Abstract
Whether the depth and healing of scalds and contact burns are similar is controversial. Due to water’s greater heat capacity, we hypothesized that when exposed to similar temperatures and durations of exposure, burns caused by hot water would be deeper than those cause by contact with hot metal. 40 standardized burns were created in two anesthetized female domestic pigs using a brass bar or circulating heated water. In one pig, the temperature was kept constant (95° C) while the duration of exposure varied (5, 10, 15 sec.) In the second pig, the exposure time was kept constant (10 sec.) while the temperature of exposure varied (70, 80, 98° C). Periodic punch biopsies were taken to determine burn depth immediately after injury, percentage burns reepithelialized within 21 days, and depth of scar at 28 days. Analysis was performed using analysis of variance. When temperature was held constant, duration of exposure (5, 10, and 15 sec.) was associated with scar depth (2.1 vs 3.8 vs 5.0 mm respectively, P=0.001) but not with burn depth (2.0 vs 2.2 vs 2.3 mm respectively, P=0.10). When exposure duration was held constant, temperature (70, 80, 98° C) was associated with scar depth (0.6 vs 1.7 vs 3.6, P<0.001) but not with burn depth (1.2 vs 1.5 vs 1.7 mm respectively, P=0.21). Burn depths were greater for scald than contact burns although not significantly greater. After controlling for temperature, the difference in scar depth between scalds and contact burns was statistically significant (marginal means 3.0 for contact burns, 4.3 for scalds, P=0.008). We conclude that burns created in swine with circulating hot water result in deeper scars than those created by contact with a brass bar when controlling for temperature and duration of exposure.
INTRODUCTION
Thermal burns are a prevalent and, often times, devastating injury that can lead to prolonged suffering and substantial morbidity. Development of effective burn therapies requires an adequate (reproducible) animal model that recreates human burn characteristics and injury progression. Porcine models are the most frequently used experimental animal model in burn studies because pig skin is anatomically and physiologically similar to human skin1, 2.
Most burns are caused by exposure to hot water, fire or a hot object (these are called contact thermal burns).3 There is considerable debate on whether scalds and contact burns are similar. Recent porcine burn studies have used either scald injuries or contact injuries to study different experimental groups. Thermal contact burns are mostly achieved using a heated metal rod.4 While studies focused on scald injuries were mostly achieved using a glass cup filled with heated water (which is not truly a scald injury) or with a heated bath. However, a recently developed scalding device, modeled after the design of Moritz and Henriques,5 allows for continuous application of hot water to the skin with a more consistent water temperature.6 It is also important to note that the current scald models replicate immersion injuries more so than splatter injuries due to the longer exposure time rather than a quick and short exposure characteristic of a splatter injury.
Although numerous studies have already been conducted with the use of thermal contact burns and scald burns models, no recent study has looked at how the results compare between the two burn models. Furthermore, no attempt has been made to standardize the two models which makes it difficult to compare results between the two groups and different experimental studies.
In a systematic review of 42 porcine burn studies describing the depth of burn injury in scald and contact burns, it was found that lower temperatures with shorter durations of exposure were required in scald burn models to achieve the same depth of injury than in thermal contact burns.4 It should be noted, however, that there is no standardization in the study and it is only a review of studies that exclusively used a contact or a scald burn.
Based on thermodynamic principles one can hypothesize that scald burns would cause more severe injuries than contact burns because of water’s higher thermal effusivity, a measure of a material’s ability to exchange thermal energy with its surroundings that takes a material’s density, specific heat capacity, and thermal conductivity into account.7
The primary purpose of this study was to compare the burn and scar depth as well as time to complete reepithelialization of similarly sized scalds and contact burns using similar temperatures and exposure times. We hypothesized that for any given set of temperatures and exposure times, scald burns would result in deeper burns and ultimately deeper scars than contact burns.
METHODS
Study Design
We performed a prospective, animal experiment comparing injury depth, time to complete reepithelialization, and scar depth, after creating burns with a variety of devices using varying temperatures and exposure times. Of all animals, the skin of pigs most closely resembles that of humans.1, 2 As a result, the pig model is generally accepted as the best model to investigate burn injuries.
Setting
The study was conducted in the Division of Laboratory Animal Research (DLAR) of a large academic university hospital under the approval of the Institutional Animal Care and Use Committee (IACUC).
Animal Handling, Sedation and Anesthesia
For this study we used two female Yorkshire pigs weighing 45–50 kg. In order to acclimate, the animals were fed standard pig chow for one week and had access to water ad lib. The animals were exposed to recurring cycles of light (12 hours) and darkness (12 hours). Following an overnight fast, the animals were sedated using an intramuscular injection that contained a combination of acepromazine 0.1 mg/kg, atropine 0.02 mg/kg, ketamine 20 mg/kg, and xylazine 2 mg/kg. General anesthesia was performed with isoflurane 0.5–5.0% in room air administered via an endotracheal tube (for painful or prolonged procedures) or nose cone (for brief dressing changes). The experimental region, flank and back, was cleared with clippers and the skin was scrubbed clean with soap and water followed by 70% isopropyl alcohol. All animal handling and care was in accordance with national guidelines established by the National Research Council’s Guide for the Care and Use of Laboratory Animals.8
Burn Creation
A variety of scald and contact burns were created with specialized devices. The scalding device was based on a device previously described by Andrews.6 It uses a circular stainless-steel pipe. While the Cuttle laboratory uses a pipe with an internal diameter of 5 cm, we used a pipe with an internal diameter of 2.5 cm in order to create more burns on each animal reducing the number of animals necessary. Furthermore, it more closely approximated the surface area of burns created with a metal bar used by our laboratory. The metal pipe is covered with an outer insulating sleeve of rubber that is folded onto itself at the distal end allowing tight contact with the underlying skin (Figure 1). In order to maintain constant contact of the pipe with the skin, two lead ring weighing 0.5 kg each were placed on top of the pipe. A continuous flow of hot water is circulated through the pipe using two plastic hoses. One attached to a pump (controlling the inflow of water) and the other attached to a vacuum (controlling the outflow of water). The plastic tube through which water flowed into the pipe was kept approximately one cm above the skin surface to ensure a constant level of the water. The temperature of the circulating hot water was controlled and held constant using a water bath. The contact burns were created with a rectangular brass bar with a contact surface area of 2.5 by 2.5 cm (Figure 1). A spring-loaded device controlled the amount of pressure applied to the skin (2 kg/6.25cm).9 The temperature of the bar was kept constant and controlled by a heating element and a temperature sensor at the distal end of the bar that comes into contact with the skin.
Figure 1.

Devices used to create scald (left) and contact (middle) burns. Representative burns on right.
In the first pig the temperature of the water and the contact bar were kept constant at 95° C while the exposure times were varied: 5, 10 and 15 seconds. In the second pig, the exposure time was kept constant (10 seconds) while the exposure temperature varied: 70, 80, and 98° C. For each exposure temperature and duration, we created between 2–4 replicates of each burn condition (Table 1). On each of the two pigs we created 20 burns. Half of the burns were scalds created with the circular pipe while the other half were contact burns created with a rectangular brass bar. In one of the pigs we created an additional single scald burn using a 5 cm internal diameter pipe, a temperature of 95° C, and an exposure time of 10 seconds, to determine whether depth of injury and healing differ between 2.5 and 5 cm diameter burns. Since there is conflicting data on the effect of burn location (both cephalic or caudal and medial or lateral) on depth of injury and healing,10 the order in which the burns were created was randomized to allow similar distribution of the various conditions in a cephalo-caudal and medial-lateral distribution.
Table 1.
Distribution of burn conditions.
| 1st Pig: Constant temperature of 95° C | Number of replicates |
|---|---|
| 5 second contact | 4 |
| 5 second scald | 4 |
| 10 second contact | 3 |
| 10 second scald | 3 |
| 15 second contact | 3 |
| 15 second scald | 3 |
| 2nd Pig: Constant exposure of 10 seconds | |
| 70° contact | 4 |
| 70° scald | 4 |
| 80° contact | 3 |
| 80° scald | 3 |
| 98° contact | 4 |
| 98° scald | 2 |
After burn creation, the necrotic epidermal layer of the burns was gently scraped away with a blunt metal instrument to simulate blister formation and rupture in humans, since the dense skin of the pigs prevents blister formation. This procedural step has been shown to result in deeper burns, slower reepithelialization and deeper scars than when the necrotic epidermis is left intact.11 All burns were then covered with a thin layer of a triple antibiotic in a petroleum base (Bacitracin Zinc, Neomycin Sulfate, Polymyxin B Sulfate; Taro Pharmaceuticals, Hawthorne, NY) and non-adherent gauze (Telfa, Kendall Healthcare Products Company, Mansfield, MA). The dressings were secured with an outer adhesive bandage (Tensoplast, BSN Medical S.A.S., Vibraye, France). Dressing changes occurred on a daily basis for the first week and then twice weekly for the remainder of the 28-day experiment. Full thickness 4-mm punch biopsies were obtained after 1, 4, 21, and 28 days at the time of a dressing change to determine burn depth, reepithelialization, and scar depth over the experimental period.
Outcomes
The primary outcome of the study was burn depth immediately (in both pigs) and four days after (in the second pig) injury. The depth of injury was determined by measuring the greatest depth of vascular injury indicated by plugging or thrombosis of the blood vessels or necrosis of the endothelial cells lining the blood vessels. This measure is best correlated with time to healing and scar formation after burn injury.12 Secondary outcomes were the percentage of burns fully reepithelialized 21 days after injury. This outcome was chosen since burns that take longer than 21 days to heal in humans are generally deep dermal or full-thickness and require excision and grafting to prevent hypertrophic scarring and contractures.13
Data Analysis
Continuous variables are summarized as means with standard deviations (SD) or 95% confidence intervals (CI) and compared across groups with analysis of variance. Categorical variables are summarized as percentage frequency of occurrence and compared across groups with Chi-square or Fisher exact tests as necessary. Multivariate analysis of variance was used to determine the association of predictor variables (temperature, exposure duration, type of device used to create injury) and outcomes (burn depth and scar depth).
RESULTS
A summary of the conditions used to create the burns is presented in Table 1. In general, burn depth and scar depth increased with increasing temperature and duration of exposure (Table 2, Fig. 2).
Table 2.
Outcomes by burn condition, absolute depth.
| Mean (SD) burn depth 1 hr. post injury, mm | Mean (SD) burn depth 4 days post injury, mm | Mean (SD) scar depth, mm | |
|---|---|---|---|
| 1st Pig: Constant temperature of 95° C | Not available | ||
| 5 second contact | 1.9 (0.4) | 1.2 (0.8) | |
| 5 second scald | 2.1 (0.3) | 3.1 (0.4) | |
| 10 second contact | 2.3 (0.3) | 3.2 (0.2) | |
| 10 second scald | 2.2 (0.3) | 4.5 (0.6) | |
| 15 second contact | 2.3 (0.4) | 4.8 (1.1) | |
| 15 second scald | 2.4 (0.4) | 5.2 (1.7) | |
| 2nd Pig: Constant exposure of 10 seconds | |||
| 70° contact | 0.9 (0.6) | 0.5 (0.1) | 0.7 (0.8) |
| 70° scald | 1.4 (0.7) | 0.9 (0.6) | 0.6 (0.4) |
| 80° contact | 1.2 (0.3) | 1.2 (0.3) | 1.8 (1.4) |
| 80° scald | 1.8 (0.6) | 1.5 (0.5) | 1.5 (0.7) |
| 98° contact | 1.6 (0.4) | 2.7 (0.4) | 3.1 (1.1) |
| 98° scald | 1.9 (0.1) | 1.9 (0.1) | 4.6 (0.4) |
Figure 2.
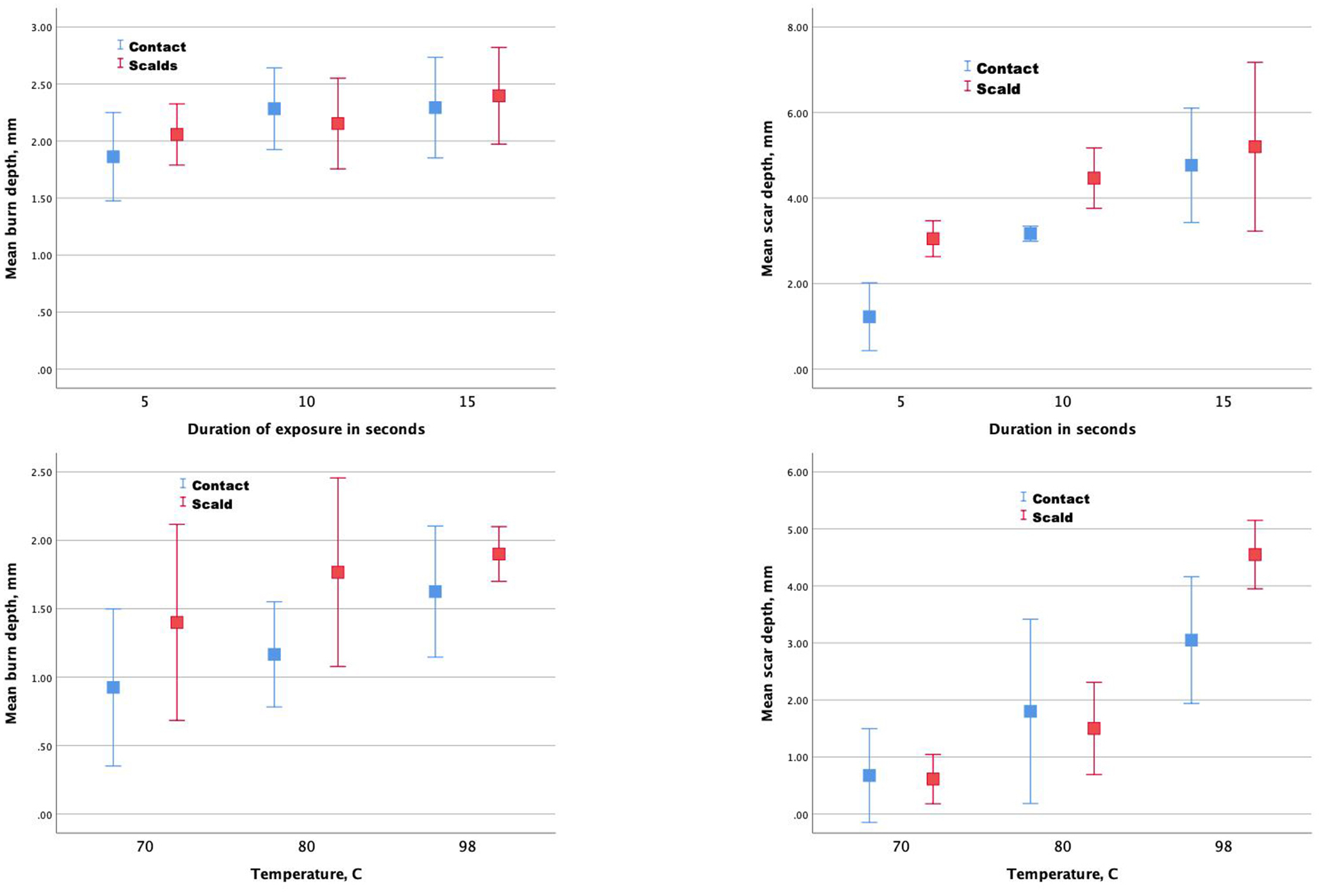
Mean burn depths (left figures) and scar depths (right figures).
Burn Depth
When temperature was held constant, duration of exposure (5, 10, and 15 sec.) was not significantly associated with burn depth measured one hour after injury (2.0 vs 2.2 vs 2.3 mm respectively, P=0.10). When exposure duration was held constant, temperature (70, 80, 98° C) was not significantly associated with the absolute burn depth (1.2 vs 1.5 vs 1.7 mm respectively, P=0.21) or the percentage of dermal thickness burned one hour after injury (47.8% vs. 60.8% vs. 72.3% respectively, P=0.23). When expressed as a percentage of the dermal depth injured, there was a significant association of temperature with burn depth four days after injury (P<0.001). Exposure to 70° C resulted in superficial partial thickness burns (extending down to one third of the dermal thickness), exposure to 80° C resulted in mid partial thickness burns (involving 62% of the dermal thickness), and exposure to 98° C resulted in full thickness burns (extending beyond the dermis into the subcutaneous layer).
Scar Depth
When temperature was held constant, duration of exposure (5, 10, and 15 sec.) was significantly associated with scar depth four weeks after injury (2.1 vs 3.8 vs 5.0 mm respectively, P=0.001). When exposure duration was held constant, temperature (70, 80, 98° C) was significantly associated with scar depth 4 weeks after injury (0.6 vs 1.7 vs 3.6, P<0.001).
Comparison of scalds and contact burns
For each pig, a comparison of burn and scar depth by device generally showed that depths were greater for scald than contact burns although not significantly greater. After controlling for temperature, the difference in scar depth between scalds and contact burns was statistically significant (marginal means 3.0 for contact burns, 4.3 for scalds, P=0.008). When combining data from both pigs and controlling for temperatures and duration, scar depths for scalds (2.7 mm) vs contacts (1.9 mm) showed a significant difference (P=0.02) while burn depths one hour after injury were not significantly different (1.7 vs 1.5 mm, P=0.07). Almost all burns were reepithelialized by day 21. Thus, no comparisons among groups were made. Representative micrographs of burns taken at 1 hour and 28 days after injury are presented in figure 3 and 4.
Figure 3.
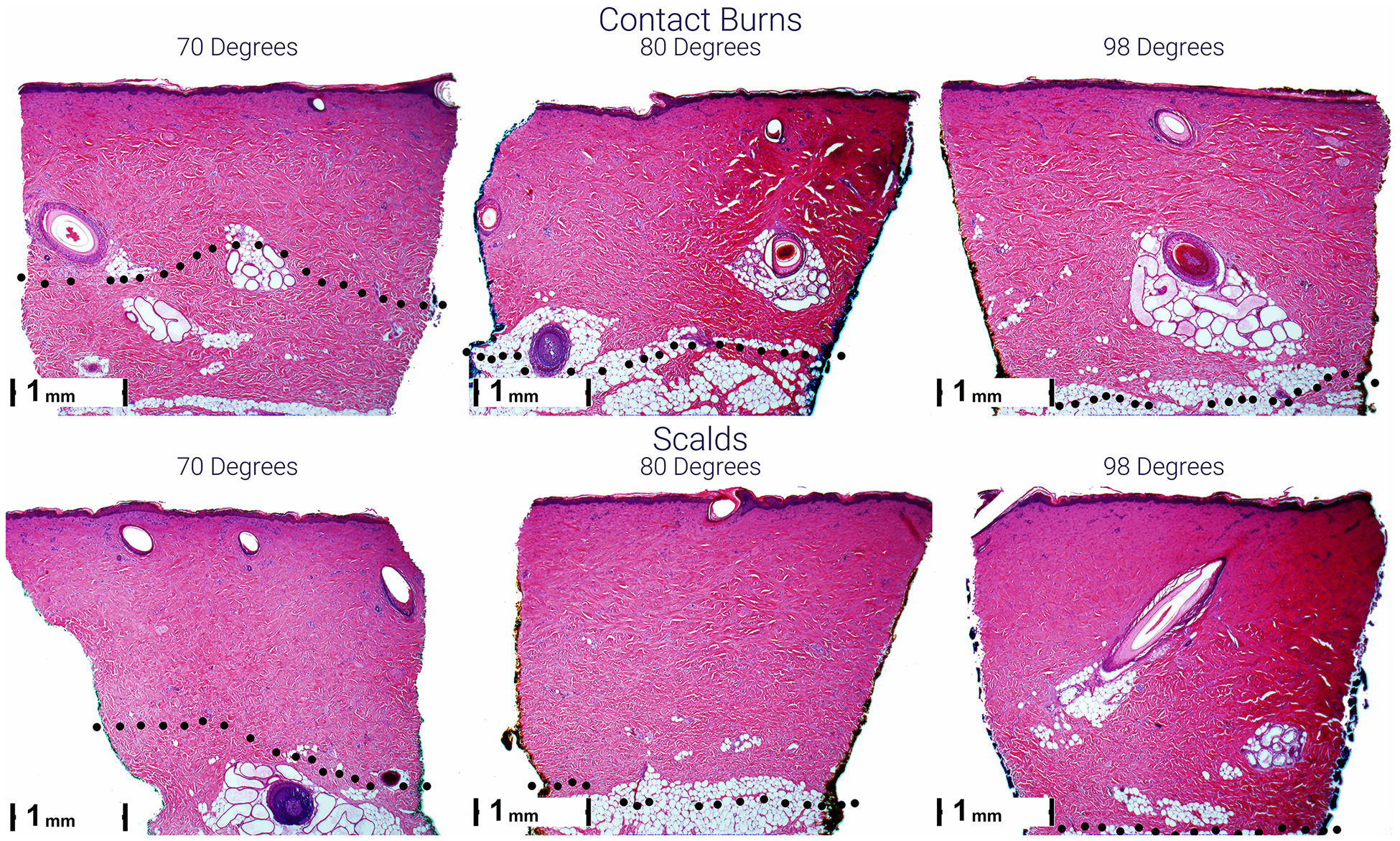
Representative micrographs of scald (lower) and contact (upper) burns 1 hour after injury. Burn depth is greater from left to right and top to bottom. The black dots indicate the lower boundary of the burn with evidence of blood vessel occlusion and/or necrosis of endothelial cells and hair follicle and sebaceous gland cells.
Figure 4.
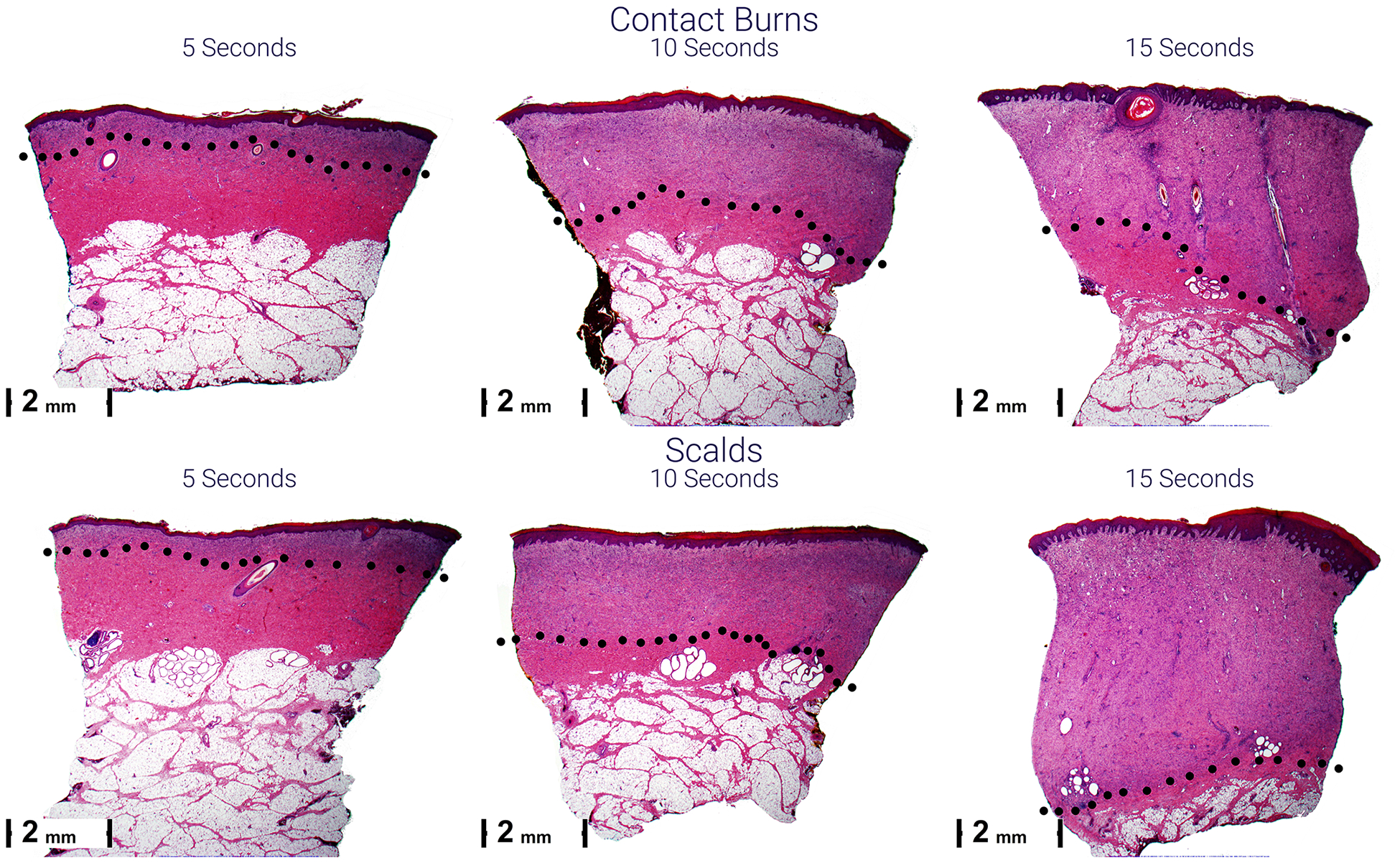
Representative micrographs of scald (lower) and contact (upper) burns 28 days after injury. Scars become thicker from left to right and top to bottom. The black dots indicate the lower boundary of the scar. The purple scars are due to the cellular infiltrate of granulation tissue composed of plump fibroblasts and new vessels. This is compared to the normal pink staining in the dermis below.
Burn morphology did not differ between scalds and contact burns. Regardless of temperature and exposure times, burns were characterized by a thin layer of homogenous, hyper-eosinophilic collagen lacking any fibrillar structure within which necrotic pyknotic fibrocytes were noted (Figure 2). Below this more superficial layer was a thicker layer of collagen containing multiple fissures but with distinct bundles of collagen. Damage to the epithelial structures was evident by pyknosis (necrosis) of the epithelial cells surrounding the hair follicles and apocrine ducts. Vascular damage was evident by the presence of vascular plugging (with RBC), extravasation of RBC, or pyknosis and necrosis of the endothelial cells in the vessel walls.
DISCUSSION
Our study demonstrates that in general, both burn depth determined immediately after injury or four days later, as well as scar depth four weeks after injury increase with increasing temperature and duration of exposure. This is consistent with numerous prior reports and is not novel.4 The fact that the differences in scar depth were generally statistically significant while the differences in burn depth immediately after injury were not is probably a result of the small sample size. In addition, since burns tend to progress over the first few days, determination of burn depth early after injury is more difficult and less representative of ultimate burn depth. Indeed, when measured four days after injury, the association of temperature of exposure and burn depth was significant. Alternatively, determination of burn depth by level of vascular injury may have underestimated burn depth. However, a prior study suggests that the level of vascular injury correlates most with scar depth.12
Most importantly our results show significantly deeper scars with scalds versus contact burns after controlling for temperature and duration of exposure. Since the heat capacity of water (liquid) is approximately 10 times higher than that of brass (solid),14 this finding is not surprising. In addition, transfer of heat by convection from circulating water but not brass, further contributes to overall heat transfer and helps explain why for any given temperature and duration of exposure scalds are worse than contact burns. Another possible explanation for the difference in depth that cannot be excluded is the fact that exposure time to scalds may have been slightly greater than contact burns since it took some additional time after stopping water inflow to completely remove all of the water from the pipe that came into contact with the skin.
In 1994 Brans et al argued that: “…there is a clear difference in the pathophysiology of scald burns as opposed to contact burns”. While they never explain what they mean by this statement they further state that: “Scald burns that seem superficial often heal with pronounced scar formation” implying that scalds result in greater tissue injury than contact burns. However, in their porcine study comparing scalds and contact burns they used very different temperatures and exposure times for the scalds (80° C for 10–40 seconds) and contact (170° C for 20 seconds) burns.15 They further argue that the contact burns result in immediate coagulation that insulates the underlying skin from further injury. In contrast, with scalds there is less homogenous injury and there is no obvious necrotic layer that can function as a barrier to stop further heat conduction to the underlying dermis, therefore resulting in greater injury. In our study we did not see clear differences in structure and homogeneity of the burns caused by scalds and contacts (Figure 2).
With regards to clinical experience, data concerning whether scalds or contact burns are more severe are conflicting. Obviously, with human injuries it generally is not possible to ascertain the temperature of exposure and exposure times making direct comparisons impossible. A recent study of injury patterns in Swiss children with burns found that scalds usually resulted in 1st and 2nd degree burns while flame injuries were more likely to be 3rd degree.16 In the same study, the rate of 3rd degree burns was higher for contacts than scalds (15.8% vs. 5.4%). Scalds and contact burns were also usually smaller in size than flame burns. Another study of non-scald burns in children from the UK found that only 6.5% of contact burns were full thickness.17 An epidemiologic study of burns and scalds in children from South Wales found that most inpatient injuries were due to scalds while most outpatient injuries were due to contact burns.18 However, they did not report burn depth and differences in outcomes may have been due to the fact that scalds were larger in size than the contact burns or much more common than contact burns in children. These results are similar to those reported several years earlier by Abeyasundara.19 Another study from Iraq found higher mortality rates with flame (27.6%) and scald (6.3%) burns while no deaths were seen in patients with contact burns.20 An earlier study conducted in the U.S. found that scalds were 2.5 times more likely to result in hospital admission than other burns.21
A major reason why clinical experience suggests that scalds may be less severe than contact burns, even though transfer of heat is greater with exposure to water for any given temperature and exposure time, is the fact that the temperature and length of exposure are probably less with scalds than contacts. In addition, considerable cooling and scatter of the liquid occurs with splash injuries further minimizing injury.22, 23 In contrast, contact with hot solid objects may be at considerably higher temperatures than even boiling water (100° C).24
Study Limitations
A major limitation of our study is the small sample size for any given combination of temperature, duration, and type of exposure. Despite this, the differences for scar depth were mostly statistically significant even though the differences in burn depth were not statistically significant. Although we attempted to tightly control exposure times, due to different methodologies of creating burns, it was not possible to completely standardize duration and temperature. Thus, with scalds, the time of exposure was slightly greater than with contact burns although cooling of the water mostly likely occurred during this brief additional exposure time. Obviously, results in pigs may not directly translate into humans. We also did not measure the temperature of the. Thermodynamic studies have shown the higher heat capacity of liquids vs. solids14 and certainly would imply that the temperature of the skin was greater after scalds. Finally, the relatively short follow up limits any conclusions regarding the long-term differences in outcomes after scalds and contact burns. While this study attempts to address the differences between scalds and contact burns, many questions remain unanswered. For example, what is the effect of type of liquid in scald burns? It is well known that burns from hot oil are more severe than from hot water.
In conclusion, when temperature and duration of exposure are tightly controlled and similar in a standardized porcine model, exposure to scalding water results in deeper injuries and scars than exposure to contact with heated brass metal.
Source of funding:
National Institute of General Medical Sciences (R01GM112693) and the Suffolk County Volunteer Firefighters Burn Center Fund.
REFERENCES
- 1.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 2.Qu M, Nourbakhsh M. Current experimental models of burns. Discovery medicine. 2017;23(125):95–103. [PubMed] [Google Scholar]
- 3. [October 2, 2019]; https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/#targetText=Over%2060%25%20of%20the%20estimated,3%20burn%20admissions%20per%20year. Accessed.
- 4.Andrews CJ, Cuttle L. Comparing the reported burn conditions for different severity burns in porcine models: a systematic review. Int Wound J. 2017;14(6):1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moritz AR, Henriques FC. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews CJ, Kempf M, Kimble R, Cuttle L. Development of a consistent and reproducible porcine scald burn model. PLoS One. 2016. September 9;11(9):e0162888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham JP, Plourde B, Vallez L, Stark J, Diller KR. Estimating the time and temperature relationship for causation of deep-partial thickness skin burns. Burns 2015;41:1741–1747. [DOI] [PubMed] [Google Scholar]
- 8.Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US). National Academy of Sciences; 2011. [Google Scholar]
- 9.Singer AJ, Hirth D, McClain SA, Crawford L, Lin F, Clark RA. Validation of a vertical progression porcine burn model. J Burn Care Res. 2011;32:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer AJ, Toussaint J, Chung WT, Thode HC, McClain S, Raut V. Effects of burn location and investigator on burn depth in a porcine model. Burns. 2016. February;42(1):184–189. [DOI] [PubMed] [Google Scholar]
- 11.Singer AJ, Thode HC Jr, McClain SA. The effects of epidermal debridement of partial-thickness burns on infection and reepithelialization in swine. Acad Emerg Med. 2000. February;7(2):114–9. [DOI] [PubMed] [Google Scholar]
- 12.Hirth D, McClain SA, Singer AJ, Clark RA. Endothelial necrosis at 1 hour postburn predicts progression of tissue injury. Wound Repair Regen. 2013. Jul-Aug;21(4):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitch EA, et al. , Hypertrophic burn scar: Analysis of variables. The Journal of Trauma, 1983. 21(10). [PubMed] [Google Scholar]
- 14. [March 24, 2020]; https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/17%3A_Thermochemistry/17.04%3A_Heat_Capacity_and_Specific_Heat Accessed.
- 15.Brans TA, Dutrieux RP, Hoekstra MJ, Kreis MJ, du Pont JS. Histopathological evaluation of scalds and contact burns in the pig model. Burns 1994;20:S48–S51. [DOI] [PubMed] [Google Scholar]
- 16.Moehrlen T, Szucs T, Landolt MA, Meuli M, Schiestl C, Moehrlen U. Trauma mechanisms and injury patterns in burn patients. Burns 2018;44:326–334. [DOI] [PubMed] [Google Scholar]
- 17.Johnson EL, Maguire S, Hollen LI, Nuttall D, Rea D, Kemp AM. Agents, mechanisms and clinical features of non-scald burns in children: a prospective UK study. Burns 2017;43:1218–1226. [DOI] [PubMed] [Google Scholar]
- 18.Battle CE, Evans V, James K, Guy K, Whitley J, Evans PA. Epidemiology of burns and scalds in children presenting to the emergency department of a regional burn unit: a 7-yeasr retrospective study. Burns and Trauma 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abeyasundara SL, Rajan V, Lam L, Harvey JG, Holland AJA. The changing pattern of pediatric burns. J Burn Care Res 2011;32:178–184. [DOI] [PubMed] [Google Scholar]
- 20.Othman N, Kendrick D, Al-Windi A. Childhood burns in Sulaimaniyah providence, Iraqi Kurdistan: A prospective study of admissions and outpatients. Burns 2015;41:394–400. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza AL, Nelson NG, McKenzie LB. Pediatric burn injuries treated in US emergency departments between 1990 and 2006. Pediatrics. 2009;124:1424–30. [DOI] [PubMed] [Google Scholar]
- 22.Alexander RT, Fowler AT. Modeling the distribution of scald type burns in a child. Acad Forensic Pathol 2016;6:638–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham JP, Nelson-Cheeseman BB, Sparrow E, Wentz JE, Gorman JM, Wolf SE. Comprehensive method to predict and quantify scald burns from beverage spills. Int J Hyperthermia 2016;32:900–910. [DOI] [PubMed] [Google Scholar]
- 24.Abraham JP, Stark J, Gorman J, Sparrow E, Minkowycz WJ. Tissue burns due to contact between a skin surface and highly conducting metallic media in the presence of inter-tissue boiling. Burns 2019;45:369–378. [DOI] [PubMed] [Google Scholar]


