Abstract
To treat coronary heart disease, coronary artery bypass grafts are used to divert blood flow around blockages in the coronary arteries. Autologous grafts are the gold standard of care, but they are characterized by their lack of availability, low quality, and high failure rates. Alternatively, tissue-engineered small-diameter vascular grafts made from synthetic or natural polymers have not demonstrated adequate results to replace autologous grafts; synthetic grafts result in a loss of patency due to thrombosis and intimal hyperplasia, whereas scaffolds from natural polymers are generally unable to support the physiological conditions. Extracellular matrix (ECM) from a variety of sources, including cell-derived, 2D, and cannular tissues, has become an increasingly useful tool for this application. The current review examines the ECM-based methods that have recently been investigated in the field and comments on their viability for clinical applications.
Impact statement
In reviewing extracellular matrix (ECM)-based small-diameter vascular grafts, we have thoroughly and concisely summarized the foundational work in the field, in addition to exploring the recent developments and approaches used. Due to the plethora of research articles for constructing vascular grafts, we dedicated this review to focus on grafts from ECM to provide a more comprehensive look at this specific technology.
Keywords: small-diameter vascular grafts, extracellular matrix, decellularized, coronary artery bypass graft
Introduction and Motivation
Cardiovascular disease (CVD) is the primary cause of death globally and kills 17 million people each year.1 CVD encompasses a range of conditions, including coronary heart disease, which is characterized by the stenosis or occlusion of blood vessels surrounding the heart.1 Treatment for CVD includes lifestyle changes to reduce risk factors, medications, and surgeries such as coronary artery bypass grafting (CABG).2 First utilized in the 1950s, CABGs divert blood flow around a blockage in the coronary artery by using a small-diameter vascular graft.3 Today, there are about 400,000 CABGs performed each year in the United States, alone.4
Autologous grafts, largely from the saphenous vein and internal mammary artery, are the current gold standard for bypasses, but they are an insufficient treatment in both the approach and the results. Autologous grafts are limited by quality and availability from the donor; ∼25% of patients requiring arterial bypass procedures do not have saphenous veins that are acceptable for implantation.5 The isolation of autologous grafts requires a secondary surgical site and risks donor site morbidity.6 In addition, the harvesting process and subsequent assessment of the graft before implantation can damage the vessel and lead to endothelial dysfunction, a pro-inflammatory response, and ultimately thrombosis and graft occlusion.7–9 Postoperative failure of saphenous vein grafts is largely due to technical failure or thrombosis within the first month, intimal hyperplasia within the first year, and atherosclerosis after the first year.10 These issues result in a 10% failure rate after the first month and a 50% failure rate at 10 years.11–13 The high failure rates render this method of treatment largely inadequate, which has led to the development of non-autologous alternatives.
Tissue engineering techniques utilizing synthetic and natural polymers fall short for small-diameter (<6 mm) vascular grafts. Commercially available synthetic grafts composed of polyethylene terephthalate/Dacron and polytetrafluoroethylene/Teflon (ePTFE) function well for large-diameter vascular grafts, but they perform poorly in small-diameter applications.14–16 Generally, small synthetic grafts are characterized by a lack of an endothelium, high modulus, and low compliance, which often lead to thrombosis and intimal hyperplasia.17–21 Alternatively, natural polymers such as collagen and fibrin have been investigated for small-diameter grafts because they are biocompatible and biodegradable and promote endothelialization.22–24 Natural polymers exhibit an elastic modulus that is more comparable to native vessels, and they do not illicit a chronic inflammatory response.22,25,26 However, they possess substantially lower strengths than physiologically required and can degrade faster than the body regenerates.2,23,26–28 These types of grafts have been reviewed extensively and will not be addressed further.28–31
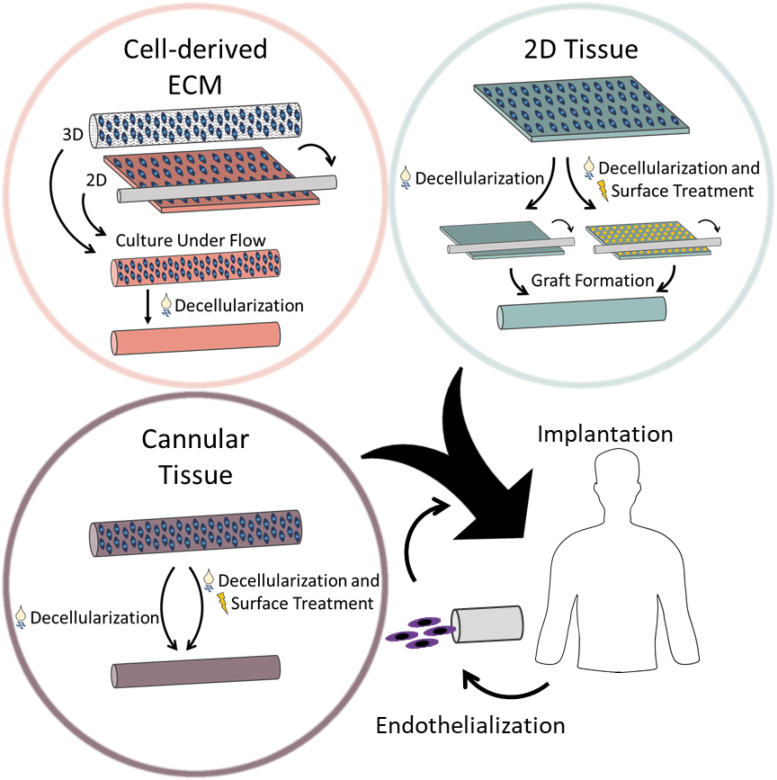
Another material that has been increasingly examined for vascular graft engineering endeavors is extracellular matrix (ECM). The composition of this three-dimensional fibrous network produced by cells depends on the type of tissue, but largely includes collagen, fibronectin, laminin, glycosaminoglycans (GAGs), and growth factors.32–36 This material is obtained through the decellularization of tissue, where the cellular and nuclear matters are removed through physical, chemical, and enzymatic approaches.36–38 The ECM provides both physical support and biological cues to cells that direct cell adhesion, proliferation, and differentiation.38,39 When utilized in tissue engineering scaffolds, ECM encourages constructive remodeling and favorable clinical outcomes.40,41 Specifically in vascular applications, collagen,42,43 fibrin,44 fibronectin,45 laminin,46,47 chondroitin sulfate,48 and vascular endothelial growth factor (VEGF)49 have been shown to encourage endothelialization. However, ECM components can also lead to platelet activation and thrombosis,50–52 so it is important to consider the individual components, as well as the overall composition of the ECM. In addition to its bioactivity and biocompatibility, the ECM has demonstrated mechanical properties that are comparable to those of native vessels.38,53–55 The ECM has been employed for tissue-engineered vascular grafts in three major ways: cell-derived ECM, extracted 2D tissues, and extracted cannular tissues (Fig. 1). Cell-derived grafts are those created from the culture of cells either in a cannular shape or in a monolayer. After the cells have produced ample ECM, the structures are decellularized. In addition, isolated 2D and cannular tissues can be used to develop grafts through the use of decellularization and surface modifications. Grafts can be implanted acellularly or endothelialized with autologous endothelial cells (ECs).
FIG. 1.
ECM-based technologies, including cell-derived ECM, 2D tissue, and cannular tissue, for small-diameter vascular graft scaffolds. Cell-derived ECM grafts are developed through weeks of cell culture before decellularization, in either a 3D or 2D geometry. The 3D geometry method employs a scaffold to support cell growth, whereas the 2D method utilizes cell sheets to wrap around a mandrel. Vascular grafts can be constructed from decellularized 2D tissue by using sutures or glue, whereas cannular tissues only need to be decellularized. ECM from 2D and cannular tissue sources frequently undergo surface treatment to improve the outcome of the graft. Vascular grafts can either be implanted directly or endothelialized with the patient's own cells before surgery. ECM, extracellular matrix.
Arteries have a relatively simple function, to carry blood away from the heart; however, they have a complex structure that can be difficult to recapitulate. To support the high pressures, flow rates, and shear stresses, arteries have a thicker wall and smaller lumen diameter than veins.56 The vessel wall is composed of three layers: tunica intima, tunica media, and tunica adventitia.57 The innermost layer, the tunica intima, contains the endothelial lining, which is imperative to the prevention of platelet adhesion and thrombosis.17,35,58 When the endothelial lining is damaged, the artery is susceptible to infection, thrombosis, and intimal hyperplasia.31,56,59,60 The tunica media, the middle layer, contains both elastin and smooth muscle and largely controls the mechanical functionality of the vessel.56,61 The smooth muscle cells (SMCs) in this layer have a contractile phenotype, characterized by the presence of smooth muscle actin (SMA), calponin, smooth muscle myosin heavy chain (SM-MHC), SM22-α, and smoothelin.62 Further, SMCs can transition between a quiescent, contractile phenotype and a proliferative, synthetic one.62,63 The outermost layer, the tunica adventitia, is predominantly composed of collagen, but it contains elastin, fibroblasts, and adipocytes.56,58 The elastin and collagen contribute to a sophisticated two-region mechanical behavior of the arterial wall.61,64 Each of these elements must be considered during the development of new approaches for constructing vascular grafts.
To create a truly viable and sustainable solution for patients, many different requirements need to be satisfied. Overall, the vascular graft must demonstrate biocompatibility, meaning it is nontoxic, nonimmunogenic, and nonthrombogenic.65 It should be easy to remodel, complete with a confluent, quiescent, and nonactivated endothelium.28,31,65 It is vital that the vascular graft mimics the complex mechanical behavior of native arteries, including the modulus, circumferential strength, burst pressure, and suture retention strength.28,65,66 Vascular grafts must be more patient friendly, in that any human-derived elements should be isolated noninvasively. In addition, grafts should have short production times, low costs, and off-the-shelf availability to maximize their clinical impact.28,67
In this review, we explore the ECM-based approaches, namely cell-derived ECM, extracted 2D tissues, and extracted cannular tissues, used to develop small-diameter vascular grafts and summarize the recent approaches in Tables 1, 2, and 3, respectively. Further, we provide our analyses on the impact of each method and the future direction of small-diameter vascular graft research. The search terms for this review included, but were not limited to, “small diameter,” “vascular graft,” “extracellular matrix,” and “decellularized.” We focused on papers published within the past 5 years that developed small-diameter grafts for applications in CABG surgeries. Articles reporting on vascular grafts that were larger than 6 mm in diameter or utilized materials that were not ECM based, such as polymers or hydrogels, were excluded from this review.
Table 1.
Summary of Recent Cell-Derived Extracellular Matrix Approaches for Small-Diameter Vascular Grafts
| Material | Cells | Maturation Length | Decellularized | Outcomes/Patency | Reference |
|---|---|---|---|---|---|
| Fibrin gel | hDFs | 2 weeks static and 3 weeks dynamic | Yes | 83% at 3 months and 60% at 6 months in baboon model | 80 |
| Needle with glue | hSMCs, hDFs, and HUVECs | 2 weeks dynamic | No | All grafts patent for 8 weeks in rat model | 86 |
| Nanograted PDMS | hMSCs and hEPCs | 3 weeks flat, 2 weeks dynamic, and 2 weeks for hEPCs | No | Functional endothelium | 78 |
| Nanograted PDMS | hDFs and hMSCs | 6 weeks hDFs, 1 week for hMSCs, and 2 weeks dynamic | Yes, after initial 6 weeks | Anisotropic mechanical behavior | 77 |
| Agarose mold | hSMCs-only and hMSCs-only | 1 week static and 1 week dynamic | No | hSMCs did not retain their contractile phenotype | 79 |
| PGA mesh | hiPSC-derived SMCs | 8–9 weeks dynamic | No | All grafts patent for 1 and 2 weeks in rat model | 84 |
| PGA mesh with fibrinogen-thrombin solution | hiPSC-derived SMCs and ECs |
1 week static, 2, 5, or 8 weeks under flow, and ECs for 3 days | No | Supported development of ECM for 9 weeks | 85 |
| PGA mesh | hiPSC-derived SMCs | 1 week static, 7 weeks dynamic | No | All grafts patent for 4 weeks in rat model | 82 |
ECM, extracellular matrix; PDMS, polydimethylsiloxane; hDF, human dermal fibroblast; PGA, polyglycolic acid; hSMC, human smooth muscle cell; hiPSC, human induced pluripotent stem cell; EC, endothelial cell.
Table 2.
Summary of Recent Two-Dimensional Tissues for Small-Diameter Vascular Grafts
| Material | Modifications and Treatments | Decellularized | Outcomes/Patency | Reference |
|---|---|---|---|---|
| Porcine SIS | Fibrinogen and thrombin, ECs with or without SMCs | Yes, pre-seeding | All grafts patent for 1 week, 1 month, and 3 months in sheep model | 96 |
| Porcine SIS | Yes | Cobblestone morphology of HUVECs | 44 | |
| Porcine SIS | Heparin and VEGF | Yes | All grafts, except 1, patent for 1 week, 1 month, and 3 months in sheep model | 93 |
| Porcine SIS | Heparin | Yes | All grafts patent for 6 weeks in canine model | 94 |
| Porcine UBM | Yes | Good adhesion and proliferation of HUVECs | 44 | |
| Porcine Aortic Sheet | Fibrin glue | Yes | All grafts patent for 3 weeks in rat model | 107 |
| Porcine Aortic Sheet | Electrospun polyurethane | Yes | Polyurethane increased stiffness | 106 |
| Human Amniotic Membrane | Thrombin | Yes | All grafts patent after 4 weeks, but 1 of 3 had thrombus in rabbit model | 100 |
| Bovine Pericardium | Poly(propylene fumarate) | Yes | All grafts patent after 2 weeks in rat model | 103 |
| Bovine Achilles Tendon | GA and elastin | Yes | Elastin reduced mechanical strength and stiffness | 97 |
| Hypophthalmichthys molitrix Swim Bladder | GA | Yes | 3/4 GA treated and 1/4 untreated patent at 4 weeks in rat model | 98 |
HUVEC, human umbilical vein endothelial cell; SIS, small intestine submucosa; GA, glutaraldehyde.
Table 3.
Summary of Recent Cannular Tissues for Small-Diameter Vascular Grafts
| Material | Treatment | Decellularized | Outcomes/Patency | Reference |
|---|---|---|---|---|
| Ostrich carotid artery | POG7G3REDV and OPG7G3REDV | Yes | 5/6 grafts were patent at 3 weeks in minipig model | 126 |
| Equine carotid artery | Yes | Decellularization decreased matrix elasticity and compliance | 112 | |
| Rat aorta | Heparin and SDF-1α | Yes | All grafts patent for 4 weeks in rat model | 124 |
| Rat aorta | Poly(1,8 octanediol citrate) and Heparin | Yes | Reduced platelet adhesion and clotting | 122 |
| Rat aorta | Poly(1,8 octanediol citrate) and Heparin | Yes | All grafts patent for 4 weeks in rat model | 66 |
| Rat aorta | Electrospun Polycaprolactone and Heparin | Yes | All grafts patent for 3 months in rat model | 113 |
| Rat aorta | TSP2 KO dermal fibroblasts from mice | Yes, before and after fibroblasts | All grafts patent for 4 weeks in rat model | 128 |
| Rat aorta | Poly(1,8 octanediol citrate) and Heparin | Yes | All grafts patent for 3 months in rat model | 123 |
| Rat aorta | VEGF-conjugated hydrogel | Yes | All grafts patent for 8 weeks in rat model | 125 |
| Rabbit aorta | Heparin, VEGF 145, and bFGF | Yes | 90% at 18 months in canine model | 121 |
| Porcine aorta | Autologous ECs | Yes | All grafts patent for 1, 3, and 6 months in canine model | 115 |
| Porcine carotid artery | Poly(ethylmethacrylate-co-diethylaminoethylacrylate) | Yes | Polymer coating recovered loss of strength from decellularization and supports ECs | 114 |
| Porcine carotid artery | Autologous ECs | Yes | 90% patency in grafts for 6 week in pig model | 116 |
| Porcine carotid artery | hMSCs | Yes | ECM, fibroblasts, and growth factors cause hMSCs to differentiate into vascular SMCs | 117 |
| Porcine carotid artery | hMSCs | Yes | ECM, growth factors, and dynamic culture can differentiate hMSCs into ECs and vascular SMCs | 118 |
| Porcine carotid artery | Heparin | Yes | Heparin crosslinking recovered loss of strength from decellularization and fights thrombus | 120 |
| Porcine vena cava | Yes | Decellularization method impacts ECM, but not HUVECs | 111 | |
| Human hand vein and artery | Yes | All grafts patent for 2 weeks in rabbit model | 129 | |
| Human internal mammary artery |
Yes | Grafts were successfully decellularized with minimal impact on the ECM | 130 | |
| Human internal mammary artery |
Yes | All grafts patent for 3 years in rabbit model | 134 | |
| Human saphenous vein | Cryopreserved and autologous ECs | De-endothelialized | 80% at 6 months and 50% at 9 months in human coronary artery bypass model | 135 |
| Human umbilical vein | Fibronectin | Yes | Decellularization damaged ECM and impacted HUVECs proliferation and attachment | 136 |
| Human Umbilical Artery | hMSCs | Yes | HLA genotype of hUA was changed with culture of hMSCs | 138 |
| Human umbilical artery | Vitrified and stored for 6 months | Yes | All grafts patent for 4 weeks in porcine model, although all had thrombus present | 137 |
| Human placenta chorion | Yes | Decellularization reduced mechanical properties, but still similar to human coronary arteries | 131 | |
| Human placenta chorion | Yes | All grafts patent for 1 month in rat model | 132 |
bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor.
Cell-Derived ECM
Niklason et al. have played a critical role in laying the groundwork for and progressing the cell-based ECM approach for small-diameter vascular grafts. Their work has utilized either SMCs (bovine,68,69 porcine,54,68 canine,70 human70,71) or human mesenchymal stem cells (hMSCs)72 to seed onto a polyglycolic acid (PGA) mesh scaffold. After 8–10 weeks of culture, these grafts demonstrated appropriate vessel wall thickness, collagen content, suture retention strength, and burst pressure.68,69 Overall, these grafts have shown good long-term patency in vivo in both baboon and canine models.70 Further, they were utilized in clinical trials for hemodialysis access in patients with end-stage renal disease with promising patency trends: 15% primary patency, 25% primary assisted patency, and 80% secondary patency at 2 years.71 Despite the positive results in animal studies and progression to hemodialysis access clinical trials, the PGA mesh foundation has caused several complications. Namely, the degradation and residual fragments of PGA trigger a shift in SMC phenotype from a contractile to a synthetic one with lower expression of myosin, SMA, and calponin.68,69,73,74 The shift to the synthetic phenotype is detrimental to the regulation of vessel diameter62 and is a precursor to intimal hyperplasia62 and atherosclerosis.63
Various methods have been implemented to eliminate the use of the polymer scaffold. Growing monolayers of cells and wrapping these cell sheets around a removable mandrel was first introduced by L'Heureux et al.75,76 This method has been applied to nanograted polydimethylsiloxane to create aligned cell sheets of hMSCs and either human dermal fibroblasts (hDFs)77 or human endothelial progenitor cells (hEPCs).78 These cell sheets were rolled and cultured under flow, which resulted in a vascular graft with circumferential cell alignment.77,78 Xing et al. demonstrated the corresponding anisotropic behavior and moduli, both similar to the behavior of native arteries.77 Further, the grafts stained positive for SMA and SM22-α, indicating contractile SMC phenotypes, and showed increased cell–cell communication.77 Jung et al. demonstrated a functional endothelium and low, but ample burst pressure, although this assembly was largely geared toward drug testing models.78 Another alternative method is culturing either hSMCs or hMSCs around pillars to form rings of cells and then culturing these rings together to allow the layers to fuse to one another.79 Although the hSMCs did not retain their contractile phenotype, the hMSC-cultured grafts did, as demonstrated by the expression of SMA and SM22-α.79 Syedain et al. developed a vascular graft from culturing hDFs in bovine fibrin gel for 5 weeks before decellularization, which resulted in grafts with burst pressure and suture retention strength comparable to human arteries.80 Grafts implanted in baboons for 3 and 6 months demonstrated patency values of 83% and 60%, respectively, but showed evidence of thrombosis on the luminal surface at explantation.80
The in vivo success of acellular grafts falls on the ability of the host cells to infiltrate the scaffold, which can be a challenge for patients with vascular cells of reduced remodeling capacity, a large demographic of CABG recipients. Previously addressed with autologous ECs, one new approach to circumvent the decellularization step is incorporating allogeneic human induced pluripotent stem cell (hiPSC)-derived cells. hiPSCs are an invaluable tool in the tissue engineering field, because they serve as a possible avenue to create a “universal” cell bank that does not induce an immune response and does not require decellularization.81,82 Although hMSCs have been investigated for this reason,83 research has expanded the use of hiPSCs due to their pluripotency. hiPSCs were differentiated into SMCs and cultured on PGA mesh for a total of 8–9 weeks.84 Although these grafts allowed blood flow and demonstrated cell infiltration in nude rats for 1 and 2 weeks, histologically the grafts appear collapsed with irregular lumens.84 A secondary study incorporated a cyclic strain during the 8 weeks of graft culture, which increased burst pressure and suture retention strength.82 These grafts were patent in nude rats over 30 days and expressed markers for a contractile and mature SMC phenotype, including SMA, calponin, and SM-MHC, as well as ECM markers for collagen I and elastin.82 The ECs can also be derived from iPSCs and have been incorporated in vascular grafts. hiPSC-derived ECs were seeded on the lumen of vascular grafts fabricated from hiPSC-derived SMCs cultured on PGA mesh for a total of 3–9 weeks.85 The walls of the grafts that underwent 6 and 9 weeks of culture were very thick, but they have visibly more SMA when compared with those at 3 weeks of culture.85
One imperative advancement to transition cell-derived scaffolds into a clinical technology is the reduction of cell culture time, as an avenue to increase production capacity and control cost. von Bornstädt et al. assembled a vascular graft by using alternating layers of SMC and fibroblast cell sheets, reinforced with Dermabond Advanced tissue glue.86 The grafts were perfused with human umbilical vein endothelial cells (HUVECs) for 24 h and then cultured for 2 weeks.86 After the 2 week culture period, grafts demonstrated ample burst pressure to support physiological conditions, which increased after 8 weeks in vivo to values comparable to native rat femoral arteries.86 A continuous endothelial lining and appropriate media and adventitia layers were confirmed, suggesting no incidence of intimal hyperplasia.86
Impact and clinical outlook
Grafts fabricated by using the cellular method are useful because they are largely patient independent, with the exception of autologous ECs, and can be fabricated ahead of time to keep patient wait times short. Although early cell-derived grafts composed from animal cells required decellularization, recent endeavors to develop these types of grafts employ human cells and largely do not undergo decellularization. We speculate that this shift is driven by the ultimate goal of mitigating an immune response without the need for decellularization through the utilization of patient-specific cells or cells from a “universal” cell bank. However, the cell culture required to produce ample cell populations, in addition to the graft culture, is extremely time-consuming, resource-intensive, and expensive. Although substantial progress has been made in improving graft function and patency of cell-based grafts, which resulted in a clinical trial for hemodialysis access, little work has been completed to improve the feasibility of clinically implementing this technology, predominately in terms of reducing culture time. We predict that by cutting the culture period, the amount of resources required and cost will decrease, rendering cell-based vascular grafts a more practical solution. In the context of the 400,000 CABG grafts implanted each year and the millions of cells necessary for each graft, there are logistics-related obstacles to investigate to scale this technique to meet the current needs of patients. Further, due to the established effects of cell source, specifically the age and health of the donor, on cell function,87–89 we anticipate that the source will affect not only cell expansion and cell sheet culture, but also graft functionality and success. Advancing the use of allogeneic hiPSCs in these grafts may serve as an alternative to the variables associated with cell donors. Despite the difficulties associated with the application of this technique, cell-derived ECM technologies provide the unique opportunity for further investigation into the largely unexplored area of the effect of ECM composition, which can be controlled through cell type and culture time, on in vivo endothelialization.
Two-Dimensional Tissue
Porcine small intestine submucosa (SIS) is a commonly used 2D tissue scaffold and it was first employed by Badylak et al. in its native state as a large-diameter vascular graft.90 Although early SIS grafts had low patency results that were comparable to autologous saphenous vein grafts, they demonstrated appropriate mechanical properties similar to those of native vessels in terms of burst pressure, elastic modulus, and compliance.91–93 The HUVECs cultured on decellularized SIS demonstrated higher proliferation and appeared more evenly spread with a cobblestone morphology, when compared with the ePTFE and Dacron alternatives.44 Although these characteristics are indicative of better endothelialization, an important mechanism in preventing platelet adhesion,17 both native and decellularized SIS grafts are prone to thrombosis.91,94
Endothelialization of the lumen and a variety of surface treatments, including the immobilization of heparin and growth factors, have been employed to prevent thrombosis and improve the patency of SIS grafts. The culture of ECs for 1 week on the lumen of a SIS graft, glued with fibrinogen and thrombin, preserved the graft patency ex vivo.95 In a follow-up study, these grafts were treated with fibrin and SMCs before endothelialization and implanted into sheep for 3 months.96 Over this time, grafts with and without SMCs were similar in their diameters, flow rates, and homogenously endothelialized lumens that were free from clotting, intimal hyperplasia, and loss of patency.96 Although cell infiltration occurred quicker in SMC grafts, by 3 months the cell densities were comparable.96 The SMC grafts had significantly more collagen, which suggests a higher maturation of cells and higher macrophage invasion.96 Han et al. used plasma treatment to immobilize heparin on the surface of decellularized SIS before it was sutured into a vascular graft and implanted into adult dogs for 6 weeks.94 The heparin treatment was vital to the patency of the grafts; all 10 treated grafts were patent and showed an endothelial lining without any signs of thrombosis, whereas scaffolds without heparin quickly occluded due to thrombi.94 Similarly, both heparin and VEGF were immobilized on laminated SIS tubes before implantation in female sheep for 1 week, 1 month, and 3 months.93 Eleven of the 12 grafts demonstrated patency without any thrombosis and developed a confluent endothelium within 1 month with SMC infiltration at 3 months.93
In addition to SIS, surface modifications have been utilized in an assortment of 2D tissues, including bovine Achilles tendon and Hypophthalmichthys molitrix-derived swim bladders. Thin layers of decellularized bovine Achilles tendon were layered around a Teflon mandrel and soaked in an elastin and glutaraldehyde (GA)-containing solution.97 The control group with GA treatment-only had a similar modulus to native arteries, whereas the addition of elastin lowered the modulus of the material.97 Further, these grafts had lower than necessary burst pressure values.97 The in vitro cell culture of SMCs and ECs showed that they aligned with the collagen fibers and demonstrated appropriate morphologies: spindle-shaped and cobblestone, respectively.97 Liu et al. utilized Hypophthalmichthys molitrix-derived swim bladders, a unique source of collagen I-rich ECM, for applications in small-diameter vascular grafts.98 After the swim bladder was isolated and decellularized, both unfixed and GA-fixed tissues showed reduced calcification with no visible pro-inflammatory M1 macrophages in a subcutaneous study in Wistar rats.98 Then, both treated and untreated swim bladder was rolled and secured with tissue adhesive to create vascular grafts.98 Grafts were implanted in rats for 4 weeks, with the GA-treated grafts demonstrating improved patency over the non-treated grafts, likely due to the increased strength and stiffness with GA treatment.98 In addition, the grafts exhibited an established endothelium without any sign of intimal hyperplasia.98
One alternative to surface treatment is to develop grafts with materials that naturally resist thrombosis. Human amniotic membrane (hAM) discourages platelet adhesion and activation.99 Amensag et al. used decellularized hAM and thrombin glue to form a six-layered graft that supported the culture of SMCs, which ultimately increased the elastic modulus and GAG content, but decreased the physiological modulus.100 All three acellular grafts displayed significant remodeling and remained patent after 4 weeks in New Zealand white rabbits, although there was thrombus formation in one graft.100 Alternatively, bovine pericardium, a nonthrombogenic source of ECM,101 is frequently used clinically as a vascular patch,102 but it has only recently been employed for small-diameter vascular grafts.103 Vascular grafts composed of decellularized bovine pericardium, coupled with poly(propylene fumarate), demonstrated a modulus similar to native vessels, with ample burst pressure and suture retention strength to support physiological pressures.103 Although both grafts remained patent for 2 weeks in Lewis nude rats without any indication of thrombosis, the new ECM present may be indicative of the onset of intimal hyperplasia.103
With a similar structure as a coronary artery, sheets of decellularized porcine aorta have also been utilized as small-diameter vascular grafts. However, human arteries are stiffer than porcine aortas and other porcine arteries.104–106 Wu et al. circumvented the mechanical mismatch across the two species by covering the porcine aorta graft with electrospun polyurethane.106 The polyurethane increased stiffness of the graft and supported the in vitro 2D cell culture of HUVECs and normal hDFs (NHDFs) for 2 weeks.106 In a study by Negishi et al., sheets of porcine aorta were decellularized by using high hydrostatic pressure since traditional detergent-based methods of decellularization, including SDS and Triton X-100, did not work.107 The decellularized tissue was fastened into a cylindrical shape with either fibrin glue or sutures and although both methods supported physiological pressures in vitro, the sutured grafts leaked in vivo.107 Glued grafts implanted into Wistar rats for 3 weeks remained patent with some endothelial-like cells present on the intima layer, but ultimately they showed low cell infiltration likely due to the higher elastin content.107
Impact and clinical outlook
Vascular grafts from 2D tissues can be readily constructed after decellularization, essentially without any patient wait times. Acellular grafts that do not contain any autologous cells must rely solely on the recipient for cell infiltration and remodeling, which may be troublesome for patients whose cells have limited regenerative capability and exacerbated by the unexplored effects of ECM composition on endothelialization for 2D ECM sources. Although pre-seeding patient-specific cells or iPSC-derived cells on the grafts may preserve patency and encourage remodeling, we believe that it limits the off-the-shelf availability of such grafts. To develop a clinically relevant small-diameter vascular graft from 2D tissue, it is crucial for the graft to match the properties of human arteries and support endothelialization.28,65,66 Therefore, we infer that the selected ECM should mimic the mechanics, structure, composition, and hemocompability of human coronary arteries, as opposed to the cell-based method earlier, in which these properties can largely be controlled through the types of cells used and the length of cell culture. Further, the incorporation of surface modifications to decrease the incidence of thrombosis, although a possible avenue forward, necessitates an additional step to treat the surface, which contributes to the complexity of the graft and can impact mechanical properties. Consequently, we propose that the effects of each processing step be carefully explored.
Cannular Tissue
The majority of recent investigations into small-diameter vascular grafts originating from cannular tissues utilize either human- or animal-derived arteries or veins. Since the geometry of the vessel and the layer of SMCs can make thorough decellularization more difficult, harsher decellularization methods may be required. Thus, the effects of decellularization protocols on ECM integrity must be addressed, as previously summarized.108–110 Simsa et al. found that regardless of the dynamics of the detergent application, porcine vena cava samples could be successfully decellularized without any cytotoxicity to HUVECs and displayed comparable values for mechanical properties, as well as elastin and GAG content.111 However, scanning electron microscopy analysis revealed the presence of remnant cell debris on the static samples and local tears and a flattened luminal surface in grafts decellularized at the highest perfusion velocity, which could be detrimental to in vivo endothelialization.111 Simsa et al. concluded that the agitation and low velocity perfusion methods of decellularization had the best overall outcomes.111 Meanwhile, Böer et al. compared a “conventional” decellularization protocol against an “intensified” one, where the “intensified” version included a mandrel to prevent tissue collapse, three times more volume of decellularization buffers, and an additional 32 h in the SDS solution, compared with the “conventional” method.112 Both protocols produced similar results, with no significant difference in fibroblast cell viability, endothelialization, burst pressure, or collagen content, although the intensified protocol produced a scaffold with significantly less sulfated glycosaminoglycan (sGAG) content.112 When compared with native equine carotid arteries, both protocols demonstrated a similar reduction in compliance.112 To compensate for the reported loss of mechanical properties during decellularization, polymers have been used as coatings. For example, polycaprolactone (PCL) was electrospun on decellularized rat aorta to improve ultimate tensile strength, suture retention strength, and burst pressure.113 Similarly, the coating of poly(ethylmethacrylate-co-diethylaminoethylacrylate) (also known as 8g7) restored the mechanical properties of decellularized porcine carotid arteries to native values.114
Comparable to SIS grafts, grafts from decellularized porcine vessels are largely unsuccessful without some type of surface treatment, whether it is pre-implantation EC-seeding or a luminal coating. Ma et al. dynamically seeded decellularized porcine aortas with autologous canine ECs and validated their patency at 1, 3, and 6 months without any signs of thrombosis in vivo.115 Similarly, the autologous endothelialization of porcine carotid arteries helped to prevent graft failure and encouraged cell recruitment and proliferation of SMCs in pigs over 6 weeks, compared with the unseeded grafts that were characterized by significant clotting, a thick media layer, and an influx of inflammatory cells.116 Li et al. demonstrated the in vitro efficacy of differentiating hMSC into ECs and SMCs with a contractile phenotype, as a means to overcome insufficient cell recruitment on decellularized porcine carotid artery scaffolds.117,118 Another method to promote endothelialization is the coating of the luminal surface with biopolymers. The 8g7 has been shown to reduce platelet adhesion, while promoting EC attachment and proliferation.119 The 8g7-coated porcine carotid arteries displayed both a significant increase in the number of attached ECs and a more homogeneous and interconnected endothelial layer.114 In addition, the fixation of heparin on grafts, largely achieved with the crosslinking agents N-(3-dimethylaminopropyl)-N’-ethyl carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), has improved graft success.66,120,121 Specifically, heparin immobilized on the surface of decellularized porcine carotid arteries showed a four-fold extension of the active partial thrombin time, indicative of an enhancement in anti-thrombogenic properties of the treated grafts.120
Heparin surface treatment is a common approach to help reduce the occurrence of thrombosis in vascular grafts composed from cannular ECM. Decellularized rat aortas covered in electrospun PCL and treated with heparin showed less platelet adhesion and activation than decellularized-only grafts.113 Similarly, rat aortas submerged in poly(1,8 octanediol citrate) solution and crosslinked before heparin immobilization showed the lowest levels of both platelet adhesion and thrombus formation, while supporting HUVEC adherence and growth in vitro.122 However, Jiang et al. reported a substantial increase in the elastic modulus of rat aortas when treated with the EDC/NHS method of heparin immobilization.66 Alternatively, grafts heparinized with the “click” method showed a formed endothelial layer, the lowest amount of intimal hyperplasia, and a reduced inflammatory response without a change in mechanical behavior.66 Despite the differences in properties that originated from the processing techniques, both types of grafts remained patent for 4 weeks in vivo.66 In a follow-up study pursuing the “click” method of treatment, all grafts were patent, but exhibited reduced blood flow, calcification, mineralization, and minimal cell infiltration into the walls.123 Zhou et al. demonstrated that heparin-coated rat aortas, also treated with SDF-1α, reduced platelet adhesion and supported the attachment, activity, and migration of bone marrow stem cells in vitro and of ECs and EPCs in vivo.124
Other types of surface treatments have been developed that not only suppress platelet adhesion and thrombosis, but also encourage endothelialization. The addition of VEGF 145 and basic fibroblast growth factor in heparinized rabbit aortas led to a substantially higher patency rate of 90% at 18 months, compared with the 10% patency of heparin-only grafts.121 Decellularized rat aortas coated with a VEGF-conjugated hydrogel (HG-VEGF) also significantly increased the development of a functional endothelium over 8 weeks; however, there was also an increase in the occurrence of intimal hyperplasia.125 Mahara et al. treated decellularized ostrich carotid arteries with two synthetic integrin α4β1 ligand peptides (REDV): POG7G3REDV and OPG7G3REDV.126 POG7G3REDV-modified grafts showed significant improvements in HUVEC adherence and EPC recruitment in vitro, whereas five of the six implanted grafts remained patent throughout the 3 week period, with no signs of thrombosis.126 Kristofik et al. cultured dermal fibroblasts from thrombospondin-2 knockout (TSP2 KO) mice on the lumens of decellularized rat aortas to encourage deposition of their naturally antithrombotic ECM.127,128 In a rat model over 4 weeks, the TSP2 KO ECM-modified grafts showed decreased platelet activation, along with increased cell recruitment and patency.128
The use of human blood vessels poses significant advantages over other tissue sources, because the structural and biochemical properties of these grafts largely mimic the target arteries. Therefore, these grafts potentially require minimal alterations beyond decellularization, which has led to investigations of their usefulness as small-diameter vascular grafts. Decellularization protocols of human vessels impact the ECM in a variety of ways, including decreasing wall thickness129 and mechanical strength,130–132 whereas collagen fibers130 and burst pressure133 are preserved. Porzionato et al. showed that decellularized arteries and veins from human hands implanted into a rabbit model remained functional, with no signs of thrombosis or inflammatory rejection.129 The vein grafts showed endothelialization of the luminal surface and sufficient cell recruitment throughout the remaining layers, whereas the arterial grafts had only partial cell infiltration at 2 weeks, likely due to the thicker vessel wall.129 However, longer in vivo studies paint a different picture. There was positive cell infiltration of ECs and SMCs in decellularized internal mammary arteries implanted into rabbit femoral arteries after 3 years,134 whereas saphenous veins endothelialized with the autologous ECs showed 80% patency at 6 months and 50% patency from 9 to 32 months in humans.135
Placental arteries and umbilical cord vessels are unique alternatives to traditional human vessels for small-diameter vascular grafts. However, placental arteries from the chorion are extremely susceptible to damage during the decellularization process, lending to a decrease in GAG content and elastic modulus, in addition to the destruction of the basement membrane.131,132 The exposure of collagen fibers during the decellularization process necessitated the use of heparin surface treatment on placental arteries by Schneider et al.132 After 4 weeks post-implantation, these grafts were free from occlusions and the mechanical properties were comparable to native tissue due to the influx of recipient cells.132 The human umbilical vein has demonstrated the deterioration of many components in the ECM, including collagen, elastin, fibronectin, and laminin, as well as a decrease in cell adhesion, as a result of a variety of decellularization techniques.136 Alternatively, in decellularized and vitrified human umbilical arteries (hUA), collagen and elastin appear to be preserved with comparable sGAGs and hydroxyproline content and without any cytotoxic impact to hMSCs.137 After 30 days in a porcine model, the ECM structures of hUA grafts mimicked those of native porcine carotids, but they lacked an organized endothelium and were prone to thrombosis.137 One method to improve the outcome of hUAs is with human leukocyte antigen (HLA)-matching, which has been investigated by Mallis et al. through recellularizing grafts of an HLA genotype with hMSCs of a differing HLA genotype.138 After 3 weeks, the repopulated grafts adopted the HLA profile of the seeded hMSCs and showed no remnants of the previous HLA genotype.138 Since the presence of residual antigens, specifically HLA, is linked to an immune response and eventual graft rejection, the ability to match the HLA genotype of the graft to the patient is imperative to the longevity of graft survival.
Impact and clinical outlook
Vascular grafts can be developed with decellularized cannular tissues from an assortment of animal sources without the need for glues or sutures. However, this technology largely focuses on arteries and veins, which provides a more limited range of tissue properties and compositions when compared with the 2D sources described earlier. Many of the decellularization techniques employed, specifically harsher techniques to target the media layer of vessels, impact graft composition and strength. Since there is such an abundance of diverse decellularization methods, it would be beneficial to compare the mechanics of different types of animal ECM for the same protocols. Although tissue remodeling may lead to the recovery of the mechanical properties, cell infiltration occurs at a much slower rate in humans than in animals. Thus, the grafts may remain susceptible to failure until regeneration occurs. Before this technology can move forward clinically, we feel that it is important to investigate the impact of decellularization techniques on human cell infiltration and remodeling, as well as the effect of the resulting ECM composition on endothelialization. In addition, a loss in strength after decellularization for a given tissue typically indicates damage incurred by the ECM. However, in some cases, these changes cause the tissue to behave more similarly to human arteries, which should be considered as well. In addition to the changes in mechanical properties, harsher decellularization methods expose collagen and lead to pro-thrombotic surfaces, which necessitates the use of surface modification. As previously mentioned, surface treatment will require supplemental processing, which can alter the ECM, but they are generally simpler adaptions than the use of cell culture when considering translation to clinical use.
Conclusions
With the incidence of CVD continuing to rise and the high failure rates of autologous grafts, it is imperative to develop an alternative to autologous vessels for small-diameter vascular graft applications. We have investigated and evaluated vascular grafts composed of cell-derived ECM, 2D tissue, and cannular tissue, focusing largely on mechanical properties, infiltration of host cells, and overall patency in vivo, as well as the feasibility of translating each of the three technologies to clinical use. Cell-based approaches require lengthy cell culture periods, but they enhance control over the graft properties when compared with 2D and cannular tissue grafts. Further, autologous endothelialization has been an important tool in preventing thrombosis and, ultimately, preserving patency in many types of grafts. However, due to the large number of grafts needed, it seems improbable that a cell-only approach is the best method forward. Two-dimensional tissues require the use of sutures or glue to form the cylindrical shape, but they provide a wider array of structures and properties than cannular tissues, which are typically arteries or veins. Further, cannular materials require more abrasive decellularization methods than cell-based ECM and 2D tissue, which damage the ECM, impair the mechanical behavior, and expose thrombosis-inducing constituents of the tissues. Both 2D and cannular grafts frequently involve a secondary treatment post-decellularization to improve in vivo success. Grafts composed of 2D or cannular ECM with a surface modification, or less likely, luminal ECs, seem to be the most feasible approaches to translate to patients. However, the effects of decellularization should be further characterized. In addition, to drive this technology toward clinical implementation, critical areas to investigate, independent of graft type, include developing a practical storage process to ensure off-the-shelf availability, examining the effects of storage on the success of the vascular graft, and constructing a human model for more relevant in vitro studies with human cells. Since all three of these technologies rely on patient cells for graft remodeling, it is imperative to explore new methods to improve host cell infiltration, as well as the impact of ECM composition on endothelialization.
Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by the NIBIB/NIH Center for Engineering Complex Tissues (Award No. P41EB023833) and the National Science Foundation CBET (Award No. 5246870).
References
- 1. Benjamin E.J., Muntner P., Alonso A., et al. . Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 139, e56–e528, 2019 [DOI] [PubMed] [Google Scholar]
- 2. Pashneh-Tala S., MacNeil S., and Claeyssens F.. The tissue-engineered vascular graft—past, present, and future. Tissue Eng Part B 22, 68 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diodato M., and Chedrawy E.G.. Coronary artery bypass graft surgery: the past, present, and future of myocardial revascularisation. Surg Res Pract 2014, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kulik A., Ruel M., Jneid H., et al. . Secondary prevention after coronary artery bypass graft surgery. Circulation 131, 927, 2015 [DOI] [PubMed] [Google Scholar]
- 5. Edwards W.S., Mohtashemi M., and Holdefer W.F.. The importance of proper caliber of lumen in femoral popliteal arterial reconstruction. J Cardiovasc Surg (Torino) 8, 195, 1967 [PubMed] [Google Scholar]
- 6. Chew D.K., W., Owens C.D., Belkin M., et al. . Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg 35, 1085, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Lawrie G.M., Weilbacher D.E., and Henry P.D.. Endothelium-dependent relaxation in human saphenous vein grafts. J Thorac Cardiovasc Surg 100, 612, 1990 [PubMed] [Google Scholar]
- 8. Angelini G.D., Breckenridge I.M., Butchart E.G., et al. . Metabolic damage to human saphenous vein during preparation for coronary artery bypass grafting. Cardiovasc Res 19, 326, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Hess C.N., Lopes R.D., Gibson C.M., et al. . Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation 130, 1445, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKavanagh P., Yanagawa B., Zawadowski G., and Cheema A.. Management and prevention of saphenous vein graft failure: a review. Cardiol Ther 6, 203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FitzGibbon G.M., Leach A.J., Keon W.J., Burton J.R., and Kafka H.P.. Coronary bypass graft fate. Angiographic study of 1,179 vein grafts early, one year, and five years after operation. J Thorac Cardiovasc Surg 91, 773, 1986 [PubMed] [Google Scholar]
- 12. Harskamp R.E., Lopes R.D., Baisden C.E., de Winter R.J., and Alexander J.H.. Saphenous vein graft failure after coronary artery bypass surgery. Ann Surg 257, 824, 2013 [DOI] [PubMed] [Google Scholar]
- 13. Fitzgibbon G.M., Kafka H.P., Leach A.J., Keon W.J., Hooper G.D., and Burton J.R.. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 28, 616, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Roll S., Müller-nordhorn J., Keil T., et al. . Dacron® vs. PTFE as bypass materials in peripheral vascular surgery – systematic review and meta-analysis. BMC Surg 8, 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green R.M., Abbott W.M., Matsumoto T., et al. . Prosthetic above-knee femoropopliteal bypass grafting: five-year results of a randomized trial. J Vasc Surg 31, 417, 2000 [PubMed] [Google Scholar]
- 16. Abbott W.M., Green R.M., Matsumoto T., et al. . Prosthetic above-knee femoropopliteal bypass grafting: results of a multicenter randomized prospective trial. J Vasc Surg 25, 19, 1997 [DOI] [PubMed] [Google Scholar]
- 17. De Visscher G., Mesure L., Meuris B., Ivanova A., and Flameng W.. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater 8, 1330, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Sarkar S., Salacinski H.J., Hamilton G., and Seifalian A.M.. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. Eur J Vasc Endovasc Surg 31, 627, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kidson I.G., and Abbott W.M.. Low compliance and arterial graft occlusion. Circulation 58, Pt 2: I1, 1978 [PubMed] [Google Scholar]
- 20. Toursarkissian B., Eisenberg P.R., Abendschein D.R., and Rubin B.G.. Thrombogenicity of small-diameter prosthetic grafts: relative contributions of graft-associated thrombin and factor Xa. J Vasc Surg 25, 730, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Lin P.H., Chen C., Bush R.L., Yao Q., Lumsden A.B., and Hanson S.R.. Small-caliber heparin-coated ePTFE grafts reduce platelet deposition and neointimal hyperplasia in a baboon model. J Vasc Surg 39, 1322, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Boccafoschi F., Rajan N., Habermehl J., and Mantovani D.. Preparation and characterization of a scaffold for vascular tissue engineering by direct-assembling of collagen and cells in a cylindrical geometry. Macromol Biosci 10, 719, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Elliott M.B., Ginn B., Fukunishi T., et al. . Regenerative and durable small-diameter graft as an arterial conduit. Proc Natl Acad Sci U S A 116, 12710, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grassl E.D., Oegema T.R., and Tranquillo R.T.. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. Am Soc Mech Eng Bioeng Div BED 60, 607, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Aussel A., Montembault A., Malaise S., et al. . In vitro mechanical property evaluation of chitosan-based hydrogels intended for vascular graft development. J Cardiovasc Transl Res 10, 480, 2017 [DOI] [PubMed] [Google Scholar]
- 26. Aussel A., Thébaud N.B., Bérard X., et al. . Chitosan-based hydrogels for developing a small-diameter vascular graft: in vitro and in vivo evaluation. Biomed Mater 12, 1, 2017 [DOI] [PubMed] [Google Scholar]
- 27. Bracaglia L.G., Messina M., Winston S., Kuo C., Lerman M., and Fisher J.P.. 3D printed pericardium hydrogels to promote wound healing in vascular applications. Biomacromolecules 18, 3802, 2017 [DOI] [PubMed] [Google Scholar]
- 28. Thottappillil N., and Nair D.P.. Scaffolds in vascular regeneration: current status. Vasc Health Risk Manag 11, 79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravi S., and Chaikof E.L.. Biomaterials for vascular tissue engineering. Regen Med 5, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kannan R.Y., Salacinski H.J., Butler P.E., Hamilton G., and Seifalian A.M.. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater 74, 570, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Isenberg B.C., Williams C., and Tranquillo R.T.. Small-diameter artificial arteries engineered in vitro. Circ Res 98, 25, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Kuo C., Guo T., Cabrera-luque J., et al. . Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J Biomed Mater Res A 106A, 1476, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hodde J.P., Badylak S.F., and Brightman A.O., and Voytik-Harbin S.L.. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 2, 209, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Taipale J., and Keski-Oja J.. Growth factors and the extracellular matrix. FASEB J 11, 51, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12, 367, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Badylak S.F., Freytes D.O., and Gilbert T.W.. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 5, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Gardin C., Ricci S., Ferroni L., et al. . Decellularization and delipidation protocols of bovine bone and pericardium for bone grafting and guided bone regeneration procedures. PLoS One 10, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pellegata A.F., Asnaghi M.A., Stefani I., et al. . Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed Res Int 2013, 1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levorson E.J., Mountziaris P.M., Hu O., Kasper F.K.. and Mikos, A.G. Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, part 1: investigation of cocultures and seeding densities for improved extracellular matrix deposition. Tissue Eng Part C Methods 20, 340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimmel H., Rahn M., and Gilbert T.W.. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: a case series on severe chronic wounds. J Am Col Certif Wound Spec 2, 55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scholl F.G., Boucek M.M., Chan K., Valdes-cruz L., and Perryman R.. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J Pediatr Congenit Hear Surg 1, 132, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Ziats N.P., and Anderson J.M.. Human vascular endothelial cell attachment and growth inhibition by type V collagen. J Vasc Surg 17, 710, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Turner N.J., Murphy M.O., Kielty C.M., et al. . α2(VIII) collagen substrata enhance endothelial cell retention under acute shear stress flow via an α2β1 integrin-dependent mechanism: an in vitro and in vivo study. Circulation 114, 820, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Coakley D.N., Shaikh F.M., O'Sullivan K., et al. . Comparing the endothelialisation of extracellular matrix bioscaffolds with coated synthetic vascular graft materials. Int J Surg 25, 31, 2016 [DOI] [PubMed] [Google Scholar]
- 45. Seeger J.M., and Klingman N.. Improved in vivo endothelialization of prosthetic grafts by surface modification with fibronectin. J Vasc Surg 8, 476, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Krüger-Genge A., Fuhrmann R., Jung F., and Franke R.P.. Effects of different components of the extracellular matrix on endothelialization. Clin Hemorheol Microcirc 64, 867, 2016 [DOI] [PubMed] [Google Scholar]
- 47. Di Russo J., Luik A., Yousif L., et al. . Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J 36, 183, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma L., Li X., Guo X., et al. . Promotion of endothelial cell adhesion and antithrombogenicity of polytetrafluoroethylene by chemical grafting of chondroitin sulfate. ACS Appl Bio Mater 3, 891, 2020 [DOI] [PubMed] [Google Scholar]
- 49. Antonova L.V., Sevostyanova V.V, Kutikhin A.G., et al. . Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(e-caprolactone) small-diameter vascular grafts in vivo. Front Pharmacol 7, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Farndale R.W., Sixma J.J., Barnes M.J., and de Groot P.G.. The role of collagen in thrombosis and hemostasis. J Thromb Haemost 2, 561, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Watson S. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des 15, 1358, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Santos M., Waterhouse A., Lee B.S.L., et al. . Simple one-step covalent immobilization of bioactive agents without use of chemicals on plasma-activated low thrombogenic stent coatings. In: Wall J.G., Podbielska H Wawrzyńska, M. eds. Functionalised Cardiovascular Stents. Cambridge, Massachusetts: Elsevier Ltd., 2018, p. 211 [Google Scholar]
- 53. Konig G., McAllister T.N., Dusserre N., et al. . Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 30, 1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Quint C., Kondo Y., Manson R.J., Lawson J.H., Dardik A., and Niklason L.E.. Decellularized tissue-engineered blood vessel as an arterial conduit. PNAS 108, 9214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cohn D., Younges H., Milgarter E., and Uretzky G.. Mechanical behaviour of isolated pericardium: species, isotropy, strain rate and collagenase effect on pericardial tissue. Clin Mater 2, 115, 1987 [Google Scholar]
- 56. Biga L.M., Dawson S., Harwell A., et al. . Anatomy and Physiology. Houston, Texas: OpenStax College, 2017 [Google Scholar]
- 57. Han D.W., Park Y.H., Kim J.K., et al. . Long-term preservation of human saphenous vei by green tea polyphenol under physiological conditions. Tissue Eng 11, 1054, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Wang D., Wang Z., Zhang L., and Wang Y.. Roles of cells from the arterial vessel wall in atherosclerosis. Mediators Inflamm 2017, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sánchez P.F., Brey E.M., and Briceño J.C.. Endothelialization mechanisms in vascular grafts. J Tissue Eng Regen Med 12, 2164, 2018 [DOI] [PubMed] [Google Scholar]
- 60. Taddei S., Virdis A., Ghiadoni L., Sudano I., and Salvetti A.. Endothelial dysfunction in hypertension. J Cardiovasc Pharmacol 38, S11–S14, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Lee K.W., Stolz D.B., and Wang Y.. Substantial expression of mature elastin in arterial constructs. Proc Natl Acad Sci USA 108, 2705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beamish J.A., He P., Kottke-Marchant K., and Marchant R.E.. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B 16, 467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamamoto M., Yamamoto K., and Noumura T.. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res 204, 121, 1993 [DOI] [PubMed] [Google Scholar]
- 64. Singh C., Wong C.S., and Wang X.. Medical textiles as vascular implants and their success to mimic natural arteries. J Funct Biomater 6, 500, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Catto V., Farè S., Freddi G., and Tanzi M.C.. Vascular tissue engineering: recent advances in small diameter blood vessel regeneration. ISRN Vasc Med 2014, 1, 2014 [Google Scholar]
- 66. Jiang B., Suen R., Wang J.J., Zhang Z.J., Wertheim J.A., and Ameer G.A.. Mechanocompatible polymer-extracellular-matrix composites for vascular tissue engineering. Adv Healthc Mater 5, 1594, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang W.G., and Niklason L.E.. A short discourse on vascular tissue engineering. NPJ Regen Med 2, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niklason L.E., Gao J., Abbott W.M., et al. . Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Niklason L.E., Abbott W., Gao J., et al. . Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg 33, 628, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Dahl S.L.M., Kypson A.P., Lawson J.H., et al. . Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 1, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Lawson J.H., Glickman M.H., Ilzecki M., et al. . Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387, 2026, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gong Z., and Niklason L.E.. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 22, 1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seifu D.G., Purnama A., Mequanint K., and Mantovani D.. Small-diameter vascular tissue engineering. Nat Rev Cardiol 10, 410, 2013 [DOI] [PubMed] [Google Scholar]
- 74. Dahl S.L.M., Rhim C., Song Y.C., and Niklason L.E.. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng 35, 348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. L'Heureux N., Pâquet S., Labbé R., Germain L., and Auger F.A.. A completely biological tissue-engineered human blood vessel. FASEB J 12, 47, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Rayatpisheh S., Heath D.E., Shakouri A., Rujitanaroj P.O., Chew S.Y., and Chan-Park M.B.. Combining cell sheet technology and electrospun scaffolding for engineered tubular, aligned, and contractile blood vessels. Biomaterials 35, 2713, 2014 [DOI] [PubMed] [Google Scholar]
- 77. Xing Q., Qian Z., Tahtinen M., Yap A.H., Yates K., and Zhao F.. Aligned nanofibrous cell-derived extracellular matrix for anisotropic vascular graft construction. Adv Healthcare Mater 6, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jung Y., Ji H., Chen Z., et al. . Scaffold-free, human mesenchymal stem cell-based tissue engineered blood vessels. Sci Rep 5, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Strobel H.A., Hookway T.A., Piola M., et al. . Assembly of tissue-engineered blood vessels with spatially controlled heterogeneities. Tissue Eng Part A 24, 1492, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Syedain Z.H., Graham M.L., Dunn T.B., et al. . A completely biological ‘off-the-shelf’ arteriovenous graft that recellularizes in baboons. Sci Transl Med 9, 1, 2017 [DOI] [PubMed] [Google Scholar]
- 81. Deuse T., Hu X., Gravina A., et al. . Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37, 252, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Luo J., Qin L., Zhao L., et al. . Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. Cell Stem Cell 26, 251, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Atoui R., and Chiu R.C.J.. Mesenchymal stromal cells as universal donor cells. Expert Opin Biol Ther 12, 1293, 2012 [DOI] [PubMed] [Google Scholar]
- 84. Gui L., Dash B.C., Luo J., et al. . Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102, 120, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Generali M., Casanova E.A., Kehl D., et al. . Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells. Acta Biomater 97, 333, 2019 [DOI] [PubMed] [Google Scholar]
- 86. von Bornstädt D., Wang H., Paulsen M.J., et al. . Rapid self-assembly of bioengineered cardiovascular bypass grafts from scaffold-stabilized, tubular bilevel cell sheets. Circulation 138, 2130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seals D.R., Jablonski K.L., and Donato A.J.. Aging and vascular endothelial function in humans. Clin Sci 120, 357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Monk B.A., and George S.J.. The effect of ageing on vascular smooth muscle cell behaviour - A mini-review. Gerontology 61, 416, 2015 [DOI] [PubMed] [Google Scholar]
- 89. Krawiec J.T., Weinbaum J.S., St. Croix, C.M., et al. A cautionary tale for autologous vascular tissue engineering: impact of human demographics on the ability of adipose-derived mesenchymal stem cells to recruit and differentiate into smooth muscle cells. Tissue Eng A 21, 426, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Badylak S.F., Lantz G.C., Coffey A., and Geddes L.A.. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 47, 74, 1989 [DOI] [PubMed] [Google Scholar]
- 91. Sandusky G.E., Badylak S.F., Morff R.J., Johnson W.D., and Lantz G.. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. Am J Pathol 140, 317, 1992 [PMC free article] [PubMed] [Google Scholar]
- 92. Roeder R., Wolfe J., Lianakis N., Hinson T., Geddes L.A., and Obermiller J.. Compliance, elastic modulus, and burst pressure of small-intestine submucosa (SIS), small-diameter vascular grafts. J Biomed Mater Res 47, 65, 1999 [DOI] [PubMed] [Google Scholar]
- 93. Koobatian M.T., Row S., Smith R.J.J., Koenigsknecht C., Andreadis S.T., and Swartz D.D.. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 76, 344, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Han B., Xue F., Fan C., and Mo X.. Surface heparinization and blood compatibility modification of small intestinal submucosa (SIS) for small-caliber vascular regeneration. Biomed Mater Eng 28, 213, 2017 [DOI] [PubMed] [Google Scholar]
- 95. Peng H., Schlaich E.M., Row S., Andreadis S.T., and Swartz D.D.. A novel ovine ex vivo arteriovenous shunt model to test vascular implantability. Cells Tissues Organs 195, 108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Row S., Peng H., Schlaich E.M., Koenigsknecht C., Andreadis S.T., and Swartz D.D.. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: the role of cells in the vascular wall. Biomaterials 50, 115, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ghazanfari S., Alberti K.A., Xu Q., and Khademhosseini A.. Evaluation of an elastic decellularized tendon-derived scaffold for the vascular tissue engineering application. J Biomed Mater Res Part A 107A, 1225, 2019 [DOI] [PubMed] [Google Scholar]
- 98. Liu J., Li B., Jing H., et al. . Swim bladder as a novel biomaterial for cardiovascular materials with anti-calcification properties. Adv Healthc Mater 9, 1, 2020 [DOI] [PubMed] [Google Scholar]
- 99. Kakavand M., Yazdanpanah G., Ahmadiani A., and Niknejad H.. Blood compatibility of human amniotic membrane compared with heparin-coated ePTFE for vascular tissue engineering. J Tissue Eng Regen Med 11, 1701, 2017 [DOI] [PubMed] [Google Scholar]
- 100. Amensag S., Goldberg L.A., O'Malley K.A., Rush D.S., Berceli S.A., and McFetridge P.S.. Pilot assessment of a human extracellular matrix-based vascular graft in a rabbit model. J Vasc Surg 65, 839, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lam M.T., and Wu J.C.. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther 10, 1039, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Oldenburg W.A., Almerey T., Selim M., Fanes H., and Hakaim A.G.. Durability of carotid endarterectomy with bovine pericardial patch. Ann Vasc Surg 50, 218, 2018 [DOI] [PubMed] [Google Scholar]
- 103. Kimicata M., Allbritton-King J.D., Navarro J., et al. . Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater 110, 68, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martin C., Pham T., and Sun W.. Significant differences in the material properties between aged human and porcine aortic tissues. Eur J Cardiothorac Surg 40, 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van Andel C.J., Pistecky P.V., and Borst C.. Mechanical properties of porcine and human arteries: implications for coronary anastomotic connectors. Ann Thorac Surg 76, 58, 2003 [DOI] [PubMed] [Google Scholar]
- 106. Wu P., Nakamura N., Morita H., et al. . A hybrid small-diameter tube fabricated from decellularized aortic intima-media and electrospun fiber for artificial small-diameter blood vessel. J Biomed Mater Res Part A 107A, 1064, 2019 [DOI] [PubMed] [Google Scholar]
- 107. Negishi J., Hashimoto Y., Yamashita A., et al. . Evaluation of small-diameter vascular grafts reconstructed from decellularized aorta sheets. J Biomed Mater Res Part A 105A, 1293, 2017 [DOI] [PubMed] [Google Scholar]
- 108. Crapo P.M., Gilbert T.W., and Badylak S.F.. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gilpin A., and Yang Y.. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int 2017, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Heath D.E. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen Eng Transl Med 5, 155, 2019 [Google Scholar]
- 111. Simsa R., Monforte Vila X., Salzer E., et al. . Effect of fluid dynamics on decellularization efficacy and mechanical properties of blood vessels. PLoS One 14, e0220743, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Böer U., Hurtado-Aguilar L.G., Klingenberg M., et al. . Effect of intensified decellularization of equine carotid arteries on scaffold biomechanics and cytotoxicity. Ann Biomed Eng 43, 2630, 2015 [DOI] [PubMed] [Google Scholar]
- 113. Gong W., Lei D., Li S., et al. . Hybrid small-diameter vascular grafts: anti-expansion effect of electrospun poly ɛ-caprolactone on heparin-coated decellularized matrices. Biomaterials 76, 359, 2016 [DOI] [PubMed] [Google Scholar]
- 114. López-Ruiz E., Venkateswaran S., Perán M., et al. . Poly(ethylmethacrylate-co-diethylaminoethyl acrylate) coating improves endothelial re-population, bio-mechanical and anti-thrombogenic properties of decellularized carotid arteries for blood vessel replacement. Sci Rep 7, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ma X., He Z., Li L., et al. . Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J Cadiothoracic Surg 12, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dahan N., Sarig U., Bronshtein T., et al. . Dynamic autologous reendothelialization of small-caliber arterial extracellular matrix: a preclinical large animal study. Tissue Eng Part A 23, 69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li N., Sanyour H., Remund T., Kelly P., and Hong Z.. Vascular extracellular matrix and fibroblasts-coculture directed differentiation of human mesenchymal stem cells toward smooth muscle-like cells for vascular tissue engineering. Mater Sci Eng C 93, 61, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li N., Rickel A.P., Sanyour H.J., and Hong Z.. Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells toward vascular cells on vascular extracellular matrix scaffold under mechanical stimulations in a rotary bioreactor. J Mater Chem B 7, 2703, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pernagallo S., Tura O., Wu M., et al. . Novel biopolymers to enhance endothelialisation of intra-vascular devices. Adv Healthc Mater 1, 646, 2012 [DOI] [PubMed] [Google Scholar]
- 120. Cai Z., Gu Y., Cheng J., et al. . Decellularization, cross-linking and heparin immobilization of porcine carotid arteries for tissue engineering vascular grafts. Cell Tissue Bank 20, 569, 2019 [DOI] [PubMed] [Google Scholar]
- 121. Kong X., Kong C., Wen S., and Shi J.. The use of heparin, bFGF, and VEGF 145 grafted acellular vascular scaffold in small diameter vascular graft. J Biomed Mater Res Part B 107B, 672, 2019 [DOI] [PubMed] [Google Scholar]
- 122. Jiang B., Akgun B., Lam R.C., Ameer G.A., and Wertheim J.A.. A polymer-extracellular matrix composite with improved thromboresistance and recellularization properties. Acta Biomater 18, 50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jiang B., Suen R., Wang J.J., Zhang Z.J., Wertheim J.A. and Ameer, G.A. Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials 144, 166, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhou J., Ye X., Wang Z., et al. . Development of decellularized aortic valvular conduit coated by heparin-SDF-1α multilayer. Ann Thorac Surg 99, 612, 2015 [DOI] [PubMed] [Google Scholar]
- 125. Iijima M., Aubin H., Steinbrink M., et al. . Bioactive coating of decellularized vascular grafts with a temperature-sensitive VEGF-conjugated hydrogel accelerates autologous endothelialization in vivo. J Tissue Eng Regen Med 12, e513, 2018 [DOI] [PubMed] [Google Scholar]
- 126. Mahara A., Somekawa S., Kobayashi N., et al. . Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 58, 54, 2015 [DOI] [PubMed] [Google Scholar]
- 127. Kristofik N., Calabro N.E., Tian W., et al. . Impaired von Willebrand factor adhesion and platelet response in thrombospondin-2 knockout mice. Blood 128, 1642, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kristofik N.J., Qin L., Calabro N.E., et al. . Improving in vivo outcomes of decellularized vascular grafts via incorporation of a novel extracellular matrix. Biomaterials 141, 63, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Porzionato A., Sfriso M.M., Pontini A., et al. . Development of small-diameter vascular grafts through decellularization of human blood vessels. J Biomater Tissue Eng 7, 101, 2017 [Google Scholar]
- 130. Kajbafzadeh A.M., Khorramirouz R., Kameli S.M., Hashemi J., and Bagheri A.. Decellularization of human internal mammary artery: biomechanical properties and histopathological evaluation. Biores Open Access 6, 74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Schneider K.H., Aigner P., Holnthoner W., et al. . Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater 29, 125, 2016 [DOI] [PubMed] [Google Scholar]
- 132. Schneider K.H., Enayati M., Grasl C., et al. . Acellular vascular matrix grafts from human placenta chorion: impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials 177, 14, 2018 [DOI] [PubMed] [Google Scholar]
- 133. Lamm P., Juchem G., Milz S., Schuffenhauer M., and Reichart B.. Autologous endothelialized vein allograft: a solution in the search for small-caliber grafts in coronary artery bypass graft operations. Circulation 104, I108, 2001 [PubMed] [Google Scholar]
- 134. Kajbafzadeh A.-M., Khorramirouz R., Kameli S.M., et al. . Three-year efficacy and patency follow-up of decellularized human internal mammary artery as a novel vascular graft in animal models. J Thorac Cardiovasc Surg 157, 1494, 2019 [DOI] [PubMed] [Google Scholar]
- 135. Herrmann F.E.M., Lamm P., Wellmann P., Milz S., Hagl C., and Juchem G.. Autologous endothelialized vein allografts in coronary artery bypass surgery – Long term results. Biomaterials 212, 87, 2019 [DOI] [PubMed] [Google Scholar]
- 136. Mangold S., Schrammel S., Huber G., et al. . Evaluation of decellularized human umbilical vein (HUV) for vascular tissue engineering – comparison with endothelium-denuded HUV. J Tissue Eng Regen Med 9, 13, 2015 [DOI] [PubMed] [Google Scholar]
- 137. Mallis P., Katsimpoulas M., Kostakis A., et al. . Vitrified human umbilical arteries as potential grafts for vascular tissue engineering. Tissue Eng Regen Med 17, 285, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mallis P., Michalopoulos E., Dinou A., et al. . Development of HLA-matched vascular grafts utilizing decellularized human umbilical artery. Hum Immunol 79, 855, 2018 [DOI] [PubMed] [Google Scholar]