Abstract
Background
A vaccine against SARS-CoV-2 is of high urgency. Here the safety and immunogenicity induced by a DNA vaccine (INO-4800) targeting the full length spike antigen of SARS-CoV-2 are described.
Methods
INO-4800 was evaluated in two groups of 20 participants, receiving either 1.0 mg or 2.0 mg of vaccine intradermally followed by CELLECTRA® EP at 0 and 4 weeks. Thirty-nine subjects completed both doses; one subject in the 2.0 mg group discontinued trial participation prior to receiving the second dose. ClinicalTrials.gov identifier: NCT04336410.
Findings
The median age was 34.5, 55% (22/40) were men and 82.5% (33/40) white. Through week 8, only 6 related Grade 1 adverse events in 5 subjects were observed. None of these increased in frequency with the second administration. No serious adverse events were reported. All 38 subjects evaluable for immunogenicity had cellular and/or humoral immune responses following the second dose of INO-4800. By week 6, 95% (36/38) of the participants seroconverted based on their responses by generating binding (ELISA) and/or neutralizing antibodies (PRNT IC50), with responder geometric mean binding antibody titers of 655.5 [95% CI (255.6, 1681.0)] and 994.2 [95% CI (395.3, 2500.3)] in the 1.0 mg and 2.0 mg groups, respectively. For neutralizing antibody, 78% (14/18) and 84% (16/19) generated a response with corresponding geometric mean titers of 102.3 [95% CI (37.4, 280.3)] and 63.5 [95% CI (39.6, 101.8)], in the respective groups. By week 8, 74% (14/19) and 100% (19/19) of subjects generated T cell responses by IFN-ɣ ELISpot assay with the median SFU per 106 PBMC of 46 [95% CI (21.1, 142.2)] and 71 [95% CI (32.2, 194.4)] in the 1.0 mg and 2.0 mg groups, respectively. Flow cytometry demonstrated a T cell response, dominated by CD8+ T cells co-producing IFN-ɣ and TNF-α, without increase in IL-4.
Interpretation
INO-4800 demonstrated excellent safety and tolerability and was immunogenic in 100% (38/38) of the vaccinated subjects by eliciting either or both humoral or cellular immune responses.
Funding
Coalition for Epidemic Preparedness Innovations (CEPI).
Keywords: SARS-CoV-2, COVID-19, DNA vaccine, INO-4800, Phase 1
Research in context.
Evidence before this study
INO-4800 is among several vaccines being tested against SARS-CoV-2, the virus that causes COVID-19 disease with the goal of inducing a protective immune response. The DNA vaccine, INO-4800, delivered intradermally with CELLECTRA® electroporation (EP) delivery system, induces a balanced immune response that includes engagement of both T cells and B cells [1], [2], [3], [4], [5].
Added value of this study
This is the first report of a clinical trial of INO-4800, a DNA vaccine targeting the SARS-CoV-2 spike antigen delivered by the ID route by CELLECTRA EP. The vaccine was safe and well tolerated with only Grade 1 AEs observed, and without increased AEs post dose two. Two doses of INO-4800, delivered by the ID route by CELLECTRA EP leads to an increased immune response without added reactogenicity. All volunteers mounted an anti-spike immune response following the second dose of the vaccine.
Implications of all the available evidence
The safety profile for INO-4800 is important as there were just 6 vaccine-related Grade 1 events in 5 subjects. In addition these were distributed similarly between the two dose groups, supporting the tolerability of this vaccine across dose groups. Therefore, INO-4800′s ability to induce broad immune responses as well as favorable tolerability and product temperature stability position this vaccine attractive especially for lower/middle income countries. Finally, the safety and tolerability profile appears supportive for expanded study in diverse individuals and those where inflammatory responses may be a concern. A study in populations 51 years old and older is now in progress (NCT04336410. ClinicalTrials.gov).
Alt-text: Unlabelled box
1. Introduction
After the original cases in China in late 2019, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus that causes CoronaVirus Disease, 2019 (COVID-19) spread quickly, the World Health Organization declared a pandemic on March 24th, 2020, and within 5 months, over 23 million cases of infection and over 800,000 deaths have been reported globally. COVID-19 typically manifests as a mild infection with symptoms that may include fever, dry cough, fatigue and headache [6]. The elderly and those with co-morbid conditions are prone to experience more severe symptoms, including pneumonia and multiorgan disease. Severe and critically-ill COVID-19 patients requiring intensive care and invasive mechanical ventilation can quickly overwhelm hospitals [7]. Despite the increasing number of infections and deaths around the globe, most people remain vulnerable to infection. There is an urgent need for safe and effective vaccines. Many are in development including nucleic acid [8,9], viral vectored [10], [11], [12], and inactivated virus vaccines [13]. Most of these vaccines target the spike protein, a class I fusion protein of SARS-CoV-2 which binds to the angiotensin converting enzyme 2 (ACE2) receptor to gain entry into the host cell.
It is believed that both arms of the immune response are likely important for control and recovery from SARS-CoV-2 infection. Preclinical animal models, while imperfect, can show impact in animal challenge due to humoral responses targeting the spike antigen, as well as those induced by the cellular response [14]. While natural infection and recovery from SARS-CoV-2 is associated with generation of binding antibodies as well as antibodies that can neutralize virus in recovered individuals [15], [16], [17], antibody responses are not detectable in all recovered patients [18], [19], [20], and these antibodies tend to wain within months [18, 21]. Studies increasingly underscore the importance of T cell responses in ameliorating the severity of disease, with immunity to the spike Ag as one important immune target [[22], [23], [24], [25]]. Therefore, eliciting a well-balanced adaptive immune response could be an important hallmark of a promising vaccine candidate.
The CELLECTRA® electroporation (EP) + DNA vaccine platform has been shown to induce both cellular and humoral immune responses to multiple infectious agents with a well-tolerated safety profile [2], [3], [4] and have demonstrated efficacy against HPV associated cervical dysplasia [5]. This technology has previously been employed in the development of a vaccine candidate (INO-4700) against another betacoronavirus: the Middle Eastern Respiratory virus (MERS), targeting its spike glycoprotein. Preclinical [26] and Phase 1 studies [2] (NCT03721718) demonstrated that INO-4700 was safe and immunogenic, and efficacious in NHP challenge studies (Patel et al., submitted).
INO-4800, a DNA vaccine which encodes the SARS-CoV-2 S-protein has been developed and tested in preclinical animal models [8], demonstrating protective impact on infection in a nonhuman primate SARS-CoV-2 challenge model (Patel et al., submitted). Here, the initial findings of the first clinical trial evaluating INO-4800 delivered by intradermal injection followed by CELLECTRA® EP, designed to generate a controlled electric field in the injection site to enhance the cellular uptake and expression of the DNA plasmid, are reported.
2. Methods
2.1. Study design and participants
The clinical trial was designed as a Phase 1, open-label, multi-center trial (NCT04336410) to evaluate the safety, tolerability and immunogenicity of INO-4800 administered intradermally (ID) followed by electroporation using the CELLECTRA® 2000 device. The trial was approved by the institutional review board of each clinical site, and all participants provided written informed consent before enrollment. Healthy participants 18 to 50 years of age without a known history of COVID-19 illness received either a 1.0 mg or 2.0 mg dose of INO-4800 in a 2-dose regimen (Weeks 0 and 4). Participants enrolled at two locations in the U.S.: The University of Pennsylvania Clinical trials Unit in Philadelphia and the Alliance for Multispecialty Research in Kansas City (Details regarding inclusion and exclusion criteria and the schedule of events are provided in the protocol and are available with the full text of this article).
2.2. DNA vaccine INO-4800
The vaccine was produced according to current Good Manufacturing Practices. INO-4800 contains plasmid pGX9501 expressing a synthetic, optimized sequence of the SARS-CoV-2 full length spike glycoprotein which was optimized as previously described [8] at a concentration of 10 mg/ml in a saline sodium citrate buffer. The optimized DNA sequence encoding SARS-CoV-2 insert was created using Inovio's proprietary in silico Gene Optimization Algorithm to enhance expression. The DNA sequence changes do not impact amino acid sequence. INO-4800 is homologous to the Wuhan strain.
2.3. Endpoints
Safety endpoints included systemic and local administration site reactions up to 8 weeks post-dose 1. Immunology endpoints include antigen-specific binding antibody titers, neutralization titers and antigen-specific interferon-gamma (IFN-γ) cellular immune responses after 2 doses of vaccine. For Live Virus Neutralization, a responder is defined as Week 6 PRNT IC50 ≥ 10, or ≥4 if a subject is a responder in ELISA. For S1+S2 ELISA, a responder is defined as a Week 6 value >1. For the ELISpot assay, a responder is defined as a Week 6 or Week 8 value that is ≥12 spot forming units per 106 PBMCs above Week 0.
2.4. Study procedures
Forty participants were enrolled into two groups; 20 participants in each of 1.0 mg and 2.0 mg dose groups that received their doses on Weeks 0 and 4. The vaccine was administered in 0.1 ml intradermal injections in the arm followed by EP at the site of vaccination. Subjects in the 1.0 mg dose group received one injection on each dosing visit. The second dose of the vaccine could be injected in the same arm or a different arm relative to the first dose. Subjects in the 2.0 mg dose group received one injection in each arm at each dosing visit. EP was performed using CELLECTRA® 2000 as previously described [3, 4]. The device delivers total four electrical pulses, each 52 ms in duration at strengths of 0.2 A current and voltage of 40–200 V per pulse.
The dose groups were enrolled sequentially with a safety run-in for each. The 1.0 mg dose group enrolled a single participant per day for 3 days. An independent Data Safety Monitoring Board (DSMB) reviewed the Week 1 safety data and based on a favorable safety assessment, made a recommendation to complete enrollment of the additional 17 participants into that dose group. In a similar fashion, the 2.0 mg dose group was subsequently enrolled.
Participants were assessed for safety and concomitant medications at all time points, including screening, Week 0 (Dose 1), post dose next day phone call, Week 1, 4 (dose 2), 6, 8, 12, 28, 40 and 52 post-dose 1. Local and systemic AEs, regardless of relationship to the vaccine, were recorded and graded by the investigator. Safety laboratory testing (complete blood count, comprehensive metabolic panel and urinalysis) were and will continue to be conducted at screening, Week 1, 6, 8, 12, 28 and 52 post-dose 1. Immunology specimens were obtained at all time points post-dose 1 except at Day 1 and Week 1. AEs were graded according to the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials guidelines that were issued by the Food and Drug Administration in September 2007. The DSMB reviewed laboratory and AE data for the participants up to 8 weeks included in this report. There were protocol-specified safety stopping rules and adverse events of special interest (AESIs). For the purpose of this report, clinical and laboratory safety assessments up to 8 weeks post the first dose are presented.
2.5. Protocol eligibility
Eligible participants must have met the following criteria: healthy adults aged between 18 and 50 years; able and willing to comply with all study procedures; Body Mass Index of 18–30 kg/m2 at screening; negative serological tests for Hepatitis B surface antigen, Hepatitis C antibody and Human Immunodeficiency Virus antibody; screening electrocardiogram (ECG) deemed by the Investigator as having no clinically significant findings; use of medically effective contraception with a failure rate of < 1% per year when used consistently be post-menopausal, or surgically sterile or have a partner who is sterile. Key exclusion criteria included the following: individuals in a current occupation with high risk of exposure to SARS-CoV-2; previous known exposure to SARS-CoV-2 or receipt of an investigational product for the prevention or treatment of COVID-19; autoimmune or immunosuppression as a result of underlying illness or treatment; hypersensitivity or severe allergic reactions to vaccines or drugs; medical conditions that increased risk for severe COVID-19; reported smoking, vaping, or active drug, alcohol or substance abuse or dependence; and fewer than two acceptable sites available for intradermal injection and electroporation.
2.6. Immunogenicity assessment methods
For this report, samples collected at screening, Week 0 (prior to dose) and at Weeks 6 and 8 were analyzed. Peripheral Blood Mononuclear Cells (PBMCs) were isolated from blood samples by a standard overlay on ficoll hypaque followed by centrifugation. Isolated cells were frozen in 10% DMSO and 90% fetal calf serum. The frozen PBMCs were stored in liquid nitrogen for subsequent analyses. Serum samples were stored at −80 C until used to measure binding and neutralizing antibody titers.
2.6.1. SARS-CoV-2 wildtype virus neutralization assays
SARS-CoV-2/Australia/VIC01/2020 isolate neutralization assays were performed at Public Health England (Porton Down, UK) [10]. Neutralizing virus titers were measured in serum samples that had been heat-inactivated at 56 °C for 30 min. SARS-CoV-2 (Australia/VIC01/2020 isolate44) was diluted to a concentration of 933 pfu/ml and mixed 50:50 in 1% FCS/MEM containing 25 mM HEPES buffer with doubling serum dilutions. After a 1 h incubation at 37 °C, the virus-antibody mixture was transferred to confluent monolayers of Vero E6 cells (ECACC 85020206; PHE, UK). Virus was allowed to adsorb onto cells at 37 °C for a further hour in an incubator, and the cell monolayer was overlaid with MEM/4% FBS/1.5% CMC. After 5 days incubation at 37 °C, the plates were fixed, stained, with 0.2% crystal violet solution (Sigma) in 25% methanol (v/v). Plaques were counted.
2.6.2. S1±S2 enzyme-linked immunosorbent assay (ELISA)
ELISA plates were coated with 2.0 µg/mL recombinant SARS-CoV-2 S1+S2 spike protein (Acro Biosystems; SPN-C52H8) and incubated overnight at 2–8 °C. The S1+S2 contains amino acids residues Val 16 – Pro 1213 of the full length spike protein, GenBank # QHD43416.1. It contains two mutations to stabilize the protein to the trimeric prefusion state (R683A, R685A) and also contains a C-terminal 10× His tag. The plates were then washed with PBS with 0.05% Tween-20 (Sigma; P3563) and blocked (Starting Block, Thermo Scientific; 37,538) for 1–3 h at room temperature. Samples were serially diluted using blocking buffer and were added in duplicate, along with prepared controls, to the washed and blocked assay plates. The samples were incubated on the blocked assay plates for one hour at room temperature. Following sample and control incubation, the plates were washed and a 1/1000 preparation of anti-human IgG HRP conjugate (BD Pharmingen; 555,788) in blocking buffer was then added to each well and allowed to incubate for 1 h at room temperature. The plates were washed and TMB substrate (KPL; 5120-0077) was then added and allowed to incubate at room temperature for approximately 10 min. TMB Stop Solution (KPL; 5150-0021) was next added and the plates read at 450 nm and 650 nm on a Synergy HTX Microplate Reader (BioTek). The magnitude of the assay response was expressed as titers which were defined as the greatest reciprocal dilution factor of the greatest dilution serial dilution at which the plate corrected optical density is 3 SD above background a subject's corresponding Week 0.
2.6.3. SARS-CoV-2 spike ELISpot assay description
Peripheral mononuclear cells (PBMCs) pre- and post-vaccination were stimulated in vitro with 15-mer peptides (overlapping by 9 residues) spanning the full-length consensus spike protein sequence. Cells were incubated overnight in an incubator with peptide pools at a concentration of 5 μg per ml in a precoated ELISpot plate, (MabTech, Human IFN-g ELISpot Plus). The next day, cells were washed off, and the plates were developed via a biotinylated anti-IFN-γ detection antibody followed by a streptavidin-enzyme conjugate resulting in visible spots. Each spot corresponds to an individual cytokine-secreting cell. After plates were developed, spots were scanned and quantified using the CTL S6 Micro Analyzer (CTL) with ImmunoCapture and ImmunoSpot software. Values are shown as the background-subtracted average of measured triplicates. The ELISpot assay qualification determined that 12 spot forming units was the lower limit of detection. Thus, anything above this cutoff is considered to be a signal of an antigen specific cellular response.
2.6.4. INO-4800 SARS-CoV-2 spike flow cytometry assay
PBMCs were also used for Intracellular Cytokine Staining (ICS) analysis using flow cytometry. One million PMBCs in 200 µL complete RPMI media were stimulated for six hours (37 °C, 5% CO2) with DMSO (negative control), PMA and Ionomycin (positive control, 100 ng/mL and 2 μg/mL, respectively), or with the indicated peptide pools (225 ug/mL). After one hour of stimulation, Brefeldin A and Monensin (BD GolgiStop and GolgiPlug, 0.001% and 0.0015%, respectively) were added to block secretion of expressed cytokines. After stimulation the cells were moved to 4 °C overnight. Next, cells were washed in PBS for live/dead staining (Life Technologies Live/Dead aqua fixable viability dye), and then resuspended in FACS buffer (0.5% BSA, 2 mM EDTA, 20 mM HEPES). Next, extracellular markers were stained, the cells were fixed and permeablized (eBioscience™ Foxp3 Kit) and then stained for the indicated cytokines (Table S2) using fluorescently-conjugated antibodies. Fig. S1A shows representative gating strategies for CD4+ and CD8+ T cells as well as examples of positive expression of IFNγ, TNFα, IL-2 and IL-4.
2.7. Statistical analysis
No formal power analysis was applicable to this trial. Descriptive statistics were used to summarize the safety end-points: proportions with AEs, administration site reactions, and AESIs through 8 weeks. Descriptive statistics were also used to summarize the immunogenicity endpoints: median responses (with 95% confidence intervals) and percentage of responders for cellular results, and geometric mean titers (with 95% confidence intervals) and percentage of responders for humoral results. Post-hoc analyses of post-vaccination minus pre-vaccination paired differences in SARS-CoV-2 neutralization responses (on the natural log-scale, with a paired t-test), ELISpot responses (with Wilcoxon signed-rank tests), and Intracellular Flow Assay responses (with Wilcoxon signed-rank tests) were performed.
2.8. Role of funding sources
The COVID19-001 Phase 1 clinical study is in part funded by the Coalition for Epidemic Preparedness Innovations (CEPI). CEPI had not role in the study design, collection, analysis, interpretation of the preliminary study data, writing of the interim report and decision to submit the manuscript for publication to EClinicalMedicine. Furthermore, all authors had full access to all the preliminary data in the study and accept responsibility to submit for publication.
3. Results
3.1. Study population demographics
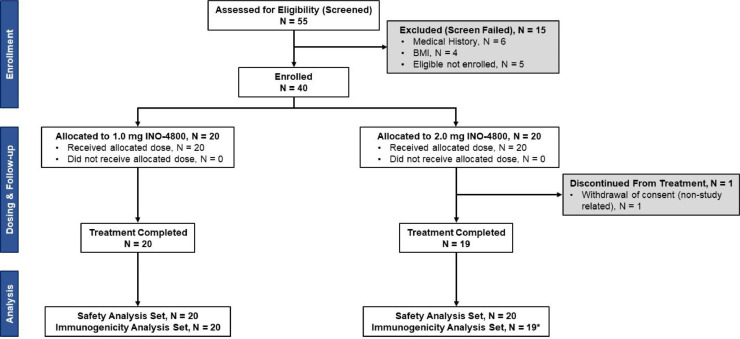
Between 06 April 2020 and 23 April 2020, a total of 55 participants were screened and 40 participants were enrolled into the initial two groups (Fig. 1). The median age was 34.5 years (range 18 to 50 years). Participants were 55% (22/40) male (Table S1). Most participants were white (82.5%, 33/40).
Fig. 1.
Consort flow diagram.
*One subject in the 2.0 mg dose group was not able to secure consistent transportation and therefore discontinued.
3.2. Vaccine safety and tolerability
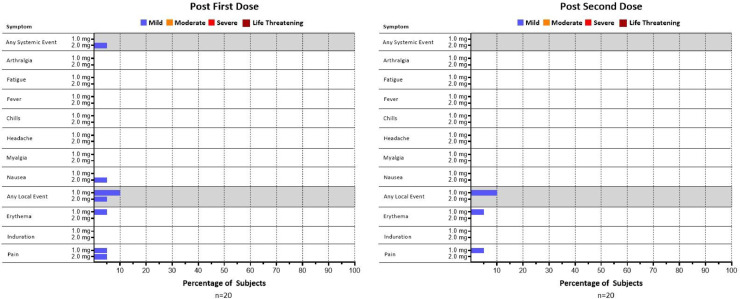
A total of 39 of 40 (97.5%) participants completed both doses; one participant in the 2.0 mg group discontinued trial participation prior to receiving the second dose due to lack of transportation to the clinical sites, and discontinuation was unrelated to the study or the dosing (Fig. 1). All 39 remaining subjects completed the visit 8 weeks post-dose 1. There was a total of 11 local and systemic adverse events (AEs) reported by 8 weeks post-dose 1; six of these were deemed related to vaccine (Table S3). All AEs were Grade 1 (mild) in severity. Five of the six related AEs were injection site reactions including injection site pain (3) and erythema (2). One Grade 1 systemic AE related to the vaccine was nausea. All related AEs occurred on the dosing day when the subjects received the first or second vaccination. There were no febrile reactions and no antipyretic medicine was used post vaccination. No subject discontinued the trial due to an AE. No serious adverse events (SAEs) nor adverse events of special interest (AESIs) were reported. There were no abnormal laboratory values that were deemed clinically significant by the Investigators throughout the initial 8-week follow-up period. There was no increase in the number of participants who experienced AEs related to the vaccine in the 2.0 mg group (10%,2/20), compared to that in the 1.0 mg group (15%, 3/20) (Fig. 2). In addition, there was no increase in frequencies of AEs with the second dose over the first dose in both dose groups.
Fig. 2.
Related systemic and local adverse events in severity of mild (Grade 1), moderate (Grad 2), severe (Grade 3) and life-threatening (Grade 4).
3.3. Immunogenicity
Thirty-eight subjects were included in the immunogenicity analyses. In addition to one subject in the 2.0 mg group who discontinued prior to completing dosing, one subject in the 1.0 mg group was deemed seropositive at baseline and was excluded. Data for this subject can be found in the Supplement (Table S5).
3.3.1. Humoral immune responses
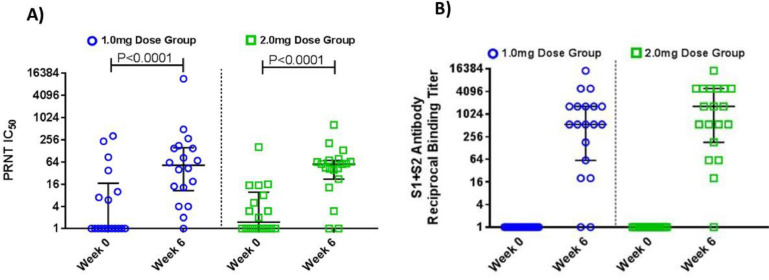
Sera was tested for the ability to bind S1+S2 spike protein. 89% (17/19) of participants in the 1.0 mg group and 95% (18/19) of participants in the 2.0 mg group had an increase in serum IgG binding titers to S1+S2 spike protein when compared to their pre-vaccination time point (Week 0), with the responder GMT of 655.5 (95% CI: 255.6,1681.0) and 994.2 (95% CI: 395.3, 2500.3) in the 1.0 mg and 2.0 mg groups, respectively (Fig. 3B, Fig. S3 and Table 1). Sera was also tested for the ability to neutralize live virus by live virus PRNT IC50 neutralization assay. The geometric mean fold-rise at Week 6 relative to baseline was 10.8 with a 95% CI of (4.4, 27.0) and 11.5 with a 95% CI of (5.3, 24.9) in the 1.0 mg and 2.0 mg groups, respectively. In each group, there was a statistically significant increase at Week 6 over baseline (P<0.0001 paired t-test, post-hoc analysis), Fig. 3A. At Week 6, the percentage of responders were 78% (14/18) and 84% (16/19) in the 1.0 mg and 2.0 mg groups, respectively (Fig. 3A and Table 1), and the responder geometric mean titer (GMT) were 102.3 (95% CI: 37.4, 280.3) and 63.5 (95% CI: 39.6, 101.8) in the 1.0 mg and 2.0 mg groups, respectively. Overall seroconversion (defined as those participants who respond with neutralization and/or binding antibodies to S protein) at Week 6 in 1.0 mg and 2.0 mg dose group were 95% (18/19) for each group (Table 1).
Fig. 3.
Humoral antibody response. The humoral response in the 1.0 mg dose group and 2.0 mg dose group was assessed for the ability to A) neutralize of live virus, (n = 18, 1.0 mg; n = 19, 2.0 mg). B) and binding to whole spike protein (S1 and S2) (n = 19, 1.0 mg; n = 19, 2.0 mg). End point titers for binding antibodies were calculated as the titer that exhibited an OD 3.0 SD above baseline, titers at baseline were set at 1 to normalize the post-baseline results. A response to live virus neutralization was a PRNT IC50 ≥ 10. In all graphs horizontal lines represent the Median and bars represent the Interquartile.
Table 1.
Immune responses.
| INO-4800 1.0 mg dose group |
INO-4800 2.0 mg dose group |
|||||
|---|---|---|---|---|---|---|
| Immuneassay | Overall value | Responder value | Respondersa (%) | Overall value | Responder value | Respondera (%) |
| Neutralization Week 6 GMT####Reciprocal Titer####[95% CI] (Range) | 44.4####[14.6, 134.8]####(1, 11,647) | 102.3####[37.4, 280.3]####(13, 11,647) | 14/18 (78%) | 34.9####[15.8, 77.2]####(1, 652) | 63.5####[39.6, 101.8]####(13,652) | 16/19 (84%) |
| S1+S2 Binding Antibody Week 6 GMT Reciprocal Titer####[95% CI] (Range) | 331.2####[91.2, 1203.2]####(1, 14,580) | 655.5####[255.6, 1681.0]####(20, 14,580) | 17/19 (89%) | 691.4####[217.5, 2197.2]####(1, 14,580) | 994.2####[395.3, 2500.3]####(20, 14,580) | 18/19 (95%) |
| Total Seroconversion (Response in S1+S2 or Neutralization) | N/A | N/A | 18/19 (95%) | N/A | N/A | 18/19 (95%) |
| IFN-gamma ELISpot Week 8####Median SFU####[95% CI] (Range) | 26.2####[10.0, 64.4]####(1, 374.4) | 45.6####[21.1, 142.2]####(16.7, 374.4) | 14/19 (74%)µ | 71.1####[32.2, 194.4]####(8.9, 615.6) | 71.1####[32.2, 194.4]####(8.9, 615.6) | 19/19 (100%)µ |
| Overall Immune Response Rate (Seroconversion or ELISpot) | N/A | N/A | 19/19 (100%) | N/A | N/A | 19/19 (100%) |
1.0 mg Dose Group excludes one subject with baseline positive NP ELISA.
Response criteria: Live Neutralization – Week 6 PRNT IC50 ≥ 10, or ≥4 if binding ELISA activity is seen; S1±S2 Binding – Week 6 value >1 ELISpot – Value ≥12 SFU over Week 0
µ – Responders based on Week 6 or Week 8 data.
3.3.2. Enzyme-linked immunospot (ELISpot)
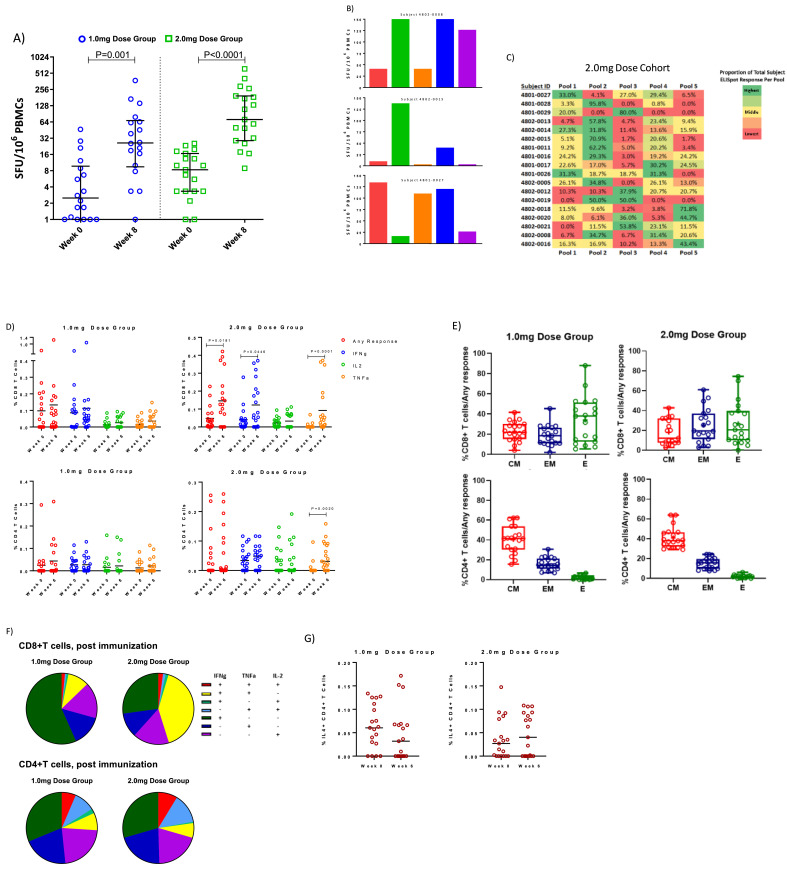
The percentage of responders at week 8 was 74% (14/19) in the 1.0 mg dose group, and 100% (19/19) in the 2.0 mg dose group. These data taken with the seroconversion data result in a 100% (19/19) overall immune response in each group (Table 1, Figs. 4A and S2). The Median SFU per 106 PBMC was 46 (95% CI: 21.1, 142.2) and 71 (95% CI: 32.2–194.4) for the responders in 1.0 mg and 2.0 mg dose groups, respectively. The median change at week 8 relative to baseline was 22.3 (95% CI: 2.2, 63.4) and 62.8 (95%CI: 22.2, 191.1) in the respective groups, and in each group, there were statistically significant increases over baseline (P = 0.001 and P<0.0001, respectively, Wilcoxon matched-pairs signed rank test, post-hoc analysis), Fig. 4A. It is also interesting to note that 3 convalescent samples (all 3 with symptoms but non-hospitalized), tested by the ELISpot assay showed lower T cell responses, with a median of 33, than the 2.0 mg dose group at Week 8 (Supplementary Fig. S3). As shown in Fig. 4B and C, the 2.0 mg group's T cell responses were mapped to 5 epitope pools. Encouragingly, T cell responses were seen in all regions of the spike protein, with the dominant pool encompassing the Receptor Binding Domain region, followed by pools covering the N Terminal Domain, as well as the Fusion Peptide, Heptad Repeat 1 and the Central Helix.
Fig. 4.
Cellular immune response: PBMCs isolated from vaccinated individuals were stimulated in vitro with SARS-CoV-2 spike antigen. The number of cells capable of secreting IFN-gamma were measured in a standard ELISpot assay for the A) 1.0 mg dose group and 2.0 mg dose group. Horizontal lines represent Medians and bars represent Interquartile Ranges. B) Peptides spanning the entirety of the spike antigen were divided into pools and tested individually in ELISpot, with pools mapped to specific regions of the antigen represented by color. Three subjects are shown exemplifying the diversity of pool responses and associated magnitude across subjects. The pie chart represents the diversity of entirety of the 2.0 mg dose group C) A heat map of each subject in the 2.0 mg dose group and the percentage of their ELISpot response dedicated to each pool covering the SARS-CoV-2 spike antigen. D) SARS-CoV-2 spike specific cytokine production was measured from CD4+ and CD8+ T cells via flow cytometry. Bars represent Mean response. E) Cytokine production is additionally broken out using CCR7 and CD45RA into Central Memory (CM), Effector Memory (EM) or Effector (E) differentiation status with data conveying what percentage of the overall cytokine response originates from what differentiated group. F) Pie charts represent the polyfunctionality of CD4+ and CD8+ T cells for each dose cohort. G) IL-4 production by CD4+ T cells for each dose cohort. Horizontal lines represent Mean response. Graphs represent all evaluable subjects. Statistical analyses were performed on all paired datasets. Those that were significant are noted within the figure; lack of notation in the figure represents lack of statistical significance.
3.3.3. Intracellular flow assay
The contribution of CD4+ and CD8+ T cells to the cellular immune response against INO-4800 was assessed by intracellular cytokine staining (ICS). In the 2.0 mg dose group, the median change from baseline to Week 6 in CD8+ T cells producing IFN-ɣ, TNF-α and/or IL-2 (Any Response) was 0.11 with a 95% CI of (−0.02, 0.23); the change was significantly increased(P = 0.0181, Wilcoxon matched-pairs signed rank test, post-hoc analysis). owing chiefly to significant increases in IFN-ɣ as well as TNF-α production (Fig. 4D). Also in the 2.0 mg dose group, the median change from baseline to Week 6 in CD4+T cells producing TNF-α was 0.02 with a 95% CI of (0.01 to 0.09); the change was also significantly increased (P = 0.0020, Wilcoxon matched-pairs signed rank test, post-hoc analysis, Fig. 4D).
The composition of CD4+ or CD8+ T cells producing any cytokine (IFN-ɣ or TNF-α or IL-2 following vaccination) was also assessed for surface markers CCR7 and CD45RA to characterize effector (CCR7−CD45RA+), effector memory (CCR7−CD45RA−), and central memory (CCR7+CD45RA−) cells (Fig. 4E). In both dose groups, CD8+T cells producing cytokine in response to stimulation with SARS-CoV-2 spike peptides were generally balanced across the three populations, whereas CD4+ T cells were predominantly of the central memory phenotype (Fig. 4E).
CD4+ and CD8+ T cells following vaccination were further explored for their ability to produce more than one cytokine at a time and were encouraged to note that nearly half (41%) of the CD8+T cells in the 2.0 mg dose group were dual producing IFN-ɣ and TNF-α (Fig. 4F). CD8+ T cells producing cytokine in the 1.0 mg dose group were primarily monofunctional IFN-ɣ producing cells (57%). The CD4+ T cell compartment was also polyfunctional in nature with 6% and 9%, in the 1.0 mg and 2.0 mg dose groups, respectively, producing all 3 cytokines, IFN-ɣ, TNF-α, and IL-2 (Table S4).
Th2 responses were also measured by assessing IL-4 production, and no statistically significant increases (Wilcoxon matched-pairs signed rank test, post-hoc analysis) were observed in either group post vaccination (Fig. 4G).
4. Discussion
This report provides initial data from a Phase 1 trial on the safety, tolerability and immunogenicity of INO-4800, a SARS-CoV-2 vaccine encoding the spike protein (S). INO-4800 was well tolerated with a frequency of product-related Grade 1 AEs of 15% (3/20 subjects) and 10% (2/20 subjects) of the participants in 1.0 mg and 2.0 mg dose group, respectively. Only Grade 1 AEs were noted in the study, which compares favorably with existing licensed vaccines. The safety profile of a successful COVID-19 vaccine is important and supports broad development of INO-4800 in at-risk populations who are at more serious risk of complications from SARS-CoV-2 infection, including the elderly and those with comorbidities.
INO-4800 also generated balanced humoral and cellular immune responses with all 38 evaluable participants displaying either or both antibody or T cell responses following two doses of INO-4800. Humoral responses measured by binding or neutralizing antibodies were observed in 95% (18/19) of the participants in each dose group. The neutralizing antibodies, measured by live virus neutralization assay, were seen in 78% (14/18) and 84% (16/19) of participants, and the corresponding GMTs were 102.3 [95% CI (37.4, 280.3)] and 63.5 [95% CI (39.6, 101.8)] for the 1.0 mg and 2.0 mg dose groups, respectively. The range overlaps that of the PRNT IC50 titers reported from convalescent patients [27,10] as well as the PRNT IC50 titers in NHPs which were protected in a SARS-CoV-2 challenge [11]. Furthermore, there was a statistically significant increase in titers. It is important to note that all but one vaccine recipient that did not develop neutralizing antibody titers responded positively in the T cell ELISpot assay, suggesting that the immune responses generated by the vaccine are registering differentially in these assays. Cellular immune responses were observed in 74% (14/19) and 100% (19/19) of 1.0 mg and 2.0 mg dose groups, respectively. Importantly, INO-4800 generated T cell responses that were more frequent and with higher responder median responses (46 [95% CI (21.1, 142.2)] vs. 71 [95% CI (32.2, 194.4)] SFU 106 PBMC) in the 1.0 mg and 2.0 mg dose groups respectively. These T cell responses in the 2.0 mg dose group were higher in magnitude than convalescent samples tested (Fig. 4A) and were similar or greater responses to those previously reported for other vaccine candidates [9, 10, 28, 29], although the results should be interpreted in the context of variability of the immunological responses after natural infection and among different trials. Furthermore, there was a statistically significant increase in SFU. In the flow cytometric assays, both the 1.0 mg and 2.0 mg Dose Groups showed increases in cytokine production from both the CD4+ and CD8+ T cell compartments, especially in the 2.0 mg group. The 2.0 mg group exhibited a number of statistically significant cytokine outputs, including IFN-ɣ and TNF-α and “any cytokine” from the CD8+ T cell compartment and TNF-α from the CD4+ T cell compartment (Fig. 4D). Of considerable importance is that CD8+ T cell responses in the 2.0 mg dose group were dominated by cells expressing both IFN-ɣ and TNF-α with or without IL-2 (Fig. 4F and Table S4). In total, these cells amounted to nearly half of the total CD8+ T cell response (42.7%, Table S4). The contribution of this set of cytokines in the context of multi-cytokine production from CD8+ T cells appears to exceed those from previously reported vaccine studies [9, 10]. The importance of such cells in mediating COVID-19 disease is underscored in a number of clinical studies [22, [30], [31], [32]] including a recent study which reported that recovered COVID-19 patients demonstrated a substantial frequency of CD8+ T cells expressing IFN-ɣ that also expressed TNF-α [22]. Additionally, a comprehensive review of currently available clinical data puts forth a model for mild vs severe COVID disease in which the presence of IFN-ɣ and TNF-α producing CD8+ T cells is proposed to be associated with a positive clinical outcome [33].
In addition to the encouraging immune responses shown this study, one attractive feature of DNA vaccines like INO-4800 is that the immunizations could be boosted without significant limitations such as anti-vector responses or dosing-incremented toxicities, and additional boosting with other DNA vaccines have resulted in higher levels of humoral and cellular immune responses without increased toxicity [2], [3], [4], [5]. The INO-4800 Phase 1 safety data further suggest that the vaccine could be a safe booster as there was no increase in frequency of side effects after the second dose compared to the first dose, an important aspect for the safety profiles of SARS-CoV-2 vaccines. Given the uncertainty about the durability of the natural infection or vaccine induced responses against COVID 19 disease, vaccine boosting by a benign approach may be an important way to maintain protection over subsequent epidemic waves of COVID 19. It is also possible that INO-4800 could serve as a useful booster shot for other S protein-targeted vaccine candidates with limitations in boosting ability. In addition, planning is underway to further test if INO-4800 could provide booster immunity for COVID-19 recovered patients whose immunity is reported to wain rapidly. Many such subjects include persons in high risk groups who would especially benefit from longer term immune protection.
A limited set of early studies in animal models of SARS-CoV and MERS-CoV infection have raised potential safety concerns about the possibility of vaccine-associated enhanced respiratory disease. Vaccines which could generate potent neutralizing antibody responses as well as Th1-biased T-cell responses might reduce the risk of vaccine-associated enhanced disease or antibody-dependent enhancement of replication. Vaccination with INO-4800 in both mouse and rhesus macaque challenge studies has demonstrated that the vaccine can generate a balanced neutralizing antibody and T cell immune response. The vaccine is protective in mouse challenge studies (manuscript in preparation). Interestingly, the memory T and B cell immune responses in rhesus macaques led to anamnestic protection against SARS-CoV-2 challenge 13 weeks from last vaccination (manuscript submitted). In this Phase 1 trial, INO-4800 vaccination led to substantial T cell responses with increased Th1 phenotype, measured by both IFN-ɣ ELISpot as well as multiparametric flow cytometry, as evidenced by increased expression of Th1-type cytokines IFN-ɣ, TNF-α, and IL-2 (Fig. 4D). Assessment of cellular responses induced by INO-4800 displayed the presence of SARS-CoV-2 specific CD4+ and CD8+ T cells exhibiting hallmarks of differentiation into both central and effector memory cells, suggesting that a persistent cellular response has been established (Fig. 4E). Importantly, this was accomplished while minimizing induction of IL-4, a prototypical Th2 cytokine (Fig. 4G), supporting that this vaccine has an immune phenotype, along with induction of protection in preclinical models, which makes it unlikely to be a risk for induction of enhanced disease.
This first-in-human trial of SARS-CoV-2 DNA vaccine has some limitations. First, this Phase 1 trial report is based on a modest sample size (40), and larger sample-sized trials may be needed to show the true immunogenicity difference between the dose groups. Second, this report only involves healthy volunteers aged from 18 to 50 years old. Severe and fatal cases of COVID-19 disproportionally affect older individuals. In this regard, the Phase 1 trial has been expanded to include cohorts of participants who are 51 years old and older. Third, only data within the first 56 days of vaccination is being reported, and this report does not include data about the durability of the vaccine-induced immunity, which was not available at the time of publication. In previous clinical trials with similar DNA vaccines, durable immune responses up to 1 year after the initial vaccination were reported [2, 3]. The clinical plan is to follow the current Phase 1 participants for 12 months for long-term safety as well as to measure the durability of immune response. Lastly, it is interesting that one of the volunteers in 1.0 mg dose group was seropositive at baseline, indicating that the person had been previously infected by the virus. This person, who had received both doses of vaccine as scheduled, did not have any AEs. A separate study of INO-4800 in seropositive individuals is planned for the future.
The development of a safe and effective vaccine remains the ultimate goal of preventive efforts against COVID-19. Multiple vaccine candidates and platforms are being tested, and it is unlikely that a single platform will prove suitably safe, effective, and logistically feasible, in terms of cold chain distribution, in all segments of the global population. Our data suggest that INO-4800 demonstrates a pristine safety profile and that immunization induces both humoral and cellular responses, supporting its further development to prevent infection, disease, and death in the global population. The safety profile could potentially make it a preferred vaccine option for high-risk populations, such as the elderly and those living with co-morbid conditions. The study of this vaccine's efficacy is planned for additional trials.
Declaration of Interests
SY, JDB, ACQ, VMA, MPM, KK, JA, AS, JP, EG, IM, PP, KS, TRFS, SR, TMcM, MD, EB, MPM, JL, MD, ASB, JES, JJK, KEB and LMH report grants from Coalition for Epidemic Preparedness Innovations, during the conduct of the study; other from Inovio Pharmaceuticals, outside the submitted work.
PT, ELR, AP, MP, FIZ, KYK, YD, DF, KB, MWC, JE and DBW report grants from Coalition for Epidemic Preparedness Innovations, during the conduct of the study.
Acknowledgments
Funding
This work is funded by Coalition for Epidemic Preparedness Innovations (CEPI).
Data sharing
The preliminary COVID19-001 Phase 1 clinical study datasets are subject to access restriction to protect subject confidentiality as the clinical study is still ongoing.
Acknowledgments
The investigators express their gratitude for the contribution of all the trial participants and the invaluable advice of the international Data Safety Monitoring Board. We also acknowledge the broader support from the various teams within Inovio: Greta Kcomt Del Rio, BS; Neiman Liu, MS, Alysia Ryan, BS; Dennis Van De Goor, MS; David Valenta, PhD; Snehal Wani, MS; EJ Brandreth, MBA; Dan Jordan, BS; Robert J. Juba Jr, MS, Stephen Kemmerrer, BSME, MBA, PE; Timothy Herring, MPH, Susan Duff, BS, the University of Pennsylvania: Sukyung Kim, RN, PhD; Alan Wanicur, BS; Zuleika Guzman, BS, the Wistar Institute: Dr. Ziyang Xu and Edgar Tello Ruiz; National Infections Service, Public Health England: Naomi Coombes, PhD; Mike Elmore, PhD and the Alliance for Multispecialty Research, Kansas City.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100689.
Appendix. Supplementary materials
References
- 1.De Rosa S.C., Edupuganti S., Huang Y. Robust antibody and cellular responses induced by DNA-only vaccination for HIV. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modjarrad K., Roberts C.C., Mills K.T. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tebas P., Kraynyak K.A., Patel A. Intradermal SynCon(R) ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis. 2019;220:400–410. doi: 10.1093/infdis/jiz132. [DOI] [PubMed] [Google Scholar]
- 4.Tebas P., Roberts C.C., Muthumani K. Safety and immunogenicity of an anti-Zika virus DNA vaccine – preliminary report. N Engl J Med. 2017;(10.1056/NEJMoa1708120) doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimble C.L., Morrow M.P., Kraynyak K.A. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith T.R.F., Patel A., Ramos S. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson L.A., Anderson E.J., Rouphael N.G. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercado N.B., Zahn R., Wegmann F. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan M.J., Lyke K.E., Kitchin N. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 13.Gao Q., Bao L., Mao H. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbink P., Mercado N.B., Nkolola J.P. Lack of therapeutic efficacy of an antibody to alpha4beta7 in SIVmac251-infected rhesus macaques. Science. 2019;365:1029–1033. doi: 10.1126/science.aaw8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotez P.J., Corry D.B., Strych U., Bottazzi M.E. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol. 2020;20:399–400. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbiani D.F., Gaebler C., Muecksch F. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seydoux E., Homad L.J., MacCamy A.J. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation. Immunity. 2020;53:98–105. doi: 10.1016/j.immuni.2020.06.001. .e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 19.Mallapaty S. What the cruise-ship outbreaks reveal about COVID-19. Nature. 2020;580:18. doi: 10.1038/d41586-020-00885-w. [DOI] [PubMed] [Google Scholar]
- 20.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection – challenges and implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 21.Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. Nat Microbiol 2020;5:1598--1607. [DOI] [PMC free article] [PubMed]
- 22.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiskopf D., Schmitz K.S., Raadsen M.P. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni L., Ye F., Cheng M.L. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. .e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-Cell 2020;183:158--68. [DOI] [PMC free article] [PubMed]
- 26.Muthumani K., Falzarano D., Reuschel E.L. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7:301ra132. doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing Antibody Responses in COVID-19 Convalescent Sera. medRxiv. J Infect Dis 2020. doi:10.1093/infdis/jiaa673. [DOI] [PMC free article] [PubMed]
- 28.Zhu F.C., Guan X.H., Li Y.H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu F.C., Li Y.H., Guan X.H. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T, Wong P, Ellingson M, et al. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. medRxiv. 2020: 2020.2006.2006.20123414.
- 32.Mazzoni A., Salvati L., Maggi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020:130. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.