Abstract
Context
Gonadotroph pituitary neuroendocrine tumors (PitNETs) can express follicle-stimulating hormone (FSH) and luteinizing hormone (LH) or be hormone negative, but they rarely secrete hormones. During tumor development, epithelial cells develop a mesenchymal phenotype. This process is characterized by decreased membranous E-cadherin and translocation of E-cadherin to the nucleus. Estrogen receptors (ERs) regulate both E-cadherin and FSH expression and secretion. Whether the hormone status of patients with gonadotroph PitNETs is regulated by epithelial-to-mesenchymal transition (EMT) and ERs is unknown.
Objectives
To study the effect of EMT on hormone expression in gonadotroph nonfunctioning (NF)-PitNETs.
Design
Molecular and clinical analyses of 105 gonadotroph PitNETs. Immunohistochemical studies and real-time quantitative polymerase chain reaction were performed for FSH, LH, E-cadherin, and ERα. Further analyses included blood samples, clinical data, and radiological images.
Setting
All patients were operated on in the same tertiary referral center.
Results
NF-PitNET with high FSH expression had decreased immunohistochemical staining for membranous E-cadherin (P < .0001) and increased staining for nuclear E-cadherin (P < .0001). Furthermore, high FSH expression was associated with increased ERα staining (P = .0002) and ERα mRNA (P = .0039). Circulating levels of plasma-FSH (P-FSH) correlated with FSH staining in gonadotroph NF-PitNET (P = .0025). Tumor size and invasiveness was not related to FSH staining, E-cadherin, or ERα. LH expression was not associated with E-cadherin or ERα.
Conclusion
In gonadotroph PitNETs, FSH staining is related to E-cadherin, ERα expression, and circulating levels of P-FSH. There was no association between FSH staining and invasiveness. The clinical significance of these findings will be investigated in ongoing prospective studies.
Keywords: pituitary adenoma, PitNET, E-cadherin, FSH
Pituitary neuroendocrine tumors (PitNETs) are among the most common intracranial tumors, with nonfunctioning (NF)-PitNETs being the most prevalent subtype (1, 2). In contrast to functioning pituitary tumors, NF-PitNETs do not hypersecrete pituitary hormones. However, immunohistochemistry shows that most NF-PitNETs produce hormones indicating their cell lineage (gonadotroph, corticotroph, thyrotroph, somatotroph, or lactotroph), and true negatives for pituitary hormones and transcription factors (null cell) are rare (3). Gonadotroph tumors are the most common subtype of NF-PitNETs and are characterized by positive staining for steroidogenic factor 1 (SF-1) and may also express follicle-stimulating hormone (FSH) and/or luteinizing hormone (LH), but are rarely functional (4). The mechanisms underlying this specter of PitNETs from hormone negative, hormone producing, to hormone hypersecreting is unknown.
PitNETs originate from specialized neuroendocrine epithelial cells. During tumor progression they may dedifferentiate to a more mesenchymal phenotype, a process called epithelial to mesenchymal transition (EMT) (5-14). EMT is characterized by altered expression and localization of E-cadherin (15, 16). In normal epithelial tissues, E-cadherin is localized on the plasma membrane where it functions as a cell to cell adhesion molecule (15). In addition, E-cadherin interacts with multiple signaling pathways (6-8) and mediates cell to cell inhibition (17). During EMT, membranous E-cadherin can be cleaved, leading to shedding of the extracellular domain and nuclear translocation of E-cadherin (5, 18-23).
Loss of membranous E-cadherin and gain of nuclear E-cadherin is associated with loss of cell differentiation, high Ki67 index, larger tumors, and increased invasiveness in PitNETs (21, 24, 25). This includes NF-PitNETs (26), somatotroph PitNETs (25, 27-31), corticotroph PitNETs (32), and also prolactinomas (33).
Gonadotroph cells in the pituitary express estrogen receptors (ERs) (34, 35), and estrogen has a direct inhibitory effect on FSH secretion from pituitary cells (36). Estrogen also inhibits FSH synthesis and secretion in pituitary cultures (37-39). Furthermore, estrogen seems to decrease E-cadherin in various tissues (40-46), including NF-PitNETs (26). We have previously found that the absence of estrogen receptor-α (ERα) (47) is a predictor of reintervention after surgery for NF-PitNETs in male patients. How this relates to the levels of FSH in the tumor tissue and the circulating counterpart has not been studied. We therefore investigated whether the degree of EMT, represented by the loss of membranous E-cadherin, is related to hormone expression in gonadotroph NF-PitNETs. Since gonadotroph tumors are mostly nonsecretory, we hypothesized that FSH expression would be higher in well-differentiated epithelial cells, and decrease as the tumors undergo EMT. This effect was expected to be attenuated by ERα expression.
Materials and Methods
We investigated 105 patients with NF-PitNETs operated at our tertiary referral center between 1998 and 2009. The majority (>90%) of operations were performed by 3 neurosurgeons. None of the patients had previously undergone surgery or radiation to the pituitary gland. Informed consent was obtained from all participants. These patients have been presented in previous publications (47-49), whereof 105 gonadotroph tumors with available tissue samples were included in this study. Of the included patients, 72 (69%) were males. Of the females, 27 were considered postmenopausal (age 53-84 years), while 6 were considered to be premenopausal (age 32-48 years). The mean age of participants was 59.9 ± 13.0 years. Plasma FSH (P-FSH) and LH (P-LH) were measured with routine laboratory assays. Mean P-FSH in the entire cohort was 6.7 ± 6.6 IU/L, in premenopausal females 7.4 ± 6.1 IU/L (normal range 1.2-21 IU/L), and in males 6.6 ± 6.6 IU/L (normal range 0.7-11.1 IU/L). Blood samples were taken preoperatively and were not timed in regard to the menstrual cycle for premenopausal women.
Immunohistochemistry
Paraffin-embedded tissue was available from all the patients. The diagnosis of PitNET was confirmed on hematoxylin and eosin stained sections. All tumors were of gonadotroph cell lineage with positive immunohistochemical staining for transcription factor SF-1. From each tumor, 2 1-mm cores from 2 different representative tumor areas were taken and used to construct tissue microarrays. The immunohistochemical staining of SF-1 (Perseus Proteomics, monoclonal, clone N1665) was performed on DAKO Autostainer Link 48, as previously described (50, 51). FSH and LH staining on samples operated until the first half of 2007 was carried out using a Ventana automatic stainer with anti-FSH (Thermo Scientific, monoclonal, Clone FSH03, Ab-3, 1:3,000) and anti-LH (Thermo Scientific, monoclonal, Clone LH01, Ab-1, 1:3,000), as reported earlier (51). Tumors operated on after the middle of 2007 were stained with anti-FSH (DAKO, monoclonal, Clone C10, Code M3504), anti-LH (DAKO, monoclonal, Clone C93, Code M3502) on a DAKO Autostainer Link 48 (47).
The expression of FSH and LH varied in intensity from weak to strong among immunolabelled cells in almost all positive tumors. Staining was considered low when less than 10% of the cells were positive independently of the staining intensity, moderate when 10% to 50% were positive, and high when >50% were positive for FSH or LH. FSH and LH expression was usually similar between the 2 tumor cores; however, when this was not the case the core with the highest percentage of positive cells was taken into consideration. Normal pituitary tissue served as control.
E-cadherin was detected using antibody against the intracellular domain (BD Transduction Laboratories, mouse monoclonal, clone 36/E-Cadherin, dilution 1:300) performed on the DAKO EnVision Flex+ system (K8012; DAKO, Glostrup, Denmark) and DAKO Autostainer as described previously (48, 51). Skin biopsy with keratinocytes was used as the positive control, while the negative control was obtained by omitting the primary antibodies. The extracellular domain of E-cadherin (Abcam ab1416, mouse monoclonal, clone HECD-1, dilution 1:100) has been investigated in earlier papers, but not considered in this study.
ERα was detected with ready-to-use monoclonal antibody SP1 (Ventana-Roche) on a Ventana Bench MarkUltra and the UltraView Universal detection kit as described earlier (47). A sample from a ductal mammary carcinoma served as the positive control. The negative control was obtained by omitting the primary antibody. All positive and negative controls gave satisfactory results.
Staining for membranous E-cadherin and ERα were quantified using an immunoreactivity score (IRS). The IRS is the product of the percentage of positively staining cells (0 = 0%; 1 = 1-10%; 2 = 10-50%; 3 = 50-80%; 4 ≥ 80%) and the staining intensity (0: no staining; 1: weak staining; 2: moderate staining; 3: strong staining), and ranges from 0 to 12. The 2 tissue cores for each tumor were assessed as 1 area in the assessment of the IRS score. In the cases with variable staining intensity within the tumor, the most prevalent intensity was used in the score. Nuclear E-cadherin was scored as either positive or negative. All immunohistochemical analyses were performed by the same pathologist (O.C.-B.), blinded to the clinical data.
Real time qPCR
Frozen tissue samples were available from 74 patients. The samples were collected during operation, frozen immediately after resection, and stored at −80°C until assayed. mRNA was extracted and quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed as described previously (52). The following primer pairs were used for PCR analyses: E-cadherin forward primer (FP) 5′-CATTGCCACATACACTCTCTTCT-3′and reverse primer (RP) 5′-CGGTTACCGTGATCAAAATCTC-3′; ERα FP 5′-GGGAAGTATGGCTATGGAATCTG-3′ and RP 5′-TGGCTGGACACATATAGTCGTT-3′; ERβ FP 5′- TTCAAAGAGGGATGCTCACTTC-3′ and RP 5′- CCTTCACACGACCAGACTCC-3. Primers for FSH and LH RT-qPCR results were standardized against the geometric mean of the reference genes GADPH and ALAS1, validated in a previous study (52).
Magnetic resonance imaging
Preoperative magnetic resonance (MR) images scans were available for 47 patients and used to evaluate tumor volume and invasiveness. Tumor volume was calculated as described previously (49). Invasiveness was defined as Knosp score of 3 or higher on either side on preoperative MR images (53). Magnetic resonance imaging (MRI) analyses were performed by 2 investigators (K.A.B.Ø. and G.R.).
Statistics
Comparisons between groups were performed with the Mann–Whitney U-test and the chi-square test. Spearman’s rank correlation was used for correlation analyses. A P < .05 was considered significant. Analyses were performed using Stata 16.0 for Windows (StataCorp LLC, College Station, TX).
Results
FSH staining
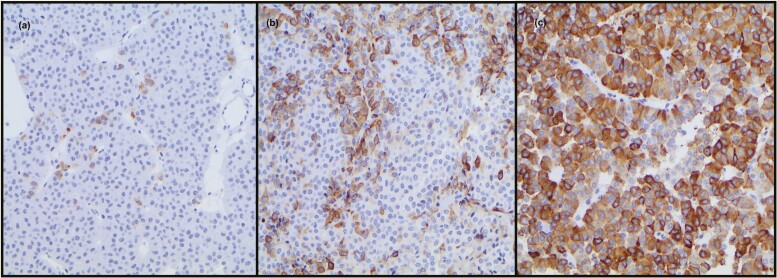
FSH staining was absent or low in tissue from 52 (49.5%) patients (27 showed no hormone staining), moderate in 22 (21%) patients, and high in 31 (29.5%) patients (Fig. 1). There was a strong correlation between FSH staining and mRNA gene expression levels (R = 0.69, R < 0.0001). FSH and LH staining were not significantly different between males and females (Table 1). FSH staining in tumors did not correlate with plasma levels of testosterone, estradiol, or prolactin (data not shown). Tumor volume or invasive behavior was not associated with FSH staining.
Figure 1.
Examples of FSH immunohistochemical staining: (A) low, (B) moderate, and (C) high score.
Table 1.
Immunohistochemical data given as median and interquartile range.
| Total (n = 105) | Female (n = 33) | Male (n = 72) | P-value | |
|---|---|---|---|---|
| Age | 59.9 ± 13.0 | 62.2 ± 13.1 | 58.8 ± 13.0 | .223 |
| FSH staining | 1.0 (0.0-2.0) | 0.0 (0.0-1.0) | 1.0 (0.0-2.0) | .508 |
| FSH mRNA | 0.56 ± 0.73 | 0.77 ± 0.95 | 0.48 ± 0.62 | .312 |
| LH staining | 0.0 (0.0-2.0) | 0.0 (0.0-1.0) | 1.0 (0.0-2.0) | .079 |
| E-cadherin IRS | 6.0 (4.0-6.0) | 6.0 (4.0-6.0) | 6.0 (4.0-6.0) | .940 |
| E-cadherin mRNA | 1.37 ± 0.74 | 1.50 ± 1.04 | 1.32 ± 0.59 | .990 |
| Nuclear E-cadherin | 73% | 70% | 75% | .568 |
| ERα IRS | 1.0 (0.0-4.0) | 2.0 (0.0-3.0) | 1.0 (0.0-4.0) | .522 |
| ERα mRNA | 1.17 ± 1.63 | 0.87 ± 1.26 | 1.30 ± 1.76 | .277 |
| ERβ mRNA | 0.41 ± 0.24 | 0.40 ± 0.22 | 0.42 ± 0.25 | .670 |
| Tumor volume cm3 | 66.1 (40.9-111.7) | 60.6 (36.5-90.4) | 82.5 (45.9-115.6) | .312 |
| Invasive (Knosp ≥ 3) | 43% | 56% | 35% | .172 |
mRNA data are given as mean ± standard deviation. Binary data given as percentages. Tumor volume and invasiveness were available from 47 patients. P-values indicate males vs females.
Abbreviations: IRS, immunoreactivity score; ER, estrogen receptor; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
FSH versus E-cadherin
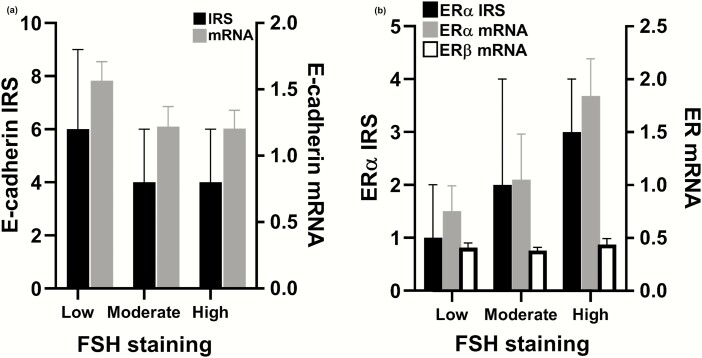
There was an inverse relationship between FSH staining and membranous E-cadherin IRS (Fig. 2A). The median membranous E-cadherin IRS was 6.0 (IQR 5.0-9.0) in tumors with low FSH staining, 4.0 (IQR 4.0-6.0) in tumors with moderate FSH staining, and 4.0 (IQR 2.0-6-0) in tumors with high FSH staining (R = −0.3842, P < 0.0001).
Figure 2.
(A) FSH staining correlated inversely with membranous E-cadherin IRS (R = −0.384; P < .0001). A similar trend was seen between FSH staining and E-cadherin mRNA (R = −0.229; P = .0502. (B) FSH staining correlated with ERα IRS (R = 0.357; P = .0002) and mRNA (R = 0.331, P = .0039). There was no difference in the expression of ERβ (R = 0.034; P = .775). IRS data shown as median and interquartile range. mRNA data shown as mean ± standard error of the mean. ER, estrogen receptor; IRS, immunoreactivity score.
Conversely, the presence of nuclear E-cadherin was seen in 56% of tumors with low FSH staining, 86% with moderate staining, and 94% with high staining (P < .0001). Tumors with nuclear E-cadherin had lower levels of membranous E-cadherin (IRS 4.3 ± 1.8 vs 8.2 ± 2.2, P < .0001). FSH staining did not correlate with E-cadherin mRNA, although there was a trend (R = −0.229, P = .0502). Neither E-cadherin staining nor mRNA was associated with tumor size or invasiveness (data not shown).
FSH versus estrogen receptors
FSH staining correlated positively with ERα IRS and mRNA levels (Fig. 2B). In tumors with low FSH staining, median ERα IRS was 1.0 (IQR 0.0-2.0), versus 2.0 (IQR 1.0-4.0) in tumors with moderate and 3.0 (IQR 1.0-4.0) in tumors with high FSH expression (R = 0.357, P = .0002). FSH staining also correlated with ERα mRNA (R = 0.331, P = .0039). There was no correlation between ERβ mRNA and FSH staining. Further, there was an inverse correlation between ERα and membranous E-cadherin staining (R = −0.31, P = .0014). Tumors with nuclear E-cadherin tended to have higher ERα staining (2.2 ± 2.2 vs 1.5 ± 2.1, P = .066). Neither ER α nor β expression was associated with age, sex, P-FSH, or P-LH levels.
Plasma FSH
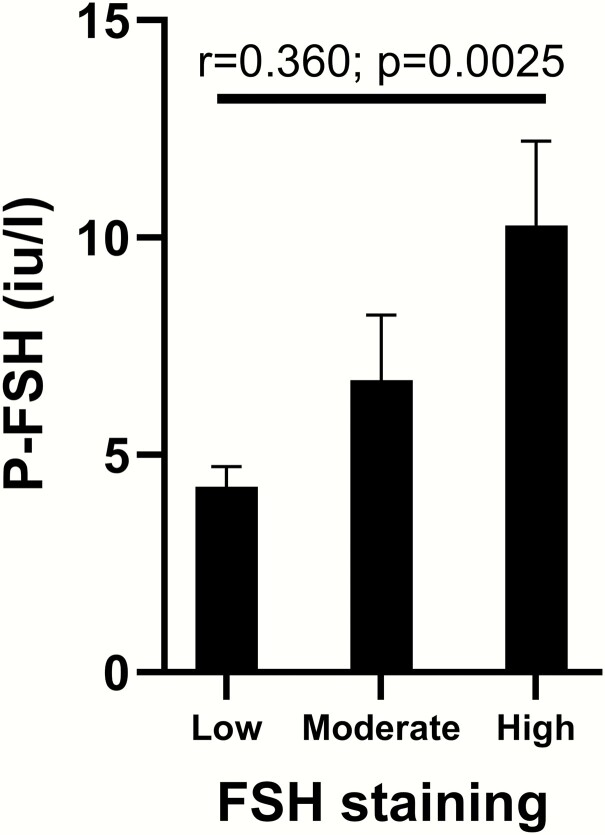
Preoperatively, P-FSH was measured in 88 patients and P-LH in 84 patients. Patients taking estrogens/testosterone and postmenopausal women were excluded, and therefore P-FSH from 62 males and 6 females was available for analysis. P-FSH correlated with tumor FSH staining (R = 0.37, P = .0004) (Fig. 3), but not FSH mRNA (R = 0.22, P = .12). There was no association between P-FSH and age, sex, plasma-estradiol, or plasma-prolactin.
Figure 3.
FSH staining in the tumor correlated with circulating levels of P-FSH. Data shown as mean ± standard error of the mean. Patients taking estrogen, testosterone and postmenopausal women were excluded from analyses.
LH staining
LH staining was low in 54 (51%) tumors, moderate in 24 (23%), and strong in 27 (26%). Samples with strong LH staining showed lower expression of membranous E-cadherin (R = −0.20, P = .040), whereas there was no relationship with E-cadherin mRNA (P = .2798) or presence of nuclear E-cadherin (P = .202). LH staining did not correlate with ERα IRS or mRNA expression, P-LH, P-testosterone, tumor volume, invasiveness, age, or sex.
Discussion
We found that FSH expression in gonadotroph NF-PitNETs was associated with E-cadherin expression and localization, both at the protein level, assessed by using immunohistochemistry, and at the gene expression level. Tumors with high FSH staining expressed less membranous and more nuclear E-cadherin, both of which are considered markers of the mesenchymal phenotype (18, 21). FSH expression and E-cadherin expression was, however, not correlated with tumor size or invasiveness. We speculated that the mesenchymal phenotype (reduced membranous E-cadherin and nuclear translocation) may affect secretory mechanisms, leading to intracellular accumulation of FSH.
Our finding of FSH and E-cadherin is in line with studies in rat lactotroph cells (54), where expression of membranous E-cadherin was related to decreased prolactin staining levels, and in studies of pancreatic islet cells where membranous E-cadherin correlates with an increase in insulin secretion (55-57). These studies suggest that hormone secretion is promoted by E-cadherin, and that hormones may accumulate intracellularly in the absence of E-cadherin. However, this does not explain the association we found between FSH staining and P-FSH levels. This could be due to increased production of FSH in dedifferentiated mesenchymal cells, but we did not examine this in our study. Compared with other PitNET subtypes, gonadotroph tumors have lower levels of membranous E-cadherin (27, 28, 32, 48), and are more commonly nonfunctional than other PitNET subtypes (4). These findings support the role of E-cadherin in hormone secretion, but the link between E-cadherin and hormone secretion is not fully understood.
Gonadotroph PitNETs have particularly low extracellular E-cadherin (48), but more frequent nuclear translocation of E-cadherin (21, 48). Loss of membranous E-cadherin and nuclear translocation is associated with changes in β-catenin and p120 expression and intracellular location (14), both of which interact with several signaling pathways and transcription factors (16-18, 20, 57-61). These changes are associated with larger and more aggressive tumors in somatotroph and corticotroph tumors (21, 24, 26, 28, 32). However, we have previously found that nuclear E-cadherin was associated with less surgical reintervention in gonadotroph PitNETs (48), although the underlying cause could not be determined.
ERα is expressed in both normal gonadotroph cells and in gonadotroph PitNETs (34, 35), and has been implicated in the development of PitNETs (26, 62-64). ERα expression is known to decrease membranous E-cadherin, increase cell proliferation, and contribute to EMT (26, 41, 42, 46), in accordance with our results. This is mediated through the Slug and Snail pathways (26, 43, 46). Estrogen also enhances pituitary tumor growth through pituitary tumor transforming gene (pttg), and this effect is counteracted by antiestrogens (62, 63). We lacked the possibility to investigate the gene expression of Slug, Snail, or pttg in this study, but this will be investigated in our newly launched prospective study.
Estrogen has also been shown to inhibit FSH secretion directly on the pituitary gland in humans (36) and in cell cultures (37-39). By inhibiting FSH secretion, estrogen may contribute to intracellular accumulation of FSH. This remains uncertain, however, since the majority of our study population was male patients and p-estrogens were not measured routinely. FSH in itself has also been shown to induce EMT in ovarian cancer cells (65). The FSH effect on ovarian cancer was shown to be dependent on FSH-receptors that was not investigated in this study.
Although circulating levels of P-FSH correlated with tumor staining for FSH, none of the patients were diagnosed with clinically functioning gonadotroph tumors preoperatively. Functioning gonadotroph tumors are considered rare (4, 66), but our data suggest that some of the FSH produced may be secreted. However, whether the circulating P-FSH was biologically active is not possible to investigate with the present study design, and will have to await prospective sampling.
Limitation
The design of the study was retrospective, but was based on a large and well-characterized population including tumor classification by immunohistochemistry for pituitary hormones and pituitary-specific transcription factors. We did not have the opportunity to evaluate the biological activity of the circulating FSH in the present study. Moreover, our cohort included both genders, as well as pre- and postmenopausal women. Tumor volume and invasiveness assessed by MRI were not available for all patients, thus representing the risk of a type 2 error. Another limitation is the tissue microarray technique used for immunohistochemical analyses, since FSH and LH expression may be heterogeneous within a tumor. This limitation has been reduced by using 2 cores from different areas from each tumor, and a high correlation between FSH staining and mRNA expression indicated that the cores were representable of the tumors.
Conclusion
Contrary to our hypothesis, loss of membranous E-cadherin and gain of nuclear E-cadherin were associated with higher staining levels of FSH in the tumors. This may suggest increased production of FSH or accumulation of FSH in gonadotroph tumors when they develop a mesenchymal phenotype. Furthermore, increased expression of FSH was related to high levels of ERα and may indicate a loss of normal feedback mechanisms. Neither FSH nor E-cadherin expression was associated with tumor volume or invasiveness. Tumor expression of FSH is associated with circulating levels of P-FSH, suggesting that some of the produced hormone is secreted without leading to overt clinical symptoms. Further prospective studies are needed to understand the mechanisms behind and the clinical significance of these findings.
Acknowledgments
Financial Support: No additional funding was received for this study.
Glossary
Abbreviations
- EMT
epithelial to mesenchymal transition
- ER
estrogen receptor
- FSH
follicle-stimulating hormone
- FP
forward primer
- IRS
immunoreactivity score
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NF
nonfunctioning
- PitNETs
pituitary neuroendocrine tumors
- qPCR
quantitative polymerase chain reaction
- RP
reverse primer
- SF-1
steroidogenic factor 1
Additional Information
Disclosure Summary: The authors have nothing to disclose and declare no conflict of interests.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Agustsson TT, Baldvinsdottir T, Jonasson JG, et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol. 2015;173(5):655-664. [DOI] [PubMed] [Google Scholar]
- 2. Tjörnstrand A, Gunnarsson K, Evert M, et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur J Endocrinol. 2014;171(4):519-526. [DOI] [PubMed] [Google Scholar]
- 3. Nishioka H, Inoshita N, Mete O, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol. 2015;26(4):349-355. [DOI] [PubMed] [Google Scholar]
- 4. Ntali G, Capatina C, Grossman A, Karavitaki N. Functioning gonadotroph adenomas. J Clin Endocrinol Metab. 2014;99(12):4423-4433. [DOI] [PubMed] [Google Scholar]
- 5. Adhikary A, Chakraborty S, Mazumdar M, et al. Inhibition of epithelial to mesenchymal transition by E-cadherin up-regulation via repression of slug transcription and inhibition of E-cadherin degradation: dual role of scaffold/matrix attachment region-binding protein 1 (SMAR1) in breast cancer cells. J Biol Chem. 2014;289(37):25431-25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Georgopoulos NT, Kirkwood LA, Walker DC, Southgate J. Differential regulation of growth-promoting signalling pathways by E-cadherin. PLoS One. 2010;5(10):e13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645-3654. [DOI] [PubMed] [Google Scholar]
- 9. Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126(Pt 2):393-401. [DOI] [PubMed] [Google Scholar]
- 10. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305-318. [DOI] [PubMed] [Google Scholar]
- 11. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190-193. [DOI] [PubMed] [Google Scholar]
- 13. Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21-45. [DOI] [PubMed] [Google Scholar]
- 17. Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108(29):11930-11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chetty R, Serra S, Asa SL. Loss of membrane localization and aberrant nuclear E-cadherin expression correlates with invasion in pancreatic endocrine tumors. Am J Surg Pathol. 2008;32(3):413-419. [DOI] [PubMed] [Google Scholar]
- 19. Chetty R, Serra S. Nuclear E-cadherin immunoexpression: from biology to potential applications in diagnostic pathology. Adv Anat Pathol. 2008;15(4):234-240. [DOI] [PubMed] [Google Scholar]
- 20. Salahshor S, Naidoo R, Serra S, et al. Frequent accumulation of nuclear E-cadherin and alterations in the Wnt signaling pathway in esophageal squamous cell carcinomas. Mod Pathol. 2008;21(3):271-281. [DOI] [PubMed] [Google Scholar]
- 21. Elston MS, Gill AJ, Conaglen JV, et al. Nuclear accumulation of e-cadherin correlates with loss of cytoplasmic membrane staining and invasion in pituitary adenomas. J Clin Endocrinol Metab. 2009;94(4):1436-1442. [DOI] [PubMed] [Google Scholar]
- 22. Ferber EC, Kajita M, Wadlow A, et al. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem. 2008;283(19):12691-12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito K, Okamoto I, Araki N, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18(50):7080-7090. [DOI] [PubMed] [Google Scholar]
- 24. Qian ZR, Sano T, Yoshimoto K, et al. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod Pathol. 2007;20(12):1269-1277. [DOI] [PubMed] [Google Scholar]
- 25. Zhou K, Jin H, Luo Y. Expression and significance of E-cadherin and β-catenins in pituitary adenoma. Int J Surg Pathol. 2013;21(4):363-367. [DOI] [PubMed] [Google Scholar]
- 26. Zhou W, Song Y, Xu H, et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J Clin Endocrinol Metab. 2011;96(8):E1237-E1245. [DOI] [PubMed] [Google Scholar]
- 27. Chauvet N, Romanò N, Meunier AC, et al. Combining cadherin expression with molecular markers discriminates invasiveness in growth hormone and prolactin pituitary adenomas. J Neuroendocrinol. 2016;28(2):12352. [DOI] [PubMed] [Google Scholar]
- 28. Fougner SL, Lekva T, Borota OC, Hald JK, Bollerslev J, Berg JP. The expression of E-cadherin in somatotroph pituitary adenomas is related to tumor size, invasiveness, and somatostatin analog response. J Clin Endocrinol Metab. 2010;95(5):2334-2342. [DOI] [PubMed] [Google Scholar]
- 29. Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol (Oxf). 2012;76(1):96-102. [DOI] [PubMed] [Google Scholar]
- 30. Lekva T, Berg JP, Fougner SL, Olstad OK, Ueland T, Bollerslev J. Gene expression profiling identifies ESRP1 as a potential regulator of epithelial mesenchymal transition in somatotroph adenomas from a large cohort of patients with acromegaly. J Clin Endocrinol Metab. 2012;97(8):E1506-E1514. [DOI] [PubMed] [Google Scholar]
- 31. Lekva T, Berg JP, Heck A, et al. Attenuated RORC expression in the presence of EMT progression in somatotroph adenomas following treatment with somatostatin analogs is associated with poor clinical recovery. PLoS One. 2013;8(6):e66927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evang JA, Berg JP, Casar-Borota O, et al. Reduced levels of E-cadherin correlate with progression of corticotroph pituitary tumours. Clin Endocrinol (Oxf). 2011;75(6):811-818. [DOI] [PubMed] [Google Scholar]
- 33. Qian ZR, Li CC, Yamasaki H, et al. Role of E-cadherin, α-, β-, and γ-catenins, and p120 (cell adhesion molecules) in prolactinoma behavior. Modern Pathology. 2002;15(12):1357-1365. [DOI] [PubMed] [Google Scholar]
- 34. Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws JER. Selective expression of estrogen receptor α and β isoforms in human pituitary tumors. J Clin Endocrinol Metab. 1998;83(11):3965-3972. [DOI] [PubMed] [Google Scholar]
- 35. Friend KE, Chiou YK, Lopes MB, Laws ER Jr, Hughes KM, Shupnik MA. Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab. 1994;78(6):1497-1504. [DOI] [PubMed] [Google Scholar]
- 36. Shaw ND, Histed SN, Srouji SS, Yang J, Lee H, Hall JE. Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J Clin Endocrinol Metab. 2010;95(4):1955-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baratta M, West LA, Turzillo AM, Nett TM. Activin modulates differential effects of estradiol on synthesis and secretion of follicle-stimulating hormone in ovine pituitary cells. Biol Reprod. 2001;64(2):714-719. [DOI] [PubMed] [Google Scholar]
- 38. Di Gregorio GB, Nett TM. Estradiol and progesterone influence the synthesis of gonadotropins in the absence of gonadotropin-releasing hormone in the ewe. Biol Reprod. 1995;53(1):166-172. [DOI] [PubMed] [Google Scholar]
- 39. Chaidarun SS, Eggo MC, Stewart PM, Barber PC, Sheppard MC. Role of growth factors and estrogen as modulators of growth, differentiation, and expression of gonadotropin subunit genes in primary cultured sheep pituitary cells. Endocrinology. 1994;134(2):935-944. [DOI] [PubMed] [Google Scholar]
- 40. Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113(2):207-219. [DOI] [PubMed] [Google Scholar]
- 41. Oesterreich S, Deng W, Jiang S, et al. Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res. 2003;63(17):5203-5208. [PubMed] [Google Scholar]
- 42. Helguero LA, Lindberg K, Gardmo C, Schwend T, Gustafsson JA, Haldosén LA. Different roles of estrogen receptors alpha and beta in the regulation of E-cadherin protein levels in a mouse mammary epithelial cell line. Cancer Res. 2008;68(21):8695-8704. [DOI] [PubMed] [Google Scholar]
- 43. Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22(9):2085-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye Y, Xiao Y, Wang W, et al. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene. 2010;29(10):1451-1462. [DOI] [PubMed] [Google Scholar]
- 45. Fan X, Gabbi C, Kim H-J, Cheng G, Andersson LC, Warner M, Gustafsson J-Å. Gonadotropin-positive pituitary tumors accompanied by ovarian tumors in aging female ERβ−/− mice. Proc Natl Acad Sci U S A. 2010;107(14):6453-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cardamone MD, Bardella C, Gutierrez A, Croce LD, Rosenfeld MG, Renzo MFD, Bortoli MD. ERα as ligand-independent activator of CDH-1 regulates determination and maintenance of epithelial morphology in breast cancer cells. PNAS 2009;106(18):7420-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Øystese KA, Casar-Borota O, Normann KR, Zucknick M, Berg JP, Bollerslev J. Estrogen receptor α, a sex-dependent predictor of aggressiveness in nonfunctioning pituitary adenomas: sstr and sex hormone receptor distribution in NFPA. J Clin Endocrinol Metab. 2017;102(9):3581-3590. [DOI] [PubMed] [Google Scholar]
- 48. Øystese KAB, Berg JP, Normann KR, Zucknick M, Casar-Borota O, Bollerslev J. The role of E and N-cadherin in the postoperative course of gonadotroph pituitary tumours. Endocrine. 2018;62(2):351-360. [DOI] [PubMed] [Google Scholar]
- 49. Øystese KA, Zucknick M, Casar-Borota O, Ringstad G, Bollerslev J. Early postoperative growth in non-functioning pituitary adenomas; A tool to tailor safe follow-up. Endocrine. 2017;57(1):35-45. [DOI] [PubMed] [Google Scholar]
- 50. Casar-Borota O, Øystese KA, Sundström M, Melchior L, Popovic V. A high-throughput analysis of the IDH1(R132H) protein expression in pituitary adenomas. Pituitary. 2016;19(4):407-414. [DOI] [PubMed] [Google Scholar]
- 51. Casar-Borota O, Fougner SL, Bollerslev J, Nesland JM. KIT protein expression and mutational status of KIT gene in pituitary adenomas. Virchows Arch. 2012;460(2):171-181. [DOI] [PubMed] [Google Scholar]
- 52. Normann KR, Øystese KAB, Berg JP, et al. Selection and validation of reliable reference genes for RT-qPCR analysis in a large cohort of pituitary adenomas. Mol Cell Endocrinol. 2016;437:183-189. [DOI] [PubMed] [Google Scholar]
- 53. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610-7; discussion 617. [DOI] [PubMed] [Google Scholar]
- 54. Kusumoto K, Kikuchi M, Fujiwara K, et al. Effect of E-cadherin expression on hormone production in rat anterior pituitary lactotrophs in vitro. Acta Histochem Cytochem. 2010;43(2):83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bosco D, Rouiller DG, Halban PA. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J Endocrinol. 2007;194(1):21-29. [DOI] [PubMed] [Google Scholar]
- 56. Rogers GJ, Hodgkin MN, Squires PE. E-cadherin and cell adhesion: a role in architecture and function in the pancreatic islet. Cell Physiol Biochem. 2007;20(6):987-994. [DOI] [PubMed] [Google Scholar]
- 57. Parnaud G, Lavallard V, Bedat B, et al. Cadherin engagement improves insulin secretion of single human β-cells. Diabetes. 2015;64(3):887-896. [DOI] [PubMed] [Google Scholar]
- 58. Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23(48):7947-7956. [DOI] [PubMed] [Google Scholar]
- 59. Semba S, Han SY, Ikeda H, Horii A. Frequent nuclear accumulation of beta-catenin in pituitary adenoma. Cancer. 2001;91(1):42-48. [DOI] [PubMed] [Google Scholar]
- 60. Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51-59. [DOI] [PubMed] [Google Scholar]
- 61. van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta. 2007;1773(1):78-88. [DOI] [PubMed] [Google Scholar]
- 62. Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest. 2002;109(2): 277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999;5(11):1317-1321. [DOI] [PubMed] [Google Scholar]
- 64. Pereira-Lima JF, Marroni CP, Pizarro CB, Barbosa-Coutinho LM, Ferreira NP, Oliveira MC. Immunohistochemical detection of estrogen receptor alpha in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology. 2004;79(3):119-124. [DOI] [PubMed] [Google Scholar]
- 65. Liu L, Zhang J, Fang C, Zhang Z, Feng Y, Xi X. OCT4 mediates FSH-induced epithelial-mesenchymal transition and invasion through the ERK1/2 signaling pathway in epithelial ovarian cancer. Biochem Biophys Res Commun. 2015;461(3):525-532. [DOI] [PubMed] [Google Scholar]
- 66. Cote DJ, Smith TR, Sandler CN, et al. Functional gonadotroph adenomas: case series and report of literature. Neurosurgery. 2016;79(6):823-831. [DOI] [PMC free article] [PubMed] [Google Scholar]