Abstract
Objective:
To evaluate the nephroprotective effect of lycopene (LPN) in acute kidney injury (AKI) regarding the oxidative stress (OS).
Materials and Methods:
Thirty Sprague Dawley male rats were divided into three groups – control group: rats treated with distilled water (orally) for 10 days (n = 10); AKI group: rats treated with distilled water and diclofenac (intraperitoneal) for 10 days (n = 10); treated group: rats treated with LPN (orally) and diclofenac for 10 days (n = 10). Body mass index (BMI) and estimated glomerular filtration rate (eGFR) were measured. Blood urea, serum creatinine (CreSerum), serum malondialdehyde (MDA), superoxide dismutase (SOD), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecules (KIM-1) were measured in the all groups on the 11th day of the experiment.
Results:
Diclofenac-induced AKI led to significant elevations of BMI, CreSerum, and blood urea compared with control (P < 0.05). In AKI model, eGFR was reduced to 11.69 ± 2.64 ml/min/1.73 compared with control group (15.88 ± 3.75 ml/min/1.73, P = 0.03). NGAL, MDA, and KIM-1 were elevated in AKI compared with control (P < 0.001). Pretreatment with LPN led to the reduction of blood urea and CreSerum as compared with AKI (P < 0.001). Similarly, eGFR was increased significantly to 14.81 ± 3.21 ml/min/1.73 compared with 11.69 ± 2.64 ml/min/1.73 in AKI (P = 0.02). Serum levels of NGAL, KIM-1, and MDA were reduced significantly in the LPN group as compared with AKI (P = 0.001), while the SOD serum level was increased to 33. 86 ± 8.61 pg/ml as compared to 22.78 ± 7.56 pg/ml in AKI (P = 0.006). As well, LPN reduced MDA/SOD ratio as compared with AKI (P = 0.00001).
Conclusion:
The finding of this study illustrated that LPN is an effective natural antioxidant that attenuates and prevents AKI through modulation of OS and lipid peroxidation. As well, LPN might be of great value in the prevention of nephrotoxicity that induced by nephrotoxic agents like diclofenac.
Key Words: Acute kidney injury, diclofenac, lycopene
INTRODUCTION
Acute kidney injury (AKI) is a sudden and abrupt reduction in kidney functions within 48 h, as reflected by the increase of blood urea nitrogen and serum creatinine (CreSerum). AKI provokes the progression of underlying chronic kidney disease and development of end-stage renal disease.[1] Mehta et al. described the diagnostic criteria of AKI, which are rapid reduction in kidney function (within 48 h), increase in CreSerum >0.3 mg/dL or an increase >50% from baseline, and reduction of urine output (UOP) <0.5 ml/kg/h.[2]
Therefore, depending on UOP and CreSerum, staging of AKI can be done in three stages – Stage I: CreSerum >0.3 mg/dL and UOP <0.5 mL/kg/h for >6 h; Stage II: Increase in CreSerum >2–3 fold from baseline and UOP <0.5 mL/kg/h for >12 h; and Stage III: CreSerum >4.0 mg/dL or >3-fold increase from baseline and UOP <0.3 mL/kg/h for >24 h or anuria for >12 h.[3] The incidence of AKI was estimated to be 550/100,000 subjects in 2003, which was higher than stroke incidence in the USA. In hospitalized patients, the incidence of AKI was 2%–7%; however, this incidence is increased in patients at the intensive care unit to 10%.[4] As well, AKI is associated with a high risk of mortality, which may exceed 50% in the hospitalized patients, due to the development of multiorgan failure.[5] The independent and modifiable risk factors for AKI are hypertension, obesity, diabetes mellitus, smoking, stroke, and heart failure. However, nonmodifiable risk factors such as elderly and inherited kidney diseases may involve in the induction of AKI postoperatively due to ischemic changes and/or anesthetic drugs.[6] It has been reported that oxidative stress (OS) is regarded as a potential factor in the initiation and progression of AKI, as OS leads to renal epithelial and tubular cell dysfunctions.[7]
OS results from the imbalance between formed oxidants and body antioxidants. Molecules such as reactive oxygen species (ROS) and reactive nitrogen species, which perform important roles in cellular activity, may contribute to OS injury when the homeostatic balance is disrupted.[8]
Different medicinal plants have been reported to be effective in the attenuation of OS-induced AKI through their antioxidant activities. The antioxidant activity of plant-derived phenolic and carotenoid compounds has been reported to attenuate OS-induced AKI.[9]
Lycopene (LPN) is one of the most commonly known naturally occurring carotenoids, mainly found in tomato and tomato-rich products. It is a highly efficient antioxidant, with free radical scavenging activity in nephrotoxicity. LPN also protects DNAs, lipids, and proteins from ROS by direct in vivo reaction and neutralization.[10]
The rational hypothesis of the present study depends on that OS and lipid perioxidation are involved in the pathogenesis of AKI, and since LPN is a natural antioxidant, an objective of the present study was to evaluate the protective effect of LPN in AKI regarding the OS.
MATERIALS AND METHODS
Study design
This experimental animal model study involved 30 Sprague Dawley male rats, aged 2–3 months with a mean and standard deviation of 2.65 ± 0.49 months and weighing 200–250 g each. The rats, prearranged from the National Animal House Centre, College of Medicine, Al-Mustansiriyia University, Baghdad-Iraq, 2018, were isolated as five rats per cage under suitable room temperature and humidity, with artificial light-dark cycle 12/12 h. The rats were given an acclimatization period of a week without any intervention and separation with free access to normal chow and water adlibitum. This study was approved in College of Medicine, Al-Mustansiriyia University, by a scientific board according to the humane care for laboratory animal, under the license number 33/7U on May 11, 2018. The rats after 1 week of acclimatization period, they were divided into three groups, 10 rats in each group. The study protocol for induction of AKI was according to Al-Kuraishy et al. method.[11] After that, the rats were randomized as placebo-controlled, open-labeled into three different groups:
Control group: Rats treated with distilled water 2.5 ml/kg orally for 10 days (n = 10)
AKI group: Rats treated with distilled water 2.5 ml/kg orally for 10 days plus diclofenac 15 mg/kg/day (voltaren 15 mg/ml ampule, Novartis, India), intraperitoneally for 5 days from the 5th to 10th day of the experiment (n = 10)
Treated group: Rats treated with LPN 5 mg/kg/day (LPN 5 mg capsule India-Mart, India) for 10 days plus diclofenac 15 mg/kg/day intraperitoneally for 5 days from the 5th to 10th day of the experiment (n = 10).
All procedures were done at 8–9 am to minimize diurnal variations in the serum levels of study biomarkers.
Chemicals
LPN capsule (5 mg, India-Mart, India), diclofenac (voltaren 15 mg/ml, ampoule, Novartis, India), and other chemicals were purchased from a private pharmaceutical company (Dar-Al-Dwa, Iraq).
Assessment of anthropometric variables
Length of rat was measured by a graduating tape in centimeters from the nose to the anus of rat that called naso–anal length. Body mass index (BMI) was measured by specific equation: BMI = body weight (g)/length (cm)2.[12] Estimated glomerular filtration rate (eGFR) was measured by Schwartz formula: eGFR = k × height (cm)/CreSerum (mg/dl), where k = 0.55.[13]
Assessment of biochemical parameters
Blood samples were taken on the 11th day after rat decapitation under anesthesia by intraperitoneal ketamine (ketamine HCL 100 mg/kg, Bionchepharma, Ireland). The blood samples were centrifuged for 10 min at 3000 rpm at room temperature. The formed samples were kept on −20C till the time of analysis. Blood urea and CreSerum were estimated using colometric method (auto-analyzer, ILab-300-Biomerieux Diagnostic, Milano, Italy). Serum malondialdehyde (MDA), superoxide dismutase (SOD), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecules (KIM-1) were measured using ELISA kit methods according to the instruction of the kit manufacturer (Myo-Bio Source, USA).
Statistical analysis
Data of the present study were presented as mean ± standard deviation; one-way ANOVA test with post hoc test was used to investigate the significance of differences among different groups. Statistical Package for the Social Sciences Software (IBM, SPSS Statistics for Windows version 20.0. 2014, Inc., Chicago, IL, USA) was used for data analysis. The levels of significance were considered when P < 0.05.
RESULTS
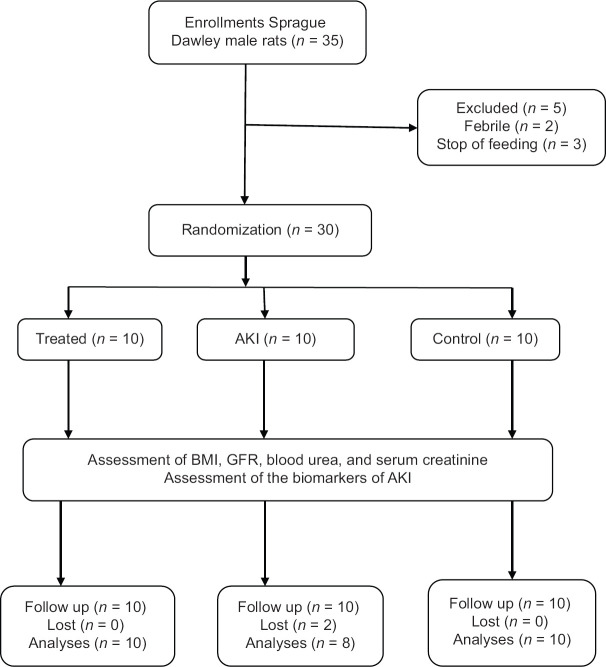
In the present study, 30 out of 35 Sprague Dawley male rats were randomized, of which five rats were excluded. During induction of AKI there was death of two rats (6.67%). Therefore, 10 rats in the control group, 10 rats in the treated group, and 8 rats in the AKI were analyzed [Figure 1].
Figure 1.
Consort flow diagram of the present study
Effects of acute kidney injury on the anthropometric and biochemical variables
The present study illustrated that diclofenac-induced AKI led to significant elevations of BMI, CreSerum, and blood urea compared with the control (P < 0.05). In AKI model, eGFR was reduced to 11.69 ± 2.64 ml/min/1.73 compared with control group 15.88 ± 3.75 ml/min/1.73 (P = 0.03). Biomarkers of tubular and glomerular injury which were serum level of NGAL and KIM-1 were elevated in AKI compared with control (P < 0.001). In AKI, the MDA serum level was increased to 298.61 ± 16.72 pg/ml while the SOD serum level was decreased to 22.78 ± 7.56 pg/ml compared with the control (P < 0.001) [Table 1].
Table 1.
Anthropometric and biochemical changes in diclofenac-induced acute kidney injury compared with control
| Parameters | Control (n=10) | AKI (n=10) | Differences | 95% CI | P |
|---|---|---|---|---|---|
| BMI (g/cm2) | 0.55±0.1 | 0.61±0.1 | 0.110 | 0.01–0.20 | 0.02* |
| CreSerum (mg/dL) | 0.88±0.1 | 1.92±0.2 | 1.040 | 0.89–1.18 | 0.001# |
| Blood urea (mg/dL) | 39.73±7.96 | 57.28±9.61 | 17.550 | 9.25–25.84 | 0.0001# |
| eGFR (ml/min/1.73) | 15.88±3.75 | 11.69±2.64 | −4.190 | −8.15–0.22 | 0.03* |
| KIM (pg/mL) | 88.68±12.39 | 122.78±18.45 | 34.100 | 19.33–48.86 | 0.0001# |
| NGAL (pg/mL) | 14.93±4.61 | 22.83±5.63 | 7.900 | 0.06–9.73 | 0.001# |
| MDA (ng/mL) | 240.52±11.72 | 298.61±16.72 | 58.090 | 44.52–71.65 | 0.0001# |
| SOD (pg/mL) | 45.78±9.69 | 22.78±7.56 | 23.000 | −31.16–14.83 | 0.0001# |
Data presented as mean±SD. *P<0.05, #P<0.01. BMI: Body mass index, eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecules, NGAL: Neutrophil gelatinase-associated lipocalin, MDA: Malondialdehyde, SOD: Superoxide dismutase, SD: Standard deviation, CreSerum: Serum creatinine, AKI: Acute kidney injury, CI: Confidence interval
Effects of lycopene on the anthropometric and biochemical variables in rat with acute kidney injury
Pretreatment with LPN in diclofenac-induced AKI reduced rat BMI insignificantly to 0.58 ± 0.02 g/cm2 compared with control (0.61 ± 0.1 g/cm2, P = 0.51). Both blood urea and CreSerum were reduced significantly in LPN group compared with AKI (P < 0.001). Similarly, eGFR was increased significantly to 14.81 ± 3.21 ml/min/1.73 compared with 11.69 ± 2.64 ml/min/1.73 in AKI (P = 0.02). Serum level of NGAL and KIM-1 was reduced significantly in LPN group as compared with AKI (P = 0.01 and = 0.002, respectively). In LPN group, MDA serum level was reduced to 250.74 ± 12.47 ng/mL as compared with AKI (298.61 ± 16.72 ng/mL, P = 0.001), while SOD serum level was increased to 33.86 ± 8.61 pg/mL as compared with 22.78 ± 7.56 pg/mL in AKI (P = 0.006) [Table 2].
Table 2.
Effects of lycopene on the anthropometric and biochemical variables in rat with acute kidney injury
| Parameters | AKI (n=10) | Lycopene (n=10) | Differences | 95% CI | P |
|---|---|---|---|---|---|
| BMI (g/cm2) | 0.61±0.1 | 0.58±0.02 | −0.030 | −0.12–0.06 | 0.51 |
| CreSerum (mg/dL) | 1.92±0.2 | 1.043±0.2 | −0.877 | −1.06–0.68 | 0.001 |
| Blood urea (mg/dL) | 57.28±9.61 | 44.84±8.66 | −12.440 | −21.03–3.84 | 0.007 |
| eGFR (ml/min/1.73) | 11.69±2.64 | 14.81±3.21 | 3.120 | 0.35–5.88 | 0.02 |
| KIM (pg/mL) | 122.78±18.45 | 96.64±13.55 | −26.140 | −41.34–10.93 | 0.002 |
| NGAL (pg/mL) | 22.83±5.63 | 15.59±6.29 | −7.240 | −12.84–1.63 | 0.01 |
| MDA (ng/mL) | 298.61±16.72 | 250.74±12.47 | −47.870 | −16.72–34.01 | 0.001 |
| SOD (pg/mL) | 22.78±7.56 | 33.86±8.61 | 11.080 | 3.46–18.69 | 0.006 |
Data presented as mean±SD. BMI: Body mass index, eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecules, NGAL: Neutrophil gelatinase-associated lipocalin, MDA: Malondialdehyde, SOD: Superoxide dismutase, SD: Standard deviation, CreSerum: Serum creatinine, AKI: Acute kidney injury, CI: Confidence interval
Effect of lycopene on the oxidative stress in acute kidney injury
Regarding the OS, MDA/SOD ratio was lower in the control group (5.25 ± 2.89) as compared with AKI (13.108 ± 3.85, P = 0.00001, difference = 7.85, 95% confidence interval [CI] 4.4397–11.2763). On the other hand, MDA/SOD ratio was low in LPN group (7.405 ± 2.31) as compared with AKI (13.108 ± 3.85, difference = −5.7030, 95% CI −9.1213–2.2847, P = 0.00001), but it was not significantly differed as compared with the control (difference = 2.155, 95% CI −1.2633–5.5733, P = 0.27) [Figure 2].
Figure 2.
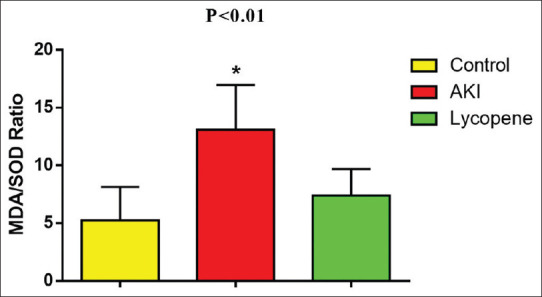
Effect of lycopene on malondialdehyde/superoxide dismutase ratio in acute kidney injury
Correlations of malondialdehyde/superoxide dismutase ratio with anthropometric and biochemical variables
In AKI, MDA/SOD ratio was significantly correlated (P < 0.05) with all anthropometric and biochemical variables, except BMI, which was not significantly correlated (P = 0.20); however, in AKI, MDA/SOD ratio was negatively correlated with SOD serum levels. In LPN-treated group, the correlations of MDA/SOD ratio were reduced; it insignificantly correlated with BMI, CreSerum, blood urea, and eGFR (P > 0.05) but significantly correlated with KIM-1, NGAL, MDA, and SOD serum levels (P < 0.05). However, in LPN group, MDA/SOD ratio was negatively correlated with SOD serum levels [Table 3].
Table 3.
Correlations of malondialdehyde/superoxide dismutase ratio with anthropometric and biochemical variables
| Parameters | AKI (n=10) |
Lycopene I (n=10) |
||
|---|---|---|---|---|
| r | P | r | P | |
| BMI (g/cm2) | 0.44 | 0.20 | 0.46 | 0.18 |
| CreSerum (mg/dL) | 0.67 | 0.03 | 0.56 | 0.09 |
| Blood urea (mg/dL) | 0.72 | 0.01 | 0.48 | 0.16 |
| eGFR (ml/min/1.73) | 0.64 | 0.04 | 0.60 | 0.06 |
| KIM (pg/mL) | 0.86 | 0.001 | 0.70 | 0.02 |
| NGAL (pg/mL) | 0.75 | 0.01 | 0.68 | 0.03 |
| MDA (ng/mL) | 0.83 | 0.002 | 0.65 | 0.04 |
| SOD (pg/mL) | −0.87 | 0.001 | −0.80 | 0.005 |
Data presented as mean±SD. BMI: Body mass index, eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecules, NGAL: Neutrophil gelatinase-associated lipocalin, MDA: Malondialdehyde, SOD: Superoxide dismutase, SD: Standard deviation, CreSerum: Serum creatinine, AKI: Acute kidney injury
DISCUSSION
AKI refers to sudden and abrupt deterioration of kidney function, which leads to elevation in blood urea and CreSerum and often associated with reduction in UOP. Only supportive treatment is available for the management of AKI, especially in critically ill patients.[14]
In the present study, diclofenac led to a significant AKI as revealed by elevated blood urea and CreSerum with significant reduction of eGFR as observed in a previous study.[15] Borghi et al. confirmed that the mechanisms of diclofenac-induced AKI are related to acute tubular necrosis and glomerular damage via the inhibition of renal prostaglandins as well as the induction of OS.[16]
These findings might explain the elevation of KIM-1, NGAL, and MDA which reflect the underling glomerular, tubular, and OS injury in the present study. It has been shown that both KIM-1 and NGAL serum levels are highly sensitive in the detection of AKI as they rise before the elevation of blood urea and CreSerum. Further, their levels are correlated with glomerular and tubular damage in AKI.[17] Recently, Rasheed et al. found that OS plays a potential role in the initiation and progression of AKI through depletion of proximal renal tubular antioxidant capacity and induction of free radicals.[18] These changes are reflected through the increase of MDA, reduction of SOD, and augmentation of MDA/SOD ratio as revealed in the present study.
Therefore, there is still an immense need to improve on the current preventive measures of AKI. Different researches have looked into the constituents of medicinal plants and their ability to prevent and attenuate AKI. In the present study, LPN was an effective agent in the attenuation of AKI through amelioration of biochemical variables in the induced AKI. LPN improved blood urea, CreSerum, OS, and glomerular/tubular damage biomarkers in AKI as documented by Ateşşahin et al.[19] Thus, pretreatment with LPN was effective in the attenuation of diclofenac-induced AKI through amelioration of eGFR and biochemical abnormalities.
Different studies show that LPN is a potent antioxidant and has free radical scavenging activity, so it prevents damage to cell membranes, proteins, lipids, and DNA. Therefore, LPN prevents OS and ischemic reperfusion-induced AKI.[20,21] Indeed, LPN attenuates cisplatin-induced AKI through upregulation of cation and anion transporters, which are reduced in AKI.[22] As well, LPN reduces inflammation and pro-inflammatory mediators during AKI; it reduced tumor necrosis factor alpha and interleukin-6 in acute xenobiotic-induced nephrotoxicity.[23] In addition, the present study illustrated that OS in AKI was significantly correlated with the biomarkers of glomersular and tubular damage in AKI. These correlations were less significant in LPN-treated group due to reduction of OS and inflammatory biomarkers. Our findings were consistent and in line with the previous researches that observed the potent antioxidant effect of LPN and its correlation with inflammatory and OS biomarkers.[24,25,26]
Study limitations
Limitations of the present study were small sample size, dose-dependent effect being not evaluated, and short duration of the study which might limit the outcomes precisely. As well, estimation of GFR by an equation that depends on the length of rat might not reflect the underlying kidney damage. Despite these limitations, this experimental study illustrated significant nephroprotective effect of LPN against AKI through modulation of OS, which is the main pathophysiological factor in the initiation and progression of AKI. However, the findings of this study are likely to translate to other species or systems, including human biology.
CONCLUSION
The finding of this study illustrated that LPN is an effective, natural antioxidant attenuates and prevents AKI through modulation of OS and lipid peroxidation. As well, LPN might be of great value in the prevention of nephrotoxicity that induced by nephrotoxic agents like diclofenac.
Research quality and ethics statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting, and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined to require Institutional Ethics Committee Review and was approved by Editorial Committee in College of Medicine, Al-Mustansiriya University in accordance with the Guide to the Care and Use of Laboratory Animal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical conduct of research
This study was approved by the Institutional Review Board / Ethics Committee. The authors followed applicable EQUATOR Network (http://www.equator-network.org/) guidelines during the conduct of this research project.
Acknowledgment
We would like to express deep thanks for all undergraduate and postgraduate medical students for their participation in this experimental novel study.
REFERENCES
- 1.Al-Kuraishy HM, Al-Gareeb AI, Hussien NR. Betterment of diclofenac-induced nephrotoxicity by pentoxifylline through modulation of inflammatory biomarkers. Asian J Pharm Clin Res. 2019;12:433–7. [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–93. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–8. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WH, Park MH, Kim HJ, Lim HY, Shim HS, Sohn JT, et al. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: A propensity score matched case-control study. Medicine (Baltimore) 2015;94:e273. doi: 10.1097/MD.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahgoub E, Kumaraswamy SM, Kader KH, Venkataraman B, Ojha S, Adeghate E, et al. Genipin attenuates cisplatin-induced nephrotoxicity by counteracting oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2017;93:1083–97. doi: 10.1016/j.biopha.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Al-Kuraishy HM, Al-Gareeb AI. Eustress and malondialdehyde (MDA): Role of panax ginseng: Randomized placebo controlled study. Iran J Psychiatry. 2017;12:194–200. [PMC free article] [PubMed] [Google Scholar]
- 9.Pektaş A, Gemalmaz H, Balkaya M, Ünsal C, Yenisey Ç, Kılıçarslan N, et al. The short-term protective effects of lycopene on renal ischemia-reperfusion injury in rats. Turk J Urol. 2014;40:46–51. doi: 10.5152/tud.2014.53765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E, et al. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum Exp Toxicol. 2015;34:487–96. doi: 10.1177/0960327114542964. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kuraishy HM, Al-Gareeb AI, Hussien NR. Synergistic effect of berberine and pentoxifylline in attenuation of acute kidney injury. Int J Crit Illn Inj Sci. 2019;9:69–74. doi: 10.4103/IJCIIS.IJCIIS_85_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Kuraishy HM, Al-Gareeb AI, Rasheed HA. Antioxidant and anti-inflammatory effects of curcumin contribute into attenuation of acute gentamicin-induced nephrotoxicity in rats. Asian J Pharm Clin Res. 2019;12:466–8. [Google Scholar]
- 13.Gao A, Cachat F, Faouzi M, Bardy D, Mosig D, Meyrat BJ, et al. Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int. 2013;83:524–30. doi: 10.1038/ki.2012.388. [DOI] [PubMed] [Google Scholar]
- 14.Bayomy NA, Elbakary RH, Ibrahim MA, Abdelaziz EZ. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat Rec (Hoboken) 2017;300:1137–49. doi: 10.1002/ar.23525. [DOI] [PubMed] [Google Scholar]
- 15.Hussien NR, Al-Kuraishy HM, Al-Gareeb AI. Reno-protective effect of berberine. J Pak Med Assoc. 2019;69(Suppl 3):S83–87. [PubMed] [Google Scholar]
- 16.Borghi SM, Fattori V, Ruiz-Miyazawa KW, Bertozzi MM, Lourenco-Gonzalez Y, Tatakihara RI, et al. Pyrrolidine dithiocarbamate inhibits mouse acute kidney injury induced by diclofenac by targeting oxidative damage, cytokines and NF-κB activity. Life Sci. 2018;208:221–31. doi: 10.1016/j.lfs.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Al-Naimi MS, Rasheed HA, Hussien NR, Al-Kuraishy HM, Al-Gareeb AI. Nephrotoxicity: Role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res. 2019;10:95–9. doi: 10.4103/japtr.JAPTR_336_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Rosuvastatin attenuates acute nephrotoxicity through modulation of oxidative stress in Sprague Dawley rats. J Pak Med Assoc. 2019;69(Suppl 3):S98–102. [PubMed] [Google Scholar]
- 19.Ateşşahin A, Ceribaşi AO, Yilmaz S. Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:372–6. doi: 10.1111/j.1742-7843.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- 20.Augusti PR, Conterato GM, Somacal S, Einsfeld L, Ramos AT, Hosomi FY, et al. Effect of lycopene on nephrotoxicity induced by mercuric chloride in rats. Basic Clin Pharmacol Toxicol. 2007;100:398–402. doi: 10.1111/j.1742-7843.2007.00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Stojiljkovic N, Ilic S, Jakovljevic V, Stojanovic N, Stojnev S, Kocic H, et al. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxid Med Cell Longev. 2018;2018:2627917. doi: 10.1155/2018/2627917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoodnia L, Mohammadi K, Masumi R. Ameliorative effect of lycopene effect on cisplatin-induced nephropathy in patient. J Nephropathol. 2017;6:144–9. doi: 10.15171/jnp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz S, Atessahin A, Sahna E, Karahan I, Ozer S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology. 2006;218:164–71. doi: 10.1016/j.tox.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Kaya C, Karabulut R, Turkyilmaz Z, Sonmez K, Kulduk G, Gülbahar Ö, et al. Lycopene has reduced renal damage histopathologically and biochemically in experimental renal ischemia-reperfusion injury. Ren Fail. 2015;37:1390–5. doi: 10.3109/0886022X.2015.1064742. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Xu Z, Liu W, Deng Y, Xu B. The protective role of procyanidins and lycopene against mercuric chloride renal damage in rats. Biomed Environ Sci. 2011;24:550–9. doi: 10.3967/0895-3988.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Xia J, Zhao HS, Hou R, Talukder M, Yu L, et al. Lycopene triggers Nrf2-AMPK cross talk to alleviate atrazine-induced nephrotoxicity in mice. J Agric Food Chem. 2018;66:12385–94. doi: 10.1021/acs.jafc.8b04341. [DOI] [PubMed] [Google Scholar]