Abstract
Introduction:
Male pattern alopecia (MPA) is a common disorder hugely impacting the quality of life of affected individuals. The meager number of options available for treatment has their own limitations. Novel therapies are continuously being researched for.
Materials and Methods:
The present study included thirty male patients with Hamilton Grade II to Grade V. All patients received four sequential treatments with microneedling (MN) on one half of the scalp and platelet-rich plasma (PRP) with MN (MN + PRP) on the other half for 4 months. Three months following the last session, evaluation was done from the vertex and temporal sites in both the groups by dermoscopic microphotographs by a blinded evaluator. In addition, the patients were asked about their satisfaction score on the basis of treatment outcome.
Results:
Overall hair thickness showed significant increase in both MN and MN + PRP group. Furthermore, the increase in thickness was almost double in the MN group as compared to MN + PRP group (0.006 and 0.003 mm, respectively). Overall hair density also increased significantly in both the study groups but more in MN + PRP group (14.6 hair/cm2) than the MN group (10.8 hair/cm2). However, the difference between the results of both the groups was not statistically significant.
Conclusion:
To the best of our knowledge, this is the first split scalp study for MPA. We conclude that MN and PRP are both effective in treatment of androgenetic alopecia and improve the hair parameters and patient satisfaction. However, no additional effect of PRP over MN was observed. Both these therapies are safe and well tolerated without any major side effects. Limitations of our study were small sample size and lack of long-term follow-up.
Key words: Male pattern alopecia, microneedling, platelet-rich plasma, split-scalp study
INTRODUCTION
Androgenetic alopecia (AGA) or common baldness is the result of progressive, patterned hair loss that occurs in genetically predisposed individuals due to an excessive response to androgens affecting both males and females.[1,2,3,4]
Currently, topical minoxidil and oral finasteride are the only US Food and Drug Administration-approved medical treatment options for the management of AGA. Various other treatments have also been tried with variable results.[5]
Microneedling (MN) and platelet-rich plasma (PRP) are such attempts which are currently being used widely for treatment of AGA.
MN is a minimally invasive procedure that utilizes multiple fine needles to create micropunctures in the skin.[6] It is widely used in conditions such as acne scars, periorbital and perioral rhytides, skin laxity, posttraumatic/burn scars, and striae distensae either alone or in combination with other treatment modalities. Recently, it is being tried for AGA.[7,8]
PRP is an autologous preparation of platelets concentrated in plasma. It contains more than twenty growth factors of which most important include platelet-derived growth factor, transforming growth factor-β, vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 along with their isoforms. PRP has already been employed in the treatment of many conditions such as wound healing, diabetic foot ulcers, osteoarthritis, and skin rejuvenation. Recently, it is being used for AGA.[2]
The present study was conducted to evaluate the safety and efficacy of microneeedling (MN) monotherapy and combination of MN and PRP in androgenetic alopecia.


MATERIALS AND METHODS
A total of 200 male patients clinically diagnosed as having AGA Stage II–V Hamilton–Norwood classification attending the Dermatology outpatient department of a tertiary level hospital in Ambala, India, were considered in this randomized, triple-blinded cohort study for a period of 1 year (February 2018–January 2019). Patient selection was done from February to May 2018 and the follow-up was done till January 2019.
A detailed history was taken, and complete dermatological examination was done to rule out confounders (other causes of hair loss for example, telogen effluvium and drug intake.) The patients with a history of heavy smoking, any systemic illness, low platelet count, and low hemoglobin were excluded from the study. The patients who were on any hair loss treatment were also excluded from the study.
Out of the 200 patients, 89 patients refused to enroll in study and 67 patients were excluded from the study for not fitting in the criteria, so the remaining 44 patients were further enrolled for the study [Figure 1].
Figure 1.
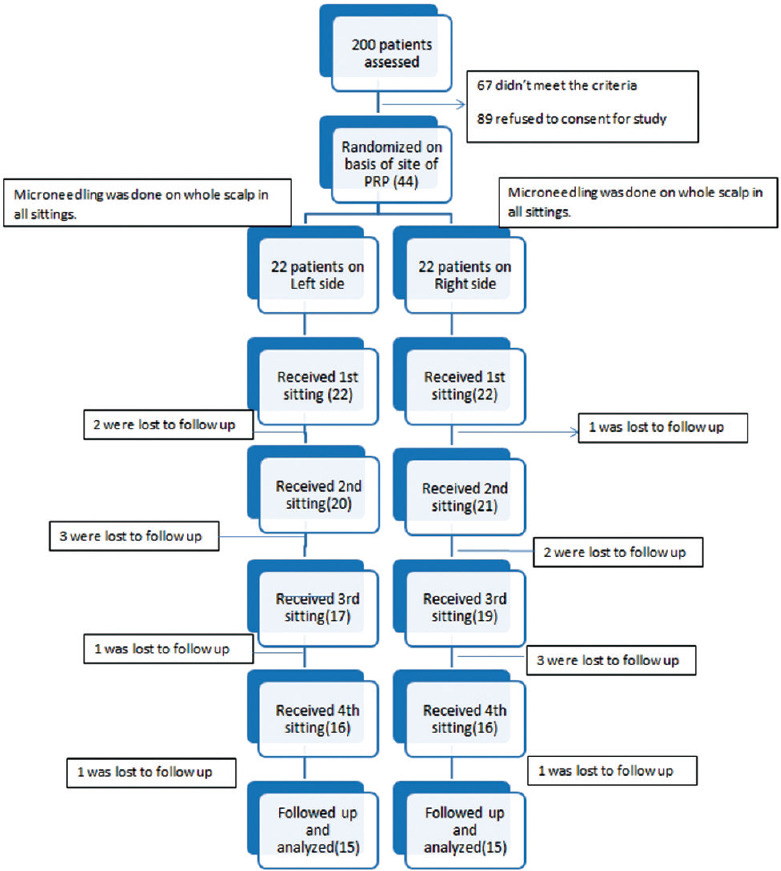
Flowchart showing study design
Fourteen patients were lost to follow-up in total. Four patients got hair transplant done and four patients were lost due to their personal problem, six patients were lost due to emigration, and the study was completed with thirty patients.
After explaining the detailed procedure to the patients, informed consent was taken. Preprocedure documentation and dermoscopic pictures were taken using Dinolite dermatoscope (AM4113ZT) by the evaluator. Photographic imaging was also done. Dermatoscopic images were taken from both halves at vertex and temporal areas. These sites were marked by tattoo ink and were remarked at each follow-up visit. The patients' hairs were trimmed to 1 cm length prior to all sessions of the therapy.
Patients were prepared for the MN procedure; in the meanwhile, venepuncture was done to prepare PRP. About 50 ml of blood was drawn in a 50-mL syringe with human-grade citrate-phosphate-dextrose solution with adenine (CPDA) as anticoagulant. Blood was transferred into sterile conical tubes, and a spin at 2500 rpm was given for 3 min. Platelet-poor plasma was extracted from supernatant and transferred into another conical tube and second spin at 3500 rpm for 15 min was given. The pellet of platelets was formed at the bottom was collected along with 2 ml of plasma, and the supernatant plasma was discarded.
After preparing the scalp area, a ring block with injection lignocaine 2% was given covering the entire scalp. A dermaroller of 1.5–2 mm sized needles was gently rolled over the affected areas of the whole scalp in to and fro and rolling movement until any pinpoint bleeding was noted. After that, the scalp was divided into two halves by an imaginary line drawn from the glabella to the occipital protuberance. The side of PRP was chosen by MS-EXCEL generated random number sequence which were concealed in sequentially numbered, opaque, sealed envelopes (SNOSE) by a third person who was not a part of the study. The PRP thus prepared was then injected at the site chosen with an insulin syringe with an amount of 0.1 ml/cm2 on the affected area. The other half (MN only side) was injected with normal saline. The contents of the insulin syringes used for the injections were not shown to the patient.
For the assessment purpose, the divided halves of scalp of each patient were allocated into the following two groups:
MN group: The side of the scalp on which MN alone was included in this group
MN + PRP group: The side of the scalp where PRP was done in addition to MN was added into this group.
Assessment was done at the start of therapy and 3 months after fourth session. The therapy was repeated at monthly interval until four sessions were completed.
To minimize bias, the patients were blinded of the PRP site. The evaluator of photomicrographs was also blinded of the treatment groups and the statistician was provided this data in coded excel sheets.
Hair thickness and density were calculated at vertex and temporal sites in both the groups before starting the therapy and 3 months after the fourth session. The hairs were counted in the dermatocopic field of 35 mm2 (7 × 5 mm) and were then standardized to hair/cm2 of the area, and thickness was calculated using the measurement tool included in Dino capture software by AnMo Electronics Corporation at 1 mm above the ostia from the same area Figure 2.
Figure 2.
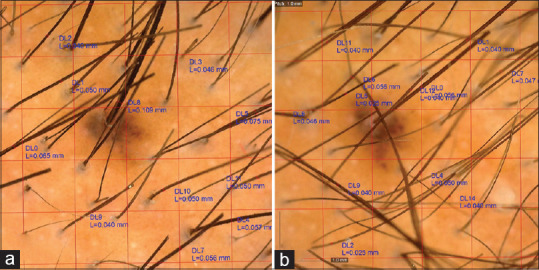
Dermoscopy of a patient – (a) before starting treatment (b) After completion of treatment
The average hair thickness and density of vertex and temporal areas was compared between two groups at the start and end of therapy. These parameters were also compared among the same group at the start and end of therapy using paired t-test. This test was applied individually in each group for comparison of posttherapy results from pretherapy results and also between the two groups at pretherapy and post therapy time point.
After 6 months of completion of study, the patients were telephonically contacted and asked for a satisfaction score ranging from 0 to 10. This score was based on their perception of decrease in bald area, decrease in hair fall, and change in thickness of hair. Patients with a score of 0 meant no improvement; 1–3 denoted mild improvement, 4–6-moderate improvement; and 7+-significant improvement [Figure 3].
Figure 3.
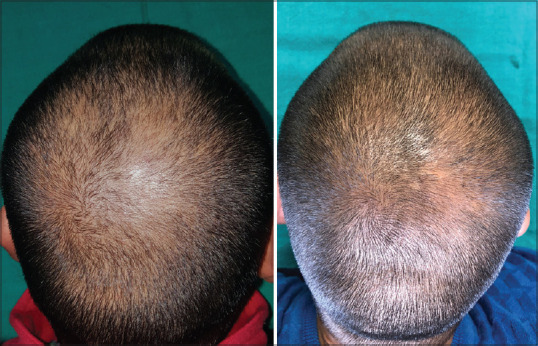
Photography of patient – before and after completion of treatment in platelet-rich plasma + microneedling group
It was planned that if any patient developed any serious adverse event during and after therapy, study would not be continued further on that patient.
RESULTS
The study was completed using thirty patients. All the thirty patients had received both MN and PRP. Fifty percent received it on the left side and 50% on the right side of the scalp. Patients were in the range of 20–44 years, with a mean age of 25.50 (±5.35) years. Twenty-one were married (70%) and nine were unmarried (30%). Majority of the patients (80%) had a positive family history of alopecia, paternal history in 65%, and maternal history was present in 25% of cases. Ten percent of the cases had a history of AGA on both paternal and maternal sides and 20% of the patients had no family history of AGA. Most of the patients (26 out of 30) were nonsmokers; however, four patients were occasional smokers. Majority of the patients (22) were non alcoholics, while eight patients had a positive history of alcohol intake. Seventy-three percent were vegetarians and only 27% of the patients were nonvegetarians.
Out of the thirty cases, 43% of the patients were undergraduates and 40% had secondary level of education. Two patients (6.7%) were matriculates and three patients (10%) were postgraduates. The minimum duration of alopecia according to the history was 3 months and the maximum duration of alopecia was 10 years, with an average duration of 25 months.
The patients were classified by modified Norwood–Hamilton grading system into Grade II to Grade V.
Seventy percent of the patients were having Norwood–Hamilton Grade III hair loss and 30% of the patients were having Grade II hair loss.
On the basis of basic and specific (BASP) classification of patterned hair loss, majority of the patients (40%) had M1V1 pattern, followed by M2V1 pattern, which was found in 33.3% patients. Nearly 13.3% of the patients had M2V2 pattern, while 10.0% of the patients fell in M1V2 pattern. Minority (3.3%) of the patients had M3V1 pattern of BASP classification.
As per protocol, hair thickness and hair density were the parameters which were compared on the basis of dermoscopic pictures at vertex and temporal sites apart from clinical photographic assessment and patient satisfaction score.
At vertex, there was an increase in hair thickness in both the groups in MN group and an in MN + PRP group (0.006 and 0.003 mm, respectively). This increase was significant in MN group and was not significant in MN + PRP group. There was no significant difference between the post therapy results of both groups. There was a significant increase in temporal thickness in both the groups, with increase of 0.006 mm in MN group and 0.004 mm in MN + PRP group. However, there was no significant difference between the posttherapy results of these two groups after 3 months of fourth session. The details of the results are depicted in Tables 1 and 2.
Table 1.
Vertex hair thickness at pretherapy and posttherapy
| Vertex Hair Thickness (mm) | Side |
Paired t-test | |||
|---|---|---|---|---|---|
| MN |
MN+PRP |
||||
| Mean | SD | Mean | SD | p | |
| Pre-Therapy | 0.070 | 0.010 | 0.072 | 0.011 | 0.136 |
| Post-Therapy | 0.076 | 0.008 | 0.075 | 0.009 | 0.522 |
| Paired t-test p | <0.001 | 0.071 | |||
Table 2.
Temporal hair thickness at pretherapy and posttherapy
| Temporal Hair Thickness (mm) | Side |
Paired t-test | |||
|---|---|---|---|---|---|
| MN |
MN+PRP |
||||
| Mean | SD | Mean | SD | p | |
| Pre-Therapy | 0.064 | 0.010 | 0.066 | 0.010 | 0.147 |
| Post-Therapy | 0.070 | 0.009 | 0.070 | 0.010 | 0.601 |
| Paired t-test p | <0.001 | 0.025 | |||
Similarly, on comparing hair density at the vertex and temporal sites, there was a significant increase in vertex hair density in both the groups, with an increase of 14 hair/cm2 and 12.2 hair/cm2 in MN group and MN + PRP group, respectively. Similarly, at the temporal site, there was a statistically significant increase in hair density in both the groups with an increase of 7.7 and 17 hair/cm2 in MN group and MN + PRP group, respectively. There was no statistically significant difference between the posttherapy results of both groups.
Details of the results are depicted in Tables 3 and 4.
Table 3.
Vertex hair density at pretherapy and posttherapy
| Vertex Hair Density (per sq-cm) | Side |
Paired t-test | ||||
|---|---|---|---|---|---|---|
| MN |
MN+PRP |
|||||
| Mean | SD | Mean | SD | p | ||
| Pre-Therapy | 98.476 | 29.907 | 101.429 | 27.299 | 0.515 | |
| Post-Therapy | 112.381 | 30.343 | 113.714 | 26.743 | 0.735 | |
| Paired t-test | p | 0.003 | 0.003 | |||
Table 4.
Temporal hair density at pretherapy and posttherapy
| Temporal Hair Density (per sq-cm) | Side |
Paired t-test | ||||
|---|---|---|---|---|---|---|
| MN |
MN+PRP |
|||||
| Mean | SD | Mean | SD | p | ||
| Pre-Therapy | 90.571 | 29.032 | 88.857 | 28.199 | 0.600 | |
| Post-Therapy | 98.285 | 27.483 | 105.905 | 26.271 | 0.067 | |
| Paired t-test | p | <0.001 | <0.001 | |||
Overall hair thickness by calculating mean thickness of vertex and temporal sites was calculated from dermoscopic images at pre-therapy and 3 months after the fourth session. There was a statistically significant increase in average hair thickness (mm) over time in both the treatment groups, MN group (0.006 mm) and MN + PRP group (0.003 mm). There was no statistically significant difference between the posttherapy results in both groups [Table 5].
Table 5.
Average hair thickness at pretherapy and posttherapy
| Average Hair Thickness (mm) | Side |
Paired t-test | ||||
|---|---|---|---|---|---|---|
| MN |
MN+PRP |
|||||
| Mean | SD | Mean | SD | p | ||
| Pre-Therapy | 0.067 | 0.009 | 0.069 | 0.009 | 0.051 | |
| Post-Therapy | 0.073 | 0.006 | 0.072 | 0.008 | 0.378 | |
| Paired t-test | p | <0.001 | 0.006 | |||
Similarly, the overall density of hair was calculated by taking mean of vertex and temporal at pretherapy and 3 months after the fourth session and was compared. There was a significant increase in average hair thickness (mm) over time in both the treatment groups MN group (10.8 hair/cm2) and MN + PRP group (14.6 hair/cm2). A statistically significant increase was there in both the group. There was no statistically significant difference between the post therapy results in both groups [Table 6].
Table 6.
Average hair density at pretherapy and posttherap
| Average Hair Density (per sq-cm) | Side |
Paired t-test | ||||
|---|---|---|---|---|---|---|
| MN |
MN+PRP |
|||||
| Mean | SD | Mean | SD | p | ||
| Pre-Therapy | 94.524 | 23.579 | 95.143 | 22.060 | 0.804 | |
| Post-Therapy | 105.333 | 23.795 | 109.809 | 20.653 | 0.177 | |
| Paired t-test | p | <0.001 | <0.001 | |||
At the end of 6 months of therapy patient's feedback on the basis of reduction in hair fall, change in texture, and change in thickness of hair was recorded. Majority of the patients (22) were moderately satisfied (73%). Six patients (20%) were mildly satisfied and two patients (7%) were not satisfied at all [Table 7].
Table 7.
Subjective improvement scale
| Score | Frequency | Percentage |
|---|---|---|
| No Improvement (0) | 2 | 7 |
| Mildly Satisfied(1-3) | 6 | 20 |
| Moderately satisfied (4-6) | 22 | 73 |
| Very Satisfied (7-10) | 0 | 0 |
All patients experienced mild tingling and aching pain following the therapy, however, no other adverse event was reported.
DISCUSSION
Hair has been significant to human civilization since time immemorial in all cultures as a symbol for identification, strength, and sexuality. Hair is a part of our body, and its condition reflects our general as well as mental health. As a part of evolutionary process and because of many other reasons, scalp hairs are regressing day by day. In the present day, hair is given undue hype because of many reasons, especially in Asian countries. Any deviation from the normal appearance of hair causes apprehension to the patient for which he/she seeks treatment. Treatment modalities for AGA are limited, and the results are not very reliable, hence it becomes very frustrating for the patient as well as for doctor. Thus, the quest for newer and effective modalities is still on.
Physiologically, at the cellular level, dermal papilla cells which are a cluster of fibroblasts regulate the activity and growth of various cells in the follicle, thus regulating the cycle. These cells signal the multipotent epidermal stem cells in the bulge region. Various attempts are being reported to stimulate and replicate these molecular signals for hair growth.[8]
MN is currently being used for scar reduction, androgenic alopecia, alopecia areata, pigmentary disorders, melasma, periobital melanosis, verruca, actinic keratosis, and transdermal drug delivery.[8]
It works by creating thousands of microtraumas through the epidermis up to the papillary dermis. These tiny wounds in the papillary dermis create a virtually confluent zone of superficial bleeding that is a powerful stimulus to initiate the normal process of wound healing. There may be overexpression of VEGF, hair growth-related genes, β catenin, Wnt3A, and Wnt10B as documented in animal studies.[9,10]
Another therapy which is currently being used widely is PRP. PRP was recognized in 1970 and was first used in 1987 in the procedure of open-heart surgery in Italy. Since then, PRP is constantly being tried in the treatment of many other diseases in different medical fields such as dentistry, cosmetic surgery, pain management, and sports medicine.[11]
PRP is an effective concentration of multiple fundamental growth factors (GFs) by virtue of platelets alone and plasma proteins, namely fibrin, fibronectin, and vitronectin. It is being used for various indications such as androgenetic alopecia, alopecia areata, skin rejuvenation, striae distensae, lichen sclerosus, wounds, and ulcers.[12]
There have been many studies to show the efficacy of MN and PRP either alone or in combination with minoxidil, however these studies have some limitation of either taking only subjective parameters or had limited no of patients. The various studies with their data and limitations are discussed in Table 8.[13,14,15,16,17,18,19,20,21,22,23]
Table 8.
Summary of clinical studies with platelet-rich plasma or microneedling
| YEAR | NO. OF PATIENT | AUTHOR | SPLIT-SCALP | INTERVENTION DONE | CONTROL PRESENT | ASSESSMENT | RESULTS | REMARKS |
|---|---|---|---|---|---|---|---|---|
| 2006 | 20 | Uebel et al13 | YES | PRP ONLY | Yes | Manual Counting by Magnifying glass | 15.1% better graft survival in grafts treated with PRP vs control. | PRP was compared for graft survival rate in receipients of Hair transplant surgery. |
| 2013 | 100 | Dhurat et al14 | NO | MN and Mx vs Mx only. | MX only group present | By PH and 7 point Sx scale. | Dermaroller along with Minoxidil treated group was statistically superior to Minoxidil treated group in promoting hair growth. | Study only took Photographic assessment which may be subjective,PRP was not done. |
| 2014 | 20 | Gkini et al15 | No | PRP only | No | HPT, DS, PH and aSX score | They concluded that PRP injections may have a positive therapeutic effect on male and female pattern hair loss without remarkable major side effects. | Beneficial effect of PRP were recorded but Microneedling was not done. |
| 2014 | 11 | Khatu et al16 | No | PRP only | No | CE,PHHPT and patient’s SX. | PRP injection is a simple, cost effective and feasible treatment option for androgenic alopecia, with high overall patient satisfaction. | Trichoscopic analysis was not done and only PRP was done. |
| 2015 | 4 | Dhurat andMathapati,17 | No | MN only | N.A | PH and patient SX scale | The authors of this study considered microneedling to be an effective, inexpensive and novel tool in the treatment of AGA warranting further study. | Dermoscopy was not done, only Microneedling was done. Patients were not on monotherapy. |
| 2016 | 24 | Alves and Grimalt18 | Yes | PRP on split scalp | Yes | PH and PTG | Hair density, hair count increased significantly as compared to control side | Microneedling was not done, only PRP was done |
| 2017 | 50 | Kachhawa et al19 | YES | PRP on split scalp | Yes | HPT, trichoscan software, patient SX. | Authors concluded that PRP injections have therapeutic effect on male pattern hair loss with no major side effects and high patient satisfaction overall. | PRP was injected on left side in all cases which could lead to bias. |
| 2017 | 93 | Jha et al20 | NO | MX, MX and PRP, MX, PRP and MN. | Yes, MX only group | HPT, DS and PH | PRP with microneedling is better in comparison with PRP alone or minoxidil monotherapy in patients with AGA | The study in all treatment groups had minoxidil which can have synergistic effects. |
| 2017 | 50 | Shah et al21 | NO | MX only and PRP+MN+MX | Yes, MX only group | Subjective assessment by patient and physician based evaluation on PH | There was a significant improvement (P < 0.05) in bothpatients’ assessment and investigator’s assessment in Group B (PRP+MN+Mx)as compared to Group A (Mx only) at the end of 6 months | No objective method was used to measure hair growth |
| 2018 | 68 | Kumar et al22 | No | MX alone and MN with MX | Yes, MX only group | DS(hair count only), PH, patients self assessment | The study showed that the combination of MN and MX treatment was superior compared to topical MX alone with regard to increase in the hair count and patient SX, although the response achieved was not cosmetically significant | PRP was not done |
| 2019 | 40 | Verma et al23 | NO | MX vs PRP | Yes MX only group | global photography, HPT, standardized hair growth questionnaire, patient satisfaction score | On Global photography: Better outcome in PRP group as compared to minoxidil group HPT negative in 75% in PRP compared to 42.8% in minoxidil group | Dermoscopic analysis was not done. |
To overcome the limitations of various studies done so far, the present study was designed on a split scalp model.
The present study is a split-head comparative study of MN with PRP and MN alone (in the current study, PRP was prepared using human-grade CPDA and average platelet counts of 15 × 105/mm3 were achieved). Three milliliters of PRP was extracted from 50 mL of blood. PRP was injected on the one half of the scalp selected by SNOSE after doing MN on complete scalp.
PRP procedure also causes micro trauma in the scalp. Thus, it is thought that PRP also stimulates wound healing similar to MN. In clinical practice, both these therapies are done in combination, and it becomes a matter of debate which therapy is more effective.
In the present study, it was observed that there is an increase in hair thickness and diameter when patients received PRP and MN. However, the increase in these parameters also occurred when patients were subjected to MN alone. The group where MN and PRP both were done has not shown any significant difference from the group where only MN was done. As far as subjective evaluation is concerned, most of the patients were moderately satisfied. The common remarks of the patients who were satisfied were that they have noticed a mark reduction in hair fall though, they have not noticed any new growth yet.
Our results have demonstrated that MN alone is equally effective as compared to MN+PRP. This goes against the conception that PRP gives additive benefits, as the group where PRP was done in addition to MN has not shown better results than where MN was done alone.
After the completion of the study, patients were contacted by telephone to record the long-term effects of therapy. Most of the patients did not report any recurrence of hair loss, but 12 patients reported that they have started having hair fall again.
The results of this study show that MN and PRP have beneficial role in patients with androgenetic alopecia. However, more studies are advocated to study the combined effect of therapies. Further studies to study the effect at different sites with more participants are advocated.
The limitations of this study were limited sample size and nonavailability of automated software.
CONCLUSION
We conclude that PRP with MN would be beneficial as an adjuvant therapy in the management of AGA, however the improvement was not that significant to make it the treatment of choice as monotherapy. The “feel-good” factor improves patient compliance and satisfaction.
Through this study, we conclude that the effect of PRP over MN as compared to MN alone is not significant. However, PRP along with MN gives better results in temporal region as compared to vertex. Thus, further studies to compare the efficacy of MN and PRP at different sites are advocated with large sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 4. UK: Blackwell Publishing Ltd; 2010. pp. 66.16–66.31. [Google Scholar]
- 2.McElwee KJ, Shapiro JS. Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 3.Alfonso M, Richter-Appelt H, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: A multinational European study. Curr Med Res Opin. 2005;21:1829–36. doi: 10.1185/030079905X61820. [DOI] [PubMed] [Google Scholar]
- 4.Leavitt M. Understanding and management of female pattern alopecia. Facial Plast Surg. 2008;24:414–27. doi: 10.1055/s-0028-1102905. [DOI] [PubMed] [Google Scholar]
- 5.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 6.Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32:564–9. doi: 10.1111/jdv.14722. [DOI] [PubMed] [Google Scholar]
- 7.Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421–9. doi: 10.1097/01.prs.0000304612.72899.02. [DOI] [PubMed] [Google Scholar]
- 8.Alster TS, Graham PM. Microneedling: A review and practical guide. Dermatol Surg. 2018;44:397–404. doi: 10.1097/DSS.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Jeong KH, Kim JE, Woo YJ, Kim BJ, Kang H. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586–92. doi: 10.5021/ad.2016.28.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong K, Lee YJ, Kim JE, Park YM, Kim BJ, Kang H. Repeated microneedle stimulation induce the enhanced expression of hair-growth-related genes. Int J Trichol. 2012;4:117. [Google Scholar]
- 11.Butt G, Hussain I, Ahmed FJ, Choudhery MS. Efficacy of platelet-rich plasma in androgenetic alopecia patients. J Cosmet Dermatol. 2019;18:996–1001. doi: 10.1111/jocd.12810. [DOI] [PubMed] [Google Scholar]
- 12.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 13.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pa-ttern baldness surgery. Plast Reconstr Surg. 2006;118:1458–66. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 14.Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: A pilot study. Int J Trichology. 2013;5:6–11. doi: 10.4103/0974-7753.114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of Androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213–9. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: Myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–10. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhurat R, Mathapati S. Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy. Indian J Dermatol. 2015;60:260–3. doi: 10.4103/0019-5154.156361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–7. doi: 10.1097/DSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 19.Kachhawa D, Vats G, Sonare D, Rao P, Khuraiya S, Kataiya R. A spilt head study of efficacy of placebo versus platelet-rich plasma injections in the treatment of androgenic alopecia. J Cutan Aesthet Surg. 2017;10:86–9. doi: 10.4103/JCAS.JCAS_50_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha AK, Udayan UK, Roy PK, Amar AK, Chaudhary RK. Original article: Platelet-rich plasma with microneedling in androgenetic alopecia along with dermoscopic pre- and post-treatment evaluation. J Cosmet Dermatol. 2018;17:313–8. doi: 10.1111/jocd.12394. [DOI] [PubMed] [Google Scholar]
- 21.Shah KB, Shah AN, Solanki RB, Raval RC. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia for the management of AGA. Int J Trichol. 2017;9:14–8. doi: 10.4103/ijt.ijt_75_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar MK, Inamadar AC, Palit A. A randomized controlled, single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211–6. doi: 10.4103/JCAS.JCAS_130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma K, Tegta GR, Verma G, Gupta M, Negi A, Sharma R. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichology. 2019;11:68–79. doi: 10.4103/ijt.ijt_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


