Abstract
Introduction: The potential use of cannabis and cannabinoid products for the treatment of low-back pain is an important area for investigation. As one of the leading reasons to visit a primary care provider, low-back pain results in a significant burden of disease in both the United States' economic and health care systems. Given the current opioid epidemic, it is important to seek novel analgesics and understand their efficacy for myriad pain conditions, including low-back pain.
Materials and Methods: A systematic review was performed using multiple online databases to assess the association of cannabis use and low-back pain in the literature.
Results: A total of 124 articles were produced via our search methods, 73 abstracts in total were screened, 16 articles underwent full-text review, and 6 articles were included in qualitative synthesis.
Discussion: This systematic literature review reveals a lack of primary research investigating cannabis as a potential treatment of low-back pain and highlights the need for further investigation with well-designed clinical trials. There remain substantial political and legal barriers to performing such research.
Conclusion: Although there is a considerable body of work on the usage of cannabinoid products for many medical conditions, including the treatment of chronic pain, more directed clinical research into their utility as an analgesic for low-back pain and related symptoms needs to be addressed.
Keywords: low-back pain, analgesia, disability, cannabis, cannabinoid, marijuana
Introduction
According to the World Health Organization, low-back pain is a leading cause of disability and diminished quality of life, as well as a major reason that individuals seek medical consultation.1 Total financial costs associated with low-back pain in the United States have been reported to exceed $100 billion per year, two-thirds of which are a result of lost wages and reduced productivity.2 Changes in cannabis policies have increased interest in its potential therapeutic usage by the medical community, and given the consequences of the opioid epidemic, there is a particular need for novel analgesics.
Pharmacological treatment options for low-back pain are limited. Currently, nonopiate analgesics are considered first-line, such as a short course of nonsteroidal anti-inflammatory drugs (NSAIDs) and/or acetaminophen.3–5 Muscle relaxants such as cyclobenzaprine are often added to the regimen.6 In the case of acute low-back pain, many providers prescribe systemic glucocorticoids to reduce inflammation; however, given the major side effect profile of these agents, the risk of long-term use outweighs the benefit.7
Cannabis plant extract contains more than 500 natural compounds, including more than 100 cannabinoids that have the potential to interact with the human body's endocannabinoid system.8 Endocannabinoids are arachidonic acid derivatives that are released by cells in injured tissues.9,10 They modulate pain pathways through the activation of cannabinoid receptors.9 Principal cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD), are the chemical structures of the cannabis plant that have been shown to have an affinity for cannabinoid receptor type 1 and cannabinoid receptor type 2. In particular, CBD, which lacks the intoxicating properties of THC, has potential to be used in the treatment of pain. The exact pharmacological mechanism for its possible analgesic effects is still unclear. Recently, research has focused on anti-inflammatory properties of cannabinoids with regard to their impact on pain relief.8
The literature for cannabis as an effective analgesic is robust. In a recent systematic review and meta-analysis, Whiting et al. concluded that there was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain.11 Specifically, in reviewing eight placebo-controlled trials, they found a reduction in pain versus placebo (37% vs. 31%; odds ratio [OR], 1.41 [95% confidence interval {CI}, 0.99–2.00]), and in reviewing another six placebo-controlled trials, they found a greater average reduction in numerical rating scale (NRS) pain assessment (−0.46 [95% CI, −0.80 to −0.11]).11 It appears that a majority of reviews have found evidence focused on neuropathic pain; however, a commonly encountered clinical complaint such as low-back pain deserves more inquiry.
It is likely that there will continue to be an increase in availability of medical cannabis and related recreational products as the political and legal landscapes change. Therefore, it is important to understand how cannabis use can be utilized in the treatment of low-back pain. The etiology of low-back pain varies greatly. Nonspecific low-back pain, most likely due to underlying musculoskeletal issues such as a lumbar paraspinal muscle strain, is frequently seen in primary care offices. Radiculopathy, spinal stenosis, and facet arthropathy are more specific etiologies and a focus of this review. A systematic review was performed to assess the use of cannabis for low-back pain.
Materials and Methods
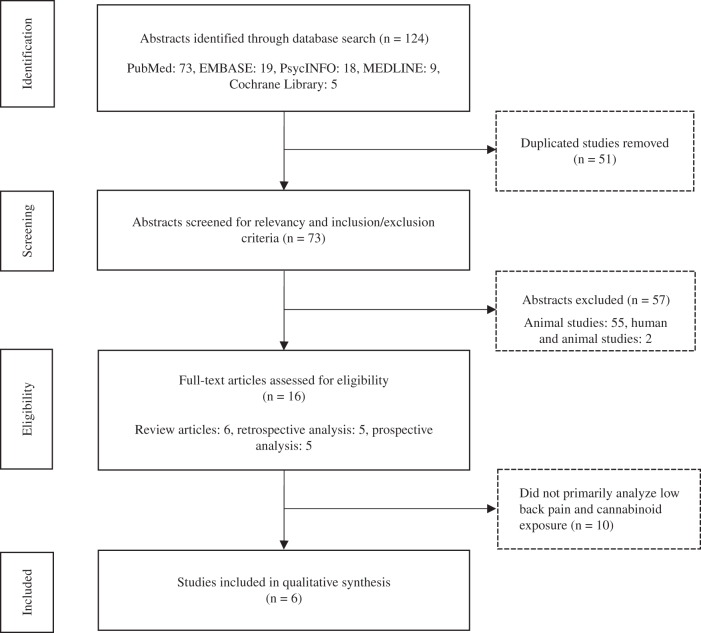
This review focuses on the association between cannabis and low-back pain by performing a systematic literature review via several online databases. Data sources included PubMed, Embase, PsycINFO, MEDLINE, and the Cochrane Library. Search strategies utilized keywords placed in specific search fields (All Fields and MeSH Terms) on October 2, 2018 (Table 1). These search results produced 124 articles (Fig. 1). Two reviewers (W.D. and B.H.) independently screened all titles and abstracts. Specific inclusion and exclusion criteria such as study design, human versus animal, and language (English) were applied and produced 16 articles for full-text review. Six studies were included in qualitative synthesis.
Table 1.
Detailed Example of Search Results in PubMed
| No. | Term | MeSH results | All Fields results | Results |
|---|---|---|---|---|
| 1 | Marijuana | 8,033 | 28,448 | |
| 2 | Cannabis | 8,033 | 18,656 | |
| 3 | Dronabinol | 6,521 | 6,650 | |
| 4 | Tetrahydrocannabinol | 6,521 | 8,577 | |
| 5 | Cannabinoids | 12,526 | 15,648 | |
| 6 | Cannabinoid | 12,526 | 24,064 | |
| 7 | Low back pain | 19,477 | 34,496 | |
| 8 | Chronic low back pain | 6,151 | 10,165 | |
| 9 | Lumbar pain | 19,477 | 33,026 | |
| 10 | Lumbar radiculopathy | 1,857 | 3,241 | |
| 11 | Lumbar stenosis | 289 | 6,583 | |
| 12 | Lumbar facet arthropathy | 485 | 754 | |
| 13 | Acute low back pain | 2,039 | 3,376 | |
| 14 | 1–6 (MeSH OR All Fields) | 45,565 | ||
| 15 | 7–13 (MeSH OR All Fields) | 58,259 | ||
| 16 | 14 AND 15 (MeSH OR All Fields) | 73 | ||
| 17 | 16 AND English (lang) | 58 | ||
| 18 | 17 AND “humans” (MeSH) | 12 |
FIG. 1.
Flowchart diagram of study identification and selection process.
Results
Using our search terms, 73 articles were screened. Of those, 16 were human studies, 2 were human and animal studies, and 55 were animal studies. The 16 human studies were categorized by the type of study as follows: 6 review articles, 5 retrospective analyses, and 5 prospective analyses. Of the 16 human studies, 6 were qualified as “relevant” if the data analyzed included specifically both low-back pain and cannabinoid exposure. These articles are briefly described below and outlined in Tables 2 and 3.12–17 The animal and human/animal subject articles were large studies of biochemical pathways involving the role of cannabinoid receptors in peripherally and/or centrally mediated pain and were not included in this analysis. There were no randomized controlled trials.
Table 2.
Detailed Description of Included Prospective Studies
| Articles | Study design | Description | Age (years) | Sample size | Outcome pain measures | Outcome intervals |
|---|---|---|---|---|---|---|
| Ramesh et al. (2018)17 | Prospective | Examines cannabinoid gene expression profiles in individuals with acute and cLBP | 18–50 | 104 | Self-reported: BPI and MPQ; experimental: QST | Baseline, 24 weeks |
| Eisenberg et al. (2014)16 | Prospective | Explores the pharmacokinetics, safety, tolerability, efficacy, and ease of use of a novel portable tMDI for cannabis in patients suffering from chronic neuropathic pain (two from lumbosacral radiculopathy) and on a stable analgesic regimen including medicinal cannabis | ≥18 | 8 | VAS | Baseline, 20 and 90 min |
| Degenhardt et al. (2007)14 | Prospective | Investigates the influence of OMT on levels of circulatory pain biomarkers in patients with cLBP, including PEA, an endogenous analogue of endocannabinoid | 24–53 | 20 | NRS | Baseline, 30 min, 24 h |
BPI, Brief Pain Inventory; cLBP, chronic low-back pain; MPQ, McGill Pain Questionnaire; NRS, numerical rating scale; OMT, osteopathic manipulative treatment; PEA, N-palmitoylethanolamide; QST, quantitative sensory testing; tMDI, thermal-metered-dose inhaler; VAS, visual analogue scale.
Table 3.
Detailed Description of Included Retrospective and Review Studies
| Articles | Study design | Description | Age (years) | Sample size | Outcome pain measures |
|---|---|---|---|---|---|
| Ko et al. (2016)12 | Retrospective: review/case report | Addresses barriers and key concerns from both the patient and physician prospective when using cannabis as a medical agent in the Canadian medical system; includes case study demonstrating the use of medical marijuana for neuropathic low-back pain | 49 | 1 | NRS, DN4, Freynhagen Pain Detect Questionnaire |
| Shmagel et al. (2016)13 | Retrospective: population-based cross-sectional survey | Compares the prevalence of illicit drug use (including cannabis) among U.S. adults with and without cLBP | 20–69 | 700 | Comprehensive back pain questionnaire in the NHANES |
| Canadian Agency for Drugs and Technologies in Health (CADTH) (2016)15 | Review | A literature review of clinical effectiveness, safety, and guidelines for cannabinoid buccal spray, which included five systematic reviews and one evidence-based guideline | N/A | N/A | N/A |
DN4, Douleur Neuropathique in 4 Questions; NHANES, National Health and Nutrition Examination Survey.
Shmagel et al. conducted a population-based cross-sectional survey that investigated the prevalence of illicit drug use among U.S. adults with versus without chronic low-back pain (cLBP).13 The cLBP was defined as “current pain in the area between the lower posterior margin of the ribcage and the horizontal gluteal fold at the time of evaluation,” with a history of the pain occurring daily for at least 3 months (N=700). Analyzing data from the 2009–2010 National Health and Nutrition Examination Survey, the authors found that 46.5% of U.S. adults with cLBP reported lifetime usage of cannabis versus 42% of those without cLBP (adjusted OR, 1.36, 95% CI, 1.06–1.74).13 The study did not differentiate between recreational and medical cannabis usage. Additionally, cLBP is a nonspecific diagnosis that may refer to a multitude of pathological conditions.
Ramesh et al. examined endocannabinoid gene expression among individuals presenting with an acute low-back pain episode (n=42), cLBP (n=42), and healthy controls (n=20).17 The authors found increased levels of endocannabinoid gene expression (e.g., CNR2 mRNA) in those with low-back pain (acute or chronic) versus healthy controls (p<0.001), and the cLBP group exhibited elevated levels of fatty acid amide hydrolase and vanilloid receptor gene (TRPV1) mRNA (p<0.05).17 The study was limited by the fact that 45% of low-back pain participants used NSAIDs (which can impact circulating endocannabinoid levels), as well as the overall small sample size of the control population. The authors conclude that while significant future research is necessary to elucidate these pathways, low-back pain-associated changes in the endocannabinoid system and expression of its components may prove to be pharmacological targets that could lead to novel analgesic treatments.17
A study by Degenhardt et al. investigated the effects of osteopathic manipulative treatment (OMT) on five pain biomarkers, including anandamide (AEA) and N-palmitoylethanolamide (PEA).14 AEA is an endocannabinoid that produces significant analgesic and anti-inflammatory effects in animal models. PEA is its endogenous analogue that has also been shown to demonstrate analgesic and anti-inflammatory properties. In 10 participants with and 10 participants without cLBP, the researchers found that biomarkers for AEA and PEA were significantly changed at 30 min and 24 h post-treatment and were significantly greater in the cLBP group at 30 min compared with the group without cLBP.14 This finding suggests that effects secondary to OMT may be mediated by endogenous opioid and endocannabinoid pathways and that OMT may cause a short-lived but greater increase in PEA concentrations in subjects with cLBP relative to the increase in subjects without cLBP. The authors acknowledge that their results are based on a small sample size with low statistical power and that while encouraging, these results are correlational rather than mechanistic.
The review article of Ko et al. discusses the history of medical cannabis, biochemical endocannabinoid pain pathways, common concerns regarding cannabis, and potential prescription consideration, and highlights a case study of a 49-year-old male with 20 years of cLBP.12 They report that his low-back pain is secondary to spinal stenosis, degenerative disc disease, neuropathic pain, and sciatica and that before intervention, he took nabilone 0.25 mg, ibuprofen 400–600 mg, baclofen 20 mg, clonazepam 0.5 mg, and pregabalin 300 mg daily. After 60 days of treatment with 1 g per day of cannabis (strain with 9% THC and 13% CBD) administered by vaporizer, his NRS pain scale decreased from a weekly average of 9/10 to 3/10, and he started to wean off oral analgesics.12
A systematic review for the usage of cannabinoid buccal spray for chronic noncancer or neuropathic pain found that THC:CBD buccal spray may be associated with reduced levels of perceived pain and is well tolerated in these patients.15 However, the authors conclude that THC:CBD buccal spray be considered a third-line therapy in the management of these patients given insufficient high-quality scientific evidence supporting its use. In this review, low-back pain was not a separate category; rather, it was included in the broad category of chronic pain. Therefore, direct associations are difficult to extrapolate.15
Finally, Eisenberg et al. explored the pharmacokinetics, safety, tolerability, efficacy, and ease of use of a novel portable thermal-metered-dose inhaler for cannabis in a cohort of eight patients suffering from chronic neuropathic pain and on a stable analgesic regimen including medicinal cannabis.16 Patients were included if they had “neuropathic pain of any type for at least 3 months,” with two of the cases due to lumbosacral radiculopathy. The authors cite a 45% reduction in pain intensity at 20 min after inhalation (p=0.001), returning to baseline within 90 min.16 Lightheadedness, lasting 15–30 min and requiring no intervention, was the only reported adverse event.
Human articles that were not included in the full review discussed the extensive history of cannabis as a medical treatment but failed to specifically mention low-back pain.18,19 In one cross-sectional analysis of a longitudinal cohort study, Patanwala et al. found that nearly 60% of homeless people surveyed experienced low-back pain, and in a multivariate analysis showed that cannabis use was associated with moderate–high physical symptom burden in the same population.20 Other review articles discussed the disappointing effectiveness of current pharmacological treatments for musculoskeletal disorders, the breadth of diseases that have the potential to be treated, and cannabinoids being used successfully for neuropathic pain.15,18,19 Several articles cited a major lack of research regarding cannabis for pain.12,18,21 Most articles reviewed noted that cannabis-based medicine is an emerging field; therefore, it is important for physicians, especially those treating pain, to understand the basic clinical application of cannabis and cannabinoid products.
Discussion
In the United States, 80% of adults experience low-back pain in their lifetimes, and low-back pain is the most common cause of job-related disability.22 Therefore, it is important to understand potential novel treatments for this problem. Cannabinoids can be expected to cause some pain relief, confirmed by one prospective trial including patients with neuropathic pain,15 as well as multiple nonsystematic reviews.18,19,21 Prior similar systematic reviews were not available for comparison. None of the six studies revealed by this search directly assessed the relationship between cannabis and low-back pain. This demonstrates that there is a paucity of data on the potential risks and benefits of using cannabinoids to treat low-back pain.
Our review has several limitations. Articles reviewed were all in English, with foreign articles excluded. Additionally, of the studies included, there is considerable heterogeneity regarding the research subject and design and therefore limited generalizability from these results. This further emphasizes the need for longitudinal studies examining the potential risks and benefits of cannabinoids for low-back pain.
There are many potential avenues for future investigation. Each state varies to some degree on how it defines and regulates the legal use, prescription, or sale of cannabis. Currently, there is no federal law allowing the legal use of medical cannabis. The National Academies of Sciences, Engineering, and Medicine recently published a report outlining some of the challenges and barriers in conducting cannabis research.23 They concluded that the classification of cannabis as a Schedule I substance serves as a significant obstacle to progression in this field of research.23
The VA Medicinal Cannabis Research Act of 2018 was introduced to the U.S. House of Representatives to “conduct and support research on the efficacy and safety of certain forms of cannabis and cannabis delivery for veterans enrolled in the VA health care system diagnosed with conditions such as chronic pain or post-traumatic stress disorder.”24 Similar bills have since been introduced. If passed, they would represent key federal legislation that allows for a uniform assessment of cannabis use and low-back pain by performing rigorous, unbiased, high-quality clinical research.
As for research design, multiple factors should be considered including minimal clinically important difference, placebo-controlled studies, appropriate blinding protocols, and relevant outcome measures. For low-back pain, quality-of-life and functional outcomes may serve as a more useful evaluation of treatment effect as opposed to changes in physical impairments, such as improved lumbar range of motion. Health-related quality-of-life instruments, which have been well studied in patients with low-back pain, include SF-36, a generic self-administered questionnaire, and more disease-specific measures such as the Roland-Morris Questionnaire and the Oswestry Low Back Pain Disability Questionnaire.25–27 None of the six articles in this review utilized these outcome measures. Furthermore, patients' history of consumption, different routes of administration, and associated adverse events should be examined more closely.
Conclusions
This systematic literature review revealed that there is a significant lack of quality evidence regarding the role of cannabinoid products in the treatment of low-back pain. Low-back pain is experienced by a majority of adults during their lifetimes and linked to significant financial burden in the United States' health care system. As the medical community at large continues to grapple with the ongoing opioid crisis, there is a need to investigate alternative analgesic treatment modalities. Greater research into the analgesic properties of cannabis could serve as an important step in the development of novel treatments. The clinical applications of cannabis and cannabinoid products, including the treatment of low-back pain, clearly deserve further exploration.
Abbreviations Used
- AEA
anandamide
- BPI
Brief Pain Inventory
- CBD
cannabidiol
- cLBP
chronic low-back pain
- DN4
Douleur Neuropathique in 4 Questions
- MPQ
McGill Pain Questionnaire
- NHANES
National Health and Nutrition Examination Survey
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PEA
N-palmitoylethanolamide
- NRS
numerical rating scale
- OMT
osteopathic manipulative treatment
- QST
quantitative sensory testing
- THC
tetrahydrocannabinol
- tMDI
thermal-metered-dose inhaler
- VAS
visual analogue scale
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Cite this article as: First L, Douglas W, Habibi B, Singh JR, Sein MT (2020) Cannabis use and low-back pain: a systematic review, Cannabis and Cannabinoid Research 5:4, 283–289, DOI: 10.1089/can.2019.0077.
References
- 1. Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81:671–676 [PMC free article] [PubMed] [Google Scholar]
- 2. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88:21–24 [DOI] [PubMed] [Google Scholar]
- 3. Saragiotto BT, Machado GC, Ferreira ML, et al. Paracetamol for low back pain. Cochrane Database Syst Rev. 2016;6:CD012230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chou R, Deyo R, Friedly J, et al. Noninvasive treatments for low back pain. AHRQ comparative effectiveness reviews. Agency for Healthcare Research and Quality (US): Rockville, MD, 2016 [PubMed] [Google Scholar]
- 5. Machado GC, Maher CG, Ferreira PH, et al. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis. 2017;76:1269–1278 [DOI] [PubMed] [Google Scholar]
- 6. van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low back pain. Cochrane Database Syst Rev. 2003;2:CD004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–530 [DOI] [PubMed] [Google Scholar]
- 8. Amin MR, Ali DW. Recent advances in cannabinoid physiology and pathology: pharmacology of medical cannabis. Springer, Cham: Switzerland, 2019 [Google Scholar]
- 9. Hill KP, Palastro MD, Johnson B, et al. Cannabis and pain: a clinical review. Cannabis Cannabinoid Res. 2017;2:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci. 2014;17:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473 [DOI] [PubMed] [Google Scholar]
- 12. Ko GD, Bober SL, Mindra S, et al. Medical cannabis – the Canadian perspective. J Pain Res. 2016;9:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shmagel A, Krebs E, Ensrud K, et al. Illicit substance use in US adults with chronic low back pain. Spine. 2016;41:1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Degenhardt BF, Darmani NA, Johnson JC, et al. Role of osteopathic manipulative treatment in altering pain biomarkers: a pilot study. J Am Osteopath Assoc. 2007;107:387–400 [PubMed] [Google Scholar]
- 15. Canadian Agency for Drugs and Technologies in Health. Cannabinoid buccal spray for chronic non-cancer or neuropathic pain: a review of clinical effectiveness, safety, and guidelines. Canadian Agency for Drugs and Technologies in Health (CADTH Rapid Response Reports): Ottawa, ON, 2016 [PubMed] [Google Scholar]
- 16. Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study. J Pain Palliat Care Pharmacother. 2014;28:216–225 [DOI] [PubMed] [Google Scholar]
- 17. Ramesh D, D'Agata A, Starkweather AR, et al. Contribution of endocannabinoid gene expression and genotype on low back pain susceptibility and chronicity. Clin J Pain. 2018;34:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curatolo M, Bogduk N. Pharmacologic pain treatment of musculoskeletal disorders: current perspectives and future prospects. Clin J Pain. 2001;17:25–32 [DOI] [PubMed] [Google Scholar]
- 20. Patanwala M, Tieu L, Ponath C, et al. Physical, psychological, social, and existential symptoms in older homeless-experienced adults: an observational study of the hope home cohort. J Gen Intern Med. 2018;33:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dosenovic S, Jelicic Kadic A, Miljanovic M, et al. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg. 2017;125:643–652 [DOI] [PubMed] [Google Scholar]
- 22. Low back pain fact sheet. National Institute of Neurological Disorders and Stroke. Available from: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Low-Back-Pain-Fact-Sheet (last accessed January20, 2019)
- 23. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press: Washington, DC, 2016 [Google Scholar]
- 24. Walz TJ. H.R.5520 – 115th Congress (2017–2018): VA Medicinal Cannabis Research Act of 2018. Available from: https://www.congress.gov/bill/115th-congress/house-bill/5520 (last accessed September18, 2019)
- 25. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483 [PubMed] [Google Scholar]
- 26. Fairbank JC, Pynsent PB. The oswestry disability index. Spine. 2000;25:2940–2952 [DOI] [PubMed] [Google Scholar]
- 27. Stratford PW, Binkley J, Solomon P, et al. Defining the minimum level of detectable change for the Roland-Morris questionnaire. Phys Ther. 1996;76:359–365 [DOI] [PubMed] [Google Scholar]