Abstract
With advanced head and neck ablative surgery comes the challenge to find an ideal reconstructive option that will optimize functional and aesthetic outcomes. Contemporary microvascular reconstructive surgery with free tissue transfer has become the standard for complex head and neck reconstruction. With continued refinements in surgical techniques, larger surgical volumes, and technological advancements, free flap success rates have exceeded 95%. Despite these high success rates, postoperative flap loss is a feared complication requiring the surgeon to be aware of potential options for successful salvage. The purpose of this article is to review free flap failure and ways to optimize surgical salvage in the scenario of flap compromise.
Keywords: free flap, complication, failure, salvage
Free flaps were first described in head and neck reconstruction in 1959 and has since become the gold standard for head and neck reconstruction. 1 The current incidence of postoperative flap loss has been reported as less than 3% and generally accepted contemporary success rates are greater than 95%. 2 Although low, free flap loss remains a potential postoperative sequela due to the complex nature of the procedure combined with preoperative medical comorbidities leading to increased morbidity, prolonged hospitalization, and increase in overall health care cost. 2 3 When faced with free flap loss, early recognition leading to early intervention with appropriate surgical revision can increase the likelihood of successful surgical salvage. Timing of the failure is important and can help determine possible underlying etiologies. Studies have shown that majority of flap failures occur within the first 48 hours and vessel thrombosis as the primary etiology with venous thrombosis being more common than arterial thrombosis. 4 This article is intended to review the risk factors and etiologies of free flap loss, as well as salvage options in the management of flap failure to optimize outcomes.
Risk Stratification
“High-risk” patients comprise 80% of postoperative deaths in major surgical procedures. 5 Las et al reviewed the risk factors related to flap loss in head and neck reconstruction and noted a 4.7 times higher risk of partial flap loss in patients with pulmonary comorbidity. 6 Seidenstuecker et al reviewed a series of studies on the physiological changes with active smoking to include thrombocytosis, activation of sympathetic nervous system, and hypoxia, all of which can compromise flap circulation. 7 While advanced age is not considered a risk factor for complications following free tissue transfer, 8 coronary artery disease was noted to be an independent predictor of overall complications following free tissue transfer in the elderly population. 9
Prior radiotherapy was not correlated with flap failure, which was consistent with multiple prior studies. 10 11 12 13 14 15 Diabetes mellitus has been reported as a risk factor for flap failure in head and neck reconstruction. 16 Analysis of relative risk for recipient vessels for flap failure is complex and difficult due to the lack of many intraoperative variables that may have led a surgeon to choose a particular vessel. In a case series of 881 patients, Zhou et al did not find choice of recipient vein or use of a coupler device to be associated with flap failure. 17 In general, the senior author (Y.D.) prefers to anastomose to the external jugular vein when available and avoids using the superior thyroid artery where possible due to increased risk of vasospasm.
Immediate Failure
Immediate flap failure represents the majority of free flap loss. In the immediate postoperative period the free flap is completely dependent on the pedicle for vascularity. This transitions as the recipient site of free tissue transfer develops vascularity and incorporates the flap. This process is a combination of neovascularization, or the generation of de novo blood vessels from the recipient bed as well as angiogenesis, or the formation of blood vessels from existing vasculature. The timing for this to occur, however, is not well agreed upon in the literature. General consensus is that the process takes at least 2 weeks. Case reports of pedicle loss after free tissue transfer does not necessarily equate to complete flap loss and have noted complete survival despite pedicle loss as early as day 8. 18 The etiology of failure can be divided into intrinsic and extrinsic factors. Generally, prolonged extrinsic factors can lead to intrinsic factors that result in flap failure even after those factors are alleviated.
Intrinsic Factors
Arterial spasm is generally considered if other flow-related factors are excluded. This is most commonly experienced intraoperatively as the vessel in question may be irrigated with vasodilatory medications to help release spasm. Identification of this as the primary cause postoperatively is extremely difficult and no systematic approach in the literature is available to address this likely due to the difficulty in diagnosing this etiology. Out of 49 take-back free flaps reported by Zhou et al, one was reported secondary to arterial vasospasm. 17
Mechanical obstruction of the vascular pedicle can occur leading to obstruction of inflow, and subsequent outflow of blood in the flap. This can occur secondary to improper vessel geometry prior to attempting microvascular anastomosis. Longer pedicles tunneled under soft tissue, vein transpositions, and grafts have a higher risk for pedicle compromise. Care should be taken when suturing the anastomosed pedicle to surrounding soft tissue in the neck as that may cause unnatural tethering and facilitate kinking. At times, specific head positioning intraoperatively can lead to what appears to be appropriate vessel geometry, but changes in head position postoperatively can lead to mechanical obstruction.
Fig. 1 highlights a case where the patient head positioning intraoperatively led to unrecognized progressive postoperative mechanical obstruction. The patient was positioned in a horseshoe for the surgical procedure. Patient underwent left orbital exenteration with anterior skull base reconstruction with pericranial flap, cranioplasty, followed by radial forearm free flap for exenteration defect. Following release of the horseshoe and return of patient head position to neutral position, progressive loss of internal Doppler was noted upon arrival to the intensive care unit (ICU). Patient was taken back immediately to the operating room (OR) where the pedicle anastomosed to the facial artery was noted to be compressed by the internal jugular vein. The pedicle anastomosis was divided with patient head in slight extension out of horseshoe and anastomosis revised with extraction of a large clot along the length of the pedicle. Complete flap salvage was achieved.
Fig. 1.
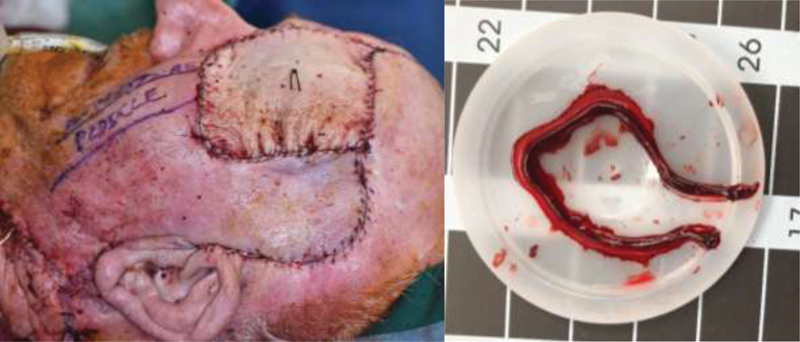
Left, patient who underwent anterior skull base resection and orbital exenteration for malignant tumor underwent pericranial flap, cranioplasty, and left neck dissection with radial forearm free flap anastomosed to facial artery and external jugular vein. Pedicle marked by pen externally to remind nursing staff to avoid external compression. Patient subsequently taken immediately postoperatively within 1 hour for loss of arterial signal from implantable Doppler. Right, large thrombectomy along the length of the pedicle removed with microforceps and Tsai irrigation. Revision of anastomosis with favorable placement of pedicle relative to the internal jugular vein resulted in complete flap salvage.
Thrombosis of the anastomosis can be secondary to a variety of reasons and these should be addressed before reanastomosis attempt. Debris within the vessel walls can propagate thrombosis not apparent during initial anastomosis intraoperatively. Vessel intimal flaps or dislodged atherosclerosis, proximity of clipped small branches near anastomosis can all affect vascular flow turbulence and promote thrombosis. As discussed in the above case, vessel geometry should be optimized for the head position the patient will be in postoperatively.
Extrinsic Factors
Recipient flap beds should be smoothed of any bony prominences or edges that may impinge on microvasculature of the flap. Prior irradiated flap beds are not known to be the cause of flap failure although this may prolong neovascularization of the flap. In the head and neck, proper insetting of the flap with water-tight closure is paramount to flap survival as leakage of salivary contents under the free flap in the recipient bed can be a source for infection that can lead to delayed failure but may not cause identifiable problems early postoperatively.
Implant devices such as drain tubing should be placed carefully and away from the anastomosis to facilitate safe removal. Insetting of the flap should be completed in proper orientation so as to minimize unnecessary tension on the pedicle and anastomosis. Overly tight skin closures should be avoided as postoperatively tissue edema, head positioning, and fluid shifts can lead to potential vessel obstruction. Tight dressings should be avoided, such as elastic wraps. Similarly, tracheostomy ties should not be used and tracheostomy tubes should be secured with skin sutures. Patient markings can be used, as shown in Fig. 1 , to remind nursing staff to avoid compression of the immediate underlying area that is the pedicle.
System hypoperfusion can result in flap hypoperfusion and mimic flap failure. Arterial spasm may be induced and lead to thrombotic propagation resulting in flap loss. Controllable factors include hypothermia, which can be addressed with heated rooms, fluid warmers, and warming blankets. Intravascular hypovolemia should be corrected to maintain adequate oxygenation of the flap. Animal studies demonstrate some degree of normovolemic hemodilution can potentially improve oxygenation in ischemic flap tissue. 19 20 There is no consensus on transfusion guidelines following microvascular reconstruction of the head and neck. Decision criteria for transfusion postoperatively should be dependent on symptoms of hypoxia and hypovolemia. Some authors recommend restrictive cutoff of hemoglobin < 7 g/dL as transfusion criteria. Vasopressors are at times unavoidable in the immediate postoperative period to maintain perfusion. In general, available studies in the literature suggest they do not harm outcomes, with more evidence supporting use of norepinephrine over other agents. 21 Massey and Gupta examined the effects of different vasopressors on pedicle artery blood flow in pigs and found phenylephrine consistently decreasing pedicle artery blood flow whereas epinephrine consistently increased both flow along the pedicle and cardiac output, which attests to the clinical differences that can be appreciated among agents. 22
Flap Monitoring
Early recognition remains key in salvage of failing free flaps. Given the majority of vessel thrombosis occur within the first 48 to 72 hours after surgery, frequent early monitoring is crucial to prompt intervention of any compromise. The senior author admits free flap patients to the ICU with hourly flap checks by the nursing staff in the first 48 hours, and then spaced out every 2 hours for the following few days, to every 4 hours in the ensuing days prior to discharge. Additionally, the author utilizes an implantable Cook–Schwartz Doppler routinely.
Clinical monitoring remains important even with the emergence of various flap monitoring technologies. A full review of available technologies is beyond the scope of this article but commonly used ones include a handheld external Doppler, implantable internal Doppler, laser Doppler, near-infrared angiography, pulse oximetry, digital photography, and surface temperature probes. Perfusion is evaluated by assessing skin color, temperature, capillary refill, and bleeding on pin prick. Arterial insufficiency is suspected if the flap skin appears pale and cool to touch, and fails to bleed with needle stick or superficial dermal cut. Venous congestion manifests as darkening of the skin with edema and rapid immediate bleed with dark blood on pin prick.
There are some promising studies utilizing cell phone photography leading to increased flap survival and decreased time to reoperation. Integration of these devices with advances in telemedicine can potentially allow experienced microsurgeons more accurate and precise real-time assessment of flaps remotely. 23 24
Delayed Failure
Delayed flap failure, defined as flap loss after the seventh postoperative day (POD), is an extremely rare complication of free tissue transfer for head and neck reconstruction. Although the etiologies of immediate flap loss were discussed above, there are significantly less reports on delayed flap loss. A recent multi-institutional retrospective review of patients undergoing free flap reconstruction of the head and neck showed a paradigm shift in timing of microvascular free tissue transfer failures beyond 72 hours. 25 Reasons for flap failure were attributed to vascular pedicle compromise, infection, defect location, and use of an osteocutaneous flap. The authors believe there will be less free flap failures due to technical aspects in the immediate postoperative period with an increase in delayed flap failures due to standardization of microsurgical technique, advanced microsurgical training, and improved microsurgical instrumentation. For these reasons, it is important for the microsurgeon to be cognizant of potential for delayed flap loss.
Reports of delayed flap loss beyond POD 7 are rare. In 2002, Salgado et al 26 reported on 10 cases in which the vascular pedicle was compromised beyond POD 7. There was complete loss of four free flaps, all of which were inset into compromised recipient beds due to radiation, ischemia, or scarring. They concluded that in cases of delayed arterial pedicle occlusion in an unhealthy recipient bed that more aggressive salvage attempts should be undertaken. In a retrospective review of free tissue transfer to the head and neck at two institutions, Wax and Rosenthal reported on 13 flaps lost beyond 7 days after transfer out of 1,530 flaps performed. Cases of flap loss were divided into three categories by time since flap transfer: within the first 2 weeks, between 30 and 90 days, and beyond 90 days. The authors determined that delayed flap loss was exceedingly rare, and possible etiologies for delayed flap loss included vascular pedicle compression, delayed infection, and residual tumor in the recipient bed. 27
In 2018, Forner et al performed a systematic review of the literature to identify cases of late flap failure after head and neck reconstruction. In addition to the previously described cohort by Wax and Rosenthal and one case from the Salgado et al, an additional 31 cases were identified. Although the etiology of delayed flap failure could not be concluded in the review, a large number of cases (50%) had delayed flap loss in the second postoperative week and may be attributed to delayed neovascularization of the flap. 1
Time from free tissue transfer to neovascularization has not been completely elucidated. In an animal model studying fasciocutaneous free tissue transfer, it was shown that an intact vascular pedicle for 7 days postoperatively ensured almost 90% flap survival after pedicle ligation. 28 In addition, there have been case reports showing free flap survival despite early loss of the vascular pedicle. 18 29 Yoon and Jones performed a review of the literature to determine the time for neovascularization needed for free flap survival despite anastomotic compromise, and concluded that flap survival was feasible after POD12 after pedicle loss. Despite these findings, a subpopulation of patients remains who undergo free tissue transfer who do not have adequate flap revascularization and experience delayed flap loss. 30
Etiologies for inadequate neovascularization include recipient site infection, malnutrition, nicotine, previous radiation therapy, and patient comorbidities such as hypertension, diabetes, and peripheral vascular disease. Multiple animal models have shown that prior radiation therapy effects free tissue transfer by increasing the risk of vascular thrombosis and delaying neovascularization. 31 However, these findings may not translate to clinical outcomes. Choi et al studied 100 consecutive patients undergoing fibula free flap for reconstruction of the mandible and studied the effects of radiation therapy on free flap outcomes, with no reports of complete flap loss who received preoperative radiation therapy. Similarly, Lin and colleagues found no difference in flap loss in patients who had prior radiation therapy compared with those who did not. On the other hand, a retrospective study of free flap reconstruction after radiation therapy found an increased number of delayed (6–15 weeks postoperatively) and late (> 15 weeks postoperatively) flap failures compared with those who did not receive prior radiation therapy. 32 Additionally, a meta-analysis by Herle et al 33 concluded that preoperative radiation was associated with increased risk of flap failure. Nevertheless, microsurgeons should expect increased complications in patients receiving prior radiation therapy, and to remember that delayed flap loss in this setting continues to be a rare entity.
Management of delayed flap loss is similar to immediate flap failure. Microsurgeons should consider the patient's current clinical status and determine the need for a second free flap. At the time that delayed flap loss is identified, patients have typically been discharged from the hospital and recovered from the initial surgery, except in the setting of recipient site infection or fistula formation. Surgeons should determine the cause for flap failure, whether due to vascular pedicle compromise without neovascularization, wound infection, or tumor recurrence. The defect to be reconstructed, availability and integrity of potential recipient vessels, and alternative donor sites are considered. Once these factors are considered and the decision is made to proceed with a second free flap, the patient is optimized to proceed with surgical intervention. Significant care is taken intraoperatively to minimize vessel injury and confirm satisfactory anastomosis and perfusion of the flap. Vigilant postoperative monitoring and care is undertaken with special attention paid to careful patient positioning to avoid pedicle compression or hematoma formation. Adjunctive treatment is instituted based on patient characteristics, including pain control, fluid management, and use of systematic anticoagulation, especially for salvage in the setting of previously radiated tissue. Ultimately, a systematic approach with optimization of extrinsic factors which could affect viability of a second free flap should yield a successful outcome.
Management
In cases of impending flap failure surgical intervention is the first line treatment. General agreement in rates of successful flap salvage in the literature is approximately 50%. Here, we describe the senior author's approach to surgical salvage. Patients and family members are counseled on the possible need for revision surgery following free flap reconstruction prior to initial procedure. When mechanical compression is suspected secondary to hematoma or tissue edema, suture release at bedside over the trajectory of the pedicle should be performed. This generally allows additional time for flap perfusion as the surgeon readies emergent takeback to the OR. At this time the nurse administers 325 mg aspirin per rectum as well as 10,000 units of heparin intravenously in anticipation to return to the OR.
Once in the OR, the arterial and venous anastomosis is explored. Visual inspection of the artery and vein can suggest presence of a clot. Confirmation of flow obstruction can be obtained using Doppler. Milking of the vein proximal and distal to the anastomosis can be helpful in confirming whether there is adequate outflow from the flap. When revision is needed, both arterial and venous anastomoses are explored. Both vessels are flushed with warmed Tsai solution, which is mixed by the pharmacist prior to use intraoperatively and comprises of 900 mL of normal saline mixed with 100 mL of 2% plain lidocaine and 20,000 units of heparin. Thrombectomy is performed using microforceps under Tsai irrigation. A Fogarty catheter may be utilized if thrombectomy is unsuccessful with microforceps.
Duration of ischemia is essential to flap salvage. Studies demonstrate mammalian skeletal muscle is much less tolerant of ischemia than skin. Irreversible damage to the microcirculation have been documented in 6 hours in humans. 34 Ischemic tolerances are also dependent on temperatures that regulate the relative metabolism of the flap tissue. In review of the literature, general windows of ischemia tolerance for skin and subcutaneous tissue, muscle, and bone are approximately 4, 2, and 3 hours, respectively. 35 These are not clear cutoffs as in many cases of failure beyond the initial 48 hours some degree of neovascularization and angiogenesis support the flap beyond the pedicle itself. In cases where recognition of flap ischemia is delayed beyond the tolerant windows, damage to the microcirculation may result in “no-flow” phenomenon even after restoration of patency of the arterial pedicle. Use of thrombolytic therapy is controversial in the literature for free flap salvage and may be theoretically useful in these “no-flow” phenomenon settings to eradicate microthrombi. These agents include urokinase, streptokinase, and recombinant tissue plasminogen activators. No agreed upon doses are available for these in the use of microsurgery flap salvage, and their success are largely limited to case reports in the literature. Systematic studies of using these agents over heparinized saline irrigations are lacking to demonstrate any superiority, although this admittedly is a difficult topic of research.
Leech Therapy
Hirudotherapy has been used for over 2,500 years in the field of medicine but has only been part of the plastic surgeons armamentarium for over 50 years. It has been utilized to treat venous insufficiency in free and pedicled flaps. Success rates reported in the literature have ranged from 65 to 85%, although this may be an overestimate. Duration of treatment ranges from 4 to 10 days as noted in a systematic review. 36 Hemoglobin monitoring is essential as a significant if not all patients undergoing leech therapy require blood transfusions. Aeromonas infection was noted to be as high as 14% in patients undergoing hirudotherapy and antibiotic prophylaxis is advised. Patient and staff aversion has been a reported issue with hirudotherapy and pharmacologic leeching with bivalirudin has been investigated in treatment of venous congestion. 37 Fig. 2 demonstrates a congested radial forearm responding to leech therapy. Fig. 3 shows leech therapy for a congested radial forearm free flap along the floor of the mouth. External flaps are ideal for leech therapy given ease of application and monitoring. Intraoral flaps are challenging as leeches tend to latch onto healthy mucosa and there is increased risk of migration. Additionally, tolerance of leeches in the oral cavity can be a significant problem. Leech migration can be addressed with suturing of the leech tail to surrounding tissue.
Fig. 2.
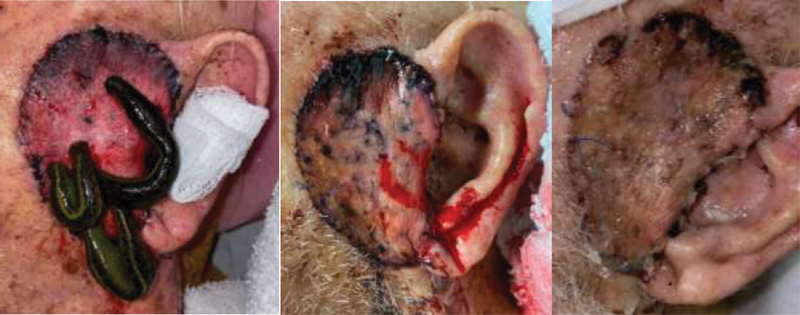
Left, venous congestion postoperatively along radial forearm free flap with leech therapy employed. Middle, improved flap congestion following several days of therapy. Right, salvaged radial forearm free flap with small amount of partial flap loss along superior rim.
Fig. 3.
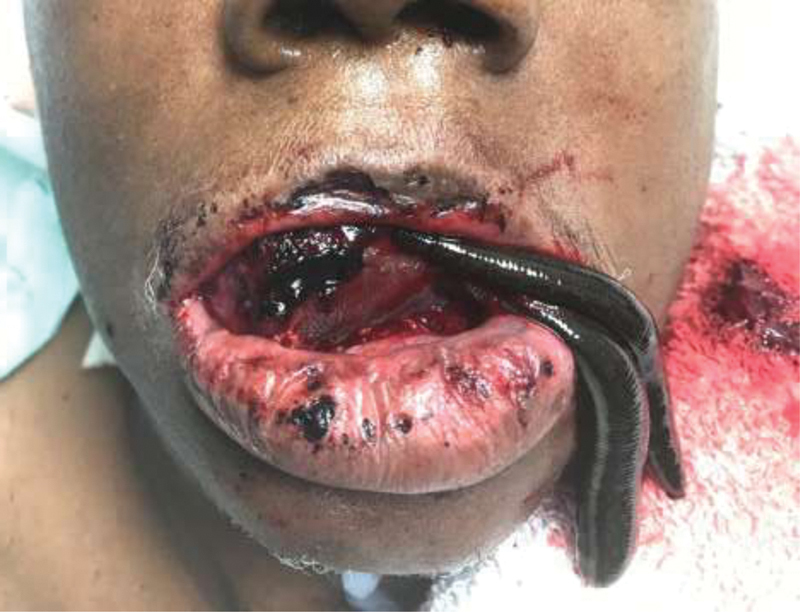
Leech therapy employed for congested radial forearm free flap along floor of mouth. Note, latching in cases of intraoral flaps can be markedly more challenging than external flaps and any leeches placed more internally or on the tongue should be sutured to adjacent tissue to avoid migration into the aerodigestive tract.
Indications for leech therapy include venous insufficiency; however, complete venous occlusion should be explored surgically. In the patient with severe comorbidities, hemodynamic instability, who requires extensive resuscitation, and is unfit for flap exploration and salvage, leech therapy presents a conservative alternative. Fig. 4 details the leech therapy protocol the senior author employs. Duration of treatment is dependent on the response of the flap tissue and adequate time needed for neovascularization to occur.
Fig. 4.
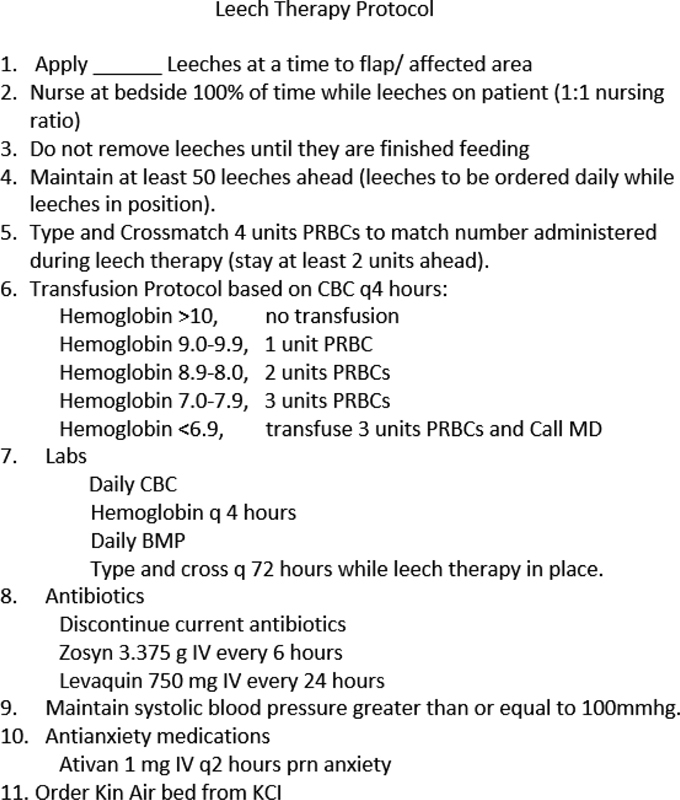
Leech therapy protocol.
Second Free Flap versus Regional Flap
In the event that a free flap cannot be salvaged, the surgeon must reevaluate the treatment plan. The decision should reflect the optimal reconstruction for the patient as well as the patient's preferences. Identification of coagulopathies and thrombogenic conditions, if unaddressed should be a contraindication for subsequent free tissue transfer. Additionally, a patient's medical status that is rapidly deteriorating should also be a contraindication for a second free flap. Cause for failure, however, cannot always be identified. Reoperation with a second free flap can be successful in these cases and success rates ranged from 85 to 94% in reports by Fearon et al, Wei et al, and Baumeister et al. Wei et al also found increased complication rates when using regional flaps followed by failed free flaps when compared with the reoperation with a second free flap group in head and neck reconstructions. 38 39 40 The senior author's preference is consistent with the recommendations from Baumeister et al and Wei et al in that a second free flap reoperation is a safe option and should be offered if the reconstructive needs of the patient have not changed. Patients should be optimized perioperatively prior to proceeding with a second free flap reconstruction. Two failed free flaps without an identifiable cause should be a contraindication to a third.
Conclusion
One of the most challenging steps to free flap salvage is in making the decision to reexplore in the OR as scenarios are fraught with uncertainty. Reexploration is a necessary adjunct to microvascular surgery and should be a built-in algorithm to any free flap protocol. When salvaging flaps, the key to successful salvage is early identification through diligent monitoring. Approaches to reexploration should be methodical and straightforward, and when indicated a second free tissue transfer is a safe option in failed primary reconstruction.
Footnotes
Conflict of Interest None declared.
References
- 1.Forner D, Williams B A, Makki F M, Trites J R, Taylor S M, Hart R D. Late free flap failure in head and neck reconstruction: a systematic review. Ear Nose Throat J. 2018;97(07):213–216. doi: 10.1177/014556131809700712. [DOI] [PubMed] [Google Scholar]
- 2.Eskander A, Kang S, Tweel B. Predictors of complications in patients receiving head and neck free flap reconstructive procedures. Otolaryngol Head Neck Surg. 2018;158(05):839–847. doi: 10.1177/0194599818757949. [DOI] [PubMed] [Google Scholar]
- 3.Hyodo I, Nakayama B, Kato H. Analysis of salvage operation in head and neck microsurgical reconstruction. Laryngoscope. 2007;117(02):357–360. doi: 10.1097/mlg.0b013e3180312380. [DOI] [PubMed] [Google Scholar]
- 4.Brady J S, Desai S V, Crippen M M. Association of anesthesia duration with complications after microvascular reconstruction of the head and neck. JAMA Facial Plast Surg. 2018;20(03):188–195. doi: 10.1001/jamafacial.2017.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Findlay G, Goodwin A, Protopapa K, Smith N, Mason M. London, UK: National Confidential Enquiry into Patient Outcome and Death; 2011. Knowing the Risk: A Review of the Perioperative Care of Surgical Patients. [Google Scholar]
- 6.Las D E, Jong T, Zuidam J M, Verweij N M, Hovius S E, Mureau M. Identification of independent risk factors for flap failure: a retrospective analysis of 1530 free flaps for breast, head and neck and extremity reconstruction. J Plast Reconstruct Aesthetic Surg. 2016;69:894–906. doi: 10.1016/j.bjps.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Seidenstuecker K, Munder B, Mahajan A L, Richrath P, Behrendt P, Andree C. Morbidity of microsurgical breast reconstruction in patients with comorbid conditions. Plast Reconstr Surg. 2011;127(03):1086–1092. doi: 10.1097/PRS.0b013e318205f255. [DOI] [PubMed] [Google Scholar]
- 8.Serletti J M, Higgins J P, Moran S, Orlando G S. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg. 2000;106(01):66–70. doi: 10.1097/00006534-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Howard M A, Cordeiro P G, Disa J.Free tissue transfer in the elderly: incidence of perioperative complications following microsurgical reconstruction of 197 septuagenarians and octogenarians Plast Reconstr Surg 2005116061659–1668., discussion 1669–1671 [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Chang D W, Miller M J, Reece G, Robb G L. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck. 2009;31(01):45–51. doi: 10.1002/hed.20927. [DOI] [PubMed] [Google Scholar]
- 11.Choi S, Schwartz D L, Farwell D G, Austin-Seymour M, Futran N. Radiation therapy does not impact local complication rates after free flap reconstruction for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130(11):1308–1312. doi: 10.1001/archotol.130.11.1308. [DOI] [PubMed] [Google Scholar]
- 12.Bozikov K, Arnez Z M. Factors predicting free flap complications in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2006;59(07):737–742. doi: 10.1016/j.bjps.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 13.le Nobel G J, Higgins K M, Enepekides D J. Predictors of complications of free flap reconstruction in head and neck surgery: analysis of 304 free flap reconstruction procedures. Laryngoscope. 2012;122(05):1014–1019. doi: 10.1002/lary.22454. [DOI] [PubMed] [Google Scholar]
- 14.Eckardt A, Meyer A, Laas U, Hausamen J E. Reconstruction of defects in the head and neck with free flaps: 20 years experience. Br J Oral Maxillofac Surg. 2007;45(01):11–15. doi: 10.1016/j.bjoms.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Tan N C, Lin P Y, Chiang Y C. Influence of neck dissection and preoperative irradiation on microvascular head and neck reconstruction-analysis of 853 cases. Microsurgery. 2014;34(08):602–607. doi: 10.1002/micr.22270. [DOI] [PubMed] [Google Scholar]
- 16.Valentini V, Cassoni A, Marianetti T M. Diabetes as main risk factor in head and neck reconstructive surgery with free flaps. J Craniofac Surg. 2008;19(04):1080–1084. doi: 10.1097/SCS.0b013e3181763531. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Zhang W B, Yu Y. Risk factors for free flap failure: a retrospective analysis of 881 free flaps for head and neck defect reconstruction. Int J Oral Maxillofac Surg. 2017;46(08):941–945. doi: 10.1016/j.ijom.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Granzow J, Li A I, Caton A, Boyd J B. Free flap survival following failure of the vascular pedicle. Ann Plast Surg. 2015;75(01):44–48. doi: 10.1097/SAP.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 19.Schramm S, Wettstein R, Wessendorf R, Jakob S M, Banic A, Erni D. Acute normovolemic hemodilution improves oxygenation in ischemic flap tissue. Anesthesiology. 2002;96(06):1478–1484. doi: 10.1097/00000542-200206000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Erni D, Wettstein R, Schramm S. Normovolemic hemodilution with Hb vesicle solution attenuates hypoxia in ischemic hamster flap tissue. Am J Physiol Heart Circ Physiol. 2003;284(05):H1702–H1709. doi: 10.1152/ajpheart.00821.2002. [DOI] [PubMed] [Google Scholar]
- 21.Motakef S, Mountziaris P M, Ismail I K, Agag R L, Patel A. Emerging paradigms in perioperative management for microsurgical free tissue transfer: review of the literature and evidence-based guidelines. Plast Reconstr Surg. 2015;135(01):290–299. doi: 10.1097/PRS.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 22.Massey M F, Gupta D K. The effects of systemic phenylephrine and epinephrine on pedicle artery and microvascular perfusion in a pig model of myoadipocutaneous rotational flaps. Plast Reconstr Surg. 2007;120(05):1289–1299. doi: 10.1097/01.prs.0000279371.63439.8d. [DOI] [PubMed] [Google Scholar]
- 23.Hwang J H, Mun G H. An evolution of communication in postoperative free flap monitoring: using a smartphone and mobile messenger application. Plast Reconstr Surg. 2012;130(01):125–129. doi: 10.1097/PRS.0b013e318254b202. [DOI] [PubMed] [Google Scholar]
- 24.Engel H, Huang J J, Tsao C K. Remote real-time monitoring of free flaps via smartphone photography and 3G wireless Internet: a prospective study evidencing diagnostic accuracy. Microsurgery. 2011;31(08):589–595. doi: 10.1002/micr.20921. [DOI] [PubMed] [Google Scholar]
- 25.Sweeny L, Topf M, Wax M K. Shift in the timing of microvascular free tissue transfer failures in head and neck reconstruction. Laryngoscope. 2020;130(02):347–353. doi: 10.1002/lary.28177. [DOI] [PubMed] [Google Scholar]
- 26.Salgado C J, Smith A, Kim S, Higgins J, Behnam A, Herrera H R, Serletti J M. Effects of late loss of arterial inflow on free flap survival. J Reconstr Microsurg. 2002;18(07):579–584. doi: 10.1055/s-2002-35095. [DOI] [PubMed] [Google Scholar]
- 27.Wax M K, Rosenthal E. Etiology of late free flap failures occurring after hospital discharge. Laryngoscope. 2007;117(11):1961–1963. doi: 10.1097/MLG.0b013e31812e017a. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen S, Bascom D A, Partsafas A, Wax M K. The effect of 2 sealants (FloSeal and Tisseel) on fasciocutaneous flap revascularization. Arch Facial Plast Surg. 2003;5(05):399–402. doi: 10.1001/archfaci.5.5.399. [DOI] [PubMed] [Google Scholar]
- 29.Wise S R, Harsha W J, Kim N, Hayden R E. Free flap survival despite early loss of the vascular pedicle. Head Neck. 2011;33(07):1068–1071. doi: 10.1002/hed.21354. [DOI] [PubMed] [Google Scholar]
- 30.Yoon A P, Jones N F. Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: a review of the literature. Microsurgery. 2016;36(07):604–612. doi: 10.1002/micr.30082. [DOI] [PubMed] [Google Scholar]
- 31.Barrera-Ochoa S, Gallardo-Calero I, Lopez-Fernandez A. Effect of previous irradiation on vascular thrombosis of microsurgical anastomosis: a preclinical study in rats. Past Reconstr Srug Glob Open. 2016;4:e1073. doi: 10.1097/GOX.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tall J, Björklund T C, Skogh A C, Arnander C, Halle M. Vascular complications after radiotherapy in head and neck free flap reconstruction. Ann Plast Surg. 2015;75(03):309–315. doi: 10.1097/SAP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 33.Herle P, Shukla L, Morrison W A, Shayan R. Preoperative radiation and free flap outcomes for head and neck reconstruction: a systematic review and meta-analysis. ANZ Journal of Surgery. 2015;85(03):121–127. doi: 10.1111/ans.12888. [DOI] [PubMed] [Google Scholar]
- 34.Wolff K-D, Stiller D. Ischemia tolerance of free-muscle flaps: an NMR-spectroscopic study in the rat. Plast Reconstr Surg. 1993;91(03):485–491. doi: 10.1097/00006534-199303000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Griffin J R, Thornton J F. Microsurgery: free tissue transfer and replantation. Selected Readings Plast Surg. 2005;10(05):1–39. [Google Scholar]
- 36.Herlin C, Bertheuil N, Bekara F, Boissiere F, Sinna R, Chaput B. Leech therapy in flap salvage: systematic review and practical recommendations. Ann Chir Plast Esthet. 2017;62(02):e1–e13. doi: 10.1016/j.anplas.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Harun A, Kruer R M, Lee A, Boahene K, Byrne P J, Richmon J D. Experience with pharmacologic leeching with bivalirudin for adjunct treatment of venous congestion of head and neck reconstructive flaps. Microsurgery. 2018;38(06):643–650. doi: 10.1002/micr.30298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei F C, Demirkan F, Chen H C.The outcome of failed free flaps in head and neck and extremity reconstruction: what is next in the reconstructive ladder? Plast Reconstr Surg 2001108051154–1160., discussion 1161–1162 [DOI] [PubMed] [Google Scholar]
- 39.Fearon J A, Cuadros C L, May J W., Jr Flap failure after microvascular free-tissue transfer: the fate of a second attempt. Plast Reconstr Surg. 1990;86(04):746–751. doi: 10.1097/00006534-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister S, Follmar K E, Zenn M R, Erdmann D, Levin L S. Strategy for reoperative free flaps after failure of a first flap. Plast Reconstr Surg. 2008;122(03):962–971. doi: 10.1097/PRS.0b013e3181811ca0. [DOI] [PubMed] [Google Scholar]


