Abstract
Disulfide-bond A oxidoreductase-like protein (DsbA-L) is a molecular chaperone involved in the multimerization of adiponectin. Recent studies have found that DsbA-L is related to metabolic diseases including gestational diabetes mellitus (GDM), and can be regulated by peroxisome proliferator-activated receptor γ (PPARγ) agonists; the specific mechanism, however, is uncertain. Furthermore, the relationship between DsbA-L and the novel adipokine chemerin is also unclear. This article aims to investigate the role of DsbA-L in the improvement of insulin resistance by PPARγ agonists in trophoblast cells cultured by the high-glucose simulation of GDM placenta. Immunohistochemistry and western blot were used to detect differences between GDM patients and normal pregnant women in DsbA-L expression in the adipose tissue. The western blot technique was performed to verify the relationship between PPARγ agonists and DsbA-L, and to explore changes in key molecules of the insulin signaling pathway, as well as the effect of chemerin on DsbA-L. Results showed that DsbA-L was significantly downregulated in the adipose tissue of GDM patients. Both PPARγ agonists and chemerin could upregulate the level of DsbA-L. Silencing DsbA-L affected the function of rosiglitazone to promote the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)/AKT pathway. Therefore, it is plausible to speculate that DsbA-L is essential in the environment of PPARγ agonists for raising insulin sensitivity. Overall, we further clarified the mechanism by which PPARγ agonists improve insulin resistance.
Keywords: Disulfide-bond A oxidoreductase-like protein (DsbA-L), Peroxisome proliferator-activated receptor γ (PPARγ), Chemerin, Insulin signaling pathway, Gestational diabetes mellitus
1. Introduction
Disulfide-bond A oxidoreductase (DsbA) is a protein disulfide isomerase that contributes to the formation of disulfide bonds in Escherichia coli (Wang and Scherer, 2008). Glutathione S‐transferase kappa (GST-κ) shares high structural similarities to DsbA, and hence it is also called “DsbA-like protein (DsbA-L).” The DsbA-L gene, located in the mitochondria, is essential to maintain mitochondrial homeostasis. The downregulation of DsbA-L could trigger the imbalanced secretion of mitochondrial DNA (mtDNA), thereby inducing mitochondrial dysfunction (Deng et al., 2020). Furthermore, it has been reported that the lack of DsbA-L could activate the cyclic guanosine-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway to enhance inflammation and insulin resistance (Bai et al., 2017). It was supposed that polymorphisms of the DsbA-L gene might be associated with metabolic diseases (Gao et al., 2009).
Gestational diabetes mellitus (GDM) is defined as a type of metabolic disease, characterized by hyperglycemia at the onset of pregnancy. The worldwide prevalence of GDM has reached approximately 15% to date (Griffith et al., 2020), with the risk of type 2 diabetes mellitus (T2DM) getting higher with the extension of follow-up time in GDM patients (Li et al., 2020). Studies have found that adiponectin expression showed constantly lower levels in GDM patients (Lorenzo-Almorós et al., 2019), which is likely related to decreased levels of chaperone DsbA-L. Insulin resistance is the major feature of GDM. The exact roles DsbA-L plays in the mechanism of insulin signaling transduction in GDM are not yet fully understood. Therefore, we established a cell model to mimic GDM placenta by adding glucose at high concentration (25 mmol/L) to the growth medium of a type of human trophoblastic cell lines, namely HTR-8/SVneo cells. The relevant methods have been verified by multiple papers in the literature (Basak et al., 2015; Kuricova et al., 2016; Lampropoulou et al., 2016; Wang et al., 2019).
As a molecular chaperone, DsbA-L has the critical role of dominating the assembly and secretion of adiponectin (Zhou et al., 2010). Interactions between DsbA-L and other adipokines, however, remain to be fully explored. An inflammatory chemokine named chemerin was firstly identified by Goralski et al. (2007), as a novel adipokine. A later review elaborated that chemerin affects lipogenesis and angiogenesis via binding to chemokine-like receptor 1 (CMKLR1) (Helfer and Wu, 2018). Beyond that, chemerin also participates in the regulation of glucose metabolism (Helfer and Wu, 2018). Previous studies by authors of the present work have shown that peroxisome proliferator-activated receptor γ (PPARγ) agonists can affect insulin signaling pathways by regulating chemerin (Zhou et al., 2020). These agonists could enhance the secretion of adiponectin by binding to the peroxisome proliferator response element (PPRE) in the promoter of DsbA-L (Jin et al., 2015). Thiazolidinediones (TZDs), such as rosiglitazone (RSG), are types of drug for the treatment of diabetes, decreasing insulin resistance through the activation of PPARγ (Lebovitz, 2019).
Given that both DsbA-L and insulin signaling pathways could be dominated by the activation of PPARγ, we investigated the effect of PPARγ on downstream molecules of the insulin pathway after silencing DsbA-L gene to determine whether DsbA-L affects PPARγ agonist function. In addition, since DsbA-L is associated with adiponectin multimerization, we explored the relationship between DsbA-L and chemerin, a novel adipokine, to better understand the role of DsbA-L in regulating adipokines other than adiponectin.
2. Materials and methods
2.1. Human samples
A total of 16 samples of subcutaneous adipose tissue were collected from pregnant women who underwent regular obstetric examination and suffered cesarean delivery in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). There were six cases of GDM patients and ten normal pregnancies among these cases. A diagnosis of GDM was determined via 75 g Oral Glucose Tolerance Test (OGTT) according to the standards set by International Association of Diabetes and Pregnancy Study Groups (IADPSG). Pregnancies accompanied by basic illnesses, infectious diseases, and other complications of pregnancy besides GDM were excluded. The present study was approved by the Ethics Committee of Tongji Hospital (No. TJ-IRB20170506).
2.2. Immunohistochemical analysis
Subcutaneous adipose tissue samples were rapidly washed in phosphate-buffered saline (PBS) and preserved in liquid nitrogen for frozen sections. For sample analysis, they were subjected to antigen retrieval after blocking with hydrogen peroxide solution, and incubated with anti-DsbA-L rabbit polyclonal antibody (ab92819; 1:100 (volume ratio); Abcam, UK) at 4 °C overnight. The next day, the secondary antibody was added to these sections and they were stained by diaminobenzidine. Images were recorded at 100-fold magnification and evaluated with Image-Pro Plus 6.0 (Media Cybernetics, Denver, USA), with the average optical densities applied for analysis.
2.3. Cell culture and small interfering RNA transfection
Cells of the HTR-8/SVneo line were obtained from Wuhan Servicebio Technology Co., Ltd., China. Dulbecco’s modified Eagle’s medium (DMEM) with high glucose concentration (about 25 mmol/L) was applied as the basic medium to mimic circulatory glucose levels of GDM. Additionally, the medium was also supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic. Cells were cultured under the conditions of 37 °C and 5% CO2. Before addition to the cell medium, small interfering RNA (siRNA) of DsbA-L (si-DsbA-L, target sequence CTGTGCC GGTATCAGAATA, Guangzhou RiboBio Co., Ltd., China) and Lipofectamine® 3000 transfection reagents were diluted separately by Opti-MEM™ Reduced Serum Medium, and mixed for 10 min.
2.4. Western blot analysis
The HTR-8/SVneo cells were lysed in radio immunoprecipitation assay (RIPA) buffer and phenylmethanesulfonyl fluoride (PMSF), and the extracted protein was denatured by boiling at high temperature. Samples were then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Thereafter, all membranes were blocked using 5% bovine serum albumin (BSA) for 1 h, and incubated overnight at 4 °C with the following primary antibodies: anti-PI3K (phosphatidylinositol 3-kinase) p110β rabbit polyclonal antibody (21739-1-AP; 1:500; Proteintech, China), anti-chemerin rabbit polyclonal antibody (10216-1-AP; 1:200; Proteintech, China), anti-DsbA-L rabbit polyclonal antibody (ab92819; 1:2000; Abcam, UK), anti-AKT2 (protein kinase B (PKB) β) rabbit monoclonal antibody (#3063; 1:1000; Cell Signaling Technology, USA), anti-p44/42 MAPK (mitogen-activated protein kinase) (extracellular signal-regulated kinase 1/2 (ERK1/2)) rabbit monoclonal antibody (#4695; 1:1000; Cell Signaling Technology, USA), anti-phospho-p44/42 MAPK (ERK1/2) rabbit monoclonal antibody (#4370; 1:2000; Cell Signaling Technology, USA), anti-β-actin mouse monoclonal antibody (66009-1-Ig; 1:5000; Proteintech, China), and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mouse monoclonal antibody (60004-1-Ig; 1:10 000; Proteintech, China). The next day, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature, and subjected to electrochemiluminescence assay with detection by G:BOX Chemi XRQ (Syngene, UK). Quantitative analysis was performed using GeneTools software (Syngene, UK).
2.5. Statistical analysis
The SPSS 25.0 software was used for statistical analysis, and GraphPad Prism 5.0 was applied to draw graphics. On the basis of conforming to the normal distribution, a Student’s t-test was utilized to compare two independent samples with the mean±standard deviation (SD) presented for description. The difference was considered statistically significant, when P<0.05.
3. Results
3.1. Clinical metabolic parameters of participants
There were no statistically significant differences in maternal age, weight, or body mass index (BMI) of pregnant women between the GDM group and the control group. However, results of OGTT for the GDM group were significantly higher than those for the control group (Table 1).
Table 1.
Clinical metabolic parameters of participants
| Group | Age (year) | Weight (kg) | BMI (kg/m2) | OGTT 0 h (mmol/L) | OGTT 1 h (mmol/L) | OGTT 2 h (mmol/L) |
| GDM (n=6) | 35.17±3.66 | 74.00±7.72 | 28.18±3.24 | 5.11±0.43 | 10.00±2.70 | 9.35±0.98 |
| Control (n=10) | 32.70±3.62 | 66.55±7.53 | 26.26±2.70 | 4.55±0.35 | 7.46±1.37 | 6.32±1.04 |
|
| ||||||
| P value | 0.210 | 0.087 | 0.222 | 0.013* | 0.024* | 0.000* |
| t value | −1.314 | −1.886 | −1.277 | −2.833 | −2.527 | −5.758 |
GDM: gestational diabetes mellitus; BMI: body mass index; OGTT: oral glucose tolerance test. All data are expressed as mean±standard deviation (SD).
P<0.05
3.2. Expression of DsbA-L in the subcutaneous adipose tissue
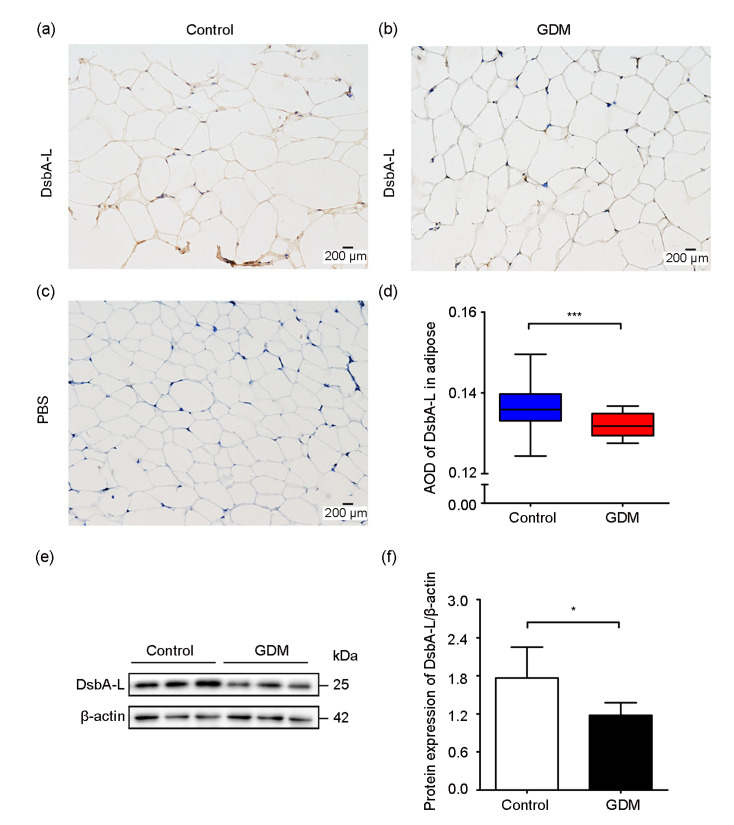
Research has revealed that DsbA-L exists mainly in the mitochondria and endoplasmic reticulum (ER) of adipocytes (Liu et al., 2015). Moreover, DsbA-L is expressed at the highest level in adipose tissue in a location where adiponectin is secreted (Wang and Scherer, 2008). In order to determine the level of DsbA-L in the subcutaneous adipose tissue of GDM patients and normal pregnant women, immunohistochemistry and western blot analyses were performed. We found that DsbA-L existed both in the control group and the GDM group (Figs. 1a and 1b) as opposed to the blank group (Fig. 1c) based on staining, while DsbA-L expression was remarkably reduced in the subcutaneous adipose tissue of GDM patients (Fig. 1d). Western blot showed the same results (Fig. 1e); quantitative analysis indicated that the GDM group showed significantly lower DsbA-L levels compared with the control group (Fig. 1f).
Fig. 1.
Expression of DsbA-L in the subcutaneous adipose tissue of GDM patients and normal pregnant women
(a) Level of DsbA-L in normal pregnant women; (b) Expression of DsbA-L in GDM patients; (c) PBS was a substitute for DsbA-L antibody in the blank group; (d) Comparison of DsbA-L levels by immunohistochemistry experiment; (e) Representative image of the immunoblots of adipose tissue samples (25 μg); (f) Relative quantitative analysis of protein expression of DsbA-L. DsbA-L: disulfide-bond A oxidoreductase-like protein; GDM: gestational diabetes mellitus; PBS: phosphate-buffered saline; AOD: average optical density. Data are shown as mean±standard deviation (SD) (n≥3). * P<0.05, *** P<0.001
3.3. Effect of chemerin on DsbA-L expression
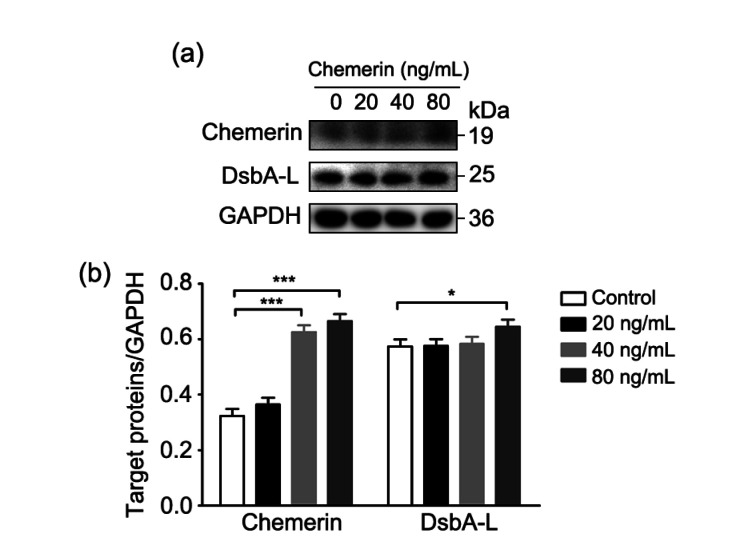
In order to investigate the effect of chemerin on DsbA-L, different concentrations of recombinant chemerin (#300-66; PeproTech, USA) were additionally supplemented to high glucose-treated HTR-8/SVneo cells, and the effects of chemerin on DsbA-L expression were observed by western blot analysis. Compared with the control group (without extra chemerin), the protein expression of chemerin was gradually elevated with increased dose, and the level of DsbA-L also improved simultaneously with raised chemerin concentration. Chemerin at 80 ng/mL led to the most obvious increase of DsbA-L expression, with a statistically significant difference compared with the control group (Fig. 2).
Fig. 2.
Expression of DsbA-L upregulated by chemerin
The chemerin concentration gradient consisted of 20, 40, and 80 ng/mL treatments, as well as a control group without additional chemerin. (a) Representative image of the immunoblots of cell samples (50 μg); (b) Relative quantitative analyses of chemerin and DsbA-L. DsbA-L: disulfide-bond A oxidoreductase-like protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. Data are shown as mean±standard deviation (SD) (n=3). * P<0.05, *** P<0.001
3.4. Effect of PPARγ agonists on DsbA-L expression
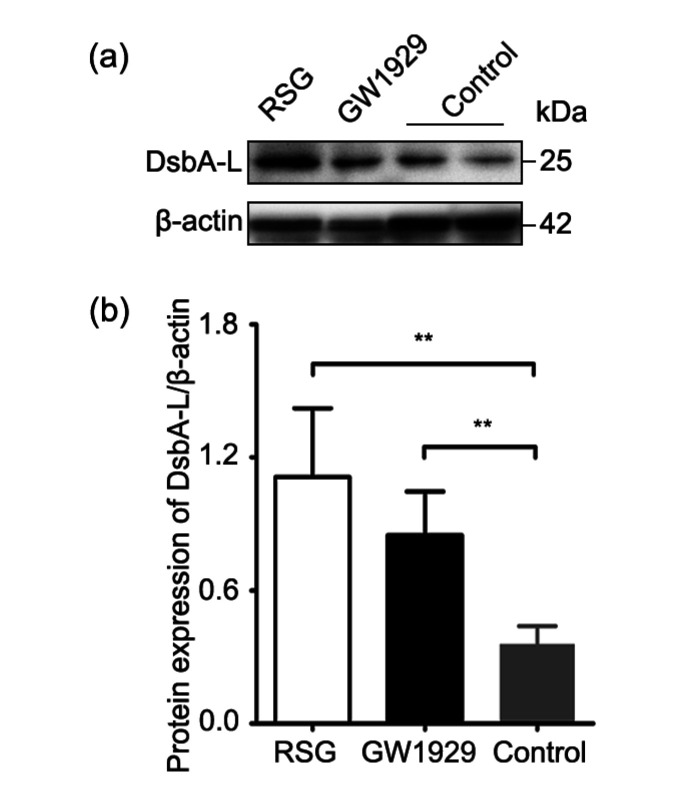
In order to examine the effect of activation of PPARγ on DsbA-L, high glucose-treated HTR-8/SVneo cells were subjected to 20 μmol/L RSG (HY-17386; MedChem Express, USA) and 20 μmol/L GW1929 (HY-15655; MedChem Express, USA), two small molecule agonists of PPARγ, and subsequent western blot analysis following 72 h of incubation. Results showed that protein levels of DsbA-L were significantly increased in the RSG and GW1929 groups (Fig. 3) compared with the control group, suggesting that PPARγ agonists had an effect of upregulating DsbA-L expression.
Fig. 3.
Protein expression of DsbA-L enhanced by PPARγ agonists, RSG, and GW1929
(a) Representative image of the immunoblots of cell samples (50 μg); (b) Relative quantitative analysis of DsbA-L. PPARγ: peroxisome proliferator-activated receptor γ; DsbA-L: disulfide-bond A oxidoreductase-like protein; RSG: rosiglitazone. Data are shown as mean±standard deviation (SD) (n=3). ** P<0.01
3.5. Effect of silencing the DsbA-L gene on PPARγ agonist action of improving insulin resistance
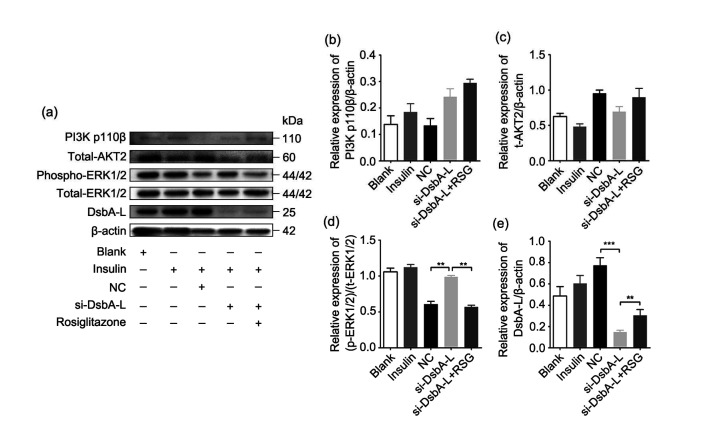
The following treatment was conducted to identify the role of DsbA-L in the process of PPARγ agonist enhancing insulin signaling pathways. Firstly, HTR-8/SVneo cells were incubated with insulin (100 nmol/L) for 48 h to induce the signaling pathways downstream of insulin. Then, we transfected siRNA into these cells targeting the DsbA-L gene (si-DsbA-L group). Six hours later, RSG (20 μmol/L) was added to the cell medium on the basis of silencing the DsbA-L gene for the group named si-DsbA-L+RSG. Finally, we carried out western blot analysis after 72 h; the protein signals of PI3K p110β, AKT2, ERK1/2, and DsbA-L are shown in Fig. 4a. There were no significant changes in the protein expression of PI3K p110β or total AKT2 (Figs. 4b and 4c). The phosphorylation of ERK1/2 was significantly increased after DsbA-L knockdown compared with the negative control (NC, cells transfected with scramble siRNA) group (Fig. 4d). Moreover, the phosphorylation of ERK1/2 was diminished in the si-DsbA-L+RSG group compared with the si-DsbA-L group. In addition, the protein level of DsbA-L was reduced in the si-DsbA-L group, whereas it was elevated in the si-DsbA-L+RSG group (Fig. 4e).
Fig. 4.
Effect of si-DsbA-L on action of PPARγ agonist of improving insulin resistance
(a) Representative image of the immunoblots of cell samples (20 μg); (b) Relative expression of PI3K p110β quantified with β-actin; (c) Relative expression of total-AKT2 quantified with β-actin; (d) Relative expression of phospho-ERK1/2 quantified with total-ERK1/2; (e) Relative expression of DsbA-L quantified with β-actin. PPARγ: peroxisome proliferator-activated receptor γ; si-DsbA-L: small interfering RNA (siRNA) of disulfide-bond A oxidoreductase-like protein (DsbA-L); PI3K: phosphatidylinositol 3-kinase; AKT2: protein kinase B (PKB) β; ERK1/2: extracellular signal-regulated kinase 1/2; NC: negative control; RSG: rosiglitazone; t-AKT2: total-AKT2; p-ERK1/2: phospho-ERK1/2; t-ERK1/2: total-ERK1/2. Data are shown as mean±standard deviation (SD) (n=3). ** P<0.01, *** P<0.001
4. Discussion
The enzyme DsbA-L was firstly found and named by Liu et al. (2008); they identified that DsbA-L could regulate the multimerization of adiponectin. Since then, DsbA-L has appeared in front of the public as a multifaceted player in adiponectin secretion (Wang and Scherer, 2008). It is of great significance to elaborate how this protein participates in the pathophysiological processes of GDM. In the present study, we found that the level of DsbA-L was significantly reduced in GDM-affected adipose tissue. Previous reports revealed that patients with obesity had a significantly decreased level of DsbA-L in contrast to normal-weight controls (Chen et al., 2017). Women with GDM are potentially associated with obesity, and most cases of GDM are related to increased BMI (Kim et al., 2010; Giannakou et al., 2019). This, to some extent, explains the low level of DsbA-L in GDM.
It was observed in previous studies that DsbA-L overexpression could improve insulin sensitivity in mice by promoting the phosphorylation of adenosine 5'-monophosphate-activated protein kinase (AMPK) and PKB/AKT (Liu et al., 2012). Adiponectin signaling primarily enhances insulin receptor substrates (IRSs) to sensitize insulin action (Achari and Jain, 2017). Promoting the biosynthesis and secretion of adiponectin may be an effective way to treat metabolic diseases (Liu and Liu, 2012). The multimerization of adiponectin is attributed to DsbA-L, which seems to suggest a potential relationship between DsbA-L and insulin sensitivity.
Adiponectin multimerization was shown to be promoted by DsbA-L through the regulation of disulfide bond formation (Achari and Jain, 2017). A study demonstrated that circulating levels of adiponectin were reduced in obesity and associated diseases (Liu et al., 2008), indicating that adiponectin was involved in the pathophysiology of these diseases. In fact, adiponectin has some beneficial properties, such as anti-inflammatory and insulin sensitization (Simpson and Whitehead, 2010). Increasing the levels of DsbA-L could improve the stability of adiponectin by inhibiting the adverse effect of ER stress (Zhou et al., 2010). Therefore, heightening the expression of DsbA-L is likely to be a promising strategy to increase insulin sensitivity (Zhou et al., 2010).
In the present study, the expression of DsbA-L was downregulated, resulting in decreased PPARγ agonist function, suggesting that DsbA-L not only participates in insulin sensitivity, but also plays a critical role in insulin signaling pathways. Several studies have indicated extensively that DsbA-L is found downstream of PPARγ (Jin et al., 2015; He et al., 2016). Consistently with these findings and furthermore, we conclude that DsbA-L is extremely significant in PPARγ action. The latter may exert an effect on the PI3K-AKT pathway by regulating the transcription of DsbA-L. The molecular process by which PPARγ agonists improve insulin resistance was further elaborated in mechanism.
Insulin resistance is associated with a damaged insulin signaling pathway (Plows et al., 2018). Phosphorylation of IRS is accompanied by the activation of two insulin signaling pathways: the PI3K-AKT pathway and the ras-MAPK pathway (Taniguchi et al., 2006). There are different negative and positive feedback loops in the AKT and MAPK pathways at the molecular level (Arkun, 2016). The PI3K-AKT signaling pathway is important in the metabolic role of insulin. In this pathway, the p110β subtype of PI3K is essential for mediating the glucose uptake of insulin sensitive tissues. Differently from AKT1 and AKT3, AKT2 is particularly enriched in the liver and adipose tissue, and is co-located with glucose transporter 4 (GLUT4), which is responsible for bringing glucose into the cell as energy supply (Plows et al., 2018). Interestingly, DsbA-L suppression stimulated the phosphorylation of ERK1/2, which might be attributed to mitochondrial dysfunction. The ERK1/2 of the MAPK pathway is mainly involved in the regulation of cell growth and survival, but not the metabolic effect of insulin. As commonly known, mitochondria provide energy for vital cell functions. Therefore, when mitochondrial function is injured via DsbA-L silencing, cells promote the activation of ERK1/2 signaling in order to adapt to new energy requirements and repair the damage (Pal et al., 2020).
We also showed that chemerin could elevate the expression of DsbA-L, which might further increase adiponectin levels. Although we did not verify this possibility via experiments, recent research supports that chemerin exerts an effect on adiponectin to regulate fat metabolism (Ferland et al., 2020), which is attributed to the interactions of adipocytokines. It has been reported that tumor necrosis factor-α (TNF-α) inhibits the multimerization and secretion of adiponectin by impairing DsbA-L (He et al., 2016). Chemerin may be a favorable adipokine, as its function is unlike that of TNF-α. Chemerin was originally discovered as an inflammatory chemokine, encoded by the retinoic acid receptor responder 2 (RARRES2) gene. Further studies have shown that it is a novel adipocytokine secreted by adipose tissue, and is involved in the pathophysiological mechanism of numerous diseases, including obesity and metabolic syndrome (Goralski et al., 2007; Helfer and Wu, 2018). In addition to that of adiponectin, the understanding of the regulatory function of DsbA-L and its interaction with other adipokines is incomplete (Liu et al., 2012). Further research is required to elucidate these mechanisms.
It should be noted that our research has some limitations. Neither the expression of GLUT4 downstream of the insulin signaling pathway is verified by our study, nor does it be clear whether HTR-8/SVneo cells cultured with high glucose in vitro are similar to the circulatory blood glucose levels of GDM patients. The role of DsbA-L in the action of PPARγ agonists in vivo remains to be further explored in animal experiments.
5. Conclusions
In summary, we confirmed the necessity of DsbA-L in PPARγ agonist function that improves insulin resistance, and for the first time we demonstrated that chemerin promotes DsbA-L expression.
Acknowledgments
We thank the Obstetric Laboratory of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China for providing the experimental platform.
Footnotes
Project supported by the National Key Research and Development Program of China (Nos. 2016YFC1000405 and 2018YFC1002903)
Contributors: Xuan ZHOU performed the experiments and prepared this manuscript. Jia-qi LI, Li-jie WEI, Jing JIA and Jing-yi ZHANG participated in carrying out the experiment. Xuan ZHOU and Jia-qi LI conducted data analysis and discussed the results. Meng-zhou HE and Li-jie WEI reviewed and edited the manuscript. Ling FENG and Shao-shuai WANG designed, organized, and supervised the project. All authors have read and approved this manuscript. Therefore, they have full access to all data in this study and take responsibility for the integrity and security of these data.
Compliance with ethics guidelines: Xuan ZHOU, Jia-qi LI, Li-jie WEI, Meng-zhou HE, Jing JIA, Jing-yi ZHANG, Shao-shuai WANG and Ling FENG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkun Y. Dynamic modeling and analysis of the cross-talk between insulin/AKT and MAPK/ERK signaling pathways. PLoS ONE. 2016;11(3):e0149684. doi: 10.1371/journal.pone.0149684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai JL, Cervantes C, Liu J, et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc Natl Acad Sci USA. 2017;114(46):12196–12201. doi: 10.1073/pnas.1708744114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basak S, Das MK, Srinivas V, et al. The interplay between glucose and fatty acids on tube formation and fatty acid uptake in the first trimester trophoblast cells, HTR8/SVneo. Mol Cell Biochem. 2015;401(1-2):11–19. doi: 10.1007/s11010-014-2287-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen HZ, Bai JL, Dong F, et al. Hepatic DsbA-L protects mice from diet-induced hepatosteatosis and insulin resistance. FASEB J. 2017;31(6):2314–2326. doi: 10.1096/fj.201600985R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng X, Yang G, Zheng X, et al. Plasma mtDNA copy numbers are associated with GSTK1 expression and inflammation in type 2 diabetes. Diabet Med. 2020;37(11):1874–1878. doi: 10.1111/dme.14132. [DOI] [PubMed] [Google Scholar]
- 7.Ferland DJ, Garver H, Contreras GA, et al. Chemerin contributes to in vivo adipogenesis in a location-specific manner. PLoS ONE. 2020;15(2):e0229251. doi: 10.1371/journal.pone.0229251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F, Fang QC, Zhang R, et al. Polymorphism of DsbA-L gene associates with insulin secretion and body fat distribution in Chinese population. Endocr J. 2009;56(3):487–494. doi: 10.1507/endocrj.k08e-322. [DOI] [PubMed] [Google Scholar]
- 9.Giannakou K, Evangelou E, Yiallouros P, et al. Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PLoS ONE. 2019;14(4):e0215372. doi: 10.1371/journal.pone.0215372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 11.Griffith RJ, Alsweiler J, Moore AE, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2020;6(6):CD012394. doi: 10.1002/14651858.CD012394.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YD, Lu LF, Wei X, et al. The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine. 2016;51(3):456–468. doi: 10.1007/s12020-015-0741-4. [DOI] [PubMed] [Google Scholar]
- 13.Helfer G, Wu QF. Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol. 2018;238(2):R79–R94. doi: 10.1530/JOE-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin D, Sun J, Huang J, et al. Peroxisome proliferator-activated receptor γ enhances adiponectin secretion via up-regulating DsbA-L expression. Mol Cell Endocrinol. 2015;411:97–104. doi: 10.1016/j.mce.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, England L, Wilson HG, et al. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100(6):1047–1052. doi: 10.2105/AJPH.2009.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuricova K, Pácal L, Šoupal J, et al. Effect of glucose variability on pathways associated with glucotoxicity in diabetes: evaluation of a novel in vitro experimental approach. Diabetes Res Clin Pract. 2016;114:1–8. doi: 10.1016/j.diabres.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Lampropoulou E, Lymperopoulou A, Charonis A. Reduced expression of ERp46 under diabetic conditions in β-cells and the effect of liraglutide. Metabolism. 2016;65(1):7–15. doi: 10.1016/j.metabol.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Lebovitz HE. Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep. 2019;19(12):151. doi: 10.1007/s11892-019-1270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ZY, Cheng YJ, Wang DY, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review and meta-analysis of 170,139 women. J Diabetes Res, 2020:3076463. 2020 doi: 10.1155/2020/3076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ML, Liu F. Up- and down-regulation of adiponectin expression and multimerization: mechanisms and therapeutic implication. Biochimie. 2012;94(10):2126–2130. doi: 10.1016/j.biochi.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu ML, Zhou LJ, Xu AM, et al. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci USA. 2008;105(47):18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ML, Xiang RH, Wilk SA, et al. Fat-specific DsbA-L overexpression promotes adiponectin multimerization and protects mice from diet-induced obesity and insulin resistance. Diabetes. 2012;61(11):2776–2786. doi: 10.2337/db12-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ML, Chen HZ, Wei L, et al. Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes. J Biol Chem. 2015;290(16):10143–10148. doi: 10.1074/jbc.M115.645416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzo-Almorós A, Hang T, Peiró C, et al. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc Diabetol, 18:140. 2019 doi: 10.1186/s12933-019-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal S, Rao GN, Pal A. High glucose-induced ROS accumulation is a critical regulator of ERK1/2-Akt-tuberin-mTOR signalling in RGC-5 cells. Life Sci, 256:117914. 2020 doi: 10.1016/j.lfs.2020.117914. [DOI] [PubMed] [Google Scholar]
- 26.Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson F, Whitehead JP. Adiponectin–it’s all about the modifications. Int J Biochem Cell Biol. 2010;42(6):785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 29.Wang XY, Pan JY, Liu H, et al. AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci. 2019;221:249–258. doi: 10.1016/j.lfs.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci USA. 2008;105(47):18077–18078. doi: 10.1073/pnas.0810027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou LJ, Liu ML, Zhang JJ, et al. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59(11):2809–2816. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Wei LJ, Li JQ, et al. The activation of peroxisome proliferator-activated receptor γ enhances insulin signaling pathways via up-regulating chemerin expression in high glucose treated HTR-8/SVneo cells. Matern-Fetal Med. 2020;2(3):131–140. doi: 10.1097/FM9.0000000000000044. [DOI] [Google Scholar]