Abstract
Alcohol use is a major risk factor associated with unprotected sexual behavior, leading to higher risk of sexually transmitted infections (STI) including the human immunodeficiency virus (HIV). Emerging largely cross-sectional data suggest functional network connectivity strength is associated with problematic alcohol use, and as evidence supports a relationship between risky sexual behaviors and alcohol use, we hypothesized that functional connectivity might be associated with both categories of risk behavior. As part of a sexual risk reduction intervention study, juvenile justice-involved adolescents (N = 239) underwent a baseline functional magnetic resonance imaging scan and completed questionnaires about their alcohol use and risky sexual behavior at 3-month intervals over 12 months of follow up. To test both cross-sectional and longitudinal relationships between alcohol use and sexual risk behaviors, we estimated a parallel process latent growth model that simultaneously modeled the trajectories of alcohol use and sexual risk behavior. Functional connectivity strength was included as an exogenous variable to evaluate its relationship with level of risk and change in risk over time in both behaviors. Associations were found between baseline alcohol use and risky sex, and between longitudinal trajectories of alcohol use and risky sex. Network functional connectivity strength of the dorsal default mode network was associated with initial and longitudinal alcohol use, which may suggest that self-awareness of the effects of alcohol could serve as a useful target to decrease subsequent risky sexual behavior in adolescence.
Keywords: Adolescence, alcohol, default mode, functional connectivity, juvenile justice, risky sex
INTRODUCTION
Many of the behaviors typical of adolescence have potentially serious negative consequences [1]. Among those behaviors is unprotected sexual intercourse. About half of all new sexually transmitted infections (STIs) worldwide, including the human immunodeficiency virus (HIV), occur among young people between the ages of 15 and 24 [2]. Youth involved with the justice system are at particularly high risk for negative outcomes as a result of risky sexual behavior, since juvenile justice-involved adolescents are younger at first intercourse, have higher rates of anal intercourse, a greater number of sex partners, and lower rates of condom use than the general adolescent population [3]. Alcohol use is a significant risk factor associated with unprotected sexual behavior [4] and the relationship of alcohol use to risky sex appears to be particularly strong for high-risk adolescents including those involved in the justice system [5-8].
Risky situations involve high-valence immediate rewards (e.g., positive social, physical, and emotional consequences of alcohol use and sexual intercourse) contrasted with the need to control behavior in the face of potentially serious but relatively distal negative consequences (e.g., unintended pregnancy, STIs). Due to the short time frame within which most of these decisions are made, decision-making about sexual activity involves the rapid consideration of a compelling reward balanced against the potential for weighty negative consequences, through the attendant involvement of widespread neural networks [9-11] which continue to develop throughout adolescence [12]. In order to gain a better understanding of the degree to which individual differences in brain functioning relate to risk taking, it is critically important to consider functional network-level correlates of risk behavior. Functional connectivity measures provide useful information regarding overall neural relationships as opposed to studying isolated regional activation, with downstream implications for development of executive functions and decision making.
Alcohol use has been shown to be associated with dysfunctional connectivity in adults, with some preliminary findings in adolescents. Compared with healthy controls, chronic alcoholic patients showed reduced task-related fronto-cerebellar [13] and fronto-striatal connectivity [14]. During resting state scans, relapsing alcoholic subjects had reduced functional connectivity in reward and executive control networks, which the authors suggest may manifest in the inability to inhibit behavior [15]. In resting state studies of risk evaluating subjects with a positive family history of alcohol use disorders compared with family history negative controls, substance-naïve youth had decreased connectivity in frontoparietal regions [16] and at-risk adolescents showed reduced connectivity between prefrontal and contralateral cerebellar regions [17]. Task-related functional connectivity has also been associated with family history of alcoholism among young adults aged 18 to 22 years, which was related to both sensation-seeking behavior and alcohol consumption [18]. These studies suggest that alterations in functional connectivity are associated not only with alcohol use, but also with the risk of problematic use. An open question is whether network connectivity might also be associated with risk behaviors associated with alcohol use, including sexual risk behavior.
The current exploratory study modeled relationships among functional connectivity networks and trajectories of alcohol use and risky sexual behavior in a sample of juvenile justice-involved adolescents. As the above studies found connectivity alterations with frontal control regions to be associated with alcohol use and risk, we hypothesized that the strength of functional connectivity within the executive control network (ECN) might be predictive of risky behaviors. Further, a growing body of research has focused on the role of the default mode network (DMN) in multiple pathologies [19, 20]. This network, associated with self-referential processes including retrieval and manipulation of episodic memories [21], also seems likely to be involved in risk. Initial correlations explored relationships among sexual risk, alcohol use, and functional connectivity strength, and a latent growth model was then applied to explore how baseline functional connectivity was related to baseline levels and trajectories of alcohol use and risky sexual behavior.
METHODS
Participants
A total of 239 adolescents were examined for these analyses. Data were taken from a longitudinal study assessing substance use and sexual risk among adolescents (N = 327) recruited from juvenile justice diversion programs in the southwestern United States. Following a baseline assessment that included a neuroimaging session and questionnaires, participants answered questions about their substance use and sexual-risk behavior every 3 months over a 1-year period. Retention rates were 84.1% (n = 201), 89.5% (n = 214), 89.5% (n = 214), and 91.2% (n = 218) at the 3-, 6-, 9-, and 12-month follow-ups, respectively.
The demography of the sample was typical of the juvenile justice system such that participants were mostly male (175 males and 64 females), were a mean age of 16.13 years (SD = 1.09, range 14-18), and were ethnically diverse (58.6% Hispanic/Latino, 21.3% Caucasian, 8.4% Mixed/Biracial, 5.4% African American, 4.2% American Indian, 1.7% Asian/Pacific Islander, and .4% Unspecified). The majority of participants reported being attracted to the opposite sex (86.8%), while only a small minority reported being attracted to people of the same sex (2.6%) or both sexes (10.6%).
Procedures
Participants were recruited through a court-ordered diversion program by research assistants visiting in person and announcing the opportunity for participation in a research study. Research assistants provided a basic description of the study including time commitment, inclusion and exclusion criteria, and compensation. Adolescents who expressed interest in the study then individually met in a private location with a research assistant to complete informed consent documents and thoroughly complete screening for the inclusion/exclusion criteria. Once adolescent assent was obtained, parent/guardian consent was obtained via telephone for each adolescent. To be eligible for the study, participants had to (1) be between the ages of 14-18 years old, (2) have verbal consent via taped-recorded phone calls from a parent or legal guardian, (3) not currently be taking psychotropic medications, and (4) be free of magnetic resonance imaging (MRI) contraindications, including a history of injury to the brain or brain-related medical conditions, non-removable metallic implants, recent tattoo, and pregnancy (if female). Juvenile justice staff were not involved in recruitment, and participation decisions had no impact on the adolescent’s juvenile justice status or treatment. Participants received a total of $185 for completing all components of the study. All procedures were reviewed and approved by the local Institutional Review Board, and a federal certificate of confidentiality was obtained from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to protect participants involved in research.
Questionnaire data were collected using Audio Computer-Assisted Self-Interviewing (ACASI) technology on individual laptop computers. Previous experience with this high-risk population indicates that poor literacy can be a limitation, and the ACASI technology helps eliminate many issues with understanding the content of questions and navigating complicated skip patterns in questionnaires [22].
Measures
Sexual History.
At baseline participants were asked a series of questions regarding their sexual history, including whether they had ever had sex, the age at which they first had intercourse, and their number of unique sexual partners. Additionally, they were asked whether they had ever been or gotten someone else pregnant, and whether they ever had a diagnosis of an STI. At each time point, we assessed frequency of condom use (“In the past 3 months, how much of the time did you use condoms when you had sexual intercourse?”) on a scale ranging from 1 (never) to 5 (always). Risky sexual behavior was examined at all time points using a composite variable calculated as how frequently a participant had sex in the past 3 months multiplied by how often they used a condom when having sex during those three months (reverse coded). Higher scores indicate riskier sexual behavior.
Alcohol Use.
At baseline, participants indicated whether they had ever used alcohol in their lifetime, and the age at which they first tried alcohol. At baseline and at each follow-up assessment they were asked a series of questions about their alcohol use in the prior 3 months. Frequency of alcohol use was assessed with a single item—“In the last 3 months, how often did you consume at least once alcoholic drink?”— with response options of 1 = never, 2 = occasionally, 3 = once a month, 4 = 2-3 times a month, 5 = 4-5 times a month, 6 = once a week, 7 = 2-3 times a week, 8 = 4-5 times a week, and 9 = every day. Frequency of alcohol use in the context of sexual behavior was measured with a single item—“In the past 3 months only, how much of the time did you use alcohol when you’ve had sexual intercourse?”—with responses ranging from 1 (never) to 5 (always). Potentially problematic levels of alcohol use were assessed at baseline using the 10-item Alcohol Use Disorders Identification Test (AUDIT; α = .80 for this sample) [23].
Image Acquisition and Processing
MRI was performed on a 3T Siemens Trio (Erlangen, Germany) whole body scanner with a 12-channel radio frequency coil. Participants were placed in the scanner and a piece of tape across each participant’s forehead reduced movement. A high-resolution T1-weighted structural image was acquired with a 5-echo multi-echo MPRAGE sequence with TE = 1.64, 3.50, 5.36, 7.22, and 9.08 ms, TR = 2.53 s, TI = 1.20 s, flip angle = 7°, NEX = 1, slice thickness = 1 mm, 33 slices, FOV = 256 × 256 mm, resolution = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm, and pixel bandwidth = 650 Hz. Whole brain resting state T2-weighted functional images were acquired using a gradient-echo echo planar imaging (EPI) sequence with TE = 29 ms, TR = 2 s, flip angle = 75°, slice thickness = 3.5 mm, 33 slices, slice gap = 1.05 mm, FOV = 240 × 240 mm, 64 × 64 matrix, voxel size = 3.75 mm × 3.75 mm × 3.5 mm. Resting-state functional connectivity scans were 5 minutes in duration. Subjects were instructed to keep their eyes open and fixate on a cross.
Functional images were preprocessed using an automated pipeline based in SPM 5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5), including realignment, slice-timing correction, unified segmentation spatial normalization, reslicing, and smoothing [24]. Time-series of cerebrospinal fluid and white-matter fluctuations, motion parameters, and first derivative of motion parameters were progressively regressed from the time-series data and then the data were band-pass filtered (0.01-0.1 Hz) using in-house scripts [25]. As motion has been shown to influence functional connectivity [26], the framewise displacement [27] for each subject was calculated across the entire resting state run from the image motion parameters for use as a covariate. Further, individuals with excessive motion (>3 mm translational or 0.053 radians rotational movement) were excluded from analyses.
Network Definition
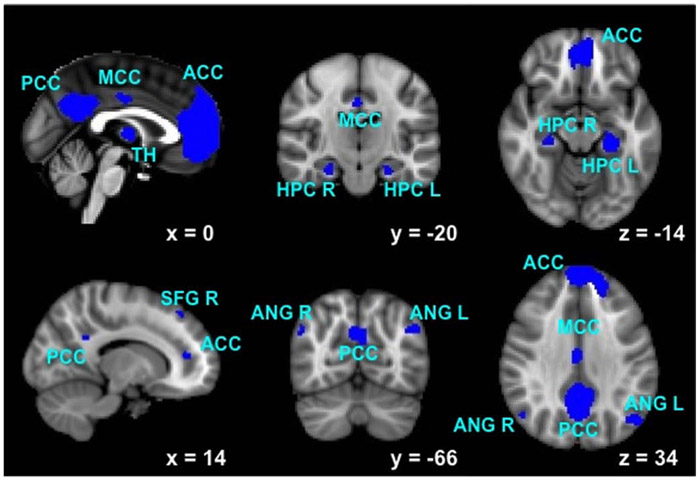
The dorsal default mode (dDMN; see Fig. 1), ventral default mode (vDMN), left executive control (LECN), and right executive control (RECN) networks were identified using publicly available, functionally-defined regions of interest [28]. The nodes included in each network are listed in Table 1. Functional connections between nodes were calculated, and a correlation matrix between all nodes within each network was created for each subject. A Fisher r-to-z transformation was applied to yield z-scores for use in subsequent analyses. Strength was calculated as the mean of the correlation z-score matrix for each network [29].
Fig. (1).
Functional regions of interest included in the dorsal default mode network. Individual node abbreviations are displayed in Table 1.
Table 1.
Definition of networks and nodes for connectivity analyses.
| Network | Area | Abbreviation |
|---|---|---|
| Left Executive Control (LECN) | ||
| 1. Left Middle Frontal Gyrus/Superior Frontal Gyrus | DLPFC L | |
| 2. Left Inferior Frontal Gyrus/Orbitofrontal Gyrus | MFG L | |
| 3. Left Superior Parietal Gyrus/Inferior Gyrus/Precuneus/Angular Gyrus | PAR L | |
| 4. Left Inferior Temporal Gyrus/Middle Temporal Gyrus | TL L | |
| 5. Right Crus I/Crus II/Lobule VI | CE R | |
| 6. Left Thalamus | TH L | |
| Right Executive Control (RECN) | ||
| 1. Right Middle Frontal Gyrus/Superior Frontal Gyrus | DLPFC R | |
| 2. Right Middle Frontal Gyrus | MFG R | |
| 3. Right Inferior Parietal Gyrus/Supramarginal Gyrus/Angular Gyrus | PAR R | |
| 4. Right Superior Frontal Gyrus | SFG R | |
| 5. Left Crus I/Crus II/Lobule VI | CE L | |
| 6. Right Caudate | CU R | |
| Dorsal Default Mode (dDMN) | ||
| 1. Medial Prefrontal Cortex, Anterior Cingulate Cortex, Orbitofrontal Cortex | ACC | |
| 2. Left Angular Gyrus | ANG L | |
| 3. Right Superior Frontal Gyrus | SFG R | |
| 4. Posterior Cingulate Cortex, Precuneus | PCC | |
| 5. Midcingulate Cortex | MCC | |
| 6. Right Angular Gyrus | ANG R | |
| 7. Left and Right Thalamus | TH | |
| 8. Left Hippocampus | HPC L | |
| 9. Right Hippocampus | HPC R | |
| Ventral Default Mode (vDMN) | ||
| 1. Left Retrosplenial Cortex, Posterior Cingulate Cortex | PCC | |
| 2. Left Middle Frontal Gyrus | MFG L | |
| 3. Left Fusiform | FF L | |
| 4. Left Middle Occipital Gyrus | MOCC L | |
| 5. Right Precuneus | PRC R | |
| 6. Right Middle Frontal Gyrus | MFG R | |
| 7. Right Middle Occipital Gyrus | MOCC R | |
| 8. Right Cerebellum | CE R | |
Statistical Analyses
We first examined baseline relationships between sexual risk behaviors and alcohol use, and then examined potential associations of functional network connectivity strength with these constructs. To test the longitudinal relationship between alcohol use and sexual risk behaviors, we estimated a parallel process latent growth model that simultaneously modeled the trajectories of alcohol use and sexual risk behavior over time. Functional connectivity strength was included as an exogenous variable to evaluate its relationship with baseline levels of risk as well as with trajectories of change in risk over time in both behaviors.
RESULTS
Baseline Levels of Risky Behavior
Table 2 depicts baseline levels of sexual behavior and alcohol use for participants. Most participants (73.6%) reported ever having sex. The mean age of first intercourse was 13.24 years (SD = 2.14) and the mean number of sexual partners was 5.10 (SD = 5.79). Alcohol use was also high in this sample of justice-involved adolescents. Most participants (86.6%) reported alcohol consumption in their lifetime, and a majority (70.1%) reported consuming alcohol at least occasionally in the last 3 months. The average frequency of use was 3.26 (SD = 2.33) on our 9-point scale, equivalent to between “2-3 times a month” and “4-5 times a month.”
Table 2.
Baseline levels of risky sexual behavior and alcohol use.
| M (SD) or % | |
|---|---|
| Sexual Behavior | |
| Ever had intercourse (% Yes) | 73.6 |
| Frequency of condom use† | 3.36 (1.21) |
| Lifetime number of partners | 5.10 (5.79) |
| Age (in years) at first intercourse | 13.24 (2.14) |
| Years sexually active | 3.02 (2.12) |
| Pregnancy event (% Yes) | 19.7 |
| Sexually Transmitted Infection (STI; % Yes) | 8.2 |
| Risky sex composite | 7.34 (7.81) |
| Alcohol Use | |
| Ever used alcohol (% Yes) | 86.6 |
| Alcohol use frequency in past 3 months‡ | 3.26 (2.33) |
| Age (in years) at first alcohol use | 12.36 (2.47) |
| Alcohol Use Disorders Identification Test (AUDIT) | 6.85 (6.94) |
Scale of 1 (never) to 5 (always)
1 = never, 2 = occasionally, 3 = once a month, 4 = 2-3 times a month, 5 = 4-5 times a month, 6 = once a week, 7 = 2-3 times a week, 8 = 4-5 times a week, 9 = every day.
Relationships Among Baseline Levels of Risky Behavior
Table 3 presents the bivariate correlations among key risky sexual behavior and alcohol use variables, and functional connectivity strength. Sexual risk and alcohol use variables were highly correlated with each other in general. For example, frequency of intercourse was positively associated with AUDIT scores. Similar relationships emerged for the remaining sexual risk variables. Of primary interest was the relationship between risky sexual behavior, frequency of alcohol use, and dDMN connectivity strength. Higher scores on the risky sexual behavior composite variable were positively related to frequency of alcohol use. The only relationship with functional connectivity to emerge was between the dDMN and frequency of alcohol use such that dDMN connectivity strength was positively associated with alcohol frequency. Therefore the subsequent parallel process growth model explored associations among dDMN connectivity strength and trajectories of alcohol use and sexual risk behavior.
Table 3.
Correlations among risky sex, alcohol use, and strength of functional connectivity measures.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Lifetime partners | ---- | |||||||||
| 2. Years sexually active | .49*** | ---- | ||||||||
| 3. STI diagnosis | .30*** | .17* | ---- | |||||||
| 4. Pregnancy | .18* | .22** | .18* | ---- | ||||||
| 5. Risky sex | .44*** | .21* | .25** | .29*** | ---- | |||||
| 6. Alcohol frequency | .26*** | .19* | .11 | .15* | .26*** | ---- | ||||
| 7. AUDIT | .31*** | .28*** | .17* | .14 | .32*** | .60*** | ---- | |||
| 8. dDMN | −.07 | .07 | −.15 | −.04 | .00 | .14* | .10 | ---- | ||
| 9. vDMN | .11 | .12 | .02 | .12 | .13 | .10 | .09 | .42*** | ---- | |
| 10. LECN | −.06 | −.01 | −.12 | −.04 | −.01 | .07 | −.01 | .42*** | .22** | ---- |
| 11. RECN | −.09 | −.10 | .00 | .10 | −.05 | .00 | −.08 | .20*** | .06 | .30*** |
STI = sexually transmitted infection; AUDIT = Alcohol Use Disorders Identification Test; dDMN = dorsal default mode network; vDMN = ventral default mode network; LECN = left executive control network; RECN = right executive control network.
Risky Sexual Behavior and Alcohol Use Over Time
We estimated a parallel process growth model [30], which allows for the simultaneous modeling of two behaviors over time, using EQS version 6.1 [31]. The trajectories of risky sexual behavior and frequency of alcohol use over time were estimated in a parallel process growth model examining the relationship between risky sex growth parameters and frequency of alcohol use growth parameters (see Fig. 2). dDMN connectivity strength was added as an exogenous variable to evaluate its association with both baseline alcohol and sexual risk behaviors as well as the trajectories of risky sexual behavior and frequency of alcohol use over time. Mean framewise displacement was originally included in the growth model as a covariate, although it had so little variability that its inclusion resulted in a model that would not converge. Given that it was only weakly related to dDMN connectivity strength [r(230) = −.17, p < .05], framewise displacement was not included in the final model. This model demonstrated adequate fit to the data: Santorra-Bentler χ2 (47, N = 239) = 60.67, p > .05; comparative fit index (CFI) = .975; root-mean-square error of approximation (RMSEA) = .034 (95% CI [0.00, 0.06]). There was a significant relationship between initial status of risky sex and alcohol use, indicating that on average those who reported higher levels of risky sex at baseline were also those who reported higher quantity and frequency of alcohol use at baseline. The slope of alcohol frequency was not significant, indicating that substance use did not substantially change over the course of the 12 months of the study. The slope of alcohol use was negatively associated with the initial status of risky sex, suggesting that young people with higher levels of risky sex at baseline experienced steeper decreases in alcohol use over time. Mean risky sex values at each time point were 6.88, 6.81, 6.75, 6.68, and 6.61 at the baseline, 3-, 6-, 9-, and 12-month assessments, respectively. The overall slope of risky sex was not significant, suggesting that on average risky sex did not substantially increase or decrease over time. Mean alcohol frequency values at each time point were 2.92, 2.82, 2.71, 2.60, and 2.49 at the baseline, 3-, 6-, 9-, and 12-months assessments, respectively. Again, the slope of alcohol frequency was not significant, suggesting that frequency of alcohol use on average did not substantially increase or decrease over time. These slopes were positively related, indicating a positive association between the two. In other words, participants whose alcohol use increased also had increases in sexual risk, and those who had decreases in alcohol use had decreases in risky sex. The key question in this analysis was to evaluate the relationship between connectivity and risk behavior both at baseline and over time. As can be seen in Fig. (2), dDMN connectivity strength was related to alcohol frequency initial status, such that those with higher connectivity strength also reported more alcohol use at baseline. However, dDMN connectivity strength had a negative relationship with changes in alcohol frequency over time, such that higher connectivity strength predicted a larger decrease in alcohol use over time.
Fig. (2).
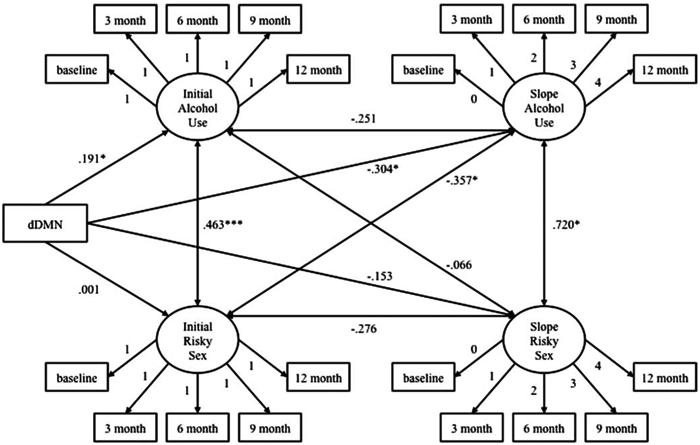
Parallel process growth model of dorsal default mode network (dDMN) functional connectivity strength, alcohol use frequency, and risky sexual behavior.
DISCUSSION
The current study utilized data from a larger intervention study to investigate the longitudinal association between risky sexual behavior and frequency of alcohol use among a population of young people involved in the juvenile justice system. We further investigated whether risky sexual behavior and frequency of alcohol use, both at baseline and in terms of their trajectory over time, was associated with functional network connectivity strength at baseline. Consistent with prior research, a latent growth model showed associations between baseline alcohol use and risky sex [4, 5], and between longitudinal trajectories of alcohol use and risky sex. A unique contribution of this work was to demonstrate a significant association of network functional connectivity strength of the dDMN with both initial alcohol use and with the longitudinal trajectory of alcohol use. Our findings also suggest that perhaps dDMN connectivity strength may not be broadly related to all forms of risk behavior, as there was no association between dDMN connectivity and sexual risk behavior. Executive control networks and the vDMN did not show associations with either alcohol use or risky sex variables.
Functional network connectivity strength of the dDMN was associated with baseline alcohol use as well as with changes in alcohol use over time. Interestingly, the dDMN was positively associated with baseline alcohol use but also associated with a steeper decline in alcohol use over time. The DMN is associated with interoception and self-referential processing [21], which may suggest that adolescents with higher awareness of bodily sensations find the experience of drinking alcohol more rewarding or pleasurable as they begin to experiment with alcohol use (i.e., early in their trajectory of alcohol use). From that perspective it may seem counterintuitive that stronger dDMN connectivity was associated with a steeper decrease in alcohol use over the 12-month follow-up period. It may be that adolescents with higher self-awareness may find alcohol use initially more pleasurable but also be more aware of potentially unpleasant effects of overuse (e.g., nausea) with greater alcohol use experience, leading to a steeper decrease in use over time. Future studies should continue to explore possible associations between interoception, the experience of alcohol use, and consequent trajectories of alcohol use over time. If such associations continue to be supported, increasing self-reference including mindfulness of bodily sensations could show promise as a possible target of prevention and intervention for future problematic alcohol use in adolescence.
A number of considerations should inform interpretation of results. Given that comorbid abuse of alcohol and marijuana or other substances is increasingly common, and existing research suggesting associations between marijuana use and risky sexual behavior [32], future studies should also explore relationships between functional network connectivity and co-occurring marijuana use and risky sexual behavior. Estimations of overall functional network strength were very broad and encompassed fairly large ROIs based on a control sample of healthy young adults [28]. Network-level analyses provide valuable insight into overall estimations of neural efficiency, but future analyses could be conducted with more specificity using more refined ROIs and with consideration of individual edges within functional networks. Finally, analyses did not include a comparison group of non-justice-involved adolescents. However, some evidence suggests that juvenile offenders may demonstrate a delay in typical neural maturation rather than a distinctly abnormal trajectory, since similar patterns of association between functional connectivity and impulsivity have been found in incarcerated adolescents and younger compared to older control participants [33]. In addition, the current sample includes adolescents with a broad range of risk behavior in both domains (alcohol use and risky sexual behavior). These adolescents were also in a diversion program and living at home, rather than in a detention or incarceration setting. To be placed in diversion reflects that these adolescents were engaging in mild to moderate risk behavior (e.g., minor in possession, truancy) associated with relatively low criminality.
Current results suggest important associations between trajectories of alcohol use and risky sexual behavior. A unique contribution of this work is our demonstration of an association between functional network connectivity strength of the dDMN and both baseline alcohol use behavior as well as changes in alcohol use over time. This suggests that intervention with the ultimate goal of decreasing alcohol-related risky sexual behavior may need to consider variability in neurocognitive function, and the implications of that variability on risk behavior and perhaps on response to intervention. From a basic science perspective, it is interesting that there were no direct linkages between network connectivity and sexual risk behavior, placing risky sex in a potentially different category in terms of its association with brain development and function. Future work should explore a broader range of neurocognitive structural and functional variables to answer the question of whether variation in these constructs has any implications for risky sexual behavior.
ACKNOWLEDGEMENTS
A.D.B. designed research. T.J.C. collected and managed data. E.M. performed statistical analyses. B.J.W. processed imaging data. R.E.T. and E.M. wrote the paper, and all authors edited and revised the manuscript. This research was supported by NIAAA grant R01 AA017390 to A.D.B.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003; 160: 1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilson CM, Wright PF, Safrit JT, et al. Epidemiology of HIV infection and risk in adolescents and youth. J Acquir Immune Defic Syndr 2010; 54 Suppl 1: S5–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Teplin LA, Mericle AA, McClelland GM, et al. HIV and AIDS risk behaviors in juvenile detainees: implications for public health policy. Am J Public Health 2003; 93: 906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bryan A, Rocheleau CA, Robbins RN, et al. Condom use among high-risk adolescents: testing the influence of alcohol use on the relationship of cognitive correlates of behavior. Health Psychol 2005; 24: 133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bryan AD, Ray L, Cooper ML. Alcohol use and protective sexual behaviors among high-risk adolescents. J Stud Alcohol Drugs 2007; 68: 327–35. [DOI] [PubMed] [Google Scholar]
- [6].Bryan AD, Schmiege SJ. HIV/STD risk reduction among detained adolescents: a randomized controlled trial. Pediatrics 2009; 124: e1180–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guo SY, Barth RP, Gibbons C. Propensity score matching strategies for evaluating substance abuse services for child welfare clients. Child Youth Serv Rev 2006; 28: 357–83. [Google Scholar]
- [8].Teplin LA, Abram KM, McClelland GM, et al. Detecting mental disorder in juvenile detainees: who receives services. Am J Public Health 2005; 95: 1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galvan A Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 2010; 72: 101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paus T Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. [DOI] [PubMed] [Google Scholar]
- [12].Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a "local to distributed" organization. PLoS Comput Biol 2009; 5: e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rogers BP, Parks MH, Nickel MK, et al. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol Clin Exp Res 2012; 36: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol 2013; 18: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex 2013; 23: 2086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wetherill RR, Bava S, Thompson WK, et al. Frontoparietal connectivity in substance-naïve youth with and without a family history of alcoholism. Brain Res 2012; 1432: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage 2011; 54: 2582–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weiland BJ, Welsh RC, Yau WY, et al. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend 2013; 128: 130–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004; 101: 4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2008; 63: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003; 100: 253–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schmiege SJ, Broaddus MR, Levin M, et al. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. J Consult Clin Psychol 2009; 77: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Allen JP, Litten RZ, Fertig JB, et al. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997; 21: 613–19. [PubMed] [Google Scholar]
- [24].Scott A, Courtney W, Wood D, et al. COINS: an innovative informatics and neuroimaging tool suite built for large heterogeneous datasets. Front Neuroinform 2011; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 2010; 36: 713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012; 59: 431–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012. ;59: 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012; 22: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci 2010; 30: 9477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York, NY: Oxford University. [Google Scholar]
- [31].Bentler PM, Wu E. EQS 6 for Windows User's Guide. Encino, CA: Multivariate Software, Inc; 2002. [Google Scholar]
- [32].Callahan TJ, Caldwell Hooper AE, Thayer RE, et al. Relationships between marijuana dependence and condom use intentions and behavior among justice-involved adolescents. AIDS Behav 2013; 17: 2715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shannon BJ, Raichle ME, Snyder AZ, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci USA 2011; 108: 11241–45. [DOI] [PMC free article] [PubMed] [Google Scholar]