Abstract
The basic concepts from the fields of biology and engineering are integrated into tissue engineering to develop constructs for the repair of damaged and/or absent tissues, respectively. The field has grown substantially over the past two decades, with particular interest in bone tissue engineering (BTE). Clinically, there are circumstances in which the quantity of bone that is necessary to restore form and function either exceeds the patient's healing capacity or bone's intrinsic regenerative capabilities. Vascularized osseous or osteocutaneous free flaps are the standard of care with autologous bone remaining the gold standard, but is commonly associated with donor site morbidity, graft resorption, increased operating time, and cost. Regardless of the size of a craniofacial defect, from trauma, pathology, and osteonecrosis, surgeons and engineers involved with reconstruction need to consider the complex three-dimensional (3D) geometry of the defect and its relationship to local structures. Three-dimensional printing has garnered significant attention and presents opportunities to use craniofacial BTE as a technology that offers a personalized approach to bony reconstruction. Clinicians and engineers are able to work together to produce patient-specific space-maintaining scaffolds tailored to site-specific defects, which are osteogenic, osseoconductive, osseoinductive, encourage angiogenesis/vasculogenesis, and mechanically stable upon implantation to prevent immediate failure. In this work, we review biological and engineering principles important in applying 3D printing technology to BTE for craniofacial reconstruction as well as present recent translational advancements in 3D printed bioactive ceramic scaffold technology.
Impact statement
Surgical reconstruction for extensive bone defects has evolved over the last 20 years toward a more customized treatment approach which fulfill functional outcomes. Additionally, the merger of surgical and microvascular principles has given rise to custom tailored patient-specific free tissue flaps which reconstruct bony maxillofacial defects while rebuilding lining, soft tissue mass, and facial subunits—all of which are key to achieving outcomes that approach normalcy. The contemporary techniques for complex boney defect reconstruction remain constrained: autologous bone transfer is complicated by limited bone stock and shape, donor site morbidity, surgical site infection, delayed healing, long operative times, and cost. Due to the shortcomings associated with autologous bone, advances in bone tissue engineering (BTE), such as 3D printing for patient and site-specific devices, have sought to restore bone deficiencies using customizable devices (scaffolds).
Keywords: bone tissue engineering, 3D printing, scaffold, biomaterials
Introduction
Three-dimensional printing in scaffold design
The ability to create personalized scaffolds based on 3D imaging data coupled with three-dimensional printing (3DP) has the capacity to offer a useful approach in fabrication of patient-specific fit-and-fill scaffolds for repair of bony defects.1 Before printing, there is an image acquisition step involving technologies such as cone beam computed tomography and computed tomography (CT), which is digitally processed to fabricate scaffolds directly or employ computer-aided design and mathematical modeling methods to produce a high-fidelity template of the defect.2–4 Within this workflow, not only is selection of the appropriate scanning system and printing technology important but also selection of an osteogenic scaffold material necessary for optimal outcome application.
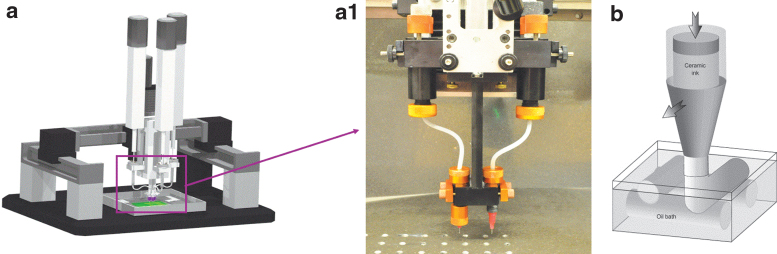
Three-dimensional printing can be categorized into four primary technologies: fused deposition modeling (FDM), stereolithography (SLA), selective laser sintering (SLS), and direct ink writing (DIW). FDM (e.g., Ultimaker [The Netherlands], MakerBot [USA], and Zortrax [Poland]) is an extrusion-based platform that utilizes a thermoplastic polymer/filament dispensed from a spool through a heated extrusion nozzle and deposited onto a build platform.5 FDM printers have minimal maintenance costs, compact size, and flexibility in printing temperature, but are constrained to a narrow range of printable polymeric materials.6 SLA is a polymerization technology whereby a platform is placed in a vat of curable resin equipped with a photopolymerizing ultraviolet (UV) laser beam, creating each subsequent layer of the composite scaffold as the platform travels through the image dataset representing the osseous defect.7 SLS technology is similar to SLA, but utilizes a high-powered CO2 laser beam to fuse layers of particles, often ceramic, to form the overall structure.8 Laser-assisted bioprinting, while less common than other printing techniques, provides increased spatial resolution along with the capacity of printing an assortment of biological materials preserving cell viability.9 DIW, commonly referred to as robocasting, utilizes a computer-controlled needle-syringe-type system (Fig. 1a) to deposit biomaterials, biologics, or cells in a layer-by-layer approach (Fig. 1b).10 It is feasible to obtain customized modifications to the scaffold design through the use of different bioinks in multicompartment-type nozzles (Fig. 1a.1), microfluidic strategies,11 and tailored colloidal gels.12
FIG. 1.
(a) Digital CAD image of a custom-built 3D printer (3D Inks LLC, Tulsa, OK), (a.1) inset of the multitip nozzle attached to the 3D printer, which allows for extrusion of scaffold materials (e.g., TCP) and fugitive support material. (b) CAD representation of the extrusion process of the colloidal gel suspension from a Luer-Lok tip into a low-viscosity oil reservoir. CAD, computer-aided design; TCP, tricalcium phosphate.
Biomaterials
Three-dimensional printed scaffold constructs provide a matrix for growth of tissues (hard or soft), supporting the integration, regeneration, and repair of the native anatomical structure. Degradable biomaterials used to produce these constructs may be divided into two broad categories: bioactive ceramics and polymers. Appropriate selection of these materials provides structural support, prevents soft tissue collapse, and tailors degradation kinetics to decrease the risk of graft extrusion and soft tissue dehiscence over the implant.13–18 The desire to balance these engineering and physiologic concerns has led to exploration of various ceramics and polymers, each with their own advantages and limitations.
Bone tissue engineering (BTE) can utilize various polymeric-based materials, either natural or synthetic.19 Naturally derived/sourced polymeric (i.e., collagen, gelatin, and hyaluronic acid) devices/products that have received FDA approval for clinical use are commonly used in craniofacial surgery.20 Degradation often depends on enzymes, which makes kinetics hard to predict. Synthetic polymers, on the other hand, can be tailored in controlled settings, so their physicochemical properties, (i.e., average molecular weight and size distribution) are conducive to material degradation. In addition, degradation typically relies on hydrolysis, reducing variation seen in enzyme-dependent degradation.21 Commonly utilized synthetic polymers in the clinical environment include poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and copolymers (PLGA).22 Of note, if the degradation rate surpasses the rate of local clearance, an accumulation of degradation products may lower the pH, damaging cells and tissue. Other important polymers are poly(ɛ-caprolactone), which can form biocompatible blends and copolymers with other polymers,23 and poly(propylene fumarate), whose mechanical properties can be tailored through a cross-linking agent or UV photoinitiation.24–26 In general, these materials promote cell adhesion and proliferation, but have reduced osseoconductivity and mechanical strength compared with bioactive ceramics.27,28
Research on bioactive ceramics has primarily concentrated on the inorganic constituent of native bone, hydroxyapatite (HA), which is both biocompatible and osseoconductive.29–31 The material, however, has the unfavorable degradation rate of 1–2% per year in vivo, limiting complete bony regeneration.32 As a result, β-tricalcium phosphate (β-TCP) ceramic was developed, with biocompatibility and osseoconductivity similar to HA, but with an increased degradation rate.32,33 Furthermore, its degradation kinetics can be tailored with respect to the macro- and mesoscale with 3D printing through changes in lattice porosity size, strut circumference, ink formulation, and sintering, which alter the surface area and porosity.34,35 Printing these bioactive ceramics allows for fabrication of personalized devices that fit and fill bony defects with complex geometries, promoting optimal osseoconduction between the scaffold and the defect interface for robust osseous healing.36–41
Bioactive molecules
Small soluble molecules such as bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor have been utilized to stimulate angiogenesis and osteogenesis.42–44 Simultaneous integration of these bioactive molecules facilitates the osteogenic, osteochondral, and vasculogenic/angiogenic capacity of 3D printed bone implants.
Transforming growth factor-beta (TGF-β) superfamily members, such as BMPs, of the secreted cysteine knot proteins play key roles in the development of limb, kidney, skin, hair, and neuronal aspects in addition to actively participating in bone and vascular homeostasis.45–48 BMPs bind to transmembrane serine/threonine protein kinase and activated type I and type II receptors.49 As one of the most investigated regenerative agents,50–56 recombinant human BMP-2 (rhBMP-2) has successfully demonstrated osseoinduction57 and angiogenesis58 in preclinical models. Both rhBMP-2 and -7 are clinically approved and available as an augmentative therapy for treatment of fractures (i.e., nonunion),45 with several groups reporting their experience with off-label use of the former agent for alveolar cleft repair.55,59–66 Although promising data exist, the family of proteins is reported to cause ectopic bone growth, impeded bone healing, edema, and premature craniofacial suture fusion.67–70 These concerns have motivated research in alternative osteogenic agents, such as those that activate the adenosine receptor pathway.
The purine nucleoside, adenosine, has an effect on almost every organ system through activation of different adenosine receptors: A1, A2A, A2B, and A3. These receptors, once activated, have been shown to regenerate bone in a murine model similar to BMP-2, but without the negative side effects.71 The A2A receptor, in particular, is known to attenuate osteoclast activity and population72–74 in addition to augmenting osteoblast differentiation.75 As an inhibitor of type 1 equilibrative nucleoside transporter (ENT1), dipyridamole indirectly activates the A2A receptor.71 The medication has a longstanding safety profile in patients, adult and pediatric, as an antithrombotic and vasodilator.75–77 This knowledge coupled with its capacity for bone regeneration makes it an alluring candidate as a safe osteogenic agent in BTE applications.19,67,78–81
Other bioactive molecules that have been studied include fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and the TGF-β family. PDGF is a polypeptide dimer with three isoforms (PDGF-AA, PDGF-BB, and PDGF-AB) with PDGF-BB the only isoform evaluated clinically and rhPDGF having potential in craniofacial regeneration.48,82 The TGF-β family comprises several isoforms whose activity is mediated by a transmembrane heterodimer receptor. New bone formation has been induced by TGF-β1, without ectopic bone growth in nonhuman primates, but undisputed evidence of rhTGF-β1's efficacy over rhBMP-2 is unavailable.48,83–86 FGF belongs to a family of polypeptides with the ability to bind heparin, with FGF-2 being the most widely researched and utilized for bone regeneration, although it does not have the capacity to regenerate bone independently.87 IGF is a polypeptide hormone with two isoforms, IGF-I and IGF-II, whose activity is mediated through the tyrosine kinase receptor, IGF-I receptor,48 which has been studied preclinically for its bone regenerative properties alone88 or in conjunction with PDGF.89
Applications of tissue engineering principles in translational preclinical models
Researchers have employed bioprinting techniques by embedding cells within an extracellular matrix, which is then 3D printed into a tissue engineering scaffold.90,91 Adipose-derived stem cells (ADSCs)92,93 and mesenchymal stem cells (MSCs)94–96 are commonly utilized multipotent cell lines in craniofacial tissue engineering, both preclinically and clinically. After isolation of ADSCs and MSCs, the cells are expanded in cell culture media and seeded onto 3D printed bioresorbable scaffolds that are then implanted into bony defects.97–101 Cell-seeded scaffolds promote the local regenerative process as pathological anomalies or clinical therapy may impede intrinsic tissue healing.
Our group has focused on the regenerative capacity of 3D printed scaffolds comprising β-TCP, with or without a bioactive molecule coating, using critical-sized bone defects in translational animal experimental designs. A critical size bone defect (CSD) is defined as the smallest intraosseous wound that will not heal by intrinsic osteogenesis within the animal's lifetime.102 Animals may be categorized into small animal models (i.e., mouse, rat, and rabbit) or large animal models (i.e., dog, goat, pig, and sheep),103 and several experimental rabbit nonhealing mandibular defect models for craniofacial tissue regeneration have been described in the literature.103–105 These animals undergo a surgical procedure in which a CSD in the mandibular bone is instrumented, which may or may not involve the alveolus. Animals are then euthanized to assess bone formation, vascular growth, and complications (infection, graft failure, and ectopic bone formation, etc.). Analysis may take the form of histology or imaging. In particular, microcomputed tomography (micro-CT) provides a robust tool to evaluate bone formation and 3D analysis of healing defects, an adjunct to conventional, two-dimensional histological methods.106 Three-dimensional models created from imaging data may be used to quantify bone regeneration, trabecular size, vessel size, and more using quantitative, 3D image-processing software.67,80
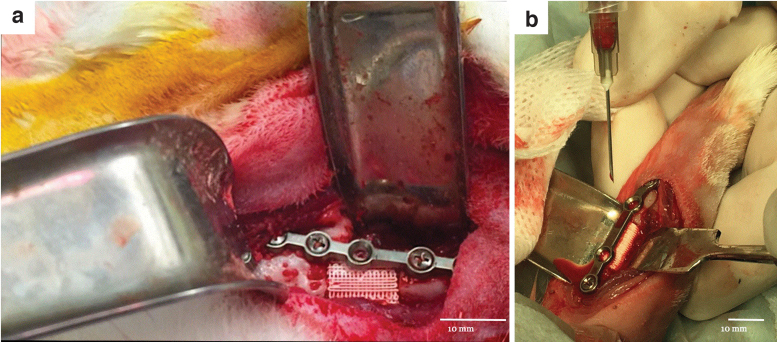
Our group was the first to report the successful deployment of 3D printed bioactive ceramic scaffolds comprising 100% β-TCP with a methodical design at various length scales in a skeletally mature rabbit model for full-thickness segmental mandible (Fig. 2a)79 and radius defects (Fig. 2b).107,108 These studies demonstrate the utility of customized, bioactive ceramic scaffolds in a fit-and-fill model to bridge CSDs with newly formed bone in vivo as early as 8 weeks, with evidence of progressive scaffold degradation and further bone regeneration and scaffold resorption over a 6-month healing period.107 Similar principles were also applied and successfully demonstrated in a larger, more clinically relevant, sheep calvarium model.78
FIG. 2.
Digital image of a custom, 3D printed β-TCP scaffold placed in (a) the mandibular body and (b) the radial diaphysis of New Zealand white rabbits with custom surgical hardware.
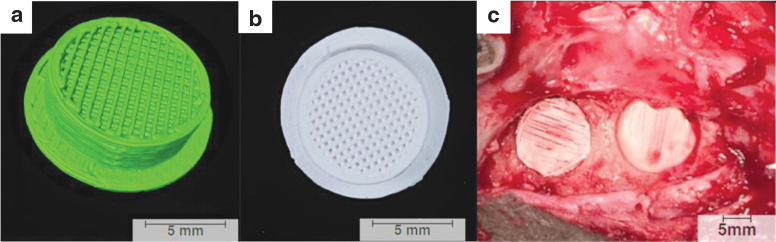
In our large, preclinical sheep model, each calvaria received two ipsilateral 11-mm-diameter defects created using a trephine along with a second set of trephine-induced defects on the contralateral side at 3 weeks, creating a two-time point study (Fig. 3). The custom 3D printed scaffolds were coated with a bovine type I collagen carrier that was bound to the scaffold through cross-linking. The experimental groups constituted either the (i) collagen carrier or (ii) collagen augmented with 100 μM dipyridamole. Each defect received a scaffold. Animals were euthanized at 6 weeks following the initial surgery, and scaffolds were subjected to microcomputed tomography (μCT) and nondecalcified histology for further evaluation. Independent of the treatment group, uncoated and dipyridamole-coated scaffolds yielded new bone formation, but dipyridamole significantly enhanced healing at both time points (i.e., 3 and 6 weeks). As has been previously observed and reported, A2AR activation by dipyridamole enhanced the intrinsic osteogenic capacity of the local dura mater.109
FIG. 3.
(a) Three-dimensional reconstruction of the scaffold created using Amira 6.1 software (Visage Imaging GmbH, Berlin, Germany). (b) Inferior surface of the scaffold showcasing the porous core with central lattice. (c) Intraoperative photograph showing scaffold placement in anterior and posterior calvarial defects.
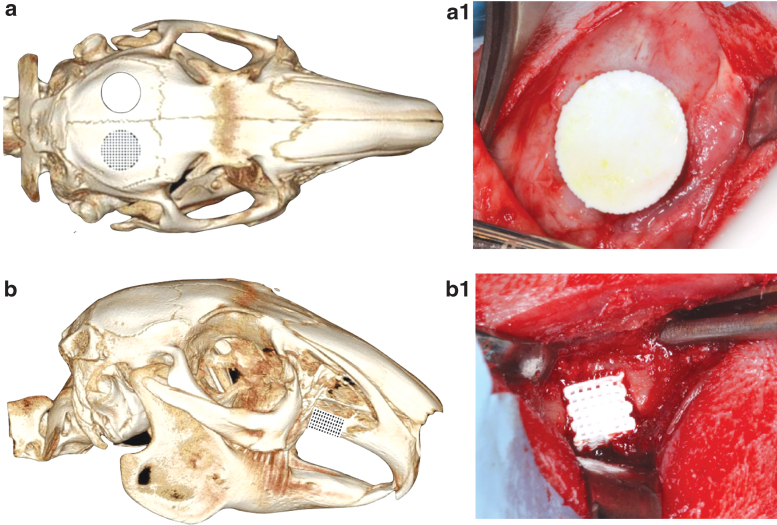
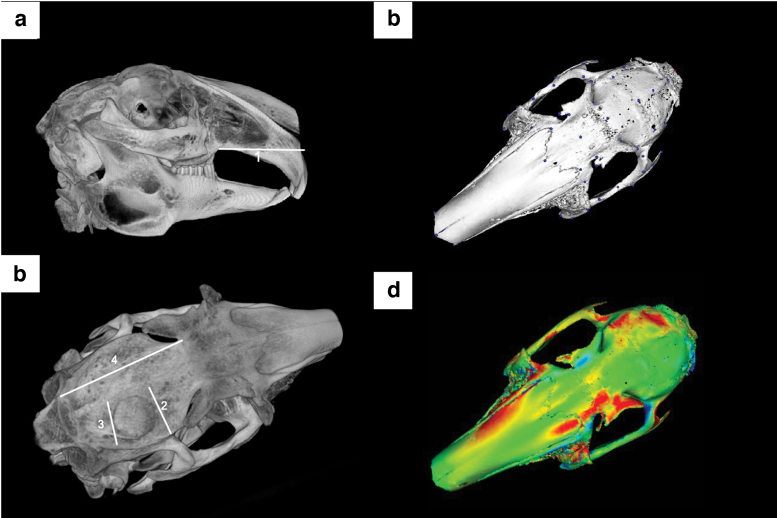
Following the success of skeletally mature models, the work further expanded our model to include skeletally immature rabbits to study a novel tissue engineering strategy in a pediatric craniofacial model by investigating both alveolar and calvarial surgical defects.67,80,81 To investigate the bone regenerative capacity of these bioceramic scaffolds through the time of facial (skeletal) maturity, immature New Zealand white rabbits were subjected to surgical procedures to create a unilateral 10-mm calvarial defect (Fig. 4a) with an ipsilateral 3.5 × 3.5-mm full-thickness alveolar defect (Fig. 4b).81 Each defect received either a 3D printed β-TCP scaffold coated with 1000 μM dipyridamole or clinical standard of care autogenous bone grafting (harvested from the respective animal's radius). Rabbits were euthanized at 8 weeks and 6 months. Bone regeneration, scaffold degradation, trabecular thickness, and trabecular spacing were calculated using micro-CT reconstruction. Vessel density, vessel diameter, osteoblast density, osteoclast density, and osteoblast-to-osteoclast ratio were calculated using histology. Elastic modulus and hardness were calculated using nanoindentation. Facial development and symmetry at facial maturity were evaluated using cephalometric measurements and analysis (Fig. 5). The 3D printed scaffolds yielded significant osteogenic regeneration, volumetrically, in both alveolar and calvarial defects in comparison with autologous bone graft and without any detriment to normal craniofacial growth, ectopic bone growth, or premature suture fusion. Similarly, newly organized and vascularized bone showed histological and mechanical properties similar to native bone, reinforcing the potential to repair the defective site and to regenerate the native form.
FIG. 4.
micro-CT image of rabbit skull, schematic depicting (a) calvarial defect and (b) alveolar cleft model, respectively. The skeletally immature rabbits underwent surgical resection of alveolar calvaria and alveolar cleft, each site receiving custom-designed and 3D printed β-TCP scaffolds. Intraoperative placement of scaffolds in (a.1) calvaria and (b.1) alveolar ridge defects using a fit-and-fill process.
FIG. 5.
micro-CT rendition of rabbit calvaria for cephalometric measurements, (a) alveolar cleft and (b) calvaria. (c) Example of model landmarking for rabbit craniofacial surface for building of a global facial model and (d) resultant heat map model when overlaying the unoperated base mesh with that of the mesh of an operated animal treated with a custom scaffold for defect repair.
Adenosine receptor ligation's bone regenerative capacity was unequivocally demonstrated through our skeletally mature and immature animal models. Using 3D printed ceramic scaffolds as carriers of dipyridamole, we leveraged localized drug delivery to the injury sites to promote bone regeneration, while avoiding unintended systemic effects. The osteogenic agent amplified the osseoconductive properties of β-TCP scaffolds without damage to craniofacial sutures or ectopic bone growth, which are documented complications of rhBMP-2,69,110,111 even with doses logarithmically increased up to 2 × more than necessary to increase bone regeneration.67,80
Conclusions and Future Directions
The clinical repair of tissues, hard or soft, is constantly evolving based on emerging technology and surgical approach. The recent progress in 3D printing technology and tissue-engineered devices will not only assist in healing but also aid in the craniofacial surgeon's approach. Therefore, a strong understanding of the biology, materials science, pharmacology, and engineering concepts involved in BTE and the growing field of regenerative medicine is paramount for researchers and clinicians alike.
Although still exploratory in the clinical setting, tissue engineering is gaining popularity among surgeons and translational researchers. Of importance is our continued exploration of the physiological microenvironment as we continue to manipulate native tissue physiology. Infection, wound dehiscence, graft failure, and premature resorption are fundamental adverse events that we must overcome. Expanding our understanding of fabrication modalities, biomaterials, degradation kinetics, biopharmaceuticals, bone remodeling, and cell harvesting will bring 3D printed bioactive scaffolds to patients as safe and efficacious devices for craniofacial skeletal reconstruction.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health [R21/R33 HD090664-01 “Use of 3D Printing for Creation of Implantable Pediatric Devices”], and Dr. Coelho was supported by grants NIH [R01 AR068593] and NYU-H+H Clinical and Translational Science Institute Grant (1UL1TR001445).
References
- 1. Langer R., and Vacanti J.P.. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Flores R.L., Liss H., Raffaelli S., et al. The technique for 3D printing patient-specific models for auricular reconstruction. J Craniomaxillofac Surg 45, 937, 2017 [DOI] [PubMed] [Google Scholar]
- 3. Witek L., Khouri K.S., Coelho P.G., et al. Patient-specific 3D models for autogenous ear reconstruction. Plast Reconstr Surg Glob Open 4, e1093, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silva N.R., Witek L., Coelho P.G., et al. Additive CAD/CAM process for dental prostheses. J Prosthodont 20, 93, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Zein I., Hutmacher D.W., Tan K.C., et al. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23, 1169, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Wu W., Geng P., Li G., et al. Influence of layer thickness and raster angle on the mechanical properties of 3D-Printed PEEK and a Comparative Mechanical Study between PEEK and ABS. Materials (Basel) 8, 5834, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhariwala B., Hunt E., and Boland T.. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng 10, 1316, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Doraiswamy A., Narayan R.J., Harris M.L., et al. Laser microfabrication of hydroxyapatite-osteoblast-like cell composites. J Biomed Mater Res A 80, 635, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Yilmaz B., Tahmasebifar A., and Baran E.T.. Bioprinting technologies in tissue engineering. Adv Biochem Eng Biotechnol 171, 279, 2020 [DOI] [PubMed] [Google Scholar]
- 10. Cui H., Nowicki M., Fisher J.P., et al. 3D bioprinting for organ regeneration. Adv Healthc Mater 6, 1601118, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu W., Zhong Z., Hu N., et al. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10, 024102, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Witek L., Shi Y., and Smay J.. Controlling calcium and phosphate ion release of 3D printed bioactive ceramic scaffolds: an in vitro study. J Adv Ceramics 6, 157, 2017 [Google Scholar]
- 13. Banwart J.C., Asher M.A., and Hassanein R.S.. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 20, 1055, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Myeroff C., and Archdeacon M.. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am 93, 2227, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Hidalgo D.A., and Rekow A.. A review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg 96, 585; discussion 97–602, 1995 [PubMed] [Google Scholar]
- 16. Hidalgo D.A. Condyle transplantation in free flap mandible reconstruction. Plast Reconstr Surg 93, 770; discussion 82–3, 1994 [PubMed] [Google Scholar]
- 17. Nguyen C., Young S., Kretlow J.D., et al. Surface characteristics of biomaterials used for space maintenance in a mandibular defect: a pilot animal study. J Oral Maxillofac Surg 69, 11, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Spicer P.P., Kretlow J.D., Henslee A.M., et al. In situ formation of porous space maintainers in a composite tissue defect. J Biomed Mater Res A 100, 827, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher M.B., and Mauck R.L.. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng Part B Rev 19, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith B.T., Shum J., Wong M., et al. Bone tissue engineering challenges in oral & maxillofacial surgery. Adv Exp Med Biol 881, 57, 2015 [DOI] [PubMed] [Google Scholar]
- 21. Thomson R.C., Wake M.C., Yaszemski M.J., et al. Biodegradable polymer scaffolds to regenerate organs. In: Peppas, N.A., and Langer, R.S., eds. Biopolymers II. Berlin, Heidelberg: Springer Berlin Heidelberg, 1995, p. 245 [Google Scholar]
- 22. Zhang Z., Ortiz O., Goyal R., et al. 13 - Biodegradable Polymers.. In: Modjarrad, K., and Ebnesajjad, S., eds. Handbook of Polymer Applications in Medicine and Medical Devices. Oxford: William Andrew Publishing, 2014, p. 303 [Google Scholar]
- 23. Gunatillake P.A., and Adhikari R.. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater 5, 1; discussion, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Timmer M.D., Ambrose C.G., and Mikos A.G.. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials 24, 571, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Fisher J.P., Timmer M.D., Holland T.A., et al. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part I. Determination of network structure. Biomacromolecules 4, 1327, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Fisher J.P., Holland T.A., Dean D., et al. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part II. In vitro degradation. Biomacromolecules 4, 1335, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Bharadwaz A., and Jayasuriya A.C.. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 110, 110698, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alizadeh-Osgouei M., Li Y., and Wen C.. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact Mater 4, 22, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kattimani V.S., Kondaka S., and Lingamaneni K.P.. Hydroxyapatite–-past, present, and future in bone regeneration. Bone Tissue Regen Insights 7, BTRI.S36138, 2016 [Google Scholar]
- 30. Kokubo T., and Yamaguchi S.. Novel bioactive materials developed by simulated body fluid evaluation: surface-modified Ti metal and its alloys. Acta Biomater 44, 16, 2016 [DOI] [PubMed] [Google Scholar]
- 31. Fernandes H.R., Gaddam A., Rebelo A., et al. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials (Basel, Switzerland) 11, 2530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore W.R., Graves S.E., and Bain G.I.. Synthetic bone graft substitutes. ANZ J Surg 71, 354, 2001 [PubMed] [Google Scholar]
- 33. Kivrak N., and Taş A.C.. Synthesis of calcium hydroxyapatite-tricalcium phosphate (HA-TCP) composite bioceramic powders and their sintering behavior. J Am Ceramic Soc 81, 2245, 1998 [Google Scholar]
- 34. Zhang W., and Yelick P.C.. Craniofacial tissue engineering. Cold Spring Harb Perspect Med 8, a025775, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez C.D., Witek L., Torroni A., et al. The role of 3D printing in treating craniomaxillofacial congenital anomalies. Birth Defects Res 110, 1055, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bauermeister A.J., Zuriarrain A., and Newman M.I.. Three-dimensional printing in plastic and reconstructive surgery: a systematic review. Ann Plast Surg 77, 569, 2016 [DOI] [PubMed] [Google Scholar]
- 37. Hutmacher D.W., Schantz J.T., Lam C.X., et al. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med 1, 245, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Steigenga J.T., al-Shammari K.F., Nociti F.H., et al. Dental implant design and its relationship to long-term implant success. Implant Dent 12, 306, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wilson C.E., de Bruijn J.D., van Blitterswijk C.A., et al. Design and fabrication of standardized hydroxyapatite scaffolds with a defined macro-architecture by rapid prototyping for bone-tissue-engineering research. J Biomed Mater Res A 68, 123, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Jimbo R., Anchieta R., Baldassarri M., et al. Histomorphometry and bone mechanical property evolution around different implant systems at early healing stages: an experimental study in dogs. Implant Dent 22, 596, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Coelho P.G., and Jimbo R.. Osseointegration of metallic devices: current trends based on implant hardware design. Arch Biochem Biophys 561, 99, 2014 [DOI] [PubMed] [Google Scholar]
- 42. Ashammakhi N., and Kaarela O.. Three-dimensional bioprinting can help bone. J Craniofac Surg 29, 9, 2018 [DOI] [PubMed] [Google Scholar]
- 43. Townsend J. Development of Biomaterials for Calvarial Bone Regeneration And Application To Traumatic Brain Injury [PhD. thesis]. Stephenson School of Biomedical Engineering, University of Oklahoma, Oklahoma, US, 2017 [Google Scholar]
- 44. Bennett P., Stewart S.K., Dretzke J., et al. Preclinical therapies to prevent or treat fracture non-union: a systematic review. PLoS One 13, e0201077, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ali I.H., and Brazil D.P.. Bone morphogenetic proteins and their antagonists: current and emerging clinical uses. Br J Pharmacol 171, 3620, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng H., Jiang W., Phillips F.M., et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85, 1544, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Jansen J.A., Vehof J.W., Ruhé P.Q., et al. Growth factor-loaded scaffolds for bone engineering. J Control Release 101, 127, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg 31, 469, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Salazar V.S., Gamer L.W., and Rosen V.. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 12, 203, 2016 [DOI] [PubMed] [Google Scholar]
- 50. Laurencin C.T., Attawia M.A., Lu L.Q., et al. Poly(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: a regional gene therapy approach to bone regeneration. Biomaterials 22, 1271, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Wehrhan F., Amann K., Molenberg A., et al. PEG matrix enables cell-mediated local BMP-2 gene delivery and increased bone formation in a porcine critical size defect model of craniofacial bone regeneration. Clin Oral Implants Res 23, 805, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Fu Y.C., Nie H., Ho M.L., et al. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng 99, 996, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Lee J.W., Kang K.S., Lee S.H., et al. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials 32, 744, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Lee S.S., Huang B.J., Kaltz S.R., et al. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 34, 452, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Francis C.S., Mobin S.S., Lypka M.A., et al. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg 131, 1107, 2013 [DOI] [PubMed] [Google Scholar]
- 56. Ribeiro F.O., Gómez-Benito M.J., Folgado J., et al. In silico mechano-chemical model of bone healing for the regeneration of critical defects: the effect of BMP-2. PLoS One 10, e0127722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Einhorn T.A., Majeska R.J., Mohaideen A., et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am 85, 1425, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Zhang F., Qiu T., Wu X., et al. Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J Bone Miner Res 24, 1224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dickinson B.P., Ashley R.K., Wasson K.L., et al. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg 121, 209, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Hammoudeh J.A., Fahradyan A., Gould D.J., et al. A comparative analysis of recombinant human bone morphogenetic protein-2 with a demineralized bone matrix versus iliac crest bone graft for secondary alveolar bone grafts in patients with cleft lip and palate review of 501 Cases. Plast Reconstr Surg 140, 318e, 2017 [DOI] [PubMed] [Google Scholar]
- 61. Chin M., Ng T., Tom W.K., et al. Repair of alveolar clefts with recombinant human bone morphogenetic protein (rhBMP-2) in patients with clefts. J Craniofac Surg 16, 778, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Herford A.S., Boyne P.J., Rawson R., et al. Bone morphogenetic protein-induced repair of the premaxillary cleft. J Oral Maxillofac Surg 65, 2136, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Fallucco M.A., and Carstens M.H.. Primary reconstruction of alveolar clefts using recombinant human bone morphogenic protein-2: clinical and radiographic outcomes. J Craniofac Surg 20 Suppl 2, 1759, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Liang F., Yen S.L., Imahiyerobo T., et al. Three-dimensional cone beam computed tomography volumetric outcomes of rhBMP-2/demineralized bone matrix versus iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg 140, 767, 2017 [DOI] [PubMed] [Google Scholar]
- 65. Alonso N., Tanikawa D.Y., Freitas Rda S., et al. Evaluation of maxillary alveolar reconstruction using a resorbable collagen sponge with recombinant human bone morphogenetic protein-2 in cleft lip and palate patients. Tissue Eng Part C Methods 16, 1183, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Melville J.C., Tran H.Q., Bhatti A.K., et al. Is reconstruction of large mandibular defects using bioengineering materials effective? J Oral Maxillofac Surg 78, 661.e1, 2020 [DOI] [PubMed] [Google Scholar]
- 67. Lopez C.D., Coelho P.G., Witek L., et al. Regeneration of a pediatric alveolar cleft model using three-dimensionally printed bioceramic scaffolds and osteogenic agents: comparison of dipyridamole and rhBMP-2. Plast Reconstr Surg 144, 358, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu S.S., Opperman L.A., and Buschang P.H.. Effects of recombinant human bone morphogenetic protein-2 on midsagittal sutural bone formation during expansion. Am J Orthod Dentofacial Orthop 136, 768.e1; discussion -9, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Kinsella C.R. Jr., Cray J.J., Durham E.L., et al. Recombinant human bone morphogenetic protein-2-induced craniosynostosis and growth restriction in the immature skeleton. Plast Reconstr Surg 127, 1173, 2011 [DOI] [PubMed] [Google Scholar]
- 70. Xu H., Ke K., Zhang Z., et al. Effects of platelet-rich plasma and recombinant human bone morphogenetic protein-2 on suture distraction osteogenesis. J Craniofac Surg 24, 645, 2013 [DOI] [PubMed] [Google Scholar]
- 71. Mediero A., Wilder T., Perez-Aso M., et al. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J 29, 1577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kara F.M., Doty S.B., Boskey A., et al. Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum 62, 534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mediero A., Frenkel S.R., Wilder T., et al. Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci Transl Med 4, 135ra65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mediero A., Kara F.M., Wilder T., et al. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol 180, 775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McGrath L.B., Gonzalez-Lavin L., Eldredge W.J., et al. Thromboembolic and other events following valve replacement in a pediatric population treated with antiplatelet agents. Ann Thorac Surg 43, 285, 1987 [DOI] [PubMed] [Google Scholar]
- 76. Ueda N., Kawaguchi S., Niinomi Y., et al. Effect of dipyridamole treatment on proteinuria in pediatric renal disease. Nephron 44, 174, 1986 [DOI] [PubMed] [Google Scholar]
- 77. Dengler R., Diener H.C., Schwartz A., et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 9, 159, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Bekisz J.M., Flores R.L., Witek L., et al. Dipyridamole enhances osteogenesis of three-dimensionally printed bioactive ceramic scaffolds in calvarial defects. J Craniomaxillofac Surg 46, 237, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lopez C.D., Diaz-Siso J.R., Witek L., et al. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. J Surg Res 223, 115, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maliha S.G., Lopez C.D., Coelho P.G., et al. Bone tissue engineering in the growing calvarium using dipyridamole-coated 3D printed bioceramic scaffolds: construct optimization and effects to cranial suture patency. Plast Reconstr Surg 145, 337e, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang M.M., Flores R.L., Witek L., et al. Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity. Sci Rep 9, 18439, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kaigler D., Avila G., Wisner-Lynch L., et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther 11, 375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Luginbuehl V., Meinel L., Merkle H.P., et al. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm 58, 197, 2004 [DOI] [PubMed] [Google Scholar]
- 84. Bonewald L.F., and Mundy G.R.. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res 250, 261, 1990 [PubMed] [Google Scholar]
- 85. Lee K., Weir M.D., Lippens E., et al. Bone regeneration via novel macroporous CPC scaffolds in critical-sized cranial defects in rats. Dent Mater 30, e199, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Simmons C.A., Alsberg E., Hsiong S., et al. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562, 2004 [DOI] [PubMed] [Google Scholar]
- 87. Kempen D.H., Creemers L.B., Alblas J., et al. Growth factor interactions in bone regeneration. Tissue Eng Part B Rev 16, 551, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Thaller S.R., Salzhauer M.A., Rubinstein A.J., et al. Effect of insulin-like growth factor type I on critical size calvarial bone defects in irradiated rats. J Craniofac Surg 9, 138, 1998 [DOI] [PubMed] [Google Scholar]
- 89. Alvarez P., Hee C.K., Solchaga L., et al. Growth factors and craniofacial surgery. J Craniofac Surg 23, 20, 2012 [DOI] [PubMed] [Google Scholar]
- 90. Ong C.S., Yesantharao P., Huang C.Y., et al. 3D bioprinting using stem cells. Pediatr Res 83, 223, 2018 [DOI] [PubMed] [Google Scholar]
- 91. Moore C.A., Shah N.N., Smith C.P., et al. 3D Bioprinting and Stem Cells. Methods Mol Biol 1842, 93, 2018 [DOI] [PubMed] [Google Scholar]
- 92. Lendeckel S., Jödicke A., Christophis P., et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32, 370, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Taylor JA. Bilateral orbitozygomatic reconstruction with tissue-engineered bone. J Craniofac Surg 21, 1612, 2010 [DOI] [PubMed] [Google Scholar]
- 94. Shang Q., Wang Z., Liu W., et al. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg 12, 586; discussion 94–5, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Levi B., Glotzbach J.P., Wong V.W., et al. Stem cells: update and impact on craniofacial surgery. J Craniofac Surg 23, 319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang L., Feng G., Wei X., et al. The effects of mesenchymal stem cells in craniofacial tissue engineering. Curr Stem Cell Res Ther 9, 280, 2014 [DOI] [PubMed] [Google Scholar]
- 97. Lin Y., Umebayashi M., Abdallah M.N., et al. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci Rep 9, 472, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Park H., Kim J.S., Oh E.J., et al. Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells. Arch Craniofac Surg 19, 181, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roskies M., Jordan J.O., Fang D., et al. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J Biomater Appl 31, 132, 2016 [DOI] [PubMed] [Google Scholar]
- 100. Ahn G., Lee J.S., Yun W.S., et al. Cleft alveolus reconstruction using a three-dimensional printed bioresorbable scaffold with human bone marrow cells. J Craniofac Surg 29, 1880, 2018 [DOI] [PubMed] [Google Scholar]
- 101. Fang D., Roskies M., Abdallah M.N., et al. Three-dimensional printed scaffolds with multipotent mesenchymal stromal cells for rabbit mandibular reconstruction and engineering. Methods Mol Biol 1553, 273, 2017 [DOI] [PubMed] [Google Scholar]
- 102. Hollinger J.O., and Kleinschmidt J.C.. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1, 60, 1990 [DOI] [PubMed] [Google Scholar]
- 103. Shah S.R., Young S., Goldman J.L., et al. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat Protoc 11, 1989, 2016 [DOI] [PubMed] [Google Scholar]
- 104. Young S., Bashoura A.G., Borden T., et al. Development and characterization of a rabbit alveolar bone nonhealing defect model. J Biomed Mater Res A 86, 182, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Piotrowski S.L., Wilson L., Dharmaraj N., et al. Development and characterization of a rabbit model of compromised maxillofacial wound healing. Tissue Eng Part C Methods 25, 160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Young S., Kretlow J.D., Nguyen C., et al. Microcomputed tomography characterization of neovascularization in bone tissue engineering applications. Tissue Eng Part B Rev 14, 295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tovar N., Witek L., Atria P., et al. Form and functional repair of long bone using 3D-printed bioactive scaffolds. J Tissue Eng Regen Med 12, 1986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Witek L., Alifarag A.M., Tovar N., et al. Repair of critical-sized long bone defects using dipyridamole-augmented 3D-printed bioactive ceramic scaffolds. J Orthop Res 37, 2499, 2019 [DOI] [PubMed] [Google Scholar]
- 109. Slater B.J., Kwan M.D., Gupta D.M., et al. The role of regional posterior frontal dura mater in the overlying suture morphology. Plast Reconstr Surg 123, 463, 2009 [DOI] [PubMed] [Google Scholar]
- 110. Shah R.K., Moncayo V.M., Smitson R.D., et al. Recombinant human bone morphogenetic protein 2-induced heterotopic ossification of the retroperitoneum, psoas muscle, pelvis and abdominal wall following lumbar spinal fusion. Skeletal Radiol 39, 501, 2010 [DOI] [PubMed] [Google Scholar]
- 111. Oetgen M.E., and Richards B.S.. Complications associated with the use of bone morphogenetic protein in pediatric patients. J Pediatr Orthop 30, 192, 2010 [DOI] [PubMed] [Google Scholar]