Abstract
Little is known about the influence of prediagnosis and postdiagnosis smoking and smoking cessation on ovarian cancer survival. We investigated this relationship in two prospective cohort studies, the Nurses’ Health Study (NHS) and NHSII. Analyses included 1,279 women with confirmed invasive, Stage I–III epithelial ovarian cancer. We used Cox proportional hazards regression models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for ovarian cancer-specific mortality by smoking status, adjusting for age and year of diagnosis, tumor stage, histologic subtype, body mass index and nonsteroidal anti-inflammatory use (postdiagnosis models only). When examining prediagnosis smoking status (assessed a median of 12 months before diagnosis), risk of death was significantly increased for former smokers (HR = 1.19, 95% CI: 1.02–1.39), and suggestively for current smokers (HR = 1.21, 95% CI: 0.96–1.51) vs. never smokers. Longer smoking duration (≥20 years vs. never, HR = 1.23, 95% CI: 1.05–1.45) and higher pack-years (≥20 pack-years vs. never, HR = 1.28, 95% CI: 1.07–1.52) were also associated with worse outcome. With respect to postdiagnosis exposure, women who smoked ≥15 cigarettes per day after diagnosis (assessed a median of 11 months after diagnosis) had increased mortality compared to never smokers (HR = 2.34, 95% CI: 1.63–3.37). Those who continued smoking after diagnosis had 40% higher mortality (HR = 1.40, 95% CI: 1.05–1.87) compared to never smokers. Overall, our results suggest both prediagnosis and postdiagnosis smoking are associated with worse ovarian cancer outcomes.
Keywords: ovarian cancer, smoking, prognosis, mortality
Introduction
Ovarian cancer is the most fatal gynecological malignancy in the US.1 Although new treatment strategies, such as anti-angiogenesis drugs (e.g., bevacizumab) and poly-adenosine diphosphate-ribose polymerase (PARP) inhibitors, which impair DNA break repair and have improved outcomes in some patients,2 the 5-year survival rate is only 48%.3 Consideration of whether lifestyle factors may influence survival in ovarian cancer patients may provide new opportunities for interventions.
Tobacco smoke is a highly proinflammatory and toxic substance,4 which is a cause of premature death from multiple conditions, including cancers.5–7 Smoking may enhance the invasive potential of cancer cells,8 increase the risk of cancer recurrence and development of second primary cancers,9,10 alter cancer drug metabolism11 and promote the development of thrombosis,12 which are all associated with an increased risk of mortality. Current smoking prior to or at diagnosis was related to worse outcomes of ovarian cancer patients in four out of five prior studies, although mixed results were noted for smoking rate, accumulative pack-years and duration since quitting in the few studies that assessed these associations.13–17 Due to limited sample sizes, most of these studies were not able to evaluate associations by histologic subtype, which have very different outcomes. Although many cancer survivors continue smoking after diagnosis, few studies have examined the impact of postdiagnosis smoking on ovarian cancer survival,17 which may result in decreased survival time due to reduced treatment effectiveness,11,18 worse side effects of treatment19 and increased risk of a second malignancy.20
To gain deeper insight into smoking as a modifiable factor that could potentially influence survival among women diagnosed with ovarian cancer, we investigated the associations of smoking before and after ovarian cancer diagnosis, as well as a change in smoking behavior from prediagnosis to postdiagnosis, with ovarian cancer-specific mortality and all-cause mortality overall and by histologic subtype. We considered associations among all participants and by tumor histology and stage as well as by patient characteristics, such as body mass index (BMI) and postdiagnosis aspirin use.
Materials and Methods
Study participants
The Nurses’ Health Study (NHS) was established in 1976 among 121,700 female registered nurses aged 30–55 years residing in 11 states in the US.21 NHSII began in 1989 among 116,429 female registered nurses aged 25–42 years from 14 US states.22 In the NHS and NHSII, participants reported detailed information about their lifestyle and medical history at study entry; this information has been updated on biennial follow-up questionnaires that were completed by participants regardless of cancer status. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Completion of the questionnaire was considered to imply informed consent.
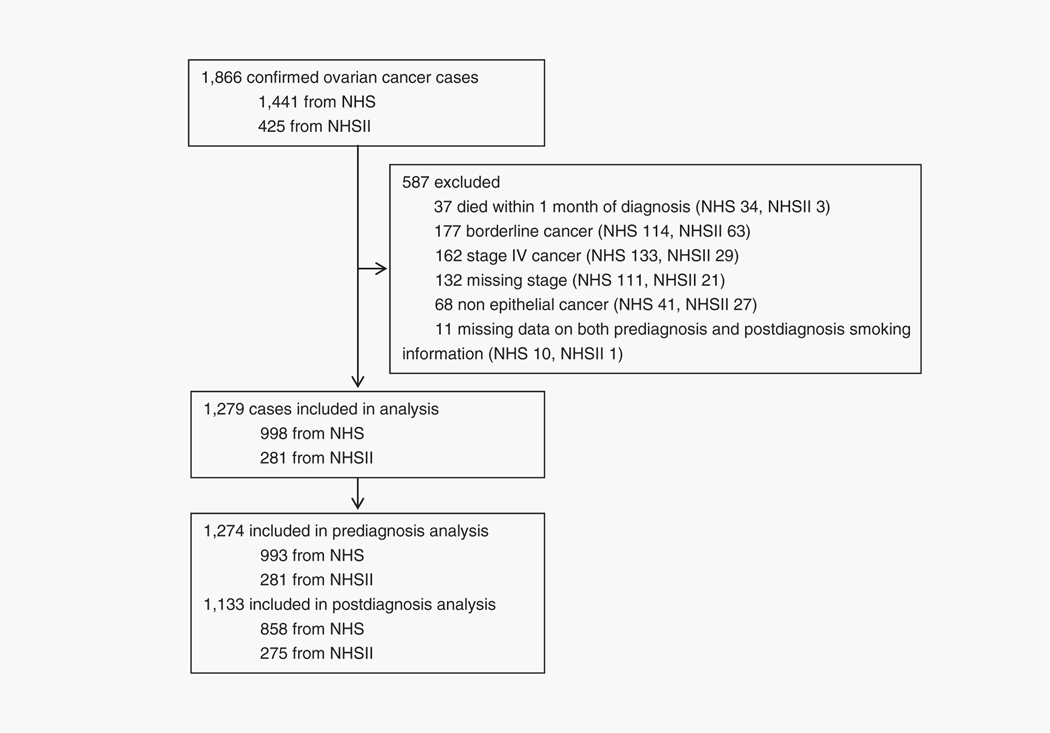
Potentially eligible participants for this analysis included women with confirmed invasive, Stage I–III epithelial ovarian cancer (based on the International Federation of Gynecology and Obstetrics classification) who had data on cigarette smoking either before or after ovarian cancer diagnosis (Fig. 1). Ovarian cancer cases were identified by self-report on the biennial questionnaires and deaths were identified via family members, the National Death Index or the US Postal Service. All ovarian cancer diagnoses were confirmed either via medical record review by a gynecological pathologist or by linkage with the relevant cancer registry. The gynecological pathologist, blinded to smoking status of study participants, abstracted data on tumor stage, histology, grade and morphology. Cause of death among confirmed ovarian cancer cases was obtained from related medical records and death certificates. Patients who died within 1 month of diagnosis (n = 37), with borderline cancer (low malignant potential disease, n = 177), Stage IV (having much lower survival rate23 and less likely to complete a postdiagnosis questionnaire, n = 162) or missing stage (n = 132), nonepithelial cancer (n = 68) or without data on both prediagnosis and postdiagnosis cigarette use (n = 11) were excluded.
Figure 1.
Study participant flow chart in the NHS and NHSII. Abbreviations: NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Assessment of smoking and other covariates
On the baseline questionnaire and every subsequent biennial questionnaire, participants were asked “Do you smoke cigarettes currently?”; those who answered yes were considered to be current smokers at that timepoint and reported the average number of cigarettes smoked per day. Former smokers were those who either reported having ever smoked cigarettes regularly in the past at baseline or those who reported currently smoking in a prior questionnaire, but no longer smoked. On the baseline questionnaire, both current and former smokers reported the age when they started smoking regularly, while former smokers additionally reported the time since quitting smoking and the average number of cigarettes smoked per day before quitting. On each questionnaire, we classified participants as never, former or current smokers, and, for ever smokers, we determined the quantity (cigarettes/day and pack-years), duration and time since quitting (former smokers only).
Prediagnosis cigarette use was assessed at the questionnaire prior to the ovarian cancer diagnosis (e.g., smoking status on the 1980 questionnaire if ovarian cancer was diagnosed in 1981) with a median assessment of 12 months before diagnosis (interquartile range [IQR]: 6–19 months). Postdiagnosis cigarette use was obtained from the first questionnaire with a return date after the date of diagnosis (median of 11 months after diagnosis, IQR: 6–18 months). The change in smoking from prediagnosis to postdiagnosis was defined by the smoking status at the closest assessments immediately prior to and after diagnosis in which the participant reported on smoking behavior (median time between two assessments were 24 months, IQR: 23–27 months), with the following categories: never smoker (never smoker at both prediagnosis and postdiagnosis assessments), former smoker (former smoker at both assessments), quit smoking after diagnosis (current smoker before diagnosis and former smoker after diagnosis) and remained current smoker (current smoker at both assessments). Too few women (n = 2) resumed smoking after diagnosis (former smoker before diagnosis and current smoker after diagnosis) to include in the analysis.
BMI and NSAID use (either aspirin or nonaspirin NSAIDs) were also queried on the biennial questionnaires. Briefly, body height and weight were queried at baseline then weight was updated on each follow-up questionnaire in both cohorts. In the NHS, the use of aspirin and nonaspirin NSAIDs was queried in 1980 and 1990, and every questionnaire thereafter, respectively. In the NHSII, both aspirin and nonaspirin NSAID use were collected in 1989, 1993, and every 2 years thereafter. Prediagnosis and postdiagnosis values of these covariates were defined from the same questionnaire as the relevant smoking assessment.
Outcomes
The primary outcome was ovarian cancer-specific mortality (International Classification of Diseases version 8 codes 1830, 1831 and 1580), while the secondary outcome was all-cause death among ovarian cancer patients. Cases of ovarian, primary peritoneal and fallopian tube cancer were included as the primary outcome since these diagnoses are typically grouped together because of common histologic subtypes and origins.
Statistical methods
Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between smoking and survival. For prediagnosis exposures, the analytic time scale was measured in months from ovarian cancer diagnosis to death or the end of follow-up (June of 2016 for NHS and June of 2017 for NHSII). For postdiagnosis exposures, the analytic time scale was measured in months from the return of the first post-diagnosis questionnaire containing smoking information to death or the end of follow-up. Primary prediagnosis and post-diagnosis exposure models were adjusted for age at diagnosis (continuous), calendar year of diagnosis (to account for potential changes in ovarian cancer treatment over time [continuous]), tumor stage (I–III), cancer histology (high-grade serous [most cases of serous cancer with unknown grade were considered as high-grade and thus were also included in this group] or poorly differentiated, low-grade serous or nonserous [e.g., mucinous, endometrioid, clear cell, transitional/Brenner, carcinosarcoma or mixed subtypes], unknown or other histology) and cohort (NHS, NHSII). We additionally assessed adjustment for BMI at prediagnosis or postdiagnosis depending on the timing of smoking exposure being assessed (<21, 21 to <23, 23 to <25, 25 to <30, 30 to <35, ≥35 kg/m2, or unknown BMI), and postdiagnosis NSAID use (never user, past user, current user of either aspirin or nonaspirin NSAIDs, current user of both aspirin and nonaspirin NSAIDs, unknown NSAID use) for analyses of postdiagnosis smoking, as we previously observed no association of prediagnosis NSAID use and survival.24 Tumor grade as well as additional prediagnosis factors, including parity, menopausal status, oral contraceptive use, postmenopausal hormone therapy, tubal ligation, hysterectomy, alcohol consumption, physical activity, family history of ovarian cancer or breast cancer and comorbidities (including cardiovascular disease, diabetes and lung cancer; assessed at the same time as the exposure), did not substantially alter the risk estimates, and thus were not included in the final models.
In analyses of change in smoking before to after diagnosis, the analytic time scale was measured from the return of the first postdiagnosis questionnaire containing smoking information to death or the end of follow-up. Covariates included age and year at diagnosis, tumor stage and histology, prediagnosis BMI, and cohort, as noted above, as well as change in BMI (<−2, −2 to <0, 0 to <2, ≥2 kg/m2, unknown) and change in NSAID usage from prediagnosis to postdiagnosis (never user, remained past user, current to past user, remained current user, never/past to current user and unknown). Due to the small sample size, participants who reported past smoking before diagnosis and current smoking after diagnosis were excluded from this analysis (n = 2).
To test the proportional hazards assumption, we computed multiplicative interaction terms between smoking and the analytic time scale (continuous) and compared models with vs. without interaction terms using the likelihood ratio test. No deviation from proportional hazards was detected. To test for heterogeneity by cohort, HRs were calculated separately in each cohort, and then pooled using random-effects meta-analysis. We carried out stratified analyses by histologic subtype (high-grade serous vs. nonserous/low-grade serous), BMI (<25 vs. ≥25 kg/m2), NSAID use (noncurrent [including never and past user] vs. current user, postdiagnosis analysis only) and stage (I/II vs. III), and tested potential effect modification by smoking status using a likelihood ratio test comparing models with vs. without interaction terms.
We conducted multiple sensitivity analyses: excluding women with smoking-related comorbidities (cardiovascular disease, diabetes and lung cancer; assessed at the same time as the exposure); excluding women diagnosed with breast or lung cancer, since these cancers are common in women and smoking is related to mortality in these patients populations10,25; including participants missing tumor stage (using a missing indicator); and excluding mucinous ovarian cancer cases, for whom smoking is an established risk factor.26,27 For prediagnosis exposures, we secondarily evaluated exposures reported at least 4 years prior to ovarian cancer diagnosis to ensure that subclinical disease and related symptomology did not affect smoking status. For postdiagnosis exposures, secondary analyses were conducted using exposures reported within 4 years after diagnosis to examine whether results were sensitive to the timing of smoking assessment. All statistical analyses were conducted with SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). p values <0.05 were considered significant and all statistical tests were two-sided.
Data availability
The data that support the findings of our study are available from the corresponding author upon reasonable request.
Results
In total, we identified 1,866 confirmed ovarian cancer cases (1,441 from NHS 1976–2016 and 425 from NHSII 1989–2017; Fig. 1). After excluding 587 participants, 1,279 (68.5%) were included in the analyses (998 from NHS and 281 from NHSII); 1,274 had data on prediagnosis smoking (993 from NHS and 281 from NHSII), 1,133 had data on postdiagnosis smoking (858 from NHS and 275 from NHSII) and 1,128 had data on both prediagnosis and postdiagnosis smoking (853 from NHS and 275 from NHSII).
Median age at ovarian cancer diagnosis was 65 years in NHS and 52 years in NHSII (Table 1). In both cohorts, the majority of study participants were diagnosed with high-grade serous tumors, Stage III cancer and were current NSAID users. Ever smoking was more common in NHS than NHSII. In the prediagnosis exposure analytic population, 892 women (70.0%) died, 759 (85.1%) of which died from ovarian cancer (median survival time was 3.8 years [IQR: 1.7–8.9] in NHS and 6.6 years [IQR: 3.3–12.1] in NHSII). In the analytic population for postdiagnosis exposures, 747 women (65.9%) died, 617 (82.6%) of which died from ovarian cancer (median survival time was 4.5 years [IQR: 2.3–11.3] in NHS and 6.6 years [IQR: 3.5–12.3] in NHSII). The characteristics of ovarian cancer patients by prediagnosis smoking status were shown in Supporting Information Table S1.
Table 1.
Selected characteristics of ovarian cancer patients in prediagnosis and postdiagnosis smoking analyses in the NHS and NHSII
| Prediagnosis analyses |
Postdiagnosis analyses |
|||
|---|---|---|---|---|
| NHS (n = 993) | NHSII (n = 281) | NHS (n = 858) | NHSII (n = 275) | |
| Deaths from ovarian cancer, n (%) | 661 (66.6) | 98 (34.9) | 525 (61.2) | 92 (33.5) |
| Deaths from all causes, n (%) | 787 (79.3) | 105 (37.4) | 648 (75.5) | 99 (36.0) |
| Age at diagnosis, years, median (IQR) | 65.0 (57.0,72.0) | 52.0 (46.0,57.0) | 65.0 (57.0,71.0) | 52.0 (46.0,57.0) |
| Calendar year of diagnosis, median (IQR) | 1998 (1989,2004) | 2006 (2001,2010) | 1998 (1989,2004) | 2006 (2001,2010) |
| Overall survival time since ovarian cancer diagnosis, years, median (IQR) | 3.8 (1.7,8.9) | 6.6 (3.3,12.1) | 4.5 (2.3,11.3) | 6.6 (3.5,12.3) |
| Tumor histologic subtype, n (%) | ||||
| High-grade serous or poorly differentiated | 688 (69.3) | 144 (51.3) | 588 (68.5) | 141 (51.3) |
| Mucinous | 37 (3.7) | 12 (4.3) | 30 (3.5) | 12 (4.4) |
| Endometrioid | 118 (11.9) | 55 (19.6) | 108 (12.6) | 55 (20.0) |
| Clear cell | 51 (5.1) | 34 (12.1) | 47 (5.5) | 32 (11.6) |
| Other or unknown | 99 (10.0) | 36 (12.8) | 85 (9.9) | 35 (12.7) |
| Stage, n (%) | ||||
| I | 201 (20.2) | 101 (35.9) | 197 (23.0) | 99 (36.0) |
| II | 79 (8.0) | 36 (12.8) | 66 (7.7) | 35 (12.7) |
| III | 713 (71.8) | 144 (51.3) | 595 (69.4) | 141 (51.3) |
| Pre-diagnosis BMI, kg/m2, n (%) | ||||
| <21 | 136 (13.7) | 32 (11.4) | 108 (12.6) | 32 (11.6) |
| 21 to <23 | 163 (16.4) | 37 (13.2) | 146 (17.0) | 35 (12.7) |
| 23 to <25 | 196 (19.7) | 37 (13.2) | 172 (20.1) | 35 (12.7) |
| 25 to <30 | 269 (27.1) | 77 (27.4) | 234 (27.3) | 76 (27.6) |
| 30 to <35 | 115 (11.6) | 46 (16.4) | 98 (11.4) | 45 (16.4) |
| ≥35 | 61 (6.1) | 45 (16.0) | 56 (6.5) | 45 (16.4) |
| Missing | 53 (5.3) | 7 (2.5) | 44 (5.1) | 7 (2.6) |
| Prediagnosis NSAID use, n (%) | ||||
| Never user | 108 (10.9) | 43 (15.3) | 88 (10.3) | 42 (15.3) |
| Past user | 206 (20.8) | 65 (23.1) | 179 (20.9) | 65 (23.6) |
| Current user to either aspirin or nonaspirin NSAIDs | 474 (47.7) | 115 (40.9) | 411 (47.9) | 113 (41.1) |
| Current user to both aspirin and nonaspirin NSAIDs | 86 (8.7) | 58 (20.6) | 79 (9.2) | 55 (20.0) |
| Missing | 119 (12.0) | 0 (0) | 101 (11.8) | 0 (0) |
| Prediagnosis smoking, n (%) 1 | ||||
| Never smoker | 438 (44.1) | 171 (60.9) | 379 (44.4) | 167 (60.7) |
| Former smoker | 417 (42.0) | 79 (28.1) | 360 (42.2) | 78 (28.4) |
| Current smoker | 138 (13.9) | 31 (11.0) | 114 (13.4) | 30 (10.9) |
Percentages may not add up to 100%.
In postdiagnosis analyses in NHS, prediagnosis smoking information was missing for five ovarian cancer cases.
Abbreviations: BMI, body mass index; IQR, interquartile range; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NSAID, nonsteroidal anti-inflammatory drug.
In analyses of prediagnosis smoking status, compared to never smokers, the risk of ovarian cancer-specific mortality was significantly increased by 19% among former smokers (HR = 1.19, 95% CI: 1.02–1.39), and was suggestively increased by 21% among current smokers (HR = 1.21, 95% CI: 0.96–1.51; Table 2). Furthermore, former (HR = 1.30, 95% CI: 1.07–1.58) and current (HR = 1.30, 95% CI: 0.98–1.73) smokers who smoked 15 or more cigarettes per day had worse ovarian cancer-specific mortality compared to never smokers. Longer smoking duration (≥20 years vs. never smoker, HR = 1.23, 95% CI: 1.05–1.45) and higher pack-years (≥20 pack-years vs. never smoker, HR = 1.28, 95% CI: 1.07–1.52) before diagnosis also were associated with greater risk of mortality, while shorter smoking duration (<20 years) and lower pack-years (<20 pack-years) were not significantly associated. No association was observed for time since quitting smoking among former smokers.
Table 2.
Epithelial ovarian cancer-specific mortality and all-cause mortality by smoking in the NHS and NHSII
| Epithelial ovarian cancer-specific mortality |
All-cause mortality |
||||
|---|---|---|---|---|---|
| Total cases, n | Deaths, n | HR (95% CI) | Deaths, n | HR (95% CI) | |
| Prediagnosis smoking | |||||
| Never smoker | 609 | 339 | 1.00 (ref) | 402 | 1.00 (ref) |
| Former smoker | 496 | 316 | 1.19 (1.02,1.39) | 361 | 1.19 (1.03,1.37) |
| Current smoker | 169 | 104 | 1.21 (0.96,1.51) | 129 | 1.30 (1.06,1.59) |
| Prediagnosis smoking rate | |||||
| Never smoker | 609 | 339 | 1.00 (ref) | 402 | 1.00 (ref) |
| Former smoker, <15 cigs/day | 259 | 157 | 1.09 (0.90,1.32) | 183 | 1.12 (0.94,1.34) |
| Former smoker, ≥15 cigs/day | 228 | 153 | 1.30 (1.07,1.58) | 170 | 1.26 (1.05,1.51) |
| Current smoker, <15 cigs/day | 66 | 39 | 1.02 (0.73,1.43) | 41 | 0.93 (0.67,1.29) |
| Current smoker, ≥15 cigs/day | 95 | 59 | 1.30 (0.98,1.73) | 81 | 1.57 (1.22,2.01) |
| Prediagnosis smoking duration | |||||
| Never smoker | 609 | 339 | 1.00 (ref) | 402 | 1.00 (ref) |
| <20 years | 258 | 154 | 1.13 (0.93,1.37) | 173 | 1.11 (0.93,1.33) |
| ≥20 years | 398 | 260 | 1.23 (1.05,1.45) | 309 | 1.27 (1.10,1.48) |
| Prediagnosis smoking pack-years | |||||
| Never smoker | 609 | 339 | 1.00 (ref) | 402 | 1.00 (ref) |
| <20 pack-years | 345 | 207 | 1.11 (0.94,1.32) | 235 | 1.11 (0.94,1.30) |
| ≥20 pack-years | 305 | 202 | 1.28 (1.07,1.52) | 242 | 1.32 (1.13,1.56) |
| Prediagnosis time since quitting smoking | |||||
| Never smoker | 609 | 339 | 1.00 (ref) | 402 | 1.00 (ref) |
| <15 years | 149 | 97 | 1.17 (0.93,1.48) | 110 | 1.20 (0.97,1.49) |
| 15 to <22 years | 105 | 62 | 1.19 (0.90,1.56) | 75 | 1.21 (0.94,1.55) |
| ≥22 years | 235 | 152 | 1.18 (0.97,1.44) | 170 | 1.14 (0.95,1.38) |
| Postdiagnosis smoking | |||||
| Never smoker | 546 | 276 | 1.00 (ref) | 339 | 1.00 (ref) |
| Former smoker | 483 | 279 | 1.26 (1.06,1.50) | 331 | 1.24 (1.06,1.45) |
| Current smoker | 104 | 62 | 1.46 (1.10,1.93) | 77 | 1.45 (1.12,1.86) |
| Postdiagnosis smoking rate | |||||
| Never smoker | 546 | 276 | 1.00 (ref) | 339 | 1.00 (ref) |
| Former smoker, <15 cigs/day | 256 | 142 | 1.24 (1.01,1.53) | 169 | 1.22 (1.01,1.48) |
| Former smoker, ≥15 cigs/day | 216 | 130 | 1.27 (1.03,1.57) | 152 | 1.24 (1.02,1.50) |
| Current smoker, <15 cigs/day | 45 | 23 | 1.08 (0.70,1.66) | 27 | 0.99 (0.67,1.48) |
| Current smoker, ≥15 cigs/day | 54 | 37 | 2.34 (1.63,3.37) | 47 | 2.42 (1.75,3.34) |
Models adjusted for age and calendar year at diagnosis, tumor histologic subtype, stage, body mass index (categorical) and cohort. Postdiagnosis models additionally adjusted for nonsteroidal anti-inflammatory drug usage.
Abbreviations: CI, confidence interval; HR, hazard ratio; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Bold values indicate p < 0.05.
In the postdiagnosis smoking analysis, increased risk of mortality was observed for both former and current vs. never smokers (HR = 1.26, 95% CI: 1.06–1.50 and HR = 1.46, 95% CI: 1.10–1.93, respectively) and for smokers of ≥15 cigarettes/day (among former smokers: HR = 1.27, 95% CI: 1.03–1.57; among current smokers: HR = 2.34, 95% CI: 1.63–3.37).
In analyses of change in cigarette smoking from prediagnosis to postdiagnosis, quitting smoking was not associated with risk of ovarian cancer death (n = 43, HR = 0.84, 95% CI: 0.54–1.31), while 20 and 40% higher risks of mortality were observed for women who were either former smokers (HR = 1.20, 95% CI: 1.01–1.43) or remained current smokers (HR = 1.40, 95% CI: 1.05–1.87), respectively (Table 3).
Table 3.
Epithelial ovarian cancer-specific mortality and all-cause mortality according to change in smoking from prediagnosis to postdiagnosis in the NHS and NHSII
| Epithelial ovarian cancer-specific mortality |
All-cause mortality |
||||
|---|---|---|---|---|---|
| Total cases, n | Deaths, n | HR (95% CI) | Deaths, n | HR (95% CI) | |
| Never smoker | 539 | 275 | 1.00 (ref) | 336 | 1.00 (ref) |
| Former smoker | 433 | 255 | 1.20 (1.01,1.43) | 299 | 1.19 (1.01,1.40) |
| Quit smoking after diagnosis | 43 | 22 | 0.84 (0.54,1.31) | 28 | 0.91 (0.62,1.35) |
| Remained current smoker | 99 | 60 | 1.40 (1.05,1.87) | 74 | 1.43 (1.11,1.86) |
Never smoker is defined as being a never smoker at both prediagnosis and postdiagnosis assessments; Former smoker is defined as being a former smoker at both assessments; Quit smoking after diagnosis is defined as being a current smoker before diagnosis and former smoker after diagnosis; Remained current smoker is defined as being a current smoker at both assessments. Models adjusted for age and calendar year at diagnosis, tumor histologic subtype, stage, prediagnosis body mass index (BMI, categorical), change of BMI (categorical) and nonsteroidal anti-inflammatory drug usage from prediagnosis to postdiagnosis and cohort. Due to the small sample size, participants who reported past smoking before diagnosis and current smoking after diagnosis were excluded (n = 2).
Abbreviations: CI, confidence interval; HR, hazard ratio; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Bold values indicate p < 0.05.
For all analyses, results for all-cause mortality were similar to those for ovarian cancer-specific mortality. Findings were similar across cohorts (all p-heterogeneity > 0.05, Supporting Information Table S2 and S3). Risk estimates from a reduced model excluding BMI and NSIADs use (postdiagnosis analysis only) were similar to those in the primary analyses (data not shown).
In stratified analyses by tumor histology, significant associations were demonstrated among women diagnosed with high-grade serous or poorly differentiated tumors, but not those with nonserous or low-grade serous tumors (Table 4). For example, current vs. never smoking in postdiagnosis analyses were associated with worse outcomes among those with high-grade serous or poorly differentiated tumor (HR = 1.71, 95% CI: 1.25–2.34), but not those with nonserous or low-grade serous histology (HR = 0.84, 95% CI: 0.39–1.79; p-heterogeneity = 0.02). Similar results were noted for change in smoking status, with those remained former and current smokers with high-grade serous or poorly differentiated histology having increased ovarian cancer-specific mortality, but no association for women with nonserous or low-grade serous histology. Findings with all-cause mortality were similar (Supporting Information Table S4).
Table 4.
Epithelial ovarian cancer-specific mortality stratified by tumor histologic subtype in the NHS and NHSII
| Epithelial ovarian cancer-specific mortality |
|||||
|---|---|---|---|---|---|
| High-grade serous or poorly differentiated histology |
Nonserous or low-grade serous histology |
p-heterogeneity | |||
| n, deaths/cases | HR (95%CI) | n, deaths/cases | HR (95% CI) | ||
| Prediagnosis smoking | |||||
| Never smoker | 250/384 | 1.00 (ref) | 80/211 | 1.00 (ref) | 0.13 |
| Former smoker | 252/339 | 1.27 (1.07,1.52) | 58/147 | 0.89 (0.63,1.27) | |
| Current smoker | 82/109 | 1.20 (0.92,1.55) | 16/54 | 1.15 (0.65,2.03) | |
| Prediagnosis smoking rate | |||||
| Never smoker | 250/384 | 1.00 (ref) | 80/211 | 1.00 (ref) | 0.046 |
| Former smoker, <15 cigs/day | 121/171 | 1.14 (0.91,1.42) | 33/82 | 0.95 (0.62,1.45) | |
| Former smoker, ≥15 cigs/day | 126/162 | 1.42 (1.14,1.77) | 24/62 | 0.82 (0.50,1.32) | |
| Current smoker, <15 cigs/day | 30/46 | 0.99 (0.67,1.45) | 5/16 | 1.01 (0.40,2.57) | |
| Current smoker, ≥15 cigs/day | 47/58 | 1.30 (0.94,1.79) | 11/36 | 1.30 (0.67,2.54) | |
| Prediagnosis smoking duration | |||||
| Never smoker | 250/384 | 1.00 (ref) | 80/211 | 1.00 (ref) | 0.06 |
| <20 years | 118/159 | 1.26 (1.01,1.58) | 31/92 | 0.73 (0.47,1.13) | |
| ≥20 years | 211/282 | 1.25 (1.04,1.51) | 42/107 | 1.15 (0.78,1.70) | |
| Prediagnosis smoking pack-years | |||||
| Never smoker | 250/384 | 1.00 (ref) | 80/211 | 1.00 (ref) | 0.19 |
| <20 pack-years | 162/222 | 1.18 (0.97,1.44) | 39/115 | 0.93 (0.63,1.38) | |
| ≥20 pack-years | 163/215 | 1.32 (1.08,1.62) | 34/83 | 0.95 (0.63,1.45) | |
| Prediagnosis time since quitting smoking | |||||
| Never smoker | 250/384 | 1.00 (ref) | 80/211 | 1.00 (ref) | 0.06 |
| <15 years | 74/96 | 1.22 (0.93,1.60) | 23/53 | 0.96 (0.60,1.56) | |
| 15 to <22 years | 49/66 | 1.36 (1.00,1.86) | 12/37 | 0.66 (0.35,1.25) | |
| ≥22 years | 125/171 | 1.25 (1.00,1.56) | 22/56 | 0.89 (0.53,1.48) | |
| Postdiagnosis smoking | |||||
| Never smoker | 202/336 | 1.00 (ref) | 66/197 | 1.00 (ref) | 0.02 |
| Former smoker | 227/324 | 1.41 (1.16,1.71) | 49/152 | 0.83 (0.56,1.23) | |
| Current smoker | 53/69 | 1.71 (1.25,2.34) | 8/34 | 0.84 (0.39,1.79) | |
| Postdiagnosis smoking rate | |||||
| Never smoker | 202/336 | 1.00 (ref) | 66/197 | 1.00 (ref) | 0.02 |
| Former smoker, <15 cigarettes/day | 111/167 | 1.32 (1.04,1.68) | 29/84 | 1.01 (0.63,1.61) | |
| Former smoker, ≥15 cigarettes/day | 109/149 | 1.44 (1.14,1.83) | 20/65 | 0.71 (0.42,1.20) | |
| Current smoker, <15 cigarettes/day | 20/33 | 1.17 (0.73,1.86) | 2/11 | 0.90 (0.21,3.81) | |
| Current smoker, ≥15 cigarettes/day | 31/33 | 2.73 (1.82,4.09) | 6/21 | 1.48 (0.60,3.65) | |
| Change in smoking from prediagnosis to postdiagnosis1,2 | |||||
| Never smoker | 196/326 | 1.00 (ref) | 65/189 | 1.00 (ref) | 0.01 |
| Former smoker | 205/285 | 1.43 (1.17,1.75) | 44/129 | 0.70 (0.46,1.06) | |
| Quit smoking after diagnosis | 18/29 | 0.85 (0.52,1.41) | 4/13 | 0.73 (0.24,2.22) | |
| Remained current smoker | 50/64 | 1.60 (1.15,2.21) | 8/30 | 1.31 (0.59,2.92) | |
Nonserous histologic subtype refers to mucinous, endometrioid, clear cell, transitional/Brenner, carcinosarcoma or mixed subtypes of ovarian cancer. Never smoker is defined as being a never smoker at both prediagnosis and postdiagnosis assessments; Former smoker is defined as being a former smoker at both assessments; Quit smoking after diagnosis is defined as being a current smoker before diagnosis and former smoker after diagnosis; Remained current smoker is defined as being a current smoker at both assessments. Models adjusted for age and calendar year at diagnosis, stage, body mass index (BMI, categorical), and cohort. Postdiagnosis models additionally adjusted for nonsteroidal anti-inflammatory drug (NSAID) usage.
Models adjusted for age and calendar year at diagnosis, stage, prediagnosis BMI (categorical), change of BMI (categorical) and NSAID usage from prediagnosis to postdiagnosis, and cohort.
Due to the small sample size, participants who reported past smoking before diagnosis and current smoking after diagnosis were excluded (n = 2).
Abbreviations: CI, confidence interval; HR, hazard ratio; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Bold values indicate p < 0.05.
In the prediagnosis analysis stratified by BMI, increased risk of ovarian cancer-specific death related to smoking status, was suggestively stronger among women whose prediagnosis BMI was lower than 25 kg/m2 (e.g., current vs. never smokers, HR = 1.44 and 0.91 for cases with BMI < 25 kg/m2 and ≥25 kg/m2, respectively; p-heterogeneity = 0.07; Supporting Information Table S5). Smoking associations after diagnosis generally were similar by BMI (e.g., current vs. never smokers, HR = 1.46 and 1.30 for cases with BMI < 25 kg/m2 and ≥25 kg/m2, respectively; p-heterogeneity = 0.84), although remaining former or current smokers from prediagnosis to postdiagnosis was associated with mortality only among those with lower BMI (compared to never smokers, for cases with BMI < 25 kg/m2, HR = 1.28 and 1.55 for former and remained current smokers, respectively; for those with BMI ≥ 25 kg/m2, HR = 1.11 and 1.12, respectively; p-heterogeneity = 0.06). In analyses by postdiagnosis NSAID use, smoking was associated with worse survival only among noncurrent users of NSAIDs. For example, the HR = 1.82 among noncurrent users of NSAIDs who remained current smokers vs. never smokers; the equivalent association was HR = 1.18 in current NSAID users (p-heterogeneity<0.01; Supporting Information Table S6). Results were similar for early and late-stage patients (data not shown).
Findings did not differ when excluding women with comorbidities, excluding those with breast or lung cancer, or including cases with unknown stage (data not shown). Secondary analyses evaluating exposure collected at least 4 years prior to diagnosis or within 4 years after diagnosis were also similar (data not shown).
Discussion
This is the largest analysis of the influence of cigarette smoking both before and after diagnosis on survival in ovarian cancer patients in two large prospective cohort studies. We observed that the risk of ovarian cancer-specific and overall mortality was elevated among former and current smokers both before and after diagnosis, especially for those with high-grade serous or poorly differentiated histology. In addition, we observed an increased risk of mortality among those who remained former or current smokers, compared to never smokers.
Consistent with our findings, results from prior studies generally observed worse outcomes for former or current prediagnosis cigarette smoking. For example, a pooled analysis of 19 case–control studies observed increased all-cause mortality among former and current smokers overall and among cases with serous histology.17 Similar results were also reported in case–control studies in Australia and Denmark,13,14 and a cohort study in Canada.16 In contrast, neither smoking status nor number of cigarettes smoked per day was associated with ovarian cancer survival among 635 women in a Swedish case–control study.15 However, this study was unable to adjust for histology, and a high proportion of participants were never smokers, compared to the other studies, which may indicate better health and life-style. Notably, prior studies only collected information about smoking before or at diagnosis, and thus could not evaluate the effect of postdiagnosis smoking. Considering the relatively small number of participants who changed their smoking status after diagnosis (n = 45, 43 cases quit smoking after diagnosis, two cases reported past smoking before diagnosis and current smoking after diagnosis), the distributions of prediagnosis and postdiagnosis smoking status were highly similar, and both were associated with increased risk of ovarian cancer-specific death and total death in our study.
Though the exact mechanisms of how smoking increases ovarian cancer mortality are not understood, several mechanisms may explain this association. Long-term cigarette smoking may result in a tumor milieu enriched with proinflammatory cytokines and chemokines, providing a preferred background for epithelial ovarian cancer genesis, growth and progression.28 Smoking may enhance the invasive potential of cancer cells, and induce ovarian cancers to develop a more aggressive phenotype facilitating metastatic spread.8 As cigarette smoking is potently tumorigenic, it was also associated with increased risk of cancer recurrence and particularly second tobacco-related primary cancers.9,10 Smoking during treatment may also alter cancer drug metabolism, leading to reduced overall and progression-free survival among ovarian cancer patients receiving adjuvant or neoadjuvant chemotherapy.11 Interestingly several studies, including ours, showed that former smoking (and remaining a former smoker after diagnosis) was also associated with an increased risk of mortality.16,17 Long duration and high smoking frequency are associated with the development of venous thrombosis, even among former smokers.12 Thrombosis is common among ovarian cancer patients and adversely impacted survival.29 Moreover, ever smokers are more likely to have additional lifestyle risk factors (e.g., alcohol drinking, less healthy diet, excessive weight and physical inactivity), which in themselves may have a negative influence on survival.30,31
Conversely, smoking cessation, either before or after cancer diagnosis, reduces tumor recurrence in multiple cancers5,6 and ultimately improves prognosis.10 Previous studies found that continuing smokers had worse overall or disease-free survival compared to those quitting smoking around the time of diagnosis among patients with lung cancer,5,32 bladder cancer33 or a mixture of multiple cancers.34 However, no study has investigated smoking cessation and ovarian cancer survival. In our study, compared to never smokers, cases that continued to smoke but not those who quit smoking near their diagnosis, had higher risk of ovarian cancer death and total death. However, the finding for smoking cessation was based on relatively few cases and given that we observed increased mortality for women who remained former smokers, these results should be interpreted with caution and evaluated in larger studies. However, these results support the importance of implementation of smoking cessation programs, even in those cancers not traditionally considered to be tobacco related.
It is also noteworthy that the association between prediagnosis smoking and mortality was primarily observed among women with BMI less than 25 kg/m2, although the same was not seen for postdiagnosis heavy smokers. Consistent with our results, two prospective cohort studies observed significantly higher mortality for current and former smokers who were underweight,35,36 while multiplicative joint effects on mortality were seen in another large cohort study.37 Smokers tend to have a lower BMI than nonsmokers, in part due to suppressed appetite.38,39 It is possible that leaner smokers may have a higher effective dose of smoking-related toxins, leading to a stronger association in the prediagnosis period. Further, given that weight loss and cachexia can occur in ovarian cancer patients,40 it is possible that overweight and obesity may confer a survival advantage and postpone the phase of cachexia, which may be potentiated by smoking.41,42
NSAIDs usage after ovarian cancer diagnosis, but not prediagnosis use, was previously found to be associated with significantly improved survival by our group.24 In the present study, we noted the hazard of death among former and current moderate smokers was not elevated among women using NSAIDs. This suggests that the adverse effect caused by moderate, but not heavy, smoking may be partly mitigated by NSAID use, although this novel finding requires replication. The main mechanism of this finding may due to the anti-inflammatory properties of NSAIDs by blocking the production of proinflammatory prostaglandins through inhibition of COX-2.43 Notably, studies suggest that smoking can increase COX-2 production in esophageal tissue and airway epithelia,44,45 although whether this extends to the ovary is unknown. Simultaneously, the antiplatelet effects of aspirin may prevent venous thrombosis,46,47 which is increased by smoking.
Our study has several limitations. Information on cigarette smoking was self-reported. It was found that nearly 10% of current smokers may categorize themselves as never or former smokers due to social desirability bias, which may underestimate the true association with current smoking.11,48 However, the prospective setting and repeated reports by biennial follow-up questionnaires in our study improve confidence in our self-reported data. Future studies should use cotinine measures to offer objective verification of smoking status. It is possible that prediagnosis smoking status was influenced by early ovarian cancer symptoms. However, secondary analyses using exposures reported at least 4 years prior to ovarian cancer diagnosis or excluding women with comorbidities did not alter the results, suggesting that reverse causation does not explain current findings. Although we adjusted for calendar year of diagnosis to account for possible changes in cancer treatment over time and deviation from proportional hazards was not detected, we were unable to evaluate potential confounding by cancer treatment or debulking status, which has been observed in prior study.11 Additionally, while we had well over 1,000 cases, there was inadequate power to conduct more detailed histology-specific analyses, especially for mucinous ovarian cancer (49 and 42 cases in prediagnosis and post-diagnosis analyses, respectively), for which cigarette smoking is an established risk factor.26,27 When we excluded mucinous tumor patients, the results were similar. Selection bias could have affected the results if women who died early and were not included in the postdiagnosis analysis, were more likely to smoke. But the distribution of smoking status was almost identical among women in the prediagnosis and postdiagnosis smoking analytic populations, in part due to the high response rates in this cohort, suggesting that selection bias did not substantially influence the results. Finally, our study population comprised of registered nurses and included few nonwhite women, which affects the generalizability of our findings.
Strengths of our study include its prospective design, long follow-up for death ascertainment, availability of cause-of-death information, and detailed biennially-updated data on cigarette use. To the best of our knowledge, our study includes the largest number of prospectively ascertained ovarian cancer patients to examine the influence of cigarette smoking both before and after diagnosis, as well as quitting smoking after diagnosis, on survival among all patients as well as by histologic subtype.
In conclusion, this large prospective study provides evidence that smoking both before and after ovarian cancer diagnosis is associated with worse prognosis. Our study also suggests that patients who continue smoking after diagnosis have an increased risk of death. If our findings are confirmed by other studies, it may support inclusion of smoking in prognostic models to predict outcome of ovarian cancer as well as future studies of the effectiveness of adding structured smoking cessation programs in standard clinical management of ovarian cancer patients. Further investigations are required to confirm these findings, better understand potential modifying factors (e.g., NSAID use, BMI), and define underlying molecular mechanisms.
Supplementary Material
What’s new?
Tobacco smoking prior to diagnosis of ovarian cancer is associated with worse patient outcome. Little is known, however, about the impact of smoking postdiagnosis on ovarian cancer survival, or whether the affect of smoking on mortality differs by histological subtype. In this study, postdiagnosis smoking was found to be associated with worse ovarian cancer survival, particularly for women who smoked 15 or more cigarettes per day and for women who had smoked for at least 20 years. Mortality was elevated especially among smokers with high-grade serous or poorly differentiated tumors. Additional investigation is needed to identify factors underlying these associations.
Acknowledgements
We would like to thank the participants and staff of the NHS and NHSII for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. We thank the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital as home of the Nurses’ Health Studies. This work was supported in part by the National Institute of Health Award Numbers U01 CA186107, U01 CA176726 and P01 CA87969. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors take full responsibility for analyses and interpretation of data.
Grant sponsor: National Cancer Institute; Grant numbers: P01CA87969, U01 CA176726, U01 CA186107
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- NHS
Nurses’ Health Study
- NHSII
Nurses’ Health Study II
- NSAID
nonsteroidal anti-inflammatory drug
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: UAM declared financial conflicts of interest with Merck and Novartis (consulting). All other authors declared no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol 2018; 23:45–51. [DOI] [PubMed] [Google Scholar]
- 3.Society AC. Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society, 2019. [Google Scholar]
- 4.Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131: 1557–66. [DOI] [PubMed] [Google Scholar]
- 5.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int 2007; 100:281–6. [DOI] [PubMed] [Google Scholar]
- 7.Parada H, Bradshaw PT, Steck SE, et al. Postdiagnosis changes in cigarette smoking and survival following breast cancer. JNCI Cancer Spectr 2017;1:pkx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner RT, Poole EM, Wentzensen NA, et al. Ovarian cancer risk factors by tumor aggressiveness: an analysis from the ovarian cancer cohort consortium. Int J Cancer 2018;145:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monica R, Afaf G. When cancer crosses disciplines: a physician’s handbook. Singapore: World Scientific, 2009. [Google Scholar]
- 10.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Available at: https://www.surgeongeneral.gov/library/reports/50-yearsof-progress/. [Google Scholar]
- 11.Kelemen LE, Warren GW, Koziak JM, et al. Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol 2016;140:124–30. [DOI] [PubMed] [Google Scholar]
- 12.Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol 2008;83:97–102. [DOI] [PubMed] [Google Scholar]
- 13.Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev 2006;15:2557–60. [DOI] [PubMed] [Google Scholar]
- 14.Kjærbye-Thygesen A, Frederiksen K, Høgdall EV, et al. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2006;15: 798–803. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Klint Å, Lambe M, et al. Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer 2008;123: 672–9. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Rosen B, Fan I, et al. Epidemiologic factors that predict long-term survival following a diagnosis of epithelial ovarian cancer. Br J Cancer 2017;116:964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Præstegaard C, Jensen A, Jensen SM, et al. Cigarette smoking is associated with adverse survival among women with ovarian cancer: results from a pooled analysis of 19 studies. Int J Cancer 2017; 140:2422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rades D, Setter C, Schild SE, et al. Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;71: 1134–42. [DOI] [PubMed] [Google Scholar]
- 19.Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment– related side effects. Oncologist 2011;16:1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garces YI, Schroeder DR, Nirelli LM, et al. Second primary tumors following tobacco dependence treatments among head and neck cancer patients. Am J Clin Oncol 2007;30:531–9. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Hankinson SE. The Nurses’ health study: lifestyle and health among women. Nat Rev Cancer 2005;5:388. [DOI] [PubMed] [Google Scholar]
- 22.Rockhill B, Willett WC, Hunter DJ, et al. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst 1998;90: 1555–160. [DOI] [PubMed] [Google Scholar]
- 23.Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst 2018;111:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt MA, Rice MS, Barnard ME, et al. Prediagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol 2018; 19:1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan W, Li S, Meng X, et al. Smoking and survival of breast cancer patients: a meta-analysis of cohort studies. The Breast 2017;33:117–24. [DOI] [PubMed] [Google Scholar]
- 26.Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol 2016;34:2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tworoger SS, Gertig DM, Gates MA, et al. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer 2008;112: 1169–77. [DOI] [PubMed] [Google Scholar]
- 28.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2011;20:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JG, Prendergast E, Geddings JE, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol 2017;146: 146–52. [DOI] [PubMed] [Google Scholar]
- 30.Minlikeeva AN, Cannioto R, Jensen A, et al. Joint exposure to smoking, excessive weight, and physical inactivity and survival of ovarian cancer patients, evidence from the ovarian cancer association consortium. Cancer Causes Control 2019;30: 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos ET, Lahti JM, Rahkonen O. Lifestyle and cancer—a joint pairwise association of lifestyle habits with subsequent cancer diagnosis. Eur J Public Health 2018;29:340–5. [DOI] [PubMed] [Google Scholar]
- 32.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med 1993;119:383–90. [DOI] [PubMed] [Google Scholar]
- 33.Fleshner N, Garland J, Moadel A, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer 1999;86:2337–45. [PubMed] [Google Scholar]
- 34.Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013;132:401–10. [DOI] [PubMed] [Google Scholar]
- 35.Luijckx E, Lohse T, Faeh D, et al. Joints effects of BMI and smoking on mortality of all-causes, CVD, and cancer. Cancer Causes Control 2019;30: 549–57. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Jemal A, Flanders WD, et al. Joint association of adiposity and smoking with mortality among US adults. Prev Med 2013;56:178–84. [DOI] [PubMed] [Google Scholar]
- 37.Pednekar MS, Gupta PC, Hebert JR, et al. Joint effects of tobacco use and body mass on all-cause mortality in Mumbai, India: results from a population-based cohort study. Am J Epidemiol 2007;167:330–40. [DOI] [PubMed] [Google Scholar]
- 38.Clair C, Chiolero A, Faeh D, et al. Dosedependent positive association between cigarette smoking, abdominal obesity and body fat: crosssectional data from a population-based survey. BMC Public Health 2011;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courtemanche C, Tchernis R, Ukert B. The effect of smoking on obesity: evidence from a randomized trial. J Health Econ 2018;57:31–44. [DOI] [PubMed] [Google Scholar]
- 40.Gadducci A, Cosio S, Fanucchi A, et al. Malnutrition and cachexia in ovarian cancer patients: pathophysiology and management. Anticancer Res 2001;21:2941–7. [PubMed] [Google Scholar]
- 41.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 2005;9:S51–63. [DOI] [PubMed] [Google Scholar]
- 42.Münstedt K, Wagner M, Kullmer U, et al. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control 2008; 19:909–16. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 2006;6:130. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T, Tang Z, Younes M, et al. Esophageal COX-2 expression is increased in Barrett’s esophagus, obesity, and smoking. Dig Dis Sci 2015;60: 65–73. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi H, Fukutomi Y, Mitsui C, et al. Smoking cessation as a possible risk factor for the development of aspirin-exacerbated respiratory disease in smokers. J Allergy Clin Immunol Pract 2018;6: 116–25. e3. [DOI] [PubMed] [Google Scholar]
- 46.Eikelboom JW, Kearon C, Guyatt G, et al. Perioperative aspirin for prevention of venous ThromboembolismThe PeriOperative ISchemia Evaluation-2 trial and a pooled analysis of the randomized trials. Anesthesiology 2016;125:1121–9. [DOI] [PubMed] [Google Scholar]
- 47.Belcaro G, Dugall M, Hu S, et al. Prevention of recurrent venous thrombosis and post-thrombotic syndrome. Minerva Cardioangiol 2018;66:238. [DOI] [PubMed] [Google Scholar]
- 48.Sandhu S, Humphris G, Whitley S, et al. Smoking habits in patient’s who have been treated for an oral cancer: validation of self-report using saliva cotinine. Oral Oncol 2004;40:576–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.