau LLH, Chan KH, Fang VJ, Leung GM, Peiris MJS, Cowling BJ. The dynamic relationship between clinical symptomatology and viral shedding in naturally acquired seasonal and pandemic influenza virus infections. Clin Infect Dis 62: 431–437, 2016. doi: 10.1093/cid/civ909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J 24, Suppl: S223–S227, 2005. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 20.Kindler E, Jónsdóttir HR, Muth D, Hamming OJ, Hartmann R, Rodriguez R, Geffers R, Fouchier RA, Drosten C, Müller MA, Dijkman R, Thiel V. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio 4: e00611–e00612, 2013. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krempl C, Schultze B, Herrler G. Analysis of cellular receptors for human coronavirus OC43. Adv Exp Med Biol 380: 371–374, 1995. doi: 10.1007/978-1-4615-1899-0_60. [DOI] [PubMed] [Google Scholar]
- 22.Künkel F, Herrler G. Structural and functional analysis of the surface protein of human coronavirus OC43. Virology 195: 195–202, 1993. doi: 10.1006/viro.1993.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA 57: 933–940, 1967. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol 20: 4–22, 2010. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 26.van der Hoek L. Human coronaviruses: what do they cause? Antivir Ther 12: 651–658, 2007. [PubMed] [Google Scholar]
- 27.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. Identification of a new human coronavirus. Nat Med 10: 368–373, 2004. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijgen L, Keyaerts E, Lemey P, Maes P, Van Reeth K, Nauwynck H, Pensaert M, Van Ranst M. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J Virol 80: 7270–7274, 2006. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Deering C, Macke M, Shao J, Burns R, Blau DM, Holmes KV, Davidson BL, Perlman S, McCray PB Jr. Human coronavirus 229E infects polarized airway epithelia from the apical surface. J Virol 74: 9234–9239, 2000. doi: 10.1128/JVI.74.19.9234-9239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, Zummo G, Holgate ST, Attia J, Thakkinstian A, Davies DE. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol 120: 586–593, 2007. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469, 2020. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 32.Woo PCY, Lau SKP, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BHL, Poon RWS, Cai JJ, Luk WK, Poon LLM, Wong SSY, Guan Y, Peiris JSM, Yuen KY. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79: 884–895, 2005. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 5: 434–435, 2020. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382: 1177–1179, 2020. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]