Abstract
Introduction: Cannabinoid hyperemesis syndrome is becoming a more prominently reported side effect of cannabis containing high-dose Δ9-tetrahydrocannabinol (THC) and designer cannabinoid drugs such as “Spice.” One active ingredient that has been found in “Spice” is 1-pentyl-3-(1-naphthoyl)indole (JWH-018), a synthetic full agonist of the cannabinoid 1 (CB1) receptor. In this study, we evaluated the potential of different doses of JWH-018 to produce conditioned gaping in rats, an index of nausea.
Materials and Methods: Rats received 3 daily conditioning trials in which saccharin was paired with JWH-018 (0.0, 0.1, 1, and 3 mg/kg, intraperitoneal [i.p.]). Then the potential of pretreatment with the CB1 antagonist, rimonabant (SR), to prevent JWH-018-induced conditioned gaping was determined. To begin to understand the potential mechanism underlying JWH-018-induced nausea, serum collected from trunk blood was subjected to a corticosterone (CORT) analysis in rats receiving three daily injections with vehicle (VEH) or JWH-018 (3 mg/kg).
Results: At doses of 1 and 3 mg/kg (i.p.), JWH-018 produced nausea-like conditioned gaping reactions. The conditioned gaping produced by 3 mg/kg JWH-018 was reversed by pretreatment with rimonabant, which did not modify gaping on its own. Treatment with JWH-018 elevated serum CORT levels compared to vehicle-treated rats.
Conclusions: As we have previously reported with high-dose THC, JWH-018 produced conditioned gaping in rats, reflective of a nausea effect mediated by its action on CB1 receptors and accompanied by elevated CORT, reflective of hypothalamic-pituitary-adrenal (HPA) activation.
Keywords: cannabinoid 1 receptor, corticosterone, emesis, HPA axis, nausea, synthetic cannabinoid
Introduction
Cannabinoid hyperemesis syndrome (CHS) is increasingly being reported as an adverse side effect of high-dose cannabis use, which is characterized by cyclical episodes of intense nausea and vomiting with severe abdominal pain.1–3 These human reports are consistent with laboratory findings using animal models, as high doses of the partial agonist of the cannabinoid 1 (CB1) receptor, Δ9-tetrahydrocannabinol (THC), produce vomiting in shrews,4,5 monkeys,6 cats,7 and dogs,8 and nausea-like conditioned gaping in rats.9 CHS can also be caused by use of “Spice,” a group of designer drugs that contain synthetic cannabinoids, such as the potent 1-pentyl-3-(1-naphthoyl)indole (JWH-018).10–15 Unlike THC, JWH-018 acts as a full agonist of CB1.10–15 Spice is associated with more severe side effects, including anxiety, panic, delusions, and hallucinations, as well as nausea and vomiting.10–12,16–20 Individuals who use Spice are more likely than cannabis users to seek emergency room treatments because of its more adverse side effects,21 including nausea and vomiting.22
The nauseating and proemetic effects of cannabinoids are paradoxical, given that cannabis and synthetic THC (i.e., Nabilone, Marinol) are recognized and approved treatments for chemotherapy-induced nausea and vomiting.23 Indeed, low doses of THC and synthetic cannabinoids reduce nausea in rats24,25 and vomiting in shrews and ferrets5,26–28 by acting on CB1 receptors of the endocannabinoid system. The findings that low doses suppress and high doses produce nausea and vomiting is another example of the typical biphasic dose effects of cannabinoids.29–32 Given that high-dose THC produces an elevation of the glucocorticoid stress hormone corticosterone (CORT) in rats,33,34 it has been suggested that cannabinoid-induced nausea and vomiting, and consequently CHS, are the result of a dysregulated endocannabinoid system producing a stress response following excessive activation of the CB1 receptor.1,35 Increased hypothalamic-pituitary-adrenal (HPA) axis activity is associated with other forms of nausea and vomiting as well.36–48 Circulating CORT in rodents and cortisol in humans, and adrenocorticotropic hormone (ACTH) are increased during nausea and vomiting,33,34 which is elevated by cannabinoid use.40
Rats are an effective animal model for studying the nauseating effects of cannabis. Although rats cannot vomit, they receive the same gastrointestinal signals that precede vomiting in ferrets, which can vomit; thus, rats have the mechanisms to detect the nauseating effects of toxins, but lack the motor output for vomiting.41–44 Considerable evidence suggests that rats display the unique behavior of gaping45 (wide open mouth with lower incisors exposed) when exposed to a flavor previously paired with an emetic drug, such as lithium chloride (LiCl), in the taste reactivity (TR) paradigm.46 Also, antiemetic drugs prevent the establishment of LiCl-induced conditioned gaping reactions, without modifying the nonselective measure of conditioned taste avoidance. Furthermore, the rat gape recruits the same orofacial muscles that are required for vomiting in emetic species.47 Thus, conditioned gaping provides a unique tool for investigating the neurobiology of nausea in rats.
In this study, we evaluated the ability of a range of doses of the synthetic cannabinoid, JWH-018, to produce conditioned gaping and evaluated the role of the CB1 receptor in this effect. Moreover, since a dysregulated stress response has been hypothesized to contribute to THC-induced nausea, circulating CORT was also measured in animals administered JWH-018.
Materials and Methods
Subjects
Animal procedures were according to the Canadian Council on Animal Care (CCAC) and the National Institute of Health guidelines. All protocols were approved by the Institutional Animal Care Committee at the University of Guelph, which is accredited by the CCAC. A total of 80 naive male Sprague Dawley rats obtained from Charles River Laboratories (St Constant, Canada) were used in all experiments. Rats were single housed and maintained as described by DeVuono et al.9 On the first day of conditioning, for experiments 1 and 2, rats weighed between 273 and 403 g and between 262 and 324 g on the day of blood collection for experiment 4. All experimental manipulations occurred during the dark phase of the cycle.
Drugs
The method of injection for all drugs used was intraperitoneal (i.p.). JWH-018 and rimonabant (SR 141716A; SR) were dissolved in ethanol and then Tween 80 (Sigma) was added to the solution in a graduated cylinder. Using a nitrogen stream, the ethanol was then evaporated off. Saline (SAL) was then added to the solution. The final vehicle (VEH) solution was composed of 1:9 Tween:SAL. JWH-018 was mixed at a concentration of 0.1, 1, and 3 mg/mL and administered i.p. at 1 mL/kg (0.1, 1, and 3 mg/kg). Rimonabant was prepared as a 1 mg/mL solution of the vehicle and was administered at volumes of 1 mL/kg (1 mg/kg).
TR apparatus
The TR chamber was a clear Plexiglas chamber (25×25×12.5 cm) with a removable, opaque Plexiglas lid that was placed on top of a table with a clear glass top and a mirror beneath the chamber at a 45° angle to record the rat's ventral surface. The intraoral infusions were delivered by an infusion pump (Model KDS100; KD Scientific, Holliston, MA) attached to intraoral cannulae. A video camera pointed toward the mirror below the chamber recorded the rat's behavior during conditioning and test trials.
Intraoral cannulation surgery
The rats in experiments 1 and 2 were implanted with intraoral cannulae as described by Limebeer et al.48 For 3 days following surgery, indices of recovery were assessed (body weight, facial swelling, activity, etc.), and the cannula was flushed daily with an oral cleansing solution (Nolvdent; Ayerst, Fort Dodge, IA).
Behavioral procedures
Experiment 1: dose–response potential of JWH-018 to produce conditioned gaping
As reported by DeVuono et al.,9 following 3 days of recovery from intraoral surgery, the rats were adapted to the TR chamber. Water was infused into their intraoral cannulae through an infusion pump for 2 min at the rate of 1 mL/min. Following the adaptation trial, the rats received a series of three consecutive conditioning trials occurring 24 h apart. For each conditioning trial, the rats were intraorally infused with 0.1% saccharin solution for 2 min at the rate of 1 mL/min. During this time, the orofacial responses were video recorded from the mirror beneath the chamber. Immediately following the saccharin infusion, the rat's intraoral cannulae were flushed with distilled water. Rats were then injected with VEH, 0.1 mg/kg JWH-018, 1 mg/kg JWH-018, or 3 mg/kg JWH-018 (n=8 rats per group) according to their random group assignment. The test trial occurred 24 h after the third conditioning trial and followed the same procedure as the conditioning trial, except rats were not injected after the saccharin infusion. Video recordings were later scored using the Observer (Noldus) event recording program. The number of gaping reactions (defined by rapid, large amplitude opening of the mouth to expose the lower incisors) that occurred in each 2-min session was counted by an observer blind to group assignment.
Experiment 2: effects of rimonabant on the establishment of JWH-018-induced conditioned gaping
The rats were treated as in experiment 1, except as indicated. Rats were randomly assigned to groups based on pretreatment drug, rimonabant (SR) or VEH, and conditioning drug, 3 mg/kg JWH-018 or VEH, resulting in four groups (VEH-VEH, SR-VEH, VEH-JWH, and SR-JWH; n=8 rats per group). On each of three trials (24 h apart), the rats were injected i.p. with either 1 mg/kg SR or VEH 30 min before being infused with 0.1% saccharin for 2 min at a rate of 1 mL/min, while orofacial reactions were recorded from the mirror beneath the TR chamber. Immediately following saccharin infusion, the rats were injected with 3 mg/kg JWH-018 or VEH and returned to their home cage. The following day, the rats received a drug-free test trial as in experiment 1.
Experiment 3: effects of JWH-018 on serum CORT levels
Rats were randomly assigned to treatment conditions (VEH or 3 mg/kg JWH-018; n=8 rats per group) and injected i.p. with their treatment every 24 h for 3 consecutive days. The rats were sacrificed 30 min following their last respective injection by rapid decapitation (restrained in a decapicone; Braintree Scientific).49 Trunk blood was collected following decapitation and separated by centrifugation, and the resulting serum was stored at −80°C before analysis. Serum samples were analyzed with an enzyme-linked immunosorbent assay kit (Arbor Assays) by following the manufacturer's instructions. Samples were tested in triplicate and diluted 1:1000 to make sure levels fit into the standard curve. Results are reported in nanograms per milliliter (ng/mL).
Statistical analysis
In experiment 1, the number of gaping reactions was entered into a 4×4 mixed-factor analysis of variance (ANOVA) with the between-group factor of group (VEH, JWH 0.1, 1, and 3) and the within-group factor of conditioning/test trial. In experiment 2, the number of gaping reactions was entered into a 4×4 mixed-factor ANOVA with the between-group factor of group (VEH-VEH, SR-VEH, and VEH-JWH SR-JWH) and the within-group factor of conditioning/testing trial. In experiment 3, mean serum CORT levels (ng/mL) were entered into an independent t-test (VEH or 3 mg/kg JWH-018). For all statistical analyses, significance was defined as p<0.05.
Results
Experiment 1: dose–response potential of JWH-018 to produce conditioned gaping
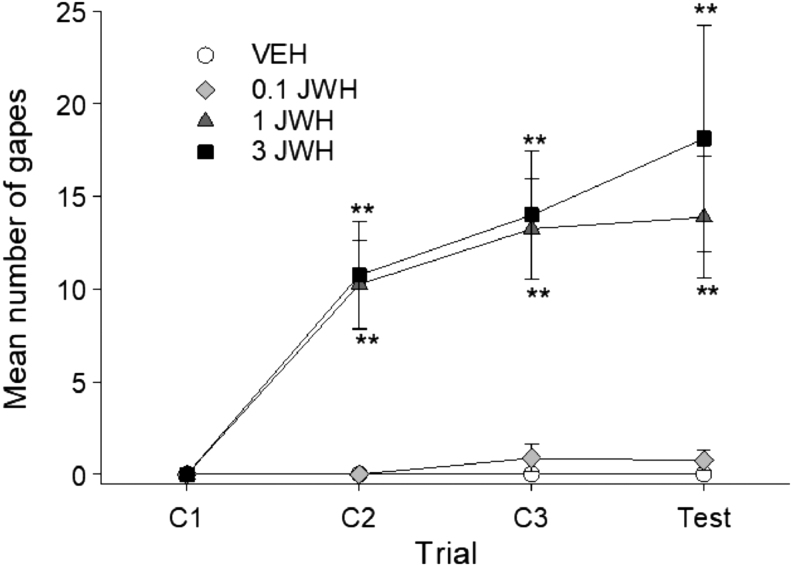
JWH-018 produced dose-dependent gaping reactions across the four conditioning/test trials. Figure 1 represents the mean number of gaping reactions produced by JWH-paired saccharin on each trial. The 4×4 mixed factor ANOVA revealed significant effects of group, F(3,28)=12.44; p<0.001, trial, F(3,84)=15.56; p<0.001, and a group×trial interaction, F(9,84)=4.88; p<0.001. Bonferroni post hoc analysis revealed that groups 1 and 3 mg/kg JWH both gaped significantly more that VEH or 0.1 mg/kg JWH on trials 2 (p's<0.01) and 3 (p's<0.01). At test, the 3 mg/kg JWH group gaped significantly more than VEH (p<0.01) or 0.1 mg/kg JWH (p<0.01). Neither the VEH and 0.1 mg/kg JWH groups, nor the 1 and 3 mg/kg group differed at any trial.
FIG. 1.
Mean (±SEM) number of gapes elicited by JWH-018-paired saccharin solution across conditioning/testing trials. JWH-018 dose-dependently produced conditioned gaping reaction. Animals conditioned with 1 or 3 mg/kg JWH-018 displayed significantly more gaping reactions than animals conditioned with 0.1 mg/kg or VEH. **p<0.01 compared to VEH, n=8 for all groups. JWH-018, 1-pentyl-3-(1-naphthoyl)indole; SEM, standard error of the mean; VEH, vehicle.
Experiment 2: effects of rimonabant on the establishment of JWH-induced conditioned gaping
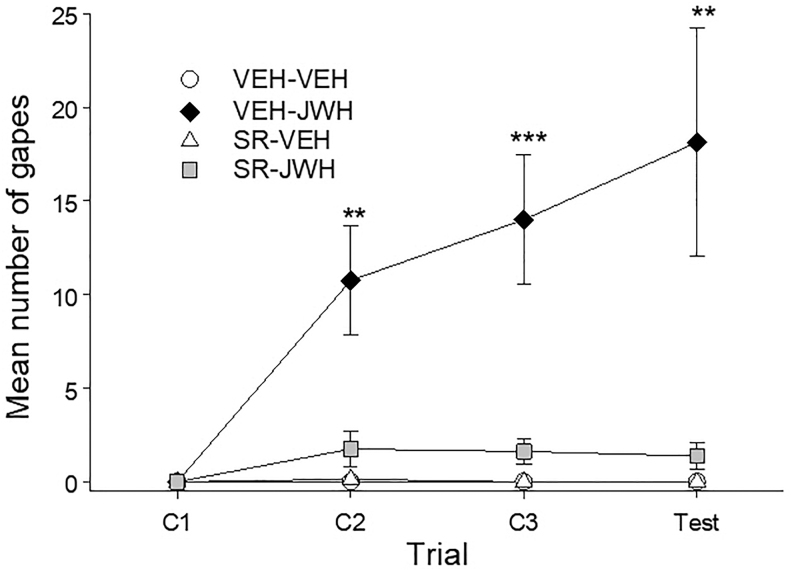
Pretreatment with rimonabant (SR; 1 mg/kg) interfered with the establishment of conditioned gaping elicited by 3 mg/kg JWH 018. Figure 2 represents the mean number of gapes displayed by the various groups on each of the trials. The 4×4 mixed-factor ANOVA for number of gapes for each trial revealed significant effects of group, F(3,28)=12.40; p<0.001, trial, F(3,84)=8.59; p<0.001, and a significant group×trial interaction, F(9,84)=6.91; p<0.001. Subsequent Bonferroni post hoc tests revealed that group VEH-JWH gaped significantly more than the VEH-VEH, SR-VEH, and SR-JWH groups at trial 2 (p's<0.01) and 3 (p's<0.001), and test (p's<0.01). The VEH-VEH, SR-VEH, and SR-JWH groups did not differ at any trial.
FIG. 2.
Mean (±SEM) number of gapes elicited by JWH-018- or VEH-paired saccharin solution when pretreated with rimonabant (SR, 1 mg/kg) or VEH across conditioning/testing trials. Pretreatment with rimonabant prevented the establishment of JWH-018-induced conditioned gaping without producing gaping on its own. **p<0.01, ***p<0.001 compared to VEH-VEH group, n=8 for all groups.
Experiment 3: effects of JWH-018 on serum CORT levels
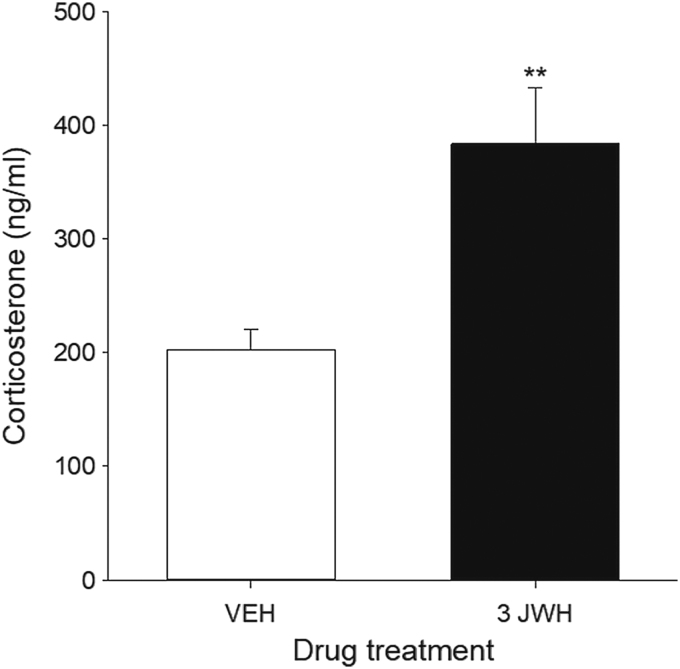
Serum CORT levels (ng/mL) were significantly increased in rats treated with 3 mg/kg JWH-018, a dose that produced conditioned gaping reactions in experiments 1 and 2. Figure 3 represents the mean serum CORT levels in rats treated with VEH or 3 mg/kg JWH-018 for 3 consecutive days. The independent sample t-test revealed a significant difference in serum CORT levels between VEH- and JWH-018-treated rats, t(14)=3.45, p=0.004.
FIG. 3.
Mean (±SEM) serum levels of corticosterone (ng/mL) of rats treated with VEH or 3 mg/kg JWH-018. Rats treated with 3 mg/kg JWH-018 had significantly elevated serum corticosterone compared to VEH-treated rats. **p<0.01 compared to VEH, n=8 for all groups.
Discussion
Consistent with high-dose THC,9 JWH-018 produced a dose-dependent increase in nausea-induced conditioned gaping reactions in rats at i.p. doses of 1 and 3 mg/kg, but not 0.1 mg/kg. This effect was mediated by the action of JWH-018 at the CB1 receptor because rimonabant prevented the conditioned gaping by 3 mg/kg JWH-018 without having any effect on its own. Daily exposure to JWH-018 elevated serum CORT, suggesting an increase of HPA tone. Activation of the HPA axis is associated with nausea and vomiting,40,50 and a dysregulated HPA axis has been proposed to contribute to nausea and vomiting disorders such as cyclical vomiting syndrome37–39,51 and CHS.40,52
The finding that 1 and 3 mg/kg JWH-018 produced conditioned gaping is consistent with our previous findings that high doses of THC (5 and 10 mg/kg, i.p.), but not a low dose (0.5 mg/kg), produce conditioned gaping in the TR test in rats.9 Nausea is a common side effect of Spice use in humans, which is reported more often than with THC use.22 JWH-018 is a full agonist at the CB1 and is more potent and efficacious than the partial CB1 receptor agonist, THC; therefore, it is not surprising that JWH-018 would produce conditioned gaping at lower doses than THC (5 and 10 mg/kg).9 The lowest dose of JWH-018 tested, 0.1 mg/kg, did not produce conditioned gaping responses, following the same dose-dependent pattern seen with THC.
Pretreatment with rimonabant, a CB1 receptor antagonist/inverse agonist, prevented the establishment of conditioned gaping produced by JWH-018. This finding suggests that the nauseating effects of JWH-018 are mediated by the drug's action at the CB1 receptor, matching what was previously found with THC-induced nausea.9 There is evidence that inverse agonists of the CB1 receptor, like rimonabant, can produce nausea and vomiting in animals26,48,53 and humans.54,55 However, the dose of rimonabant used in this experiment (1 mg/kg, i.p.) has been previously shown not to produce conditioned gaping,9,25 nor impact learning of the association of nausea paired with a taste.9 Indeed, in this experiment, rimonabant pretreatment did not produce conditioned gaping on its own.
A prominent hypothesis about the etiology of CHS is that cannabinoid-induced nausea and vomiting are the result of a dysregulated endocannabinoid system.1,35 We previously found that repeated administration of a high dose of THC that produces conditioned gaping also produced an upregulation of the genes for the degrading enzyme of the endocannabinoid anandamide (AEA) and 2-arachidonoylglycerol (2-AG), fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively, in the hypothalamus, a region critical for regulation of the HPA axis.9 Tai et al.56 found that repeated exposure to THC or the full CB1 agonist JWH-018 produced downregulation, and desensitization of hypothalamic CB1 receptors in mice; however, they did not measure expression of genes for enzymes that degrade endocannabinoids. The endocannabinoid system in the hypothalamus is required for allostasis of the HPA axis.57–59 The endocannabinoids, AEA and 2-AG, inhibit the HPA axis by inhibiting glutamate release in the hypothalamus by acting on CB1 receptors on glutamatergic terminals.60,61 High doses of CB1 receptor agonists, on the other hand, activate the HPA axis33,34,62; however, the mechanism for how this occurs remains unknown. It is unlikely that high doses of cannabinoids activate the HPA axis by directly acting on the hypothalamus, as agonism of the CB1 receptor in this region inhibits the HPA axis. Indeed, hypothalamic deafferented rats do not show increased ACTH or CORT following a high dose of cannabinoids.34 Therefore, it is thought that cannabinoids activate the HPA axis indirectly by altering input to the hypothalamus.62
Activation of the HPA axis is associated with nausea and vomiting,40,50 and a dysregulated HPA axis has been proposed to cause nausea and vomiting disorders such as cyclical vomiting syndrome37–39,51 and CHS.40,52 The relationship between the HPA axis and nausea is still correlational and more research is needed to understand the direction and causality of this association. Indeed, a significant increase in serum CORT levels was found in rats treated with 3 mg/kg JWH-018, indicating activation of the HPA axis. Future experiments that directly measure endocannabinoid levels in regions regulating the HPA activation may elucidate the relationships between the endocannabinoid system, stress systems, and nausea.
Conclusion
Overall, these experiments show that JWH-018, a potent CB1 receptor agonist found in recreational synthetic cannabinoids drugs, produces dose-dependent nausea by acting at the CB1 receptor and results in an elevation of serum CORT, suggestive of activation of the HPA axis.
Acknowledgment
The authors would like to thank David M. Mutch, Department of Human Health Research, University of Guelph, for assistance with this article.
Abbreviations Used
- 2-AG
2-arachidonoylglycerol
- ACTH
adrenocorticotropic hormone
- AEA
anandamide
- ANOVA
analysis of variance
- CB1
cannabinoid 1
- CCAC
Canadian Council on Animal Care
- CHS
cannabinoid hyperemesis syndrome
- CORT
corticosterone
- HPA
hypothalamic-pituitary-adrenal
- i.p.
intraperitoneal
- JWH-018
1-pentyl-3-(1-naphthoyl)indole
- LiCl
lithium chloride
- SAL
saline
- THC
Δ9-tetrahydrocannabinol
- TR
taste reactivity
- VEH
vehicle
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Information
This research was supported by research grants from the Natural Sciences and Engineering Research Council of Canada (NSERC-03629) and the Canadian Institutes of Health Research (CIHR-388239) to L.A.P.
Cite this article as: DeVuono MV, Hrelja KM, Petrie GN, Limebeer CL, Rock EM, Hill MN, Parker LA (2019) Nausea-induced conditioned gaping reactions in rats produced by high dose synthetic cannabinoid, JWH-018, Cannabis and Cannabinoid Research 5:4, 298–304, DOI: 10.1089/can.2019.0103.
References
- 1. Allen JH, De Moore G, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venkatesan T, Sengupta J, Lodhi A, et al. An internet survey of marijuana and hot shower use in adults with cyclic vomiting syndrome (CVS). Exp Brain Res. 2014;232:2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sorensen CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J Med Toxicol. 2017;13:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cluny NL, Naylor RJ, Whittle BA, et al. The effects of cannabidiol and tetrahydrocannabinol on motion-induced emesis in Suncus murinus. Basic Clin Pharmacol Toxicol. 2008;103:150–156 [DOI] [PubMed] [Google Scholar]
- 5. Darmani NA. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol Biochem Behav. 2001;69:239–249 [DOI] [PubMed] [Google Scholar]
- 6. Scheckel CL, Boff E, Dahlen P, et al. Behavioral effects in monkeys of racemates of two biologically active marijuana constituents. Science. 1968;160:1467–1469 [DOI] [PubMed] [Google Scholar]
- 7. Hockman CH, Perrin RG, Kalant H. Electroencephalographic and behavioral alterations produced by delta-9-tetrahydrocannabinol. Science. 1971;172:968–970 [DOI] [PubMed] [Google Scholar]
- 8. Shannon HE, Martin WR, Silcox D. Lack of antiemetic effects of delta-9-tetrahydracannabinoil in apomorphine-induced emesis in the dog. Life Sci. 1978;23:49–53 [DOI] [PubMed] [Google Scholar]
- 9. DeVuono MV, Hrelja KM, Sabaziotis L, et al. Conditioned gaping produced by high dose Δ9-tetrahydracannabinol: dysregulation of the hypothalamic endocannabinoid system. Neuropharmacology. 2018;141:272–282 [DOI] [PubMed] [Google Scholar]
- 10. Forrester MB, Kleinschmidt K, Schwarz E, et al. Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol. 2012;31:1006–1011 [DOI] [PubMed] [Google Scholar]
- 11. Seely KA, Lapoint J, Moran JH, et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmermann US, Winkelmann PR, Pilhatsch M, et al. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Dtsch Arztebl Int. 2009;106:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopkins CY, Gilchrist BL. A case of cannabinoid hyperemesis syndrome caused by synthetic cannabinoids. J Emerg Med. 2013;45:544–546 [DOI] [PubMed] [Google Scholar]
- 14. Bick BL, Szostek JH, Mangan TF. Synthetic cannabinoid leading to cannabinoid hyperemesis syndrome. Mayo Clin Proc. 2014;89:1168–1169 [DOI] [PubMed] [Google Scholar]
- 15. Ukaigwe A, Karmacharya P, Donato A. A gut gone to pot: a case of cannabinoid hyperemesis syndrome due to K2, a synthetic cannabinoid. Case Rep Emerg Med. 2014:167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zawilska JB. “Legal highs”—new players in the old drama. Curr Drug Abuse Rev. 2011;4:122–130 [DOI] [PubMed] [Google Scholar]
- 17. Vandrey R, Dunn KE, Fry JA, et al. A survey study to characterize use of Spice products (synthetic cannabinoids). Drug Alcohol Depend. 2012;120:238–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller HH, Kornhuber J, Sperling W. The behavioral profile of spice and synthetic cannabinoids in humans. Brain Res Bull. 2016;126:3–7 [DOI] [PubMed] [Google Scholar]
- 19. Spaderna M, Addy PH, D'Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology. 2013;228:525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol. 2012;8:62–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Amsterdam J, Brunt T, van den Brink W. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J Psychopharmacol. 2015;29:254–263 [DOI] [PubMed] [Google Scholar]
- 22. Ford BM, Tai S, Fantegrossi WE, et al. Synthetic pot: not your grandfather's marijuana. Trends Pharmacol Sci. 2017;38:257–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Limebeer CL, Parker LA. Delta-9-tetrahydrocannabinol interferes with the establishment and the expression of conditioned rejection reactions produced by cyclophosphamide: a rat model of nausea. Neuroreport. 1999;10:3769–3772 [DOI] [PubMed] [Google Scholar]
- 25. Parker LA, Mechoulam R, Schlievert C, et al. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology. 2003;166:156–162 [DOI] [PubMed] [Google Scholar]
- 26. Darmani NA. Delta-9-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24:198–203 [DOI] [PubMed] [Google Scholar]
- 27. Van Sickle MD, Oland LD, Ho W, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774 [DOI] [PubMed] [Google Scholar]
- 28. Van Sickle MD, Oland LD, Mackie K, et al. Delta-9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576 [DOI] [PubMed] [Google Scholar]
- 29. Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacology. 1998;59:347–352 [DOI] [PubMed] [Google Scholar]
- 30. Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Be. 2005;81:331–342 [DOI] [PubMed] [Google Scholar]
- 31. Taylor DA, Fennessy MR. Biphasic nature of the effects of delta9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur J Pharmacol. 1977;46:93–99 [DOI] [PubMed] [Google Scholar]
- 32. Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsycopharmacol. 2013;16:2273–2284 [DOI] [PubMed] [Google Scholar]
- 33. Murphy LL, Muñoz RM, Adrian BA, et al. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446 [DOI] [PubMed] [Google Scholar]
- 34. Puder M, Weidenfeld J, Chowers I, et al. Corticotrophin and corticosterone secretion following Δ9-tetrahydrocannabinol, in intact and in hypothalamic deafferentated male rats. Exp Brain Res. 1982;46:85–88 [DOI] [PubMed] [Google Scholar]
- 35. Darmani NA. Cannabinoid-induced hyperemesis: a conundrum-from clinical recognition to basic science mechanisms. Pharmaceuticals. 2010;3:2163–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2009;117:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taché Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Digest Dis Sci. 1999;44(8 Suppl):79S–86S [PubMed] [Google Scholar]
- 38. Levinthal DJ, Bielefeldt K. Adult cyclical vomiting syndrome: a disorder of allostatic regulation? Exp Brain Res. 2014;232:2541–2547 [DOI] [PubMed] [Google Scholar]
- 39. Li BUK, Misiewicz L. Cyclic vomiting syndrome: a brain-gut disorder. Gastroenterol Clin North Am. 2003;32:997–1019 [DOI] [PubMed] [Google Scholar]
- 40. Venkatesan T, Zadvornova Y, Raff H, et al. Endocannabinoid-related lipids are increased during an episode of cyclic vomiting syndrome. Neurogastroent Motil. 2016;28:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Endo T, Takahashi M, Minami M. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biogenic Amines. 1995;11:399–407 [Google Scholar]
- 42. Horn CC, Richardson EJ, Andrews PLR, et al. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81 [DOI] [PubMed] [Google Scholar]
- 43. Bernstein IL, Chavez M, Allen D, et al. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res. 1992;575:132–137 [DOI] [PubMed] [Google Scholar]
- 44. Eckel LA, Ossenkopp K-P. Area postrema mediates the formation of rapid, conditioned palatability shifts in lithium-treated rats. Behav Neurosci. 1996;110:202–212 [PubMed] [Google Scholar]
- 45. Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279 [DOI] [PubMed] [Google Scholar]
- 46. Parker LA. Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol. 2014;722:122–133 [DOI] [PubMed] [Google Scholar]
- 47. Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci. 1986;100:544–555 [DOI] [PubMed] [Google Scholar]
- 48. Limebeer CL, Vemuri VK, Bedard H, et al. Inverse agonism of cannabinoid CB 1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rock EM, Moreno-Sanz G, Limebeer CL, et al. Suppression of acute and anticipatory nausea by peripherally restricted fatty acid amide hydrolase inhibitor in animal models: role of PPARα and CB1 receptors. Br J Pharmacol. 2017;174:3837–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Otto B, Riepl RL, Klosterhalfen S, et al. Endocrine correlates of acute nausea and vomiting. Auton Neurosci Basic. 2006;129:17–21 [DOI] [PubMed] [Google Scholar]
- 51. Hayes WJ, VanGilder D, Berendse J, et al. Cyclic vomiting syndrome: diagnostic approach and current management strategies. Clin Exp Gastroenterol. 2018;11:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richards JR. Cannabinoid hyperemesis syndrome: pathophysiology and treatment in the emergency department. J Emerg Med. 2018;54:354–363 [DOI] [PubMed] [Google Scholar]
- 53. McLaughlin PJ, Winston KM, Limebeer CL, et al. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293 [DOI] [PubMed] [Google Scholar]
- 54. Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:43–65 [DOI] [PubMed] [Google Scholar]
- 55. Pi-sunyer FX, Aronne LJ, Heshmati H, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA. 2006;295:761–776 [DOI] [PubMed] [Google Scholar]
- 56. Tai S, Hyatt WS, Gu C, et al. Repeated administration of phytocannabinoid Δ9-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res. 2015;102:22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel S, Roelke CT, Rademacher DJ, et al. Endocannabinoid signaling negatively modulates stress- induced activation of thehypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438 [DOI] [PubMed] [Google Scholar]
- 59. Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Behav Rev. 2008;32:1152–1160 [DOI] [PubMed] [Google Scholar]
- 60. Evanson NK, Tasker JG, Hill MN, et al. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Di S, Malcher-Lopes R, Halmos KC, et al. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2009;624:71–76 [DOI] [PubMed] [Google Scholar]