Abstract
Objective: To synthesize the best evidence surrounding the efficacy of cannabinoids for acute pain in the clinical setting based on subjective pain scores and observed adverse effects.
Design: Systematic review with meta-analysis.
Data Sources: PubMed, Embase, Cochrane Databases, and Google Scholar.
Eligibility Criteria: English-language randomized-controlled clinical trials comparing cannabinoids with placebo in patients with acute pain.
Data Extraction and Synthesis: Study quality was assessed using the Cochrane risk of bias tool. All stages were conducted independently by a team of three reviewers. Data were pooled through meta-analysis and stratified by route of administration.
Primary Outcomes and Measures: Patient-reported pain and adverse events (AEs).
Results: Six trials (678 participants) were included examining oral (5 trials) and intramuscular (1 trial) cannabinoids. Overall, there was a small but statistically significant treatment effect favoring the use of cannabinoids over placebo (−0.90, 95% confidence interval [CI] −1.69 to −0.1, i2=65%, p=0.03). When stratified by route of administration, intramuscular cannabinoids were found to have a significant reduction in pain relative to placebo (−2.98, 95% CI −4.09 to −1.87, i2=0%, p<0.0001). No difference in effect was observed between oral cannabinoids and placebo (−0.21, 95% CI −0.64 to 0.22, i2=3%, p=0.34). Serious AEs were rare, and similar across the cannabinoid (14/374, 3.7%) and placebo groups (8/304, 2.6%).
Conclusions: There is low-quality evidence indicating that cannabinoids may be a safe alternative for a small but significant reduction in subjective pain score when treating acute pain, with intramuscular administration resulting in a greater reduction relative to oral. Higher quality, long-term randomized-controlled trials examining whether there may be a role for cannabinoids in treating acute pain are required.
Keywords: acute pain, cannabinoids, meta-analysis, systematic review, surgical pain
Introduction
With the recent legalization of cannabis in Canada, cannabinoids have been gaining mainstream acceptance for potential efficacy in pain management. Typically, the term “cannabinoids” refers to a heterogeneous group of chemicals that are both naturally occurring and can be synthetically derived. There has been a growing dialogue about cannabinoids and their role as an effective tool in the management of pain.1–4 Based on self-reported data by cannabis users, cannabinoids provide relief similar to opioids and decrease opioid consumption.5 Furthermore, there is a growing body of evidence demonstrating the efficacy of cannabinoids for chronic pain conditions.6
There is a paucity of evidence from the scientific literature to support the efficacy of cannabinoid medications in treating acute pain in the clinical setting. To our knowledge, there is only one systematic review and no meta-analyses investigating the effects of cannabinoids in the clinical setting for acute pain.7 Stevens and Higgins concluded that, “on the basis of the available randomized controlled trial evidence, cannabinoids have no role in the management of acute pain.” However, it seems that the conclusions may have been overstated given the limited number of studies included in their review and lack of meta-analysis performed. A meta-analysis offers a consolidated and quantitative review, allowing for a more precise estimate of the effect of the treatment. With that said, our goal was to reassess this subject using systematic review and meta-analysis methodology.
The aim of this systematic review and meta-analysis was to synthesize the best evidence surrounding the efficacy of cannabinoids for acute pain using subjective pain scores, and observed adverse effects.
Methods
We conducted a systematic review and meta-analysis on the efficacy and adverse events (AEs) associated with using cannabinoids for the treatment of acute pain. This review adhered to the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses and Cochrane Collaboration guidelines for the performance and reporting of systematic reviews and meta-analysis.8,9
Literature search
A systematic literature search of PubMed, Embase, Cochrane Databases, and Google Scholar from inception to January 21, 2019, was performed. We utilized a structured search strategy with keywords related to “cannabinoids,” “cannabis” or “marijuana” AND “acute pain” or “postoperative pain,” utilizing MeSH terms where possible. A manual search of related citations was also performed.
Study eligibility
Peer-reviewed randomized-controlled clinical trials comparing cannabinoids with placebo in patients with acute pain were eligible for inclusion. We defined acute pain as a distressing experience secondary to actual or potential tissue damage lasting <3 months. We excluded any studies where the duration of pain was >3 months, or the condition being studied was associated with chronic pain (i.e., diabetic neuropathy, multiple sclerosis). Although there are a number of human experimental acute pain studies, we excluded them for several reasons.10–24 First, there are significant differences in trial design and patient population compared with clinical trials. As well, cannabinoid impact on experimental acute pain was recently explored as the primary topic of a well-done systematic review and meta-analysis.25 Pain outcomes of interest consisted of validated subjective pain scores, including the visual analog scale (VAS), McGill Pain Questionnaire, or the numeric rating scale (NRS).
Exclusion criteria included the following: (1) trials focusing on chronic pain or conditions associated with chronic pain; (2) nonhuman trials or nonclinical trials; (3) abstracts or posters from conferences; and (4) trials with incomplete or absent pain outcome data. Titles and abstracts were then screened for eligibility by two independent reviewers (N.N., K.G.) utilizing the Rayyan QCRI software.26 Any disagreements on eligibility led to inclusion of the study for full-text review by the primary author (A.G.).
Data extraction
Two investigators (N.N., K.G.) collected all relevant information regarding study and patient population, including the study design, patient demographic information, and sample size. Intervention details abstracted included the type, dosage, and route of cannabinoid utilized. Outcomes collected included the type of pain scale used, as well as the mean score and standard deviation (SD) at baseline, and change from baseline or postoperative pain score and SD when available. The following decisions were made regarding the calculation of effect size for the data. When studies utilized multiple pain scores (i.e., on movement vs. rest), a mean pooled effect size was computed for the overall meta-analysis. When multiple cannabinoid types or doses were examined within a single study, these were handled as individual comparisons and the placebo group was divided by the number of comparators. When pain measurements were taken at multiple time points following the pain stimulus, the raw pain score from the largest single time point contrast between the active and placebo conditions was taken (peak effect). If raw pain scores at specific time points were unavailable, we utilized the average pain scores over the study duration, if provided. Pain scores were recorded as mean and SD when available. When not reported, SD was calculated from the other available data in the article. For data presented exclusively in graph format, we utilized a validated data extraction software (WebPlotDigitizer, version 4.1; Ankit Rohatgi) to record outcomes. AEs were recorded by type and overall incidence for each study and classified as serious or nonserious if reported.
Study appraisal
Three independent reviewers (A.G., N.N., K.G.) assessed the methodological quality and study validity utilizing the Cochrane risk of bias tool through Review Manager 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen, Denmark) software.27 Bias was assessed utilizing the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Risk of bias for each of these domains was determined to be low risk, high risk, or unclear risk. The included studies were then given a grade of high risk, moderate risk, or low risk.
The quality of the evidence and the confidence in the estimate of the effect across outcomes were assessed utilizing the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.28,29 Accordingly, data from randomized-controlled trials are considered high-quality evidence, but the quality can be rated down due to risk of bias, imprecision, indirectness, imprecision, and publication bias.29
Data analysis
Pain scores were reported as the mean pain scores following the pain stimulus along with 95% confidence intervals (CIs). Our index instrument of choice was the VAS, which utilizes a scale from 0 to 10, with higher scores representing increased pain. All subjective pain scores were transformed to match our index instrument leading to mean pain scores between 0 and 10. The mean pain scores were analyzed through a random effects model based on the inverse variance method. Total and serious AEs were pooled and odds ratios (ORs) were calculated between the two groups.
Heterogeneity was quantified utilizing the I2 statistic, which estimates the proportion of total variability among studies due to heterogeneity as opposed to chance alone.30,31 The I2 values were then interpreted according to the Cochrane Handbook; I2 0–40% representing low heterogeneity, I2 30–60% representing moderate heterogeneity, I2 50–90% representing substantial heterogeneity, and I2 75–100% representing considerable heterogeneity.27 We planned to stratify our analysis by type of cannabinoid and route of administration to explore results with moderate or substantial heterogeneity. Forest plots were visually inspected to assess for publication bias.
Results
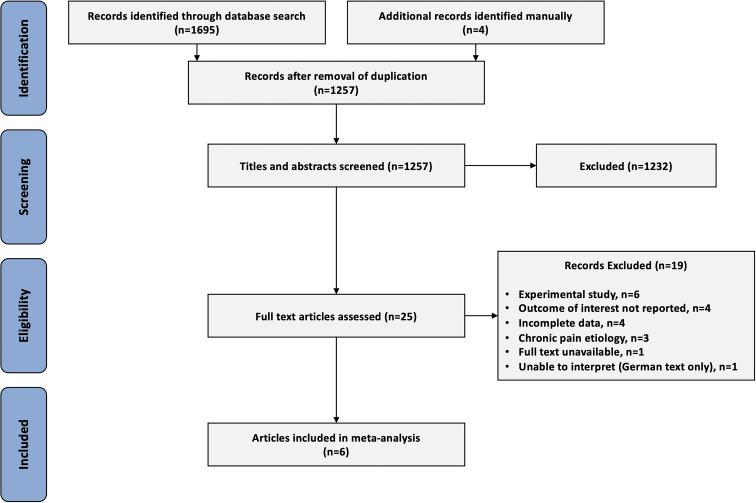
Our search identified 1257 citations after exclusion of duplicates. Twenty-five articles underwent full-text review, and six articles were assessed to be eligible after reviewing the inclusion and exclusion criteria (Fig. 1).32–37 Publication years ranged from 1981 to 2017.
FIG. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Study characteristics
Relevant study characteristics and treatment information are provided in Table 1. The study sample ranged from 30 to 340 participants, for a total of 678 participants. Of the six studies included, two were conducted in the United States, two in Canada, one in the United Kingdom, and one multinational trial completed in the United Kingdom, Italy, and Germany.
Table 1.
Studies Included in the Meta-Analysis
| Study | Total no. of patients (males) | Pain stimulus | Type and dosage of cannabinoid used | Route of administration | Length of follow-up |
|---|---|---|---|---|---|
| Beaulieu32 | 30 (3) | Surgery (gynecologic [46%], orthopedic [44%], other [10%]) | Nabilone (1 or 2 mg) | Oral | 24 h |
| Buggy et al.33 | 40 (0) | Surgery (elective total abdominal hysterectomy) | THC (5 mg) | Oral | 6 h |
| Jain et al.34 | 56 (51) | Acute postoperative, trauma, or fracture pain | Levonantradol (1.5, 2, 2.5, or 3 mg) | Intramuscular | 6 h |
| Kalliomaki et al.35 | 120 (120) | Lower third molar surgical removal | AZD1940 (800 μg) | Oral | 8 h |
| Levin et al.36 | 340 (0) | Elective surgery | Nabilone (0.5 mg) | Oral | 120 min |
| Ostenfeld et al.37 | 92 (49) | Third molar tooth extraction | GW842166 (100 or 800 mg) | Oral | 10 h |
THC, Δ9-tetrahydrocannabinol.
The six included studies were randomized, placebo-controlled, parallel-group trials (n=678). Three studies examined multiple cannabinoid doses, leading to a total of 11 cannabinoid intervention versus placebo comparisons.32–37
Table 2 summarizes the route of administration utilized for the included studies. Oral cannabinoids were examined in 5/6 (83%) of the included studies. Three of these trials examined synthetic Δ9-tetrahydrocannabinol (THC) in the form of nabilone (2) and AZD1940 (1).32,35,36 One study examined the effects of a synthetic CB2-selective agonist, GW842166.37 One study examined a plant-based cannabis oil extract in capsule form.33 Finally, one study analyzed the effects of levonantradol, a synthetic liquid THC, administered intramuscularly.34
Table 2.
Route of Cannabinoid Administration
Risk of bias
Reviewers assessed the risk of bias utilizing the Cochrane risk of bias tool for all included studies (Fig. 2). Two studies were found to be at a low risk of bias, three studies were found to be at moderate risk of bias, and one was found to be at high risk of bias.
FIG. 2.
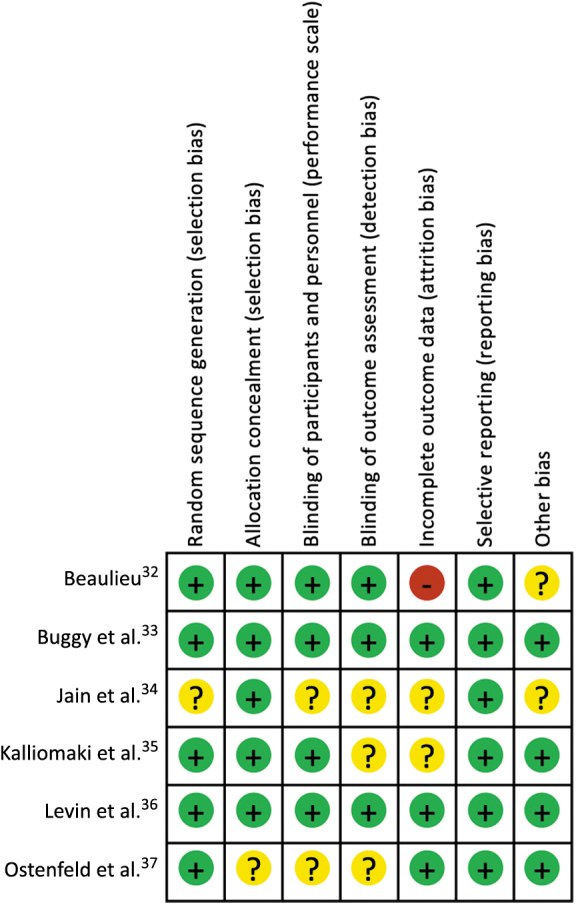
Risk of bias summary.
Reviewers also rated the quality of evidence for included trials utilizing the GRADE approach. The evidence for pain reduction in the included trials was downgraded to “low” given the serious risk of bias and inconsistency noted (Table 3).
Table 3.
Grade Evidence
| Subjects (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | Quality |
|---|---|---|---|---|---|---|
| 678 (6) | Serious | Serious | No serious indirectness | No serious imprecision | None | Low |
Pain outcomes
Meta-analysis results for pain scores are summarized in Table 4. When reviewing all 11 comparisons (678 participants) of the included studies (6), there was a statistically significant treatment effect for the use of cannabinoids (−0.90, 95% CI −1.69 to −0.10, i2=65%, p=0.002). There was significant heterogeneity within this analysis due to one study with a large treatment effect, which utilized a different route of administration (intramuscular vs. oral) when compared with the rest of the clinical trials included.34
Table 4.
Pain Scores
| Route | No. of Studies | No. of Patients | Mean difference [95% CI] |
|---|---|---|---|
| All | 6 | 678 | –0.90 [−1.69, −0.10] |
| Oral | 5 | 622 | –0.21 [−0.64, 0.22] |
| Intramuscular | 1 | 56 | –2.98 [−4.09, −1.87] |
CI, confidence interval; IV, inverse variance.
Given this marked difference, we stratified results based on route of administration. There were five studies (7 comparisons and 622 patients) that reported on oral cannabinoids and found no significant treatment effect when compared with placebo (−0.21, 95% CI −0.64 to 0.22, i2=3%, p=0.41. There was one trial (4 comparisons, 56 participants) that evaluated intramuscular administration of cannabinoids and found a significant treatment effect favoring the use of cannabinoids over placebo (−2.98, 95% CI −4.09 to −1.87, i2=39%, p<0.0001).
Adverse events
All six studies reported AEs experienced in both the placebo and cannabinoid arms. Examining these studies, there was a total of 437 events in 374 patients (1.17 AE/patient) in the cannabinoid group and 309 side effects in 304 patients in the control group (1.02 AE/patient). In the cannabinoid group, there was a significantly higher incidence of dizziness and hypotension (Table 5).
Table 5.
Common and Serious Adverse Events
| Event | Cannabis | Placebo | OR (CI) | p |
|---|---|---|---|---|
| Nausea | 102 | 80 | 1.05 (0.75–1.48) | 0.78 |
| Sedation | 66 | 61 | 0.85 (0.58–1.26) | 0.42 |
| Dizziness | 58 | 26 | 1.96 (1.20–3.20) | 0.007* |
| Dry mouth | 49 | 49 | 0.78 (0.51–1.20) | 0.27 |
| Headache | 41 | 31 | 1.08 (0.66–1.78) | 0.75 |
| Increased awareness/psychological high | 9 | 2 | 3.72 (0.80–17.40) | 0.09 |
| Hypotension | 13 | 3 | 3.61 (1.02–12.80) | 0.047* |
| Red eyes | 1 | 0 | 2.44 (0.10–60.10) | 0.59 |
| Malaise/fatigue | 10 | 8 | 1.02 (0.40–2.60) | 0.97 |
| Vomiting | 9 | 3 | 2.47 (0.66–9.22) | 0.17 |
| Serious adverse reaction | 14 | 8 | 1.44 (0.60–3.48) | 0.42 |
Indicates statistical significance between groups (p<0.05).
There was no significant difference in serious adverse effects (OR 1.44 [0.60–3.48]).
Overall, 22/678 (3.2%) of the patient encounters included in the meta-analysis had serious adverse effects. There were 14/374 (3.7%) serious AEs in the cannabinoid arms and 8/304 (2.6%) in the control group. Serious adverse effects in the treatment groups consisted of nausea (5), headache (4), sedation (3), dysphagia (1), and pharyngolaryngeal pain (1). Side effects in the placebo group consisted of headache (3), nausea (2), myalgia (1), pain (1), and swelling (1).
Discussion
Systematic review and meta-analysis of the available literature revealed that there was a small but significant reduction in subjective pain scores when compared with placebo in patients experiencing acute pain.
There was a significant difference in the effect size between oral and nonoral routes of administration. This indicated that there may be differences in efficacy for acute pain based on route of administration. Varying results based on route of administration have been well documented in the chronic pain population.3 These differences are not surprising given that pharmacokinetics varies as a function of the route of absorption.38 Specifically, oral absorption of cannabinoids is slow and variable with maximal plasma concentrations occurring 60–120 min postingestion but can be delayed upward of 6 h.38 Cannabinoids are subjective to significant first-pass liver metabolism, which further reduces the bioavailability.39 Utilizing transdermal, inhaled, or oral transmucosal formulations allows for direct plasma uptake and avoidance of the first-pass effect. Inhaled cannabinoids reach peak effect in 10 min and plasma levels are maintained for several hours.40
There was a high rate of AEs in both groups. This is not surprising given that all subjects involved underwent a pain stimulus making it difficult to attribute side effects to the cannabinoids versus the pain stimulus or a combination of the two. However, there was a significant increase in nonserious AEs in the cannabinoid group. There was no evidence of increase in serious AEs, suggesting an overall favorable safety profile for cannabinoids in this population.
Given the well-documented harms of prescribing opioids, patients and providers alike are searching for safer nonopioid analgesics for acute pain.41 With the recent legalization of recreational cannabis in Canada and the legalization of medical cannabis in 33 U.S. states and Washington, District of Columbia, as of January 2020, there is significant interest surrounding the potential benefits and harms of cannabinoids as an analgesic agent. A recently published retrospective analysis of postoperative cannabinoid use supports our data suggesting that there may be a role for cannabinoids in the management of acute pain.42 Hickernell et al. found that postoperative dronabinol led to significantly reduced length of stay and trended toward lower opioid consumption in primary total joint arthroplasty patients.42 Interestingly, they found that VAS pain scores remained the same between the two groups, indicating that we may need different outcome measures to better understand how cannabinoids effect acute pain. Holdcroft et al. utilized a dose-escalation protocol for postoperative oral THC, and demonstrated significantly improved subjective pain scores and a reduced need for rescue analgesia in postoperative patients.43 However, other level III evidence suggested that utilization of cannabinoids perioperatively leads to worse pain scores postoperatively. Prabhu performed a retrospective review of patients undergoing major orthopedic surgery44 and found that patients on preoperative cannabinoids had higher postoperative pain scores when compared with a propensity-matched cohort that did not utilize cannabinoids.
Shi45 evaluated the State Inpatient Databases for both cannabis- and opioid-related hospitalizations before and after legalization of medical cannabis. Regardless of legalization status, hospitalizations related to both cannabis and opioids increased significantly over the time period analyzed (1997–2004). However, states with legalization demonstrated that in the years following legalization there was a 23% reduction in hospitalizations related to opioid dependence and a 13% reduction in opioid-related overdoses.45 Legalization had no association with cannabis-related hospitalizations.
Our understanding of the antinociceptive effects of cannabinoids is evolving with the rapidly growing body of preclinical and clinical data. THC acts on various components of the endogenous cannabinoid system (ECS) to exert anti-inflammatory and antinociceptive effects.46–48 THC interacts with the CB1 and CB2 receptors in the ECS and has been found to downregulate proinflammatory markers in preclinical trials.49,50 Several animal model trials have found that both agonists of the CB1 and CB2 receptors play a role in acute antinociception.51 Cannabidiol (CBD), the major nonpsychoactive cannabis compound, may elicit analgesic effects through different cellular pathways.52,53 CBD has been found to be an agonist to several cell surface receptors associated with anti-inflammatory and analgesic pathways.53–55
Interestingly, of the studies included in this review, all of them examined the effects of trans-THC predominately or exclusively. However, there is a growing body of literature that suggests that CBD has analgesic and anti-inflammatory pathways with a more favorable side effect profile.56 There have been several animal studies showing reductions in pain and inflammation in osteoarthritis and neuropathic pain models.53,57,58 Given the promising animal studies and favorable safety profile, further human trials examining the efficacy of CBD for the management of acute pain and inflammation are warranted.
Strengths and limitations
This study was an exhaustive review of the literature and includes recent studies that have not previously been assessed and represents the most current literature on the evidence for use of cannabinoids for acute pain. We followed conduct and reporting guidelines for the systematic reviews and meta-analysis, including duplicate assessment of study eligibility and a risk of bias evaluation. To our knowledge, this is the first study to perform a meta-analysis on cannabinoids in acute pain in clinical trials.
This study has several limitations. Our review was limited by the overall quality and quantity of the available trials, as well as inconsistency in the reporting of outcome and AE reporting. The majority of studies included in this review were of relatively small sample size and largely underpowered. There was significant heterogeneity in the studies included. Variation existed in the type, dosage, timing, duration, and route of cannabinoid used.
Given the current available evidence, there is low-quality evidence that cannabinoids have a small, but statistically significant reduction in acute pain in the clinical setting. At the time of this publication, there remain few randomized-controlled trials for the use of cannabinoids for acute pain. Our review highlights the need for further research to investigate the optimal route and composition of cannabinoids in the acute pain setting, including large, high-quality randomized clinical trials to better understand the risks and benefits of cannabinoids in this patient population.
Abbreviations Used
- AEs
adverse events
- CBD
cannabidiol
- CI
confidence interval
- ECS
endogenous cannabinoid system
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- IV
inverse variance
- NRS
numeric rating scale
- ORs
odds ratios
- SD
standard deviation
- THC
Δ9-tetrahydrocannabinol
- VAS
visual analog scale
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Cite this article as: Gazendam A, Nucci N, Gouveia K, Abdel Khalik H, Rubinger L, Johal H (2020) Cannabinoids in the management of acute pain: a systematic review and meta-analysis, Cannabis and Cannabinoid Research 5:4, 290–297, DOI: 10.1089/can.2019.0079.
References
- 1. Aggarwal SK. Cannabinergic pain medicine: a concise clinical primer and survey of randomized-controlled trial results. Clin J Pain. 2013;29:162–171 [DOI] [PubMed] [Google Scholar]
- 2. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiting PF, Wolff RF, Deshpande S, et al. . Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473 [DOI] [PubMed] [Google Scholar]
- 4. Madden K, van der Hoek N, Chona S, et al. . Cannabinoids in the management of musculoskeletal pain: a critical review of the evidence. JBJS Rev. 2018;6:e7. [DOI] [PubMed] [Google Scholar]
- 5. Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20:E755–E796 [PubMed] [Google Scholar]
- 7. Stevens A, Higgins M. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand. 2017;61:268–280 [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons: Chichester, UK, 2008 [Google Scholar]
- 10. Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38:1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalliomäki J, Philipp A, Baxendale J, et al. . Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol. 2012;39:336–342 [DOI] [PubMed] [Google Scholar]
- 12. Kraft B, Frickey NA, Kaufmann RM, et al. . Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101–110 [DOI] [PubMed] [Google Scholar]
- 13. Lee MC, Ploner M, Wiech K, et al. . Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain. 2013;154:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redmond WJ, Goffaux P, Potvin S, et al. . Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin. 2008;24:1017–1024 [DOI] [PubMed] [Google Scholar]
- 15. Rukwied R, Watkinson A, McGlone F, et al. . Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain. 2003;102:283–288 [DOI] [PubMed] [Google Scholar]
- 16. Cooper ZD, Bedi G, Ramesh D, et al. . Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 2018;43:2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper ZD, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend. 2016;167:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esfandyari T, Camilleri M, Busciglio I, et al. . Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol. 2007;293:G137–G145 [DOI] [PubMed] [Google Scholar]
- 19. Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275 [DOI] [PubMed] [Google Scholar]
- 20. Naef M, Curatolo M, Petersen-Felix S, et al. . The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88 [DOI] [PubMed] [Google Scholar]
- 21. Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58 [DOI] [PubMed] [Google Scholar]
- 22. van Amerongen G, Siebenga P, de Kam ML, et al. . Effect profile of paracetamol, Δ9-THC and promethazine using an evoked pain test battery in healthy subjects. Eur J Pain. 2018;22:1331–1342 [DOI] [PubMed] [Google Scholar]
- 23. Wallace M, Schulteis G, Atkinson JH, et al. . Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:785–796 [DOI] [PubMed] [Google Scholar]
- 24. Walter C, Oertel BG, Felden L, et al. . Brain mapping-based model of Δ(9)-tetrahydrocannabinol effects on connectivity in the pain matrix. Neuropsychopharmacology. 2016;41:1659–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Vita MJ, Moskal D, Maisto SA, et al. . Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouzzani M, Hammady H, Fedorowicz Z, et al. . Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. John Wiley & Sons: Chichester, UK, 2011 [Google Scholar]
- 28. Welch VA, Akl EA, Guyatt G, et al. . GRADE equity guidelines: 1. Considering health equity in GRADE guideline development: introduction and rationale. J Clin Epidemiol. 2017;90:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balshem H, Helfand M, Schünemann HJ, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JPT, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 32. Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth. 2006;53:769–775 [DOI] [PubMed] [Google Scholar]
- 33. Buggy DJ, Toogood L, Maric S, et al. . Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106:169–172 [DOI] [PubMed] [Google Scholar]
- 34. Jain AK, Ryan JR, McMahon FG, et al. . Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol. 1981;21:320S–326S [DOI] [PubMed] [Google Scholar]
- 35. Kalliomaki J, Segerdahl M, Webster L, et al. . Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand J Pain. 2013;4:17–22 [DOI] [PubMed] [Google Scholar]
- 36. Levin DN, Dulberg Z, Chan A, et al. . A randomized controlled trial of nabilone for the prevention of postoperative nausea and vomiting in elective surgery. Can J Anaesth. 2017;64:385–395 [DOI] [PubMed] [Google Scholar]
- 37. Ostenfeld T, Price J, Albanese M, et al. . A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668–676 [DOI] [PubMed] [Google Scholar]
- 38. Bruni N, Della Pepa C, Oliaro-Bosso S, et al. . Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23:2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360 [DOI] [PubMed] [Google Scholar]
- 40. Hartman RL, Brown TL, Milavetz G, et al. . Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem. 2015;61:850–869 [DOI] [PubMed] [Google Scholar]
- 41. Understanding the Epidemic. Centers for Disease Control and Prevention: 2017. Available at: https://www.cdc.gov/drugoverdose/epidemic/index.html (last accessed January6, 2020).
- 42. Hickernell TR, Lakra A, Berg A, et al. . Should cannabinoids be added to multimodal pain regimens after total hip and knee arthroplasty? J Arthroplasty. 2018;33:3637–3641 [DOI] [PubMed] [Google Scholar]
- 43. Holdcroft A, Maze M, Dore C, et al. . A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology. 2006;104:1040–1046 [DOI] [PubMed] [Google Scholar]
- 44. Prabhu AJ. Weeding out the problem: the impact of preoperative cannabinoid use on pain in the perioperative period. Pain. 2018;16:17. [DOI] [PubMed] [Google Scholar]
- 45. Shi Y. Medical marijuana policies and hospitalizations related to marijuana and opioid pain reliever. Drug Alcohol Depend. 2017;173:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donvito G, Nass SR, Wilkerson JL, et al. . The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nagarkatti P, Pandey R, Rieder SA, et al. . Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB 2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:1–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001;81:715–723 [DOI] [PubMed] [Google Scholar]
- 50. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400. [DOI] [PubMed] [Google Scholar]
- 51. Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Urits I, Borchart M, Hasegawa M, et al. . An update of current cannabis-based pharmaceuticals in pain medicine. Pain Ther. 2019;8:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ward SJ, McAllister SD, Kawamura R, et al. . Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodríguez-Muñoz M, Onetti Y, Cortés-Montero E, et al. . Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol Brain. 2018;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li H, Kong W, Chambers CR, et al. . The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell Immunol. 2018;329:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, et al. . Safety and side effects of cannabidiol, a cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249 [DOI] [PubMed] [Google Scholar]
- 57. Hammell D, Zhang L, Ma F, et al. . Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Philpott HT, O'Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]