Abstract
Objective:
To evaluate the effects of thioctic acid (TA) add-on metformin therapy on glycemic indices and associated inflammatory reactions induced-endothelial dysfunction (ED) in patients with type 2 diabetes mellitus (T2DM).
Methods:
In this case–control clinical study, a total number of 70 patients with T2DM compared with 30 healthy controls were divided into three groups: Group A (n = 30), healthy controls; Group B (n = 36), T2DM patients on metformin and Group C (n = 34), T2DM patients on metformin plus TA 600 mg/day. Anthropometric measurements, lipid profile, and routine biochemical variables were estimated. Serum human vascular cell adhesion molecule-1 (VCAM-1) and E-selectin were measured before and after 10 consecutive week's therapy with metformin and/or TA.
Results:
Metformin therapy led to significant reduction of fasting insulin and insulin resistance (IR) with an increment in the insulin sensitivity (P < 0.01). Metformin therapy improved lipid profile compared to the baseline (P < 0.01) with significant reduction of atherogenic index. Metformin plus TA therapy reduced fasting blood glucose, glycated hemoglobin, and IR and showed increment in the insulin sensitivity (P < 0.01) with insignificant effect on fasting insulin (P = 0.09) compared with metformin monotherapy. sVCAM-1 level was high in patients with T2DM (3.74 ± 1.34 ng/ml) at baseline, which decreased by metformin monotherapy to 2.32 ± 0.67 ng/ml or metformin plus TA to 1.98 ± 0.31 ng/ml (P < 0.01), but metformin plus TA illustrated insignificant difference compared to metformin alone (P = 0.29).
Conclusion:
TA add on metformin therapy improves glycemic indices and associated inflammatory mediators in patients with T2DM through modulation of IR , IS , and direct direct anti-inflammatory effect.
Key Words: Diabetes mellitus, endothelial dysfunction, metformin, thioctic acid
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is an endocrine disorder characterized by chronic hyperglycemia and glucometabolic disorders, due to defect in insulin secretion and/or action.[1]
Hyperglycemia in T2DM is linked to oxidative stress and induction of free radicals causing injury to the cell structures and activation of multiple signaling responses which are involved in the induction of endothelial dysfunction (ED). ED is developed due to the reduction of nitric oxide (NO), activation of endothelial cell leukocyte adhesion, induction of oxidative stress, and inflammation.[2] The endothelium plays a chief role in the regulation of blood flow through the production of NO, prostacyclin, endothelium-derived hyperpolarizing factor, and vasoconstrictor mediators such as endothelin-1 and angiotensin II. ED is a principal sign of atherosclerosis, which can be perceived before the development of macrovascular complications of T2DM.[3]
Normally, NO prevents leukocyte adhesion, but in the presence of risk factors, the endothelium is activated by adhesion molecules, such as vascular cell adhesion molecule-1 (sVCAM-1) and intercellular adhesion molecule-1, which provoke the adhesion of leukocytes to the endothelial surface.[4]
VCAM-1 is an endothelial ligand for integrins, which expressed on the platelets and leukocytes, which facilitate the adhesion of circulating leukocytes. The expression of VCAM-1 is correlated to the concentration of sVCAM-1 in patients with T2DM.[5] Besides, E-selectin which is a Ca+2-dependent cell surface glycoprotein recruits leukocyte under proinflammatory condition. It has been reported that high level of E-selectin is linked with different diseases, including T2DM, metabolic syndrome, vasculitis, rheumatoid arthritis, and cancer.[6]
Thioctic acid (TA) is a potent antioxidant and has the capability to regenerate other antioxidant such as glutathione. TA has the ability to scavenge oxygen free radicals.[7] TA is used in the treatment of ED, neuropathies, atherosclerosis, Alzheimer's disease, and multiple sclerosis. Similarly, TA enhances GLUT-4 translocation, leading to the improvement of glycemic control and prevention of diabetic complications in T2DM.[8] It is well-known that T2DM-induced complications, mainly ED, are linked to the oxidative stress injury and induction of proinflammatory reactions. In addition, TA may improves ED and proinflammatory reactions in patients with T2DM.
Therefor, the aim of the present study was to evaluate the effects of thioctic acid (TA) add-on metformin therapy on glycemic indices and associated inflammatory reactions induced-endothelial dysfunction (ED) in patients with T2DM.
METHODS
This prospective study was completed and permitted by explicit controlled Ethical Committee under Principled Clearance Number RTD13MF 4/8/2018 in reverence to the Declaration of Helsinki.[9] All patients and enrolled participants gave informed verbal consent for their participation in this study. Inclusion criteria included patients with T2DM with an age range of 47–65 years of metformin therapy. Exclusion criteria included psychological diseases, neurological diseases, hypothyroidism, end-stage kidney disease, hepatic dysfunction, connective tissue disorders, history of intake of dopamine receptor agonist and antagonist agents, malignant disorders, and sexual dysfunction.
Study design
A total numbers of 70 patients with T2DM compared with 30 healthy controls were divided into three groups:
Group A (n = 30): Healthy controls
Group B (n = 36): T2DM on metformin 1500 mg/day
Group C (n = 34): T2DM patients on metformin 1500 mg/day plus TA 600 mg/day.
The duration of the study was 10 consecutive weeks. All drugs were purchased from the private pharmaceutical company, metformin (metformin HCL 500 mg, Actavis, India), and TA (thiotacid 300 mg, Eva Pharma, Egypt).
Anthropometric measurements
Body mass index (BMI) was estimated by specific equation, BMI = weight (kg)/height (cm2).[10] Blood pressure measurements were also determined by a digital sphygmomanometer at supine position. Pulse pressure = SBP-DBP, mean arterial pressure (MAP), 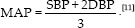 .
.
Biochemical assay
After an overnight fast, 5 ml of venous blood was obtained, which was centrifuged at 3000 rpm and stored at −20°C for later analysis. Fasting blood glucose (FBG) was determined by glucose oxidase method; glycated hemoglobin (HbA1c) was measured by specific kit (human HbA1c AIi1c, GHbA1c, MBS702379). Total cholesterol (TC) and total triglyceride (TG) were estimated by enzyme-linked immunosorbent assay (ELISA) kit method. High-density lipoprotein (HDL), low-density lipoprotein (LDL), and atherogenic index of plasma (AIP) were determined by modified Friedewald's formula (AIP = log (TG/HDL)).[12] Fasting insulin was measured by ELISA kit method (insulin human ELISA kit, Catalog number: KAQ1251). Insulin resistance (IR) and pancreatic β-cell function were determined by homeostatic model assessment (HOMA-2). Serum human vascular cell adhesion molecule-1 and serum human E-selectin were measured by ELISA kit methods (Catalog # BMS232, Kono biotech, China, and Lot number 130676075, Kono biotech, China, respectively); all kit procedures were done according to the kit instructions.
Statistical analysis
Data analysis was done using SPSS (IBM SPSS Statistics version 22.0 [IBM Corp., Armonk, USA]). The data presented as mean ± standard deviation, and either unpaired or paired Student's t-test was used to determine the level of significance differences. Analysis of variance followed by Bonferroni post hoc test was used to compare the results of the study variables among different groups. The level of significance was regarded when P < 0.05.
RESULTS
In the present study, 70 out of 73 patients with T2DM continue this prospective study, due to withdrawal of three patients because of noncompliance and complications [Figure 1]. The gender difference was high, 65.62% male compared with 34.37% females. 56.25% of our patients were smokers. The duration of T2DM was 7.81 ± 3.34 years, and most of those patients were associated with other metabolic disorders, including hypertension, dyslipidemia, and ischemic heart diseases. Other criteria of this study are illustrated in Table 1.
Figure 1.
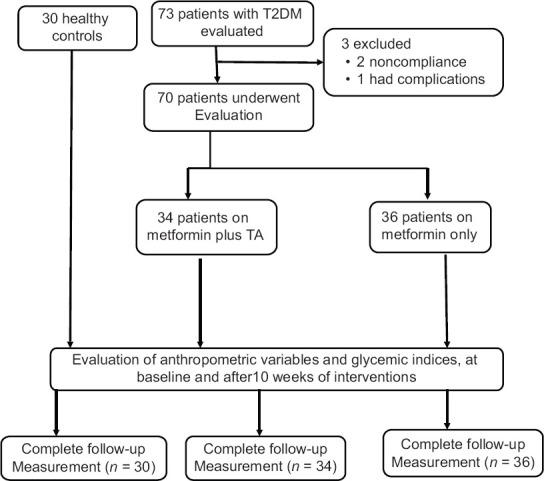
Consort flow diagram of the study
Table 1.
Characteristics of patients with type 2 diabetes mellitus
| Variables | Mean±SD, n (%) |
|---|---|
| n | 70 |
| Age (years) | 61.56±7.83 |
| Gender (male:female ratio) | 42 (65.62):22 (34.37) |
| Smoking | 36 (56.25) |
| Duration of T2DM (years) | 7.81±3.43 |
| Dyslipidemia | 48 (75.00) |
| Hypertension | 51 (79.68) |
| Previous MI | 6 (9.37) |
| Previous stroke | 2 (3.13) |
| Peripheral neuropathy | 29 (45.31) |
| Diabetic retinopathy | 23 (35.39) |
| Diabetic nephropathy | 27 (42.18) |
| Current therapy | |
| Metformin | 36 (42.85) |
| Metformin + TA | 34 (48.57) |
| Fenofibrate | 41 (64.06) |
| Statins | 10 (15.62) |
| Omega-3-fatty acids | 19 (29.68) |
| Anti-platelets | 11 (17.18) |
| ACEIs | 34 (53.13) |
| ARBs | 23 (35.93) |
| Anticoagulants | 6 (9.37) |
Data are expressed as mean±SD, n (%). MI: Myocardial infarction, ACEIs: Angiotensin-converting enzyme inhibitors, ARBs: Angiotensin receptor blockers, TA: Thioctic acid, T2DM: Type 2 diabetes mellitus, SD: Standard deviation
The present study illustrated significant differences between control and patients with T2DM, except in BMI (P = 0.45). Blood pressure profile, lipid profile, IR, FBG, and HbA1c were higher in patients with T2DM compared with controls (P < 0.01). Insulin sensitivity (IS) was low in patients with T2DM compared with controls (P < 0.01) [Table 2].
Table 2.
Baseline glucometabolic differences in patients with type 2 diabetes mellitus compared with the healthy control
| Variables | Control (n=30) | T2DM (n=70) | Differences | 95% CI | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | 29.62±4.32 | 30.56±6.12 | 0.940 | −1.5276-3.4076 | 0.45 |
| SBP (mmHg) | 122.45±7.88 | 138.34±8.94 | 15.890 | 12.10191-9.6781 | 0.0001* |
| DBP (mmHg) | 77.53±6.34 | 83.74±8.98 | 6.210 | 2.6022-9.8178 | 0.0009* |
| PP (mmHg) | 44.65±6.88 | 54.6±7.63 | 9.950 | 6.6973-3.2027 | 0.0001* |
| MAP (mmHg) | 92.51±7.49 | 101.94±8.52 | 9.430 | 5.8224-3.0376 | 0.0001* |
| FBG (mg/dL) | 86.43±6.94 | 139.88±9.66 | 53.450 | 49.5420-57.3580 | 0.0001* |
| HbA1c | 5.6±1.44 | 8.65±3.78 | 3.050 | 1.6302-4.4698 | 0.0001* |
| FSI (µIU/mL) | 6.78±1.86 | 27.66±8.67 | 20.880 | 17.6939-24.0661 | 0.0001* |
| HOMA-IR | 0.87±0.02 | 3.83±1.08 | 2.960 | 2.5672-3.3528 | 0.0001* |
| IS % | 114.6±12.82 | 26.1±5.12 | −88.500 | −92.1661-84.8339 | 0.0001* |
| TC (mg/dL) | 155.98±11.84 | 211.97±11.63 | 55.990 | 50.8499-61.1301 | 0.0001* |
| TG (mg/dL) | 100.22±11.69 | 332.81±23.29 | 232.590 | 223.6429-241.5371 | 0.0001* |
| HDL (mg/dL) | 51.49±5.91 | 34.73±4.33 | −16.760 | −18.9061-4.6139 | 0.0001* |
| LDL (mg/dL) | 84.4±7.93 | 110.7±9.41 | 26.300 | 22.3582-30.2418 | 0.0001* |
| VLDL (mg/dL) | 20.044±4.28 | 66.56±7.91 | 46.516 | 43.4518-49.5802 | 0.0001* |
| AI | 0.07±0.0001 | 0.621±0.12 | 0.551 | 0.0001* |
*P<0.01. Data are presented as mean±SD, unpaired t-test. BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PP: Pulse pressure, MAP: Mean arterial pressure, FBG: Fasting blood glucose, FSI: Fasting serum insulin, IS: Insulin sensitivity, TC: Total cholesterol, TG: Triglyceride, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very LDL, AI: Atherogenic index, T2DM: Type 2 diabetes mellitus, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostasis model assessment-insulin resistance, CI: Confidence interval, SD: Standard deviation
Effects of metformin therapy on the metabolic profile
Metformin therapy led to a minor and insignificant reduction in BMI compared to the baseline (P = 0.09). Metformin therapy improved SBP (P = 0.001), but not DBP (P = 0.34). In addition, metformin therapy decreased FBG and HbA1c significantly (P = 0.001 and P = 0.02, respectively). In addition, metformin therapy led to significant reduction of fasting serum insulin (FSI) and IR with an augmentation in the IS (P < 0.01). Furthermore, metformin therapy significantly ameliorated lipid profile through reduction of TC, TG, VLDL, LDL, and of the AI with increment in HDL-cholesterol (HDL-c) serum levels compared to the baseline (P < 0.01).
Effects of metformin plus thioctic acid therapy on the metabolic profile
Metformin plus TA therapy led to insignificant effect on BMI compared to the baseline data (P = 0.17). Combination therapy reduced SBP, DBP, and MAP (P = 0.001), but not PP (P = 0.35). In addition, combination therapy decreased FBG and HbA1c significantly (P = 0.001). In addition, this combination led to significant reduction of FSI and IR with an increment in IS (P < 0.01). Furthermore, this therapy significantly improved lipid profile through reduction of TC, TG, VLDL, LDL, and of the AI with increment in HDL-c serum levels compared to the baseline (P < 0.01) [Table 3].
Table 3.
Effects of metformin alone or in combination with thioctic acid on glucometabolic profile in patients with type 2 diabetes mellitus
| Variables | Metformin alone (n=36) |
Metformin plus TA (n=34) |
||||
|---|---|---|---|---|---|---|
| Before (n=36) | After (n=36) | P | Before (n=34) | After (n=34) | P | |
| BMI (kg/m2) | 31.56±7.12 | 28.56±7.55 | 0.09 | 30.56±6.12 | 28.33±6.52 | 0.17 |
| SBP (mmHg) | 139.34±7.94 | 130.34±6.94 | 0.001 | 138.34±8.94 | 129.21±7.66 | 0.001* |
| DBP (mmHg) | 84.74±8.98 | 82.74±8.34 | 0.34 | 83.74±8.98 | 72.78±7.66 | 0.001* |
| PP (mmHg) | 54.6±7.63 | 47.6±6.11 | 0.001 | 54.6±7.63 | 56.43±7.55 | 0.35 |
| MAP (mmHg) | 101.94±8.52 | 98.6±7.94 | 0.09 | 101.94±8.52 | 91.59±7.29 | 0.001* |
| FBG (mg/dL) | 139.88±9.66 | 126.86±10.56 | 0.001 | 139.88±9.66 | 119.67±8.94 | 0.001* |
| HbA1c % | 8.65±3.78 | 6.99±1.21 | 0.02 | 8.65±3.78 | 6.01±1.11 | 0.001* |
| FSI (µIU/mL) | 27.66±8.67 | 7.81±2.34 | 0.001 | 27.66±8.67 | 6.81±2.34 | 0.001* |
| HOMA-IR | 3.83±1.08 | 1.10±0.12 | 0.001 | 3.83±1.08 | 0.95±0.046 | 0.001* |
| IS % | 26.1±5.12 | 91.1±8.99 | 0.001 | 26.1±5.12 | 105.6±6.75 | 0.001* |
| TC (mg/dL) | 211.97±11.63 | 187.55±10.77 | 0.001 | 211.97±11.63 | 180.55±9.77 | 0.001* |
| TG (mg/dL) | 332.81±23.29 | 188.31±12.95 | 0.001 | 332.81±23.29 | 178.31±10.97 | 0.001* |
| HDL (mg/dL) | 34.73±4.33 | 44.63±5.81 | 0.001 | 34.73±4.33 | 49.63±6.81 | 0.001* |
| LDL (mg/dL) | 110.7±9.41 | 105.30±8.39 | 0.01 | 110.7±9.41 | 95.30±8.39 | 0.001* |
| VLDL (mg/dL) | 66.56±7.91 | 37.66±6.23 | 0.001 | 66.56±7.91 | 35.66±6.23 | 0.001* |
| AI | 0.621±0.12 | 0.256±0.09 | 0.001 | 0.621±0.12 | 0.195±0.03 | 0.001* |
*P<0.01. Data are presented as mean±SD, unpaired t-test. BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PP: Pulse pressure, MAP: Mean arterial pressure, FBG: Fasting blood glucose, FSI: Fasting serum insulin, IS: Insulin sensitivity, TC: Total cholesterol, TG: Triglyceride, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very LDL, AI: Atherogenic index, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostasis model assessment-insulin resistance, SD: Standard deviation, TA: Thioctic acid
The therapeutic effects of metformin plus TA were more effective than metformin alone on the cardiometabolic profile in patients with T2DM (P < 0.05), with exception of BMI, SBP, FSI, and VLDL (P > 0.05) [Table 4].
Table 4.
Posttreatment differences between metformin and metformin plus thioctic acid on patients with type 2 diabetes mellitus
| Variables | Group І (n=36) | Group ІІ (n=34) | Differences | 95% CI | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | 28.56±7.55 | 28.33±6.52 | −0.230 | −3.8757-3.4157 | 0.89 |
| SBP (mmHg) | 130.34±6.94 | 129.21±7.66 | −1.130 | −4.8913-2.6313 | 0.54 |
| DBP (mmHg) | 82.74±8.34 | 72.78±7.66 | −9.960 | −14.0985-5.8215 | 0.0001* |
| PP (mmHg) | 47.6±6.11 | 56.43±7.55 | 8.830 | 5.4143-12.2457 | 0.0001* |
| MAP (mmHg) | 98.6±7.94 | 91.59±7.29 | −7.010 | −10.8369-3.1831 | 0.0001* |
| FBG (mg/dL) | 126.86±10.56 | 119.67±8.94 | −7.190 | −12.1149-2.2651 | 0.004* |
| HbA1c % | 6.99±1.21 | 6.01±1.11 | −0.980 | −1.5630-0.3970 | 0.0001* |
| FSI (µIU/mL) | 7.81±2.34 | 6.81±2.34 | −1.000 | −2.1717-0.1717 | 0.09 |
| HOMA-IR | 1.10±0.12 | 0.95±0.046 | −0.150 | −0.1966-0.1034 | 0.0001* |
| IS % | 91.1±8.99 | 105.6±6.75 | 14.500 | 10.4840-18.5160 | 0.0001* |
| TC (mg/dL) | 187.55±10.77 | 180.55±9.77 | −7.000 | −12.1646-1.8354 | 0.008* |
| TG (mg/dL) | 188.31±12.95 | 178.31±10.97 | −10.000 | −16.0409-3.9591 | 0.0001* |
| HDL (mg/dL) | 44.63±5.81 | 49.63±6.81 | 5.000 | 1.8467-8.1533 | 0.002* |
| LDL (mg/dL) | 105.30±8.39 | 95.30±8.39 | −10.000 | −14.2011-5.7989 | 0.0001 |
| VLDL (mg/dL) | 37.66±6.23 | 35.66±6.23 | −2.000 | −5.1195-1.1195 | 0.20 |
| AI | 0.256±0.09 | 0.195±0.03 | −0.061 | −0.0954-0.0266 | 0.0008* |
*P<0.01. Data are presented as mean±SD, unpaired t-test. Group І: Metformin alone, Group ІІ: Metformin plus ALA, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PP: Pulse pressure, MAP: Mean arterial pressure, FBG: Fasting blood glucose, FSI: Fasting serum insulin, IS: Insulin sensitivity, TC: Total cholesterol, TG: Triglyceride, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very LDL, AI: Atherogenic index, HbA1c: Glycated hemoglobin, HOMA-IR: Homeostasis model assessment-insulin resistance, CI: Confidence interval, SD: Standard deviation
Effects of metformin and/or thioctic acid therapy on the biomarkers of endothelial dysfunction
Regarding the biomarkers of ED, the sVCAM-1 level was high in patients with T2DM (3.74 ± 1.34 ng/ml) at baseline, which decreased by metformin monotherapy to 2.32 ± 0.67 ng/ml or metformin plus TA to 1.98 ± 0.31 ng/ml (P < 0.01). However, metformin plus TA illustrated insignificant difference compared to metformin alone (P = 0.29). E-selectin level was high in patients with T2DM (8.55 ± 2.56 ng/L) at baseline, which decreased by metformin monotherapy to 6.71 ± 2.85 ng/L or metformin plus TA to 3.91 ± 1.72 ng/L (P < 0.01), but metformin plus TA illustrated significant difference compared to metformin alone (P < 0.01) [Figure 2].
Figure 2.
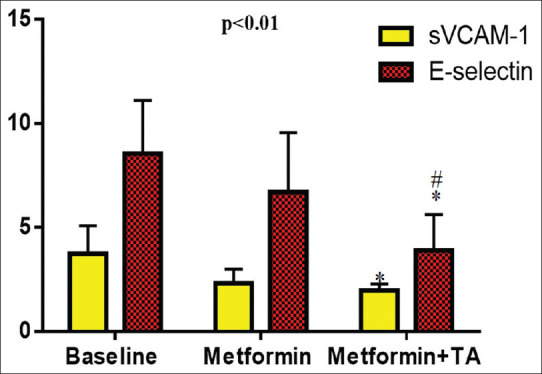
Effects of metformin and/or TA on the biomarkers of endothelial dysfunction in patients with type 2 diabetes mellitus *P< 0.01 versus baseline; #P< 0.05 versus metformin
DISCUSSION
The results of the present study showed insignificant effect of metformin alone or metformin plus TA on BMI; this finding is in harmony with a previous study that illustrated that metformin acts as an insulin sensitizer and improves the anabolic effect of insulin without significant effect on the body weight in both diabetic and diabetic patients.[12] Likewise, findings of the present study illustrated that TA add-on metformin therapy led to insignificant effect on BMI, which might due to the smallest effective dose or short duration of therapy. Koh et al. confirmed that TA at 1800 mg/day leads to modest weight loss in obese patients; thus, it may be regarded as adjuvant therapy for obesity.[13] The antiobesity effect of TA is related to inhibition of hypothalamic AMP-activated protein kinase, which is important in regulation of feeding.[14]
Moreover, the findings of the present study confirmed that metformin alone or metformin with TA led to significant reduction of blood pressure due to activation of MAPK-dependent insulin-signaling pathways, which regulate the secretion of the vasoconstrictor ET-1.[15] Besides, TA improves mitochondrial dysfunction and prevents oxidative stress induced-vasoconstriction, since mitochondrial dysfunction generates free radicals and causing oxidative injury, ED, and peripheral vasoconstrictions.[16]
Therefore, coadministration of TA and metformin leads to vasodilatation and reduction of blood pressure as both of those agents are regarded as potent antioxidants that reverse ED in patients with T2DM.[17,18]
In addition, TA add-on metformin therapy in the present study led to significant improvement of glycemic indices through reduction of FBG, HbA1c, and FSI, as reported by Kamenova study that illustrated oral administration of TA improves glycemic indices in patients with T2DM through enhancement of peripheral IS.[19] Likewise, TA activates insulin signaling pathway, increase peripheral glucose utilization, and reduction of oxidative stress, with potent antioxidant effects, which together improve the outcome of T2DM.[20]
What's more, our study showed that TA add-on metformin therapy reduced serum insulin level due to the synergistic effect of TA on improving insulin level when used in combination with metformin. Since; metformin improves insulin tissue sensitivity to insulin, and enhances peripheral glucose uptake.[21]
In addition, TA improves IS, pancreatic β-cell function, and reduction of IR through modulation of pancreatic MAPK.[22] A recent study by Nobakht-Haghighi et al. confirmed that long-term therapy with TA leads to noteworthy improvement of pancreatic β-cell function through the regulation of oxidative stress and p53 and/or p38 induced pancreatic β-cell apoptosis.[23]
Indeed, TA-metformin combination has an optimistic effect on lipid profiles, primarily on LDL and AI, due to inhibition of hepatic acetyl CoA-Carboxylase activity, reduce fatty acid synthesis, antioxidant effect, upregulation of hepatic LDL receptors, and elevation of mitochondrial fatty acid oxidation.[24]
It has been reported that ED is a common cardiovascular complication in patients with T2DM due to chronic hyperglycemia and/or related oxidative stress.[25] Bretón-Romero et al. showed that ED in T2DM is due to the upregulation of proinflammatory signals which implicated in the pathogenesis of ED and IR.[26]
The present study showed that both sVCAM-1 and E-selectin were elevated in patients with T2DM, which indicates ED as established by Al-Rubeaan et al.'s study.[27]
In the present study, metformin monotherapy led to significant reduction of biomarkers of ED following 10 weeks of therapy, since metformin therapy has a beneficial effect on the endothelial function through modulation of endothelial NO and reduction of oxidative stress.[28]
Recently, an animal model study by Ahuja et al. showed that coadministration of TA with metformin in the experimental diabetes leads to a significant amelioration of IR, inflammatory reactions, oxidative stress, and ED, via suppression of endothelial TLR-2 receptors.[29]
The potential mechanism of TA in the attenuation of ED is related to different pathways, including phosphorylation of protein kinase B (AKT), which attenuates the expression and adhesion of cellular molecules to the vascular endothelium during chronic inflammatory conditions, antioxidant effects, free radical scavenging effect, improvement of endothelial NO, anti-inflammatory, and antithrombotic effects.[30]
Moreover, most of previous studies reported no or slight effect of TA on the ED and proinflammatory changes in T2DM;[31,32] thus, the novelty of the present study confirmed the beneficial antioxidant effect of TA in amelioration of oxidative stress and/or inflammatory reaction-induced ED in patients with T2DM.
Therefore, TA plays an important role in the prevention and attenuation of diabetic induced-ED and should be concerned in the management of T2DM.
The chief strengths of the present study are the parallel group, randomized prospective study, careful clinical characterization of recruited patients, and meticulous conduct of the biomarkers of ED. In spite of these strengths, the present study has some limitations, including small sample size which may limit the interpretation of the results, short duration of TA therapy since 10 consecutive weeks duration of therapy is commonly and frequently used in clinical trials with dietetic supplements, dose-dependent effect of TA was not done, and biomarkers of oxidative stress were not evaluated. Nevertheless, our study is regarded as a preliminary step for large-scale study to assess dose-dependent effect of TA add-on metformin and other antidiabetic medications.
CONCLUSION
TA add on metformin therapy improves glycemic indices and associated inflammatory mediators in patients with T2DM through modulation of IR, IS, and direct direct anti-inflammatory effect.
Research quality and ethics statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting, and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined to require Institutional Ethics Committee, Research Cell, King George's Medical University, Lucknow and appropriate approval (84th ECM II-B-Thesis) was granted by the Research Cell, King George's Medical University, Lucknow.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical conduct of research
This study was approved by the Institutional Review Board / Ethics Committee. The authors followed applicable EQUATOR Network (http://www.equator-network.org/) guidelines during the conduct of this research project.
Acknowledgments
We thank the registry database team and all medical staff members in the Department of Clinical Endocrinology for their cooperation.
REFERENCES
- 1.Al-Kuraishy HM, Al-Gareeb AI, Waheed HJ, Al-Maiahy TJ. Differential effect of metformin and/or glyburide on apelin serum levels in patients with type 2 diabetes mellitus: Concepts and clinical practice. J Adv Pharm Technol Res. 2018;9:80–6. doi: 10.4103/japtr.JAPTR_273_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J Clin Diagn Res. 2016;10:FC21–26. doi: 10.7860/JCDR/2016/19971.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadhim SS, Al-Windy SA, Al-Kuraishy HM, Al-Gareeb AI. Endothelin-1 is a surrogate biomarker link severe periodontitis and endothelial dysfunction in hypertensive patients: The potential nexus. Journal of International Oral Health. 2019;11:369–76. [Google Scholar]
- 4.Kim MJ, Kim T, Suh GJ, Kwon WY, Kim KS, Jung YS, et al. Association between the simultaneous decrease in the levels of soluble vascular cell adhesion molecule-1 and S100 protein and good neurological outcomes in cardiac arrest survivors. Clin Exp Emerg Med. 2018;5:211–8. doi: 10.15441/ceem.17.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brevetti G, Martone VD, de Cristofaro T, Corrado S, Silvestro A, Di Donato AM, et al. High levels of adhesion molecules are associated with impaired endothelium-dependent vasodilation in patients with peripheral arterial disease. Thromb Haemost. 2001;85:63–6. [PubMed] [Google Scholar]
- 6.Telen MJ. Cellular adhesion and the endothelium: E-selectin, L-selectin, and pan-selectin inhibitors. Hematol Oncol Clin North Am. 2014;28:341–54. doi: 10.1016/j.hoc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kuraishy HM, Al-Gareeb AI. Co-administration effects of α-lipoic acid and nucleo CMP on arousal and sensory cortical activity. J Young Pharma. 2016;8:12–7. [Google Scholar]
- 8.Scaramuzza A, Giani E, Redaelli F, Ungheri S, Macedoni M, Giudici V, et al. Alpha-lipoic acid and antioxidant diet help to improve endothelial dysfunction in adolescents with type 1 diabetes: A pilot trial. J Diabetes Res. 2015;2015:474561. doi: 10.1155/2015/474561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 10.Alkuraishy HM, Al-Gareeb AI. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Cross-sectional study. Emerg Med Int. 2015;2015:283021. doi: 10.1155/2015/283021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkuraishy HM, Al-Gareeb AI, Waheed HJ. Lipoprotein-associated phospholipase A2 is linked with poor cardio-metabolic profile in patients with ischemic stroke: A study of effects of statins. J Neurosci Rural Pract. 2018;9:496–503. doi: 10.4103/jnrp.jnrp_97_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with Omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH, Jeong E, et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am J Med. 2011;124:85.e1–8. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–33. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Liu H, Wen X, Peng Y, Tian Y, Zhao L. Effects of metformin on blood pressure in nondiabetic patients: A meta-analysis of randomized controlled trials. J Hypertens. 2017;35:18–26. doi: 10.1097/HJH.0000000000001119. [DOI] [PubMed] [Google Scholar]
- 16.McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, et al. Effect of combined treatment with alpha-lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9:249–55. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Tian Y, Berr SS, French BA. Therapeutic efficacy of alpha-lipoic acid against acute myocardial infarction and chronic left ventricular remodeling in mice. Cardiol Res Pract. 2020;2020:ID 6759808. doi: 10.1155/2020/6759808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Morales N, Rovira-Llopis S, Bañuls C, Lopez-Domenech S, Escribano-Lopez I, Veses S, et al. Does metformin protect diabetic patients from oxidative stress and leukocyte-endothelium interactions? Antioxid Redox Signal. 2017;27:1439–45. doi: 10.1089/ars.2017.7122. [DOI] [PubMed] [Google Scholar]
- 19.Kamenova P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones (Athens) 2006;5:251–8. doi: 10.14310/horm.2002.11191. [DOI] [PubMed] [Google Scholar]
- 20.Dworacka M, Chukanova G, Iskakova S, Kurmambayev Y, Wesołowska A, Frycz BA, et al. New arguments for beneficial effects of alpha-lipoic acid on the cardiovascular system in the course of type 2 diabetes. Eur J Pharm Sci. 2018;117:41–7. doi: 10.1016/j.ejps.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Jin P, Min HU, Zhou X, Zhang J, Jie LI. Metformin improves glucose metabolism and serum levels of lipoprotein derived hormones in obese children with hyperinsulinemia. Chin J Biochem Pharma. 2017;37:213–4. [Google Scholar]
- 22.Targonsky ED, Dai F, Koshkin V, Karaman GT, Gyulkhandanyan AV, Zhang Y, et al. α-Lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia. 2006;49:1587–98. doi: 10.1007/s00125-006-0265-9. [DOI] [PubMed] [Google Scholar]
- 23.Nobakht-Haghighi N, Rahimifard M, Baeeri M, Rezvanfar MA, Moini Nodeh S, Haghi-Aminjan H, et al. Regulation of aging and oxidative stress pathways in aged pancreatic islets using alpha-lipoic acid. Mol Cell Biochem. 2018;449:267–76. doi: 10.1007/s11010-018-3363-3. [DOI] [PubMed] [Google Scholar]
- 24.Al-Kuraishy HM, Al-Gareeb AI, Shams HA, Al-Mamorri F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. Journal of Laboratory Physicians. 2019;11:317–22. doi: 10.4103/JLP.JLP_123_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suganya N, Bhakkiyalakshmi E, Sarada DV, Ramkumar KM. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br J Nutr. 2016;116:223–46. doi: 10.1017/S0007114516001884. [DOI] [PubMed] [Google Scholar]
- 26.Bretón-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016;36:561–9. doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Rubeaan K, Nawaz SS, Youssef AM, Al Ghonaim M, Siddiqui K. IL-18, VCAM-1 and P-selectin as early biomarkers in normoalbuminuric Type 2 diabetes patients. Biomark Med. 2019;13:467–78. doi: 10.2217/bmm-2018-0359. [DOI] [PubMed] [Google Scholar]
- 28.Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI, Hussien NR, Al-Nami MS. Effects of diabetic pharmacotherapy on prolactin hormone in patients with type 2 diabetes mellitus: Bane or Boon. Journal of advanced pharmaceutical technology & research. 2019;10:163–69. doi: 10.4103/japtr.JAPTR_65_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahuja S, Uniyal A, Akhtar A, Sah SP. Alpha lipoic acid and metformin alleviates experimentally induced insulin resistance and cognitive deficit by modulation of TLR2 signalling. Pharmacol Rep. 2019;71:614–23. doi: 10.1016/j.pharep.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Tromba L, Perla FM, Carbotta G, Chiesa C, Pacifico L. Effect of alpha-lipoic acid supplementation on endothelial function and cardiovascular risk factors in overweight/obese youths: A double-blind, placebo-controlled randomized trial. Nutrients. 2019;11:pii: E375. doi: 10.3390/nu11020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammadi V, Khorvash F, Feizi A, Askari G. Does alpha-lipoic acid supplementation modulate cardiovascular risk factors in patients with stroke? A randomized, double-blind clinical trial. Int J Prev Med. 2018;9:34. doi: 10.4103/ijpvm.IJPVM_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porasuphatana S, Suddee S, Nartnampong A, Konsil J, Harnwong B, Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac J Clin Nutr. 2012;21:12–21. [PubMed] [Google Scholar]


