Key Points
High but variable mortality for hospitalized MM patients (27% to 57%).
Uncontrolled MM in the setting of COVID-19 infection was associated with an increased risk of death.
Abstract
The primary cause of morbidity and mortality in patients with multiple myeloma (MM) is an infection. Therefore, there is great concern about susceptibility to the outcome of COVID-19–infected patients with MM. This retrospective study describes the baseline characteristics and outcome data of COVID-19 infection in 650 patients with plasma cell disorders, collected by the International Myeloma Society to understand the initial challenges faced by myeloma patients during the COVID-19 pandemic. Analyses were performed for hospitalized MM patients. Among hospitalized patients, the median age was 69 years, and nearly all patients (96%) had MM. Approximately 36% were recently diagnosed (2019-2020), and 54% of patients were receiving first-line therapy. Thirty-three percent of patients have died, with significant geographic variability, ranging from 27% to 57% of hospitalized patients. Univariate analysis identified age, International Staging System stage 3 (ISS3), high-risk disease, renal disease, suboptimal myeloma control (active or progressive disease), and 1 or more comorbidities as risk factors for higher rates of death. Neither history of transplant, including within a year of COVID-19 diagnosis, nor other anti-MM treatments were associated with outcomes. Multivariate analysis found that only age, high-risk MM, renal disease, and suboptimal MM control remained independent predictors of adverse outcome with COVID-19 infection. The management of MM in the era of COVID-19 requires careful consideration of patient- and disease-related factors to decrease the risk of acquiring COVID-19 infection, while not compromising disease control through appropriate MM treatment. This study provides initial data to develop recommendations for the management of MM patients with COVID-19 infection.
Visual Abstract
Introduction
In the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 pandemic, known as COVID-19, cancer represents a major risk factor2-4 for COVID-19–associated death. Cancer patients with COVID-19 represented 8.3% of deaths in the New York city area, and 7.6% of deaths from the Wuhan area in China.5,6 An even higher fatality rate (20.3%) was observed in Italy.7 A recent study aimed at identifying risk factors for death in cancer patients developing COVID-19 interestingly (but not surprisingly) reported age over 65 years as a risk factor. In this study, treatment with checkpoint inhibitors was a risk factor, but not ongoing chemotherapy.5
Multiple myeloma (MM) is a hematological cancer involving plasma cells, mostly within the bone marrow.8 Apart from the specific cancer-related symptoms, most patients display immunosuppression9 involving both the B- and T-cell compartments. Infections are common disease complications,10 and unfortunately remain a major cause of death. Furthermore, corticosteroids, and especially dexamethasone, are used as treatment throughout the disease course, usually at high doses.11,12 This MM therapy may increase the immunosuppression observed in patients with MM, though low doses seem to improve mortality in hospitalized patients. MM usually affects the elderly population, a more vulnerable group of patients due to immunosenescence together with other comorbidities. In addition, younger MM patients are usually treated with high-dose chemotherapy followed by autologous stem cell transplant,13 with high infection susceptibility during the 3-month period following transplant.14 For all of these reasons, MM could theoretically represent a high risk factor for poor outcomes with COVID-19.15
In this international study, we have collected data and investigated the risk and outcome of COVID-19 infection in MM patients globally, both to evaluate the death rate and to identify potential risk factors that could be modified to improve patient outcomes during the current pandemic and in the future. With this in mind, we have predominantly focused our analysis on patients requiring hospitalization for COVID-19 infection.
Methods
Patient cohort
The international MM COVID-19 data set created by the International Myeloma Society (IMS) has retrospectively collected data for 650 patients with a plasma cell disorder from 10 different countries and multiple centers. All patients in the study had confirmed positive SARS-CoV-2 tests, according to the protocols in their respective countries. A questionnaire created by IMS was shared with participant institutes/investigators, and all required information was collected by the participating investigators. Data cleaning, preprocessing, and quality control were completed before the data analysis. COVID-19 outcome is defined as recovery from the virus and discharge from hospital or death due to COVID-19. Those patients who required an ongoing treatment at the time of data collection, unknown COVID-19 outcomes, and not hospitalized, were excluded from the cohort for statistical analysis. The IMS COVID-19 data set is reviewed by the New England Institutional Review Board based on federal regulation 45 CFR 46 and associated guidance; it is determined to be human subjects research exempt and approved for a waiver of authorization. All data captured are deidentified and comply with Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor regulations.
Statistical analysis
All statistical analyses were performed using R (www.r-project.org). Descriptive statistics for demographic information and clinical variables are reported. Parametric 2-group comparison was used for age, univariate logistic regression was used to evaluate the association between COVID-19 outcome and variables, and odds ratios (ORs) with 95% confidence intervals (CIs) were estimated. Multivariate analysis was performed using only variables that were associated with the outcome on univariate analysis.
Results
Overall, 650 patients with a plasma cell disorder and COVID-19 infection are included in this study, with the majority of patients being from Spain (28.62%), France (28.46%), the United States (19.38%), and the United Kingdom (14.77%) (Table 1; Figure 1A; supplemental Table 1 [available on the Blood Web site]). Median age was 69 years (range, 34-92 years), and 58.5% of patients were male (Table 2). The vast majority of patients (95.5%) had MM, whereas 29 patients (4.5%) had another plasma cell disorder. The MM immunoglobulin subtype included 55% immunoglobulin G (IgG), 21% IgA, and 20% light chain (Table 2). Patients were equally distributed between International Staging System (ISS) stages 1 to 3, with 32.1% of patients having high-risk cytogenetics and 26.5% having renal dysfunction. Fifty-four percent of patients were receiving first-line therapy, whereas 23.5% of patients had 3 or more lines of therapy. Additional demographic data are presented in Table 1, Table 2, and supplemental Tables 1 and 2.
Table 1.
Total number of patients and their COVID-19 outcomes recorded in the IMS COVID-19 data set by country and diagnosis
| All patients, n = 650 | Hospitalized | Hospitalized with invasive ventilation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Died (%) | Died (%) | Recovered (%) | Unknown (%) | Total | Died (%) | Recovered (%) | Unknown (%) | Total | |
| Country | ||||||||||
| Total | 650 (100) | 222 (34.1) | 139 (31.10) | 300 (67.11) | 8 (1.79) | 447 | 73 (80.22) | 16 (17.58) | 2 (2.20) | 91 |
| Spain | 186 (28.62) | 56 (30.1) | 46 (30.26) | 105 (69.08) | 1 (0.66) | 152 | 9 (60.00) | 5 (33.33) | 1 (6.67) | 15 |
| France | 185 (28.46) | 69 (37.2) | 35 (26.52) | 97 (73.48) | 132 | 31 (83.78) | 6 (16.22) | (0.00) | 37 | |
| USA | 126 (19.38) | 31 (24.6) | 11 (21.57) | 37 (72.55) | 3 (5.88) | 51 | 17 (94.44) | 1 (5.56) | (0.00) | 18 |
| UK | 96 (14.77) | 53 (55.2) | 44 (53.66) | 37 (45.12) | 1 (1.22) | 82 | 7 (100.00) | (0.00) | (0.00) | 7 |
| Other | 57 (8.77) | 13 (22.8) | 3 (10.00) | 24 (80.00) | 3 (10.00) | 30 | 9 (64.29) | 4 (28.57) | 1 (7.14) | 14 |
| Diagnosis | ||||||||||
| Total | 646 (100) | 222 (34.3) | 139 (31.24) | 295 (66.29) | 10 (2.25) | 445 | 73 (80.22) | 16 (17.58) | 2 (2.20) | 91 |
| MM | 617 (95.51) | 212 (34.3) | 136 (31.85) | 283 (66.28) | 8 (1.87) | 427 | 67 (78.82) | 16 (18.82) | 2 (2.35) | 85 |
| MGUS/SMM | 19 (2.94) | 5 (26.3) | 1 (8.33) | 9 (75.00) | 2 (16.67) | 12 | 3 (100) | 3 | ||
| Amyloid | 10 (1.55) | 5 (50) | 2 (33.33) | 4 (66.67) | 6 | 3 (100) | 3 | |||
All patients (n = 650) refers to all of the patients including, hospitalized patients and outpatients without any exclusion, in our data set.
MGUS, monoclonal gammopathy of undetermined significance; SMM, smoldering MM; UK, United Kingdom; USA, United States of America.
Figure 1.
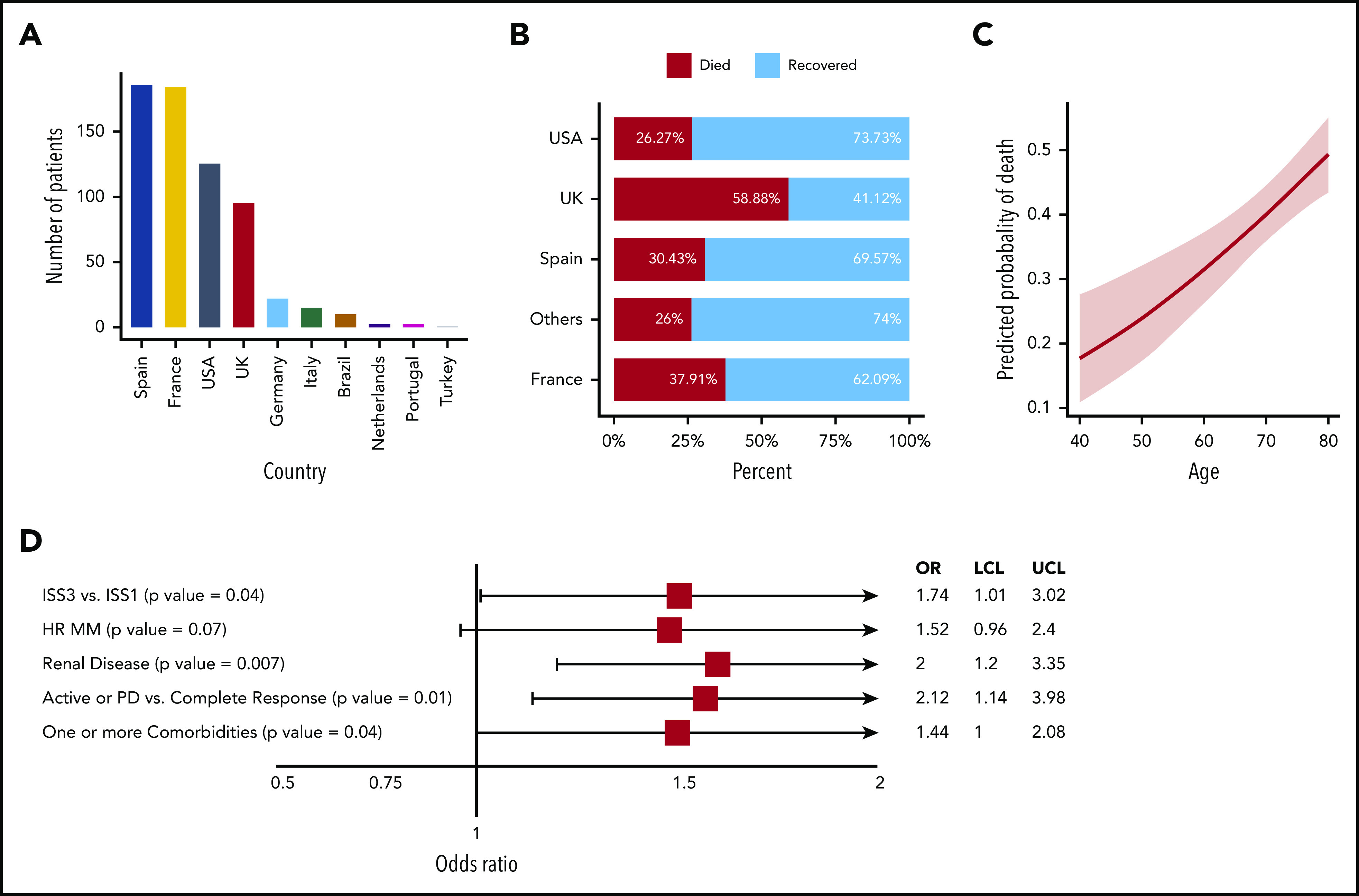
Patient origin, mortality, and associated risk factors. (A) Number of patients in the IMS COVID-19 data set with plasma cell disorders. (B) Overall (outpatient and hospitalized) COVID-19 death rates in the data set by contributing countries. (C) Predicted COVID-19 outcome for MM patients by age. (D) A forest plot for risk factors for MM patients from univariate analysis. HR, high risk; LCL, lower confidence level; PD, progressive disease; UCL, upper confidence level; UK, United Kingdom; USA, United States of America.
Table 2.
Patient characteristics for hospitalized MM patients and overall data set
| MM hospitalized recovered, n = 299 | MM hospitalized died, n = 203 | All MM patients, n = 617 | |
|---|---|---|---|
| Age, y | |||
| Median [min-max] | 70 [35-92] | 72 [47-92] | 69 [34-92] |
| Sex, n (%) | |||
| Female | 126 (42.14) | 76 (37.43) | 270 (41.53) |
| Year of diagnosis, n (%) | |||
| 2020 and 2019 | 114 (38.64) | 67 (33) | 226 (35.59) |
| 2018 and 2017 and 2016 | 86 (29.15) | 69 (34) | 200 (31.50) |
| 2015 or before | 95 (32.20) | 67 (33) | 209 (32.91) |
| MGUS/MM type, n (%) | |||
| IgG | 127 (57.72) | 59 (47.96) | 255 (55.19) |
| IgA | 50 (22.72) | 30 (24.39) | 100 (21.64) |
| Light chain | 38 (17.27) | 34 (27.64) | 93 (20.12) |
| ISS stage | |||
| ISS1/2 | 164 (69.49) | 88 (61.53) | 331 (68.39) |
| ISS3 | 72 (30.50) | 55 (38.46) | 153 (31.61) |
| High-risk disease by FISH [del 17p, t(4;14), amp 1q, or t(14;16)], n (%) | |||
| Yes | 57 (23.36) | 47 (30.51) | 136 (32.07) |
| Renal disease, n (%) | |||
| Yes | 43 (21.71) | 41 (35.65) | 113 (26.52) |
| Line of treatments, n (%) | |||
| 1 or less | 156 (54.74) | 101 (51.27) | 331 (54) |
| 2 | 63 (22.10) | 48 (24.36) | 138 (22.51) |
| 3 or more | 66 (23.16) | 48 (24.36) | 144 (23.49) |
| Patient receiving active treatment, n (%) | |||
| Yes | 225 (87.20) | 131 (86.75) | 456 (83.57) |
| Prior transplant, n (%) | |||
| Yes | 118 (40.54) | 60 (32.78) | 241 (39.12) |
| Disease status, n (%) | |||
| Newly diagnosed | 134 (50.95) | 86 (44.55) | 282 (48.53) |
| MM status at COVID-19, n (%) | |||
| Active or PD | 37 (14.57) | 34 (22.97) | 87 (16.66) |
| Partial response | 143 (56.30) | 82 (55.40) | 290 (55.55) |
| Complete response | 74 (29.13) | 32 (21.62) | 145 (27.77) |
All MM patients includes hospitalized patients and outpatients regardless of their COVID-19–associated outcome.
FISH, fluorescence in situ hybridization; ISS, International Staging System; max, maximum; min, minimum. See Table 1 for expansion of other abbreviations.
Thirty-three percent of patients died following COVID-19 diagnosis. The death rate increased from 4% for those who were outpatients to 31% for hospitalized patients not on ventilator support to 80% for patients on ventilator support (Table 1; supplemental Table 1). The variability in death rates across 4 major countries is shown in Figure 1B and Table 1. The death rate in patients with other plasma cell disorders was 31% (9 of 29).
We have further focused on analyzing the hospitalized patients, for whom the mortality rates ranged from 27% in Germany, Italy, Brazil, The Netherlands, Portugal, and Turkey to 57% in the United Kingdom (Table 2). Age was significantly associated with COVID-19 outcome (P < .001). The estimated probability of death for 40-, 60-, and 80-year old patients was 17.76%, 31.43%, and 49.3%, respectively (Figure 1C). Forty percent of hospitalized patients were female, and in contrast to prior reports, sex was not associated with outcome. Of note, the mean age for male patients (69 years) was significantly lower than female patients (71.5 years) (P = .01).
Of the patients with available data, those diagnosed with MM in 2019 or 2020 accounted for 35.6% of the cohort, and those with ≤1 line of therapy accounted for 54% of the cohort; 32.9% of patients were diagnosed on or before 2015. Neither time from diagnosis nor number of prior lines of treatment had any impact on outcome of COVID-19 infection. Immunoglobulin type distribution was similar to the general MM population, and isotypes were not associated with outcome. Univariate analysis identified ISS3 vs ISS1 (P = .04), high-risk disease [del 17p, t(4;14), amp 1q or t(14;16)] (P = .07), renal disease (P = .007), inadequate MM control (active disease or progressive disease [PD] vs complete response) (P = .01), and 1 or more comorbidities (P = .04) correlating with higher rates of death (Figure 1D).
Eighty-seven percent of patients who had a known treatment status were on active MM therapy at the time of COVID-19 diagnosis, and 89% patients had their therapy held during COVID-19 diagnosis and management (Table 2; supplemental Table 2). A history of prior transplant or transplant within a year of COVID-19 diagnosis did not impact outcome. In fact, patients with a history of stem cell transplant within a year of COVID-19 diagnosis had a lower death rate; however, this difference was cofounded by a 10-year age difference between transplant and nontransplant patients, and was not observed when adjusted for age. Similarly, we did not observe any significant difference in outcome from COVID-19 infection whether patients underwent transplant within 6 months or >6 months before their COVID-19 diagnosis. Approximately 86% of patients had prior exposure to proteasome inhibitors (PIs), 80% to immunomodulatory (IMiD) agents, and 30% to anti-CD38 antibody. In univariate analysis, prior PI, IMiD, or anti-CD38 treatment was not associated with outcome. Although univariate analysis showed that patients who were receiving IMiD treatment at the time of COVID-19 diagnosis had decreased mortality compared with patients not on IMiDs, multivariate analysis failed to identify IMiD or any of these features as being related with outcome (Table 3). We did not observe any significant correlation between active PI, IMiD, anti-CD38 monoclonal antibody, alkylating agents, steroids, or other treatments (venetoclax, 96-hour infusional regimens, bispecific T-cell engagers, belantamab, chimeric antigen receptor T cell, elotuzumab, histone deacetylase) and the COVID-19 outcome.
Table 3.
Estimated COVID-19 outcome predictors based on multivariate analysis and their ORs for MM patients
| Variable | P | OR (95% CI) |
|---|---|---|
| Age | .006 | 1.04 (1.01-1.08) |
| ISS3 | .899 | 1.05 (0.49-2.22) |
| High-risk disease | .013 | 2.35 (1.20-4.66) |
| Renal disease | .014 | 2.71 (1.23-6.08) |
| Active disease or PD | .063 | 1.91 (0.96-3.81) |
| Comorbidities | .711 | 0.88 (0.44-1.75) |
| Prior anti-CD38 | .558 | 0.77 (0.31-1.85) |
| Active anti-CD38 | .262 | 1.68 (0.68-4.21) |
| Active IMiD | .769 | 1.10 (0.59-2.07) |
OR for age is calculated by increments of 1 year. High-risk disease includes patients with del 17p, t(4;14), amp 1q or t(14;16) alterations detected by FISH. Renal disease is defined as creatinine clearance <40 mL/min, creatinine >2 mg/dL, or on dialysis. Active disease or PD refers to newly diagnosed or relapsed patients whose MM was not responsive to any treatment or not controlled at the time of COVID-19 diagnosis. Comorbidities refers to 1 or more condition associated with cardiac, neurological, pulmonary, or renal disease, diabetes, and/or hypertension. Prior anti-CD38 refers to anti-CD38 monoclonal antibody usage any time before COVID-19 diagnosis. Active anti-CD38 and active-IMiD refer to using these treatments at the time of COVID-19 diagnosis. Variables with P < .1 were considered statistically significant and are shown in bold.
See Table 2 for expansion of abbreviations.
The treatments of COVID-19 were very heterogeneous, with the most frequent therapies including combination strategies (70%), antibiotics (14%), and hydroxychloroquine (10%). No therapies for COVID-19 appeared to be protective or associated with worse outcomes.
Of the aforementioned variables that were associated with an increased risk of death by univariate analysis, only age (OR = 1.04; 95% CI, 1.01-1.08), high-risk MM [del 17p, t(4;14), amp 1q or t(14;16)] (OR = 2.35; 95% CI, 1.20-4.66), renal disease (OR = 2.71; 95% CI, 1.23-6.08), and active or progressive MM (OR = 1.91; 95% CI, 0.96-3.81) remained as independent predictors of adverse outcome on multivariate analysis (Table 3).
Discussion
The COVID-19 infection has affected patients globally, with high incidence in Europe and the Americas. The disease has involved patients of all age groups; however, heterogeneity in outcome of COVID-19 infection has been observed to be associated with comorbidities, racial differences, as well as individual characteristics such as smoking.16-18 Of note, the presence of comorbidities has been extensively studied to identify patients at greater risk of infection and those with worse outcome. In this regard, our current study focuses on a single type of cancer, MM, to understand both impact and outcome of patients when they develop COVID-19 infection. As MM patients have hallmark immunosuppression, it is of great interest to understand the impact of both the disease and its treatment, that is, the immunosuppressive effects of high-dose therapy with autologous transplantation, as well as novel targeted therapies.
Here, we report data primarily from 4 countries (Spain, France, the United Kingdom, and the United States) having a high prevalence of COVID-19 infection and with the highest frequencies of COVID-19 in MM patients. Differing access to testing likely led to the majority of outpatients (55%) coming from the United States.
Interestingly, recent data from institutions in New York City showed that ∼19% of 127 patients with COVID-19 actually had MM precursor conditions (plasmacytoma, monoclonal gammopathy of undetermined significance, smoldering MM).19 Based on the fact that data collection was feasible predominantly in patients who were hospitalized, our study has focused on hospitalized patients and their outcomes, and is unable to provide insight into a question regarding susceptibility and outcome of COVID-19 in patients with MM precursor conditions.
According to Surveillance, Epidemiology, and End Results Program (SEER) data, 22% of the patients with myeloma had their diagnosis of MM in 2019 or 2020. In our cohort, 36% of the patients with COVID-19 infection were diagnosed with MM in 2019 or 20, suggesting higher susceptibility even in earlier stages of the disease. Even if we account for variability in diagnosis and selection of cases, there seems to be no evidence of increased hospitalizations in advanced multiply relapsed MM, as was initially postulated. However, differing access to COVID-19 testing or care between newly diagnosed and established patients at academic hospitals collaborating with the IMS may impact these results. Of note, a prior report noted that hypogammaglobulinemia (IgG < 700 mg/dL) was not associated with outcomes of COVID-19 infection, but severe hypogammaglobulinemia (IgG < 400 mg/dL) was associated with death.20 The impact of disease stage and clinical immunoparesis is being evaluated in prospective studies. The sex distribution in this analysis was similar to the general incidence of MM, and thus suggests a similar susceptibility to COVID-19 infection between male and female patients with MM. A clear association between age and outcome was confirmed in these patients, as is true in other settings.
The differences reported here between various countries reflects, at least in part, differing diagnostic and management practices, as well as the resources available and used in management of COVID-19 at the height of the pandemic, as well as referral patterns. Health system differences between countries may influence the ability to seek or obtain SARS-CoV-2 testing and health care, including admission to hospital. Health care providers should always consider local COVID-19 prevalence and local guidelines, and recommendations made here should only be used as a reference. For example, the number of patients who received ventilator support differed from 7% to 31%, and there are also differences in outpatient management vs hospitalization. Nonetheless, hospitalized patients can be considered to have more severe COVID-19–related complications requiring more intensive support.
Our data suggest higher mortality in hospitalized patients with MM and COVID-19 infection than nonmyeloma patients. A recent study from Spain found a higher mortality rate in MM patients with COVID-19 (34%) compared with age- and sex-matched non-MM patients with COVID-19 (23%).21 A recent publication from France confirmed overall mortality in all hospitalized patients with COVID-19 to be 16%, which is significantly lower than mortality observed in hospitalized MM patients in France (39%).22 Our data clearly suggest a lack of relationship between prior lines of therapy or prior type of therapy, or those receiving active MM therapy at the time of diagnosis with COVID-19 and outcome. Interestingly, neither past nor recent high-dose therapy had significant impact on outcome. These data indicate that postponing indicated MM therapies, including high-dose therapy, may not be necessary during the COVID-19 pandemic. Within the limitation of our sample size and retrospective nature of the study, there is no clear suggestion for the need to avoid any specific MM treatment. Importantly, patients with good MM control (complete response) had superior outcome compared with those with relapsed disease or partial response. A similar finding was observed in a study of 928 cancer patients with COVID-19, in which patients with active cancer (progressing vs remission) had an adjusted OR of 5.2 for 30-day mortality, but there was no association with recent noncytotoxic therapy nor recent cytotoxic systemic chemotherapy.2 As most patients receive dexamethasone as part of combination therapy, judging its independent impact on outcome was not possible, which is important because a recent report suggests superior survival for those COVID-19 patients given dexamethasone as part of their COVID-19 therapy.23
Our multivariate model identifies age, high-risk or progressive MM, and presence of renal disease as indicators of poor outcome. MM therapy to achieve deep response may therefore also protect patients from adverse outcome from COVID-19 infection. Although little is known about the recovery of patients with MM from COVID-19 infection, Wang et al found that the median time to polymerase chain reaction (PCR) negativity was 43 days (range, 19-68 days) from initial positive PCR.20 Interestingly, 96% of MM patients (22 of 23) developed antibodies to SARS-CoV-2 at a median of 32 days after initial diagnosis.
Based on the observations reported here, young patients with high-risk and/or active MM need to receive therapies to control their disease, which will also improve their outcome if infected with COVID-19. For elderly patients with a higher death rate from COVID-19, disease control is also beneficial, but may be achieved using regimens that decrease frequency of office visits (eg, oral drug) in order to avoid exposure to the virus. Importantly, continued therapy including steroids and high-dose therapy are not contraindicated, and in fact should be continued to achieve better MM control, which is associated with improved outcome even with COVID-19 infection.
In conclusion, this collaborative international effort provides the first large-scale analysis and initial IMS suggestions on the management of, and outcomes for, patients with MM during the current COVID-19 pandemic (Table 4). As the pandemic and data accumulation rapidly increase, we need prospective studies on treatment options and additional patient characteristics to further understand the variables associated with COVID-19–associated death in MM patients. The high mortality noted in MM patients highlights the critical importance of measures to prevent contracting COVID-19, such as social distancing and wearing masks, in patients with MM.
Table 4.
Recommendations for management of MM patients in the era of a global COVID-19 pandemic
| Recommendations for management of MM patients in the COVID-19 era |
|---|
| • Measures to prevent contracting COVID-19 including social distancing, wearing masks, and personal hygiene are critically important for MM patients |
| • COVID-19 PCR testing should be considered once in all newly diagnosed MM patients before starting therapy and also in patients prior to high-dose or cellular therapies; however, the testing of other MM patients as well as the frequency of repeat testing should be guided by symptoms and prevalence of COVID-19 in the environs |
| • MM patients diagnosed with COVID-19 and having any of the following characteristics: age >60 y, high-risk cytogenetics, active disease or PD, or renal disease should be monitored more closely for COVID-19 complications |
| • Currently, there are no data to support avoiding any specific MM treatments, including corticosteroids and high-dose therapy; this is particularly important in those patients with active disease or PD |
| • The risk/benefit of MM therapy should be weighed against an individual’s risk factors for COVID-19 complications and the prevalence of COVID-19 at a given time |
| o Young patients, especially those with high-risk and/or active MM, should receive optimal MM therapies to control their disease |
| o MM disease control is also important for elderly patients; however, consideration should be given to using regimens that result in decreased frequency of office visits to decrease the risk of COVID-19 exposure |
| • Data regarding the safety of continuing MM therapy in COVID-19 PCR+ patients are lacking; as with any MM patient with an active infection, the risks/benefit of MM therapy must be weighed carefully, and consideration should be given to at least ensuring clinical stability |
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank all medical centers and health workers for their fight against the global pandemic.
This work was supported by the efforts of the International Myeloma Society and its members globally.
Explanation of novelty: This study investigated the characteristics and outcomes of MM patients with COVID-19 infection in 10 countries globally.
Footnotes
For original data, please contact the International Myeloma Society at adminassistant@myelomasociety.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.C., M.K.S., K.C.A., J.S.-M., N.C.M., and H.A.-L. designed the research and analyzed the data; and all authors contributed to data collection and wrote the manuscript.
Conflict-of-interest disclosure: A.C. is consultant to/on the advisory boards for Janssen, Celgene, Novartis, Amgen, Bristol Myers Squibb, Karyopharm, Sanofi Genzyme, Seattle Genetics, Oncopeptides, Millennium/Takeda, Antengene, GlaxoSmithKline, and Secura Bio, and has received research funding from Janssen, Celgene, Novartis, Amgen, Pharmacyclics, Seattle Genetics, and Millennium/Takeda. J.M.-L. has received honoraria for participation in advisory boards from Novartis, Roche, Bristol Myers Squibb, Adaptive Biotech, Incyte, Amgen, and Janssen-Cilag. K.W. has received honoraria for participation in advisory boards from Adaptive Biotech, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Sanofi, and Takeda, and has received research funding from Amgen, Celgene, Sanofi, and Janssen. C.R. has received honoraria from Akcea and Celgene and research funding from Amgen. K.C.A. is a consultant for Bristol Myers Squibb, Millennium-Takeda, Janssen, Sanofi, Tolero, and Precision Biosciences, and is a scientific founder of OncoPep and C4 Therapeutics. P.M. has received honoraria for participation in advisory boards from Janssen, Celgene/Bristol Myers Squibb, Amgen, Sanofi, and AbbVie. M.-V.M. has received honoraria for lectures and participation in advisory boards from Janssen, Celgene–Bristol Myers Squibb, Amgen, Takeda, AbbVie, GlaxoSmithKline, Adaptive Biotech, Roche, Seattle Genetics, Pfizer, and Regeneron. J.S.-M. has received honoraria for lectures and participation in advisory boards from Amgen, Bristol Myers Squibb, Celgene, Janssen, Merck, Novartis, Takeda, Sanofi, and Roche. N.C.M. is a consultant for Bristol Myers Squibb, Janssen, OncoPep, Amgen, Karyopharm, Legend Biotech, AbbVie, Takeda, and GlaxoSmithKline, and is a scientific founder of OncoPep. The remaining authors declare no competing financial interests.
Correspondence: Hervé Avet-Loiseau, Centre de Recherche en Cancérologie de Toulouse, INSERM U1037, Toulouse, France; e-mail: avetloiseau.herve@iuct-oncopole.fr.
REFERENCES
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer NM, Choueiri TK, Shah DP, et al. ; COVID-19 and Cancer Consortium . Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LY, Cazier JB, Angelis V, et al. ; UK Coronavirus Monitoring Project Team . COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson A, Gyawali B, Booth CM. Risk of COVID-19 in patients with cancer. JAMA Oncol. 2020;6(9):1471-1472. [DOI] [PubMed] [Google Scholar]
- 5.Robilotti EV, Babady NE, Mead PA, et al. . Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J, Yuan X, Xiao J, et al. . Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. [DOI] [PubMed] [Google Scholar]
- 8.Avet-Loiseau H. Introduction to a review series on advances in multiple myeloma [editorial]. Blood. 2019;133(7):621. [DOI] [PubMed] [Google Scholar]
- 9.Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138(5):563-579. [DOI] [PubMed] [Google Scholar]
- 10.Blimark C, Holmberg E, Mellqvist UH, et al. . Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szalat R, Munshi NC. Novel agents in multiple myeloma. Cancer J. 2019;25(1):45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9(3):135-143. [DOI] [PubMed] [Google Scholar]
- 13.Corre J, Montes L, Martin E, et al. . Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk. Haematologica. 2020;105(9):e480-e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waszczuk-Gajda A, Drozd-Sokołowska J, Basak GW, et al. . Infectious complications in patients with multiple myeloma after high-dose chemotherapy followed by autologous stem cell transplant: nationwide study of the Infectious Complications Study Group of the Polish Adult Leukemia Group. Transplant Proc. 2020;52(7):2178-2185. [DOI] [PubMed] [Google Scholar]
- 15.Terpos E, Engelhardt M, Cook G, et al. . Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN). Leukemia. 2020;34(8):2000-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark A, Jit M, Warren-Gash C, et al. ; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group . Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003-e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Yu J, He W, et al. . Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34(8):2173-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esai Selvan M. Risk factors for death from COVID-19 [published correction appears in Nat Rev Immunol. 2020;20(7):448]. Nat Rev Immunol. 2020;20(7):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultcrantz M, Richter J, Rosenbaum C, et al. . COVID-19 infections and clinical outcomes in patients with multiple myeloma in New York City: a cohort study from five academic centers [published correction appears in Blood Cancer Discov. 1(3):290]. Blood Cancer Discov. 2020;1(3):234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B, Van Oekelen O, Mouhieddine TH, et al. . A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-López J, Mateos M-V, Encinas C, et al. . Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J. 2020;10(10):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H, Tran Kiem C, Lefrancq N, et al. . Estimating the burden of SARS-CoV-2 in France. Science. 2020;369(6500):208-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. . Dexamethasone in hospitalized patients with Covid-19: preliminary report [published online ahead of print 17 July 2020]. N Engl J Med. doi:10.1056/NEJMoa2021436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.