Abstract
The clinical and public health utility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serologic testing requires a better understanding of the dynamics of the humoral response to infection. To track seroconversion of IgG and IgM antibodies in patients with SARS-CoV-2 infection and its association with patient and clinical factors and outcomes. Residual patient specimens were analyzed on the Abbott ARCHITECT i2000 instrument using the Abbott SARS-CoV-2 IgG assay and prototype SARS-CoV-2 IgM assay. Age, sex, comorbidities, symptom onset date, mortality, and specimen collection date were obtained from electronic medical records. Three hundred fifty-nine longitudinal samples were collected from 89 hospitalized patients 0 to 82 days postsymptom onset. Of all, 51.7% of the patients developed IgG and IgM antibodies simultaneously; 32.8% seroconverted for IgM before IgG. On average, patients seroconverted for IgG by 8 days and for IgM by 7 days postsymptom onset. All patients achieved IgG seropositivity by 19 days and IgM seropositivity by 17 days. Median time to IgG and IgM seroconversion was prolonged and initial levels of IgG were lower in immunocompromised patients and patients <65 years of age compared to immune competent patients and those ≥65 years of age. Immunocompromised patients also had persistently lower levels of IgM that peaked on day 17.6 and decreased thereafter compared to immune competent patients. IgM seroconversion in patients who died reached significantly higher levels later after symptom onset than in those who recovered. SARS-CoV-2 infected patients have similar time to seroconversion for IgG and IgM. However, differences in immune status and age alter time to seroconversion. These results may help guide serologic testing application in COVID-19 management.
Keywords: SARS-CoV-2 serology, Seroconversion, Serologic assay, COVID-19, Humoral immune response
1. Introduction
Widespread, rapid, and accurate diagnostic testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical for tracking infection incidence and informing mitigation strategies to limit the spread of infection (Centers for Disease Control and Prevention, 2020). The utility of serologic testing to detect humoral immune responses to the virus—namely, the presence and levels of immunoglobulin (Ig)G, IgM, and total Ig—is an area of intense investigation. SARS-CoV-2 serologic testing may be useful for confirming diagnosis in symptomatic patients presenting outside of the window of positivity for polymerase chain reaction (PCR)-based SARS-CoV-2 tests, identifying convalescent plasma donors, evaluating immune responses and the efficacy of vaccine candidates, and establishing seroprevalence at the population level (Centers for Disease Control and Prevention, 2020; Havers et al., 2020; Infectious Diseases Society of America, 2020; Long et al., 2020).
Several studies have examined the timing of SARS-CoV-2 antibody seroconversion relative to symptom onset with discrepant results (Guo et al., 2020; Long et al., 2020; Zhao et al., 2020). Differences in SARS-CoV-2 seroconversion dynamics across studies are related to assay design, including the selection of the antigen target, infection severity, or other comorbidities. Furthermore, few studies have assessed the utility of seroconversion profiles to predict infection severity or outcomes following SARS-CoV-2 infection. More information about the dynamics of the early humoral immune response is needed to realize the full potential of serological testing for SARS-CoV-2 (Farnsworth and Anderson, 2020; Theel et al., 2020).
We examined IgG and IgM seroconversion in a cohort of SARS-CoV-2‒infected patients. Patient residual serum and plasma samples were collected at multiple timepoints postsymptom onset and SARS-CoV-2 IgG and IgM seroconversion profiles were compared based on immune status, survival, age, and sex.
2. Materials and methods
2.1. Specimens
Montefiore and Washington University Institutional Review boards approved the use of deidentified residual specimens and patient data. Specimens were collected as part of routine clinical care between March 21, 2020 and July 20, 2020, from patients hospitalized with a PCR-confirmed Coronavirus Disease 19 (COVID-19) diagnosis at Montefiore Medical Center (Bronx, NY) or Washington University Medical Center (St. Louis, MO). All specimens were collected in speckled red top serum tubes (BD, Franklin Lakes, NJ) or EDTA plasma tubes (BD). Specimens were stored at 4 °C for up to 5 days. Specimens were then centrifuged, and serum or plasma aliquoted and frozen at −80 °C until analysis. Residual specimens were deidentified and transported to Abbott Diagnostics (Abbott Park, IL) for serological testing. Age, sex, comorbid conditions, immune status, symptom onset date, mortality, and collection date were obtained from electronic medical records. Patients were considered immunocompromised if they had a history of solid organ transplant, bone marrow transplant, HIV, prolonged use of corticosteroids or other immune altering medications noted in the electronic medical records (Centers for Disease Control and Prevention, 2020).
2.2. Diagnostic testing
Diagnosis of SARS-CoV-2 infection was adjudicated by PCR-based testing using various tests approved under Emergency Use Authorization (EUA), listed in Supplementary Table 1.
2.3. Serological testing
All patient specimens were analyzed on the Abbott ARCHITECT i2000 instrument using the EUA Abbott SARS-CoV-2 IgG assay and the Abbott prototype SARS-CoV-2 IgM assay, per the manufacturer's instructions. A minimum of 100 µL of residual specimen was used for each assay. While both assays are intended for qualitative determination of antibodies to SARS-CoV-2, they report a semiquantitative signal using chemiluminescent microparticles to detect IgG and IgM binding to the SARS-CoV-2 nucleocapsid protein and spike protein, respectively. Assay results are reported as an index value of the ratio of specimen to calibrator absorbance (S/C or S/CO). Per the manufacturer's recommendations, an index value of ≥1.4 S/C indicates IgG seropositivity and ≥1.0 S/CO indicates IgM positivity. The sensitivity and specificity of the ARCHITECT IgG test (Bryan et al., 2020; Tang et al., 2020; Theel et al., 2020) and prototype IgM test (Ng et al., 2020) have been reported previously.
2.4. Data and statistical analysis
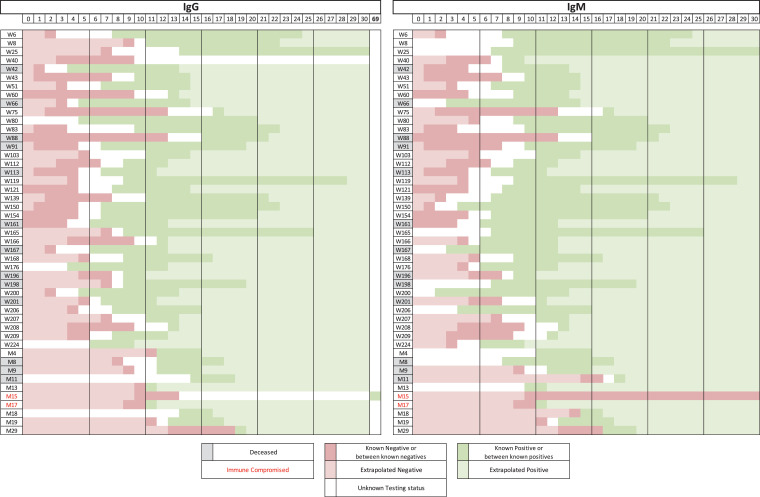
Seroconversion was calculated by plotting seropositive and negative timepoints in heatmaps (Fig. 1 ). Presumed seropositive days were defined as days beyond positive tests, up to 30 days. Presumed seronegative days were defined as days between symptom onset and first negative day. Percent seroconversion was calculated by totaling the number of patients with known or presumed seropositivity divided by the total number of patients. Seroconversion was calculated from the date of symptom onset. Time from PCR-positive to seroconversion was not assessed since previous studies have demonstrated a wide range from symptom-onset to ED presentation and diagnostic testing, grossly overestimating the sensitivity and time to seroconversion at early timepoints (Tang et al., 2020).
Fig. 1.
Heat map showing time to IgG and IgM seroconversion plots for each patient. Known positive days (days between positive tests) are shown in dark green and presumed seropositive days (days beyond positive tests up to 30 days) are shown in light green. Known seronegative days (days between known negative days) are shown in dark red and presumed seronegative days (days between symptom onset and first negative day) are shown in light red. Deceased patients are highlighted in grey and immune compromised patients are in red text.
Because serological status was not assessed daily for each patient, the total number of patients with serological assay data varied each day. To plot seroconversion curves, data was transformed by multiplying the percent seroconverted for each day by the total number of patients in each group.
Seroconversion plots and individual groups were compared and analyzed by Log-Rank (Mantel-Cox) test. Differences between IgG and IgM antibody levels were calculated using one-way ANOVA with a Kruskal-Wallis test and a Dunn multiple-comparison test. Normality was assessed using the D'Agostino & Pearson test. All statistical analyses were performed using Excel or GraphPad Prism (version 8.3.1, San Diego, CA).
3. Results
Three hundred fifty-nine residual samples were obtained from 89 hospitalized patients with PCR-confirmed SARS-CoV-2 infection (51 patients from Washington University and 38 from Montefiore; Table 1 ). Samples were collected from patients over a mean of 12.0 days ± 10.7 (range 0–82 days). An average of 4.1 ± 1.3 (range 1–9) samples were collected from each patient. All patients in the study were symptomatic except for one asymptomatic patient who was hospitalized for an injury related to a fall, and was PCR positive upon admission. The average age was 63 years (range 14–93) and 59% were male (Supplementary Fig. 1A). At the conclusion of the study, 69 patients had recovered and 20 had died. 51.7% developed IgG and IgM antibodies simultaneously, and a greater proportion of patients exhibited IgM seroconversion before IgG seroconversion (32.8%; Fig. 1, Supplementary Fig. 1B). Of all, 15.5% of the patients seroconverted IgG before IgM.
Table 1.
Summary of days to seroconversion and patient outcomes.
| n | Days to seroconversion |
P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | Min | Max | IgG v IgM | ||||
| All | IgG | 89 | 8 | 7–9 | 3 | 27 | 0.4194 | |
| IgM | 88 | 7 | 6–8 | 2 | 76 | |||
| Day of seroconversion caught | IgG | 41 | 8 | 7–10 | 4 | 19 | 0.9533 | |
| IgM | 34 | 8 | 7–10 | 4 | 17 | |||
| Male | IgG | 53 | 8 | 6–10 | 3 | 24 | 0.1705 | Male v Female IgG |
| IgM | 52 | 7 | 6–8 | 2 | 74 | 0.0757 | ||
| Female | IgG | 36 | 8 | 6–9 | 3 | 15 | 0.4768 | Male v Female IgM |
| IgM | 36 | 6 | 5–8 | 3 | 15 | 0.2358 | ||
| Survived | IgG | 69 | 8 | 6–10 | 3 | 27 | 0.2938 | Survived v Died IgG |
| IgM | 68 | 6 | 6–8 | 2 | 76 | 0.5517 | ||
| Deceased | IgG | 20 | 7.5 | 6–9 | 5 | 18 | 0.6993 | Survived v Died IgM |
| IgM | 19 | 8 | 6–10 | 3 | 18 | 0.4941 | ||
| Immune competent | IgG | 80 | 7.5 | 6–8 | 3 | 27 | 0.1974 | Competent v Compromised IgG |
| IgM | 79 | 6 | 6–7 | 2 | 27 | 0.0004 | ||
| Immune compromised | IgG | 9 | 15 | 11–20 | 11 | 20 | 0.2531 | Competent v Compromised IgM |
| IgM | 8 | 17.5 | 11–76 | 11 | 76 | <0.0001 | ||
| <65 years old | IgG | 40 | 11 | 9–12 | 4 | 27 | 0.5442 | Older v younger IgG |
| IgM | 39 | 8.5 | 7–12 | 3 | 27 | <0.0001 | ||
| ≥65 years old | IgG | 49 | 6 | 5–8 | 3 | 14 | 0.6322 | Older v younger IgM |
| IgM | 49 | 6 | 4–7 | 2 | 76 | 0.0008 | ||
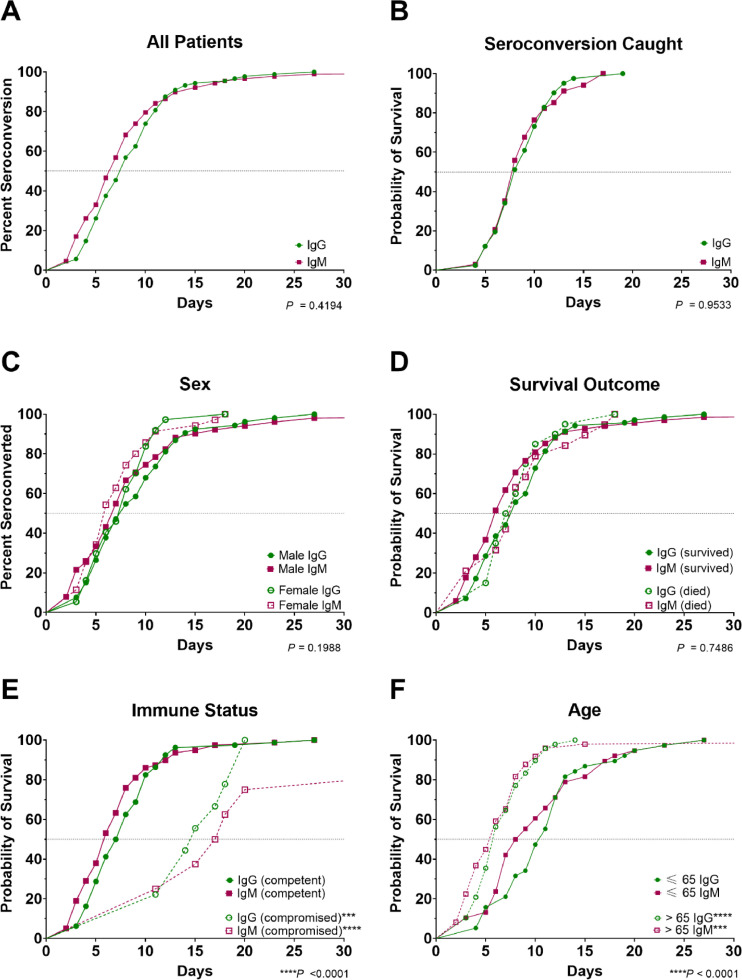
Overall, patients were positive for IgG by 8 days (95% CI: 7–9) and positive for IgM by 7 days (95% CI: 6–8) postsymptom onset (Table 1, Fig. 2 A). Time of seroconversion was captured for 41 patients. Their median time to conversion was 8 days for both IgG (95% CI: 7–10) and IgM (95% CI: 7–10; Fig. 2B). The proportion of IgG and IgM seropositivity rate increased similarly over time, with 100% of patients achieving IgG and IgM seropositivity by 19 days (Fig. 2B).
Fig. 2.
Time course of IgG and IgM seroconversion for all patients (A), patients where seroconversion was caught (B), all patients grouped by sex (C), survival outcomes (D), immune status (E), and age (years) (F). P values were calculated by one-way ANOVA. Overall group comparison P values are listed in each graph. For comparisons between individual groups, ***P < 0.001 and ****P < 0.0001 compared to the matched IgG or IgM of the opposite group.
No significant differences were observed in time to seroconversion between males and females (IgG: P = 0.0757, IgM: P = 0.2358; Fig. 2C). Similarly, no significant differences were observed in the time to IgG or IgM seroconversion between patients who survived versus died (P = 0.5517 and P = 0.4941, respectively; Fig. 2D). The median time to seroconversion was prolonged, however, for immunocompromised patients (IgG: 15 days, IgM: 17.5 days) compared to immune-competent patients (IgG: 7.5 days [P = 0.0004], IgM: 6 days [P < 0.0001]; Fig. 2E).
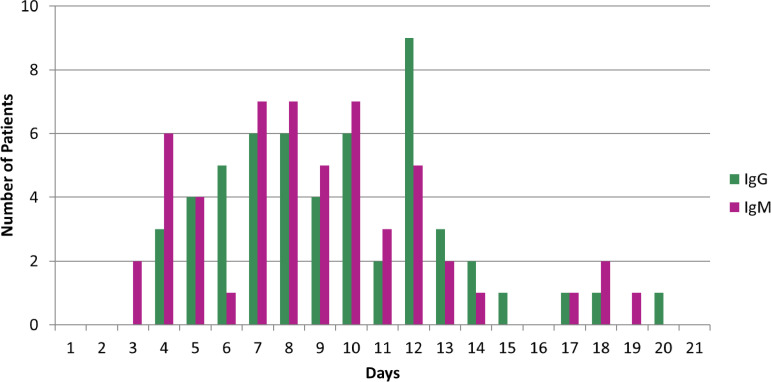
Older patients (≥65 years of age) seroconverted significantly faster for IgG (P < 0.0001; Fig. 2F) and IgM (P = 0.0008; Fig. 2F). Patients at or older than 65 years seroconverted for IgG and IgM 6 days postsymptom onset, whereas patients younger than 65 years seroconverted for IgG and IgM 11 days and 8.5 days postsymptom onset, respectively. There was considerable heterogeneity in the time to seroconversion postsymptom onset for IgG and IgM in patients where seroconversion was captured (Fig. 3 , Supplementary Fig. 2). Some immune-competent patients were positive for IgM as early as 2 to 3 days postsymptom onset while others were not positive until after day 12. Similarly, some patients seroconverted for IgG as early as 4 to 5 days postsymptom onset while others did not seroconvert until day 14.
Fig. 3.
First day of positive seroconversion after symptom onset for each patient.
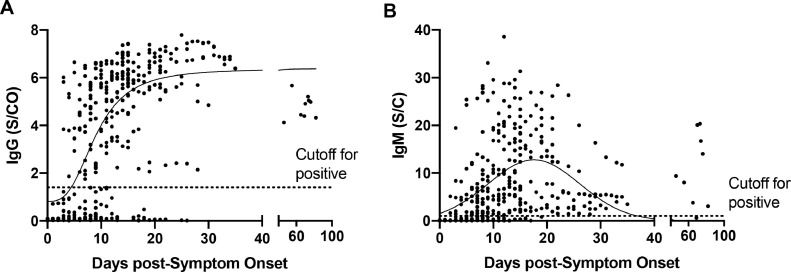
Mean peak IgG levels in immunocompetent patients was 5.4 S/C (95% CI: 5.7–6.1) and occurred at day 15.0 postsymptom onset (95% CI: 13.3–17.2; Fig. 4 A). Mean peak IgM levels was 12.77 S/CO (95% CI: 11.2–14.4) and occurred at day 17.6 (95% CI; 16.0–19.5; Fig. 4B). In contrast to IgG, which remained high past 30 days, IgM levels decreased and were predicted to go below the limit of detection for the IgM assay by day 36.9 (95% CI 31.7–40.2) using the best fit line. Three persistently hospitalized patients were tested >60 days postsymptom onset and had IgG levels exceeding an S/C of 4.0 (n = 9 specimens).
Fig. 4.
Dot plots of the level of IgG (A) and IgM (B) over time for each study patient.
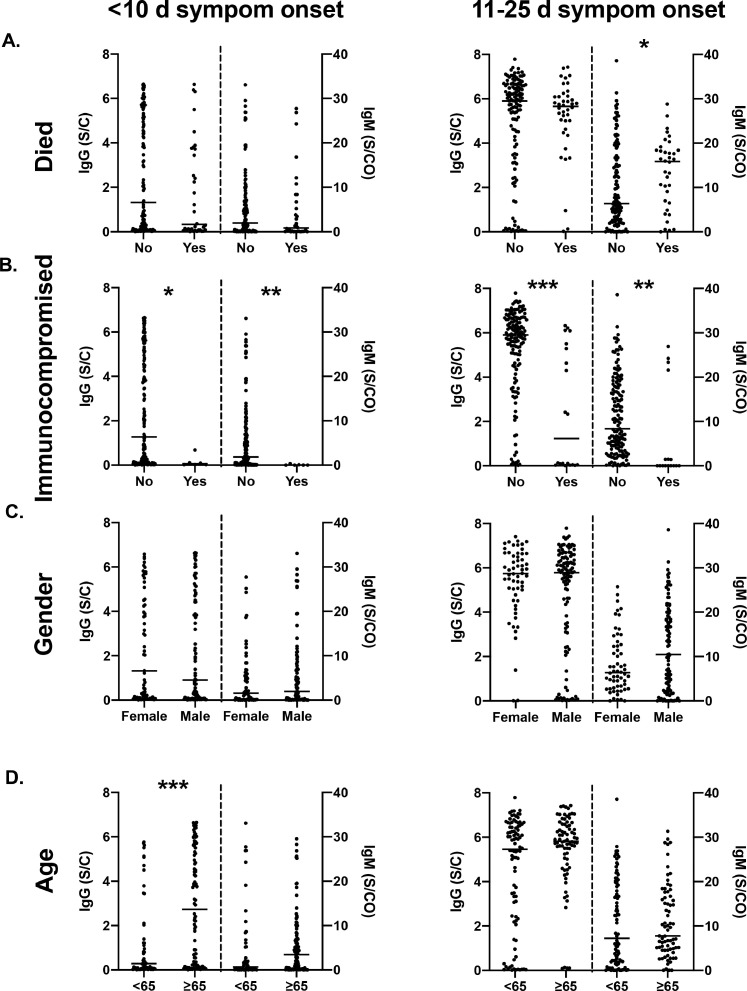
Specimens were next stratified by days postsymptom onset (early, ≤10 days or late, 11–25 days). A modest but insignificant difference in mean IgG levels was observed early postsymptom onset in patients who survived (1.80 S/C, 95%CI: 1.03–2.56 vs 2.47 S/C, 95% CI: 2.03–2.92; Fig. 5 A). Patients who died had significantly higher IgM signal later postsymptom onset (13.8 S/CO, 11.2–12.5) compared to those who survived (10.0 S/CO, 8.4–11.6; Fig. 5A). Immunocompromised patients had significantly lower levels of both IgG (2.36 S/C, 2.0–2.79 vs 0.16 S/C, 0.0–0.43) and IgM (5.46 S/CO, 4.19–6.73 vs 0.78 S/CO, 0.0–1.19) compared to immune-competent patients, which persisted over time (Fig. 5B). No significant differences were observed in IgG or IgM levels between males and females (Fig. 5C). Early postsymptom onset, older patients ≥65 had higher levels of IgG signal relative to those <65 (2.88, 2.38–3.38 vs 1.29, 0.77–1.8); however, by days 11 to 25, there was no significant difference in IgG or IgM levels.
Fig. 5.
Comparison of IgG and IgM levels in patient specimens collected ≤10 days from symptom onset (early) and 11 to 25 days from symptom onset (late), categorized based on (A) outcome (recovery or death), (B) immune status (immunocompromised or immune-competent), (C) sex, and (D) age (<65 or ≥65 years). *P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001 when comparing between groups.
4. Discussion
Limited data is available assessing the kinetics of IgG and IgM seroconversion following SARS-CoV-2 infection using commercial, high-throughput assays and specimens collected longitudinally. In this study, we observed that the majority of patients developed IgG and IgM antibodies simultaneously or seroconverted for IgM slightly earlier than IgG. Our findings confirm previous studies (Guo et al., 2020; Long et al., 2020; Ng et al., 2020; Zhao et al., 2020), further define the humoral immune response to SARS-CoV-2 infection, and may have important implications for the clinical utility of IgM antibodies to SARS-CoV-2.
Studies have reported asynchronous seroconversion of IgG and IgM following SARS-CoV-2 infection with somewhat conflicting results. Guo et al. found a median time to IgM detection of 5 days and IgG detection of 14 days postsymptom onset in a cohort of 43 PCR-positive patients using a laboratory developed ELISA (Guo et al., 2020). In a study of 173 PCR-positive, hospitalized patients, Zhao et al. also reported sequential seroconversion of IgM then IgG at days 12 and 14, respectively, postsymptom onset (Zhao et al., 2020). Conversely, Long et al. (2020) measured IgM and IgG in serum samples collected at 3-day intervals in 70 PCR-positive patients and reported seroconversion for both antibodies 13 days postsymptom onset using the Bioscience magnetic chemiluminescence enzyme immunoassay (MCLIA). Furthermore, they observed approximately equal numbers of patients with synchronous and asynchronous seroconversion, with 10/26 patients seroconverting to IgG before IgM. Using high-throughput IgG and IgM assays, we also observed asynchronous seroconversion of IgM and IgG; however, the median time to IgG and IgM positivity were 8 and 7 days, respectively. Similar to these findings, in a study using the same ARCHITECT IgG and prototype IgM assays, Ng et al. (2020) reported simultaneous increases in IgG and IgM antibody levels in 43 PCR-positive patients. This implies that contrary results are likely related to differences in study design, sample collection, patient population, and assay design. Nonetheless, near simultaneous seroconversion of IgG and IgM is consistent with studies assessing immunity to the previous SARS-CoV during the 2002-2004 outbreak (Hsueh et al., 2004). Interestingly, we confirmed previous findings (Long et al., 2020) that a subset of patients (15.5% observed here) paradoxically seroconvert IgG before IgM. We did observe a trend towards earlier IgG and later IgM seroconversion in patients who were immune compromised as well as in patients who subsequently died, implying that differences in seroconversion may be due in part to infection severity. Few studies have examined the link between immune response and disease outcomes; our observation of a potential link between IgM seroconversion rate and signal persistence late after symptom onset in patients who subsequently died requires further study.
Previous studies have assessed the antibody response to SARS-CoV-2; however, the majority have used laboratory-developed assays or clinical assays not widely available in most countries. Therefore, the findings from previous studies may not be generalizable. Our finding of higher SARS-CoV-2 antibodies in older (≥65 years of age) versus younger (<65 years of age) adults confirms a previous report that found a correlation between older age and higher SARS-CoV-2 antibody levels (Klein et al., 2020). Similarly, Gorse et al. (2020) examined IgG antibodies to 4 human coronaviruses in older (60-85 years old) and younger (21-40 years old) individuals 5 days and 3 to 4 weeks postsymptom onset. The authors observed significantly higher antibody concentrations in the older group during both stages, suggesting that older participants would have had a greater number of previous exposures to coronaviruses, which would prompt a larger and earlier immune response compared to younger participants. Interestingly, we also observed that patients younger than 65 years of age had a prolonged time to seroconversion and lower levels of IgG signal early (<10 days) postsymptom onset, though this difference in IgG signal did not persist over time.
We also observed differences in seroconversion rate and antibody signal level over time based on patient and clinical factors, including immune status. Ng et al. reported a delay in IgG seroconversion in immunosuppressed patients (kidney transplant recipients); however, this delay was not seen in all patients who were immunosuppressed (Ng et al., 2020). We also found a delayed time to IgG/ IgM seroconversion and lower mean levels of IgG and IgM signal in immunocompromised patients, with IgG levels peaking on day 15 and remaining elevated, while IgM signal levels decreased after peaking on day 17.6. Interestingly, of the seronegative immunocompromised patients, only 1 died from complications due to SARS-CoV-2 infection. This implies that factors secondary to antibody-mediated clearance are crucial for resolution of primary SARS-CoV-2 infections.
Our study adds to a growing body of literature supporting a role for IgM testing to confirm SARS-CoV-2 infection in symptomatic patients in lieu of RNA testing, either because a patient presents after the window for PCR-based testing or because PCR testing is unavailable. Given the lack of evidence in the peer reviewed literature, the Infectious Diseases Society of America (IDSA) makes no recommendations for or against the use of SARS-CoV-2 IgM serological assays to detect past infection (Infectious Diseases Society of America, 2020). However, the IDSA does advocate for the use of serological testing in symptomatic patients that are persistently PCR negative (Infectious Diseases Society of America, 2020). Serological testing for anti-SARS-CoV-2 IgG was sufficient in the early phase of the COVID-19 pandemic to confirm diagnosis in such cases. However, as the prevalence of SARS-CoV-2 infections increases and as other seasonal respiratory infections simultaneously rise, it will be important to distinguish acute infections from past infections. To this end, the presence of IgM antibodies to SARS-CoV-2 may help reduce diagnostic uncertainty in this clinical scenario. In support of this, the majority of patients in our study had low or decreasing IgM levels 30 days postsymptom onset and 16/47 patients were trending downward before day 14 postsymptom onset. While larger studies are necessary to confirm these findings, the best fit curve predicted that patients would be negative for IgM antibodies by day 37 postsymptom onset while remaining persistently positive for IgG using the assays described here. In clinical practice, the simultaneous presence of anti-SARS-CoV-2 IgM and IgG may provide evidence of recent infection (i.e., within the past 40 days), whereas the presence of IgG without IgM may imply infection >40 days prior. IgM screening may also be useful in low prevalence populations. The CDC recommends that when screening low prevalence populations, an orthogonal approach is used by which serological positives are tested with a second method to confirm the presence of anti-SARS-CoV-2 antibodies (Centers for Disease Control and Prevention, 2020). Most studies to date have focused on orthogonal testing for total Ig or IgG SARS-CoV-2 antibodies (Ripperger et al., 2020; Xu et al., 2020). To this end, an approach by which IgM and IgG are screened simultaneously and separately on a single high-throughput instrument may provide benefit (Risch et al., 2020). Furthermore, while caution should be used when interpreting anti-SARS-CoV-2 IgM results to diagnose acute infections in the absence of IgG (Infectious Diseases Society of America, 2020), the high specificity reported for the assays used in this study (Bryan et al., 2020; Ng et al., 2020; Tang et al., 2020; Theel et al., 2020) imply that this approach may provide clinical value when testing low prevalence populations.
A growing body of literature has reported associations between results by commercial serological assays and neutralizing antibody titers. Similar to the kinetics of seroconversion demonstrated here, neutralizing antibody titers rise ∼2 weeks after symptom onset followed by a decline (Brochot et al., 2020; Ng et al., 2020; Sun et al., 2020; Tang et al., 2020; Wu et al., 2020). Studies have also demonstrated associations between neutralizing antibody titers correlated with those of IgG in patients with more severe COVID-19 symptoms (Sun et al., 2020; Tang et al., 2020) and in elderly and middle-aged patients relative to younger patients (Wu et al., 2020). Interestingly, a recent study implied a crucial role of circulating IgM for SARS-CoV-2 neutralization (Gasser et al., 2020). While further studies are needed, the seroconversion kinetics demonstrated here may have important implications for demonstrating duration and efficacy of immunity, evaluation of convalescent plasma, and vaccine development (Wu et al., 2020).
One limitation of our study is that we examined a hospitalized study population, most of whom had relatively severe symptoms, which precluded analysis of variations in immune response based on symptom severity. Further, the number of immune compromised individuals we assessed was relatively small. Lastly, the IgG and IgM assays used are qualitative; therefore, analyses based on changes in level refer to a relative increase in signal over background rather than antibody titers. Future SARS-CoV-2 serology studies with larger patient cohorts should further evaluate differences in IgG and IgM dynamic profiles based on symptom severity, comorbid conditions, and other patient and clinical factors. As PCR-based SARS-CoV-2 testing expands to include asymptomatic individuals, future studies should also include PCR-positive asymptomatic patients to examine differences in immune response dynamics and evaluate the role of serologic testing in diagnosis and prognosis.
In conclusion, we demonstrate similar time to seroconversion for IgG and IgM in SARS-CoV-2‒infected patients measured longitudinally. Furthermore, we identify that differences in immune status and age alter time to seroconversion. These results may help guide the effective application of serologic testing in the management of COVID-19.
Conflicts of Interest
MR, AO, JP, MG, RT, and GC are employees of Abbott Laboratories.
Acknowledgments
Acknowledgments
The authors thank Stacey Tobin for assistance in preparation of this manuscript.
Author contributions
CWF - Conception, formal analysis, provision of study specimens, manuscript preparation. KH - Provision of study materials, manuscript preparation. MST - Provision of study materials, manuscript preparation. EO - Conception, formal analysis, provision of study specimens, manuscript preparation. MR - Conception, formal analysis, manuscript preparation. AO, JP, MG, RT - Data collection, manuscript review. GC - Conception, manuscript review.
Funding
The study was funded by Abbott Diagnostics.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.diagmicrobio.2020.115300.
Appendix. Supplementary materials
References
- Brochot E, Demey B, Touze A, Belouzard S, Dubuisson J, Schmit JL. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. If You Are Immunocompromised, Protect Yourself From COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/immunocompromised.html [Updated May 14. Accessed December 8, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing. interim guidelines for COVID-19 antibody testing in clinical and public health settings. [Updated August 1, 2020]. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed September 7, 2020.
- Centers for Disease Control and Prevention. CDC guidance for expanded screening testing to reduce silent spread of SARS-CoV-2. [Updated December 1, 2020]. https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/expanded-screening-testing.html. Accessed December 3, 2020.
- Farnsworth CW, Anderson NW. SARS-CoV-2 serology: much hype, little data. Clin Chem. 2020;66:875–877. doi: 10.1093/clinchem/hvaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R, Cloutier M, Prévost J, Fink C, Ducas É, Ding S. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.10.09.333278. 2020.10.09.333278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol. 2020;92:512–517. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infectious Diseases Society of America . 2020. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Serologic Testing.https://www.idsociety.org/practice-guideline/covid-19-guideline-serology/ [Updated. Accessed September 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infectious Diseases Society of America . 2020. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Serologic Testing. [Updated August 18 https://www.idsociety.org/practice-guideline/covid-19-guideline-serology/ Accessed December 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infectious Diseases Society of America . 2020. ISDA COVID-19 Antibody Testing Primer.https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-covid-19-antibody-testing-primer.pdf [Updated May 4. Accessed September 7, 2020. [Google Scholar]
- Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020 doi: 10.1172/jci142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Ng D, Goldgof G, Shy B, Levine A, Balcerek J, Bapat SP. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood from the San Francisco Bay Area. medRxiv. 2020 doi: 10.1101/2020.05.19.20107482. 2020.05.19.20107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DL, Goldgof GM, Shy BR, Levine AG, Balcerek J, Bapat SP. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun. 2020;11:4698. doi: 10.1038/s41467-020-18468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933. doi: 10.1016/j.immuni.2020.10.004. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch M, Weber M, Thiel S, Grossmann K, Wohlwend N, Lung T. Temporal course of SARS-CoV-2 antibody positivity in patients with COVID-19 following the first clinical presentation. Biomed Res Int. 2020;2020 doi: 10.1155/2020/9878453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Tang X, Bai R, Liang C, Zeng L, Lin H, et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020;26:1690 e1–1690 e4. doi: 10.1016/j.cmi.2020.08.043 [DOI] [PMC free article] [PubMed]
- Tang MS, Case JB, Franks CE, Chen RE, Anderson NW, Henderson JP. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MS, Hock KG, Logsdon NM, Hayes JE, Gronowski AM, Anderson NW. Clinical performance of two SARS-CoV-2 serologic assays. Clin Chem. 2020;66:1055–1062. doi: 10.1093/clinchem/hvaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel ES, Harring J, Hilgart H, Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/jcm.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol. 2020;58:e00797. doi: 10.1128/JCM.00797-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang A, Liu M, Wang Q, Chen J, Xu S. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Lancet Preprint. 2020 doi: 10.2139/ssrn.3566211. [Updated March 28, 2020]. http://dx.doi.org/Accessed December 8. [DOI] [Google Scholar]
- Xu G, Emanuel AJ, Nadig S, Mehrotra S, Caddell BA, Curry SR. Evaluation of orthogonal testing algorithm for detection of SARS-CoV-2 IgG antibodies. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.