Abstract
Abnormal sex hormone levels in utero have been associated with child behavioral problems, but it is unclear if normal variation in prenatal sex hormones is associated with subsequent behavior in childhood. We assessed maternal sex hormones, including serum estrone (E1), estradiol (E2), estriol (E3), free testosterone (FT), and total testosterone (TT), during early pregnancy (gestational week 6–21 (mean = 11.1)) and evaluated child behavior at ages 4–5 using the Behavioral Assessment System for Children (BASC-2) and Social Responsiveness Scale (SRS-2) in 404 mother/child pairs (211 girls, 193 boys) within The Infant Development and Environment Study, a multi-site pregnancy cohort study. Associations between hormones and composite scores were evaluated using multiple linear regressions in both sexes combined, and separate models assessed effect modification by sex with the addition of interaction terms. A 10-fold increase in maternal FT or TT was associated in both sexes with a 4.3-point (95% CI: 0.5, 8.2) or 4.4-point (0.8, 8.0) higher BASC-2 internalizing composite T score, respectively. In addition, a 10-fold increase in FT or TT was associated with a 3.8-point (0.04, 7.5) or 4.0-point (0.5, 7.5) higher behavioral symptoms index composite score. In models evaluating effect modification by sex, a 10-fold increase in E1 was associated with a 4.3-point (1.2, 7.4) decrease in adaptive skills composite score in girls only (interaction p=0.04). We observed associations between testosterone and internalizing behaviors and behavioral symptoms index in both sexes, as well as a female-specific association between E1 and adaptive skills. Sex hormones during pregnancy may play a key role in influencing later-life behavior, and additional studies should further examine different periods of susceptibility to hormonal signals.
Keywords: Estrogen, Testosterone, Child Behavior, Neurodevelopment
1. Introduction
Sex hormones play an important role in regulating the development of sex-differentiated brain structures and sex-specific reproductive behaviors (Cohen-Bendahan 2005, Auyeung 2013, Martel 2013). Their role in child behavioral problems and psychiatric disorders is suggested by sex differences in the prevalence of these disorders, with some disorders found to be more common in males (e.g., autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD)) (Werling and Geschwind 2013, Arnett 2015) and others more common in females (e.g., anxiety disorders) (Eaton 2012, Altemus 2014). Given that sex hormone levels remain low until puberty (Sisk and Zehr 2005), pre-pubescent behavioral effects of sex hormones are likely programmed during fetal development (Miranda and Sousa 2018). A key period for this programming is likely in early pregnancy, which is thought to be a critical period for neurological sex differentiation as this is when male fetal testosterone surges, driving subsequent masculinization of brain structures and gender-stereotyped behavior (Hines 2002, McCarthy 2016).
Prenatal sex hormone associations with child pathologic behavior have primarily been evaluated in the context of androgens (Gore 2014), with several studies observing associations between second trimester amniotic fluid testosterone and a variety of problematic behaviors, including attention problems in boys (Korner 2019) and autistic traits in both sexes (Auyeung 2012). Studies of disorders of sex development such as congenital adrenal hyperplasia (CAH), a disorder that results in abnormally high androgen concentrations in utero and throughout life, have observed higher scores for assessments of later-life autism-like behaviors (Knickmeyer 2006) or increased incidences of anxiety and depression-related disorders and substance abuse in later life in both males and females with CAH (Falhammar 2014, Engberg 2015). Maternal hyperandrogenic endocrinopathies such as polycystic ovarian syndrome (PCOS) have also been associated with neurobehavioral outcomes in children lacking CAH or any other salient endocrine disorders, including higher odds of ASD, ADHD, and their comorbidity in children (Kosidou 2017, Cherskov 2018, Cesta 2019). Another study evaluating behavioral assessments in the context of maternal PCOS and directly measured amniotic testosterone levels found that increased autism-like behaviors and lower empathetic behaviors were associated with higher testosterone in both boys and girls and with maternal PCOS in girls (Palomba 2012). In contrast to these findings related to hyperandrogenism, boys with reproductive disorders associated with lower prenatal testosterone levels, such as hypospadias and cryptorchidism, have also been shown to have higher odds for ASD (Rotem 2018).
Compared with androgens, fewer studies have examined the relationship between prenatal estrogens and child behavior problems. Although estrogen levels have been associated with inhibition and related internalizing features, most of these studies focus on activational effects in adolescence, when sex differences in internalizing disorders tend to emerge (Martel 2013). In the adult brain, estrogens are considered neuroprotective, in part due to neuronal trophic and anti-apoptotic effects (Kajta and Beyer 2003). Increased ASD risk was observed in association with decreases in maternal serum E3 sampled between gestational weeks 15–20, which coincides with the protective effect of E3 observed for birth outcomes like pre-term birth (Windham 2016). By contrast, a recent pilot study observed the opposite, with greater maternal serum E2 in gestational weeks 15–18 associated with an increased ASD risk in offspring (Bilder 2019). Though an earlier study of second trimester amniotic E2 found no association with autistic traits in toddlers (Auyeung 2012), a more recent male-only study of second trimester amniotic estrogens found increased risk of ASD associated with E1, E2, E3, and progesterone but not testosterone; however, those models did not adjust for covariates (Baron-Cohen 2019). Beyond direct estrogen measures, recent studies of fetal single nucleotide polymorphisms (SNPs) in sex hormone receptors have yielded mixed results and some evidence of effect modification by sex (Miodovnik 2012).
Most of the existing research on the relationship between psychopathologic behaviors and sex hormones has either been conducted in animal models or in the context of maternal conditions known to affect prenatal sex hormones. Despite the evidence for the role of prenatal sex hormones in the development of child behavior (Auyeung 2013), little is known about the organizational role maternal sex hormones during pregnancy play in behaviors related to psychopathologic disorders, and few studies have evaluated the relationship between these aspects of child behavior and levels of maternal prenatal sex hormones in healthy individuals. The current study examines the relationship between maternal sex hormone levels measured during early pregnancy in healthy women and behavioral outcomes in children aged 4–5 years old. These outcomes encompass several childhood behavioral dimensions, broadly categorized as autism-related, externalizing, internalizing, adaptive behaviors, and other behavior problems as measured by the behavioral symptoms index. Based on existing literature, we hypothesized that prenatal maternal sex hormones would be associated with child behaviors, with prenatal testosterone expected to be associated with externalizing behaviors, autism-related behaviors, and behavioral symptoms index and prenatal estrogen predicted to be associated with internalizing behaviors. Given the potential sex-specificity of sex hormone associations with behavior, we also examined whether these associations are modified by sex.
2. Methods
2.1. Study participants
From 2010 to 2012, pregnant women were recruited in their first trimester at the University of California San Francisco (UCSF), University of Minnesota (UMN), University of Rochester Medical Center (URMC), and Seattle Children’s Hospital/University of Washington (UW) to take part in The Infant Development and Environment Study (TIDES). Eligibility criteria included that participants were ≥18 years of age, English speaking, and <13 weeks pregnant, as well as that they had no severe threat to pregnancy and intended to deliver at one of the study hospitals. TIDES was approved prior to recruitment by the institutional review boards at each center, and informed consent was obtained from all participants. Questionnaires containing demographic, medical history, physiological, and behavioral questions (e.g., pertaining to alcohol and tobacco use) were administered in early pregnancy, usually on the same day as serum collection or completed online at the participants’ convenience. Gestational dating was based on the first ultrasound in the medical record. 626 women completed screening and had serum samples drawn during early pregnancy. This study is limited to 403 mothers and 404 children for whom complete early pregnancy serum sex hormone, child 4–5-year behavioral assessment, and covariate data were available (including data from both members of a single twin pair).
2.2. Serum sex hormone measurements
A single serum sample was obtained in early pregnancy between gestational week 6 and 21, with most samples drawn in the first trimester (median = 11.6 weeks gestation), and stored at −80 °C until analysis. The Endocrine and Metabolic Research Laboratory at the Los Angeles Biomedical Research Institute (now renamed Lundquist Institute) at the Harbor-UCLA Medical Center performed all serum hormone assays using validated methods. Serum E1, E2, and E3 were separated on a column with a gradient profile from 63% to 100% methanol using a Shimadzu (Columbia, MD) high performance liquid chromatography (HPLC) system and a triple quadrupole mass spectrometer (Applied Biosystems (Foster City, CA) API5000 liquid chromatography tandem mass spectrometer (LC-MS/MS)) operated in the negative mode using multiple-reaction monitoring. For both E1 and E2, the calibration curve was linear over a range of 2 to 2000 pg/mL with the lower limit of quantification (LLOQ) was 2.0 pg/mL, and the calibration curve for E3 was linear from 50 to 5000 pg/mL with a LLOQ of 50 pg/mL. The within-run and between-run precisions (% coefficient of variation) were 2.6%−5.6% and 3.9%−4.6% for E1, 4.3%−5.0% and 4.6%−5.2% for E2, and 4.1%−5.7% and 5.2% - 8.7% for E3, respectively. The accuracy ranged from 91.9 – 101.2 for E1, 93.9 – 100.3 for E2, and 87.2 – 104.3 for E3.
Serum TT LC-MS/MS runs were performed as previously described (Shiraishi 2008) with a Shimadzu HPLC system attached to an Applied Biosystems API5500 LC-MS/MS equipped with a turbo-ion-spray source and operated in the positive mode. The calibration curve was linear from 2 ng/dL to 2000 ng/dL, the LLOQ for TT was 2.0 ng/dL, the within- and between-run precisions were less than 5%, and the accuracy of spiked samples was between 100% and 113%. Percent FT, representing unbound testosterone, was measured by equilibrium dialysis using radiolabeled testosterone as described previously, and the LLOQ is 0% (Qoubaitary 2006). Then FT was calculated by multiplying the percent FT with the TT concentration. Both FT and TT measurements were included in our analyses because evidence suggests that FT may be a more clinically relevant measure of testosterone activity (Tsujimura 2012, Antonio 2016), but it remains unclear if FT is the only bioactive form of testosterone (Goldman 2017). Therefore, analyzing both provides a more comprehensive examination of testosterone effects.
2.3. Neurobehavioral Assessments
TIDES children completed study visits between 4 and <6 years of age (median = 4.5 years). Mothers were asked to complete a study questionnaire in person or, when this was not possible, by mail. Mothers completed the Behavioral Assessment System for Children, 2nd Edition (BASC-2), and Social Responsiveness Scale, 2nd Edition (SRS-2). For the BASC-2, Likert scale (“never”, “sometimes”, “often”, or “always”) responses on 138 items were combined into raw subscale scores, which were again summed to get four raw composite scores: externalizing, internalizing, behavioral symptoms index, and adaptive skills composite scores. The BASC-2 externalizing composite score is comprised of hyperactivity and aggression subscale scores; the internalizing composite is comprised of anxiety, depression, and somatization subscale scores; the behavioral symptoms index (BSI) composite is comprised of attention problems, atypicality, and withdrawal subscale scores; and the adaptive skills composite is comprised of activities of daily living, adaptability, functional communication, and social skills subscale scores.
The SRS-2 is comprised of 65 questions on a 0–3 Likert scale, which are summed to become one of the subscale scores of social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behaviors (RRB). The SRS-2 has been validated to distinguish children with ASD from healthy children (Frazier et al., 2012). The SRS-2 total score is calculated as the sum of all subscale scores. For both BASC-2 and SRS-2, raw scores were converted into sex-standardized, norm-referenced T scores (mean = 50, SD = 10) based on each assessment’s manualized procedures. As per the conventions of both tests, higher values indicate more problematic behaviors for all scores except for the BASC-2 adaptive skills composite score.
2.4. Statistical methods
The BASC-2 and SRS-2 composite scores were chosen as the primary outcomes for this analysis, and they were analyzed as sex-standardized T scores rather than raw scores because T scores, not raw scores, are utilized in clinical applications. Summary statistics were computed for all relevant measures. The twins were treated as independent participants since including a random intercept or other means of nesting this single pair of twins within the same mother did not change results. Maternal sex hormone concentrations were right-skewed and log10-transformed in all models. Hormone concentrations below the LLOQ were imputed using the LLOQ divided by . Multivariable linear regression was used to examine associations between maternal hormones and BASC-2 and SRS-2 outcomes using the “HC0” Huber-White heteroskedasticity-consistent standard error sandwich estimator to avoid potential heteroskedasticity-induced bias (White 1980). To examine potential effect modification by sex, multiple linear regressions including a maternal sex hormone by child sex interaction term were also evaluated. Each model only evaluated one sex hormone predictor and one behavioral outcome at a time. Due to issues with the theory behind p-value adjustment (Rothman 1990), the novelty of the relationships we are examining, and the exploratory nature of our analysis, we decided not to adjust for multiple comparisons. All models adjusted for the a priori-selected covariates maternal and child age, study center, race, income and education categories, child sex, and alcohol and cigarette consumption in the week prior to the serum draw. Covariates were chosen a priori because of their potential confounding effect on one or more outcome measures (Fox 1995, Herrmann 2008, O’Connor and Paley 2009). We chose to analyze composite behavior scores rather than subscale scores due to their increased reliability and clinical relevance, but we calculated subscale T score summary statistics and regression results for reference.
3. Results
3.1. Study Participants
Table 1 summarizes the demographic and pregnancy characteristics for study participants. Participants were recruited in similar numbers across study centers. Mothers were primarily white (75%) and most had some level of college or graduate education (89%). Few mothers reported using tobacco cigarettes (3%) or drinking alcohol (4%). Self-reported pre-pregnancy and early pregnancy (median 11.6 weeks gestation) BMI were similar, with 19% of mothers reporting an obese pre-pregnancy BMI > 30 and 22% reporting a pre-pregnancy BMI of 25–30. Most serum samples (92%) were drawn in the first trimester. Few children (5%) were born with a low birth weight (≤ 2.5 kg), and only 1 (0.25%) was born with an extremely low birth weight (≤ 1 kg). Forty (9.9%) of the births were preterm (<37 weeks), and 1 (0.25%) was extremely preterm (<28 weeks). Twenty-eight (7%) mothers reported having polycystic ovary syndrome (PCOS). Of the original 626 women recruited, those included in the final analysis were more likely to be slightly older, white, higher income, more educated, and from the UCSF or UMN study sites (see Table A.3).
Table 1:
Demographic Characteristics of 404 Mother-Child Dyads in the TIDES Study with Maternal Sex Hormone and Child Neurobehavioral Outcome Measures
| Characteristic | Mean (SD) | Median (Range) |
| Maternal age (years) | 31.50 (5.08) | 31.87 (18.25 – 44.26) |
| Pre-pregnancy BMIa | 25.57 (6.15) | 23.65 (17.36 – 50.65) |
| Early-pregnancy BMI | 26.00 (6.10) | 23.65 (17.36 – 50.65) |
| Gestational age at serum draw (weeks) | 11.11 (2.64) | 11.57 (5.71 – 20.29) |
| Gestational age at birth (weeks) | 39.27 (1.88) | 39.57 (25.00 – 42.43) |
| Birth weight (kg) | 3.37 (0.57) | 3.35 (0.55 – 5.15) |
| Child age (years) | 4.53 (0.34) | 4.53 (3.93 – 5.95) |
| Characteristic | N (%) | |
| Child sex | ||
| M | 193 (47.77%) | |
| F | 211 (52.23%) | |
| Center | ||
| UCSF | 99 (24.50%) | |
| UMN | 131 (32.43%) | |
| URMC | 92 (22.77%) | |
| UW | 82 (20.30%) | |
| Race | ||
| Asian | 20 (4.95%) | |
| Black | 34 (8.42%) | |
| Other | 47 (11.63%) | |
| White | 303 (75.00%) | |
| Income | ||
| ≤ $25000 | 78 (19.31%) | |
| $25001 – $75000 | 108 (26.73%) | |
| > %75000 | 218 (53.96%) | |
| Education | ||
| High school | 43 (10.64%) | |
| College | 158 (39.11%) | |
| Graduate school | 203 (50.25%) | |
| Early pregnancy alcohol consumption | ||
| No | 390 (96.53%) | |
| Yes | 14 (3.47%) | |
| Early pregnancy cigarette use | ||
| No | 386 (95.54%) | |
| Yes | 18 (4.46%) | |
One participant was missing pre-pregnancy BMI data.
3.2. Sex Hormone Levels
Table 2 shows maternal sex hormone summary statistics and the results of t-tests comparing hormones by fetal sex. Sex hormone levels fell within typical ranges (Oleary 1991, Schock 2016) and did not differ significantly by child sex. Log10-transformed E1 and E2 were strongly correlated (r = 0.89), as were FT and TT (r = 0.82) (see Figure A.1). Hormone measurements were below the LLOQ only for E3 (34%).
Table 2:
Maternal Serum Sex Hormone Concentrations between Mothers of Female versus Male Children
| Hormone | Fetal Sex | Mean (SD) | Median (Range) | N missing (%) | N < LLOQ (%) | t-test p-valuea |
|---|---|---|---|---|---|---|
| Total | 1031 (952) | 736 (62.7 – 6590) | 2 (0.50%) | 0 (0%) | 0.21 | |
| E1 (pg/mL) | M | 1000 (964) | 690 (87.3 – 5110) | 2 (1.04%) | 0 (0%) | |
| F | 1060 (942) | 774 (62.7 – 6590) | 0 (0.00%) | 0 (0%) | ||
| E2 (pg/mL) | Total | 1763 (1354) | 1440 (100 – 9300) | 2 (0.50%) | 0 (0%) | 0.42 |
| M | 1784 (1498) | 1310 (170 – 9300) | 2 (1.04%) | 0 (0%) | ||
| F | 1744 (1214) | 1460 (100 – 6060) | 0 (0.00%) | 0 (0%) | ||
| E3 (pg/mL) | Total | 183 (313) | 89.3 (50 – 2360) | 4 (0.99%) | 136 (34.00%) | 0.26 |
| M | 202 (340) | 94.1 (50 – 2230) | 2 (1.04%) | 62 (32.46%) | ||
| F | 167 (285) | 86.1 (50 – 2360) | 2 (0.95%) | 74 (35.41%) | ||
| FT (ng/dL) | Total | 0.33 (0.21) | 0.27 (0.04 – 1.69) | 5 (1.24%) | 0 (0%) | 0.43 |
| M | 0.33 (0.23) | 0.27 (0.04 – 1.69) | 3 (1.55%) | 0 (0%) | ||
| F | 0.33 (0.20) | 0.28 (0.06 – 1.11) | 2 (0.95%) | 0 (0%) | ||
| TT (ng/dL) | Total | 71.7 (43.6) | 61.8 (12.9 – 288) | 0 (0.00%) | 0 (0%) | 0.22 |
| M | 70.9 (46.7) | 61.2 (12.9 – 263) | 0 (0.00%) | 0 (0%) | ||
| F | 72.5 (40.6) | 64.2 (15.9 – 288) | 0 (0.00%) | 0 (0%) | ||
t-tests evaluated differences in hormone concentrations between mothers of female and male children. Hormones were log10-transformed prior to analysis with the t-test due to right skew. Percent values for missing data counts reflect the percent of the total for that fetal sex group. Percent values below the lower limit of quantification (LLOQ) are out of the non-missing total for that fetal sex group. E1 = estrone, E2 = estradiol, E3 = estriol, FT = free testosterone, TT = total testosterone.
3.3. Neurobehavioral Assessment Outcomes
Sex-standardized BASC-2 and SRS-2 composite T scores are summarized in Table 3. The distributions for all T scores were similar to the normative sample (i.e., mean of 50 and SD of 10). Though BASC-2 and SRS-2 T scores are sex-standardized, there were still significant differences in T scores between sexes among the study participants. Males had significantly lower BASC-2 internalizing and higher SRS-2 total composite T scores.
Table 3:
BASC-2 and SRS-2 Composite T Scores in Male and Female Children
| Score | Child Sex | Mean (SD) | Median (Range) | N missing (%) | t-test p-valuea |
|---|---|---|---|---|---|
| BASC Externalizing | Total | 49.4 (8.0) | 48 (34 – 78) | 1 (0.25%) | 0.89 |
| M | 49.4 (7.9) | 48 (35 – 73) | 0 (0.00%) | ||
| F | 49.3 (8.1) | 48 (34 – 78) | 1 (0.47%) | ||
| BASC Internalizing | Total | 48.9 (9.1) | 48 (30 – 78) | 1 (0.25%) | 0.02* |
| M | 47.8 (8.9) | 47 (30 – 77) | 0 (0.00%) | ||
| F | 49.9 (9.2) | 49.5 (31 – 78) | 1 (0.47%) | ||
| BASC BSI | Total | 48.9 (8.3) | 48 (30 – 85) | 1 (0.25%) | 0.76 |
| M | 48.7 (8.2) | 48 (30 – 77) | 0 (0.00%) | ||
| F | 49.0 (8.4) | 49 (34 – 85) | 1 (0.47%) | ||
| BASC Adaptive Skills | Total | 51.1 (8.7) | 52 (20 – 72) | 1 (0.25%) | 0.14 |
| M | 51.8.91) | 52 (20 – 70) | 0 (0.00%) | ||
| F | 50.5 (8.6) | 51 (25 – 72) | 1 (0.47%) | ||
| SRS Total | Total | 44.9 (6.9) | 44 (35 – 81) | 12 (2.97%) | 0.03* |
| M | 45.6 (7.1) | 44 (35 – 74) | 6 (3.11%) | ||
| F | 44.2 (6.6) | 43 (35 – 81) | 6 (2.84%) | ||
t-tests evaluated differences in BASC-2 and SRS-2 composite T scores between female and male children. Percent values for missing data counts reflect the percent of the total for that child sex group. BSI = behavioral symptoms index.
Pearson correlation coefficients between BASC-2 and SRS-2 composite T scores are shown in Figure A.2. The strongest correlations were between BASC-2 externalizing and BSI scores (r = 0.84) and BSI and SRS-2 total score (r = 0.72), and correlations between the other scores were moderate, ranging in magnitude from r = −0.32 between BASC-2 internalizing and adaptive skills scores to r = 0.69 between BSI and internalizing score.
3.4. Linear Regressions
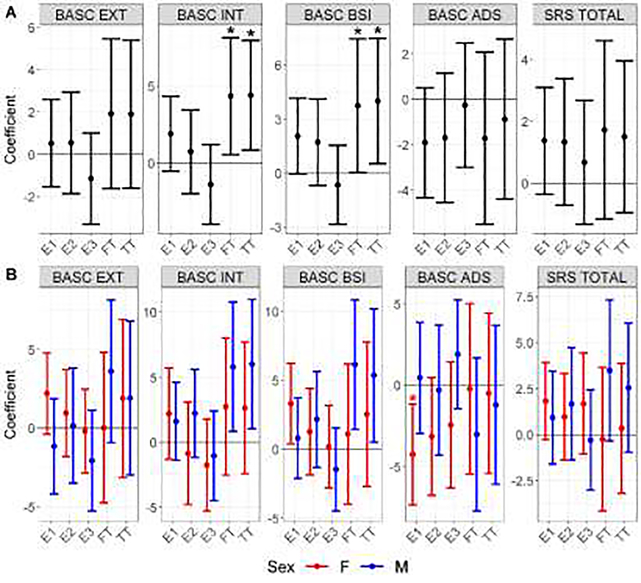
The results of linear regressions associating maternal sex hormone levels and behavioral test composite scores are shown in Figure 1A and Table A.1 for models without the hormone by sex interaction term and in Figure 1B and Table A.2 for models with the interaction term. In both sexes combined, each ten-fold increase in TT is significantly associated with a 4.40 (95% CI: 0.83, 7.97) point and 4.01 (95% CI; 0.52, 7.50) point increase in BASC-2 internalizing and BSI composite scores, respectively. A ten-fold increase in FT is significantly associated with a 4.35 (0.54, 8.16) point increase in internalizing composite score and a 3.75 (0.05, 7.46) point increase in BSI composite score. In the hormone by sex interaction models (Figure 1B), the only association with a significant sex-specific hormone main effect coefficient and a significant hormone by sex interaction term is the female-specific adverse association between E1 and BASC-2 adaptive skills composite score (−4.26 (−7.37, −1.16); interaction p=0.035). Subscale score summary statistics and regression results are shown in Section 5 of Appendix A.
Figure 1: Mean and 95% CI Changes in Child BASC and SRS Composite Scores Associated with Maternal Sex Hormones.
Figure 1A shows results from linear regressions without a hormone by sex interaction term, and Figure 1B shows the sex-specific marginal coefficients from linear regressions including the interaction term. All coefficients are point changes in scores associated with a ten-fold increase in hormones. Estimates with an asterisk above them indicate p<0.05 in Figure 1A and that both marginal sex-specific and relevant interaction term p-values are <0.05 in Figure 1B. EXT: Externalizing composite score, INT: internalizing composite score, BSI: behavioral symptoms index composite score, ADS: adaptive skills composite score, SRS TOTAL: SRS total composite score.
4. Discussion
The findings of this study suggest that maternal testosterone levels during pregnancy are positively associated with internalizing behaviors and behavioral symptoms index in 4–5-year-old children of both sexes. Our results are somewhat inconsistent with findings from the literature observing associations between PCOS and ASD (Kosidou 2016, Cherskov 2018, Cesta 2019) or ADHD (Kosidou 2017, Cesta 2019), although there was a significant association between FT and TT and BSI score, which includes the attention problems subscale score. Although the estimates were imprecise and not statistically significant, male-specific FT associations of a similar nature were observed with SRS-2 total score and BSI. Associations between behavior and the highly correlated FT and TT measures were similar in effect size, suggesting that these measures both similarly indicate prenatal testosterone activity. For the estrogens, there was a sex-specific association between prenatal E1 and worse adaptive skills in girls, but not boys. All significant associations were in the adverse direction, and the magnitudes of these associations were all around 4 points per 10-fold increase in hormone. Given that each T score unit can be interpreted as 0.1 SD of the population distribution for that outcome and each sex hormone vary over a range of 1–2 log10 units, these results suggest that natural variation in early pregnancy maternal sex hormones could have meaningful impacts on behavioral outcomes in children when considered at the population-level.
Few previous studies support the observed associations in both sexes between maternal testosterone and internalizing behaviors and BSI score, which encompasses some behavior problems related to both internalizing problems (e.g., social withdrawal) and externalizing problems (e.g., attention problems). However, rodent models of maternal hyperandrogenism suggest female-specific internalizing behavioral effects of testosterone. Female mice born to dams treated with dihydrotestosterone (DHT) during pregnancy exhibited increased adrenoceptor α1B expression in the amygdala and corticotropin-releasing hormone in the hypothalamus, possible markers of anxiety (Manti 2018), and female rats born to dams injected with testosterone during pregnancy exhibited anxiety-like behaviors and changes in amygdala receptor gene expression consistent with anxiety (Hu 2015). Social vocalizations decreased for both sexes of rat pups born to dams treated with the aromatase inhibitor letrozole, but only female offspring also exhibited decreased social and sexual interaction (Xu 2015). In contrast, in humans it was male infants with higher amniotic testosterone levels during pregnancy who exhibited greater fear responses, not females (Bergman 2010). Both males and females with CAH exhibited greater lifetime rates of psychiatric disorders related to anxiety and depression (Falhammar 2014, Engberg 2015), though this may not reflect the influence of prenatal hyperandrogenic exposures specifically since CAH may also affect these outcomes through impaired adrenaline production and other mechanisms. In relation to other psychopathological behavioral outcomes, studies suggest that testosterone associations with behavior are not sex-specific, as this was the case for amniotic testosterone associations with autism-related behaviors (Kosidou 2016, Kosidou 2017, Cherskov 2018), and maternal PCOS associations with child ASD and ADHD (Kosidou 2016, Kosidou 2017, Cherskov 2018). There is also a lack of sex-specificity observed for correlations between maternal plasma testosterone and fetal plasma testosterone and cortisol later in pregnancy (Gitau 2005), as well as between amniotic testosterone and cortisol from gestational week 15 to 37 (Sarkar 2008). These correlations suggest that maternal testosterone directly or indirectly plays a role in regulation of the fetal hypothalamic-pituitary-adrenal (HPA) axis, which has already been suggested in adult studies showing crosstalk between the HPA and hypothalamic-pituitary-gonadal (HPG) axes, such as testosterone and E2 increasing arginine vasopressin signaling that leads to increased adrenal cortisol release (Grassi 2013). Dysregulation of the fetal HPA has been associated with later-life anxiety and depression (Ansorge 2007), and so perhaps our observed maternal testosterone associations with internalizing and BSI-related behaviors may result, in part, through testosterone effects on the fetal HPA axis. We also used more accurate methods to measure TT and FT, LC-MS and equilibrium dialysis, respectively, than other groups who largely used immunoassays and equations to measure TT and FT, respectively. This may have contributed to some of the differences between our observed testosterone associations and those of previous studies.
Few studies have examined maternal estrogens in relation to child behavioral outcomes, and none thus far have observed the sex-specific association between E1 and adaptive skills that we observed in our results. Perhaps reflecting a similar mechanism, we also observed a suggestive but nonsignificant association between E1 and BSI, with some suggestion of a greater association in females. Miodovnik et al. observed adverse associations with adaptive skills in males born to mothers with a functional CYP1B1 SNP, and studies have shown that a functional increase in CYP1B1 leads to decreased testosterone signaling and increased E2 (Miodovnik 2012). These results suggest that males, but not females, may have increased behavioral problems with increasing maternal estrogen exposure. The adaptive skills composite score covers some behaviors that may overlap with autistic traits such as social skills, and the recent finding of an increased risk of autism in association with second trimester maternal E2 may reflect similar processes to our observations regarding E1 (Bilder 2019). The observed estrogen association with these adverse behavioral outcomes may be a compensatory response given the neuroprotective and uteroprotective role of estrogens, including maintaining normal development of the fetal adrenal cortex, which is vital in assuring placental steroid production and fetal survival (Kaludjerovic and Ward 2012). A similar compensatory mechanism may also underlie the amniotic E2 associations with ASD risk observed by Baron-Cohen et al. (Baron-Cohen 2019). However, these authors suggest that prenatal estrogen increases GABAergic signaling, which subsequently increases cerebrocortical excitatory synapses, a theorized mechanism of autism (Sellers 2015). It is unclear why we only observe the adverse association with E1 in females. E2 is the predominant form of estrogen in women until menopause and is the most biologically active form of estrogen (Rothman 2011), and so it is surprising that we would see an association with E1 rather than E2. The primary role of E1 is to act as a pro-estrogen, able to be converted into the more potent E2 or to a lesser extent E3 (Kuhl 2005). In addition, 17β-hydroxysteroid dehydrogenase 2 prevents excess active estrogen delivery to the fetus by catalyzing the conversion of E2 back into E1 in the placental fetal endothelial cells (Drolet 2007). If the observed association with E1 reflects a compensatory response, perhaps E1 is being upregulated as a pro-estrogen pool, ready to be rapidly converted to E2, though it is also possible that these associations instead reflect adverse neurological effects of estrogens.
Our results differ from several studies evaluating hormones in amniotic fluid, and this may be due to a difference in hormone action or transport between the maternal and fetal compartments. Amniotic fluid levels are thought to more directly reflect fetal exposure than maternal serum, and the two compartments have been shown to be uncorrelated for FT and TT and moderately correlated for E2 in the second trimester (van de Beek 2004). However, direct sampling of paired fetal and maternal blood samples from the mid-second to late third trimester demonstrated correlations and similar concentrations between maternal and fetal blood TT in both sexes (Gitau 2005), and our own group previously found increased maternal testosterone in mothers of male fetuses later in pregnancy, suggesting testosterone transfer between the fetus and mother (Sathyanarayana 2014). Fetal testosterone is primarily synthesized by the fetus itself, either primarily by the testes in males or the fetal adrenal cortex in females, and protective mechanisms such as aromatase exist to prevent excessive maternal testosterone delivery to the fetus by transforming it to estrogen (Makieva 2014). It is possible that observed maternal testosterone associations with neurobehavioral outcomes are at least partially mediated by aromatization of maternal testosterone into fetal estrogen after crossing the placenta, which could explain differences in associations between maternal serum and amniotic testosterone, as well as the recent Baron-Cohen et al. 2019 findings regarding amniotic estrogen and ASD. Another possibility is that maternal testosterone may influence development without directly translocating to the fetus, such as through reductions in uterine blood flow and placental amino acid transport observed in rodent experiments (Sathishkumar 2011, Gopalakrishnan 2016). As E2 has been shown to be moderately correlated between amniotic fluid and maternal serum, maternal estrogen levels may more directly reflect fetal exposure. During pregnancy, maternal and fetal estrogens are both primarily synthesized in the placenta with approximately equal contribution of the estrogen precursor dehydroepiandrosterone from both the mother and the fetus (Siiteri and MacDonald 1966), and placental E1 and E2 are secreted preferentially into the fetus and mother, respectively (Gurpide 1982). Despite this common source for maternal and fetal estrogen, maternal estrogen may still act on fetus via indirect pathways, such as by enhancing uteroplacental blood flow as suggested by animal studies (Albrecht and Pepe 2010). Future studies further examining maternal versus amniotic hormone level associations with child behavioral problems are necessary to determine if they differ in magnitude, direction, or sex-specificity.
Strengths of this study include the prospective, multi-site design; a relatively large sample, allowing for examination of sex-specific effects of sex hormones; examination of both prenatal maternal estrogen and testosterone concentrations during a critical period of gestation in healthy pregnancies; and assessment of both behavioral problems and adaptive behaviors using standardized, norm-referenced measures. As with any observational study, our findings do not necessarily imply a causal association between prenatal sex hormones and child behavior. Other limitations include the low rates of child neurobehavioral problems in this largely healthy and typically developing cohort, such as only 4% of participants having SRS-2 total scores exceeding the clinical T score threshold of 60. This may explain the limited associations observed and failure to replicate previously reported associations between testosterone and disruptive behavior and ASD characteristics. The healthy nature of the pregnancies in our cohort also precluded us from examining sex hormone levels in amniotic fluid, as this is typically evaluated in samples derived from amniocentesis, which tends to be performed only for at-risk pregnancies. The TIDES cohort also includes a high percentage of well-educated, white, and higher-income populations and low rates of prenatal tobacco and alcohol exposure. This may affect the generalizability of our findings in other, more diverse populations. This effect was compounded by the fact that participants without complete data who were excluded from this analysis were more likely to be black, lower income, and less educated. Ideally, our analyses will be replicated in larger and more diverse populations.
Maternal sex hormone concentrations were assessed relatively early in pregnancy (i.e., 11 weeks gestation on average), a period during which sex hormones are thought to influence neurobehavioral development. However, measuring hormone concentrations at multiple time points throughout pregnancy might have allowed us to identify critical exposure windows for these developmental outcomes. Although our group previously found that gestational age in early pregnancy was associated with changing levels of each of the measured sex hormones (Barrett 2019), we did not observe evidence of effect modification of associations by gestational age at blood draw. Therefore, we do not believe that variation in sampling time within the early pregnancy period this study evaluated influenced the observed hormone and behavior associations. Furthermore, we did not observe any correlations or associations between time of day of sample collection and sex hormone levels, suggesting that the timing of sample collection did not influence our results. Although multiple blood measurements within the same short developmental window may have provided more stable estimates of maternal circulating hormone levels than relying on a single spot measurement, previous longitudinal evidence has shown single serum hormone measurements to be reliable predictors of levels throughout pregnancy (Schock 2016). This study did have 34% of E3 samples below the LLOQ, likely due to E3 remaining low in early pregnancy. If more samples were drawn further into the second trimester, we may have been able to better examine potential protective or adverse behavioral effects of E3 as suggested by the findings on ASD risk observed by Windham et al. and Baron-Cohen et al., respectively. Finally, our study cannot address the possibility of parental behavior and postnatal care effects on child behavior, such as mothers with higher endogenous sex hormones potentially exhibiting different parenting strategies that predispose their children to behavioral problems, and this may be a fruitful area for future research.
5. Conclusions
In one of the only studies to examine maternal sex hormone levels in early pregnancy in relation to child behavioral outcomes in early childhood, we find associations between testosterone and internalizing and BSI-related behaviors in both sexes and a negative association between E1 and adaptive skills in girls. Maternal sex hormones may influence child neurobehavioral outcomes, and so it is important to understand factors that affect hormones during pregnancy as these could have lasting impacts on neurobehavioral programming.
Supplementary Material
Highlights.
Early pregnancy maternal serum sex hormones are associated with 4–5y child behavior
Maternal testosterone was associated with internalizing and behavioral symptoms
Maternal estrone was associated with poorer adaptive skills in girls
Acknowledgements
We thank the TIDES Study Team for their contributions. Coordinating Center: Fan Liu, Erica Scher; UCSF: Marina Stasenko, Erin Ayash, Melissa Schirmer, Jason Farrell, Mari-Paule Thiet, Laurence Baskin; UMN: Heather L. Gray, Chelsea Georgesen, Brooke J. Rody, Carrie A. Terrell, Kapilmeet Kaur; URMC: Erin Brantley, Heather Fiore, Lynda Kochman, Lauren Parlett, Jessica Marino, William Hulbert, Robert Mevorach, Eva Pressman; UW/SCH: Kristy Ivicek, Bobbie Salveson, Garry Alcedo and the families who participated in the study. In addition, we thank Christina Wang for sex hormone analysis, the TIDES families for their participation, and the residents at URMC and UCSF who assisted with birth exams.
Role of Funding Source
Funding for TIDES was provided by the following grants from the National Institute of Environmental Health Sciences: R21ES023883, R01ES016863-04, and R01ES016863-02S4. The NIEHS had no role in the study design, data collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit this article for publication.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht ED and Pepe GJ (2010). “Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy.” Int J Dev Biol 54(2–3): 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N and Neill Epperson C (2014). “Sex differences in anxiety and depression clinical perspectives.” Front Neuroendocrinol 35(3): 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Hen R and Gingrich JA (2007). “Neurodevelopmental origins of depressive disorders.” Curr Opin Pharmacol 7(1): 8–17. [DOI] [PubMed] [Google Scholar]
- Antonio L, Wu FC, O’Neill TW, Pye SR, Ahern TB, Laurent MR, Huhtaniemi IT, Lean ME, Keevil BG, Rastrelli G, Forti G, Bartfai G, Casanueva FF, Kula K, Punab M, Giwercman A, Claessens F, Decallonne B, Vanderschueren D and European G Male Ageing Study Study (2016). “Low Free Testosterone Is Associated with Hypogonadal Signs and Symptoms in Men with Normal Total Testosterone.” J Clin Endocrinol Metab 101(7): 2647–2657. [DOI] [PubMed] [Google Scholar]
- Arnett AB, Pennington BF, Willcutt EG, DeFries JC and Olson RK (2015). “Sex differences in ADHD symptom severity.” J Child Psychol Psychiatry 56(6): 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Ahluwalia J, Thomson L, Taylor K, Hackett G, O’Donnell KJ and Baron-Cohen S (2012). “Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age.” Molecular Autism 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV and Baron-Cohen S (2013). “Prenatal and postnatal hormone effects on the human brain and cognition.” Pflugers Arch 465(5): 557–571. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Tsompanidis A, Auyeung B, Norgaard-Pedersen B, Hougaard DM, Abdallah M, Cohen A and Pohl A (2019). “Foetal oestrogens and autism.” Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Mbowe O, Thurston SW, Butts S, Wang C, Nguyen R, Bush N, Redmon JB, Sheshu S, Swan SH and Sathyanarayana S (2019). “Predictors of Steroid Hormone Concentrations in Early Pregnancy: Results from a Multi-Center Cohort.” Matern Child Health J 23(3): 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Glover V, Sarkar P, Abbott DH and O’Connor TG (2010). “In utero cortisol and testosterone exposure and fear reactivity in infancy.” Horm Behav 57(3): 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder DA, Esplin MS, Coon H, Burghardt P, Clark EAS, Fraser A, Smith KR, Worsham W, Chappelle K, Rayner T and Bakian AV (2019). “Early Second Trimester Maternal Serum Steroid-Related Biomarkers Associated with Autism Spectrum Disorder.” J Autism Dev Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta CE, Oberg AS, Ibrahimson A, Yusuf I, Larsson H, Almqvist C, D’Onofrio BM, Bulik CM, Fernandez de la Cruz L, Mataix-Cols D, Landen M and Rosenqvist MA (2019). “Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding?” Psychol Med: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherskov A, Pohl A, Allison C, Zhang H, Payne RA and Baron-Cohen S (2018). “Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory.” Transl Psychiatry 8(1): 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C and Berenbaum SA (2005). “Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings.” Neurosci Biobehav Rev 29(2): 353–384. [DOI] [PubMed] [Google Scholar]
- Drolet R, Simard M, Plante J, Laberge P and Tremblay Y (2007). “Human type 2 17 beta-hydroxysteroid dehydrogenase mRNA and protein distribution in placental villi at mid and term pregnancy.” Reprod Biol Endocrinol 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE, Grant BF and Hasin DS (2012). “An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample.” J Abnorm Psychol 121(1): 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg H, Butwicka A, Nordenstrom A, Hirschberg AL, Falhammar H, Lichtenstein P, Nordenskjold A, Frisen L and Landen M (2015). “Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: A total population study.” Psychoneuroendocrinology 60: 195–205. [DOI] [PubMed] [Google Scholar]
- Falhammar H, Butwicka A, Landen M, Lichtenstein P, Nordenskjold A, Nordenstrom A and Frisen L (2014). “Increased Psychiatric Morbidity in Men With Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency.” Journal of Clinical Endocrinology & Metabolism 99(3): E554–E560. [DOI] [PubMed] [Google Scholar]
- Fox RA, Platz DL and Bentley KS (1995). “Maternal factors related to parenting practices, developmental expectations, and perceptions of child behavior problems.” J Genet Psychol 156(4): 431–441. [DOI] [PubMed] [Google Scholar]
- Gitau R, Adams D, Fisk NM and Glover V (2005). “Fetal plasma testosterone correlates positively with cortisol.” Arch Dis Child Fetal Neonatal Ed 90(2): F166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM and Jasuja R (2017). “A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications.” Endocr Rev 38(4): 302–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M, Saade GR, Hankins GD and Sathishkumar K (2016). “Elevated Testosterone Reduces Uterine Blood Flow, Spiral Artery Elongation, and Placental Oxygenation in Pregnant Rats.” Hypertension 67(3): 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K and Pfaff D (2014). “Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism.” Endocr Rev 35(6): 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi D, Bellini MJ, Acaz-Fonseca E, Panzica G and Garcia-Segura LM (2013). “Estradiol and Testosterone Regulate Arginine-Vasopressin Expression in SH-SY5Y Human Female Neuroblastoma Cells Through Estrogen Receptors-alpha and -beta.” Endocrinology 154(6): 2092–2100. [DOI] [PubMed] [Google Scholar]
- Gurpide E, Marks C, de Ziegler D, Berk PD and Brandes JM (1982). “Asymmetric release of estrone and estradiol derived from labeled precursors in perfused human placentas.” Am J Obstet Gynecol 144(5): 551–555. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K and Weitzman M (2008). “Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment.” Curr Opin Pediatr 20(2): 184–190. [DOI] [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston KJ, Golding J, Avon P Longitudinal Study of and T. Children Study (2002). “Testosterone during pregnancy and gender role behavior of preschool children: a longitudinal, population study.” Child Dev 73(6): 1678–1687. [DOI] [PubMed] [Google Scholar]
- Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP and Stener-Victorin E (2015). “Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring.” Proc Natl Acad Sci U S A 112(46): 14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M and Beyer C (2003). “Cellular strategies of estrogen-mediated neuroprotection during brain development.” Endocrine 21(1): 3–9. [DOI] [PubMed] [Google Scholar]
- Kaludjerovic J and Ward WE (2012). “The Interplay between Estrogen and Fetal Adrenal Cortex.” J Nutr Metab 2012: 837901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, Brook CG and Hines M (2006). “Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia.” Horm Behav 50(1): 148–153. [DOI] [PubMed] [Google Scholar]
- Korner LM, Pause BM, Meinlschmidt G, Tegethoff M, Frohlich S, Kozlowski P, Rivet N, Jamey C, Reix N, Kintz P, Raul JS and Heil M (2019). “Prenatal testosterone exposure is associated with delay of gratification and attention problems/overactive behavior in 3-year-old boys.” Psychoneuroendocrinology 104: 49–54. [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C and Gardner RM (2016). “Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden.” Molecular Psychiatry 21(10): 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C and Gardner RM (2017). “Maternal Polycystic Ovary Syndrome and Risk for Attention-Deficit/Hyperactivity Disorder in the Offspring.” Biol Psychiatry 82(9): 651–659. [DOI] [PubMed] [Google Scholar]
- Kuhl H (2005). “Pharmacology of estrogens and progestogens: influence of different routes of administration.” Climacteric 8 Suppl 1: 3–63. [DOI] [PubMed] [Google Scholar]
- Makieva S, Saunders PT and Norman JE (2014). “Androgens in pregnancy: roles in parturition.” Hum Reprod Update 20(4): 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manti M, Fornes R, Qi XJ, Folmerz E, Hirschberg AL, Barbosa TD, Maliqueo M, Benrick A and Stener-Victorin E (2018). “Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring.” Faseb Journal 32(8): 4158–4171. [DOI] [PubMed] [Google Scholar]
- Martel MM (2013). “Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders.” Psychol Bull 139(6): 1221–1259. [DOI] [PubMed] [Google Scholar]
- McCarthy MM (2016). “Sex differences in the developing brain as a source of inherent risk.” Dialogues in Clinical Neuroscience 18(4): 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Diplas AI, Chen J, Zhu C, Engel SM and Wolff MS (2012). “Polymorphisms in the maternal sex steroid pathway are associated with behavior problems in male offspring.” Psychiatr Genet 22(3): 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A and Sousa N (2018). “Maternal hormonal milieu influence on fetal brain development.” Brain Behav 8(2): e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ and Paley B (2009). “Psychiatric conditions associated with prenatal alcohol exposure.” Dev Disabil Res Rev 15(3): 225–234. [DOI] [PubMed] [Google Scholar]
- Oleary P, Boyne P, Flett P, Beilby J and James I (1991). “Longitudinal Assessment of Changes in Reproductive Hormones during Normal-Pregnancy.” Clinical Chemistry 37(5): 667–672. [PubMed] [Google Scholar]
- Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R and La Sala GB (2012). “Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study.” Clin Endocrinol (Oxf) 77(6): 898–904. [DOI] [PubMed] [Google Scholar]
- Qoubaitary A, Meriggiola C, Ng CM, Lumbreras L, Cerpolini S, Pelusi G, Christensen PD, Hull L, Swerdloff RS and Wang C (2006). “Pharmacokinetics of testosterone undecanoate injected alone or in combination with norethisterone enanthate in healthy men.” J Androl 27(6): 853–867. [DOI] [PubMed] [Google Scholar]
- Rotem RS, Chodick G, Davidovitch M, Hauser R, Coull BA and Weisskopf MG (2018). “Congenital Abnormalities of the Male Reproductive System and Risk of Autism Spectrum Disorders.” Am J Epidemiol 187(4): 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (1990). “No adjustments are needed for multiple comparisons.” Epidemiology 1(1): 43–46. [PubMed] [Google Scholar]
- Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VHH, Ridgway EC and Wierman ME (2011). “Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry.” Steroids 76(1–2): 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O’Connor TG and Glover V (2008). “Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming.” J Neuroendocrinol 20(4): 489–496. [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Chinnathambi V, Gao HJ, Hankins GDV and Yallampalli C (2011). “Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport.” Reproductive Biology and Endocrinology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang C and Swan SH (2014). “Phthalate exposure and reproductive hormone concentrations in pregnancy.” Reproduction 147(4): 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso HA, Idahl A, Lehtinen M, Surcel HM and Fortner RT (2016). “Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study.” BMC Pregnancy Childbirth 16(1): 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers KJ, Erli F, Raval P, Watson IA, Chen D and Srivastava DP (2015). “Rapid modulation of synaptogenesis and spinogenesis by 17beta-estradiol in primary cortical neurons.” Front Cell Neurosci 9: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS and Wang C (2008). “Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry.” Clin Chem 54(11): 1855–1863. [DOI] [PubMed] [Google Scholar]
- Siiteri PK and MacDonald PC (1966). “Placental estrogen biosynthesis during human pregnancy.” J Clin Endocrinol Metab 26(7): 751–761. [DOI] [PubMed] [Google Scholar]
- Sisk CL and Zehr JL (2005). “Pubertal hormones organize the adolescent brain and behavior.” Frontiers in Neuroendocrinology 26(3–4): 163–174. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Yamamoto R, Okuda H, Yamamoto K, Fukuhara S, Yoshioka I, Kiuchi H, Takao T, Miyagawa Y, Nishida M, Yamauchi-Takihara K, Moriyama T and Nonomura N (2012). “Low serum free testosterone level is associated with carotid intima-media thickness in middle-aged Japanese men.” Endocr J 59(9): 809–815. [DOI] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM and Buitelaar JK (2004). “Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: What is the best source of information to investigate the effects of fetal hormonal exposure?” Hormones and Behavior 46(5): 663–669. [DOI] [PubMed] [Google Scholar]
- Werling DM and Geschwind DH (2013). “Sex differences in autism spectrum disorders.” Curr Opin Neurol 26(2): 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H (1980). “A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity.” econometrica 48(4): 817–838. [Google Scholar]
- Windham GC, Lyall K, Anderson M and Kharrazi M (2016). “Autism Spectrum Disorder Risk in Relation to Maternal Mid-Pregnancy Serum Hormone and Protein Markers from Prenatal Screening in California.” J Autism Dev Disord 46(2): 478–488. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Zhang HF, Shou XJ, Li J, Jing WL, Zhou Y, Qian Y, Han SP, Zhang R and Han JS (2015). “Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring.” Physiology & Behavior 138: 13–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.