Abstract
Background:
Transthyretin amyloidosis cardiomyopathy (ATTR-CM) is an underappreciated cause of heart failure that results from misfolded TTR (prealbumin) protein. Diflunisal is an approved non-steroidal anti-inflammatory drug that stabilizes TTR, with limited data available regarding effects on cardiac structure and function.
Methods and Results:
ATTR-CM patients (n=81, 41% treated with 250 mg twice-daily diflunisal by clinical practice) were retrospectively identified with baseline and follow-up (median interval 1 year) serum biomarker and echocardiographic data compared, including global longitudinal strain (GLS). Chi-squared and Wilcoxon tests assessed differences between subjects, divided by treatment group, and univariable and multivariable linear regression was performed. At baseline, patients treated with diflunisal were younger (68 vs 77 years, P = .0001), with lower B-type natriuretic peptide (BNP; 249 vs 545 pg/mL, P = .009) and serum creatinine (1.1 vs 1.2 mg/dL, P = .04), but similar TTR concentration (P = .31), cardiac troponin I (P = .06), and GLS (P = .67). At follow-up, diflunisal untreated versus treated patients showed differences in TTR concentration (19 vs 33 mg/dL, P = .01) and favorable differences in left atrial volume index (+4.6 vs −1.4 mL/m2, P = .002) and cardiac troponin I (+0.03 vs −0.01 ng/mL, P = .01) for the entire cohort. Among the subset with wild-type ATTR (n=53), diflunisal treatment was associated with differences in GLS (+1.2% untreated vs +0.1% treated, P = .03). Changes in wall thickness (P = .2), left ventricular ejection fraction (P = .71), and BNP (P = .42) were similar between groups.
Conclusions:
In ATTR-CM, diflunisal treatment resulted in measurable differences in some parameters of cardiac structure and function after only 1 year of administration. Further longer-term analysis is warranted.
Keywords: Ventricular strain, cardiac amyloidosis, transthyretin, diflunisal
Transthyretin cardiac amyloidosis (ATTR-CM) is an underappreciated cause of heart failure in the elderly that confers significant morbidity and mortality. ATTR-CM is caused by myocardial accumulation of misfolded TTR protein. TTR (also known as prealbumin) circulates in tetramer form, however, in the context of TTR mutation and/or aging, TTR dissociates into monomers that then misfold into amyloid fibrils that accumulate in the myocardium. Amyloid deposition ultimately results in progressively increased myocardial stiffness, manifest as a restrictive cardiomyopathy with resultant heart failure and ultimately death. Recent large case series have found that the median survival in ATTR-CM is highly dependent upon the degree of amyloid accumulation as manifest by clinical symptoms and measured by cardiac biomarkers, with a median survival of approximately 2–6 years from diagnosis,1,2 depending upon disease stage. Two types of ATTR-CM exist: mutant or hereditary ATTR-CM (ATTRv or hATTR) caused by a single nucleotide polymorphism in TTR that favors misfolding, and the likely more common wild-type ATTR-CM (ATTRwt) in which the TTR gene sequence is normal. Recent data indicate that ATTR amyloidosis, particular ATTRwt, is much more common than has previously been appreciated.3,4
Previously felt to be treatable only by orthotopic liver and heart transplantation, novel therapeutics have recently emerged that leverage strategies directed toward stabilization of TTR misfolding or suppression of TTR expression.5–8 The recently reported randomized clinical trial of tafamidis (Pfizer, Inc) in ATTR-CM demonstrated a reduction in a composite endpoint of all-cause mortality and cardiovascular hospitalization, validating the hypothesis that TTR tetramer stabilization can improve clinical outcomes.6 Accordingly, the U.S. FDA approved tafamidis in May 2019 as the only currently approved therapy for ATTR-CM without polyneuropathy. The list price for tafamidis is $225,000 per year.
Diflunisal is an FDA-approved generic nonsteroidal anti-inflammatory drug (NSAID) that has high binding affinity for TTR tetramers, stabilizing the tetramer by increasing its dissociation barrier. Diflunisal has been shown effective in ATTR polyneuropathy,5 can be well tolerated in selected patients with ATTR-CM with appropriate monitoring of renal function,10,11 and appears to prolong survival.12 Thus, diflunisal may represent an available, safe, and cost-effective alternative treatment for ATTR-CM in selected individuals. Because of the widespread availability and cost advantage of diflunisal, we sought to determine whether administration of this agent would result in measurable changes in cardiac structure and function. We hypothesized that diflunisal treatment would result in stabilization or improvement in measured cardiac and serum parameters, whereas untreated patients would demonstrate a detectable decline.
Methods
Patient Selection
Following Institutional Review Board approval, we identified 173 sequential patients with ATTR-CM seen at the Boston University Amyloid Center between 2011 and 2016 of which 81 had complete baseline and follow-up (median interval 1 year) echocardiographic data and were not on other investigational TTR active medications through clinical trial. We selected patients who by definition returned for at least 1 follow-up visit. At baseline all patients were diflunisal naive. Patients were treated with diflunisal as per usual clinical care (n=33) at our Center, and treatment was verified for at least 6 months between the first and second follow-up by chart review. At our Center, patients were treated with diflunisal 250 mg by mouth twice daily and a proton pump inhibitor if they met the following criteria: creatinine clearance > 45 mL/min/1.73m2, New York Heart Association (NYHA) class 3 heart failure or better, and euvolemia on physical examination. Following initiation, renal function was repeated at 1 to 2 weeks to assure stability in creatinine.
Echocardiography
Transthoracic echocardiograms were analyzed consistent with American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines13 and were assessed for left ventricular ejection fraction (LVEF), inter-ventricular (IVSd), and left ventricular posterior wall thickness (PWd) in diastole, left atrial volume index (LAVI; left atrial volume divided by body surface area), early (E) and late (A) mitral inflow Doppler velocities, tissue Doppler velocity of the mitral annulus during early diastole (e′), and tricuspid regurgitant jet velocity. Left ventricular systolic longitudinal strain analysis was performed using the TomTec 2D Cardiac Performance Analysis package (Unterschleissheim, Germany) by 2 readers blinded to treatment group and outcomes. The reported global longitudinal strain (GLS) was calculated as an average of the peak endocardial and myocardial GLS values, with the reference point taken at the start of the QRS complex. A random sample of 20 studies was selected for inter-observer reproducibility of GLS (intra-class correlation coefficient = 0.88).
Biochemical Data
Biochemical data were collected at baseline and follow-up from the electronic medical record. Completeness of data was 96% for B-type natriuretic peptide (BNP), 94% for cardiac troponin I (4th generation assay with reference range: <0.013 ng/mL), 96% for creatinine, and 24% for TTR/prealbumin (the latter of which was routinely measured at our Center starting in 2014 through our hospital clinical laboratory). Comparisons were made with actual data, no imputation of missing data values was performed. Estimated GFR (eGFR) was calculated by the MDRD formula.
Statistical Analysis
Descriptive statistics (median, interquartile range [IQR]) were calculated for parameters at baseline. To determine the effect of diflunisal treatment, only patients that had both complete baseline and follow-up parameters were included in the statistical analysis for a given variable. Chi-squared (or Fisher’s exact for small groups) and two-sample Wilcoxon rank sum (Mann–Whitney U) tests were utilized to compare differences between subjects treated and not treated with diflunisal. Benjamini–Hochberg correction was applied to account for multiple comparisons for the whole cohort at an alpha < 0.05, whereas exploratory subgroup analyses by TTR genetic status were performed without correction. Univariable and limited multivariable linear regression analyses were performed to account for baseline differences. For multivariable modeling, we selected a priori variables that are established predictors of disease progression including age, eGFR, and measures of left ventricular wall thickness and ejection fraction. Two separate models were generated to avoid overfitting of data including: Model 1: Age and eGFR, Model 2: LV wall thickness and LVEF. A P value < .05 was considered as statistically significant. Multivariable linear regression models were tested for normality of residuals, linearity, multicollinearity, autocorrelation, and homoscedasticity. Power and log transformations were applied to variables in models with significantly skewed residuals. All analyses were performed using the R software package v1.0.143 (The R Foundation).
Results
Baseline Characteristics
Baseline characteristics, categorized by treatment assignment, are displayed in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | Untreated (N=48) | Treated (N=33) | Total (N=81) | P |
|---|---|---|---|---|
| Demographics | ||||
| ATTRwt, n (%) | 37 (77%) | 16 (48%) | 53 (65%) | .02 |
| Age, y | 77 (71–81) | 68 (65–73) | 74 (68–79) | .0001 |
| Female, n (%) | 3 (6.3%) | 4 (12.1%) | 7 (8.6%) | .60 |
| BSA, m2 | 2.0 (1.9–2.1) | 2.0 (1.9–2.1) | 2.0 (1.9–2.1) | .96 |
| Caucasian race, n (%) | 34 (71%) | 23 (70%) | 57 (70%) | 1.00 |
| Atrial fibrillation, n (%) | 16 (33%) | 7 (21%) | 23 (28%) | .35 |
| Follow-up interval, y | 1.0 (1.0–1.3) | 1.0 (1.0–1.2) | 1.0 (1.0–1.3) | .97 |
| Echocardiography | ||||
| LVEF, % | 42 (38–49) | 43 (40–50) | 43 (38–50) | .32 |
| GLS, % | −9.1 (−11.1 to −7.5) | −9.6 (−11.7 to −7.8) | −9.5 (−11.7 to −7.5) | .68 |
| Apical/basal ratio | 2.1 (1.8–2.7) | 2.1 (1.7–2.5) | 2.1 (1.7–2.6) | .79 |
| IVSd, mm | 16(15–17) | 16(15–18) | 16(15–17) | .15 |
| PWTd, mm | 16(15–17) | 16(15–18) | 16(15–18) | .21 |
| LAVI, mL/m2 | 42.0 (35.6–51.5) | 43.1 (36.1–50.3) | 42.4 (35.8–51.2) | .91 |
| E, cm/s | 86 (76 – 97) | 83 (68–94) | 85 (72–97) | .18 |
| A, cm/s | 35 (26–59) | 47 (30–69) | 44 (29–63) | .23 |
| E/A | 2.5 (1.4–3.5) | 1.7 (1.4–2.5) | 2.0 (1.4–3) | .04 |
| Mean e′, cm/s | 5 (4–6) | 5 (4–5) | 5 (4–5.3) | .54 |
| E/e′ | 18 (14.5–22) | 18 (14–21) | 18 (14–22) | .81 |
| TR velocity, m/s | 2.6 (2.5–3.1) | 2.4 (2.1–2.7) | 2.6 (2.3–2.9) | .03 |
| Biomarkers | ||||
| BNP, pg/mL | 545 (264–739) | 249 (145–499) | 441 (196–703) | .009 |
| Troponin I, ng/mL | 0.1 (0.07–0.15) | 0.08 (0.04–0.11) | 0.09 (0.06–0.13) | .06 |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.1 (0.9–1.3) | 1.1 (1.0–1.4) | .04 |
| eGFR, mL/min/1.73 m2 | 60.8 (49.0–79.7) | 72.7 (60.5–84.8) | 68.7 (53.3–83.0) | .04 |
| Albumin, mg/dL | 4.0 (3.8–4.3) | 4.1 (3.9–4.4) | 4.1 (3.9–4.3) | .14 |
| TTR,* mg/dL | 18 (17–22) | 22 (20–26) | 21 (19–26) | .31 |
Values listed as median (IQR) for continuous variables and number (%) for categorical variables, P < .05 considered statistically significant for differences between treated and untreated groups.
BSA, body surface area; PWTd: posterior wall thickness in diastole; mean e′, average (septal and lateral) early mitral annular tissue velocity.
TTR: n=15, 12 were treated with diflunisal and 3 untreated.
The median age was 74 years (IQR, 68–79) with ATTRwt diagnosed in n=53 (65%) of the total cohort. A total of n=33 (41%) were treated with diflunisal. Of those treated, 48% had ATTRwt and 52% had ATTRv genotypes (mutations as follows: V1221=14, T60A=9, S77T=1, A97S=1, F33L=1, G47E=1, T59K=1). The majority of patients were Caucasian (70%), and most of the patients were male (91%). A total of n=23 (28%) patients had atrial fibrillation, with no difference between diflunisal treatment groups (odds ratio [OR]: 0.55, 95% confidence interval [CI]: 0.18–1.51, P = .35). Patients who received diflunisal were more likely to be younger (median 68 vs 77 years, P = .0001), with a lower median BNP (249 vs 545 pg/mL, P = .009), TR velocity (2.4 vs 2.6 m/s, P = .03), transmitral E/A ratio (1.7 vs 2.5, P = .04), serum creatinine (1.1 vs 1.2 mg/dL, P = .04), and a higher eGFR (72.7 vs 60.8 mL/min/1.73 m2, P = .04). Baseline median GLS was −9.5% (IQR, −11.7% to −7.5%), which did not differ between treatment groups at baseline. For the entire cohort, median cardiac troponin I (0.09 ng/mL [IQR, 0.06 – 0.13]) and TTR concentration (21 mg/dL [IQR, 19 – 26]) were similar between the 2 groups, as were non-deformational echocardiographic parameters, such as LVEF (43% [IQR, 38 – 50]), left IVSd (16 mm [IQR, 15 – 17]), PWd (16 mm [IQR, 15 – 18]), and E/e′ ratio (18 [IQR, 14 – 22]). When patients were compared by TTR genetic status (ATTRwt vs ATTRv), differences in age, eGFR, LVEF, and TTR concentration were identified (Supplementary Table S1).
Diflunisal Associated Changes in Total Cohort
The median time interval between baseline and follow-up studies was 1 year (IQR, 1.0 – 1.3). Table 2 illustrates the differences between baseline and follow-up for treated and untreated patients for the entire cohort. Data completeness for this analysis can be found in Supplementary Table S2.
Table 2.
Median Differences Between All Treated and Untreated Patients With Diflunisal
| ATTR-CM (N=81) | Untreated (N=48) | Treated (N=33) | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | P | P* | |
| Echocardiography | ||||||
| LVEF, % | 42 | 41 | 43 | 41 | .71 | .71 |
| GLS, % | −9.1 | −8.2 | −9.6 | −8.6 | .36 | .56 |
| Apical/basal ratio | 2.1 | 2.0 | 2.1 | 1.9 | .58 | .62 |
| IVSd, mm | 15.5 | 17 | 16 | 17 | .20 | .47 |
| PWd, mm | 16 | 17 | 16 | 16 | .05 | .14 |
| LAVI, mL/m2 | 42.0 | 46.6 | 43.1 | 41.7 | .002 | .03 |
| E/A | 2.5 | 2.5 | 1.7 | 2.2 | .48 | .56 |
| Mean e′, cm/s | 5 | 4 | 5 | 5 | .04 | .14 |
| E/e′ | 18 | 21 | 18 | 17 | .42 | .56 |
| Biomarkers | ||||||
| BNP, pg/mL | 545 | 478 | 249 | 256 | .42 | .56 |
| Troponin I, ng/mL | 0.1 | 0.13 | 0.08 | 0.07 | .01 | .047 |
| Creatinine, mg/dL | 1.2 | 1.3 | 1.1 | 1.3 | .44 | .56 |
| eGFR, mL/min/1.73 m2 | 60.8 | 57.4 | 72.7 | 65.7 | .36 | .56 |
| TTR, mg/dL | 18 | 19 | 22 | 33 | .01 | .047 |
P values reflect change differences between follow-up and baseline for treated versus untreated patients. TTR: n=15 with complete baseline and follow-up TTR data.
P values with Benjamini–Hochberg correction applied.
Mean e′, average (septal and lateral) early mitral annular tissue velocity.
Diflunisal treatment resulted in a significant increase in levels of TTR concentration (treated: increase of 11.0 mg/dL, untreated: increase of 0.1 mg/dL, P = .01, though only 15 patients had complete baseline and follow-up data, 12 treated and 3 untreated). In addition, diflunisal resulted differences in troponin I between treatment groups (treated: decrease of 0.01 ng/mL, untreated: increase of 0.02 ng/mL, P = .01), and measurable decrease in left atrial volume index (LAVI; treated: reduction 1.4 mL/m2, untreated: increase in 4.6 mL/m2, P = .002). Univariable linear regression analysis also demonstrated that LAVI, mean e′, and TTR concentration were different between treated and untreated patients (Supplementary Table S3). To determine the robustness of these observations, we performed a limited multivariable analysis utilizing a priori selected variables that confer established prognostic significance to adjust the observed differences. Differences in LAVI and TTR concentration remained significant after adjusting for age and baseline eGFR (Model 1) or IVSd and LVEF (Model 2) in linear regression models (Supplementary Table S4). Because of significantly skewed residuals in models that included LAVI and troponin I, these variables were power and log transformed respectively and analysis re-run with no change in significance of results.
Diflunisal Associated Changes in ATTRwt Subgroup
As patients with ATTRwt amyloidosis constituted the predominant type of ATTR-CM in this cohort, we performed additional exploratory analyses in this subgroup. Among patients with ATTRwt (n=53; Table 3), differences in median TTR concentration (treated: increase of 9 mg/dL, P = .04, untreated: decrease of 3 mg/dL) and cardiac troponin I (treated: no change of 0.00 ng/mL, P = .02, untreated: increase of 0.02 ng/mL) were observed (Fig. 1). Differences in GLS and TTR concentration remained significant following multivariable linear regression modeling adjusting for age and baseline eGFR (Model 1) or IVSd and LVEF (Model 2) in linear regression models (Supplementary Tables S5 and Table S6).
Table 3.
ATTRwt Subgroup: Median Differences Between Treated and Untreated Patients With Diflunisal
| ATTRwt (N = 53) | Untreated (N=37) | Treated (N=16) | |||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | P | |
| Echocardiography | |||||
| LVEF, % | 40 | 42 | 42 | 41 | .32 |
| GLS, % | −9.7 | −8.5 | −9.4 | −9.3 | .03 |
| Apical/basal ratio | 1.9 | 2.0 | 2.2 | 2.1 | .63 |
| IVSd, mm | 15 | 17 | 16 | 17 | .31 |
| PWd, mm | 16 | 17 | 16 | 16 | .94 |
| LAVI, mL/m2 | 42.3 | 46.6 | 43.7 | 42.7 | .16 |
| E/A | 2.4 | 2.3 | 1.7 | 2.2 | .46 |
| Mean e′, cm/s | 5 | 4 | 5 | 5 | .09 |
| E/e′ | 18 | 20 | 17 | 15 | .10 |
| Biomarkers | |||||
| BNP, pg/mL | 494 | 407 | 207 | 230 | .56 |
| Troponin I, ng/mL | 0.10 | 0.12 | 0.07 | 0.07 | .02 |
| Creatinine, mg/dL | 1.3 | 1.3 | 1.1 | 1.3 | .41 |
| eGFR, mL/min/1.73 m2 | 59.9 | 56.1 | 68.7 | 54.5 | .21 |
| TTR, mg/dL | 22 | 19 | 24 | 33 | .04 |
P values reflect change differences between follow-up and baseline for treated versus untreated patients. TTR: n=12 with complete baseline and follow-up TTR data.
Mean e′, average (septal and lateral) early mitral annular tissue velocity.
Fig. 1.
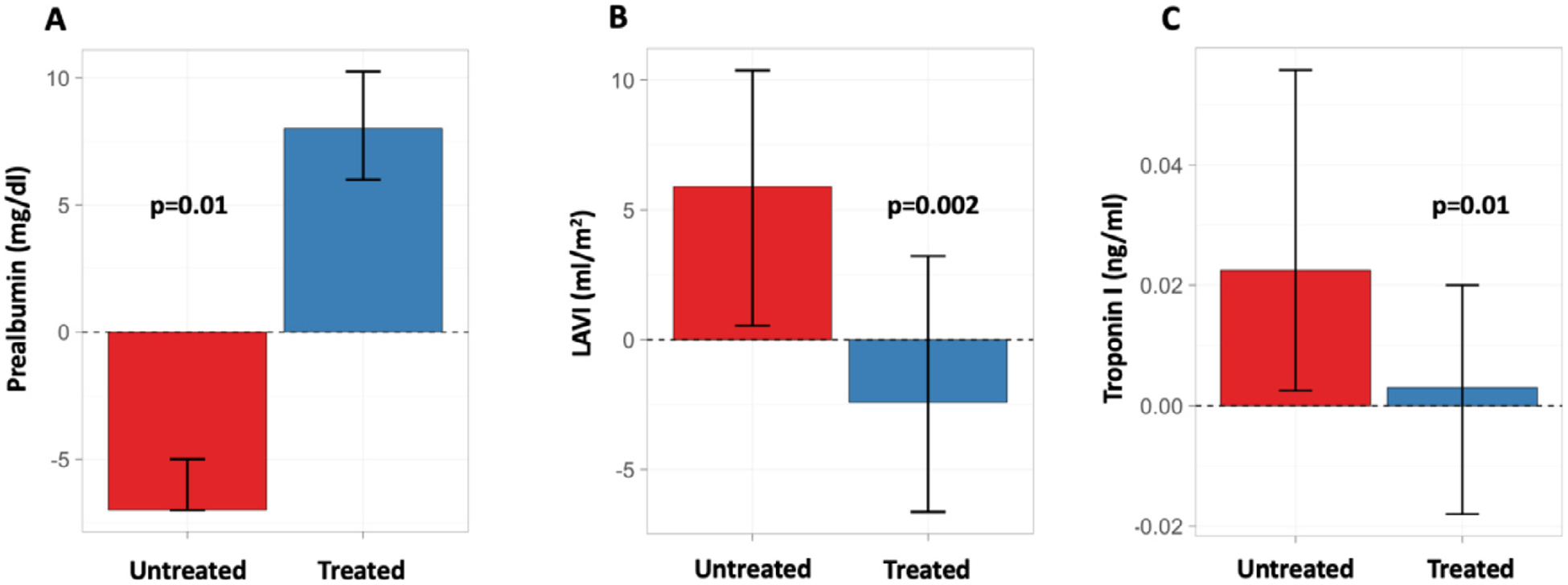
Effect of diflunisal treatment for ATTR-CM on changes in median (A) TTR concentration (n=15), (B) LAVI (n=78), and (C) cardiac troponin I (n=73) at a median follow-up of 1 year. Untreated and treated patients with complete data are shown in red and blue, respectively. Error bars indicate IQR.
In the ATTRwt subgroup, upon follow-up, differences in median GLS was also observed with diflunisal treatment (treated worsening of 0.1%, untreated: worsening of 1.2%, P = .04). Baseline and follow-up changes in GLS for treated and untreated patients, with mean trendlines, can be found in Fig. 2.
Fig. 2.
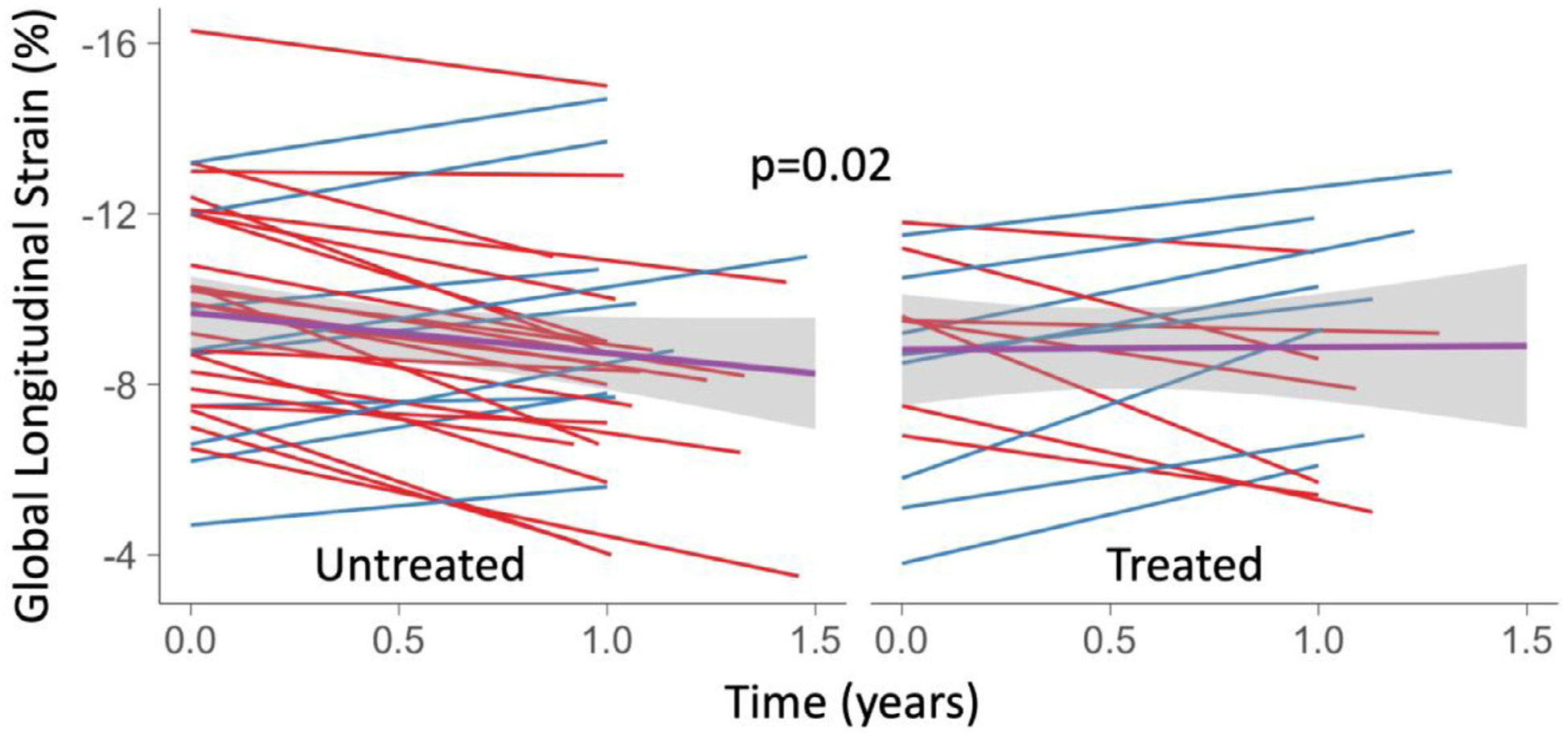
Spaghetti plot of GLS over time for ATTRwt patients untreated (left) and treated (right) with diflunisal. Blue lines indicate improvement and red lines indicate decline in GLS over time. A linear model of changes between baseline and follow-up compared between treated and untreated patients is displayed (black line) with a 95% CI (grey).
There was no significant difference in creatinine change or eGFR with diflunisal treatment (P = .41). Changes for the smaller ATTRv cohort (n=28) can be found in Supplementary Table S7.
Discussion
In this retrospective cohort of patients with ATTR-CM, we confirm that the currently available, inexpensive, oral TTR stabilizer diflunisal can be safely administered to selected patients with ATTR-CM, and that treatment appears to attenuate increase in cardiac biomarkers (troponin I) as well as elicit measurable improvement in cardiac structure and function, including GLS. These data lend support to the observation of improved survival that has been reported in ATTR-CM patients treated with diflunisal as well as other TTR stabilizers.
ATTR-CM is an under-diagnosed but increasingly recognized cause of congestive heart failure. In 2018, the U. S. FDA approved 2 TTR suppressive therapies, patisiran (Onpatro, Alnylam) and inotersen (Tegsedi, Akcea), for hATTR/ATTRv polyneuropathy. ATTR cardiomyopathy in the absence of neuropathy is not an approved indication and further, the most common TTR mutation in the United States, V122I, has been historically associated with cardiomyopathy predominantly. Moreover, approvals for these agents did not extend at all to ATTRwt, thought to be most common type. The TTR stabilizer tafamidis reduced all-cause mortality and the frequency of cardiovascular hospitalizations in patients with ATTR-CM,6 accordingly resulting in its approval by FDA for ATTR-CM irrespective of genotype in May 2019 at a list price of $225,000 per year.
Diflunisal is an inexpensive, FDA approved, NSAID that has been repurposed and shown effective in hATTR/ATTRv neuropathy5 through binding of TTR and stabilization of misfolding, similar in mechanism to tafamidis. Multiple reports that describe the tolerability of diflunisal have been published when administered to selected patients with ATTR-CM with careful monitoring.10,11 Diflunisal was found in early preclinical studies of normal subjects to bind to TTR tetramers in a manner that affords stability to the tetramer structure, thereby preventing the formation of monomers that have the propensity to misfold and aggregate into amyloid fibrils.9 A clinical assessment of TTR stabilization is inferred from increases in serum TTR concentrations with treatment, which we have recently shown to be prognostic in ATTR-CM patients.14 Thus, lower TTR concentrations in ATTR-CM can be seen as indicative of unstable TTR protein, whereas increases in concentration can be inferred as a marker of stabilization. In our current study, we demonstrate that increased TTR concentration associated with diflunisal treatment (albeit shown in a smaller subset of patients) appears to be associated with positive effects on cardiac function.
The clinical benefit of TTR stabilization in ATTR is inferred from 2 randomized clinical trials that evaluated the effect of the 2 available stabilizer medications, tafamidis and diflunisal, on hATTR/ATTRv polyneuropathy. Tafamidis administration was shown to stabilize TTR and delay neuropathy progression.15,16 These data prompted the European Medicines Agency to approve tafamidis for hATTR polyneuropathy. Similarly, our Amyloidosis Center directed a multicenter, randomized, placebo-controlled trial sponsored by NIH/FDA in 130 hATTR polyneuropathy patients for 2 years that demonstrated delayed neurological progression with diflunisal treatment.5
Smaller cohort studies have also suggested the benefit of diflunisal in ATTR-CM. In 40 Japanese ATTRv patients treated with diflunisal, there was no progression in LV wall thickness or ejection fraction after 2 years.17 Koyama et al18 showed that 34 ATTRv patients treated with diflunisal had improvement in apical rotation, but no change in GLS after 1 year. As is our study, neither of these studies was a placebo-controlled trial. Finally, a recent single-center retrospective study comparing 29 ATTR-CM patients on stabilizer medications (tafamidis in 16 patients and diflunisal in 13 patients) to 91 ATTR-CM patients not on stabilizer medications, showed a reduced risk of death in patients treated with stabilizer medications, even after adjusting for markers of an earlier stage of disease (Cox HR: 0.37, P = .003).12
Given the limitations of this retrospective study, our results with diflunisal are quantitatively similar to those seen with the TTR suppressive medication patisiran when studied in the prespecified hATTR/ATTRv cohort of the APOLLO study.19 In this analysis, patisiran was associated with a statistically significant reduction in NT-proBNP and LV wall thickness compared with untreated patients, with differences noted in GLS over 18 months between treatment groups. In specific, GLS did not measurably change in the patisiran treated subjects, whereas untreated controls demonstrated worsening of GLS by 1.46% over 18 months. We report very similar results in our treated patients (minimal change of 0.1% after a median of 1 year) versus untreated patients (worsening of 1.2% after a median of 1 year or 1.8% if extrapolating over 18 months). Likewise, in the ATTR-ACT study, baseline GLS was similar to our cohort (−9.3%), and there was a measurable absolute difference of 0.7% in GLS at 30 months between treated and untreated patients.6 Although our study is a retrospective, uncontrolled cohort, the general concept holds that treatment appears to attenuate the decline in measurable cardiac parameters. We would hypothesize that, similar to the ATTR-ACT study, a measureable decline in cardiac parameters including GLS would be detectable over 12 to 18 months among patients treated with diflunisal but that the trajectory of decline would be attenuated compared with untreated patients.
Our study has several limitations. First, this was a retrospective study subject to selection bias. The decision of diflunisal treatment was made by the treating clinician, and the tolerance of diflunisal almost definitely would have excluded those with more advanced cardiac or renal disease. Thus, baseline differences in the population may have influenced the trajectory of change and results observed. However, it is notable that baseline troponin, GLS, LVEF, E/e′ ratio and wall thickness were similar between treated and untreated patients. Further, one cannot generalize our results to a more advanced ATTR-CM population with more severe chronic kidney disease, NYHA class 4 heart failure, or other factors that would preclude administration of an NSAID. It is important to note that although creatinine clearance decreased at follow-up in both treated and untreated patients, treatment with diflunisal was well tolerated. Second, by design, our study was not able to determine clinical outcomes including survival. Only patients who attended a follow-up visit were included in this study. Because many patients at our Center are referred nationally, data addressing interval heart failure admissions or clinic visits were not sufficiently reliable so as to accurately assess the outcomes effect of diflunisal treatment in this short duration. Third, we excluded patients enrolled in a clinical trial (including those treated with patisiran, tafamidis, or inotersen) and those without follow-up, introducing potential selection bias. Finally, because of the orphan status of the disease, our sample size is rather small, increasing the possibility of a type 2 error. Additionally, there was substantial missing data for TTR concentration owing to routine measurement of this variable only starting midway through our study period. For all of these reasons, our data should be seen as hypothesis generating. We submit that larger studies with longer duration follow-up assessing clinical measurable changes and outcomes are now required to better determine efficacy of diflunisal in ATTR-CM.
Conclusion
Our findings suggest that the widely available, inexpensive oral NSAID diflunisal can be safely administered to selected patients with ATTR-CM and that treatment may attenuate some parameters of cardiac functional decline after 1 year of administration. Diflunisal may therefore represent a viable and inexpensive alternative to novel therapeutics among selected patients with ATTR-CM who are unable to afford or have access to the newest FDA-approved therapies. Further analysis with larger cohorts is warranted to determine longer-term treatment efficacy of diflunisal and its relation to clinical outcomes.
Supplementary Material
Acknowledgment
We greatly acknowledge the statistical support and advising provided by Jitendra Ganju, PhD.
Funding: AHA Fellow-to-Faculty Award 17FTF33670369 (to D.M.G.). This work was supported by a research grant from Eidos Therapeutics (to F.L.R.) and NIH R01HL139671.
Footnotes
Disclosures
G.L., A.P., R.M., L.H.C., N.V., O.K.S.: none; M.S.M., Consultant: Pfizer, Eidos Therapeutics, and serving on Advisory Boards for Pfizer, Alnylam, Eidos, Akcea; F.L.R., Consultant: Pfizer, Inc, Research grant: Eidos Therapeutics, Pfizer, Inc; D.M.G., Eidos Therapeutics; J.F., employment Eidos Therapeutics; J.H., prior employment, Eidos Therapeutics; J.L.B., Consultant: Alnylam Pharmaceutical, Ionis Pharmaceutical/Akcea Therapeutics, Intellia Therapeutics.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2019.11.024.
References
- 1.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016;68:1014–20. [DOI] [PubMed] [Google Scholar]
- 2.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39:2799–806. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL, Dispenzieri A, Grogan M, Johnson G, Bokhari S, Maurer MS. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol 2016;1:880–9. [DOI] [PubMed] [Google Scholar]
- 5.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, Diflunisal Trial Consortium. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA 2013;310:2658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–16. [DOI] [PubMed] [Google Scholar]
- 7.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 8.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 9.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 2006;13:236–49. [DOI] [PubMed] [Google Scholar]
- 10.Ikram A, Donnelly JP, Sperry BW, Samaras C, Valent J, Hanna M. Diflunisal tolerability in transthyretin cardiac amyloidosis: a single center’s experience. Amyloid 2018;25:197–202. [DOI] [PubMed] [Google Scholar]
- 11.Castãno A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail 2012;18:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenblum H, Castano A, Alvarez J, Goldsmith J, Helmke S, Maurer MS. TTR (Transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail 2018;11:e004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the AmericanSociety of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. e14. [DOI] [PubMed] [Google Scholar]
- 14.Hanson JLS, Arvanitis M, Koch CM, Berk JL, Ruberg FL, Prokaeva T, Connors LH. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail 2018;11:e004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 2012;79:785–92002E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barroso FA, Judge DP, Ebede B, Li H, Stewart M, Amass L, Sultan MB. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid 2017;24:194–204. [DOI] [PubMed] [Google Scholar]
- 17.Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid 2015;22:79–83. [DOI] [PubMed] [Google Scholar]
- 18.Koyama J, Minamisawa M, Sekijima Y, Ikeda SI, Kozuka A, Ebisawa S, Miura T, Motoki H, Okada A, Izawa A, Ikeda U. Left ventricular deformation and torsion assessed by speckle-tracking echocardiography in patients with mutated trans-thyretin-associated cardiac amyloidosis and the effect of diflunisal on myocardial function. Int J Cardiol Heart Vasc 2015;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon SD, Adams D, Kristen A, Grogan M, González-Duarte A, Maurer MS, et al. Effects of patisiran, an rna interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis: an analysis of the APOLLO study. Circulation 2019;139:431–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


