Abstract
Background:
Immune-related adverse events (irAEs) are common during immune checkpoint inhibitor (ICI) treatment and reported to be associated with good survival. This study evaluated the association between onset timing of irAEs and survival of cancer patients treated with ICIs.
Methods:
Databases including PubMed, Embase, and the Cochrane library were systematically searched to retrieve clinical studies assessing the relationship between irAEs and survival in cancer patients with ICIs. The overall response rate for treatment response and hazard ratio (HR) for overall survival (OS) and progression-free survival (PFS) were calculated using RevMan 5.3. Subgroup analysis in terms of cancer type, ICIs type, region, specific irAEs, accordingly.
Results:
A total of 34 studies were included. The HRs for OS and PFS in cancer patients with versus without irAEs were 0.57 [95% confidence interval (CI): 0.44, 0.74; p < 0.0001], and 0.50 (95% CI: 0.37, 0.67; p < 0.00001), respectively. The odds ratio for overall response in cancer patients with irAEs was 4.72 (95% CI: 3.48, 6.40; p < 0.00001) compared with those without irAEs. Subgroup analyses suggested that the prognostic role of irAEs was associated with cancer types and region, but not irAEs types. The landmark analysis of OS revealed that there is a non-proportional (early) effect of irAEs on OS in ICI-treated cancer patients (landmark >12 weeks, HROS = 1.08; 95% CI: 0.89, 1.30; p = 0.46).
Conclusion:
Our findings suggest that the occurrence of irAEs could be a prognostic factor for cancer patients who were treated with ICIs.
Keywords: biomarker, immune-related adverse events, immunotherapy, meta-analysis, survival
Introduction
Immunotherapy is one of the most promising treatment strategies against various tumors.1,2 Immune checkpoint inhibitors (ICIs) have been widely used in clinical practice,2 with improved response rates and prolonged survival of cancer patients.2,3 However, not all the patients gain benefits due to numerous difficulties, such as tumor heterogeneity and host immunity status.3,4 Therefore, it is essential to explore potential prognostic factors to predict who could have better outcome from immunotherapy.
As a result of enhanced or improved host immunity by ICIs, immune-related adverse events (irAEs) are often reported in clinical trials.5–11 Results of several meta-analyses suggest that the commonly affected organs are skin, endocrine, gastrointestinal tract and liver.12–14 Recent research attention has focused on the relation between irAEs and treatment outcomes.10,11,15 Results from these trials show that cancer patients with irAEs have better efficacy than those without irAEs.10,16 However, there are also other views against a positive relation between irAEs and overall survival (OS) of ICI-treated cancer patients.17–19 In addition, the onset time of irAEs varied between individual patients and ICI agents, making it difficult to examine the actual prognostic role of irAEs in survival of cancer patients. Therefore, whether irAEs can serve as a biomarker for immunotherapy is still in debate.
In this study, we systematically searched databases to identify clinical studies assessing the effects of irAEs on treatment outcomes and survival of cancer patients treated with ICIs, and aimed to evaluate the relation between irAEs and efficacy and survival in cancer patients receiving ICIs.
Material and methods
This study was performed according to the PRISMA and the Cochrane handbook guidelines. This study was not registered.
Search strategy
Electronic databases including Embase, PubMed, and the Cochrane library were searched until February 2019. Search terms were “Immune-related adverse events or irAEs or irAE or treatment related adverse events,” “cancer or tumor or neoplasm,” “immune checkpoint inhibitors or immune checkpoint blockades or PD-1 inhibitors or PD-L1 inhibitors or CTLA-4 inhibitors.” These terms were used in different combinations. There were no language restrictions during the search.
Inclusion and exclusion criteria
Clinical studies including randomized controlled trials, and retrospective studies were included. Inclusion criteria: (1) Cancers were diagnosed by sufficient clinical evidence, such as pathology or cytology; (2) patients were treated with ICIs including PD-1, PD-L1, and/or CTLA-4 inhibitors alone or in combination; (3) survival data [OS, progression-free survival (PFS), and/or time to treatment failure (TTF)] in cancer patients with versus without irAEs were reported; treatment response measures were reported in the included studies; (4) if results from the same patient sources were published by different journals, the study with the most complete or up-to-date data was included; (5) eligible studies were not only full-text, but also abstract, conference meeting presentation, and unpublished literature.
Exclusion criteria: Studies were excluded if (1) insufficient data on baseline information, efficacy or survival; (2) reviews, animal studies, comments, survey, and guidelines.
Study selection and data extraction
The screen for eligible studies was conducted by two researchers (HX and DC), independently. Any inconsistency was solved through discussion. The following information was extracted: first name of the first author, publication time, region, number of patients, sex (male), age, cancer type, immunotherapy agent, reported specific irAEs, objective response rate (ORR), hazard ratios (HRs) of irAEs versus no irAEs for OS, PFS, and TTF based on landmark analysis or not.
Quality assessment
To evaluate the quality of the retrospective studies, the Newcastle–Ottawa scale (NOS) method was introduced.20 According to the protocol of the NOS, three major aspects are focused on during evaluation: selection, comparability of the cohort, and evaluation of the results. According to the instruction of the NOS, a maximum of four stars, two stars, and three stars can be given to the selection, the comparability, and the results assessment, respectively. A good quality study was defined as having six or more stars.
Statistical analysis
The RevMan 5.3 software was used to combine the individual HR and its related 95% confidence interval (CI). GraphPad Prism 6 was used to draw plots. Engauge software was used to extract survival rate at various time-points from survival plots. Q test and I2 statistic were introduced to calculate the heterogeneity among the included studies. A significant heterogeneity was considered if p < 0.1 or I2 >50%, and the random-effects model was used. If p > 0.1 or I2 <50%, the fixed-effect model was used. For the pooled estimate, it was considered statistically significant if p < 0.05.
To detect the impact of time on the prognostic role of irAEs, we used landmark data from individual studies to perform the meta-analysis. We also calculated the odds ratio (OR) of survival rates at 2, 4, 6, 8, 10, 12, 18, 24, and 30 months using the individual data (number of death, number at risk) of the included studies. The OR was calculated as following: (death events/number at risk)non-irAE/(death events/number at risk)irAE.
The individual HRs of irAEs versus no irAEs were extracted. For studies that did not present HRs directly, reported methods21,22 were used to calculate the HR. The overall HR <1 indicated that appearance of irAEs was associated with better outcomes for cancer patients treated with ICIs. If HR >1, it indicated patients with irAEs had poor outcomes. Treatment response rates after ICIs treatment were also extracted to determine the influence of irAEs on treatment efficacy of ICIs. Subgroup analyses of survival were performed with regard to cancer type, ICIs type, region, specific irAEs, and number of irAEs. Funnel plot was used to detect the publication bias, and a p < 0.05 suggested that there was a significant publication bias.
Results
Search results
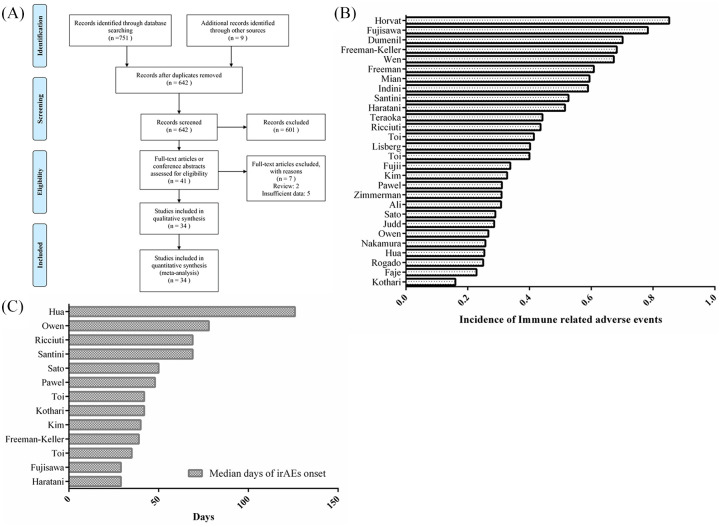
A total of 760 relevant articles were retrieved after the preliminary search. After removing 118 duplications, the title and abstract of the remaining 642 studies were screened; 601 of them were discarded as they were animal studies, comments, reviews, and brief reports. After reading the full text, a further seven articles were excluded because of insufficient data, and the remaining 34 studies with 5840 patients were considered as eligible studies.10,11,15–19,23–49 Figure 1A shows the details of the literature screen and selection.
Figure 1.
Flow chart of identifying eligible studies and characteristics of irAEs. (A) Flow chart of identifying eligible studies; (B) individual incidence of irAEs among included studies; and (C) onset time (median days) of irAEs in individual studies.
Baseline characteristics of included studies
Table 1 presents the baseline characteristics of the included studies. Of these studies 94% were retrospective and only two were prospective studies. The publication years ranged from 2013 to 2019. Most of the cases were diagnosed with non-small-cell lung carcinoma (NSCLC) or melanoma. The incidences of irAEs among included studies ranged from 16% to 85% (Figure 1B), with an overall incidence of 44.89% (1994/4442). The median days of irAEs onset ranged from 29 to 126 (Figure 1C). The reporting regions included Asia (n = 10), North America (n = 17), and Europe (n = 7). Fourteen trials10,15–17,19,23,25,26,32–36,48,49 reported outcomes of tumor response, 20 studies11,16–19,23,26–28,31,33–40 showed OS data, and 15 studies exhibited PFS data.10,11,15–18,23,28,31,33–36,47,48
Table 1.
Baseline characteristics of included studies.
| Author name | Design | irAEs+ | irAEs– | Region | Age | Sex, male | Patient type | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ali et al.25 | retrospective | 7 | 33 | Europe | 66 (46–88) | 22 | advanced NSCLC | Nivolumab | efficacy, irAE |
| Arbour et al.29 | retrospective | 640 | North America | 29–93 | 232 | advanced NSCLC | Single-agent PD-(L)1 inhibitor | efficacy, OS, PFS, irAE | |
| Dumenil et al.43 | retrospective | 47 | 20 | Europe | 69 | 46 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Faje et al.26 | retrospective | 64 | 216 | North America | 63 | 67 | Melanoma | Ipilimumab | OS, TTF, irAE |
| Freeman and Weber38 | retrospective | 87 | 56 | North America | NA | NA | advanced melanoma | Nivolumab | OS, irAE |
| Freeman-Keller et al.19 | retrospective | 101 | 47 | North America | 17–90 | 87 | Melanoma | Nivolumab | efficacy, OS, PFS, irAE |
| Fucà et al.44 | retrospective | 151 | Europe | 65 | 89 | advanced NSCLC | PD-1 or PD-L1 inhibitors | efficacy, OS, PFS, irAE | |
| Fujii et al.31 | retrospective | 98 | 192 | North America | 59 (19–86) | 136 | advanced cancer | Immunotherapy drug | efficacy, OS, PFS, irAE |
| Nakamura et al.35 | retrospective | 9 | 26 | Asia | 40–85 | 18 | Melanoma | Nivolumab | efficacy, OS, PFS, irAE |
| Haratani et al.11 | retrospective | 69 | 65 | Asia | 68 (33–85) | 90 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Horvat et al.18 | retrospective | 254 | 44 | North America | 65 (21–93) | 182 | Melanoma | Ipilimumab | OS, TTF, irAE |
| Hua et al.49 | prospective | 17 | 50 | Europe | 54 (20–74) | NA | advanced melanoma | Pembrolizumab | efficacy, OS, irAE |
| Indini et al.47 | retrospective | 102 | 71 | Europe | 62 (18–85) | 107 | advanced melanoma | Nivolumab or Pembrolizumab | efficacy, OS, PFS, irAE |
| Judd et al.17 | retrospective | 64 | 160 | North America | 65 | 101 | advanced cancer | Nivolumab or Pembrolizumab | efficacy, OS, PFS |
| Kim et al.33 | retrospective | 19 | 39 | Asia | 63 (49–68) | 43 | advanced NSCLC | Nivolumab or Pembrolizumab | efficacy, OS, PFS, irAE |
| Kothari et al.28 | retrospective | 28 | 147 | North America | 68 (33–88) | NA | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Lisberg et al.34 | retrospective | 39 | 58 | North America | 32–83 | 50 | NSCLC | Pembrolizumab | efficacy, OS, PFS, irAE |
| Margiotta et al.46 | retrospective | 163 | North America | NA | NA | advanced cancer | PD-1 or PD-L1 inhibitors | efficacy | |
| Mian et al.27 | retrospective | 510 | 348 | North America | 69 | NA | Melanoma | Ipilimumab | OS, irAE |
| Fujisawa et al.37 | retrospective | 47 | 13 | Asia | 63 (31–85) | 30 | advanced melanoma | Nivolumab, followed with Ipilimumab | efficacy, OS, irAE |
| Owen et al.30 | retrospective | 24 | 66 | North America | 67 | NA | NSCLC | Nivolumab | OS, irAE |
| Pawel et al.40 | retrospective | 132 | 293 | North America | NA | NA | advanced NSCLC | Atezolizumab | OS |
| Ricciuti et al.48 | retrospective | 85 | 110 | Europe | 63 (30–84) | 128 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Rogado et al.16 | retrospective | 10 | 30 | Europe | NA | NA | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Santini et al.24 | retrospective | 20 | 18 | North America | 42–84 | 20 | advanced NSCLC | PD-1 or PD-L1 inhibitors | efficacy, OS, PFS, irAE |
| Sato et al.15 | retrospective | 11 | 27 | Asia | 69 (49–86) | 28 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Scott and Pennell41 | retrospective | 210 | North America | 68 (60–74) | 113 | advanced NSCLC | Nivolumab | OS, irAE | |
| Shah et al.45 | retrospective | 141 | North America | NA | NA | advanced NSCLC | PD-1 or PD-L1 inhibitors | efficacy | |
| Taniguchi et al.42 | retrospective | 201 | Asia | 68 (27–87) | 135 | NSCLC | Nivolumab | efficacy, OS, PFS, irAE | |
| Teraoka et al.23 | prospective | 19 | 24 | Asia | 70 (50−82) | 27 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Toi et al.36 | retrospective | 29 | 41 | Asia | 68 | 61 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Toi et al.10 | retrospective | 28 | 42 | Asia | 68 (36–88) | 61 | advanced NSCLC | Nivolumab | efficacy, OS, PFS, irAE |
| Wen et al.39 | retrospective | 35 | 17 | Asia | 53 (20–78) | 31 | advanced melanoma | Nivolumab, Ipilimumab | efficacy, OS, PFS, irAE |
| Zimmerman et al.32 | retrospective | 39 | 87 | North America | 58 | NA | advanced melanoma | Ipilimumab | efficacy, OS, PFS, irAE |
irAE, immune-related adverse events; NA, not available; NSCLC, non-small-cell lung cancer; OS, overall survival; PD-1, programmed cell death protein-1; PD-L1, Programmed death-ligand 1; PFS, progression-free survival.
NOS quality assessment result
As most of the included trials were retrospective studies, the NOS method was applied to assess the overall quality of these studies. As shown in Table 2, five studies27,30,32,40,46 had five stars, showing high risk of bias and were considered as low to moderate quality. The main reasons lowering the overall quality were selection and outcome bias. The data of incidence of irAEs from these studies were used for overall calculation of irAEs occurrence, but excluded from meta-analysis.
Table 2.
Quality assessment of included studies.
| Author | Selection |
Comparability |
Outcome |
Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||
| Ali et al.25 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Arbour et al.29 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Dumenil et al.43 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Faje et al.26 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Freeman and Weber38 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Freeman-Keller et al.19 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Fucà et al.44 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Fujii et al.31 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Fujisawa et al.37 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Haratani et al.11 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Horvat et al.18 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Hua et al.49 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Indini et al.47 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Judd et al.17 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Kim et al.33 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Kothari et al.28 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Lisberg et al.34 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Margiotta et al.46 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Mian et al.27 | ☆ | ☆ | ☆☆ | ☆ | ☆ | 6 | |||
| Nakamura et al.35 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Owen et al.30 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Pawel et al.40 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Ricciuti et al.48 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Rogado et al.16 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Santini et al.24 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Sato et al.15 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Scott and Pennell41 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Shah et al.45 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Taniguchi et al.42 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Teraoka et al.23 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Toi et al.10 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Toi et al.36 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Wen et al.39 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Zimmerman et al.32 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
Note: “Selection” part includes A: representativeness of cases, B: selection of controls, C: exposure ascertainment, and D: no death when investigation begin. “Comparability” part includes E: comparable on confounders. “Outcome” part includes F: outcome assessment, G: adequate follow-up, and H: loss to follow-up rate. The total score is equal to the total number of stars.
Results of meta-analysis
OS
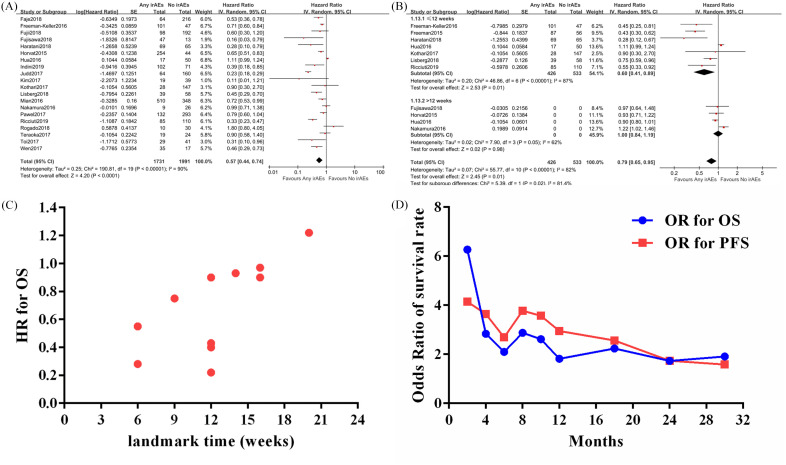
The impact of irAEs on OSin cancer patients treated with ICIs was assessed in 20 studies.11,16–19,23,26–28,31,33–40 Among them, 1731 cancer patients presented at least one of the reported irAEs, and 1991 cases were absent from irAEs. The random-effect model was applied (p < 0.01, I2 = 90%). The combined result (Figure 2A) showed that patients with irAEs had a significantly reduced risk of mortality compared with no irAEs group (HR = 0.57; 95% CI: 0.44, 0.74; p < 0.0001).
Figure 2.
Assessment of the prognostic role of any immune-related adverse events (irAEs) versus no irAEs on overall survival (OS) in cancer patients treated with immune checkpoint inhibitors. A, The association between irAEs and OS; B, Assessing the association between irAEs and OS based on landmark analysis results; C, The distribution of hazard ratios from different landmark analyses with regard to OS in cancer patients with versus without irAEs; D, The estimated odds ratios for OS and progression-free survival in cancer patients with versus without irAEs at different time-point.
The prognostic role of irAEs is dependent on onset time of irAEs
Next, we examined the impact of onset time of irAEs on the prognostic role of irAEs. The timings of landmark analyses in the included studies ranged from 6 weeks to 20 weeks, and we classified the studies into two groups (⩽12 and >12 weeks). As shown in Figure 2B, the patients with any irAEs still had a better OS (HR = 0.60; 95% CI: 0.41, 0.89; p = 0.01) than those without irAEs in the ⩽12 subgroup analysis. However, when the landmark timing extended to >12 weeks, there was no significant difference between OS in patients with any irAEs versus no irAEs (HR = 1.00; 95% CI: 0.84, 1.19; p = 0.98).
To further evaluate the impact of time on prognostic role of irAEs, we used individual HRs from included studies with landmark analysis to draw a scatter plot (Figure 2C), and found that the HR of irAE versus no irAE was increasing over time (linear regression, R2 > 0.4). Next, the number of death and number at risk at various time-points extracted from the survival curve of the included studies were used to calculate a series of OR for OS and PFS, aiming to assess the influence of time on the prognostic effect of irAEs. As shown in Figure 2D, the prognostic effects of irAEs with regard to OS and PFS were decreased over time. At 2 months, the ORs of irAEs versus no irAEs for OS and PFS were 6.27 and 4.15, whereas they were 1.91 and 2.95 at 12 months, respectively. Together, these results showed that the association between irAEs and survival in cancer patients treated with ICIs was changing over time.
Specific irAEs on OS
Next, we assessed the prognostic effect of specific irAEs on OS in cancer patients treated with ICIs. There were five studies11,26,33,35,48 that reported the impact of endocrine adverse events on survival, and seven studies11,19,23,35,39,48,49 showed survival data of patients suffering skin and vitiligo events. As shown in Supplemental Figure 1A, the random-effect model was used (p < 0.1). The pooled results showed that patients with endocrine adverse events had a 61% reduction in risk of death (HR = 0.39; 95% CI: 0.27, 0.56; p < 0.0001) compared with patients without these events. Patients that presented with skin rash or vitiligo also had a significantly lower risk of mortality (HR = 0.48; 95% CI: 0.28, 0.84; p = 0.009) compared with no irAEs group. When combining the data of these two groups, the overall HR was 0.43 (p = 0.0003) with a low risk of heterogeneity (p = 0.50, I2 = 0%).
Number of irAEs on OS
Two studies19,34 evaluated the impact of number of irAEs on OS. The results from these studies suggested that increased number of irAEs may be associated with better survival when comparing with those without irAEs or lower number of irAEs. As there were differences in the statistical methods, it was not appropriate to perform meta-analysis. By performing the Cox proportional hazards regression models, the study by Lisberg et al. found that increasing numbers of irAEs was associated with a trend toward improved OS (unadjusted HR = 0.77; p = 0.079 and adjusted HR = 0.72; p = 0.088).34 The study of Freeman-Keller et al. showed that compared with patients who reported two or fewer irAEs, OS benefit was observed in those with three or more irAEs (HR = 0.53; p < 0.001).19
High-grade irAEs and OS
Only two studies17,31 reported survival data of patients with high-grade irAEs (grade 3 or higher) versus no irAEs. These patients were diagnosed with various types of cancer. However, the heterogeneity between these two studies was low, as indicated by the test (p = 0.92, I2 = 0%). The pooled result failed to determine a significant role for high-grade irAEs in predicting the survival of cancer patients treated with ICIs, though there was a trend (HR = 0.61; 95% CI: 0.38, 1.00; p = 0.05).
PFS
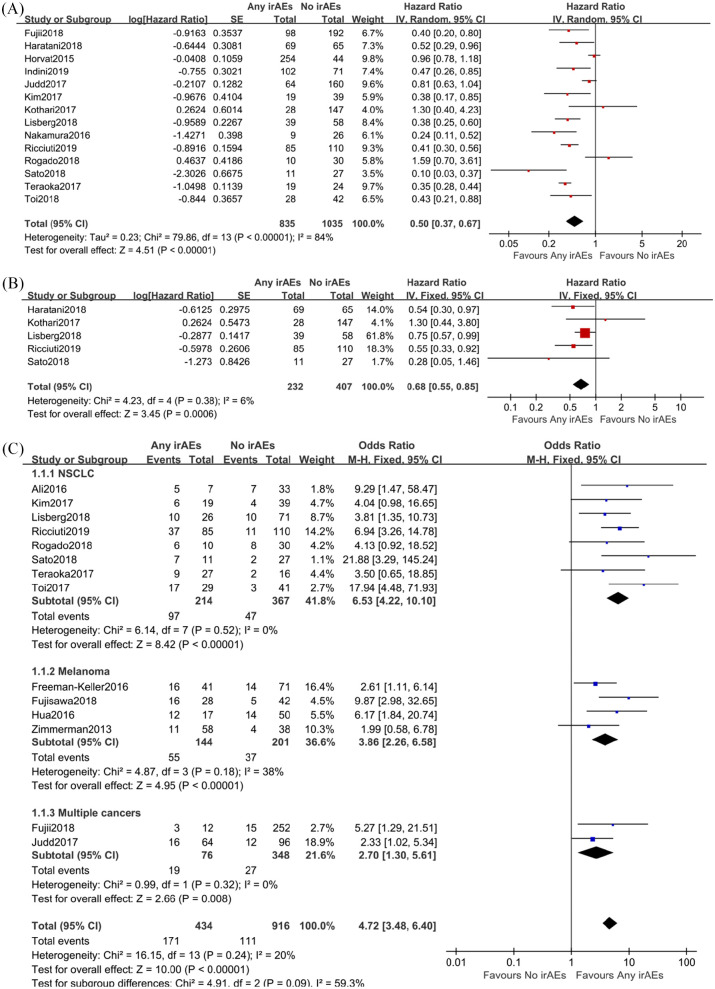
Fifteen studies10,11,15–18,23,28,31,33–36,47,48 reported the PFS of cancer patients with or without irAEs (Figure 3A). A significant heterogeneity between included studies was found (p < 0.01, I2 = 84%), and the random-effect model was introduced to minimize the impact of differences. The overall result showed that patients with irAEs had a lower risk of disease progression (HR = 0.50; 95% CI: 0.37, 0.67; p < 0.00001) when compared with those without irAEs. We also performed a meta-analysis based on the data from landmark analysis (Figure 3B). The pooled result showed that occurrence of irAEs was associated with better PFS (HR = 0.68; 95% CI: 0.55, 0.85; p = 0.0006) in patients receiving ICIs, though some of the included studies showed negative conclusions.
Figure 3.
Combined analysis of prognostic effect of any immune-related adverse events (irAEs) versus no irAEs on progression-free survival (PFS) and efficacy in cancer patients treated with immune checkpoint inhibitors. A, The association between irAEs and PFS; B, Assessing the association between irAEs and PFS based on landmark analysis results; C, Objective response rates in cancer patients with or without irAEs when treated with immune checkpoint inhibitors. Subgroup analysis was performed with regard to cancer types (non-small-cell lung carcinoma, melanoma, and other cancers).
ORR
Fourteen studies10,15–17,19,23,25,26,32–36,48,49 reported ORRs in ICIs-treated cancer patients presenting irAEs versus no irAEs. The random-effect model was used as the subgroup I2 was 59.3%. As presented in Figure 3C, appearance of irAEs in cancer patients was associated with an improved ORR when compared with those without irAEs (OR = 4.72; 95% CI: 3.48, 6.40; p < 0.00001), indicating the ICIs efficacy was almost five times better in patients with irAEs.
We introduced subgroup analysis to evaluate whether the prognostic role of irAEs related to treatment response was independent of cancer types or not. The results showed that the relation of irAEs and efficacy was independent of cancer types. The HRs for NSCLC, melanoma and other cancers were 6.53 (95% CI: 4.22, 10.10; p < 0.0001), 3.86 (95% CI: 2.26, 6.58; p < 0.00001), and 2.70 (95% CI: 1.30, 5.61; p = 0.008), respectively.
Subgroup analysis of OS and PFS
Cancer type
The prognostic role of irAEs on OS (HR = 0.58; 95% CI: 0.39, 0.87; p = 0.008) and PFS (HR = 0.45; 95% CI: 0.33, 0.61; p < 0.00001) were significant in NSCLC patients treated with ICIs (Supplemental Figure 2A and 2B). Similar results were observed for OS (HR = 0.68; 95% CI: 0.53, 0.87; p = 0.002), but not for PFS (HR = 0.51; 95% CI: 0.23, 1.15; p = 0.10) in melanoma patients.
Drug type
The common agents were Nivolumab and Ipilimumab. The subgroup analysis showed that irAEs was a significant predictor for OS (HR = 0.58 for Nivolumab; HR = 0.64 for Ipilimumab; HR = 0.48 for other, p < 0.05 for all) and PFS (HR = 0.45 for Nivolumab; HR = 0.57 for other, p < 0.01 for all), suggesting the prognostic role of irAEs was not dependent on the types of the immune checkpoint blockades (Supplemental Figure 3A and 3B).
Region
The studies were reported from Asia, North America and Europe. The subgroup analysis showed that OS of cancer patients from Asia (HR = 0.51; 95% CI: 0.31, 0.83; p = 0.006) and North America (HR = 0.57; 95% CI: 0.41, 0.78; p = 0.0004) was better if they had irAEs, but not Europe (HR = 0.70; 95%CI: 0.32, 1.57; p = 0.39), indicating that the predictive role of irAEs on OS was dependent on region (Supplemental Figure 4A). As shown in Supplemental Figure 4B, the prognostic role of irAEs still worked on PFS in patients in Asia (HR = 0.35; 95% CI: 0.27, 0.46; p < 0.00001), but the prognostic role of irAEs on PFS was not significant in cancer patients from North America (HR = 0.87; 95% CI: 0.65, 1.16; p = 0.33) and Europe (HR = 0.62; 95% CI: 0.31, 1.23; p = 0.17).
Assessment of publication bias
The funnel analysis of the included studies was conducted using OS, PFS and ORR data. The symmetry of the funnel graph is good (Supplemental Figure 5), suggesting that the results are less likely to be affected by publication bias.
Discussion
This meta-analysis confirmed that patients with irAEs had better survival and treatment response from immune checkpoint blockades when compared with patients without irAEs. The results also indicated that the predictive value of irAEs may be dependent on onset timing of irAEs, cancer type, region, but independent of ICI type. With regard to specific irAEs, we found skin reaction and endocrine adverse events were associated with better OS than those without these events. For number and grade of irAEs, it was suggested that patients with more irAEs and higher grade irAEs may have better OS.
In recent years, several biomarker candidates have emerged and some of them are promising, such as PD-1,1,2 tumor mutation burden,50 sex,51 and baseline neutrophil to lymphocyte ratio (NLR).20 Biomarkers such as PD-1 and tumor mutation burden are accurate and reliable, as supported by high-quality clinical studies.1,2,52 But these biomarkers are usually expensive and complex. Sex and NLR are recently suggested to be prognostic markers of immunotherapy in cancer patients. A meta-analysis by Conforti et al. found that the difference in efficacy between men and women treated with ICIs was significant (p = 0.0019), and they concluded that the magnitude of immunotherapy benefit was sex-dependent.51 Our previous study suggested that baseline NLR was also a reliable and feasible biomarker in advanced NSCLC patients treated with Nivolumab, though this finding was limited to NSCLC.20 In this study, we found that irAEs was associated with better outcomes of ICIs, and this relation was dependent on time, cancer types and region. The degree and number of irAEs also impacted the OS of patients treated with ICIs.
To our knowledge, this is the first landmark-based meta-analysis evaluating the relation between any irAEs and efficacy of ICIs and survival in various cancers with positive findings. Previously, there was a meta-analysis53 evaluating the prognostic role of vitiligo development on survival of patients with stage III–IV melanoma receiving immunotherapy. They found that vitiligo development was significantly related with both better OS (HR = 0.25, p = 0.003) and PFS (HR = 0.51, p = 0.005), when compared with those without vitiligo development.53 In 2016, an abstract by Prince et al.54 suggested that AEs with checkpoint inhibitors did not predict for improved OS. It seems that there are still disagreements even after pooling individual data, and the connection between irAEs and survival fails to reach an agreement. The former study only focused on the specific vitiligo event in melanoma patients, while the latter abstract did not mention the survival data. In this study, we included eligible studies as much as possible, without limitations to cancer type, ICIs type, and region. Instead of focusing on vitiligo, we checked the differences between OS of patients with any irAEs versus no irAEs, and found the occurrence of any irAEs was a beneficial indicator for ICIs treatment in terms of OS, PFS and ORR. These findings demonstrate that irAEs are associated with better efficacy of immunotherapy, indicating irAEs could serve as an indicator of immunotherapy efficacy. Interestingly, we found that the prognostic role of irAEs may be dependent on region. The better survival benefit for Asian patients may be explained by the incidence of irAEs in this population. The study by Yang et al. suggested that the incidence of irAEs in Asian patients could be as high as 90%, possibly related to T-cell aggregation.55 Of note, we also aimed to address the association between specific irAEs and prognosis of patients receiving ICIs treatments. However, we focused only on skin and endocrine toxicities in particular due to lack of sufficient data on other irAEs.
Though a positive link is found between irAEs and efficacy and survival of cancer patients treated with ICIs, it is still not convincing in determining irAEs as a prognostic factor for immunotherapy. The timing of occurrence of irAEs varied between individuals. Patients may already exhibit favorable benefits from ICIs before the appearance of irAEs, or experience irAEs after several cycles of treatments. A treatment landmark-based study may reduce the influence of the above factor. Indeed, after performing meta-analyses based on data from landmark analysis, we find the prognostic role of irAEs is not significant when a longer timing (>12 weeks) is applied, suggesting irAEs may be a time-dependent prognostic factor.
However, a better way to assess the effect of onset time of irAEs on survival outcomes is accessing and evaluating individual data. It is suggested that irAEs are associated with antibody production and memory immune responses. Both early and late onset of irAEs should be associated with better survival upon immunotherapy. In contrast, our results found that irAEs that occurred after 12 weeks were not significantly associated with improved outcomes. We suggest that there is a non-proportional (early) effect of irAEs on PFS and OS, and this might partially explain the lack of effect of irAEs on OS when the landmark time is >12 weeks. Another reason may be the limited number of studies reporting >12 weeks landmark analysis. With regard to time-point, there were a few time-points used for landmark analysis in the included studies, such as 6 weeks (n = 2), 9 weeks (n = 1), 12 weeks (n = 4), 14 weeks (n = 1), 16 weeks (n = 2), and 20 weeks (n = 1). The time-point of 12 weeks was used as a cut-off as it is located in the middle of multiple cut-off values and may represent the actual impact of irAEs on survival (with relevantly sufficient studies for analysis). In fact, there is currently a lack of best cut-off time-point for landmark analysis in these patients. The impact of discontinuous ICIs on survival is not well known, though there is evidence supporting that re-challenge with ICIs is still an effective way to control malignant diseases.24,56 For these re-treated patients, it is also not known whether irAEs are still associated with improved outcomes or not.
There are several limitations within our study. First, although we included 34 studies, they were almost all retrospective trials with small numbers of participants, making inevitable baseline differences. Indeed, the baseline characteristics of these eligible studies differed from each other, such as number, age, sex of participants, disease type and stage, treatments, irAE definition, and outcome measurement. To minimize the impacts of these factors on survival, we used subgroup analyses in terms of cancer types, ICIs types, and region. Second, there may be publication bias. This may be related to the published literature that was mostly positive results. The ones that the analysis failed to include could be gray literature, such as unpublished literature, unpublished results due to negative results, special reports, etc. Third, risks of selection, reporting, and outcome bias existed within the included studies. Patients with treatment response experienced more cycles of ICIs, which may result in increased risk of irAEs. In addition, patients with rapid progression and irAEs after short-term ICIs treatment may be not included in the original study. Of note, there are differences between anti-PD-1 and anti-CTLA-4 in terms of the safety profile, outcomes and mechanisms. In general, anti-CTLA-4 therapy alone yields higher toxicity compared with anti-PD-1/L1 therapy alone. When the two combined together, there generally has been an even higher rate of irAEs as well as higher response. These factors were bound to affect the results of our study. In view of the above defects and problems, it is suggested that the results of this study should be applied with caution.
Conclusion
Our findings suggest that irAEs is a time-dependent prognostic factor for cancer patients treated with ICIs. Further research and clinical trials are needed to verify our findings.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835920980546 for The association between immune-related adverse events and the prognosis of solid cancer patients treated with immunotherapy: a systematic review and meta-analysis by Huilin Xu, Ximing Xu, Wei Ge, Jinju Lei and Dedong Cao in Therapeutic Advances in Medical Oncology
Footnotes
Authors’ contributions: XX performed the study selection, data extraction and analysis, and manuscript writing.
HX performed the study selection, evaluated the quality, data extraction and analysis.
WG conducted the data extraction and quality assessment.
JL conducted the data extraction and quality assessment.
DC designed, examined the data and monitored and supervised the process of this study.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Fund of China (grant number 81700208 and 31971166).
Role of the funding source: The funding provides partial financial support during the process of study selection, and data extraction.
Availability of data and material: All data generated or analyzed during this study are included in this published article and its supplementary information files.
ORCID iD: Dedong Cao  https://orcid.org/0000-0002-5777-4176
https://orcid.org/0000-0002-5777-4176
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Huilin Xu, Department of Oncology, The Fifth Hospital of WuHan, WuHan, Hubei, China.
Ximing Xu, Department of Oncology, RenMin Hospital of Wuhan University, Wuhan, China.
Wei Ge, Department of Oncology, RenMin Hospital of Wuhan University, Wuhan, China.
Jinju Lei, Department of Oncology, RenMin Hospital of Wuhan University, Wuhan, China.
Dedong Cao, Department of Oncology, RenMin Hospital of Wuhan University, Jiefang Road #238 Wuchang District, Wuhan, 430000, China.
References
- 1. The Lancet Oncology. Immunotherapy: hype and hope. Lancet Oncol 2018; 19: 845. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 3. The Lancet Respiratory Medicine. Lung cancer immunotherapy biomarkers: refine not reject. Lancet Respir Med 2018; 6: 403. [DOI] [PubMed] [Google Scholar]
- 4. Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 2018; 50: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Betzalel G, Baruch EN, Boursi B, et al. Possible immune adverse events as predictors of durable response to BRAF inhibitors in patients with BRAF V600–mutant metastatic melanoma. Eur J Cancer 2018; 101: 229–235. [DOI] [PubMed] [Google Scholar]
- 6. Anderson R, Rapoport BL. Immune dysregulation in cancer patients undergoing immune checkpoint inhibitor treatment and potential predictive strategies for future clinical practice. Front Oncol 2018; 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): approaches on special subgroups and unresolved burning questions. Cancer Treat Rev 2018; 64: 21–29. [DOI] [PubMed] [Google Scholar]
- 8. Sattar J, Kartolo A, Hopman WM, et al. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J Geriatr Oncol. Epub ahead of print 10 August 2018. DOI: 10.1016/j.jgo.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 9. Miranda Poma J, Ostios Garcia L, Villamayor Sanchez J, et al. What do we know about cancer immunotherapy? Long-term survival and immune-related adverse events. Allergol Immunopathol (Madr). Epub ahead of print 6 July 2018. DOI: 10.1016/j.aller.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 10. Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist 2018; 23: 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan M, Lin J, Liao G, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018; 97: e11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017; 119: 1–12. [DOI] [PubMed] [Google Scholar]
- 14. Nishijima TF, Shachar SS, Nyrop KA, et al. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist 2017; 22: 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018; 115: 71–74. [DOI] [PubMed] [Google Scholar]
- 16. Rogado J, Pacheco Barcia V, Vera B, et al. 187P Nivolumab-related immune-related adverse events in advanced NSCLC predict therapeutic objective response. J Thorac Oncol 2018; 13: S112. [Google Scholar]
- 17. Judd J, Zibelman M, Handorf E, et al. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 2017; 22: 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 2015; 33: 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016; 22: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao D, Xu H, Xu X, et al. A reliable and feasible way to predict the benefits of nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. OncoImmunology 2018; 7: e1507262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002; 21: 3337–3351. [DOI] [PubMed] [Google Scholar]
- 22. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 23. Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 2017; 12: 1798–1805. [DOI] [PubMed] [Google Scholar]
- 24. Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 2018; 6: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. OncoImmunology 2016; 5: e1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018; 124: 3706–3714. [DOI] [PubMed] [Google Scholar]
- 27. Mian I, Yang M, Zhao H, et al. Immune-related adverse events and survival in elderly patients with melanoma treated with ipilimumab. J Clin Oncol 2016; 34: 3047. [Google Scholar]
- 28. Kothari S, Bagley S, Aggarwal C, et al. Immune-related adverse events and their effect on outcomes in patients (PTS) with non-small cell lung cancer (NSCLC) treated with nivolumab. J Thorac Oncol 2017; 12: S1290. [Google Scholar]
- 29. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018; 36: 2872–2878. [DOI] [PubMed] [Google Scholar]
- 30. Owen DH, Wei L, Villalona-Calero MA, et al. Impact of immune-related adverse events (irAE) on overall survival (OS) in patients treated with immunotherapy for non-small cell lung cancer (NSCLC). J Clin Oncol 2017; 35: 9080. [Google Scholar]
- 31. Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs 2018; 36: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimmerman ZF, Storer B, Godara A, et al. Outcomes and clinical markers associated with benefit from ipilimumab (Ipi) in patients with advanced melanoma: a retrospective single-institution study. J Clin Oncol 2013; 31: e20048. [Google Scholar]
- 33. Kim HI, Kim M, Lee S-H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. OncoImmunology 2017; 7: e1375642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lisberg A, Tucker DA, Goldman JW, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res 2018; 6: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 2017; 44: 117–122. [DOI] [PubMed] [Google Scholar]
- 36. Toi Y, Sugawara S, Kawashima Y, et al. Immune-related adverse events (IRAES) of nivolumab predicts clinical benefit in advanced lung cancer patients. J Thorac Oncol 2017; 12: S2417. [Google Scholar]
- 37. Fujisawa Y, Yoshino K, Otsuka A, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci 2018; 89: 60–66. [DOI] [PubMed] [Google Scholar]
- 38. Freeman M, Weber J. Subset analysis of the safety and efficacy of nivolumab in elderly patients with metastatic melanoma. J ImmunoTher Cancer 2015; 3(Suppl. 2): P133. [Google Scholar]
- 39. Wen X, Ding Y, Li J, et al. The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: a retrospective case series. Cancer Immunol Immunother 2017; 66: 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Pawel J, Syrigos K, Mazieres J, et al. 1314PAssociation between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol 2017; 28(Suppl. 5). [Google Scholar]
- 41. Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol 2018; 13: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 42. Taniguchi Y, Tamiya A, Isa SI, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res 2017; 37: 5857–5862. [DOI] [PubMed] [Google Scholar]
- 43. Dumenil C, Massiani MA, Dumoulin J, et al. Clinical factors associated with early progression and grade 3-4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS One 2018; 13: e0195945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019; 4: e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shah N, Kelly W, Ma B, et al. P2.07-050 Impact of steroid use for immune related adverse events on outcomes in Non-Small Cell Lung Cancer (NSCLC) treated with checkpoint inhibitors. J Thorac Oncol 2017; 12: S2148. [Google Scholar]
- 46. Margiotta P, Caldararo M, Altman D, et al. Effect of pretreatment steroids on the development of immune related adverse events. J Clin Oncol 2018; 36: e15095. [Google Scholar]
- 47. Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019; 145: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019; 145: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016; 152: 45–51. [DOI] [PubMed] [Google Scholar]
- 50. Devarakonda S, Rotolo F, Tsao M-S, et al. Tumor mutation burden as a biomarker in resected non-small-cell lung cancer. J Clin Oncol 2018; 36: 2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 2018; 19: 737–746. [DOI] [PubMed] [Google Scholar]
- 52. Offin M, Rizvi H, Tenet M, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. Epub ahead of print 25 July 2018. DOI: 10.1158/1078-0432.CCR-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015; 33: 773–781. [DOI] [PubMed] [Google Scholar]
- 54. Prince RM, Dãez L, Alcaraz-Sanabria A, et al. Treatment-related side effects as predictors of efficacy of check-point inhibitors (CPIs). J Clin Oncol 2016; 34: 3062. [Google Scholar]
- 55. Seeruttun SR. Equibalancing immune-related adverse events and anticancer activity of immune checkpoint inhibitors. Thorac Cancer 2019; 10: 1855–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martini DJ, Hamieh L, McKay RR, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD-1/PD-L1 therapy for immune-related adverse events. Cancer Immunol Res 2018; 6: 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835920980546 for The association between immune-related adverse events and the prognosis of solid cancer patients treated with immunotherapy: a systematic review and meta-analysis by Huilin Xu, Ximing Xu, Wei Ge, Jinju Lei and Dedong Cao in Therapeutic Advances in Medical Oncology