Abstract
Facial paralysis is a devastating condition, encompassing a spectrum of disorders, with resultant psychosocial, functional, and aesthetic sequelae. With this in mind, an individualized treatment approach based on the cause, pattern, and duration of palsy is necessary. Treatment options include pharmacologic agents, corneal protective interventions, physical therapy, and surgical procedures. The use of steroids and antivirals in the setting of idiopathic facial paralysis or virus-associated facial paralysis is well supported. Despite the diversity of surgical interventions described, there is a lack of consensus regarding optimal treatment. This article provides an overview of the current management of facial paralysis. Medical, surgical, and physical treatment options are discussed with a review of the relevant literature.
Keywords: facial paralysis, facial reanimation, synkinesis, smile dysfunction, modified selective neurectomy, botulinum toxin
Introduction: Initial Considerations in Evaluation of the Patient with Facial Paralysis
Facial palsy (FP) is a debilitating condition with both aesthetic and functional impairment ultimately resulting in significant impact on a patient's quality of life. 1 2 3 4 Patient outcomes may range from complete and persistent acute flaccid facial paralysis (FFP) to complete return of normal facial function. Within this spectrum of extremes exist zonal permutations of both hypoactivity and hyperactivity often referred to as postparalytic facial nerve syndrome. 5
It is imperative that the treating physician establish a time course of FP onset and progression. The potential underlying causes of acute facial paralysis vary broadly and may include Bell's palsy (BP), Lyme disease, systemic infection, otic infections or cholesteatomas, Ramsay-Hunt syndrome (RHS; varicella-zoster virus), iatrogenic insult, autoimmune conditions, granulomatous diseases (sarcoidosis, Melkersson–Rosenthal syndrome), trauma, congenital malformation, pontine infarct, or benign or malignant tumor. 6 A full review of these underlying causes is beyond the scope of this article; however, clinicians should familiarize themselves with these possible underlying disorders and their individualistic treatment protocols. For a more expansive review readers are referred to more comprehensive texts. 7
A complete and thorough history is invaluable in facilitating diagnosis and the importance of this cannot be overstated. The most common cause of FP is viral-associated BP. 8 However, in the setting of acute paralysis, the following “red flags” may suggest an alternative diagnosis to BP: bilateral paralysis, indolent onset of paresis (BP is often associated with a prodrome and fully evolves within 1–3 days), constitutional symptoms, or presence of focal neurologic deficits, or absence of partial recovery of movement or improved facial tone within 3 to 4 months of onset. 6 9 10 BP manifests over the first 72 hours with variance in the degree of hemifacial paresis. However, recovery of facial movement and overall tone should occur within 3 to 4 months. Approximately 70% of patients suffering from BP will display full recovery. The remainder will undergo varying degrees of recovery with aberrant nerve regeneration and resultant postfacial paralysis synkinesis (PFPS). 11 Facial synkinesis presents following injury to the facial nerve and manifests as involuntary movement during volitional or spontaneous movement. This phenomenon may become clinically apparent 3 to 4 months following facial nerve injury. 12 13 Several theories regarding the underlying pathophysiology of synkinesis have been postulated. The most widely accepted mechanism is that of aberrant regeneration of the facial nerve following injury with resultant rerouting of proximal nerve axons leading to contraction of antagonistic facial musculature 14 15 ( Fig. 1 ). The aesthetic and functional sequelae of facial synkinesis have a significantly detrimental impact on patients' quality of life. 16 Treatment goals in PFPS aim to address oral competence, facial and cervical tightness, oral commissure position, and smile symmetry and spontaneity. 11 17
Fig. 1.
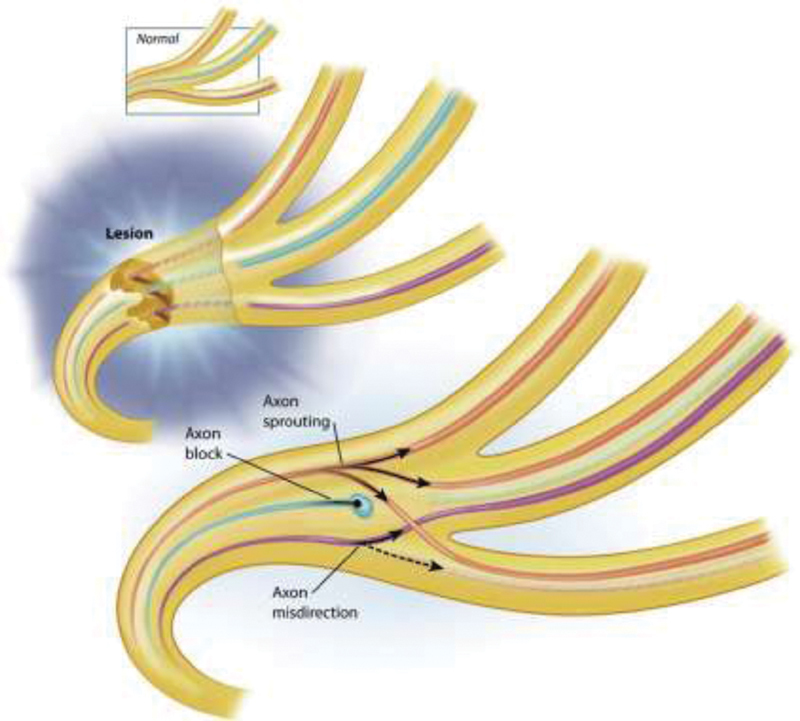
Aberrant regeneration leading to synkinesis. Following injury to the facial nerve, there is rerouting of proximal axons along multiple distal pathways leading to reinnervation of both correct and incorrect muscles. (Courtesy of The Facial Paralysis Institute, Beverly Hills, CA.)
Factors associated with poor outcome in BP include hyperacusis, age greater than 60, diabetes mellitus, hypertension, and severe radicular pain. 9 In particular, the patient's age at presentation should be noted, as zoster-associated FP (RHS) is prevalent within the older patient demographic. Patients with RHS may display periauricular skin vesicles, otalgia, ipsilateral lingual hypoesthesia, or decreased taste. In contrast to BP, individuals suffering from RHS demonstrate increased severity of symptoms as well as hyperacusis, hearing loss, uveitis, or keratoconjunctivitis. The diagnosis is often challenging and patients are often underdiagnosed on the basis of clinical presentation as the vesicular rash may appear in a delayed fashion, following initial facial paralysis. When present, the timing of cutaneous vestibular eruption may portend prognostic significance. In approximately 25% of patients, the cutaneous eruption will precede paralysis; this clinical presentation has shown an association with higher likelihood of facial nerve recovery. 18 19 Overall, this particular etiology has shown to present with hearing loss, pain, and longer recovery times with a greater proportion of patients displaying residual paresis and synkinesis. 18
A complete and thorough head and neck examination should be performed on all patients including examination of cranial nerves and otoscopy. A systematic approach toward patient evaluation is crucial. Zonal evaluation of facial function both at rest and with movement is therefore critical. Evaluation of the face may be performed in horizontal thirds with comparison to the unaffected, nonparalyzed, side. 20
FP may present in many different forms but it is important to differentiate flaccid paralysis from partial paralysis. It is also important to note whether patients with partial paralysis demonstrate synkinesis or weakness of movement from decreased neural input or muscular atrophy.
In patients with acute FFP, examination of the upper third should note absence of forehead wrinkles and brow ptosis. Symmetry of the upper third with concomitant zonal paresis of the lower face may suggest a central cause of FP and should prompt advanced imaging (magnetic resonance imaging, computed tomography) to rule out possible intracranial pathology. 21 The periocular complex should be evaluated with particular attention to eye closure, corneal reflex, and protective Bell phenomenon (superior rotation of the globe with attempted eye closure). Eye closure is almost always incomplete with resultant lagophthalmos and increased height of the palpebral fissure. The tone of the orbicularis oculi muscle may be assessed, as well as the position of the lower eyelid relative to the iris, with a snap test of the lower lid skin to evaluate for elasticity. 22 23 Individuals with PFPS will have some similarities but significant differences from flaccid patients. The frontalis muscle is viable but may elevate normally due to simultaneous activation of orbicularis oculi muscle and corrugators. Certain patients will have paradoxical elevation of the brows. Although the blink rate may be slower, patients often have complete closure with narrowing of the palpebral fissure.
The middle third of the face may display an effaced nasolabial fold, unveiling or exaggeration of tear trough deformity, ptosis of the malar soft tissue, and fat pad in cases of FFP. The nasal ala will also be displaced inferomedially and the philtrum may be pulled to the contralateral side as a result of unopposed contralateral facial mimetic muscle contraction. Patients may report nasal congestion due to external nasal valve obstruction of the affected side and the nasolabial fold may be effaced. PFPS patients have paradoxical deepening of nasolabial folds, increased tension of midface muscles, and limited nasal airway dysfunction.
When evaluating the lower third of the face, weakening of the lower lip may result in oral incompetence and the oral commissure may be displaced inferiorly. The degree of paralysis (complete flaccidity, partial flaccidity with tone, or a combination of flaccid paralysis and hyperkinesis) should be noted as this will have importance in both diagnostic evaluation and future targeted treatment. In FFP, individuals experience drooling and biting of lower lip and buccal mucosa due to lack of tone and support. In PFPS, drooling is less prevalent but buccinator hypertrophy may lead to biting of inner buccal mucosa and chronic irritation. In both flaccid and synkinetic cases, pursing of the lips and articulation are compromised.
Smile dysfunction can vary significantly in individuals with flaccid paralysis versus synkinesis. In FFP, there is no movement of oral commissure and dental show is only present on the contralateral unaffected side of the face. In PFPS, oral commissure is not always displaced inferiorly. Often times, it is neutral with the contralateral side and may even be elevated in rare cases of hypercontracted zygomaticus muscles. Upon volitional or spontaneous smile, the oral commissure typically will remain neutral and not elevate resulting in significant asymmetry due to overactivity of depressor anguli oris (DAO), buccinator, platysma, and orbicularis oris muscles (OOMs). Upper and lower teeth dental show on the affected side is variable. Severe cases of PFPS, will reveal only central incisors in the upper dentition and no visibility of lower teeth due to hyperactive OOM.
Documentation via photography and/or videography to document the appearance of the face at rest and with volitional movement on initial presentation, and subsequently on follow-up, is essential. This will allow for continued evaluation of the patient's progress with staged interventions over time. 24 25 Several tools have been developed to analyze spontaneous smile and emotional expressivity. However, no standardized measure has been uniformly recognized due in part to the difficult in both eliciting and quantitatively assessing facial expressivity. 26 27
Diagnostic Evaluation
When history and physical examination findings are most consistent with BP, investigation, with regard to further laboratory assessment or imaging, may not be required. However, further investigation, in the form of serologic testing, is recommended in Lyme endemic regions. 1 28 29 Imaging should be considered in the setting of abnormal otoscopic examination, tuning fork testing, or audiometric evaluation suggesting an otogenic source. Physical exam findings of palpable parotid or cervical lesions, slow onset of FP with continued progression, unilateral recurrent facial paralysis, or an absence of recovery at 3 to 4 months also warrant radiographic evaluation. 30 Thin-cut high-resolution computed tomography of the temporal bone without contrast or gadolinium-enhanced magnetic resonance imaging of the temporal bones or neck may therefore be indicated depending on the level of suspicion. Laboratory evaluation of FP is indicated in the setting of suspected autoimmune conditions or in patients with recurrent episodes. Laboratory testing should include: complete blood count, rheumatoid factor, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibody, angiotensin-converting enzyme, antiphospholipid antibodies, and antineutrophil cytoplasmic antibody. 31 32
Electrodiagnostic Testing
Patients with acute complete FFP, due to suspected BP, or temporal bone fractures should be considered for electrodiagnostic testing. This battery of testing includes electroneuronography (ENoG) and electromyography (EMG). Although controversial, an ENoG showing greater than 90% decrease in the maximum amplitude of a suprathreshold evoked compound muscle action potential with absent motor unit action potentials on EMG within a 4- to 14-day time period, following onset of paralysis, is an indication for surgical decompression of the facial nerve. 33 34
Medical Treatment of Acute Facial Paralysis
Corticosteroid Therapy
Medical management for patients presenting with acute FP, presenting within the first 72 hours to 2 weeks from onset, should be multimodal with management involving a multidisciplinary approach. In the setting of suspected BP, administration of high-dose corticosteroid therapy within the first 72 hours of symptom onset has some shown benefit in reduction of overall time to recovery. 35 A Cochrane review evaluated the use of steroids with that of a placebo and demonstrated high-quality evidence from several randomized controlled trials in support of the use of corticosteroid therapy. The same review also showed moderate evidence in support of corticosteroid use with resultant decreases in PFPS and epiphora at 6-month follow-up when compared with placebo. No serious adverse outcomes from steroid treatment were documented. 36
A randomized double-blind placebo-controlled trial, with 496 patients distributed to four treatment arms demonstrated the beneficial effect of prednisolone when compared with placebo alone, antiviral therapy alone, or combined therapy. 37 When evaluated at 3-, 6-, and 9-month follow-up patients receiving prednisolone demonstrated statistically significant improvement in FP when compared with those in the placebo or antiviral therapy groups. Combined therapy, employing acyclovir and prednisolone, showed similar improvement to that of prednisolone alone. Another randomized trial compared the utility of prednisone and valacyclovir, indicating a significant reduction in time to recovery in those treated with steroid therapy.
When reviewing the primary literature, the most commonly utilized dosing was 60 mg per day for approximately 5 to 7 days followed by a 5-day taper. 37 38 Within the pediatric population, weight-based dosing should be implemented with regimens such as: prednisolone 0.5 to 1.0mg/kg/day with a 5-day taper 39 or prednisone 1 to 2 mg/kg/day for 10 days followed by a 3- to 5-day taper 40 previously showing efficacy.
The American Academy of Otolaryngology – Head and Neck Surgery (AAO-HNS) clinical practice guidelines for BP recommend the use of oral corticosteroids over a 10-day course with at least 5 days at a high dose (i.e., prednisone 60 mg for 5 days or prednisolone 50 mg for 10 days followed by a 5-day taper). Steroids are most efficacious if started within 72 hours of symptom onset. The clinical guidelines also recommend consideration of the use of steroids in the pediatric population, despite low level evidence, due to similarities in the pathophysiology of the disease process and the low risk-to-benefit ratio of the drug. 41
Although corticosteroid use is associated with various potential adverse effects, short-term dosing, defined as less than 10 days, has previously shown to be well tolerated. 36 Patients should be appropriately counseled regarding potential side effects and receive prophylactic H 2 receptor antagonist therapy for potential dyspepsia and gastric ulceration.
Antiviral Therapy
The use of combination therapy, in the form of antiviral and corticosteroid treatment, in BP has shown potential added benefit, particularly in patients afflicted with severe to complete paralysis. 42 43 Antiviral drugs frequently used include acyclovir and valacyclovir, a 1-valine ester prodrug of acyclovir with three to four times the bioavailability. 44 45 46
The efficacy of antiviral as monotherapy is however limited. Studies comparing the benefit of steroids to antiviral agents have shown a clearly delineated benefit in use of corticosteroid as single agent therapy. A recent Cochrane review analyzing the role of antiviral treatment in BP, showed a summative benefit when combination therapy, utilizing antiviral with steroids, was employed when compared with steroid monotherapy for patients with varying severity of paralytic severity. 47 Over 10 randomized controlled trials were included in this review. Combination therapy showed benefits in recovery rates and reducing the incidence of PFPS, with limited adverse effects. 35 37 41 44 48 There is no consensus in regard to the optimal dosage of antiviral agents employed in treatment of facial paralysis. Dosage ranges reported within the literature include: acyclovir 1.6 to 3.0 g/day, or valacyclovir 1.0 to 3.2 g/day, for 5 to 7 days. 47 Antiviral therapy has not been extensively studied within the pediatric population and therefore are not typically recommended in this population. 41 Patients should be counseled regarding potential adverse effects from antiviral use including gastrointestinal related symptoms, allergic reactions, angioedema, and organ failure. 41 47 Pregnancy, renal or liver dysfunction, and immunocompromised represent contraindications to antiviral therapy.
Adjuvant Treatment and Ocular Management
Facial paralysis associated with Lyme disease is treated with antibiotics in the form of intravenous ceftriaxone or oral doxycycline. 49 50 Adjuvant corticosteroid treatment in the setting of Lyme disease is commonly prescribed although its efficacy is unclear. 28 51 52 FP associated in the setting of otitis media is treated with wide myringotomy with or without mastoidectomy, topical otic and parenteral antibiotics, and corticosteroid therapy. 53 54 55
In addition to pharmacologic treatment of FP, adjuvant therapies treatment modalities have been found to be effective. Other therapeutic interventions that have been described include acupuncture, soft tissue mobilization or massage, biofeedback, facial neuromuscular retraining, thermal therapy, and electrotherapy. 56 57 58 59
Soft tissue mobilization, or massage, has been shown to increase tissue perfusion with resultant increase in oxygenation. It has shown utility both when implemented on the affected and unaffected, nonparalyzed, contralateral face when compensatory hyperkinesis results in muscle tightness, and pain. Active exercises involving digital manipulation of the facial musculature facilitate muscle activity and delay atrophy until volitional movement returns. 60 Mime therapy employs real-time feedback with mirror-stimulation of facial exercises. 61 62 Upon return of muscle activity, facial neuromuscular retraining and rehabilitation exercises are commonly employed and have been shown to be one of the most important nonsurgical treatment modalities. 57 58 59 62 Although extremely useful in PFPS, the clinical significance of physical therapy is difficult to objectively analyze in the recovery of acute FP and differentiate from outcomes due to spontaneous recovery. 63 Based on the body of literature, the AAO-HNS clinical practice guidelines currently make no recommendation for physical rehabilitation in cases of acute FP. 41 However, due to the low-risk profile associated with physiotherapy and the psychosocial sequelae of FP, neuromuscular retraining should be considered in all patients with chronic facial paralysis and synkinesis.
Similarly, a Cochrane review of acupuncture evaluated 6 randomized controlled trials with 537 patients and found a lack of high-quality evidence although anecdotally studies suggested a beneficial effect following acupuncture. 64 Electrical stimulation delivers electrical impulses to promote muscle tone by providing electrical impulses, deprived to muscles in the context of FP, to stimulate muscle tone and mitigate risk of atrophy. Studies, utilizing animal models, have demonstrated that proximal motor nerve stimulation improves recovery. Although there is limited evidence suggesting that electrical stimulation may reduce time to recovery, none have shown a significant difference after 6 months. Furthermore, the literature suggests that the deleterious effects following this treatment modality, namely increased synkinesis, may outweigh any potential benefit and therefore electrical stimulation is currently not recommended. 57 63 65
It is also important for the clinician to consider that patients suffering from facial paralysis experience significant decreases in quality-of-life with increased rates of depression and anxiety. 66 67 68 Patients may be overwhelmed with the uncertainty and grief associated with FP and may therefore benefit from individual counseling or referral to a FP support group.Lastly, while patients are evaluated and treated for facial paralysis, special attention is given to appropriate ocular management as the potential complications associated with paralytic corneal exposure can be permanent and devastating. This is probably the single most important issue which must be acutely addressed to avoid long-term morbidity associated with acute facial nerve decompensation. Resultant lagophthalmos, poor blink, and incomplete eye closure, coupled with lower lid retraction and/or ectropion individually or collectively can compromise corneal coverage and protection. In addition, reduced orbicularis oculi function, coupled with unopposed and normal levator palpebrae superioris (innervated by the oculomotor nerve) action typically leads to upper eyelid retraction; and tear production, under neuro-control of the autonomic arm of the facial nerve can be reduced. Both these deficits can further compromise corneal integrity and potentially lead to exposure keratitis, corneal abrasion, or frank ulceration. Finally, and often unrecognized measures of how the corona will withstand eyelid paresis is the presence of both a normal Bell's response and normal corneal sensation. These are critical cornel protective mechanisms which often tip the scale of corneal health in one direction or another. When cornel integrity is compromised, initial treatment includes the use of preservative-free artificial tears during the day and ointment during the night. Nocturnal eyelid taping, the use of ocular moisture chambers, and an in-room humidifier are also of benefit if the most conservative measures do not relieve symptoms. Soft tissue mobilization with eyelid stretching exercises have also shown utility in facilitating recovery in eye closure. 69 These exercises are postulated to mechanically disturb crosslinking of myosin fibers within the levator palpebrae. As a next measure, protective bandage contact lenses, scleral lenses, or PROSE lenses are often disease altering in these patients and should be considered when symptoms warrant. 70 Surgery is considered in patients who respond poorly to these measures. Surgical treatments such as punctal plugging, canthoplasty, and reversible upper lid loading procedures are often appropriate. Temporary tarsorrhaphy is a simple, in-office, and reversible procedure, which should only be employed in cases where the vision is at immediate risk. This can be converted to a similar permanent procedure if needed.
Surgical Management of Facial Paralysis
Therapeutic surgical options in the management of FP vary and may be classified on the basis of onset of facial paralysis, timing of presentation, and status of the facial nerve and musculature. Acute FFP with intact nerve continuity, for example, in the setting of vestibular schwannoma resection with facial nerve continuity confirmed via stimulation, portends a good prognosis with potential for spontaneous recovery within 6 to 12 months. In this context, patients may benefit from close follow-up, every 3 to 4 months, to ensure recovery of function while mitigating the sequelae of facial nerve palsy with medical management as described previously.
In the setting of a poor prognosis for spontaneous recovery, either with nerve discontinuity or absent recovery within a 6- to 12-month time frame and viable facial musculature, procedures involving reinnervation of native facial musculature should be attempted. Although no definitive consensus has yet to be established, evidence indicates that facial musculature remains viable, and therefore receptive to reinnervation, for up to 24 months following denervation. 71 72 73 74 Primary neurorrhaphy or interposition grafts should always be considered as soon as facial nerve transection is suspected and the proximal and distal branches of the facial nerve are accessible. Although the smile mechanism will generally not be restored back to perfect normalcy, primary nerve repairs or interposition grafts preserves and revives muscle function and does restore the neural pathway between brain and facial musculatures that is of paramount importance in restoration of an emotional spontaneous smile. Delayed procedures such as Modified Selective Neurectomy as described by the senior author (B.A.) may be able to further improve the reanimation outcome. 11 17
If the proximal portion of the facial nerve is not accessible, cranial nerve substitution technique using the masseteric and/or hypoglossal nerve should be considered if the patient's FFP occurred less than 2-year prior. 73 75 76 77 78 Cranial nerve substitution is intended to restore muscle tone and viability which is very important to the overall facial appearance and function. These techniques, however, do not restore spontaneous facial reanimation and adjunctive procedures such as gracilis muscle transfer motorized by cross-facial nerve graft is needed to improve a patient's emotional smile mechanism. 79 80 81 82 These techniques may be considered as early as 6 months following vestibular schwannoma extirpation in patients with minimal improvement. 83
Patients with spontaneous recovery often exhibit various degrees of synkinetic facial movement due to aberrant nerve regeneration. Synkinesis, as described earlier, is debilitating and can limit patients both functionally and psychologically. In these patients, neuromuscular retraining, chemodenervation, and Modified Selective Neurectomy of the distal branches of the buccal and cervical branches of the facial nerve have shown significant promise in improving functional deficits and spontaneous smile function. Unlike patients with FFP, synkinetic patients have viable muscles of facial expression with neural input. Uncoordinated and simultaneous overactivity of frowning muscles (DAO, platysma), buccinator, and orbicularis oris prevent the normal smile muscles (zygomatic major, zygomatic minor, depressor labii inferioris, levator labii alaeque nasi) to work appropriately. Azizzadeh et al demonstrated that Modified Selective Neurectomy can independently improve smile function and synkinesis scores as well as reduce the chemodenervation requirements 11 17 ( Figs. 2 and 3 ).
Fig. 2.
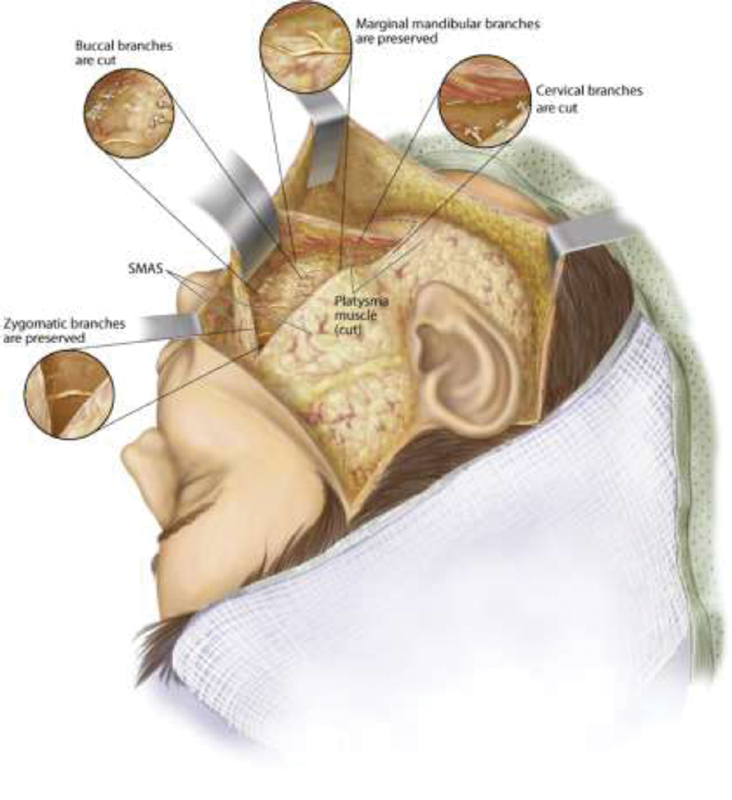
Modified selective neurectomy. Illustration depicting approach deep to the superficial musculoaponeurotic system (SMAS) and platysma, multiple distal nerves are identified and tagged. Stimulation of each nerve is first performed, and cervical and buccal branches that cause downward or lateral excursion of the oral commissure and upper lip are transected. The marginal mandibular branches are identified and preserved along with zygomatic branches. (Courtesy of The Facial Paralysis Institute, Beverly Hills, CA; with permission.)
Fig. 3.

( A ) Preoperative and ( B ) postoperative photodocumentation 1 month following right modified selective neurectomy, platysma myotomy, revision bilateral rhytidectomy, autologous fat grafting, and periorbital botulinum toxin A. ( C ) Preoperative and ( D ) postoperative photodocumentation 10 months following modified selective right neurectomy, platysma myotomy, bilateral rhytidectomy, autologous fat grafting, and fractionated carbon dioxide laser resurfacing. (Courtesy of The Facial Paralysis Institute, Beverly Hills, CA; with permission.)
In the setting of nonviable facial musculature due to long-term denervation (> 24 months), distal perineural spread of malignancy, congenital absence, or resection of musculature in the setting of malignancy, cranial nerve substitutions techniques, or repair are no longer feasible. Chemodenervation of the contralateral facial muscles and surgical interventions on the affected side can be utilized in this patient population. Targeted static procedures with suspension of the upper brow, periocular complex, midface, nasolabial folds, nasal valve, and oral commissure have been extensively described in the literature and may be performed. 84 85 Lagophthalmos and lower lid malposition must be addressed to prevent long-term complications as well as improve function and aesthetics. 86 87 88 However, restoration of dynamic facial animation in patients with long-standing paralysis requires more in-depth considerations. Dynamic facial reanimation has been described through antidromic or orthodromic temporalis muscle transfer or microvascular free microneurovascular muscle transfer. 89 90 91 92 93 94 Locoregional muscle transposition, with use of the temporalis muscle tendon unit, provides a bite-activated volitional smile and has shown great utility in patients that may not be candidates for free muscle transfer due to either personal preference or significant medical comorbidities precluding extensive surgical intervention. Free muscle transfer, however, remains the gold standard for facial reanimation. Innervation of transferred muscle has been described with use of one or more donor cranial nerves. 79 89 91 The gracilis muscle motorized by contralateral cross-facial nerve graft and/or ipsilateral masseteric nerve remains the most commonly used muscle in facial reanimation. When gracilis flap reconstruction is employed, a two-stage approach is typically necessary with antecedent cross-face nerve grafting to decrease the overall time for flap innervation. 95 The sural nerve is most commonly used for cross-face grafting due to its length and low donor site morbidity. 96 The utilization of cross-face nerve graft is of paramount importance in this patient population to allow some level of spontaneous and emotional facial movement.
Isolated dynamic procedures, addressing paralysis of the lower lip, have also been described with use of anterior digastric transfer or fascia graft. 97 98 99 The co-senior author (B.A.), however, prefers to perform chemodenervation of contralateral depressor labii inferioris or selective neurectomy of contralateral marginal mandibular nerve to improve symmetry of lower lip movement and dental show. 17 100 Ultimately, facial reanimation outcomes may be optimized with multimodal approaches utilizing a combination of chemodenervation as well as static and dynamic procedures to address functional and aesthetic impairment. 101
Conclusion
Management of facial paralysis can be daunting. Therefore, a comprehensive approach with systematic assessment of functional and aesthetic deficits is necessary. A thorough history and physical examination is critical in establishing a diagnosis and appropriate treatment plan. A multidisciplinary approach should be implemented with multimodal therapy including medical treatment, physical therapy, meticulous ocular care, and appropriate surgical intervention dependent on the zonal permutation of paralysis and underlying pathology.
Conflict of Interest None declared.
Disclosure Statement
Dr. Azizzadeh receives royalties from Wiley, Thieme, Elsevier, and Springer. Dr. Ducic and Dr. Shokri have no financial or conflicts of interest to disclose. No funding was received for this article.
References
- 1.Ho A L, Scott A M, Klassen A F, Cano S J, Pusic A L, Van Laeken N. Measuring quality of life and patient satisfaction in facial paralysis patients: a systematic review of patient-reported outcome measures. Plast Reconstr Surg. 2012;130(01):91–99. doi: 10.1097/PRS.0b013e318254b08d. [DOI] [PubMed] [Google Scholar]
- 2.Ishii L E, Godoy A, Encarnacion C O, Byrne P J, Boahene K D, Ishii M. What faces reveal: impaired affect display in facial paralysis. Laryngoscope. 2011;121(06):1138–1143. doi: 10.1002/lary.21764. [DOI] [PubMed] [Google Scholar]
- 3.Pavese C, Cecini M, Camerino N. Functional and social limitations after facial palsy: expanded and independent validation of the Italian version of the facial disability index. Phys Ther. 2014;94(09):1327–1336. doi: 10.2522/ptj.20130254. [DOI] [PubMed] [Google Scholar]
- 4.VanSwearingen J M, Cohn J F, Turnbull J, Mrzai T, Johnson P. Psychological distress: linking impairment with disability in facial neuromotor disorders. Otolaryngol Head Neck Surg. 1998;118(06):790–796. doi: 10.1016/S0194-5998(98)70270-0. [DOI] [PubMed] [Google Scholar]
- 5.Valls-Solé J. Facial palsy, postparalytic facial syndrome, and hemifacial spasm. Mov Disord. 2002;17 02:S49–S52. doi: 10.1002/mds.10059. [DOI] [PubMed] [Google Scholar]
- 6.Hohman M H, Hadlock T A. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. Laryngoscope. 2014;124(07):E283–E293. doi: 10.1002/lary.24542. [DOI] [PubMed] [Google Scholar]
- 7.Slattery W H, Azizzadeh B. 1st ed. New York, NY: Thieme Medical Publishers, Inc.; 2014. The Facial Nerve. [Google Scholar]
- 8.Peitersen E. Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4–30. [PubMed] [Google Scholar]
- 9.Adour K K, Byl F M, Hilsinger R L, Jr, Kahn Z M, Sheldon M I. The true nature of Bell's palsy: analysis of 1,000 consecutive patients. Laryngoscope. 1978;88(05):787–801. doi: 10.1002/lary.1978.88.5.787. [DOI] [PubMed] [Google Scholar]
- 10.Devriese P P, Schumacher T, Scheide A, de Jongh R H, Houtkooper J M. Incidence, prognosis and recovery of Bell's palsy. A survey of about 1000 patients (1974-1983) Clin Otolaryngol Allied Sci. 1990;15(01):15–27. doi: 10.1111/j.1365-2273.1990.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 11.Azizzadeh B, Frisenda J L. Surgical management of postparalysis facial palsy and synkinesis. Otolaryngol Clin North Am. 2018;51(06):1169–1178. doi: 10.1016/j.otc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Beurskens C H, Oosterhof J, Nijhuis-van der Sanden M W. Frequency and location of synkineses in patients with peripheral facial nerve paresis. Otol Neurotol. 2010;31(04):671–675. doi: 10.1097/MAO.0b013e3181d8d84d. [DOI] [PubMed] [Google Scholar]
- 13.Celik M, Forta H, Vural C. The development of synkinesis after facial nerve paralysis. Eur Neurol. 2000;43(03):147–151. doi: 10.1159/000008154. [DOI] [PubMed] [Google Scholar]
- 14.Kimura J, Rodnitzky R L, Okawara S H. Electrophysiologic analysis of aberrant regeneration after facial nerve paralysis. Neurology. 1975;25(10):989–993. doi: 10.1212/wnl.25.10.989. [DOI] [PubMed] [Google Scholar]
- 15.Wetzig P. Aberrant regeneration of oculomotor and facial nerves. Rocky Mt Med J. 1957;54(04):347–348. [PubMed] [Google Scholar]
- 16.Brach J S, VanSwearingen J, Delitto A, Johnson P C. Impairment and disability in patients with facial neuromuscular dysfunction. Otolaryngol Head Neck Surg. 1997;117(04):315–321. doi: 10.1016/S0194-5998(97)70119-0. [DOI] [PubMed] [Google Scholar]
- 17.Azizzadeh B, Irvine L E, Diels J. Modified Selective Neurectomy for the treatment of post-facial paralysis synkinesis. Plast Reconstr Surg. 2019;143(05):1483–1496. doi: 10.1097/PRS.0000000000005590. [DOI] [PubMed] [Google Scholar]
- 18.Devriese P P, Moesker W H. The natural history of facial paralysis in herpes zoster. Clin Otolaryngol Allied Sci. 1988;13(04):289–298. doi: 10.1111/j.1365-2273.1988.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 19.Robillard R B, Hilsinger R L, Jr, Adour K K.Ramsay Hunt facial paralysis: clinical analyses of 185 patients Otolaryngol Head Neck Surg 198695(3 Pt 1):292–297. [DOI] [PubMed] [Google Scholar]
- 20.Marenda S A, Olsson J E. The evaluation of facial paralysis. Otolaryngol Clin North Am. 1997;30(05):669–682. [PubMed] [Google Scholar]
- 21.Gupta S, Mends F, Hagiwara M, Fatterpekar G, Roehm P C. Imaging the facial nerve: a contemporary review. Radiol Res Pract. 2013;2013:248039. doi: 10.1155/2013/248039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen R C. Controversies in periocular reconstruction for facial nerve palsy. Curr Opin Ophthalmol. 2018;29(05):423–427. doi: 10.1097/ICU.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 23.Joseph S S, Joseph A W, Smith J I, Niziol L M, Musch D C, Nelson C C. Evaluation of patients with facial palsy and ophthalmic sequelae: a 23-year retrospective review. Ophthalmic Epidemiol. 2017;24(05):341–345. doi: 10.1080/09286586.2017.1294186. [DOI] [PubMed] [Google Scholar]
- 24.Kleiss I J, Eviston T J, Hadlock T A. Nijmegen, Netherlands: Radboud University Nijmegen; 2015. Quantitative assessment of facial function in patients with peripheral facial palsy: a systematic review. In: Assessment of Facial Function in Peripheral Facial Palsy; pp. 97–122. [Google Scholar]
- 25.Sir Charles Bell Society . Fattah A Y, Gurusinghe A DR, Gavilan J. Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plast Reconstr Surg. 2015;135(02):569–579. doi: 10.1097/PRS.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 26.Iacolucci C M, Banks C, Jowett N. Development and validation of a spontaneous smile assay. JAMA Facial Plast Surg. 2015;17(03):191–196. doi: 10.1001/jamafacial.2015.0083. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Akizuki T, Ozawa T, Yoshimura K, Agawa K, Ota T. Dual innervation method using one-stage reconstruction with free latissimus dorsi muscle transfer for re-animation of established facial paralysis: simultaneous reinnervation of the ipsilateral masseter motor nerve and the contralateral facial nerve to improve the quality of smile and emotional facial expressions. J Plast Reconstr Aesthet Surg. 2009;62(12):1589–1597. doi: 10.1016/j.bjps.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Jowett N, Gaudin R A, Banks C A, Hadlock T A. Steroid use in Lyme disease-associated facial palsy is associated with worse long-term outcomes. Laryngoscope. 2017;127(06):1451–1458. doi: 10.1002/lary.26273. [DOI] [PubMed] [Google Scholar]
- 29.Smouha E E, Coyle P K, Shukri S. Facial nerve palsy in Lyme disease: evaluation of clinical diagnostic criteria. Am J Otol. 1997;18(02):257–261. [PubMed] [Google Scholar]
- 30.Su B M, Kuan E C, St John M A. What is the role of imaging in the evaluation of the patient presenting with unilateral facial paralysis? Laryngoscope. 2018;128(02):297–298. doi: 10.1002/lary.26825. [DOI] [PubMed] [Google Scholar]
- 31.Gaudin R A, Jowett N, Banks C A, Knox C J, Hadlock T A. Bilateral facial paralysis: a 13-year experience. Plast Reconstr Surg. 2016;138(04):879–887. doi: 10.1097/PRS.0000000000002599. [DOI] [PubMed] [Google Scholar]
- 32.Jowett N, Hadlock T A. Contemporary management of Bell palsy. Facial Plast Surg. 2015;31(02):93–102. doi: 10.1055/s-0035-1549040. [DOI] [PubMed] [Google Scholar]
- 33.Remenschneider A K, Michalak S, Kozin E D. Is serial electroneuronography indicated following temporal bone trauma? Otol Neurotol. 2017;38(04):572–576. doi: 10.1097/MAO.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 34.Yadav S, Panda N K, Verma R, Bakshi J, Modi M. Surgery for post-traumatic facial paralysis: are we overdoing it? Eur Arch Otorhinolaryngol. 2018;275(11):2695–2703. doi: 10.1007/s00405-018-5141-y. [DOI] [PubMed] [Google Scholar]
- 35.Engström M, Berg T, Stjernquist-Desatnik A. Prednisolone and valaciclovir in Bell's palsy: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008;7(11):993–1000. doi: 10.1016/S1474-4422(08)70221-7. [DOI] [PubMed] [Google Scholar]
- 36.Madhok V B, Gagyor I, Daly F. Corticosteroids for Bell's palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2016;7:CD001942. doi: 10.1002/14651858.CD001942.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan F M, Swan I R, Donnan P T. Early treatment with prednisolone or acyclovir in Bell's palsy. N Engl J Med. 2007;357(16):1598–1607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- 38.Austin J R, Peskind S P, Austin S G, Rice D H. Idiopathic facial nerve paralysis: a randomized double blind controlled study of placebo versus prednisone. Laryngoscope. 1993;103(12):1326–1333. doi: 10.1288/00005537-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Pitaro J, Waissbluth S, Daniel S J. Do children with Bell's palsy benefit from steroid treatment? A systematic review. Int J Pediatr Otorhinolaryngol. 2012;76(07):921–926. doi: 10.1016/j.ijporl.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 40.Unüvar E, Oğuz F, Sidal M, Kiliç A. Corticosteroid treatment of childhood Bell's palsy. Pediatr Neurol. 1999;21(05):814–816. doi: 10.1016/s0887-8994(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 41.Baugh R F, Basura G J, Ishii L E.Clinical practice guideline: Bell's palsy Otolaryngol Head Neck Surg 2013149(03, Suppl):S1–S27. [DOI] [PubMed] [Google Scholar]
- 42.de Almeida J R, Al Khabori M, Guyatt G H. Combined corticosteroid and antiviral treatment for Bell palsy: a systematic review and meta-analysis. JAMA. 2009;302(09):985–993. doi: 10.1001/jama.2009.1243. [DOI] [PubMed] [Google Scholar]
- 43.McAllister K, Walker D, Donnan P T, Swan I. Surgical interventions for the early management of Bell's palsy. Cochrane Database Syst Rev. 2013;(10):CD007468. doi: 10.1002/14651858.CD007468.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Hato N, Yamada H, Kohno H. Valacyclovir and prednisolone treatment for Bell's palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol. 2007;28(03):408–413. doi: 10.1097/01.mao.0000265190.29969.12. [DOI] [PubMed] [Google Scholar]
- 45.Perry C M, Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996;52(05):754–772. doi: 10.2165/00003495-199652050-00009. [DOI] [PubMed] [Google Scholar]
- 46.Wagstaff A J, Faulds D, Goa K L. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47(01):153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 47.Gagyor I, Madhok V B, Daly F. Antiviral treatment for Bell's palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2015;CD001869(11):CD001869. doi: 10.1002/14651858.CD001869.pub9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adour K K, Ruboyianes J M, Von Doersten P G. Bell's palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol. 1996;105(05):371–378. doi: 10.1177/000348949610500508. [DOI] [PubMed] [Google Scholar]
- 49.Cadavid D, Auwaerter P G, Rumbaugh J, Gelderblom H. Antibiotics for the neurological complications of Lyme disease. Cochrane Database Syst Rev. 2016;12:CD006978. doi: 10.1002/14651858.CD006978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wormser G P, Dattwyler R J, Shapiro E D. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(09):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 51.Clark J R, Carlson R D, Sasaki C T, Pachner A R, Steere A C. Facial paralysis in Lyme disease. Laryngoscope. 1985;95(11):1341–1345. [PubMed] [Google Scholar]
- 52.Quality Standards Subcommittee of the American Academy of Neurology . Halperin J J, Shapiro E D, Logigian E. Practice parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2007;69(01):91–102. doi: 10.1212/01.wnl.0000265517.66976.28. [DOI] [PubMed] [Google Scholar]
- 53.Ellefsen B, Bonding P. Facial palsy in acute otitis media. Clin Otolaryngol Allied Sci. 1996;21(05):393–395. doi: 10.1046/j.1365-2273.1996.00810.x. [DOI] [PubMed] [Google Scholar]
- 54.Redaelli de Zinis L O, Gamba P, Balzanelli C. Acute otitis media and facial nerve paralysis in adults. Otol Neurotol. 2003;24(01):113–117. doi: 10.1097/00129492-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Makeham T P, Croxson G R, Coulson S. Infective causes of facial nerve paralysis. Otol Neurotol. 2007;28(01):100–103. doi: 10.1097/01.mao.0000232009.01116.3f. [DOI] [PubMed] [Google Scholar]
- 56.Brach J S, VanSwearingen J M. Physical therapy for facial paralysis: a tailored treatment approach. Phys Ther. 1999;79(04):397–404. [PubMed] [Google Scholar]
- 57.Teixeira L J, Valbuza J S, Prado G F. Physical therapy for Bell's palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2011;CD006283(12):CD006283. doi: 10.1002/14651858.CD006283.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Diels H J. Facial paralysis: is there a role for a therapist? Facial Plast Surg. 2000;16(04):361–364. doi: 10.1055/s-2000-15546. [DOI] [PubMed] [Google Scholar]
- 59.Diels H J, Combs D. Neuromuscular retraining for facial paralysis. Otolaryngol Clin North Am. 1997;30(05):727–743. [PubMed] [Google Scholar]
- 60.Skouras E, Merkel D, Grosheva M. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor Neurol Neurosci. 2009;27(03):237–251. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- 61.Beurskens C H, Heymans P G. Positive effects of mime therapy on sequelae of facial paralysis: stiffness, lip mobility, and social and physical aspects of facial disability. Otol Neurotol. 2003;24(04):677–681. doi: 10.1097/00129492-200307000-00024. [DOI] [PubMed] [Google Scholar]
- 62.Pereira L M, Obara K, Dias J M, Menacho M O, Lavado E L, Cardoso J R. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clin Rehabil. 2011;25(07):649–658. doi: 10.1177/0269215510395634. [DOI] [PubMed] [Google Scholar]
- 63.Manikandan N. Effect of facial neuromuscular re-education on facial symmetry in patients with Bell's palsy: a randomized controlled trial. Clin Rehabil. 2007;21(04):338–343. doi: 10.1177/0269215507070790. [DOI] [PubMed] [Google Scholar]
- 64.Chen N, Zhou M, He L, Zhou D, Li N. Acupuncture for Bell's palsy. Cochrane Database Syst Rev. 2010;(08):CD002914. doi: 10.1002/14651858.CD002914.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foecking E M, Fargo K N, Coughlin L M, Kim J T, Marzo S J, Jones K J. Single session of brief electrical stimulation immediately following crush injury enhances functional recovery of rat facial nerve. J Rehabil Res Dev. 2012;49(03):451–458. doi: 10.1682/jrrd.2011.03.0033. [DOI] [PubMed] [Google Scholar]
- 66.Coulson S E, O'dwyer N J, Adams R D, Croxson G R. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004;25(06):1014–1019. doi: 10.1097/00129492-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 67.Fu L, Bundy C, Sadiq S A. Psychological distress in people with disfigurement from facial palsy. Eye (Lond) 2011;25(10):1322–1326. doi: 10.1038/eye.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nellis J C, Ishii M, Byrne P J, Boahene K DO, Dey J K, Ishii L E. Association among facial paralysis, depression, and quality of life in facial plastic surgery patients. JAMA Facial Plast Surg. 2017;19(03):190–196. doi: 10.1001/jamafacial.2016.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindsay R W, Robinson M, Hadlock T A. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Phys Ther. 2010;90(03):391–397. doi: 10.2522/ptj.20090176. [DOI] [PubMed] [Google Scholar]
- 70.Dimit R, Gire A, Pflugfelder S C, Bergmanson J P. Patient ocular conditions and clinical outcomes using a PROSE scleral device. Cont Lens Anterior Eye. 2013;36(04):159–163. doi: 10.1016/j.clae.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Conley J.Hypoglossal crossover–122 casesTrans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol 1977;84(4 Pt 1): ORL-763-8 [PubMed]
- 72.Gavron J P, Clemis J D.Hypoglossal-facial nerve anastomosis: a review of forty cases caused by facial nerve injuries in the posterior fossa Laryngoscope 198494(11 Pt 1):1447–1450. [PubMed] [Google Scholar]
- 73.Kunihiro T, Kanzaki J, Yoshihara S, Satoh Y, Satoh A. Hypoglossal-facial nerve anastomosis after acoustic neuroma resection: influence of the time anastomosis on recovery of facial movement. ORL J Otorhinolaryngol Relat Spec. 1996;58(01):32–35. doi: 10.1159/000276791. [DOI] [PubMed] [Google Scholar]
- 74.Wu P, Chawla A, Spinner R J. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen Res. 2014;9(20):1796–1809. doi: 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borschel G H, Kawamura D H, Kasukurthi R, Hunter D A, Zuker R M, Woo A S. The motor nerve to the masseter muscle: an anatomic and histomorphometric study to facilitate its use in facial reanimation. J Plast Reconstr Aesthet Surg. 2012;65(03):363–366. doi: 10.1016/j.bjps.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 76.Stennert E. I. Hypoglossal facial anastomosis: its significance for modern facial surgery. II. Combined approach in extratemporal facial nerve reconstruction. Clin Plast Surg. 1979;6(03):471–486. [PubMed] [Google Scholar]
- 77.Slattery W H, III, Cassis A M, Wilkinson E P, Santos F, Berliner K. Side-to-end hypoglossal to facial anastomosis with transposition of the intratemporal facial nerve. Otol Neurotol. 2014;35(03):509–513. doi: 10.1097/MAO.0b013e3182936bcf. [DOI] [PubMed] [Google Scholar]
- 78.May M, Sobol S M, Mester S J. Hypoglossal-facial nerve interpositional-jump graft for facial reanimation without tongue atrophy. Otolaryngol Head Neck Surg. 1991;104(06):818–825. doi: 10.1177/019459989110400609. [DOI] [PubMed] [Google Scholar]
- 79.Peng G L, Azizzadeh B. Cross-facial nerve grafting for facial reanimation. Facial Plast Surg. 2015;31(02):128–133. doi: 10.1055/s-0035-1549046. [DOI] [PubMed] [Google Scholar]
- 80.Razfar A, Lee M K, Massry G G, Azizzadeh B. Facial paralysis reconstruction. Otolaryngol Clin North Am. 2016;49(02):459–473. doi: 10.1016/j.otc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Undavia S, Azizzadeh B. San Diego, CA: Plural Publishing; 2014. Facial Reanimation With Free Tissue Transfer; pp. 209–230. [Google Scholar]
- 82.Azizzadeh B, Pettijohn K J. The gracilis free flap. Facial Plast Surg Clin North Am. 2016;24(01):47–60. doi: 10.1016/j.fsc.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Rivas A, Boahene K D, Bravo H C, Tan M, Tamargo R J, Francis H W. A model for early prediction of facial nerve recovery after vestibular schwannoma surgery. Otol Neurotol. 2011;32(05):826–833. doi: 10.1097/MAO.0b013e31821b0afd. [DOI] [PubMed] [Google Scholar]
- 84.Alex J C, Nguyen D B. Multivectored suture suspension: a minimally invasive technique for reanimation of the paralyzed face. Arch Facial Plast Surg. 2004;6(03):197–201. doi: 10.1001/archfaci.6.3.197. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y M, Sherris D A. Static procedures for the management of the midface and lower face. Facial Plast Surg. 2008;24(02):211–215. doi: 10.1055/s-2008-1075836. [DOI] [PubMed] [Google Scholar]
- 86.Anderson R L, Gordy D D. The tarsal strip procedure. Arch Ophthalmol. 1979;97(11):2192–2196. doi: 10.1001/archopht.1979.01020020510021. [DOI] [PubMed] [Google Scholar]
- 87.Joseph S S, Joseph A W, Douglas R S, Massry G G. Periocular reconstruction in patients with facial paralysis. Otolaryngol Clin North Am. 2016;49(02):475–487. doi: 10.1016/j.otc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Yoo D B, Griffin G R, Azizzadeh B, Massry G G. The minimally invasive, orbicularis-sparing, lower eyelid recession for mild to moderate lower eyelid retraction with reduced orbicularis strength. JAMA Facial Plast Surg. 2014;16(02):140–146. doi: 10.1001/jamafacial.2013.2401. [DOI] [PubMed] [Google Scholar]
- 89.Boahene K D. Dynamic muscle transfer in facial reanimation. Facial Plast Surg. 2008;24(02):204–210. doi: 10.1055/s-2008-1075835. [DOI] [PubMed] [Google Scholar]
- 90.Gillies H. Experiences with fascia lata grafts in the operative treatment of facial paralysis: (section of otology and section of laryngology) Proc R Soc Med. 1934;27(10):1372–1382. [PMC free article] [PubMed] [Google Scholar]
- 91.Harii K, Ohmori K, Torii S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis. A preliminary report. Plast Reconstr Surg. 1976;57(02):133–143. doi: 10.1097/00006534-197602000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Harii K, Asato H, Yoshimura K, Sugawara Y, Nakatsuka T, Ueda K. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998;102(04):941–951. doi: 10.1097/00006534-199809040-00001. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin C R.Permanent facial paralysis; the role of surgical support Lancet 19522(6736):647–651. [DOI] [PubMed] [Google Scholar]
- 94.Shindo M. Facial reanimation with microneurovascular free flaps. Facial Plast Surg. 2000;16(04):357–359. doi: 10.1055/s-2000-15551. [DOI] [PubMed] [Google Scholar]
- 95.O'Brien B M, Franklin J D, Morrison W A. Cross-facial nerve grafts and microneurovascular free muscle transfer for long established facial palsy. Br J Plast Surg. 1980;33(02):202–215. doi: 10.1016/0007-1226(80)90013-2. [DOI] [PubMed] [Google Scholar]
- 96.Coert J H, Dellon A L. Clinical implications of the surgical anatomy of the sural nerve. Plast Reconstr Surg. 1994;94(06):850–855. doi: 10.1097/00006534-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 97.Edgerton M T. Surgical correction of facial paralysis: a plea for better reconstructions. Ann Surg. 1967;165(06):985–998. doi: 10.1097/00000658-196706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan S T. Anterior belly of digastric muscle transfer: a useful technique in head and neck surgery. Head Neck. 2002;24(10):947–954. doi: 10.1002/hed.10150. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe Y, Sasaki R, Agawa K, Akizuki T. Bidirectional/double fascia grafting for simple and semi-dynamic reconstruction of lower lip deformity in facial paralysis. J Plast Reconstr Aesthet Surg. 2015;68(03):321–328. doi: 10.1016/j.bjps.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 100.Azizzadeh B.Selective neurectomy with platysmal myotomy for partial facial paralysis. International Facial Nerve Symposium 2017. Los Angeles, CA
- 101.Cabin J A, Massry G G, Azizzadeh B. Botulinum toxin in the management of facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2015;23(04):272–280. doi: 10.1097/MOO.0000000000000176. [DOI] [PubMed] [Google Scholar]


