Key Points
Question
What is the tolerability and efficacy of pazopanib and gemcitabine compared with pazopanib alone in pretreated soft tissue sarcoma?
Findings
In this randomized clinical trial with 86 eligible patients, the combination of gemcitabine and pazopanib showed a significantly higher progression-free survival rate at 12 weeks (primary end point) compared with pazopanib alone.
Meaning
The combined regimen of gemcitabine and pazopanib seems to have clinical activity and should be further evaluated.
Abstract
Importance
Pazopanib and gemcitabine have shown good tolerability, albeit modest single-agent activity in pretreated soft tissue sarcoma. A combined regimen to improve outcomes is required.
Objective
To determine the efficacy of gemcitabine and pazopanib compared with pazopanib alone.
Design, Setting, and Participants
This multicenter, randomized phase 2 clinical trial was conducted in Germany from September 2011 to July 2014 and included patients with an Eastern Cooperative Oncology Group performance status score of 0 to 2, adequate organ function, measurable lesion, and progression after at least 1 prior treatment with anthracyclines and/or ifosfamide. Data analysis was performed during 2019 and 2020.
Interventions
Patients were randomized to pazopanib with gemcitabine (A) or without gemcitabine (B).
Main Outcomes and Measures
The primary end point was progression-free survival rate (PFSR) at 12 weeks; secondary end points included toxicity, quality of life, overall survival, and response rates.
Results
A total of 90 patients were randomized, and 86 eligible patients (43 women [50%]) were evaluable, with a median age of 57 (range, 22-84) years and Eastern Cooperative Oncology Group performance status score of 0/1 in 77 participants (90%). The predominant histological subtypes were leiomyosarcoma (22 [26%]) and liposarcoma (16 [19%]). After a median follow-up of 12.4 (range, 1-48) months, the primary end point was met, with a PFSR at 12 weeks of 74% (A) vs 47% (B) (hazard ratio [HR], 1.60; 90% CI, 1.15-2.23; P = .01). In the combination arm, PFSR was significantly longer, with a median of 5.6 vs 2.0 months (HR, 0.58; 95% CI, 0.36-0.92; P = .02) compared with single-agent pazopanib, whereas overall survival was similar, with 13.1 vs 11.2 months (HR, 0.98; 95% CI, 0.60-1.58; P = .83). The objective response rate was overall low, with 11% (A) vs 5% (B) (P = .10). The toxicity of the combination of pazopanib and gemcitabine was increased, but it was manageable and mainly hematological.
Conclusions and Relevance
This phase 2 randomized clinical trial of patients with soft tissue sarcoma found that the addition of gemcitabine to pazopanib was tolerable, and PFSR at 12 weeks was significantly higher compared with pazopanib alone. These results suggest clinical activity of the combination, but they should be confirmed in a phase 3 trial in a more homogeneous population (eg, leiomyosarcoma).
Trial Registration
German Clinical Trials Identifier: DRKS00003139
This randomized clinical trial examines the efficacy of gemcitabine and pazopanib compared with pazopanib alone for German patients with soft tissue sarcoma.
Introduction
For patients with advanced soft tissue sarcoma (STS) that is not amenable to surgery, chemotherapy is the standard of care. Besides the established first-line agents doxorubicin and ifosfamide, several agents have shown histology-specific efficacy in later line settings in randomized phase 3 trials (eg, pazopanib, trabectedin or eribulin).1,2,3 Despite these developments, outcomes of metastatic STS remain poor. Thus, a tolerable, as well as more efficacious, combination regimen are required. Single-agent pazopanib has demonstrated efficacy and feasibility in a phase 3 trial.1 Gemcitabine was evaluated in several mostly single-arm phase 2 trials and showed moderate efficacy, with an overall good tolerability of the drug.4,5,6,7,8,9,10 Therefore, based on the good tolerability, albeit modest single-agent activity, of both drugs and the good feasibility of the full-dose combination in a phase 1 trial, pazopanib and gemcitabine were chosen for randomized comparison vs single-agent pazopanib.11
Pazopanib is a multityrosine kinase licensed after failure of standard chemotherapy in patients with metastatic nonadipocytic STS in the phase 3 PALETTE trial.1,12 Because of remaining uncertainties on the role of pazopanib in liposarcoma when designing this trial, liposarcomas were included in this and further trials.13,14
This trial was conducted within the framework of the German sarcoma working group of the Arbeitsgemeinschaft Internistische Onkologie (AIO). The data were partially presented at the 2016 Annual Meeting of the American Society of Clinical Oncology (June 3-7, 2016; Chicago, Illinois).15
Methods
Design and Participants
This was a multicenter, 2-arm, open-label, randomized phase 2 clinical trial conducted at 14 German sites. Eligible patients were at least age 18 years with histologically confirmed advanced STS who had relapsed or progressed after prior chemotherapy, including an anthracycline, ifosfamide/trofosfamide, or both. Patients with chondrosarcoma, osteosarcoma, Ewing sarcoma, and gastrointestinal stromal tumor were excluded. Eligible patients had measurable disease (RECIST, version1.1),16 Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, normal baseline hematologic parameters, bilirubin levels less than 1.5 times the upper limit of normal, and transaminase levels less than 2.5 times the upper limit of normal.
The study was conducted according to the standards of principles of good clinical practice, all applicable regulatory requirements, and the Declaration of Helsinki. Approval of the protocol was obtained from an independent ethics committee at the University of Halle (Supplement 1). All patients provided written informed consent before enrollment and before undergoing any study-specific procedures.
Random Assignment and Procedures
After informed consent was obtained, eligible patients were centrally randomized and stratified for liposarcoma (local diagnosis) in a 1:1 ratio to pazopanib plus gemcitabine (A) or pazopanib (B). Pazopanib was administered orally, once daily, 800 mg, beginning on day 1 of cycle 1, and gemcitabine was administered intravenously, 1000 mg/m2, over 30 minutes on days 1 and 8 of each 21-day cycle. Dose modifications were based on toxicities causally related to the respective drug. Treatment was administered until progression, intolerable toxicity, or a delay in treatment for more than 2 weeks.
Screening assessments were completed within 14 days before the first dose of study treatment, including medical history, physical examination, laboratory assessments, electrocardiogram, and echocardiogram. Patients were monitored for adverse events (AEs) throughout the study. Disease assessment was performed within 14 days before the first dose of study treatment and every 6 weeks thereafter until week 12, followed by assessment every 8 weeks, according to RECIST, version 1.1.16 Quality of life was assessed at baseline and every 3 weeks using the European Organisation for Research and Treatment of Cancer QLQ C30 questionnaire.
Efficacy and Safety Assessment
The primary end point was progression-free survival rate (PFSR) at 12 weeks, defined as the rate of patients being progression free and alive 12 weeks after randomization. Secondary end points were response rate, progression-free and overall survival, time to progression, safety, and quality of life. The frequency, severity, and relationship to treatment for AEs that occurred during study treatment and up to 30 days after the last dose of study drug were evaluated and coded according to the Common Terminology Criteria for Adverse Events, version 4.0 (National Cancer Institute).
Statistical Analysis
The sample size calculation was based on a 1-sided χ2 test for 2 independent groups, with PFSR at 12 weeks as the primary end point. A PFSR at 12 weeks of 40% was supposed to be reached by the monotherapy arm based on the results of a pivotal phase 2 trial.12 In the combination arm (A), the PFSR at 12 weeks was expected to be at least 60% to prove superiority with 60% power. The study had 60% power at a 1-sided statistical significance value of P < .05 to test this hypothesis. Thus, 45 evaluable participants were required per treatment group.
The efficacy parameters were determined in the modified intent-to-treat (ITT) population (excluding noneligible or untreated patients). All patients who received at least 1 dose of study drug were included in the safety analyses. The primary end point was determined by comparing the 2 treatment arms using the 1-sided Cochran-Mantel-Haenszel test with control for the strata liposarcoma (yes vs no) and a statistical significance level of P < .05. Relative risk was given with a corresponding 2-sided 90% CI. The secondary efficacy analyses were done in an exploratory way without adjustment of P values for multiple testing. Survival data were analyzed by Cox regression that was adjusted for the previously mentioned strata. Hazard ratios (HRs) and their 2-sided 95% CIs were estimated. In addition, Kaplan-Meier estimates were presented together with associated median event times. Statistical analyses were performed using SAS, version 9.4 (SAS Institute).
Results
Patient Characteristics
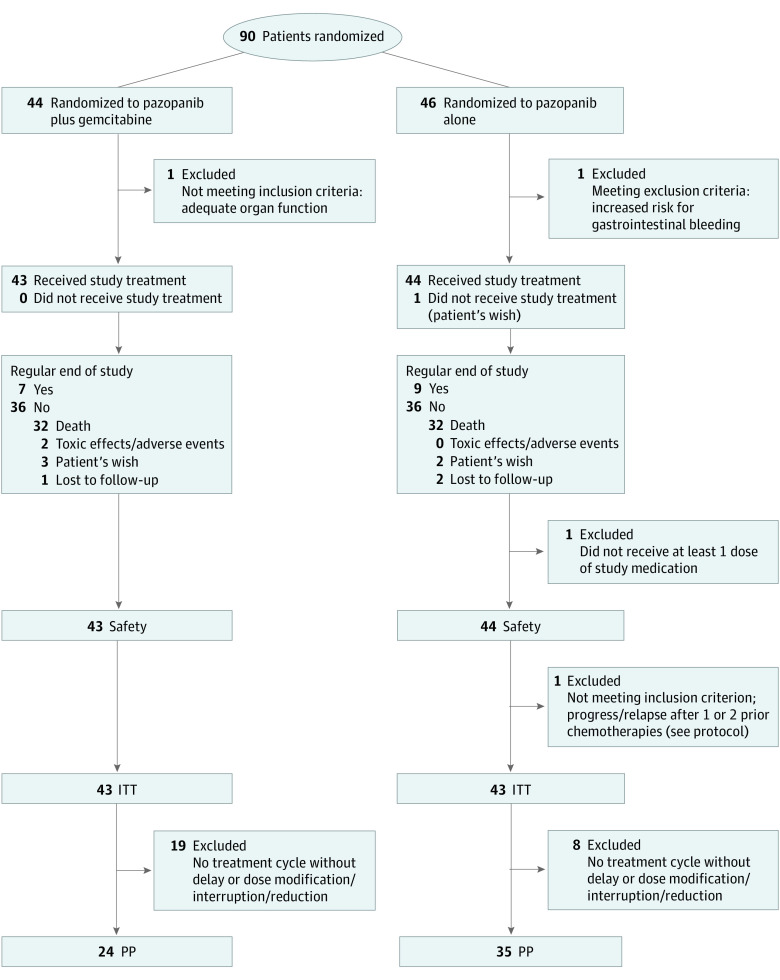
Between September 2011 and June 2014, 90 patients with advanced STS were enrolled and randomized to pazopanib with or without gemcitabine. Before treatment, 3 patients dropped out because of screening failure (n = 2) and withdrawal of consent (n = 1). Thus, 87 patients received treatment and form the safety population. Because of violation of inclusion criteria, another patient had to be excluded from the ITT population, which ultimately comprised 86 patients who were eligible and evaluable (Figure 1). Baseline characteristics of the ITT population are displayed in Table 1. The median (range) age was 57 (23-84) and 59 (22-81) years, 23 (53.5%) and 20 patients (46.5%) were women, and 38 (88.4%) and 39 patients (90.7%) had an ECOG PS of 0/1 for pazopanib plus gemcitabine compared with pazopanib alone, respectively. The median number of prior treatment lines was 2 (range, 1-7), with more than 2 prior lines for 13 (30%; A) vs 16 (37%; B) participants (Table 1). Central pathology review was conducted to correctly classify patients. Rare sarcoma subtypes with a frequency of 1 or 2 were defined as other. The most frequent histological subtypes were leiomyosarcoma (22 [25.6%]) and liposarcoma (16 [18.6%]). Twenty-three patients (26.7%) received anthracyclines, 2 (2.3%) ifosfamide/trofosfamide alone, 60 (69.8%) a combination of both, and 21 (24.4%) gemcitabine as a prior line treatment. In arm B, 16 patients (37%) received subsequent gemcitabine poststudy.
Figure 1. Consolidated Standards of Reporting Trials Diagram.
Modified intent to treat (ITT) is defined as eligible patients and excludes 1 patient with withdrawal of consent before any treatment. PP indicates per protocol.
Table 1. Patient Characteristics in the ITT Groups.
| Characteristic | No. (%) | |
|---|---|---|
| Arm A: pazopanib/gemcitabine (n = 43) | Arm B: pazopanib (n = 43) | |
| Age, median (range), y | 57 (23-84) | 59 (22-81) |
| Sex | ||
| Female | 23 (53) | 20 (47) |
| Male | 20 (47) | 23 (53) |
| ECOG | ||
| 0/1 | 38 (88) | 39 (91) |
| 2 | 5 (12) | 4 (9) |
| Prior treatment lines | ||
| 1 | 11 (26) | 13 (30) |
| 2 | 19 (44) | 14 (33) |
| >2 | 13 [range, 3-7] (30) | 16 [range, 3-7] (37) |
| Anthracycline | 10 (23) | 13 (30) |
| Ifos−/trofosfamide | 2 (4) | 0 |
| Anthracycline + Ifos−/trofosfamide | 31 (72) | 29 (67.5) |
| Gemcitabine | 10 (23) | 11 (26) |
| Posttrial gemcitabine | 16 (37) | |
| Histology (central review) | ||
| Angiosarcoma | 2 (4) | 2 (4) |
| Leiomyosarcoma | 13 (30) | 9 (21) |
| High + dedifferentiated liposarcoma | 8 (19) | 2 (5) |
| Myxoid liposarcoma | 1 (2) | 5 (12) |
| Malignant peripheral nerve sheath tumor | 1 (2) | 3 (7) |
| Synovial sarcoma | 5 (12) | 3 (7) |
| Undifferentiated sarcoma (NOS) | 5 (12) | 10 (23) |
| Others | 5 (12) | 6 (14) |
| Not available for central review | 3 (7) | 3 (7) |
| Grading | ||
| Low | 3 (7) | 4 (9) |
| Intermediate | 15 (35) | 12 (28) |
| High | 17 (39) | 17 (39) |
| Missing | 8 (19) | 10 (23) |
| Concomitant proton pump inhibitors | 27 (63) | 33 (77) |
Abbreviations: ECOG: Eastern Cooperative Oncology Group performance status; Ifos-, ifosfamide: ITT, intent to treat; NOS, not otherwise specified.
Treatment Received
The median (range) dose intensity of pazopanib was equal in both arms, (90% [28%-100%; A] and 89% [14%-100%; B], respectively). In the combination arm, most patients needed dose reductions of gemcitabine, reaching the median dose level of 75% (750 mg/m2). At least 1 cycle without dose delay or modifications could be applied in 24 (56%; A) and 35 (81%; B) patients.
Efficacy
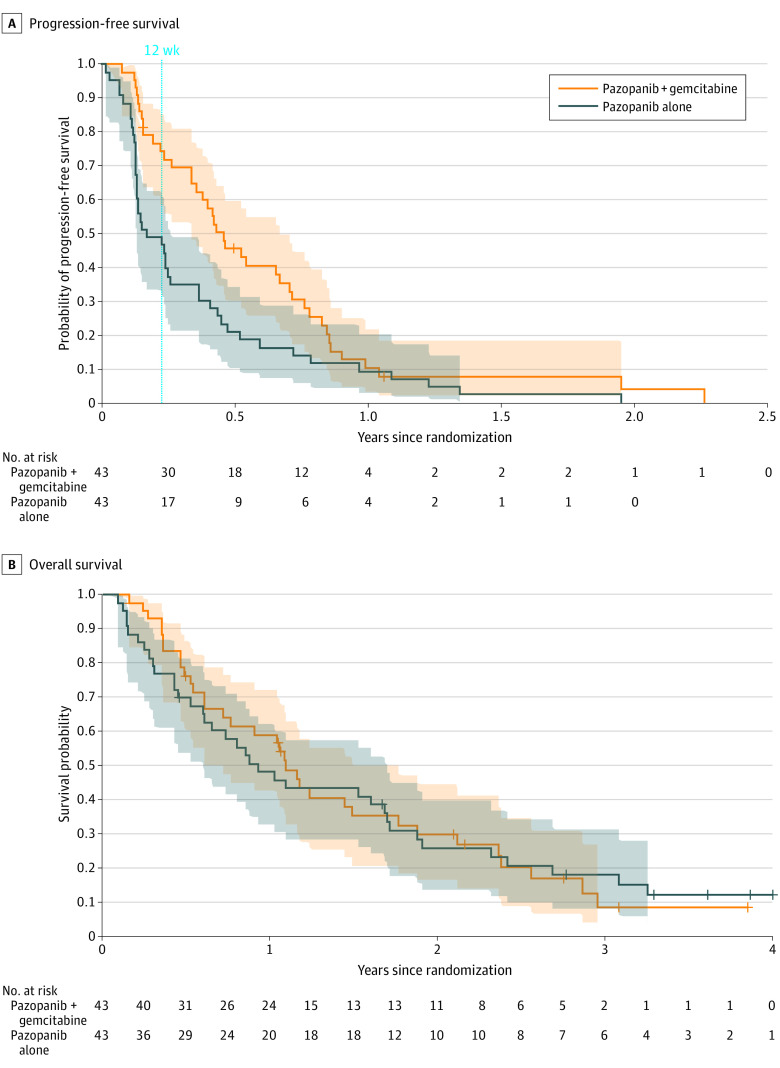
The efficacy results were determined in the ITT population of eligible patients who received at least 1 treatment (n = 86), after a median follow up of 39 (range, 21-54) months. The PFSR at 12 weeks was 74% in the combination arm (A) compared with 47% with single agent pazopanib (B) (P = .01; adjusted relative risk estimate, 1.60; 2-sided 90% CI, 1.15-2.23) (Table 2). Thus, the primary end point was reached. The progression-free survival was significantly higher, with a median of 5.6 vs 2 months (HR, 0.58; 95% CI, 0.36-0.92; P = .02) in the combination compared with the control arm (Figure 2A), whereas overall survival was similar, with a median of 13.1 vs 11.2 months (HR, 0.98; 95% CI, 0.60-1.58; P = .83) (Figure 2B). The objective response rate was overall low, with 5 of 43 (11%) vs 2 of 43 (5%) in the combination compared with the control arm (P = .10; adjusted relative risk estimate, 2.65; 90% CI, 0.71-9.90).
Table 2. Efficacy According to RECIST, Version 1.1.
| Efficacy parameters | Arm A: pazopanib/gemcitabine (n = 43) | Arm B: pazopanib (n = 43) | HR (95% CI) | P value |
|---|---|---|---|---|
| PFS rate at 12 wk, % | 74 | 47 | NA | .01 |
| PFS, mo | 5.6 | 2.0 | 0.58 (0.36-0.92) | .02 |
| TTP, mo | 5.6 | 2.0 | 0.57 (0.36-0.91) | .02 |
| OS, mo | 13.1 | 11.2 | 0.98 (0.60-1.58) | .83 |
| ORR (CR/PR), No. (%) | 5 (11) | 2 (5) | ||
| CR | 1 (2) | 0 | NA | .10 |
| PR | 4 (9) | 2 (5) | ||
| SD | 27 (63) | 20 (47) |
Abbreviations: CR, complete response; HR, hazard ratio; NA, not applicable; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease; TTP, time to progression.
Figure 2. Progression-Free Survival and Overall Survival.
Kaplan-Meier estimates with pointwise 95% CIs.
Exploratory subgroup analyses regarding the effect of adding gemcitabine to pazopanib compared with single-agent pazopanib in the largest 2 histological subgroups (leiomyosarcoma [22 (25.6%)] and liposarcoma [16 (18.6%)]) showed a similar effect in leiomyosarcoma for progression-free survival, with a median of 8.5 vs 3.0 months (HR, 0.41; 95% CI, 0.16-1.07) and for overall survival, with a median of 20.2 vs 24.2 months (HR, 1.2; 95% CI, 0.43-3.4); however, there was a pronounced effect of the combination regimen in liposarcoma (16 [18.6%]) for progression-free survival, with a median of 8.6 vs 1.5 months (HR undefined because of the extreme difference; eFigure 1 in Supplement 2) and for overall survival, with a median of 25.4 vs 5.4 months (HR, 0.39; 95% CI, 0.54-1.87). Prior gemcitabine had no effect on the efficacy of the combination (HR, 0.58; 95% CI, 0.35-0.96 without and HR, 0.58; 95% CI, 0.23-1.45 with prior gemcitabine) (eFigure 1 in Supplement 2). Further exploratory subgroups are displayed in Figure 2. Beside the noted difference in liposarcoma and some diminished effect in patients older than 65 years, the effect of the addition of gemcitabine to pazopanib did not differ for sex or ECOG PS. In the 16 patients in arm B who received posttrial gemcitabine, there was no difference in survival compared with patients without posttrial gemcitabine (HR, 0.97; 95% CI, 0.49-1.92) (eFigure 1 in Supplement 2).
Safety
The combination was generally well tolerated in an outpatient setting. Adverse events are summarized in Table 3. The most frequently observed grade 3/4 AEs in the combination arm were leukopenia, anemia, thrombocytopenia, and fatigue related to gemcitabine. Although rates of infections that were grade 3/4 were numerically higher in the combination arm (12% vs 5%), only 1 case of febrile neutropenia occurred. Pazopanib-related AEs (eg, hypertension, liver enzyme elevations, hemorrhage, or thromboembolic events) did not relevantly differ between the 2 treatment arms.
Table 3. Toxicity According to National Cancer Institute Common Toxicity Criteria, Version 4.0.
| Grade 3/4 adverse events (safety population n = 87) | Patients, No. (%) | |
|---|---|---|
| Arm A: pazopanib/gemcitabine (n = 43) | Arm B: pazopanib (n = 44) | |
| All | 34 (79) | 25 (57) |
| Leucopenia | 14 (33) | 1 (2) |
| Febrile neutropenia | 1 (2) | 0 |
| Anemia | 4 (9) | 0 |
| Thrombocytopenia | 17 (40) | 0 |
| Infection | 5 (12) | 2 (5) |
| Fatigue | 3 (7) | 0 |
| Diarrhea | 2 (5) | 1 (2) |
| Stomatitis | 1 (2) | 0 |
| Vomiting | 2 (5) | 0 |
| Transaminase elevation | 4 (9) | 3 (7) |
| Pleural effusion | 3 (7) | 0 |
| Pneumothorax | 1 (2) | 1 (2) |
| Hemoptysis | 1 (2) | 0 |
| Gastrointestinal hemorrhage | 0 | 1 (2) |
| Hypertension | 5 (12) | 2 (5) |
| Events | ||
| Thromboembolic | 2 (5) | 1 (2) |
| Severe adverse | 26 (60) | 22 (50) |
| SAEs | ||
| Treatment related | 22 (51) | 8 (18) |
| Fatal | 3 (7) | 5 (11) |
Abbreviation: SAEs, severe adverse events.
In the combination arm, 3 patients (7%) discontinued treatment because of an AE compared with 3 (7%) in the single agent arm. Overall, 64 patients (74.4%) discontinued treatment because of progression. In the combination arm, 24 and 13 severe AEs (SAEs) related to pazopanib and gemcitabine occurred in 15 (34.9%) and 7 patients (16.3%), respectively. For single-agent pazopanib, 9 related SAEs in 8 patients were noted. Overall, 8 patients had SAEs with fatal outcome, with 5 in the single-agent arm and 3 in the combination arm, with 6 of 8 unrelated to pazopanib. The 2 fatal SAEs related to pazopanib were acute respiratory distress syndrome and general health deterioration.
Quality of Life
Quality of life questionnaires were available from 35 (81.4%) and 38 patients (88.4%) at baseline and 17 (39.5%) and 16 (37.2%) after 12 weeks for the combination and single-agent pazopanib arms, respectively. The median (range) global health score was 58.3 (8-100) and 66.7 (0-100) at baseline, 50.0 (33-92) and 58.0 (8-83) after 6 weeks, and 50.0 (17-100) and 50.0 (0-83) after 12 weeks for the combination and single-agent pazopanib arms, respectively. In addition, functional and symptom scale scores were similar for both arms, except for fatigue, with increasing (and thus worse) scores after 6 weeks (55.6; range, 0-100) over baseline (33.3; range, 0-89) in the combination arm, compared with similar scores in the single-agent arm for these points (both 33.3).
Discussion
In the PAPAGEMO phase 2 randomized clinical trial, the combination of gemcitabine and pazopanib showed a higher PFSR at 12 weeks (primary end point), progression-free survival, and time to progression compared with pazopanib alone. Overall survival was similar in both arms, which was likely because of the high rate of subsequent treatments.
Overall, the obtained results are comparable with others achieved in current second- to third-line trials. Notably, eribulin or trabectedin vs dacarbazine showed similar median progression or overall survival times in a preselected leiomyosarcoma and liposarcoma cohort, which also form the largest cohort in our trial.2,3 Compared with the pivotal PALETTE trial, which had a median progression-free survival of 4.6 months, the control arm in the PAPAGEMO trial with single-agent pazopanib showed a lower median progression-free survival of 2 months.1 Other than differences in the histological subgroups included (particularly the limited activity of pazopanib in liposarcoma), a numerically higher rate of patients (71% in PAPAGEMO vs 56% in PALETTE) received at least 2 lines before trial inclusion, which was associated with a shorter progression-free survival of pazopanib in the PALETTE trial.17 Furthermore, the rate of concomitant use of proton pump inhibitors that are known to limit the efficacy of pazopanib of 70% (n = 60) was high in this trial compared with 18% in the PALETTE trial18 (eFigure 1 in Supplement 2).
Recently, a French group reported results of combined pazopanib and gemcitabine in a nonrandomized, single-arm trial of 106 patients with leiomyosarcoma.19 Despite comparable results with our randomized cohort that showed a higher progression-free survival for the combination, the authors concluded a lack of efficacy compared with historical data derived from a trial comparing gemcitabine alone with gemcitabine and docetaxel in second line.10 This preceding trial showed relatively good results for single-agent gemcitabine and no added benefit for the combination, in contrast to available first-line data.6,10
The effect of gemcitabine in addition to pazopanib seemed to be pronounced in liposarcoma, with an improvement in median progression-free and overall survival, although this was limited by the small number of patients (n = 16). The relatively poor results of single-agent pazopanib in liposarcoma align with prior data from the pivotal pazopanib trial by Sleijfer et al.12 The limited data on the efficacy of single-agent gemcitabine in liposarcoma only show a moderate efficacy, with 2 of 12 patients achieving disease stabilization for more than 24 weeks.6 Thus, the PAPAGEMO trial may indicate synergistic activity of the combination. However, some subtypes of liposarcoma might be more amenable to gemcitabine, and the imbalance of the prognostically different liposarcoma subtypes (high and dedifferentiated vs myxoid) might have contributed to the pronounced survival difference.20 In line with other current investigations, and thus further underlining the cautious use of concomitant medications, patients receiving concomitant proton pump inhibitors (70%) had worse outcomes in this trial (eFigure 1 in Supplement 2).18 The overall low number of patients and the retrospective, unplanned nature of the subgroup analyses limits the relevance of these findings.
Overall, the combination regimen was well tolerated and required dose modifications that were mainly caused by bone marrow toxicity. The main patient-relevant grade 3/4 AEs of the combination were infections, fatigue, and pleural effusion. Other than fatigue, quality of life did not seem to differ between the treatment arms, although the availability of questionnaires decreased over time. Thus, overall, this efficacious combination can be safely administered in an at least second-line sarcoma population.
Limitations
The limitations of this trial are the exploratory phase 2 design with a small number of patients, heterogenous patient population, lack of central radiology, imbalance between both study arms in rather gemcitabine-sensitive sarcoma (eg, leiomyosarcoma), and the lack of an available central review of radiological imaging.
Conclusions
In this phase 2 randomized clinical trial of patients with advanced STS, refractory to anthracycline and/or, the combination of pazopanib and gemcitabine showed a higher PFSR at 12 weeks compared with single-agent pazopanib, an association with acceptable tolerability, and no detrimental effect on quality of life. Further validation in a more homogenous population (eg, in patients with leiomyosarcoma) seems to be justified.
Trial Protocol.
eFigure 1. Exploratory subgroup analyses for Overall survival in patients receiving subsequent gemcitabine or not (A), progression free survival (PFS) in liposarcoma patients (B), according to the use of prior gemcitabine (C) or not (D) and effect of concomitant use of protone pump inhibitors (PPI) in arm A (E) and B (F)
eFigure 2. Forest plot exploratory subgroup analyses for progression free survival (PFS) and overall survival (OS)
References
- 1.van der Graaf WT, Blay JY, Chawla SP, et al. ; EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group . Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879-1886. doi: 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 2.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637. doi: 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793. doi: 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann JT, Oechsle K, Huober J, et al. An open label, non-comparative phase II study of gemcitabine as salvage treatment for patients with pretreated adult type soft tissue sarcoma. Invest New Drugs. 2006;24(3):249-253. doi: 10.1007/s10637-005-3537-1 [DOI] [PubMed] [Google Scholar]
- 5.Look KY, Sandler A, Blessing JA, Lucci JA III, Rose PG; Gynecologic Oncology Group (GOG) Study . Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) Study. Gynecol Oncol. 2004;92(2):644-647. doi: 10.1016/j.ygyno.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 6.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25(19):2755-2763. doi: 10.1200/JCO.2006.10.4117 [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19(15):3483-3489. doi: 10.1200/JCO.2001.19.15.3483 [DOI] [PubMed] [Google Scholar]
- 8.Svancárová L, Blay JY, Judson IR, et al. Gemcitabine in advanced adult soft-tissue sarcomas: a phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2002;38(4):556-559. doi: 10.1016/S0959-8049(01)00408-7 [DOI] [PubMed] [Google Scholar]
- 9.Okuno S, Edmonson J, Mahoney M, Buckner JC, Frytak S, Galanis E. Phase II trial of gemcitabine in advanced sarcomas. Cancer. 2002;94(12):3225-3229. doi: 10.1002/cncr.10602 [DOI] [PubMed] [Google Scholar]
- 10.Pautier P, Floquet A, Penel N, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study). Oncologist. 2012;17(9):1213-1220. doi: 10.1634/theoncologist.2011-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer R, Madi A, Jeffels M, et al. A Phase I study of pazopanib in combination with gemcitabine in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(1):93-101. doi: 10.1007/s00280-012-1982-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126-3132. doi: 10.1200/JCO.2008.21.3223 [DOI] [PubMed] [Google Scholar]
- 13.Valverde CM, Broto JM, Lopez-Martin JA, et al. Phase II clinical trial evaluating the activity and tolerability of pazopanib in patients (pts) with advanced and/or metastatic liposarcoma (LPS): a joint Spanish Sarcoma Group (GEIS) and German Interdisciplinary Sarcoma Group (GISG) study—NCT01692496. J Clin Oncol. 2016;34(15):11039-11039. doi: 10.1200/JCO.2016.34.15_suppl.11039 [DOI] [Google Scholar]
- 14.Grünwald V, Karch A, Schuler M, et al. Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 years or older: results of a German intergroup study. J Clin Oncol. 2020;38(30):3555-3564. doi: 10.1200/JCO.20.00714 [DOI] [PubMed] [Google Scholar]
- 15.Schmoll H-J, Ruessel J, Reichardt P. Pazopanib vs pazopanib + gemcitabine in refractory soft tissue sarcoma: a randomized phase II trial of the AIO. J Clin Oncol. 2016;34(15):11004-11004. doi: 10.1200/JCO.2016.34.15_suppl.11004 [DOI] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Cesne AL, Bauer S, Demetri GD, et al. Safety and efficacy of pazopanib in advanced soft tissue sarcoma: PALETTE (EORTC 62072) subgroup analyses. BMC Cancer. 2019;19(1):794. doi: 10.1186/s12885-019-5988-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mir O, Touati N, Lia M, et al. Impact of concomitant administration of gastric acid-suppressive agents and pazopanib on outcomes in soft-tissue sarcoma patients treated within the EORTC 62043/62072 Trials. Clin Cancer Res. 2019;25(5):1479-1485. doi: 10.1158/1078-0432.CCR-18-2748 [DOI] [PubMed] [Google Scholar]
- 19.Pautier P, Penel N, Ray-Coquard I, et al. A phase II of gemcitabine combined with pazopanib followed by pazopanib maintenance, as second-line treatment in patients with advanced leiomyosarcomas: a unicancer French Sarcoma Group study (LMS03 study). Eur J Cancer. 2020;125:31-37. doi: 10.1016/j.ejca.2019.10.028 [DOI] [PubMed] [Google Scholar]
- 20.Braas D, Ahler E, Tam B, et al. Metabolomics strategy reveals subpopulation of liposarcomas sensitive to gemcitabine treatment. Cancer Discov. 2012;2(12):1109-1117. doi: 10.1158/2159-8290.CD-12-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eFigure 1. Exploratory subgroup analyses for Overall survival in patients receiving subsequent gemcitabine or not (A), progression free survival (PFS) in liposarcoma patients (B), according to the use of prior gemcitabine (C) or not (D) and effect of concomitant use of protone pump inhibitors (PPI) in arm A (E) and B (F)
eFigure 2. Forest plot exploratory subgroup analyses for progression free survival (PFS) and overall survival (OS)