Abstract
Vagus nerve stimulation (VNS) paired with rehabilitative training enhances recovery of function in models of stroke and is currently under investigation for use in chronic stroke patients. Dosing is critical in translation of pharmacological therapies, but electrical stimulation therapies often fail to comprehensively explore dosing parameters in preclinical studies. Varying VNS parameters has non-monotonic effects on plasticity in the central nervous system, which may directly impact efficacy for stroke. We sought to optimize stimulation intensity to maximize recovery of motor function in a model of ischemic stroke. The study design was preregistered prior to beginning data collection (DOI: 10.17605/OSF.IO/BMJEK). After training on an automated assessment of forelimb function and receiving an ischemic lesion in motor cortex, rats were separated into groups that received rehabilitative training paired with VNS at distinct stimulation intensities (sham, 0.4 mA, 0.8 mA, or 1.6 mA). Moderate intensity VNS at 0.8 mA enhanced recovery of function compared to all other groups. Neither 0.4 mA nor 1.6 mA VNS was sufficient to improve functional recovery compared to equivalent rehabilitation without VNS. These results demonstrate that moderate intensity VNS delivered during rehabilitation improves recovery and defines an optimized intensity paradigm for clinical implementation of VNS therapy.
Keywords: vagus, stroke, dosing, motor, stimulation
Introduction
Stroke is a leading cause of long-term acquired disability [1]. The development of post-stroke interventions to improve recovery of upper-limb function and reduce disability is of clear importance. Interventions that combine rehabilitation with techniques that augment synaptic plasticity in spared networks hold promise to enhance recovery after stroke [2].
Recently, closed-loop vagus nerve stimulation (VNS) paired with task-specific rehabilitative training has emerged as one such strategy [2]. VNS drives neuromodulator release that enhances neuroplasticity when precisely timed with rehabilitative exercises [3]. Preclinical studies demonstrate that VNS paired with rehabilitative training improves motor function and enhances reorganization of task-specific neurocircuitry in models of stroke and other neurological injuries [3, 4]. Highlighting the clinical potential of this strategy, two completed clinical studies in chronic stroke patients demonstrate that VNS therapy is safe and provide initial evidence of improved upper-limb function, and a Phase 3 pivotal trial is ongoing [5, 6].
While these results are promising, the Stroke Therapy Academic Industry Roundtable (STAIR) recommends that novel treatments develop adequate dose-response curves during preclinical testing [7]. Although such investigations are often conducted for pharmacological treatments, there is less emphasis on dose-response investigation in electrical stimulation studies, despite a well-defined effect of stimulation parameters on outcomes. Dosing studies present challenges for active neurostimulation therapies, as dose parameters include stimulation intensity, frequency, train duration, and inter-train interval. In the present study, we chose to focus on evaluation of stimulation intensity. This parameter has the most well-defined effects on VNS-dependent plasticity, which is believed to underlie its therapeutic action for stroke [8, 9]. We assessed stimulation intensities (0.4, 0.8, and 1.6 mA) that produce an approximately fourfold range of spike activity in the locus coeruleus, a neuromodulatory nucleus required for the pro-plasticity actions of VNS [10, 11]. Our findings reveal that moderate intensity VNS delivered during rehabilitative training significantly enhances recovery after ischemic lesion in rats, whereas low and high intensity stimulation fail to improve recovery compared to rehabilitative training without stimulation. These findings provide information to guide the selection of VNS intensity to improve recovery after stroke.
Methods
This study was pre-registered on Open Science Framework before beginning data collection (DOI: 10.17605/OSF.IO/BMJEK).
Subjects
One-hundred twenty-one female Sprague-Dawley rats weighing approximately 250 g were used for this study (Charles River Laboratories). Rats were housed in a reverse 12:12 hour light cycle to increase daytime activity. Rats were food deprived to no less than 85% of their normal body weight during behavioral testing. On weekends, when no behavioral training was performed, rats had access to food ad libitum. All experimental procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
We performed an a priori power analysis to determine the number of animals required for the study, and we defined exclusion criteria in the study registration before beginning data collection. Using the G*Power program (Universität Dusseldorf), and based off of data from previously published work [3], we calculated the estimated sample size to conduct an ANOVA of all experimental groups at the final week of therapy. Given an estimated Cohen’s f effect size of 0.86, we estimated the sample size for each group to be 7 animals. Therefore, we aimed to assign 13 to 15 subjects to each experimental group in an effort to offset attrition. To minimize subjective bias, rats were dynamically allocated into balanced experimental groups based on behavioral task performance after receiving an ischemic lesion. Thirty-seven rats died after the ischemic lesion and were excluded from the remainder of the study. Additionally, 24 rats failed to demonstrate a forelimb deficit after the lesion, as defined by an average post-lesion baseline performance with at least 30% of trials exceeding 60 degrees on the supination task and were excluded. Three rats were excluded because they failed to perform the supination task after the stroke surgery. Four rats were excluded due to a failed cuff electrode before being randomly assigned to an experimental group. Fifty-three rats were assigned to an experimental group. Of the rats that were assigned to a group, two rats were excluded due to a mechanical failure of the head-mounted connector used for delivering VNS. Nineteen rats were excluded due to a nonfunctioning implanted cuff electrode during the therapy stage of the study, as determined by cuff impedance exceeding 10 kΩ during therapy. Of the twenty-one total rats that were excluded after having been assigned an experimental group, 6 belonged to the 0.4 mA group, 8 belonged to the 0.8 mA group, and 7 belonged to the 1.6 mA group. There was no difference in the proportion of animals excluded across experimental VNS groups (X2 = 0.33, p = 0.84). Because the No VNS group did not receive stimulation and thus were not reliant on a functioning cuff, no rats were excluded from this group. The final number of animals included in the results was 32.
Supination task assessment
Rats trained daily on the supination task which required animals to use their right forelimb to reach, grasp, and rotate a spherical knob past an adaptively scaled angle threshold [3]. If the angle exceeded that threshold within 2 seconds, the trial was recorded as a success and a reward was delivered (45 mg dustless precision pellet, BioServ, Frenchtown, NJ). Each day consisted of two 30 minute training sessions spaced 2 hours apart. On the first day of training, the knob device was positioned at 0.5 cm from the inner cage wall. As rats acquired the task and performance increased, the knob device was retracted from the behavioral booth so that rats were required to reach farther to access the manipulandum. After reaching a distance of 1.25 cm from the inner cage wall, three successive weights were used to increase the force necessary to turn the knob (3 g, 5 g, and 6 g). The weight was increased to the next level after the rat successfully performed 50 trials per behavior session in one day.
The threshold turn angle to receive a food reward was maintained at 5 degrees throughout the initial training period. Once rats reached the 5 g resistance stage, the angle threshold was progressively scaled by the software to adapt the difficulty of the task as previously described [3, 12]. The threshold for a given trial was calculated as the median of the maximal turn angle from the previous 10 trials, up to a maximum of 60 degrees.
Rats were considered proficient at the task when they exceeded a 60-degree turn angle on 75% of trials over a 3-day period. Once proficient, rats received an ischemic lesion in left motor cortex and a vagus nerve cuff implant. One week later, rats returned to behavioral training and completed a 5-day post-lesion performance assessment before being dynamically allocated into balanced experimental groups based on performance. Rats then received rehabilitative training paired with the appropriate VNS intensity for six weeks. No VNS was delivered on the final week to assess effects lasting after the cessation of stimulation.
Vagus nerve cuff construction
Stimulating cuff electrodes were constructed as previously described [13, 14]. In brief, two Teflon-coated multistranded platinum-iridium (0.006”) wires were connected to a 4-mm section of Micro-Renethane tubing (1.8 mm inner diameter). The wires were spaced 2 mm apart along the length of the tubing. An 8 mm region of the wires lining the inside circumference of the tube was stripped of the insulation. A cut was made lengthwise along the tubing to allow the cuff to be wrapped around the nerve and then closed with silk threads. This configuration resulted in the exposed wires being wrapped around the vagus nerve at points separated by 2 mm, while the leads exiting the cuff remained insulated.
Ischemic lesion and vagus nerve cuff implant surgery
The ischemic lesion and cuff implant were performed similar to previous studies [3, 4]. Rats were deeply anesthetized with ketamine hydrochloride (50 mg/kg), xylazine (20 mg/kg), and acepromazine (5 mg/kg) injected intramuscularly. After placing the rat in a stereotaxic frame, a two-channel connector was attached to the skull using acrylic. A craniotomy was performed to expose the left motor cortex using either a microdrill or surgical rongeurs, and the underlying dura was removed. Eight 2 μL injections of Endothelin-1 (ET-1, Bachem, Torrance, CA, 1 mg/mL in saline) were performed into the forelimb region of left motor cortex (1.8 mm depth, mediolateral (ML) coordinates of 2.5 and 3.5 mm, anteroposterior (AP) coordinates of 2.5, 1.5, 0.5, and −0.5 m) followed by a ninth sub-cortical striatal injection (6 mm depth, 0.0 AP, 3.0 ML). Each injection was performed over a period of 2 minutes, and the needle was left in the brain for an additional 5 minutes to allow diffusion of the ET-1 solution. After the final injection, the craniotomy was covered with a thin layer of Kwik-Cast sealant (World Precision Instruments, Sarasota, FL).
The rat was then removed from the stereotaxic frame and placed in a supine position. An incision was made in the neck, and blunt dissection of the muscles was performed to expose the left cervical vagus nerve. After isolating the nerve from the carotid artery, the nerve was placed inside the stimulating cuff, and the cuff was sutured closed. Cuff leads were tunneled subcutaneously and attached to the two-channel connector atop the skull. All incisions were then sutured and the exposed two-channel connector was encapsulated in acrylic.
Immediately after implantation, stimulation efficacy of the implanted cuff electrode was verified by assessing activation of the Hering-Breuer reflex, as previously described [15]. If stimulation trains failed to evoke a reliable reduction in blood oxygen saturation, the cuff was repositioned or replaced.
Upon completion of the surgery, topical antibiotic cream was applied to both incision sites. Rats received subcutaneous administration of Buprenorphine (0.3 mg/kg) and 4 mL of 1:1 0.9% saline and 5% dextrose. Each rat received one oral tablet of Baytril after surgery (2 mg/tablet, BioServ, Frenchtown, NJ). Behavioral training resumed one week after surgery.
Stimulation parameters
VNS was delivered coincident with forelimb movement during rehabilitative training, as in previous studies [3, 4]. Software-controlled stimulation was triggered upon successful supination attempts. Each stimulation consisted of a 500 ms train of 100 μs biphasic pulses at 30 Hz. The stimulation intensities used were 0.4 mA, 0.8 mA, and 1.6 mA, as appropriate for each group.
Tracking voltages of vagus nerve cuff implants
Cuff voltage was recorded daily during behavioral training sessions using ScopeVNS, a custom-built software application (https://github.com/davepruitt/ScopeVNS), connected to PicoScope 2204A digital oscilloscopes (Pico Technology, Tyler, TX). Voltage was used to calculate cuff impedance using the equation V = IR. Rats with an average cuff impedance exceeding 10 kΩ throughout VNS therapy were excluded from the study.
Statistical methods
The primary outcome measure of this study was performance on the supination task. Histological analysis was performed by an individual blind to each rat’s experimental group and quantified using Olympus CellSens. An experimenter blinded to condition traced the lesion in each tissue section, and the area traced was then combined with other sections to calculate the lesion volume. To calculate the percent recovery at the end of therapy, we used each subject’s week 6 performance normalized to the range of its pre and post performance. This was calculated using the following equation:
We used standard parametric statistical tests (ANOVA with Bonferroni-corrected post-hoc tests) to assess differences over time and across experimental groups. A cut-off value of p = 0.05 was used to determine statistical significance for ANOVA. Post hoc comparisons between groups used a Bonferroni-corrected alpha value of 0.017. All results are written as mean ± standard error of the mean (SEM), and a “*” denotes statistical significance in figures. Error bars in figures represent SEM.
Results
Moderate Intensity VNS Significantly Improves Recovery
All groups displayed comparable performance prior to lesion (one-way ANOVA, F(3, 28) = 1.05, p = 0.39) and displayed similar deficits after the lesion (one-way ANOVA, F(3, 28) = 0.32, p = 0.81). To investigate the effect of stimulation intensity on the degree of recovery, each group received rehabilitative training paired with a distinct VNS intensity for 5 weeks (Figure 1). A repeated-measures ANOVA revealed a significant effect of VNS intensity (Figure 2A, F(3, 26) = 3.18, p = 0.0405). During the course of therapy, an effect of 0.8 mA VNS on recovery of motor function emerged. This effect was significant by the third week of therapy (F(3, 28) = 3.43, p = 0.03). At the end of therapy, the 0.8 mA VNS group performed significantly better than other experimental groups (one-way ANOVA, F(3, 26) = 3.18, p = 0.0406; post-hoc Bonferroni-corrected unpaired t-tests; 0.8 mA VNS vs No-VNS, p = 0.011; 0.8 mA VNS vs 0.4 mA VNS, p = 0.016; 0.8 mA VNS vs 1.6 mA VNS, p = 0.019), and achieved a higher percent recovery (Figure 2B, one-way ANOVA, F(3, 26) = 3.48, p = 0.03). The 0.8 mA group was the only group to achieve significantly better performance at the completion of therapy than at the beginning (repeated-measures ANOVA, F(6, 30) = 4.36, p = 0.0028). No difference in task performance was observed between the other groups (post-hoc tests after mixed-model repeated-measures ANOVA; No VNS vs 0.4 mA, p = 0.91; No VNS vs 1.6 mA, p = 0.78; 0.4 mA vs 1.6 mA, p = 0.99). Consistent with previous results, the group receiving rehabilitative training without VNS demonstrated no significant improvements in forelimb performance [3]. All groups performed a similar number of trials (one-way ANOVA, F(3, 28) = 0.27, p = 0.84) and received a comparable number of stimulations (one-way ANOVA, F(3, 28) = 0.54, p = 0.66). These results suggest that a moderate stimulation intensity confers the greatest benefit to motor function when pairing VNS with rehabilitative training.
Figure 1:
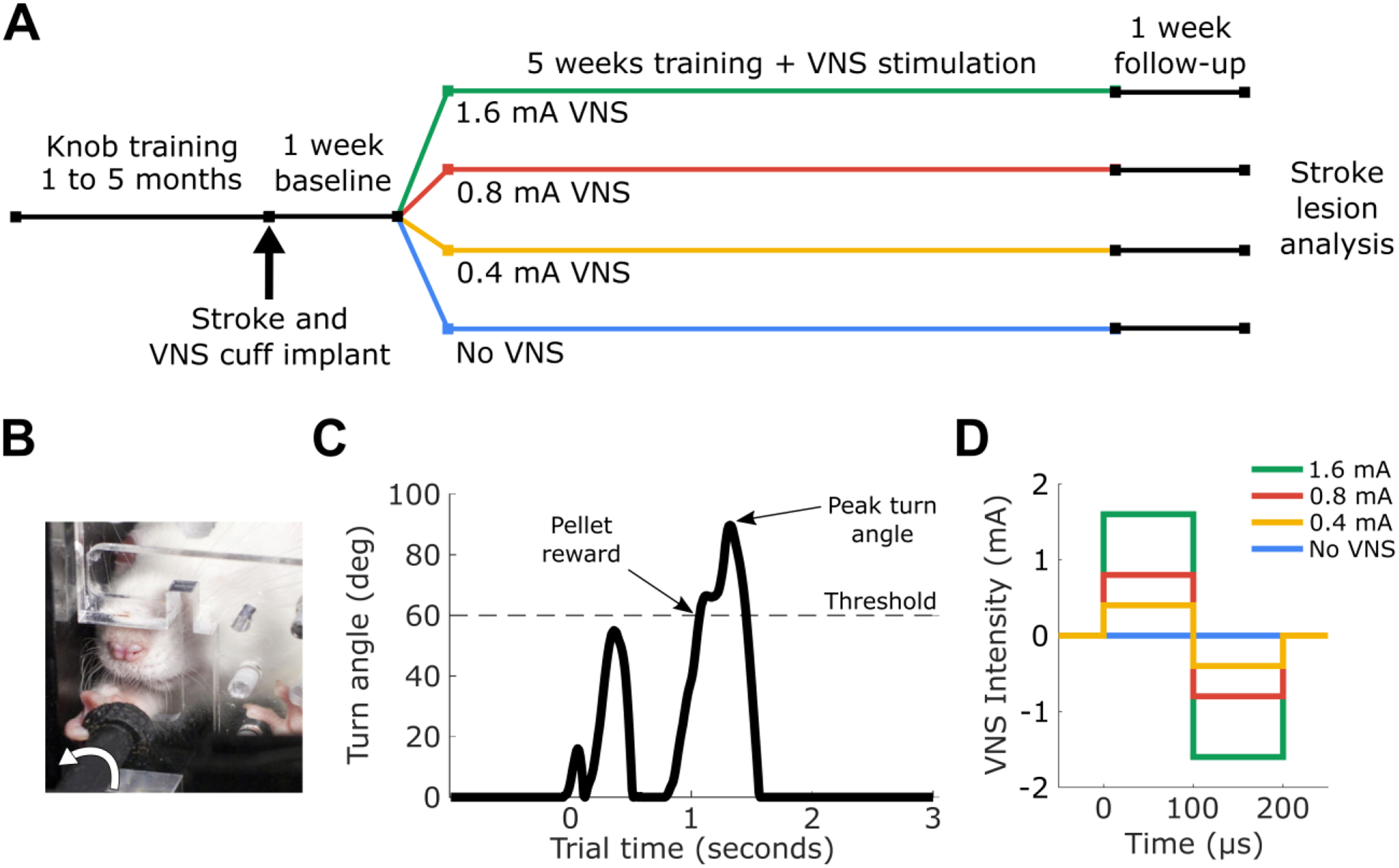
(A) The experimental design and timeline. (B) Rats were trained to turn a knob using a supination motion. (C) An example trial from the supination task. (D) An illustration of the stimulation intensities used in this experiment.
Figure 2:
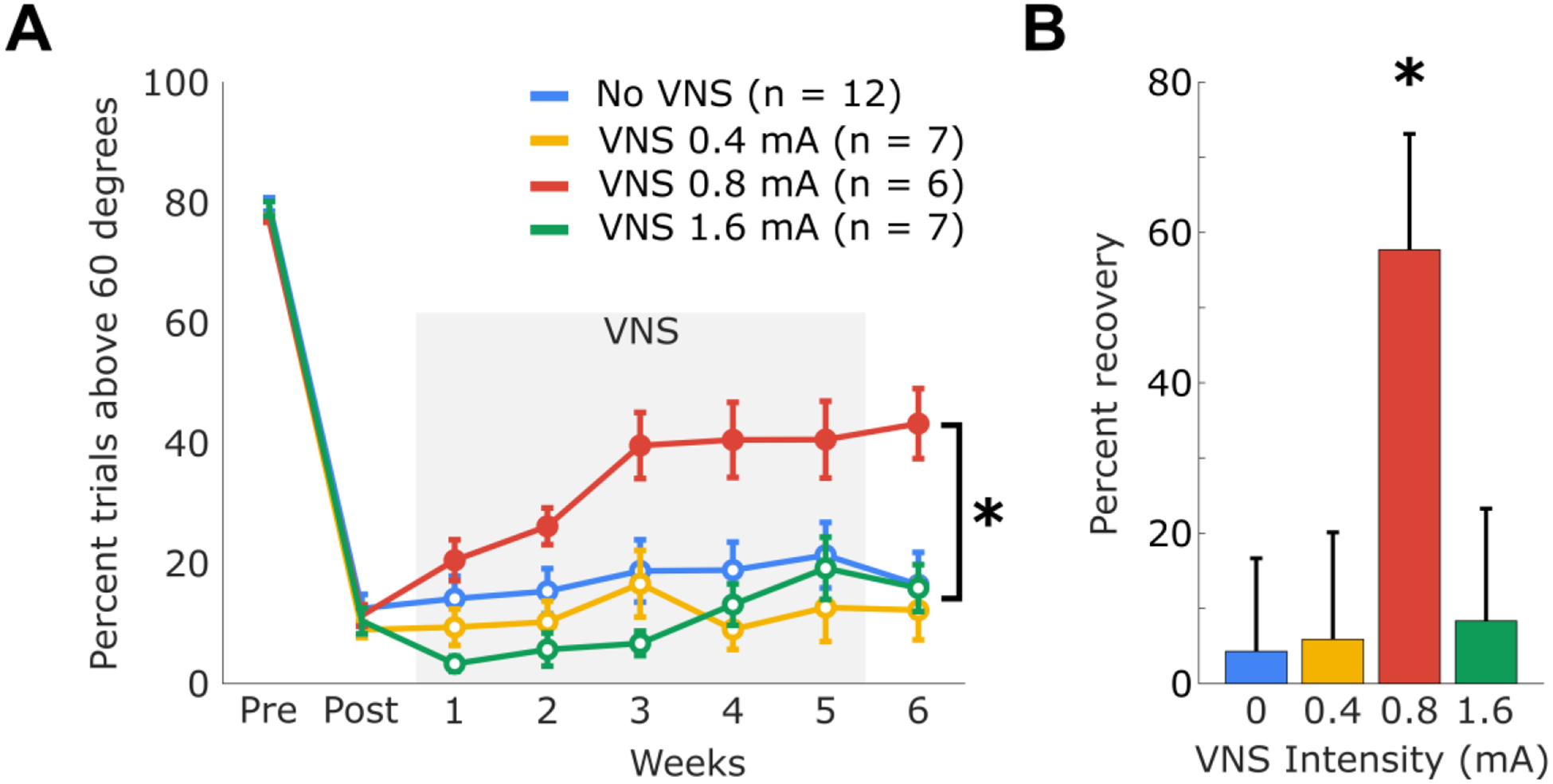
(A) Percent of trials performed that exceeded 60 degrees. Solid circles during therapy weeks indicate performance significantly better than Post. (B) The overall percent recovery achieved by each experimental group. Percent recovery is calculated as performance at the end of therapy normalized to the difference pre-lesion and post-lesion performance. Error bars represent SEM.
VNS Does Not Change Lesion Size
Histological analysis revealed no significant difference in lesion size across groups, suggesting that differences in task performance are not due to differences in stroke severity (Figure 3; one-way ANOVA, F(3, 24) = 1.69, p = 0.19). These findings are consistent with previous studies [3] and indicate that changes in lesion size cannot account for differences in recovery observed across groups.
Figure 3:
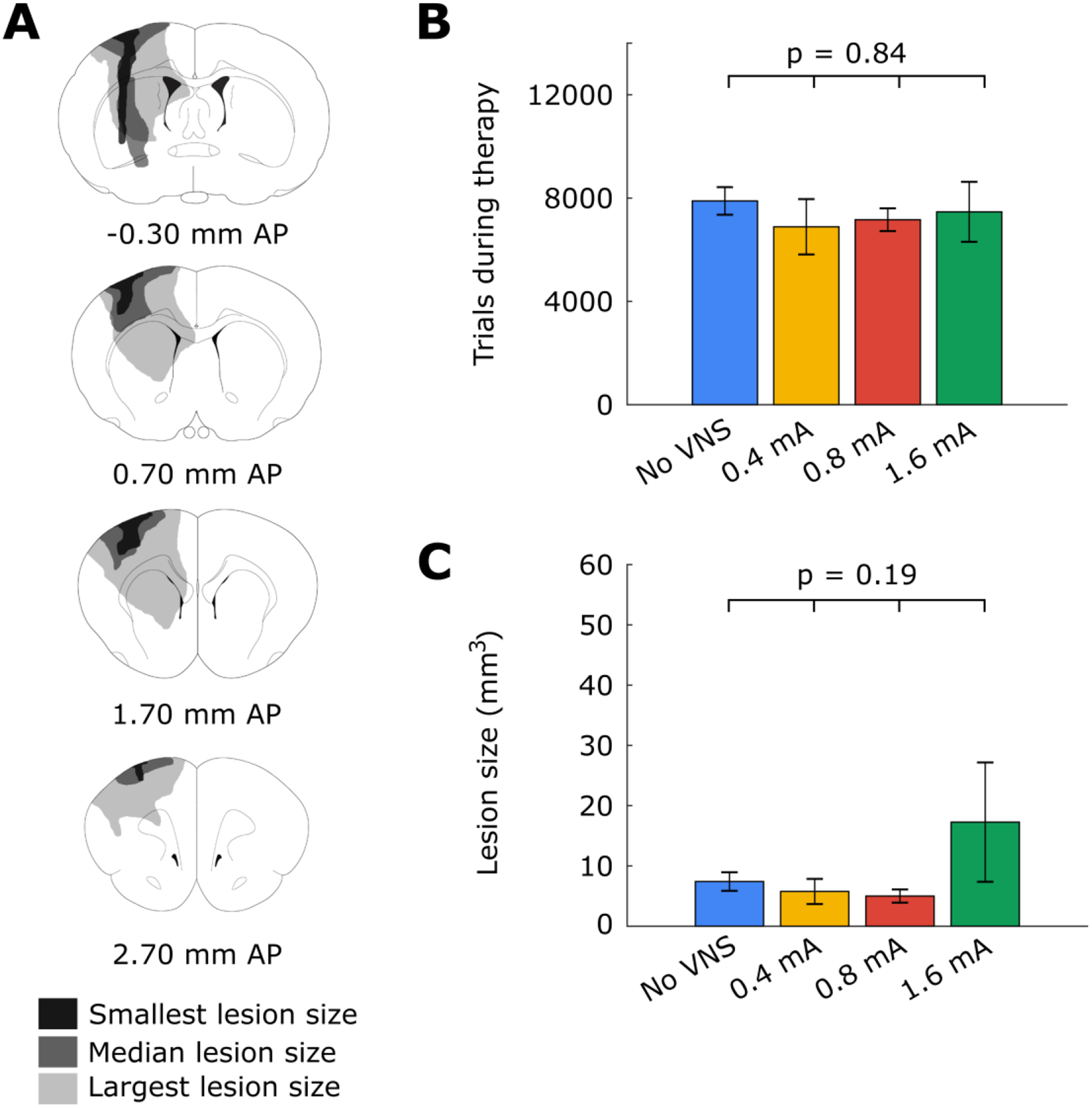
(A) Illustrated coronal sections demonstrating the smallest, median, and largest motor cortex lesions observed in the study. (B) There was no significant difference in the number of trials initiated across all experimental groups. (C) There was no significant difference in lesion volume across all experimental groups. Error bars represent SEM.
Discussion
In this study, we sought to determine the VNS intensity that yields the greatest recovery of forelimb function in a model of stroke. Moderate intensity VNS at 0.8 mA significantly enhanced recovery, corroborating previous preclinical and clinical findings [5, 6, 9]. Lower and higher stimulation intensities (0.4 and 1.6 mA) both failed to yield significant improvements. It is significant to note that simply increasing stimulation intensity alone did not increase effectiveness of VNS therapy. Rats receiving 1.6 mA stimulation intensity received equivalent rehabilitative training and a comparable number of stimulations as rats in the other experimental groups, yet failed to demonstrate improved recovery compared to rehabilitative training without VNS.
Identifying optimal stimulation parameters for stroke recovery is key for effective clinical translation of neuromodulation therapies. Recent clinical trials have investigated both the safety and efficacy of moderate intensity 0.8 mA VNS in stroke patients [5, 6]. The success of these trials are consistent with the results of this study and provides further justification for continued use of moderate intensity VNS. Modeling studies reveal that this stimulation intensity produces comparable levels of fiber activation in the vagus nerve in rats and humans, suggesting that the activation of a particular class of fibers is responsible for VNS-dependent enhancement of recovery, but further studies are required [16]. Moreover, the relatively narrow range of effective intensities also raises the importance of ensuring reliable stimulation at a prescribed level, which can be especially challenging for non-invasive interventions in which electrode placement and surface contact, as well as underlying anatomy, are variable [17, 18].
The present study reports an inverted-U relationship between stimulation intensity and functional recovery, in which moderate intensity yields greater benefits than lower or higher intensities. This relationship has been well-characterized in studies that evaluate enhancement of synaptic plasticity with VNS [9]. The precise mechanisms that underlie the inverted-U relationship of VNS intensity and recovery are not fully understood. VNS acts via monotonic engagement of noradrenergic circuits, which are required for VNS-dependent enhancement of plasticity [10, 11]. Although stimulation intensity follows a monotonic relationship with activation of the noradrenergic system, the observed inverted-U relationship with functional recovery and cortical plasticity may derive from differential activation of noradrenergic receptors. At moderate stimulation intensities, VNS may induce release of norepinephrine at concentrations sufficient to activate higher-affinity α-adrenergic receptors, which promote plasticity. At higher stimulation intensities, VNS-dependent release of norepinephrine may activate lower-affinity β-adrenergic receptors, which can produce opposite effects on plasticity and promote stability [19]. This raises the possibility that pharmacological manipulations may provide a means to intervene and enhance the range of effective VNS intensities, which has implications for clinical implementation. For example, drugs that inhibit β-adrenergic receptors may allow higher stimulation intensities to promote stroke recovery. In practical terms, an inverted-U effect makes dosing more challenging, as more stimulation does not necessarily produce greater benefits. Thus, clinicians managing active stimulation interventions should consider both increasing as well as decreasing stimulation parameters in patients that fail to respond to initial therapy.
Vagus nerve stimulation represents an emerging neurostimulation technique to modulate neural activity and subsequently influence stroke recovery. VNS provides rapid, phasic activation of neuromodulatory networks that serve to enhance plasticity in circuits engaged by rehabilitation [3, 10, 20]. Other neurostimulation techniques have been investigated to promote post-stroke recovery, including electrical epidural motor cortex stimulation (EECS), deep brain stimulation (DBS), repetitive transcranial magnetic stimulation (rTMS), and transcranial direct current stimulation (tDCS) [21–24]. While the targets of these strategies are varied, the general goal of each is to modulate excitability within spared circuits to promote reorganization and support recovery. Indeed, the lack of efficacy in clinical trials of rTMS and EECS points to the need for optimization and selection of stimulation parameters. The present study provides initial insight into the range of stimulation parameters that produce effective post-stroke recovery.
A number of limitations of the study merit consideration. First, this study focused on three fixed VNS intensities. These intensities were chosen based on their degree of activation of the locus coeruleus and modulation of VNS-dependent cortical plasticity in previous studies. It is possible that finer exploration of stimulation intensities, or biomarker-based individualization of stimulation intensities, may identify stimulation intensities that may provide even greater benefit. Additionally, the dose of neuromodulation therapies also encompasses other stimulation parameters, such as frequency, pulse width, train duration, and inter-stimulation interval. Indeed, all of these parameters have been linked to the magnitude of VNS-dependent enhancement of plasticity [25–29]. Future studies may explore the effect of varying these parameters on VNS paired with rehabilitation to improve stroke recovery. Finally, the present study restricted evaluation to female animals. While there are no known differences in the effects of VNS between males and females, future studies should consider incorporating assessment of VNS-dependent enhancement of recovery in both sexes.
Acknowledgments
We thank Jaimee Nguyen, Ardalan Naghian, Veda Nashi, Kishan Thomala, Ankita Shankar, Joel Wright, Sangavi Manalan, Priyanka Vayalali, Anya Ali, Emmanuel Aykara, Medhi Zaidi, Jennifer Le, Nidhi Desai, Riley Dickson, Tony Castillo, Jenitta Kunjammattil, Tyler Short, Amber Ho, Shashi Obulasetty, Emad Sidiqi, and Ahmed Alshaikhsalama who assisted with rodent behavioral training.
Funding
This project was supported by the National Institute of Health R01 NS085167 (MPK) and R01 NS094384 (SAH).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data and code availability
All data and analysis code from this study has been made available on Github (https://github.com/davepruitt/OptimalDosingIntensityVNS).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
Conflicts of Interest
MPK is a consultant for MicroTransponder which develops VNS-related technologies.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hays SA. Improving Stroke Rehabilitation with Vagus Nerve Stimulation. Springer, Singapore, pp. 503–515. [Google Scholar]
- [3].Meyers EC, Solorzano BR, James J, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018; STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014; 45: 3097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dawson J, Pierce D, Dixit A, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2015; STROKEAHA.115.010477-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kimberley TJ, Pierce D, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper Limb Rehabilitation After Chronic Stroke. Stroke; 49 Epub ahead of print 2018. DOI: 10.1161/STROKEAHA.118.022279. [DOI] [PubMed] [Google Scholar]
- [7].Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–8. [DOI] [PubMed] [Google Scholar]
- [8].Borland MS, Vrana WA, Moreno NA, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul. Epub ahead of print 9 September 2015. DOI: 10.1016/j.brs.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morrison RA, Hulsey DR, Adcock KS, et al. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul. Epub ahead of print 3 November 2018. DOI: 10.1016/J.BRS.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hulsey DR, Riley JR, Loerwald KW, et al. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017; 289: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hulsey DR, Shedd CM, Sarker SF, et al. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol; 320 Epub ahead of print 1 October 2019. DOI: 10.1016/j.expneurol.2019.112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meyers E, Sindhurakar A, Choi R, et al. The supination assessment task: an automated method for quantifying forelimb rotational function in rats. J Neurosci Methods. Epub ahead of print March 2016. DOI: 10.1016/j.jneumeth.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470: 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rios M, Bucksot J, Rahebi K, et al. Protocol for Construction of Rat Nerve Stimulation Cuff Electrodes. Methods Protoc 2019; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bucksot JE, Castelan KM, Skipton SK, et al. Parametric characterization of the rat Hering-Breuer reflex evoked with implanted and non-invasive vagus nerve stimulation. Exp Neurol 2020; 327: 113220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bucksot JE, Wells AJ, Rahebi KC, et al. Flat electrode contacts for vagus nerve stimulation. PLoS One 2019; 14: e0215191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hammer N, Löffler S, Cakmak YO, et al. Cervical vagus nerve morphometry and vascularity in the context of nerve stimulation - A cadaveric study. Sci Rep 2018; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Planitzer U, Hammer N, Bechmann I, et al. Positional Relations of the Cervical Vagus Nerve Revisited. Neuromodulation 2017; 20: 361–368. [DOI] [PubMed] [Google Scholar]
- [19].Salgado H, Köhr G, Trevĩo M. Noradrenergic tone determines dichotomous control of cortical spike-timing-dependent plasticity. Sci Rep; 2 Epub ahead of print 2012. DOI: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Engineer ND, Kimberley TJ, Prudente CN, et al. Targeted vagus nerve stimulation for rehabilitation after stroke. Frontiers in Neuroscience; 13 Epub ahead of print 1 March 2019. DOI: 10.3389/fnins.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Claflin ES, Krishnan C, Khot SP. Emerging treatments for motor rehabilitation after stroke. The Neurohospitalist 2015; 5: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elias GJB, Namasivayam AA, Lozano AM. Deep brain stimulation for stroke: Current uses and future directions. Brain Stimulation 2018; 11: 3–28. [DOI] [PubMed] [Google Scholar]
- [23].Levy RM, Harvey RL, Kissela BM, et al. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil Neural Repair 2016; 30: 107–19. [DOI] [PubMed] [Google Scholar]
- [24].Harvey RL, Edwards D, Dunning K, et al. Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 2018; 49: 2138–2146. [DOI] [PubMed] [Google Scholar]
- [25].Buell EP, Loerwald KW, Engineer CT, et al. Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul. Epub ahead of print 18 July 2018. DOI: 10.1016/J.BRS.2018.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Borland MS, Engineer CT, Vrana WA, et al. The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity. Neuroscience 2018; 369: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Loerwald KW, Buell EP, Borland MS, et al. Varying Stimulation Parameters to Improve Cortical Plasticity Generated by VNS-tone Pairing. Neuroscience 2018; 388: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Loerwald KW, Borland MS, Rennaker RL, et al. The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation. Brain Stimul. Epub ahead of print 15 November 2017. DOI: 10.1016/J.BRS.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buell EP, Borland MS, Loerwald KW, et al. Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity. Neuroscience 2019; 406: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]


