Abstract
Radiation therapy is an important and commonly used treatment modality for head and neck cancers. Osteoradionecrosis (ORN) is a potential debilitating complication of treatment, which most commonly affects the mandible. Management strategies are tailored to the severity of disease. Medical management including oral rinses, irrigations, antibiotics, and pharmacological treatments is viable for mild-to-moderate ORN. More severe disease is best addressed with a combination of medical management and surgical intervention aimed at aggressively removing devitalized tissue until bleeding bone is encountered and reconstructing the soft tissue and bone defect. Reconstruction with either regional vascularized flaps or vascularized osteocutaneous free flaps in case of larger full-thickness bone defects (greater than 6 cm) or anterior mandible (medial to mental foramen) is most appropriate. Maxillary ORN complications can present with a wide range of functional problems and facial disfigurement. Life-threatening and time-sensitive problems should be treated first, such as skull base bone coverage or correction of severe ectropion, to avoid blindness from exposure keratopathy. Then, less time-sensitive issues can be addressed next, such as nasal obstruction, velopharyngeal insufficiency, and chronic tearing. It may require a combination of specialists from different disciplines to address various issues that can arise from maxillary ORN.
Keywords: osteoradionecrosis, mandible reconstruction, midface reconstruction, maxillary reconstruction, fibula free flap, scapula free flap, nasal reconstruction, hyperbaric oxygen, PENTOCLO, pentoxifylline, tocopherol, clodronate
Radiation Therapy
Radiation therapy is an important and commonly utilized treatment modality for head and neck cancers. Radiation therapy is generally subdivided into external and internal (also called brachytherapy). Most head and neck cancers are treated with external beam radiation therapy using high energy photons generated by a linear accelerator. 1 Mechanistically, the process involves targeting ionizing radiation toward pathological tissue, which causes tissue damage. The ionizing radiation creates free radicals, which cause genetic degradation on a cellular level and ultimately result in a loss of cellular reproduction and tumor cell death. Normal cells are generally better able to withstand the effects of the ionizing radiation; however, even cells that survive may have impaired mechanisms for the production of collagen and regulatory enzymes. This ultimately results in a progressive loss of vascularity, cellularity, and tissue integrity. 2 3
Radiated tissue is often unable to revascularize spontaneously, leading to one of the well-known complications of osteoradionecrosis (ORN). One of the well-established risk factors for ORN is an increased radiation dose, usually doses of 65 to 80 Gy, which diminishes capillary density to only 20 to 40% of that of nonirradiated tissue. 4 There appears to be an increased risk of ORN when the radiation dose was ≥ 65 Gy. Side effects of radiation therapy are generally classified as acute or chronic in nature. Acute effects of radiation therapy include mucositis, thickened secretions, mucosal infection, pain, and sensory disruptions. Chronic effects typically include tissue fibrosis, salivary gland dysfunction, increased susceptibility to mucosal infection, neuropathic pain, sensory disorders, ORN, dental caries, and periodontal disease. 1 5
Osteoradionecrosis
Etiology
ORN is defined as exposed devitalized irradiated bone that fails to heal over a period of 3 to 6 months in the absence of local neoplastic disease. Radiographically, it is described by decreased bone density, lytic areas, cortical interruption, soft tissue thickening, and sometimes pathological fractures ( Fig. 1 ). Patients with ORN typically present with complaints of poorly controlled, persistent orofacial pain and chronic bone exposure, and go on to develop pathological fractures, nonunion with chronic infection, and/or sinonasal or orocutaneous fistulas. These pathologies may ultimately affect their swallowing, respiration, and speech function. 6 ORN of the facial skeleton is one of the most devastating complications of head and neck radiation therapy. Historically, it was thought to be related to radiation exposure, trauma, and infection. More recently, identified risk factors for the development of ORN include the presence of dental disease, the need for preirradiation dental surgery, poor oral hygiene, smoking and drinking habits, total radiation dosage (> 5,000 Gy), and the extent of original tumor size. 4 7
Fig. 1.
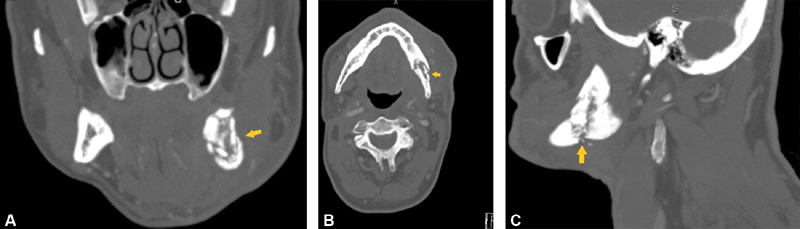
Radiographic images of left-sided mandibular osteoradionecrosis. Left mandibular angle pathological fracture ( yellow arrow ) can be seen with bony resorption and loss of cortical bone. ( A ) Coronal view. ( B ) Axial view. ( C ) Sagittal view.
In a systematic review by Teng and Futran, the prevalence of ORN was 7.4% in conventional radiation therapy, 5.1% in intensity-modulated radiotherapy, 5.3% in concurrent chemo-radiation therapy, and 5.3% in brachytherapy. 6 The mandible is more commonly affected by ORN than other parts of the facial skeleton due to differences in blood supply and anatomical structure. Mandibular ORN is encountered far more commonly than maxillary ORN and is thought to occur in two distinct patterns. Close to 60% are considered posttraumatic, whereas the other 40% are thought to be spontaneous in nature. 8 Spontaneous cases typically appear between 6 and 24 months after irradiation, with the incidence decreasing after 2.5 years. Trauma-related mandibular ORN shows a bimodal distribution, with a peak at 3 months and the other peak occurring at 5 years. 2 ORN of the maxilla and skull base has also been well described and are more commonly seen in combined therapies. Between 70 and 94% of ORN cases occur within the first 3 years after radiotherapy. 6
Postirradiation dental extractions are thought to be among the most common causes of ORN, with an estimated incidence between 2 and 18% following extractions. Studies have shown an increasing risk of ORN related to dental extractions in the first 4 to 5 years postradiation treatment. Hence, it is best to avoid extractions during this postradiation period as much as possible with all necessary extractions ideally being performed prior to the start of radiation therapy. Timing is also relatively important with respect to preirradiation dental extractions. Ideally, extractions can be performed at least 3 to 4 weeks prior to radiation therapy, and if timing constraints exist, at least 10 to 14 days should be allotted to allow recovery. The goal is to allow sufficient time for healing prior to the strain that occurs during radiation therapy. 9 10
Pathogenesis
The pathophysiology of ORN was first described by Marx in 1983, in which he proposed that radiation causes endarteritis and results in tissue hypoxia, hypocellularity, and hypovascularity. 11 It also reduces the proliferation of bone marrow, collagen, and periosteal and endothelial cells. Radiation-induced fibrosis theory has become a three-phase proposed mechanism to describe the molecular events leading to the clinical manifestations of the disease. The initial prefibrotic phase involves the presence of endothelial cells with an accompanied acute inflammatory response. The constitutive organized phase follows, which is defined by abnormal fibroblastic activity and a loss of extracellular matrix organization. The final late fibroatrophic phase involves remodeling of the tissues with the formation of fragile healed tissues at risk of reinjury. Histologically, in ORN, there is an obvious destruction of osteocytes and an absence of osteoblasts from bone margins. 12 13
Staging
Although there is currently no uniform staging system for classifying ORN, several have been proposed over the years. The original staging system proposed by Marx outline three stages to classify and organize disease manifestations and their response to hyperbaric oxygen (HBO). 11 14 Since 1983, multiple new staging systems have been proposed each with different guidelines and management strategies ( Table 1 ). 4 11 15 16 17 18
Table 1. Different staging systems of ORN.
| Study | Year | Stages | |
|---|---|---|---|
| Marx 11 14 | 1983 | Stage I | Exposed alveolar bone without pathological fracture, which responds to HBO therapy |
| Stage II | Not responsive to HBO therapy and requires sequestrectomy and saucerization | ||
| Stage III | Most severe and involves full-thickness bone damage or pathological fracture and requires resection and reconstruction with free tissue | ||
| Glanzmann and Grätz 16 | 1995 | Stage 1 | Bone exposure without signs of infection and persisting for at least 3 mo |
| Stage 2 | Bone exposure with signs of infection or sequester and without the signs of grades 3–5 | ||
| Stage 3 | Bone necrosis treated with mandibular resection with a satisfactory result | ||
| Stage 4 | Bone necrosis with persisting problems despite mandibular resection | ||
| Stage 5 | Death from ORN | ||
| Støre and Boysen 17 | 2000 | Stage 0 | Mucosal defect only |
| Stage 1 | Radiological evidence of necrotic bone with intact mucosa | ||
| Stage 2 | Positive radiological findings with denuded bone intraorally | ||
| Stage 3 | Clinically exposed radionecrotic bone, verified by imaging techniques, along with skin fistulae and infection | ||
| Schwartz and Kagan 15 | 2002 | Stage I | Minimal soft tissue ulceration and limited exposed cortical bone; patients are treated with conservative management |
| Stage II | Localized involvement of the mandibular cortex and underlying medullary bone | ||
| Stage III | Full-thickness involvement of the bone, including the inferior border; pathological fractures may be present | ||
| Notani et al 18 | 2003 | Stage I | ORN confined to the alveolar bone |
| Stage II | ORN limited to the alveolar bone and/or mandible above the level of the inferior alveolar canal | ||
| Stage III | ORN involving the mandible below the level of the inferior alveolar canal and/or skin fistula and/or pathological fracture | ||
Abbreviations: HBO, hyperbaric oxygen; ORN, osteoradionecrosis.
Prevention
Over recent years, our ability to identify risk factors for ORN and devise preventative strategies has improved. Current protocols are divided into three phases: before radiation exposure, during radiation exposure, and after radiation exposure. The first phase is focused on screening and initiation of preventative measures. During radiation therapy, the preventative measures are continued along with treatment and counseling for acute complications. In the postradiation phase, prevention is important along with treatment of chronic and late complications, and therefore close follow-up during this period is extremely important ( Table 2 ).
Table 2. Outlines preventative measures before, during, and after radiation therapy.
| Preradiation therapy |
| • Thorough assessment of dentition, periodontium, and oral hygiene (obtain radiographic information as needed) • Extraction of nonsalvageable teeth (allow 3–4 wk for wound healing) • Initiation of preventative measures (brushing teeth, topical fluoride application, oral rinses, trismus prevention exercises) • Nutritional consultation (optimization of nutritional status) |
| During radiation therapy |
| • Continue preventative measures (brushing teeth, topical fluoride application, oral rinses, trismus prevention exercises, avoiding denture use) • Appropriate follow-up with the dental team per institutional policy • Employ treatment measures as needed for mucositis, plaque removal, pain relief, oral dryness, and trismus |
| Postradiation therapy |
| • Close follow-up with the dental team • Continue preventative measures (brushing teeth, topical fluoride application, oral rinses, trismus prevention exercises, wait for 3 mo before denture use) • Ideally no extractions needed; however, if needed, it should be carried with appropriate measures taken |
Common treatment methods to combat radiation-related side effects include daily sprays or lozenges for mucositis, chlorhexidine mouth rinse for plaque removal, pain relief rinses (e.g., viscous lidocaine or sucralfate suspension), salivary substitutes (e.g., Biotene) for oral dryness, and trismus prevention with mouth opening exercises. Extractions postradiation therapy pose a significant risk for the development of ORN. Ideally, with pretreatment screenings and evaluations, dental extractions will not be needed; however, occasionally such circumstances may arise. If extractions are necessary, they should be performed with careful soft tissue handling and primary wound closure. Prophylactic high-dose broad-spectrum antibiotic coverage is important, ideally continued for at least 2 weeks. Preventative HBO therapy has also been proposed in high-risk patients. 19
Management of Osteoradionecrosis
Medical Management
Early stage ORN, which is described by small areas of exposed bone, is traditionally managed with conservative measures. These measures include thorough wound care involving oral rinses (e.g., chlorhexidine), saline irrigations, and a strong focus on oral hygiene. Acute exacerbations are managed with broad-spectrum antibiotics and appropriate analgesia. With conservative measures, complete resolution of ORN is estimated to occur in 8 to 33% of patients within 1 year. 4 18 It is important to understand the limits of medical management and utilize appropriate adjuncts or surgical options before additional worsening complications arise.
Hyperbaric Oxygen
HBO therapy has been described for the treatment of ORN since the 1960s; however, its use remains controversial. The purpose of HBO therapy is to increase the oxygen gradient and enhance diffusion of oxygen into hypoxic tissues. The increased oxygen content stimulates angiogenesis, collagen formation, and fibroblast proliferation. The increased oxygen gradient is also bactericidal and bacteriostatic, which is important in the setting of ORN as it is frequently complicated by chronic infection.
Historically, HBO therapy was considered an integral component of ORN prevention and treatment, but the paradigm has shifted in recent years. 2 14 Recently a randomized, placebo-controlled, double-blind study from France was conducted to assess the efficacy and safety of HBO for the treatment of mandibular ORN. The trial was ultimately terminated prematurely because of a failure to demonstrate any beneficial effect. Best practice guidelines from the otolaryngology literature in 2013 demonstrates there is no clear evidence to suggest that HBO therapy is effective in the prevention or treatment of ORN. 20 Prophylactic HBO following salvage surgery has also not been shown to decrease the rates of postoperative complications, length of hospital stays, or the need for additional surgical intervention. 6
In regard to the impact of prophylactic HBO prior to dental extraction, the data have been divided. Fritz et al in a systematic review in 2010 concluded that there was no reliable evidence to support or refute the efficacy of HBO in the prevention of postextraction ORN. 21 In 2011, Nabil and Samman found a 7% incidence of ORN after tooth extraction, and when extraction was performed along with HBO, the incidence was 4%. They concluded that prophylactic HBO may be effective in reducing the risk of ORN after postradiation dental extractions. 22 Ultimately, the utility and efficacy of HBO therapy appear to be controversial and limited at best, and other adjunctive treatment modalities should be considered.
Pentoxifylline/Tocopherol/Clodronate
Pharmacological treatments have been proposed to counteract the molecular mechanisms associated with ORN development. These molecular mechanisms include free radical formation, endothelial dysfunction, inflammation, microvascular thrombosis, fibrosis, and tissue necrosis. Several drugs have been proposed in the management of ORN including pentoxifylline, tocopherol, and clodronate, together referred to as PENTOCLO.
Pentoxifylline is a methylxanthine derivative that is thought to have beneficial effects in the setting of ORN by inducing vascular dilation and increased erythrocyte flexibility. This, in turn, improves blood viscosity and flow, increasing vascularity of affected tissues. Tocopherols are a vitamin E analog with antioxidant properties including inhibition of platelet aggregation, production of nitric oxide in the endothelium, and production of superoxide in immune cells. The thought is that tocopherols scavenge reactive oxygen species that are involved in the pathogenesis of ORN. Clodronate is a bisphosphonate that inhibits bone resorption by directly acting on osteoclast activity. It also acts directly on osteoblasts to increase the formation of bone and reduce fibroblast proliferation. However, some are hesitant to use clodronate as a single or combination therapy due to the mandibular ORN risk associated with bisphosphonate use. 23
The PENTOCLO combination as described by Delanian et al recommends initial treatment with antibiotics and corticosteroids for a month to control active infection, reduce inflammation, and allow for drug penetration. The treatment regimen consisted of pentoxifylline 400 mg twice daily plus 1,000 IU vitamin E daily and 1,600 mg of clodronate for 5 consecutive days per week. The clodronate was alternated with 20 mg prednisone and 1,000mg ciprofloxacin 2 days per week. Treatment duration was dependent on the healing course, with a median treatment time of 9 months. 24
A combination therapy with pentoxifylline and tocopherol has been reported to be effective in reducing progressive changes associated with ORN particularly in early stage disease. Breik et al showed a 45% response rate to the combination therapy, demonstrating improvement in wound healing. It may also play a role in the conservative management of patients with more advanced disease that refuse surgery or are nonoperative candidates. Combination therapy with PENTOCLO has also been reported to have beneficial effects; however, definitive evidence is limited for these pharmacological modalities and further analysis is needed. 25
Alternative Treatment Modalities
Other alternative treatment modalities have been proposed for the treatment of ORN with the limited body of literature available. Ultrasound therapy is an older treatment modality that was first described in 1992. It was proposed to induce local angiogenesis and revascularization. Current literature is very limited, and thus it remains an area of continued investigation. 26 Recent studies have investigated the use of low-power lasers associated with photodynamic therapy (PDT) as a treatment modality for ORN. It aims to promote disinfection in pathological areas by stimulating synthesis of collagen and fibroblasts. A recent retrospective study showed the combined use of low-power laser (Therapy XT–diode laser at 660 nm and 808 nm, DMC Group) with PDT led to remission of ORN and repair of mucosal damage. 27 However, studies evaluating this treatment modality are limited in the current literature, and its reproducibility remains to be seen.
Other studies have mentioned both ozone and platelet-rich fibrin as alternative therapies for ORN. Ozone is a molecule with three atoms of oxygen, which is thought to have multiple beneficial properties including antimicrobial, analgesic, anti-inflammatory, and stimulating the circulatory system. It has been shown in case reports to potentially possess tissue healing properties in ORN, but most authors state further investigation is needed. 28 Platelet-rich fibrin has been proposed to aid in the healing process. After patients undergo surgical debridement of necrotic bone to achieve healthy bone margins, platelet-rich fibrin is placed in the bone defect. The platelet-rich fibrin is obtained from the patient's own blood and collected by centrifugation. 29 Although there are many alternative therapies that have been proposed in the literature, evidence of these therapies remains limited, and further investigation is needed prior to widespread use. Furthermore, it is likely these treatment modalities will have limited efficacy in severe ORN cases.
Surgical Management of Mandibular Osteoradionecrosis
Surgical interventions have remained a cornerstone of mandibular ORN treatment throughout the years. Surgical treatment is typically reserved for patients who have failed the aforementioned established medical therapy or if they have presented with severe complications of ORN. These complications include pathological fractures of the mandible and large soft tissue defects with exposed bone or hardware. The exact surgical treatment will depend on a multitude of factors and must be individualized for each patient. These factors include the amount of mandibular bone exposed, depth of the bony defect (partial vs. full-thickness mandibular defect), location of the bone exposure along the mandible, quality of surrounding soft tissue that will be used for reconstruction, and the presence of concurrent facial skin or intraoral mucosal defects that will also need to be reconstructed. There is currently no generalized consensus treatment algorithm for ORN. We present our treatment algorithm used by senior authors (T. L. and Y. D.). ( Fig. 2 )
Fig. 2.
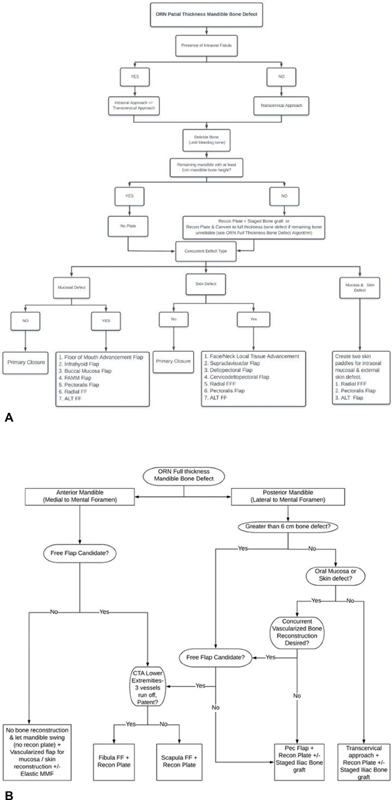
Mandible ORN surgical treatment algorithm. ( A ) Partial-thickness mandibular bone defect algorithm. ( B ) Full-thickness mandibular bone defect algorithm. ALT, anterolateral thigh; CTA, computed tomography angiography; FAMM, facial artery musculomucosal; FF, free flap; FFF, forearm free flap; MMF, maxillomandibular fixation; ORN, osteoradionecrosis.
The overall goals of surgical treatment include the following:
Aggressively debride mandibular bone affected by osteomyelitis until bleeding bone is encountered.
Cover exposed mandible as it may act as a nidus for intraoral microbial flora and can lead to ongoing chronic infection.
Similarly, any exposed hardware must be replaced (if appropriate) and covered with robust vascularized tissue to remove biofilm formation on the surface of the exposed hardware, which may lead to an ongoing source of infection that may foster antimicrobial resistance to even intravenous antibiotic therapy.
In the setting of a postradiation pathological fracture, provide rigid fixation to achieve bony union.
In the setting of segmental bone defect, restore mandible continuity and premorbid occlusion with rigid fixation.
Reconstruct concurrent intraoral mucosal defect and/or external skin defect with vascularized tissue. In the setting of large segmental mandibulectomy bone defects with concurrent intraoral and/or external skin defects, a vascularized osteocutaneous free flap may be needed for reconstruction.
Remove compromised dentition at the time of the definitive treatment surgery. If there are signs of dental infection in close proximity (∼1 cm) to the ORN site, one should consider extraction of the involved tooth and aggressively debride the alveolar bone until bleeding bone edge is encountered as it can be a source of future infection.
Proceed with dental rehabilitation once ORN has been successfully treated.
Salivary leakage at the site of ORN must be avoided at all cost as it may lead to recurrent osteomyelitis or even a free flap loss. As such, one must be aware of the limitation of regional local flaps and potentially compromised flap vascularity to tolerate salivary exposure as the wound heals. When designing a local tissue advancement flap in the radiated area, the advancement flap should be raised relatively thick (if possible, 2–4 mm thick in either submuscular or submucosal plane while protecting the facial nerve innervation) to further optimize distal tip flap survival. When raising an intraoral, regional flap overlying the mandible, the flap can be raised superficial to the periosteum of the bone to tolerate salivary exposure if it were to occur. Bone with periosteum generally tends to heal better than bone without any periosteum attached as vascularity to the bone itself has been decreased due to the lack of periosteum. If there is severely compromised intraoral lining, it may be beneficial to consider an external approach with limited intraoral incision to minimize the tissue burden on the intraoral incision and avoid a salivary leak. With the external approach to the mandible, dissection along the bone can be performed in the subperiosteal tissue plane to provide further vascular support to the intraoral lining.
Planning of surgical incisions is extremely important when working in a radiated field. Regional flap vascularity should be optimized by designing wider tissue flaps to allow for increased vascularity to the distal tip of the flap. Distal tips necrosis of a radiated regional flap can be relatively common, and this may lead to a host of complications including salivary fistulas. One may use distal tip bleeding as a sign of how robust the tissue will be during the healing process. Aggressive soft tissue debridement is extremely important during the initial interventions, particularly in the setting of chronic infections. One must avoid overestimating the area of reliable soft tissue and, instead, air toward being more aggressive in soft tissue debridement to ensure that any regional flap will be in contact with healthy soft tissue. Additionally, if there are any signs of infection surrounding implanted hardware, it is important to completely remove all compromised tissue while utilizing bleeding tissue edges as a healthy margin. Lastly, one may consider the use of Dermabond (Ethicon) or a fibrin glue intraorally to create a watertight seal along the mucosal incision line as an added layer of protection.
Small concurrent oral mucosal defects overlying the exposed mandible can be reconstructed with vascularized local flaps, such as the facial artery musculomucosal (FAMM) flap, infrahyoid flap, buccal mucosal flap, or floor of mouth advancement flaps, among others. 30 31 32 33 Due to previous radiation history, one should assume poor intraoral closure healing potential and be cautious of placing a significant amount of hardware when an osseous free flap is not used or when insufficient soft tissue is present.
With respect to hardware placement, it is typically best to avoid hardware placement whenever possible as the hardware has a potential for both exposure and biofilm formation. With that said, in the presence of a pathological fracture or a vascularized free flap placement, hardware placement is unavoidable and even necessary. To avoid the feared complication of hardware eroding through radiated skin externally, placement of a well-vascularized soft tissue flap with tissue bulk directly overlying the bone and hardware is recommended. The radiated skin will often have severe fat atrophy, which results in extremely thin skin and becomes poorly tolerant of a reconstruction plate. If there is regional muscle or relatively thick soft tissue present, the soft tissue/muscle can be used to cover the bone and hardware. If there is no reliable regional muscle or soft tissue present, a de-epithelialized skin flap from an osteocutaneous free flap may be used to cover the bone and hardware while the skin paddle can be buried under the native external skin.
A pathological fracture of the mandible after radiation therapy poses a special challenge as these fractures may be less inclined to achieve bony union than nonirradiated bone. It appears that radiation dose that is ≥ 60 Gy is associated with a higher risk of pathological fracture. The most commonly involved sites include the mandibular body and angle. Kim et al reported successful treatment of postradiation pathological mandible fractures with open reduction and internal fixation (ORIF) in patients presenting with severe bone deviation, malocclusion, or relatively small mandibular bone height. They noted delayed bone healing in all ORIF patients, with bone healing ranging from 5 to 19 months. Patients who underwent closed reduction had significantly delayed bone union occurring after 5 years of observation. In their case series, two patients went on to have ORIF performed with miniplates and concurrent nonvascularized iliac bone grafting. These two patients had suffered plate fractures but went on to achieve bony union. The authors attributed plate fractures to small mandibular bone height resulting in a load-bearing situation (instead of the load sharing) and delayed bone healing. One patient was treated with a reconstruction plate without bone grafting and went on to achieve bony union without any plate fracture. Assuming one can achieve reliable, watertight seal intraorally, they recommend concurrent nonvascularized iliac bone grafting at the time of ORIF to increase bone healing potential. They additionally noted that reconstructive plates should be used whenever possible instead of miniplates due to the risk of plate fracture, particularly if limited mandibular bone height exists. 34
Although vascularized bone grafts are regarded as superior in the setting of ORN, nonvascularized bone grafts may also play a role when there is adequate soft tissue coverage and reliable mucosal closure to avoid a salivary leak. Nonvascularized bone grafts are generally restricted to smaller mandibular defects, less than 5 to 6 cm in size, and defects of the posterior mandible (lateral/posterior to mental foramen). These grafts are less reliable due to uneven blood supply, smaller bone stock, and limited resistance to infection in the setting of a salivary leak. This can be disadvantageous if bone stock is needed for stabilization or dental implant placement. 35 In some cases, a bulky muscular flap, such as pectoralis major flap, can be used to wrap the reconstruction plate especially if one is planning for a concurrent or a staged nonvascularized bone graft placement. Myocutaneous pectoralis flap with a deepithelialized skin paddle can be used in this setting to minimize plate exposure. Once the patient has achieved a watertight seal without a fistula, one may consider staged nonvascularized bone grafting using an external approach. A salivary leak on top of the nonvascularized bone graft will likely result in near-total graft loss and a problematic infection.
One must be cautious about reconstruction plate placement along the anterior mandible (medial to the mental foramen) with a full-thickness bony defect without any form of bone grafting as this will likely lead to delayed plate exposure. Generally, the posterior mandible (lateral/posterior to the mental foramen) can better tolerate plate placement without bony reconstruction particularly if there is sufficient soft tissue bulk surrounding the plate.
In the setting of severe ORN, vascularized osseous or osteocutaneous free flaps may be necessary. These instances include those with large segmental defects (> 6 cm) or full-thickness bony defects along the anterior mandible (between the mental foramen) with or without concurrent intraoral or external skin defects. Common free flaps used for mandible reconstruction include fibula, scapula, iliac crest, and radial bone. Fibula free flap is used most widely due to relative ease with dental implant placement and favorable bone length ( Fig. 3 ). Fibula free flap can provide up to 22 cm of bone length, which essentially allows for the reconstruction of near-total mandibular defect. 36 Concurrent dental implantation has been described extensively in the literature and allows concurrent dental rehabilitation. However, if the patient lacks adequate fibula due to vasculopathy, scapula free flap is an alternative option for mandibular reconstruction, although its bone length is typically limited (8–12 cm). Although not used as widely, using iliac crest and radial forearm free flaps have also been well established for mandible reconstruction in the literature. Iliac crest free flap harvest in an overweight patient can be a challenge, and the flap may end up with excess soft tissue bulk that may be hard to inset. Radial forearm free flaps are typically limited in terms of the amount of bone length and height that can be obtained. Nonetheless, iliac crest and radial forearm free flaps may be used successfully in select patients and depending on patient anatomy and surgeon preference. Our treatment algorithm is outlined below ( Table 3 ).
Fig. 3.
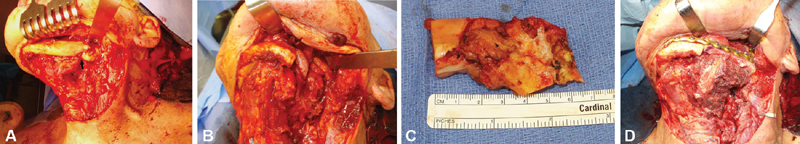
( A ) The patient in Fig. 1 had undergone radiation therapy for mandibular squamous cell carcinoma and subsequently developed left mandibular osteoradionecrosis. A pathological fracture can be seen upon subperiosteal dissection of the mandible bone ( arrow ). ( B ) A 7-cm segmental mandibulectomy was performed spanning from the midbody to the midramus with resection of unreliable intraoral mucosa as well as external skin that had gotten infected at the site of the pathologic fracture. ( C ) An approximately 7-cm excised mandibular segment. ( D ) An osteocutaneous fibula free flap with a reconstruction plate was used for the reconstruction. Portion of the skin paddle was used for the intraoral mucosal defect reconstruction, and the rest of the skin flap was deepithelialized and buried on top of the reconstruction plate and bone to provide soft tissue coverage. The external skin defect was closed with neck local tissue advancement flap.
Table 3. Summary of mandible ORN treatment recommendations by authors.
| Medical management |
| • Oral rinses (e.g., chlorhexidine) and saline irrigations • Antibiotic therapy (broad spectrum) for acute infections • Appropriate analgesia • Steroids may play a role in acute inflammatory response |
| Hyperbaric oxygen therapy |
| • No clear evidence of utility in prevention or treatment; however, side effect profile is relatively low if used in select settings |
| PENTOCLO |
| • No general consensus; preliminary studies appear promising, but further investigation is needed • Published regimen: 400 mg pentoxifylline twice a day, 1,000 IU vitamin E, and 1,600 mg clodronate for 5 consecutive days alternating with 20 mg prednisone and 1,000 mg ciprofloxacin 2 d per week • Treatment duration depending on clinical improvement |
| Surgical management |
| • Mild-to-moderate disease - Aggressive bony debridement and vascularized LTA options include buccal mucosal flap, floor of mouth advancement flap, FAMM flap, and infrahyoid flap • Severe disease: failed conservative therapy or previous debridement with LTA - Avoid using nonvascularized bone graft especially if mucosal closure is unreliable - Perform aggressive bone resection including segmental mandibulectomy until healthy bleeding bone edge is encountered - Provide adequate rigid fixation with hardware and provide adequate soft tissue coverage on top of the bone and hardware to prevent exposure - If the bone defect is relatively small (< 6 cm) and posterior (posterior to mental foramen), one could consider a vascularized pedicled flap (such as pectoralis flap) with reconstruction plate placement with staged nonvascularized bone graft (iliac bone graft once the oral lining has sealed up). However, reconstruction plates in this setting may cause a delayed complication of either intraoral or external exposure. Wrapping the pectoralis muscle around the plate may minimize the risk of plate exposure. - If the bone defect is relatively large (> 6 cm) and/or involves anterior mandible (anterior to mental foramen), typically a vascularized bone flap will be necessary to span the bone defect while rigid fixation is performed with a reconstruction plate. Osseous or osteocutaneous fibula or scapula free flap can be used. Skin from the free flap can be used to reconstruct intraoral and/or concurrent external skin defect, whereas deepithelialized portion of the skin flaps can be used for coverage for hardware and bone. |
Abbreviations: FAMM, facial artery musculomucosal; LTA, local tissue advancement; ORN, osteoradionecrosis; PENTOCLO, pentoxifylline/tocopherol (vitamin E)/clodronate.
Surgical Management of Maxillary Osteoradionecrosis
Maxillary ORN is relatively rare when compared with mandibular ORN. Cordeiro et al and Brown et al have created classification schemes for describing various maxillectomy defects often resulting from cancer resection defects. 37 38 Depending on the location and size of the defect, there is a wide range of reconstruction options available for the maxillary reconstruction addressing facial trauma or cancer defects. 39 Maxillary ORN complications can be diverse, and wound healing challenges during regional or microvascular free flap reconstruction present as a formidable challenge to even the most experienced free flap reconstruction specialist.
Postradiation-related complications of the maxilla can be diverse and can present with challenging wound healing issues as well as irreversible scar contracture issues that can significantly distort normal skin and cartilage and result in severely compromised function and facial disfigurement. The multitude of functional problems can involve the orbit, sinus, nasal airway, skull base, and intraoral region. Depending on the location involved, these patients may present with a combination of problems that may require a combination of different specialists (skull base specialist, rhinologist, oculoplastic surgeon, rhinoplasty specialist, microvascular surgeon).
When a maxillary ORN patient presents with a combination of problems, it is important to prioritize what needs to be addressed first and stage future surgeries to address lesser important issues at a later point. For example, if a patient presents with a sinonasal fistula at the site of Weber Ferguson incision after getting radiated to the ipsilateral maxilla, such a patient may present with a host of problems including (1) sinonasal fistula, (2) localized sinusitis, (3) nasal obstruction as a result of severe contracture of the nasal tip toward the side of the radiation, (4) osteomyelitis along the skull base or orbital bone if exposed, (5) diplopia if there is an issue with the orbital floor reconstruction (infected or exposed orbital floor hardware), (6) exposure keratopathy from severe ectropion, resulting from lower eyelid scar contracture, (7) chronic tearing due to loss of lacrimal duct, and (8) palatal defect resulting in salivary leak and velopharyngeal insufficiency (VPI).
In general, first priority should be given to addressing life-threatening or time-sensitive issues such as surgical treatment of the skull base osteomyelitis by debriding the dead bone and covering the exposed bone with a vascularized tissue. Preserving vision should receive a special priority by surgically treating significant exposure keratopathy with lower eyelid reconstruction and correcting the severe ectropion. Once life-threatening and time-sensitive problems are addressed first, the next set of surgeries will focus on improving “lesser” important functional problems such as performing dacryocystorhinostomy to address chronic tearing or addressing the nasal obstruction and disfigurement caused by severe vestibular stenosis and twisting of the nasal tip.
Postradiation-related complications may not appear for 3 to 6 months or up to several years after the completion of radiation therapy. During the first 3 to 6 months after radiation therapy, one must be cautious of raising skin flaps or making incisions in the radiated field, as the skin flaps may poorly tolerate these maneuvers and may result in skin flap failure. Seniors authors generally delay operating in a radiated field for at least 6 months postradiation to minimize the risk of poor wound healing and distal tip skin flap failure. The precise surgical plan for these patients should be guided by both the anatomical/cosmetic and functional deficits that may exist. Additionally, it is important to medically optimize these patients prior to undertaking surgical interventions. This includes restoring normal thyroid function and correcting nutritional deficits that may exist.
Some of the common postradiation-related issues that are encountered include oral cutaneous or sinonasal cutaneous fistulas resulting in adjacent bony exposure and unnatural communication along the orbital bone, anterior skull base, or maxillary bone. The following complications are also relatively common: (1) VPI resulting from a palatal defect in a situation where a flap placed intraorally failed to heal with an air and watertight seal along the entire palate, (2) nasal obstruction and VPI with a concurrent large septal perforation and palatal defect, (3) nasal obstruction from severe twisting of the nasal tip or total collapse of the nasal cartilage, or nasal vestibular scarring from asymmetric radiation effect of treating only one side of the face (ipsilateral to the tumor side), (4) massive nasal cutaneous fistula resulting from postradiation full-thickness tissue breakdown along the Weber Fergusson incision/external lateral rhinotomy incision, and (5) orbital or skull base bony exposure.
Management of radiation-related complications is a challenging process. The treatment of oronasal fistulas and VPI is one such example that depends on a multitude of factors including palatal defect size and severity of the functional deficit. Depending on the reliability of the local tissue a regional flap, such as a FAMM flap, may be a consideration. Alternatively, one may choose to follow cleft palate reconstruction principles with the understanding that radiated mucosa may poorly tolerate extensive dissection. Regardless of the reconstructive option chosen, in general, it is best to avoid relying heavily on radiated, local skin flaps and raise thicker flaps to optimize vascularity as the radiated tissue can be notoriously unreliable. It is typically safer to recruit additional nonirradiated vascularized tissue for the reconstruction. In the case of a palatal fistula, another treatment option would be to consider a customized obturator, which can obliterate the fistula with the added benefit of concurrent dental rehabilitation.
Complications related to nasal obstruction, vestibular stenosis, and cosmetic nasal deformities secondary to radiation side effects can be extremely challenging to address for even the most experienced rhinoplasty surgeons. Often times, achieving perfect nasal symmetry can be extremely challenging as the radiated side will behave differently than the nonirradiated side, and the radiated side will have severely compromised nasal skin envelope as well as nasal cartilage atrophy and loss of structural support ( Fig. 4 ). As such, proper patient counseling and management of their expectations are important. Surgically, attempts should be made to restore lost nasal cartilage structural support with placement of large spanning lateral strut and columellar strut grafts. To address severely contracted nasal skin flap, carefully planned local tissue advancement skin flap may be needed to provide sufficient skin envelope to cover the autogenous cartilage grafts on the radiated skin flap side. In some cases, a melolabial fold flap, a paramedian forehead flap (PMFF), or even a radial forearm free flap may be required depending on the size of the nasal skin defect and concurrent involvement of the upper lip or medial cheek skin defect. Lastly, radiated nasal skin flap may not tolerate significant separation from the underlying structure, as often is done in an open rhinoplasty approach, and may require a creative incision approach to optimize skin flap vascularity. It may take a multistage surgical approach to achieve the desired result.
Fig. 4.
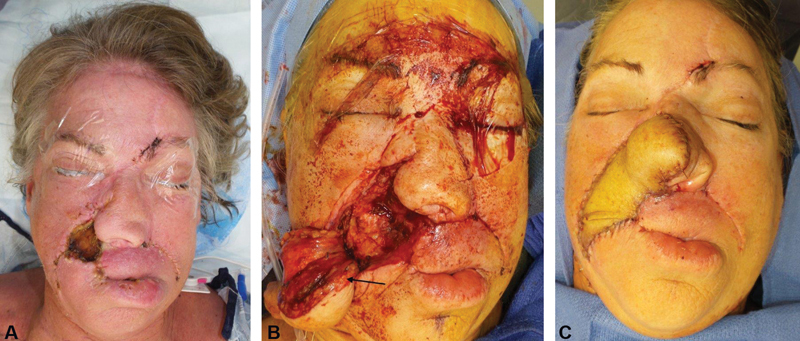
( A ) A patient with a right-sided maxillary squamous cell carcinoma underwent medial maxillectomy and partial rhinectomy of the entire right ala and right nasal wall. During the initial resection, the patient underwent rib cartilage grafting and melolabial flap surgery to address the partial rhinectomy defect. However, after postoperative radiation therapy, the patient developed osteoradionecrosis, which was likely related to an odontogenic infection arising from the ipsilateral maxillary canine and incisor. This led to extensive maxillary bone osteomyelitis and a cutaneous fistula formation with a communication into the nasal cavity as well as the oral cavity through a defect along the upper lip region. The severe twisting of the nasal tip is typical of unilateral radiation therapy. Attempts at using medial cheek advancement and supraclavicular skin flap led to a persistent fistula formation and ongoing infection. ( B ) The patient eventually required a radial forearm free flap ( arrow ) to provide sufficient soft tissue bulk to cover the maxillary bone and to provide a large skin envelope to reconstruct the nasal ala and upper lip defect. ( C ) The nasal tip required extensive rib cartilage grafting with large lateral strut grafts and a columellar strut graft to correct the severe nasal tip twisting. The patient will undergo staged vestibular stenosis repair of the right side to connect to the right nasal cavity at a later time once the radial forearm free flap has had a chance to atrophy and heal for at least 6 months.
Another radiation-related nasal deformity we encounter relatively commonly is associated with the use of an external lateral rhinotomy incision or Weber Ferguson approach. In the setting of radiation, surgeons should be aware of the poor wound healing capability of these incisions and the potential for large nasocutaneous fistulas to occur along the incision after the radiation therapy in a delayed fashion. Therefore, it is the senior authors' opinion that lateral rhinotomy or Weber Ferguson incisions should be avoided whenever possible if postoperative radiation is required. Instead, a midfacial degloving approach can be used for access to the entire midface with less likelihood of fistula formation along the incision. Once a nasal fistula has developed along the previous lateral rhinotomy incision, one should consider reconstruction from both a cosmetic standpoint and a functional one. The PMFF can be a versatile option for the reconstruction of nasocutaneous defects. To properly seal off the fistula, the PMFF should be raised with an underlying pericranial flap attached at the base to increase the flap thickness to provide an additional layer of vascularity ( Fig. 5 ). If the PMFF is inadequate or too small for the reconstruction of the defect, utilizing a radial forearm free flap may provide an ideal reconstruction option as it provides relatively thin, nonirradiated, vascularized donor tissue.
Fig. 5.
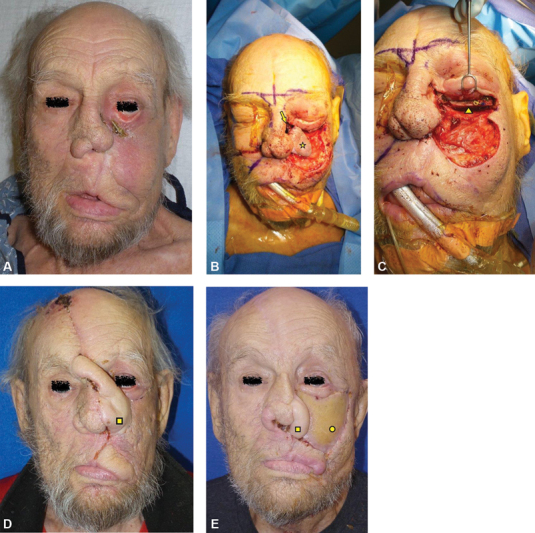
( A ) A patient initially presented with an advanced maxillary squamous cell carcinoma that required left maxillectomy including the orbital floor and unilateral palate resection. The patient had the resection performed with a Weber Ferguson incision. At that time, the patient had a scapula tip free flap for the palate and anterior maxilla reconstruction, and the orbital floor was reconstructed with an orbital floor implant. At 6 months postradiation, the patient presented with a large nasocutaneous fistula at the site of Weber Ferguson incision that eventually enlarged further and exposed the orbital floor implant. Nasal tip is severely twisted as typically seen with unilateral radiation therapy. The previously placed scapula flap along the palate and anterior maxilla was in good condition and uninvolved. ( B ) To address the nasocutaneous fistula ( arrow ) and severe ectropion resulting from radiation-induced skin contracture, a medial cheek skin flap ( star ) was raised thick and pedicled medially to be used as the inner nasal lining. ( C ) The patient underwent an orbital floor implant removal, and an iliac bone graft was used to recreate the orbital floor ( triangle ). A paramedian forehead flap (square) was used to provide a second layer closure to seal off the nasocutaneous fistula, while also reconstructing the missing left nasal sidewall and ala. ( D ) The fistula had sealed off, and the patient had undergone paramedian forehead flap division surgery around 6 weeks later. After the division surgery, the patient unfortunately had multiple medical issues including sudden hypothyroidism, and the patient presented with a recurrence of the fistula at the same site along the previous lateral rhinotomy incision approximately 3 months after the division. The paramedian forehead flap did survive and remained as the left nasal ala. ( E ) Once his thyroid function had returned to normal, the patient underwent a radial forearm free flap reconstruction, which was used to provide the inner nasal lining and the medial cheek and lower eyelid skin to successfully seal off the nasal fistula without recurrence. Arrow, fistula; star, cheek advancement flap for nasal lining; triangle, Iliac bone graft for orbital floor reconstruction; square, paramedian forehead flap; circle. radial forearm free flap.
During maxillectomy, orbital exenteration is performed if there is gross cancer invasion of the orbital content. Postoperatively, the orbit will be covered with radiation treatment in these malignant cancer patients. The reconstruction of the orbit should be able to tolerate a full dose of radiation therapy, and the underlying orbital bone and anterior skull base bone should remain covered under a vascularized flap. In these situations, we recommend placing a large musculocutaneous free flap in the orbital socket at the time of the initial resection with the expectation that radiation therapy will result in significant flap volume loss. Despite appropriate preventative precautions, orbital exenteration poses a particular reconstructive challenge as these patients can develop a fistula to the sinus or the anterior skull base. To seal off the fistula, a secondary free flap may be needed for additional volume. Alternatively, local regional flaps may provide adequate reconstructive options in situations when a free flap is deemed too big for the size of the defect. We have had success reconstructing such orbital fistula postradiation using a combination of pericranial flap and a temporalis muscle flap. The pericranial flap is used to line the anterior skull base and orbital roof once the devitalized bone has been drilled away. The temporalis muscle flap is then raised and tunneled into the orbital socket by making a large bony opening immediately posterior to the lateral orbital rim and removing the lateral orbital wall. One may also consider creating an osteoplastic bone flap involving the lateral orbital rim to improve exposure while drilling along the lateral orbital wall, although that is not always necessary. Any skin defects that arise can be closed with either local tissue advancement or skin grafting depending on local factors and the size of the skin defect ( Fig. 6 ).
Fig. 6.
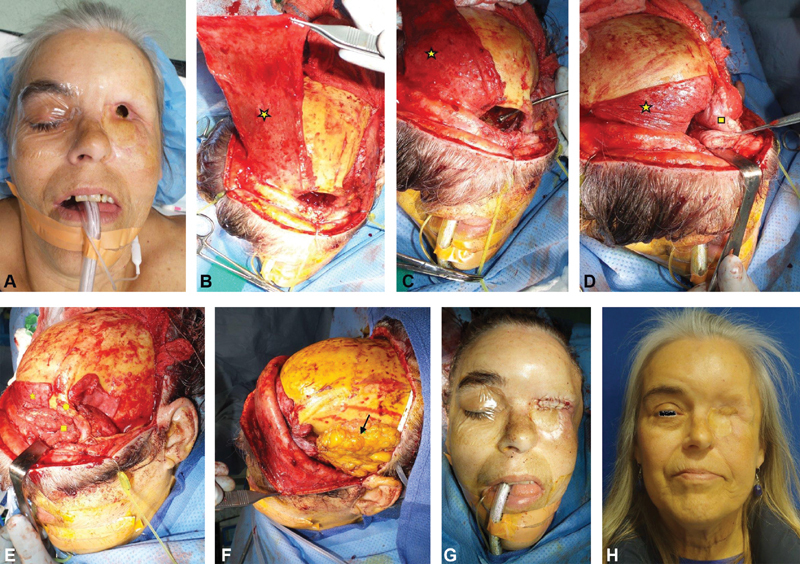
( A ) A patient initially presented with an aggressive, mucoepidermoid carcinoma of the lacrimal duct with gross skin and ipsilateral eye involvement. The patient underwent medial maxillectomy with orbital exenteration. At that time, osteocutaneous radial forearm free flap was used to restore the resected medial buttress and to provide skin coverage along the orbit and medial cheek. Four years after completion of postoperative radiation, the patient presented with a fistula in the orbital region. The fistula had communication to the sinus and the orbital roof. (B) The patient underwent a bicoronal incision approach to expose the orbital roof. The orbital roof and anterior skull base were gently drilled down until bleeding bone was encountered. A large pericranial flap was developed based on the contralateral blood supply and draped along the exposed orbital roof and anterior skull base bone. ( C ) Temporalis muscle flap was tunneled into the orbit by creating a large opening immediately behind the lateral orbital rim. A suction can be seen traversing the large bone opening. ( D,E ) The temporalis muscle was placed on top of the pericranial flap and was used to obliterate the dead space. ( F ) To address the temporal hallowing, composite abdominal fat graft was placed along the temporalis flap donor site to correct the volume defect. ( G ) Small remaining skin defect was addressed by skin grafting on top of the underlying pericranial/temporalis muscle flap. ( H ) The patient healed without fistula recurrence and shows no significant temporal hallowing. Star, pericranial flap; square, temporalis flap; arrow, abdominal fat graft; circle, lateral orbital rim.
The exact type of flap reconstruction required for maxilla depends largely on the amount of full-thickness bone or soft tissue flap that will need to be reconstructed. Coskunfirat et al published a case series of microvascular reconstructive options for maxillary defects. They utilized a combination of flap options including anterolateral thigh, radial forearm, rectus femoris musculocutaneous, and supracondylar chimeric flaps. They concluded the anterolateral thigh was the most versatile option for obliterating large dead space and for soft tissue defect reconstruction without needing bone reconstruction. 40 However, if the anterior arch of the maxillary bone along the hard palate is involved with a full-thickness bone defect (between the canines) and if bony reconstruction is desired to maintain proper skeletal support to prevent irreversible midfacial skin contracture that can occur with radiation or for dental rehabilitation, the scapular free flap may be an ideal option as reported by Miles and Gilbert. 41 Contrary to common belief, scapular free flaps can tolerate osteotomies by maintaining the periosteal attachments and also allows dental implant placement depending on the bone thickness. Similarly, a fibular free flap has also been utilized for the reconstruction of the bony maxilla. The fibula provides several advantages including a long vascular pedicle, which may be preferable in patients with depleted cervical donor vessels for optimal vessel reach, and easier dental implantation due to its thicker bone when compared with a scapula flap. 42
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Yeh S A. Radiotherapy for head and neck cancer. Semin Plast Surg. 2010;24(02):127–136. doi: 10.1055/s-0030-1255330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maier A, Gaggl A, Klemen H. Review of severe osteoradionecrosis treated by surgery alone or surgery with postoperative hyperbaric oxygenation. Br J Oral Maxillofac Surg. 2000;38(03):173–176. doi: 10.1054/bjom.1999.0285. [DOI] [PubMed] [Google Scholar]
- 3.Epstein J B, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23(05):389–398. doi: 10.1002/hed.1049. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson A S, Buchbinder D, Hu K, Urken M L. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46(11):795–801. doi: 10.1016/j.oraloncology.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Sroussi H Y, Epstein J B, Bensadoun R J. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6(12):2918–2931. doi: 10.1002/cam4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng M S, Futran N D. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13(04):217–221. doi: 10.1097/01.moo.0000170527.59017.ff. [DOI] [PubMed] [Google Scholar]
- 7.Nabil S, Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(01):54–69. doi: 10.1016/j.tripleo.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 8.Goyal S, Mohanti B K. Bilateral mandibular fracture related to osteoradionecrosis. Indian J Dent. 2015;6(02):107–109. doi: 10.4103/0975-962X.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T H, Liu C J, Chao T F, Chen T J, Hu Y W. Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: a national-based cohort study. Head Neck. 2017;39(07):1313–1321. doi: 10.1002/hed.24761. [DOI] [PubMed] [Google Scholar]
- 10.Koga D H, Salvajoli J V, Alves F A. Dental extractions and radiotherapy in head and neck oncology: review of the literature. Oral Dis. 2008;14(01):40–44. doi: 10.1111/j.1601-0825.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 11.Marx R E. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(05):283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 12.Westermark A, Sindet-Pedersen S, Jensen J. Osteoradionecrosis, pathogenesis, treatment and prevention. Tandlaegebladet. 1990;94(16):669–673. [PubMed] [Google Scholar]
- 13.Bras J, de Jonge H K, van Merkesteyn J P. Osteoradionecrosis of the mandible: pathogenesis. Am J Otolaryngol. 1990;11(04):244–250. doi: 10.1016/0196-0709(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 14.Marx R E. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41(06):351–357. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz H C, Kagan A R. Osteoradionecrosis of the mandible: scientific basis for clinical staging. Am J Clin Oncol. 2002;25(02):168–171. doi: 10.1097/00000421-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Glanzmann C, Grätz K W. Radionecrosis of the mandibula: a retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36(02):94–100. doi: 10.1016/0167-8140(95)01583-3. [DOI] [PubMed] [Google Scholar]
- 17.Støre G, Boysen M. Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin Otolaryngol Allied Sci. 2000;25(05):378–384. doi: 10.1046/j.1365-2273.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Notani K, Yamazaki Y, Kitada H. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck. 2003;25(03):181–186. doi: 10.1002/hed.10171. [DOI] [PubMed] [Google Scholar]
- 19.Jansma J, Vissink A, Bouma J, Vermey A, Panders A K, Gravenmade E J. A survey of prevention and treatment regimens for oral sequelae resulting from head and neck radiotherapy used in Dutch radiotherapy institutes. Int J Radiat Oncol Biol Phys. 1992;24(02):359–367. doi: 10.1016/0360-3016(92)90692-b. [DOI] [PubMed] [Google Scholar]
- 20.Lubek J E, Hancock M K, Strome S E. What is the value of hyperbaric oxygen therapy in management of osteoradionecrosis of the head and neck? Laryngoscope. 2013;123(03):555–556. doi: 10.1002/lary.23496. [DOI] [PubMed] [Google Scholar]
- 21.Fritz G W, Gunsolley J C, Abubaker O, Laskin D M. Efficacy of pre- and postirradiation hyperbaric oxygen therapy in the prevention of postextraction osteoradionecrosis: a systematic review. J Oral Maxillofac Surg. 2010;68(11):2653–2660. doi: 10.1016/j.joms.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Nabil S, Samman N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int J Oral Maxillofac Surg. 2011;40(03):229–243. doi: 10.1016/j.ijom.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Rivero J A, Shamji O, Kolokythas A. Osteoradionecrosis: a review of pathophysiology, prevention and pharmacologic management using pentoxifylline, α-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(05):464–471. doi: 10.1016/j.oooo.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Delanian S, Chatel C, Porcher R, Depondt J, Lefaix J L. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80(03):832–839. doi: 10.1016/j.ijrobp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Breik O, Tocaciu S, Briggs K, Tasfia Saief S, Richardson S. Is there a role for pentoxifylline and tocopherol in the management of advanced osteoradionecrosis of the jaws with pathological fractures? Case reports and review of the literature. Int J Oral Maxillofac Surg. 2019;48(08):1022–1027. doi: 10.1016/j.ijom.2019.03.894. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, Lang M, Fan W. Prevention of osteoradionecrosis of the jaws by low-intensity ultrasound in the dog model. Int J Oral Maxillofac Surg. 2016;45(09):1170–1176. doi: 10.1016/j.ijom.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro G H, Minamisako M C, Rath I BDS. Osteoradionecrosis of the jaws: case series treated with adjuvant low-level laser therapy and antimicrobial photodynamic therapy. J Appl Oral Sci. 2018;26:e20170172. doi: 10.1590/1678-7757-2017-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrillo A, Filiaci F, Ramieri V. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci. 2012;16(12):1741–1747. [PubMed] [Google Scholar]
- 29.Chen Y T, Chang Y C. Use of platelet-rich fibrin and surgical approach for combined treatment of osteoradionecrosis: a case report. J Int Med Res. 2019;47(08):3998–4003. doi: 10.1177/0300060519862468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi A, Rajendraprasad J S, Shetty K. Reconstruction of intraoral defects using facial artery musculomucosal flap. Br J Plast Surg. 2005;58(08):1061–1066. doi: 10.1016/j.bjps.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 31.Hatoko M, Kuwahara M, Tanaka A, Yurugi S. Use of facial artery musculomucosal flap for closure of soft tissue defects of the mandibular vestibule. Int J Oral Maxillofac Surg. 2002;31(02):210–211. doi: 10.1054/ijom.2002.0235. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y F, Zhang W F, Zhao J H. Reconstruction of intraoral defects after cancer surgery using cervical pedicle flaps. J Oral Maxillofac Surg. 2001;59(10):1142–1146. doi: 10.1053/joms.2001.26713. [DOI] [PubMed] [Google Scholar]
- 33.Tezel E. Buccal mucosal flaps: a review. Plast Reconstr Surg. 2002;109(02):735–741. doi: 10.1097/00006534-200202000-00048. [DOI] [PubMed] [Google Scholar]
- 34.Kim C M, Park M H, Yun S W, Kim J W. Treatment of pathologic fracture following postoperative radiation therapy: clinical study. Maxillofac Plast Reconstr Surg. 2015;37(01):31. doi: 10.1186/s40902-015-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster R D, Anthony J P, Sharma A, Pogrel M A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21(01):66–71. doi: 10.1002/(sici)1097-0347(199901)21:1<66::aid-hed9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Santamaria E, Wei F C, Chen H C. Fibula osteoseptocutaneous flap for reconstruction of osteoradionecrosis of the mandible. Plast Reconstr Surg. 1998;101(04):921–929. doi: 10.1097/00006534-199804040-00006. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro P G, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000;105(07):2331–2346. doi: 10.1097/00006534-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Brown J S, Shaw R J. Reconstruction of the maxilla and midface: introducing a new classification. Lancet Oncol. 2010;11(10):1001–1008. doi: 10.1016/S1470-2045(10)70113-3. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy C M, Cordeiro P G. Microvascular reconstruction of oncologic defects of the midface. Plast Reconstr Surg. 2010;126(06):1947–1959. doi: 10.1097/PRS.0b013e3181f446f1. [DOI] [PubMed] [Google Scholar]
- 40.Coskunfirat O K, Wei F C, Huang W C, Cheng M H, Yang W G, Chang Y M. Microvascular free tissue transfer for treatment of osteoradionecrosis of the maxilla. Plast Reconstr Surg. 2005;115(01):54–60. [PubMed] [Google Scholar]
- 41.Miles B A, Gilbert R W. Maxillary reconstruction with the scapular angle osteomyogenous free flap. Arch Otolaryngol Head Neck Surg. 2011;137(11):1130–1135. doi: 10.1001/archoto.2011.187. [DOI] [PubMed] [Google Scholar]
- 42.Futran N D, Wadsworth J T, Villaret D, Farwell D G. Midface reconstruction with the fibula free flap. Arch Otolaryngol Head Neck Surg. 2002;128(02):161–166. doi: 10.1001/archotol.128.2.161. [DOI] [PubMed] [Google Scholar]


