Introduction
Cannabinoid preparations are available across the globe as regulatory agency-approved medicines, through medical cannabis programs, and as hemp-derived products. Many regions, including most provinces in Canada, have designated cannabis businesses as “essential” services during the coronavirus disease 2019 (COVID-19) pandemic, and sales of cannabis remain strong in an otherwise economically challenging time. In light of the potential increased use of cannabis and a recent surge in research to rapidly identify medications to treat COVID-19, it is critical to delineate possible pharmacokinetic (PK) and pharmacodynamic (PD) drug–drug interactions (DDIs) between cannabinoids and such experimental medications. Delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are lipophilic, highly protein bound, have a large volume of distribution, a long half-life, bioaccumulate, and share common metabolic pathways within the cytochrome P450 (CYP450) family (e.g., 3A4, 2C9, and 2C19), drug transporters (e.g., breast cancer resistance protein), and plasma protein-binding substrates. Both THC and CBD have been shown to have clinically significant PK (e.g., warfarin and clobazam)1,2 and PD interactions (e.g., valproic acid).2 The severity of some cannabinoid DDIs, such as potential hepatocellular injury and sedation,2,3 further highlights the importance of early identification of possible interactions between cannabinoids and medications that may be used to treat COVID-19.
Methods
Pharmaceutical candidates for COVID-19 were identified through reviews4 and clinicaltrials.gov, Google, and PubMed searches using keywords: (COVID-19, COVID-2) research, drug, investigational product, and clinical trial. The initial search was conducted during March 24–26, 2020 and was updated on August 06, 2020. COVID-19 drug candidates were considered relevant if there was a published mechanism, planned or ongoing clinical trial, and/or off-label use (vaccines and contraindicated medications were excluded). Package inserts, product monographs, and drug submissions were collected from regulatory bodies (U.S. Food and Drug Administration [FDA], Ministry of Health of the Russian Federation, Pharmaceuticals and Medical Devices Agency [PMDA, Japan]) and reviewed to identify PK (i.e., interaction with CYP450s, drug transporters, or protein binding) or PD (i.e., side effects and additive drug effects) sources of DDIs. If not available in regulatory documents, PK and PD sources of DDIs were identified using PubMed searches (keywords: [name of drug], DDI, CYP450, transporter, protein binding, side effect, and adverse effect). DDIs between relevant COVID-19 drug candidates and CBD and THC were identified as “possible” (overlapping sources of DDIs) or “unlikely” (no overlapping sources of DDIs).
Results
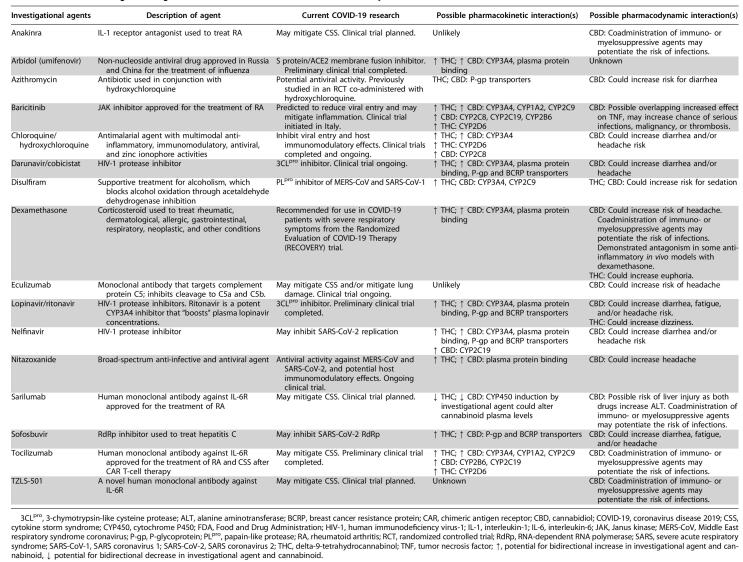
Of the 28 identified existing or novel pharmaceutical candidates for COVID-19 treatment, 12 (i.e., bevacizumab, brilacidin, convalescent plasma, favipiravir, galidesivir [BCX4430], griffithsin, intravenous immunoglobulin, niclosamide, REGN3048, remdesivir, vitamin C, and XueBiJing) had unknown or unlikely DDIs due to either unidentified or unrelated metabolic, transporter, or protein-binding pathways. Ten candidates were found to possibly result in DDIs through CYP interaction, five through drug transporter interactions, six through protein binding, and 15 that may cause PD DDIs, including exacerbate side effect profiles (see Table 1). Increased risk for diarrhea, headache, dizziness, somnolence, infection, and liver injury were identified in many pharmaceutical candidates for COVID-19 and are known side effects of THC and/or CBD.2,3
Table 1.
COVID-19 Investigational Agents with Possible Pharmacokinetic or Pharmacodynamic Cannabinoid Interactions
Discussion
The majority of candidate drugs under examination for COVID-19 treatment could have DDIs with THC and/or CBD. Although, to our knowledge, there are no case reports on such DDIs, physicians treating patients with COVID-19 and/or those patients enrolled in clinical trials of candidate drugs should assess for cannabis/cannabinoid use and monitor physiological responses to COVID-19 that could be influenced by cannabis/cannabinoid use (e.g., potentiation of side effects, changes in exposure of candidate drugs, and/or levels of cytokines5,6). Although concomitant binding of THC/CBD and candidate drugs for COVID-19 to plasma proteins, transporters, or metabolism do not necessarily produce a clinical effect, the importance of cannabinoid screening is highlighted by the common use of cannabis/cannabinoids for conditions that may function as risk factors for COVID-19 complications or mortality (e.g., human immunodeficiency virus and autoimmune disorders such as multiple sclerosis). The importance of closely monitoring physiological responses to COVID-19 (e.g., cytokines) is supported by research that has identified increased infection potential and immunosuppressive effects associated with CBD2,7; concomitant administration of immunosuppressants may further reduce levels of their intended cytokine target(s). Although more research is needed, these additive effects could result in primary and/or secondary infections. In addition, a recent in vitro study demonstrated that CBD can antagonize some dexamethasone effects, overriding the anti-inflammatory potential of steroids when used in combination.8 Until a priori research determines whether (and to what extent) successful COVID-19 treatments interact with cannabis/cannabinoids, it seems prudent to assess current cannabis/cannabinoid use among suspected or confirmed COVID-19 patients and consider additional monitoring in the event that modified treatment approaches are needed to avoid unwanted DDIs.
Acknowledgments
The authors thank Dr. Mark A. Ware, MD, for his contribution to the conceptualization of this project. The authors also thank Dr. Jessica Irons, PhD, and Irina Mosesova, BSc, for their review of the article.
Abbreviations Used
- 3CLpro
3-chymotrypsin-like cysteine protease
- ALT
alanine aminotransferase
- BCRP
breast cancer resistance protein
- CAR
chimeric antigen receptor
- CBD
cannabidiol
- COVID-19
coronavirus disease 2019
- CSS
cytokine storm syndrome
- CYP450
cytochrome P450
- DDI
drug–drug interaction
- FDA
Food and Drug Administration
- HIV-1
human immunodeficiency virus-1
- IL-1
interleukin-1
- IL-6
interleukin-6
- JAK
Janus kinase
- MERS-CoV
Middle East respiratory syndrome coronavirus
- PD
pharmacodynamic
- P-gp
P-glycoprotein
- PK
pharmacokinetic
- PLpro
papain-like protease
- RA
rheumatoid arthritis
- RCT
randomized controlled trial
- RdRp
RNA-dependent RNA polymerase
- SARS
severe acute respiratory syndrome
- SARS-CoV-1
SARS coronavirus 1
- SARS-CoV-2
SARS coronavirus 2
- THC
delta-9-tetrahydrocannabinol
- TNF
tumor necrosis factor
- ↑
potential for bidirectional increase in investigational agent and cannabinoid
- ↓
potential for bidirectional decrease in investigational agent and cannabinoid
Author Disclosure Statement
M.H.L. reports personal fees, nonfinancial support, and other from Canopy Growth Corporation and GW Pharmaceuticals outside the submitted study. L.M. reports personal fees, nonfinancial support, and other from Canopy Growth Corporation and Canopy Health Innovations Inc., outside the submitted study. B.F.T. reports personal fees, nonfinancial support, and other from Canopy Growth Corporation, personal fees and nonfinancial support from Elsevier and Research Triangle Institute, nonfinancial support from Curaleaf, outside the submitted study. E.N.P. reports personal fees, nonfinancial support, and other from Canopy Growth Corporation, and personal fees from Battelle outside the submitted study. M.O.B.-M. reports personal fees, nonfinancial support, and other from Canopy Growth Corporation and Zynerba Pharmaceuticals, personal fees from Tilray, personal fees and nonfinancial support from AusCann Group Ltd., Realm of Caring Foundation, and The Lambert Center for the Study of Medicinal Cannabis and Hemp, outside the submitted study.
Funding Information
This study did not receive funding. Although all authors serve as employees of Canopy Growth Corporation, the company played no part in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Cite this article as: Land MH, MacNair L, Thomas BF, Peters EN, Bonn-Miller MO (2020) Letter to the Editor: Possible drug–drug interactions between cannabinoids and candidate COVID-19 drugs, Cannabis and Cannabinoid Research 5:4, 340–343, DOI: 10.1089/can.2020.0054.
References
- 1. Foster BC, Abramovici H, Harris CS. Cannabis and cannabinoids: Kinetics and interaction. Am J Med. 2019;132:1266–1270 [DOI] [PubMed] [Google Scholar]
- 2. Epidiolex Package Insert. In: GB, Inc., ed. FDA approved 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf Accessed March24, 2020
- 3. Marinol Package Insert. In: AbbieVie, Inc., ed. FDA approved 1985, revised 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf Accessed March24, 2020
- 4. McCreary EK, Pogue JM. COVID-19 treatment: A review of early and emerging options. Open Forum Infect Dis. 2020;7:ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nichols JM, Kaplan BLF. Immune responses modulated by cannabidiol. Cannabis Cannabinoid Res. 2020;5:12–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kusher DI, Dawson LO, Taylor AC, et al. . Effect of the psychoactive metabolites of marijuana, delta 9-tetrahydrocannabinol (THC), on the synthesis of tumor necrosis factor by human large granular lymphocytes. Cell Immunol. 1994;154:99–108 [DOI] [PubMed] [Google Scholar]
- 7. Vuolo F, Petronilho F, Sonai B, et al. . Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015;2015:538670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muthumalage T, Rahman I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol Appl Pharmacol. 2019;382:114713. [DOI] [PMC free article] [PubMed] [Google Scholar]