Abstract
Flavors in electronic cigarette (ECIG) liquids may increase ECIG aerosol toxicity via intact distillation or chemical transformation. For this report, we performed a meta-analysis of the literature to categorize the compounds found in flavored ECIG liquids into a few chemical classes and to predict their possible chemical transformations upon ECIG liquid aerosolization. This analysis allowed us to propose specific correlations between flavoring chemicals and aerosol toxicants. A literature search was conducted in November 2019 using PubMed. Keywords included terms related to ECIGs and flavors. Studies were included if they reported chemical ingredients of flavored liquids and clearly stated the commercial names of these liquids. The obtained data were visualized on a network diagram to show the common chemical compounds identified in flavored ECIG liquids and categorize them into different chemical classes. The systematic literature review included a total of 11 articles. Analysis of the data reported gave a total of 189 flavored liquids and 173 distinct chemical compounds that were categorized into 22 chemical classes according to their functional groups. The subsequent prediction of chemical transformations of these functional groups highlighted the possible correlation of flavor compounds to aerosol toxicants.
Introduction
Electronic cigarette (ECIG) prevalence has increased dramatically in recent years, and ECIGs now rank as the most used tobacco product category among U.S. middle and high school students.1 Most ECIG users say that flavor is a key factor in their initiation and continued use.2,3 Flavors are pleasant and rewarding, and also influence user perception of ECIG safety, thus prolonging use among smoking-naïve individuals.4−7 Flavors also are reported to affect smokers’ decision to switch to ECIGs,7 and therefore may assist in reducing tobacco smoking. At the same time, the myriad flavored liquids on the market (e.g., >15 000 in the U.S. market and >20 000 in the Dutch market) pose a daunting public health challenge to regulatory bodies, as flavor diversity increases the attractiveness of ECIG use and complicates the largely unknown toxicity profiles of ECIG aerosols.8−10
The contribution of flavors to aerosol toxicity was recognized several years ago.11 This contribution can be due to toxic ingredients initially present in the flavored liquid, or toxicants formed when the parent liquid is heated and vaporized.12−14 Some reports in the literature have found that flavors are a dominant source of toxic aldehyde emissions.15,16 Others found that flavors affect the emission of radicals and reactive oxygen species (ROS).17,18 Only a limited number of studies noted a direct correlation between specific flavor ingredients and the formation of specific aerosol toxicants.19−21
In general, studies that analyze toxicant emissions in ECIG aerosols usually target toxicants commonly found in combustible cigarette smoke.22 Only one study performed a non-targeted screening of ECIG aerosols to check for other toxicants.23 In contrast, this screening or chemical profiling is more commonly done on flavored ECIG liquids.24−28 Knowing the chemical constituents of flavored liquids is important, as it may give an insight into the toxicity of the generated aerosols based on the transfer efficiency of these chemical compounds and their reactivity under ECIG conditions.14,29,30 In this report, we performed a meta-analysis of the published literature to identify all reported chemical compounds in flavored ECIG liquids and classify these compounds into chemical classes based on their functional groups (FGs). Next, we predicted their possible chemical reactivity upon ECIG aerosolization based on reported literature.
Methodology
Search Method
A literature search on the PubMed database with no time restriction was conducted in November 2019, using the following terms: (flavor OR flavour OR flavoring OR flavouring OR flavored OR flavoured OR flavorant OR flavourant) AND (electronic cigarette OR e-cigarette OR e-cig OR e-liquid OR electronic nicotine delivery system OR ENDS OR vape OR e-hookah). The only limits applied were that “hits” needed to be published online and written in English.
Inclusion Criteria
Studies were included in the meta-analysis if they (1) reported a general chemical profiling of flavored liquids (screening of chemical ingredients of flavored liquids) and (2) clearly stated the commercial names of these liquids. These names are needed for the graphical representation of the data. To determine eligibility, two reviewers independently examined the title and abstract of each reference from the literature. Studies that did not meet the inclusion criteria upon review of titles and abstracts were excluded. The papers that did meet the study requirements were collected and stored in a shared folder. The two reviewers read each of these reports and removed irrelevant articles due to mismatch with the above inclusion criteria. Each reviewer extracted information from the text of the remaining articles, and they cross-validated the data they found.
Network Representation of the Results
To visualize the most common FGs of the identified chemical compounds in ECIG liquids, we used the open-source network analysis and visualization software Gephi (version 0.9.2). The extracted information from the included articles was compiled in a Microsoft Excel file, which included article title, commercial names of the studied liquids, identified chemical compounds, chemical structures, and determined functional groups. Each liquid, chemical, and functional group was given a unique code to be used later in the input to the Gephi software. The data were sorted in the following hierarchy: commercial name of flavored liquid, name of the chemical compound, and chemical class according to FGs. The FGs were determined after careful examination of the chemical structure of the identified compound. These FGs were verified by an online tool (ACE functional group finder).31 Note that one chemical could have two or more FGs, and thus could be linked in the étoile (star) to more than one chemical class. The three categories were taken as nodes connected by undirected links: flavored liquids are linked to chemical compounds, and the latter are linked to chemical classes—the size of a spherical node changes with the number of connections it has to the other nodes.
Results
Included Studies
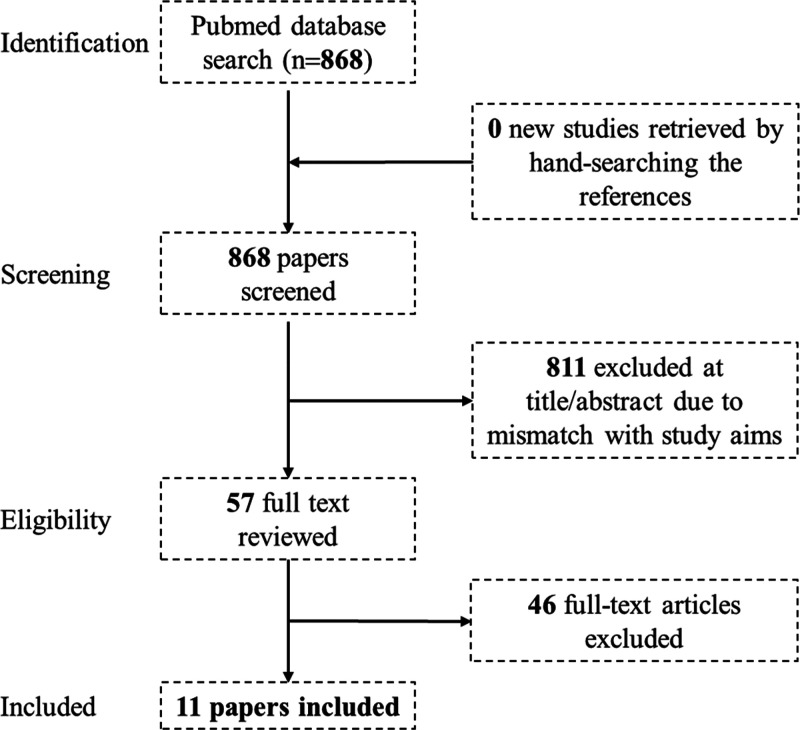
After removal of duplicates and non-English articles, the search retrieved 868 items. Studies were included in the meta-analysis if they matched the inclusion criteria. Upon review of the article titles and abstracts, 811 articles were excluded due to irrelevance to the study aims. A review of the full text of the remaining 57 articles removed 46 items that were excluded due to a lack of relevance/failure to meet the inclusion criteria. Thus, a total of 11 articles was included in the meta-analysis. Figure 1 shows the PRISMA diagram that illustrates the steps that accompanied the selection process.
Figure 1.
A PRISMA diagram illustrating the process of literature selection.
Classification of Flavor Chemicals
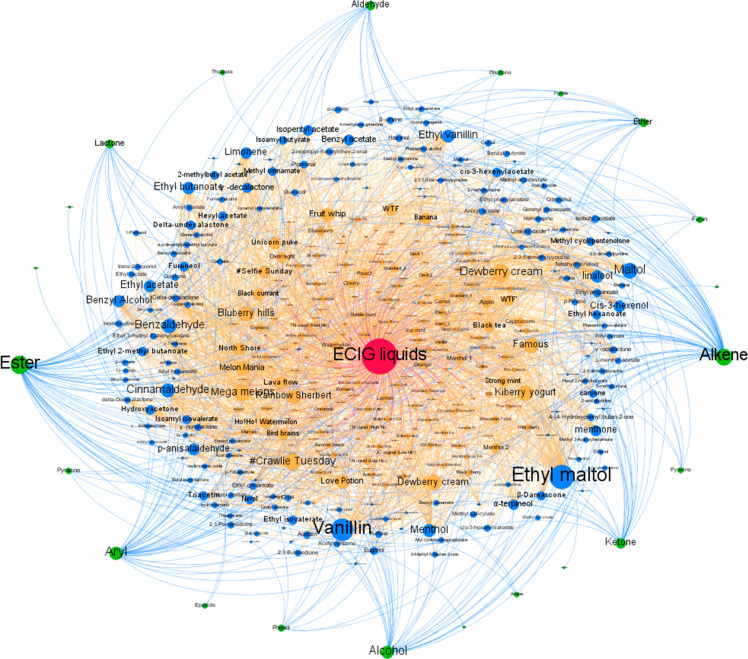
The data were extracted from the included 11 articles.14,17,24−27,29,32−35 Analysis revealed 189 flavored ECIG liquids containing some combination of 173 chemical compounds (8.4 ± 9.5 chemical compounds per ECIG liquid). Figure 2 shows the Gephi diagram, or the chemical class étoile, of the collected data with the 173 distinct chemical compounds detected (Supporting Information). The sphere in the center of the diagram represents flavored ECIG liquids in general. Analysis of the structures of these chemical compounds allowed their classification into 22 chemical classes: alcohol, aldehyde, alkene, amide, amine, aryl (or aromatics), diketone, epoxide, ester, ether, furan, hydrazone, imidazole, ketone, lactone, phenol, pyrazine, pyrazole, pyridine, pyrimidine, pyrrole, and thiazole (Supporting Information). This representation shows that, in principle, a large number of flavored ECIG liquids and their corresponding chemical ingredients could be reduced to a manageable number of chemical classes. Indeed, some of the studies included in this Review mentioned the frequency of chemical classes present in their sample set.14,24,25,35
Figure 2.
A chemical class étoile of 173 chemical compounds found in 189 flavored liquids and their classification into 22 chemical classes. Color code: red = flavored ECIG liquids in general, orange = commercial flavored liquid, blue = chemical compound, green = chemical class.
The étoile shows that some chemical compounds are common among the tested flavored ECIG liquids, like ethyl maltol (n = 89, 47%), vanillin (n = 69, 37%), menthol (n = 54, 29%), ethyl vanillin (n = 43, 23%), linalool (n = 43, 23%), benzaldehyde (n = 41, 22%), benzyl alcohol (n = 39, 21%), maltol (n = 38, 20%), cinnamaldehyde (n = 37, 20%), ethyl butanoate (n = 35, 19%), and hydroxyacetone (n = 31, 16%). This observation is in agreement with the results of a recent report that analyzed the flavor ingredients in ECIG liquids marketed in The Netherlands using information provided by the manufacturers.36
Discussion
Reactivity of Chemical Classes
The étoile shows that ester (n = 58, 33%) and alkene (n = 57, 33%) are the most frequent chemical classes, followed by aryl (n = 41, 24%), alcohol (n = 31, 18%), ketone (n = 25, 14%), aldehyde (n = 17, 10%), and lactone (n = 15, 9%). Considering the ECIG heating element as a pyrolysis reactor operating within an oxygen-containing atmosphere,53−38 the reactions taking place during ECIG use can be classified as oxidation, thermal degradation, radical generation/addition, and adduct formation. Under such conditions, esters are prone to break down into carboxylic acids and small alkenes.39 Ethyl butanoate, for example, will decompose to give ethylene and butanoic acid. Alkenes may undergo oxidation to give diols, carbonyls, or peroxides.40,41 The aryl class in our classification includes chemical compounds that have a benzene ring next to a functional group (except for p-cymene). The aryl class comprises chemical compounds like benzyl alcohol, benzaldehyde, cinnamaldehyde, and other related chemical compounds that can undergo oxidation to carboxylic acids followed by decarboxylation to give benzene, toluene, ethylbenzene, xylene (BTEX), and styrene.42 Alcohols under thermal conditions undergo oxidation via radical intermediates to give carbonyl compounds and/or carboxylic acids.43 Lactones exhibit transformations similar to esters.44 Aldehydes can react with ECIG carriers (propylene glycol (PG) and vegetable glycerol (VG)) to give hemiacetals,45 or oxidize to give carboxylic acids.46 Ketones can undergo Baeyer–Villiger oxidation to give esters or lactones.47 In summary, under typical ECIG operating conditions, predominant chemical classes are predicted to produce mainly carbonyls, carboxylic acids, alkenes, and volatile organic compounds like BTEX (Table 1).15,48−50
Table 1. Summary of Predicted Chemical Transformations of Flavor Chemical Compounds Identified in the Literature.
| esters | → | carboxylic acids + alkenes |
| alkenes | → | diols + carbonyls + peroxides |
| aryls | → | BTEX + styrene |
| alcohols | → | carbonyls + carboxylic acids |
| lactones | → | carboxylic acids + alkenes |
| aldehydes | → | hemiacetals + carboxylic acids |
| ketones | → | esters + lactones |
Other less frequent chemical classes, like epoxide and hydrazine, could be highly reactive under ECIG pyrolysis conditions. Also, the presence of more than one FG in a chemical and the presence of multiple components in the same reaction medium can lead to more than the identified products through secondary reactions. Moreover, as many ECIGs today contain nicotine salts and have low-pH liquids, those innovations may affect the outcome of the depicted chemical reactions. Nevertheless, predicting the primary reaction pathways that may take place in flavored ECIG liquids when aerosolized may help guide future research on the assessment of ECIG aerosol toxicant profile. Importantly, ECIG operating parameters (e.g., power output and puff duration) can highly impact the generation of toxicants in the aerosols and should be considered in any prediction of chemical transformations of flavor ingredients. Also, there is a possibility that chemical transformations of liquid ingredients can be catalyzed on the surface of the heating element and this effect may complicate such a prediction.38 An antecedent to this can be traced back to the pioneering work of Uchiyama on carbonyl emissions from the thermal degradation and oxidation of PG and VG in ECIG liquid.51,52
This work could help economize the efforts of targeted analysis of toxicants in ECIG aerosols as it highlights potential analytes of interest. Also, the predicted reactivity can inform the design of clinical trials so that researchers avoid flavored liquids that are expected to emit highly toxic compounds, and it may aid in the development of specific biomarkers of exposure to certain flavors. This work will be extended by subsequent empirical validation of some of the predicted reaction pathways of flavor chemicals. For example, future research might focus on the transformation of alcohol and aryl FGs, under different ECIG operating conditions, to give carbonyl and BTEX emissions, respectively.
Limitations
This work has several limitations that may restrict its generalizability. Due to the inclusion criterion that a published report needed to state explicitly the commercial names of flavored ECIG liquids, which was required for our graphical presentation, we may have missed some important chemical compounds in the excluded reports. However, the idea behind our approach is not to provide a comprehensive list of all identified chemical compounds, but to build correlations between liquid chemicals and aerosol toxicants. Also, chemical compounds that have more than one FG may present a challenge to our predictions due to competing reactivities of the different FGs, or the detrimental effect of one FG on the reactivity of the other. This structure specificity of FG reactivity is to be assessed as we strive to validate our predictions by empirical data. Of course, the influence of ECIG operating parameters on the formation of toxicants should be taken into consideration. In addition, our reactivity prediction highlights the possible detection of toxicants under specific conditions and that result must be determined experimentally.
Conclusion
This report highlights the importance of categorizing flavor ingredients into a few chemical classes and correlating their chemical reactivity with the toxicant formation in the aerosols. This work can be used to construct a conceptual framework that may help in enhancing knowledge on how flavor compounds in ECIG liquids contribute to toxicant emissions in ECIG aerosols. This database will be made available online for other researchers to identify aerosol toxicants to be studied and to allow researchers to understand the possible provenances of their analytical discoveries.
Glossary
Abbreviations
- ECIG
electronic cigarette
- PG
propylene glycol
- VG
vegetable glycerol
- ROS
reactive oxygen species
- FG
functional group
- ENDS
electronic nicotine delivery system
Biographies
Sally Salam is a graduate student at the Department of Chemistry at the American University of Beirut (AUB). She received her B.S. degree in 2018 from the Beirut Arab University. She is pursuing her Master’s degree under the supervision of Dr. Najat A. Saliba and Dr. Ahmad El-Hellani. Her research focuses on the assessment of the toxicity of electronic cigarette (ECIG) emissions.
Najat A. Saliba is a Professor of analytical chemistry at the Department of Chemistry and the Director of the Nature Conservation Center at AUB. She received her Ph.D. degree in 1999 from the University of Southern California. She specializes in the chemistry of airborne particles, including tobacco smoke. She has adapted analytical methods for examining toxicants in waterpipe tobacco and combustible cigarette smoke and in ECIG aerosols. She has published more than 80 peer-reviewed publications, of which 30 address tobacco products. She has frequently advised the WHO on tobacco control.
Alan Shihadeh is a Professor of mechanical engineering and the Dean of the Maroun Semaan Faculty of Engineering and Architecture at AUB. He received his Sc.D. in 1998 from the Massachusetts Institute of Technology. He has developed technologies to record human puffing behavior in natural settings and reproduce this behavior in the analytical lab to measure toxicant emissions from ECIGs and waterpipes. Also, he has developed a mathematical model to predict ECIG aerosol emissions. He has co-authored more than 70 peer-reviewed tobacco-related publications and has advised WHO, ISO, and regulatory agencies on tobacco matters.
Thomas Eissenberg is a Professor of psychology and the Director of the Center for the Study of Tobacco Products (CSTP) at the Virginia Commonwealth University (VCU). He received his Ph.D. in 1994 from McMaster University. His research focuses on novel tobacco products, namely waterpipe and ECIG. He has used a variety of methods, including analytical lab, aerosol research lab, clinical lab, and surveys, to assess nicotine delivery, toxicant emissions, and subjective effect profiles of these products. His work has been extensively funded by NIH, and he has co-authored over 240 peer-reviewed publications.
Ahmad El-Hellani is an Assistant Research Professor at the Department of Chemistry at AUB. He received his Ph.D. in 2012 from Université Paris Sud. He joined CSTP in 2014 as a postdoc trainee, and since 2018 he is a senior analytical chemist at the center. His research focuses on understanding the influence of different operating parameters of ECIG (i.e., device power, liquid composition, and user puffing topography) on nicotine delivery and toxicant emission. Also, he focuses on understanding the underlying chemical mechanisms that take place upon ECIG activation, including the association of some flavoring ingredients with toxicants in the generated aerosols. He has co-authored more than 20 peer-reviewed tobacco-related publications and has advised WHO on tobacco products regulation.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.0c00247.
List of chemical compounds identified in the meta-analysis with structural information (PDF)
Author Contributions
The first and the corresponding authors conducted the literature search. The corresponding author drafted the manuscript. All authors approved the final version of the manuscript.
This research is supported by grant number U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
The authors declare the following competing financial interest(s): Drs. Eissenberg and Shihadeh are paid consultants in litigation against the tobacco industry and the electronic cigarette industry. They are named on a patent for a device that measures the puffing behavior of electronic cigarette users. In addition, Dr. Eissenberg is named on another patent for a smartphone app that determines electronic cigarette device and liquid characteristics.
Supplementary Material
References
- Cullen K. A.; Gentzke A. S.; Sawdey M. D.; Chang J. T.; Anic G. M.; Wang T. W.; Creamer M. R.; Jamal A.; Ambrose B. K.; King B. A. (2019) e-Cigarette Use Among Youth in the United States. JAMA 322 (21), 2095–2103. 10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry R. L.; Groom A. L.; Vu T.-H. T.; Stokes A. C.; Berry K. M.; Kesh A.; Hart J. L.; Walker K. L.; Giachello A. L.; Sears C. G.; McGlasson K. L.; Tompkins L. K.; Mattingly D. T.; Robertson R. M.; Payne T. J. (2019) The role of flavors in vaping initiation and satisfaction among U.S. adults. Addictive Behaviors 99, 106077. 10.1016/j.addbeh.2019.106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall P.; Hoek J. (2020) Role of flavours in vaping uptake and cessation among New Zealand smokers and non-smokers: a cross-sectional study. Tobacco Control 10.1136/tobaccocontrol-2019-055469. [DOI] [PubMed] [Google Scholar]
- Zare S.; Nemati M.; Zheng Y. (2018) A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One 13 (3), e0194145 10.1371/journal.pone.0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. M.; Goldenson N. I.; Cho J.; Kirkpatrick M. G.; McConnell R. S.; Stone M. D.; Pang R. D.; Audrain-McGovern J.; Barrington-Trimis J. L. (2019) Flavored E-cigarette Use and Progression of Vaping in Adolescents. Pediatrics 144, e20190789 10.1542/peds.2019-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Sankey J. C.; Kong G.; Choi K. (2019) Perceived ease of flavored e-cigarette use and e-cigarette use progression among youth never tobacco users. PLoS One 14 (2), e0212353 10.1371/journal.pone.0212353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijnders K. A.; Krusemann E. J.; Boesveldt S.; de Graaf K.; de Vries H.; Talhout R. (2019) E-Liquid Flavor Preferences and Individual Factors Related to Vaping: A Survey among Dutch Never-Users, Smokers, Dual Users, and Exclusive Vapers. Int. J. Environ. Res. Public Health 16 (23), 4661. 10.3390/ijerph16234661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu G.; Sun J. Y.; Zhu S.-H. (2018) Evolution of Electronic Cigarette Brands From 2013–2014 to 2016–2017: Analysis of Brand Websites. Journal of Medical Internet Research 20 (3), e80 10.2196/jmir.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüsemann E. J.; Visser W. F.; Cremers J. W.; Pennings J. L.; Talhout R. (2018) Identification of flavour additives in tobacco products to develop a flavour library. Tobacco Control 27 (1), 105–111. 10.1136/tobaccocontrol-2016-052961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havermans A.; Krüsemann E. J. Z.; Pennings J.; De Graaf K.; Boesveldt S.; Talhout R. (2019) Nearly 20 000 e-liquids and 250 unique flavour descriptions: an overview of the Dutch market based on information from manufacturers. Tobacco Control 10.1136/tobaccocontrol-2019-055303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R. Z.; Davis B.; Wang Y.; Bahl V.; Lin S.; Talbot P. (2014) Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. In Vitro 28 (2), 198–208. 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Vas C. A.; Porter A.; McAdam K. (2019) Acetoin is a precursor to diacetyl in e-cigarette liquids. Food Chem. Toxicol. 133, 110727. 10.1016/j.fct.2019.110727. [DOI] [PubMed] [Google Scholar]
- Kosmider L.; Sobczak A.; Prokopowicz A.; Kurek J.; Zaciera M.; Knysak J.; Smith D.; Goniewicz M. L. (2016) Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 71 (4), 376–377. 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh N. J.; Lawton R. I.; Hershberger P. A.; Goniewicz M. L. (2016) Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control 25 (Suppl 2), ii81–ii87. 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A.; Samburova V. (2016) Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 50 (23), 13080–13085. 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- Qu Y.; Kim K.-H.; Szulejko J. E. (2018) The effect of flavor content in e-liquids on e-cigarette emissions of carbonyl compounds. Environ. Res. 166, 324–333. 10.1016/j.envres.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Bitzer Z. T.; Goel R.; Reilly S. M.; Elias R. J.; Silakov A.; Foulds J.; Muscat J.; Richie J. P. (2018) Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radical Biol. Med. 120, 72–79. 10.1016/j.freeradbiomed.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y.; Mishin V.; Laskin J. D.; Mainelis G.; Wackowski O. A.; Delnevo C.; Schwander S.; Khlystov A.; Samburova V.; Meng Q. (2019) Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem. Res. Toxicol. 32 (6), 1087–1095. 10.1021/acs.chemrestox.8b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeke S.; Peyton D. H.; Strongin R. M. (2018) Triacetin Enhances Levels of Acrolein, Formaldehyde Hemiacetals, and Acetaldehyde in Electronic Cigarette Aerosols. ACS Omega 3 (7), 7165–7170. 10.1021/acsomega.8b00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage R.; El-Hellani A.; Haddad C.; Salman R.; Talih S.; Shihadeh A.; Eissenberg T.; Aoun Saliba N. (2019) Toxic emissions resulting from sucralose added to electronic cigarette liquids. Aerosol Sci. Technol. 53 (10), 1197–1203. 10.1080/02786826.2019.1645294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussy S.; El-Hellani A.; Baalbaki R.; Salman R.; Shihadeh A.; Saliba N. A. (2016) Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tobacco Control 25 (Suppl 2), ii88–ii93. 10.1136/tobaccocontrol-2016-053220. [DOI] [PubMed] [Google Scholar]
- Goniewicz M. L.; Knysak J.; Gawron M.; Kosmider L.; Sobczak A.; Kurek J.; Prokopowicz A.; Jablonska-Czapla M.; Rosik-Dulewska C.; Havel C.; Jacob P.; Benowitz N. (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23 (2), 133–139. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson C.; Martin S.; Frosina J.; Wright C. (2017) Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. Journal of Chromatography A 1497, 144–154. 10.1016/j.chroma.2017.02.050. [DOI] [PubMed] [Google Scholar]
- Tierney P. A.; Karpinski C. D.; Brown J. E.; Luo W.; Pankow J. F. (2016) Flavour chemicals in electronic cigarette fluids. Tobacco Control 25 (e1), e10–e15. 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye E. E.; McWhirter K. J.; Luo W.; Tierney P. A.; Pankow J. F.; Talbot P. (2019) High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 9 (1), 2468. 10.1038/s41598-019-39550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszyk J.; Kubica P.; Kot-Wasik A.; Namieśnik J.; Wasik A. (2017) Comprehensive determination of flavouring additives and nicotine in e-cigarette refill solutions. Part I: Liquid chromatography-tandem mass spectrometry analysis. Journal of Chromatography A 1519, 45–54. 10.1016/j.chroma.2017.08.056. [DOI] [PubMed] [Google Scholar]
- Aszyk J.; Woźniak M. K.; Kubica P.; Kot-Wasik A.; Namieśnik J.; Wasik A. (2017) Comprehensive determination of flavouring additives and nicotine in e-cigarette refill solutions. Part II: Gas-chromatography-mass spectrometry analysis. Journal of Chromatography A 1517, 156–164. 10.1016/j.chroma.2017.08.057. [DOI] [PubMed] [Google Scholar]
- Krüsemann E. J. Z.; Pennings J. L. A.; Cremers J. W. J. M.; Bakker F.; Boesveldt S.; Talhout R. (2020) GC-MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 188, 113364. 10.1016/j.jpba.2020.113364. [DOI] [PubMed] [Google Scholar]
- Gerloff J.; Sundar I. K.; Freter R.; Sekera E. R.; Friedman A. E.; Robinson R.; Pagano T.; Rahman I. (2017) Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. In Vitro Toxicol 3 (1), 28–40. 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J. F.; Kim K.; Luo W.; McWhirter K. J. (2018) Gas/Particle Partitioning Constants of Nicotine, Selected Toxicants, and Flavor Chemicals in Solutions of 50/50 Propylene Glycol/Glycerol As Used in Electronic Cigarettes. Chem. Res. Toxicol. 31 (9), 985–990. 10.1021/acs.chemrestox.8b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACE functional group finder, https://epoch.uky.edu/ace/public/fnalGroups.jsp.
- Peace M. R.; Mulder H. A.; Baird T. R.; Butler K. E.; Friedrich A. K.; Stone J. W.; Turner J. B. M.; Poklis A.; Poklis J. L. (2018) Evaluation of Nicotine and the Components of e-Liquids Generated from e-Cigarette Aerosols. J. Anal. Toxicol. 42 (8), 537–543. 10.1093/jat/bky056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko J. G.; Tran H.; Stanfill S. B.; Blount B. C.; Watson C. H. (2015) Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob. Res. 17 (10), 1270–1278. 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar R. Z.; Luo W.; McWhirter K. J.; Pankow J. F.; Talbot P. (2018) Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 8 (1), 8288. 10.1038/s41598-018-25575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M.; Omaiye E. E.; Luo W.; McWhirter K. J.; Pankow J. F.; Talbot P. (2019) Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci. Rep. 9 (1), 2782. 10.1038/s41598-019-38978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüsemann E. J. Z.; Havermans A.; Pennings J. L. A.; de Graaf K.; Boesveldt S.; Talhout R. (2020) Comprehensive overview of common e-liquid ingredients and how they can be used to predict an e-liquid’s flavour category. Tobacco Control 10.1136/tobaccocontrol-2019-055447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hellani A.; El-Hage R.; Salman R.; Talih S.; Zeaiter J.; Eissenberg T.; Shihadeh A.; Saliba N. A. (2020) Electronic Cigarettes Are Chemical Reactors: Implication to Toxicity. Chem. Res. Toxicol. 33 (10), 2489–2490. 10.1021/acs.chemrestox.0c00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Chen W.; Liao J.; Matsuo T.; Ito K.; Fowles J.; Shusterman D.; Mendell M.; Kumagai K. (2017) A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS One 12 (1), e0169811 10.1371/journal.pone.0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba N. A.; El Hellani A.; Honein E.; Salman R.; Talih S.; Zeaiter J.; Shihadeh A. (2018) Surface chemistry of electronic cigarette electrical heating coils: Effects of metal type on propylene glycol thermal decomposition. J. Anal. Appl. Pyrolysis 134, 520–525. 10.1016/j.jaap.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R.; Kralert P. G.; Kagi R. I. (1992) Kinetics and mechanism of the thermal decomposition of esters in sediments. Org. Geochem. 19 (1), 133–140. 10.1016/0146-6380(92)90032-S. [DOI] [Google Scholar]
- Wang A.; Jiang H. (2010) Palladium-Catalyzed Direct Oxidation of Alkenes with Molecular Oxygen: General and Practical Methods for the Preparation of 1,2-Diols, Aldehydes, and Ketones. J. Org. Chem. 75 (7), 2321–2326. 10.1021/jo100125q. [DOI] [PubMed] [Google Scholar]
- Lim S. Y.; Kang M.; Kim J.; Lee I.-M. (2005) Epoxidation of Simple Alkenes with O2 and Isobutyraldehyde Catalyzed by Ni Catalysts Deposited on Nanoporous Carbon. Bull. Korean Chem. Soc. 26 (6), 887. 10.5012/bkcs.2005.26.6.887. [DOI] [Google Scholar]
- Manion J. A.; McMillen D. F.; Malhotra R. (1996) Decarboxylation and Coupling Reactions of Aromatic Acids under Coal-Liquefaction Conditions. Energy Fuels 10, 776–788. 10.1021/ef950219c. [DOI] [Google Scholar]
- Vanoye L.; Favre-Reguillon A.; Aloui A.; Philippe R.; de Bellefon C. (2013) Insights in the aerobic oxidation of aldehydes. RSC Adv. 3 (41), 18931–18937. 10.1039/c3ra42385a. [DOI] [Google Scholar]
- Bailey W. J.; Bird C. N. (1977) Pyrolysis of esters. 27. Pyrolysis of lactones. J. Org. Chem. 42 (24), 3895–3899. 10.1021/jo00444a021. [DOI] [Google Scholar]
- Balashov A. L.; Danov S. M.; Krasnov V. L.; Chernov A. Y.; Ryabova T. A. (2002) Association of Formaldehyde in Aqueous-Alcoholic Systems. Russ. J. Gen. Chem. 72 (5), 744–747. 10.1023/A:1019512419592. [DOI] [Google Scholar]
- McNesby J.; Heller C. Jr (1954) Oxidation of Liquid Aldehydes by Molecular Oxygen. Chem. Rev. 54, 325. 10.1021/cr60168a004. [DOI] [Google Scholar]
- Bolm C.; Schlingloff G.; Weickhardt K. (1993) Use of molecular oxygen in the Baeyer-Villiger oxidation the influence of metal catalysts. Tetrahedron Lett. 34 (21), 3405–3408. 10.1016/S0040-4039(00)79167-2. [DOI] [Google Scholar]
- El-Hellani A.; Al-Moussawi S.; El-Hage R.; Talih S.; Salman R.; Shihadeh A.; Saliba N. A. (2019) Carbon Monoxide and Small Hydrocarbon Emissions from Sub-ohm Electronic Cigarettes. Chem. Res. Toxicol. 32 (2), 312–317. 10.1021/acs.chemrestox.8b00324. [DOI] [PubMed] [Google Scholar]
- Ogunwale M. A.; Li M.; Ramakrishnam Raju M. V.; Chen Y.; Nantz M. H.; Conklin D. J.; Fu X.-A. (2017) Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2 (3), 1207–1214. 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J. F.; Kim K.; McWhirter K. J.; Luo W.; Escobedo J. O.; Strongin R. M.; Duell A. K.; Peyton D. H. (2017) Benzene formation in electronic cigarettes. PLoS One 12 (3), e0173055 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekki K.; Uchiyama S.; Ohta K.; Inaba Y.; Nakagome H.; Kunugita N. (2014) Carbonyl Compounds Generated from Electronic Cigarettes. Int. J. Environ. Res. Public Health 11 (11), 11192–11200. 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S.; Inaba Y.; Kunugita N. (2010) Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. Journal of Chromatography A 1217 (26), 4383–4388. 10.1016/j.chroma.2010.04.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.