Abstract
Background:
Thalamic pathology is a marker for neurodegeneration and MS disease progression.
Objective:
To 1) characterize the morphology of thalamic lesions, their relation to 2) cortical and white matter (WM) lesions and 3) clinical measures; 4) assess the imaging correlates of thalamic atrophy.
Methods:
Ninety MS patients and 44 healthy controls, underwent acquisition of 7-Tesla images for lesion segmentation, 3-Tesla scans for atrophy evaluation. Thalamic lesions were classified according to shape and presence of a central venule. Regression analysis identified the predictors of 1) thalamic atrophy, 2) neurological disability and 3) information processing speed.
Results:
Thalamic lesions were mostly ovoid than periventricular, and for the great majority (78%) displayed a central venule. Lesion volume in the thalamus, cortex, and WM did not correlate with each other. Thalamic atrophy was only associated with WM lesion volume (p=0.002). Subpial and WM lesion volume were associated with neurological disability (p=0.016; p<0.001); WM and thalamic lesion volumes related with cognitive impairment (p<0.001; p=0.03).
Conclusion:
Thalamic lesions are unrelated to those in cortex and WM, suggesting that they may not share common pathogenic mechanisms, and do not contribute to thalamic atrophy. Combined WM, subpial and thalamic lesion volumes at 7-Tesla contribute to disease severity.
Keywords: multiple sclerosis, thalamus, atrophy, central venule, ovoid, periventricular
INTRODUCTION
Multiple sclerosis is a chronic demyelinating and neurodegenerative disease that affects both the gray and white matter (WM) of the CNS1. Previous pathologic and MRI studies have shown that gray matter (GM) pathology in MS is often an early phenomenon with significant correlation to clinical disability, cognition and disease progression2–6. The thalamus is an interconnected deep GM structure frequently affected in the course of MS. Thalamic abnormalities including focal demyelinating lesions, atrophy, and microstructural tissue changes appear early in the disease and contribute to neurological and cognitive impairment7–9.
Local neuronal loss, demyelination, and axonal damage are frequently reported as the substrates of thalamic atrophy10. Morphological features of thalamic lesions, including shape and location, can also help explain the mechanisms underlying the pathogenesis of focal thalamic demyelination. It has been reported that thalamic lesions typically line the ventricular surface11. Lesions in proximity to the lateral ventricle may be influenced by the CSF and therefore more susceptible to inflammatory cells and mediators including activated microglia and macrophages1,12. Local inflammatory activity, characterized by cytotoxicity and axonal transection, can further drive axonal injury or loss with resulting neurodegeneration12. The thalamus is also particularly prone to pathological processes due to its interconnectivity with other brain structures and regions13.
With its high prevalence, studies are supporting the notion that thalamic degeneration can be used as a predictor for disease evolution7,14 and even serve as a biomarker for neurodegeneration in MS10, 15. Initial data at 7 Tesla (T) MRI of MS patients have indeed found a positive correlation between thalamic and cortical lesion volume, suggesting that thalamic lesion assessment may be an estimate of overall GM lesion burden in the disease16. Other studies, however, have reported that thalamic degeneration and atrophy are more closely related to WM pathology because of axonal transection in WM tracts17, 18. While ultra-high resolution 7T MRI demonstrates increased sensitivity and detection of cortical and thalamic lesion volume19–21, the knowledge regarding thalamic involvement in MS at 7T mainly stems from small patient cohorts.
The main objective of this study was to take advantage of a relatively large MS cohort of 90 patients at different stages of the disease imaged at 7T to better characterize different aspects of thalamic pathology, assess to which extent those are linked to cortical and WM pathology and could be considered as a rapid estimate of overall brain gray matter disease burden in MS.
Specifically, we aimed to 1) characterize the morphology of thalamic lesions, their relation to 2) cortical and WM lesions and 3) clinical outcome measures; 4) to assess the imaging correlates of thalamic atrophy.
MATERIALS AND METHODS
Study participants
Our Institution Ethics Committee approved all study procedures and a written informed consent was signed by all participants enrolled in the study.
Ninety MS patients (61 RRMS, 29 SPMS) and 44 age-matched healthy controls were prospectively enrolled in the study between 2011–2016. Inclusion criteria for MS patients were: age between 18–60 years, ≥ 8 years of schooling, and stable treatment with disease-modifying agents for at least 3 months or no treatment. Exclusion criteria included a relapse in prior 3 months, and cortisone treatment in prior one month. Four patients’ data were discarded due to the motion artifacts on 7T acquisitions.
Subjects underwent a clinical examination to assess neurological disability using the Expanded Disability Status Scale (EDSS), and information processing speed, a domain frequently and early affected in MS, using the Symbol Digit Modalities Test (SDMT). Standard Z-scores for this test were obtained based on age and education of each participant.
MRI protocol and image analysis
All participants underwent two scanning sessions within approximately a week on 7T and 3T scanners (Siemens, Germany) using a 32-channel phased array coil to acquire: a) 7T single-echo FLASH T2*- weighted spoiled gradient-echo pulse sequence (resolution = 0.33 × 0.33 × 1 mm3) for lesion segmentation at the cortical, WM, and thalamic levels; b) 3T 3D magnetization-rapid acquisition with multiple gradient echoes (resolution = 0.9 × 0.9 × 0.9 mm3) T1-weighted structural image for cortical surface reconstruction using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) with cortical thickness estimation, co- registration with 7T data. Thalamic volume was obtained using FSL-FIRST (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST) and normalized to total intracranial volume.
All topological defects in cortical surfaces were corrected by lesion inpainting method. Cortical, WM and thalamic lesions were segmented by consensus of two experienced raters using Slicer v4.2.0 (http://www.slicer.org). Cortical lesions that extended for at least three voxels across two consecutive slices were classified as 1) type I (leukocortical) lesions that extend across both WM and the cortical GM; 2) type II (intracortical) lesions located within the cerebral cortex; 3) type III and type IV (subpial) lesions, extending from pial surface to cortical layers 3 and 4 or through the entire width of the cortex but without involving subcortical WM (Figure 1). Lesion counts and volumes were quantified using FreeSurfer and FSL (version 5.0, http://fsl.fmrib.ox.ac.uk) tools.
Figure 1. Examples of cortical lesion subtypes on 7 Tesla T2*-weighted MRI images.
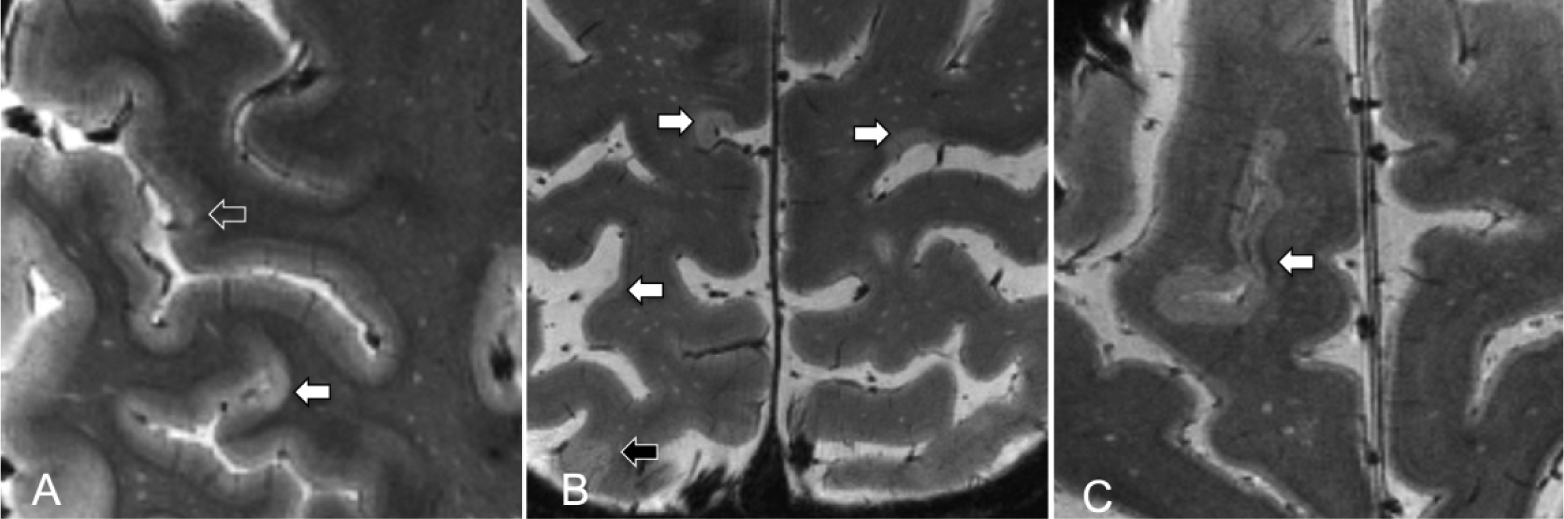
(A-C) Images show examples of multiple MS lesions (white arrows showing subpial lesions; open arrow showing type 2 intracortical lesion; black arrow showing leukocortical lesion)
Thalamic lesions subtypes were classified according to MRI appearance: ovoid lesions (small discrete ovoid areas) and periventricular lesions (diffuse lesions lining the periventricular surface)17. The presence of a central vein was acknowledged inside thalamic lesions according to the North American Imaging in Multiple Sclerosis Cooperative criteria22 as follows: 1) could be visualized a single linear hypointensity/dot in at least two perpendicular planes 2) the vein must be located centrally regardless of the lesion shape.
STATISTICAL ANALYSIS
Statistical analysis was performed using JMP Pro v13 software. As a result of the data not being normally distributed, Spearman’s rank correlation test was used for assessing bivariate correlations between thalamic and either cortical or WM lesion volumes. Regression analysis was used to quantify the association between thalamic volume and lesion volumes (WM lesions, cortical lesions and thalamic lesions) along with cortical thickness, age, and gender. The independent contribution of imaging metrics (thalamic volume, thalamic lesion volume, cortical lesion volume types, white matter lesion volume, and cortical thickness) to EDSS and SDMT scores were assessed by using stepwise linear regression. The models were adjusted for potential confounds, such as age and gender for EDSS score and only gender for SDMT (age was accounted for in the Z-score calculation and thus not included as a covariate).
The variables that were not normally distributed were logarithmically transformed while EDSS score was ranked before entering into the regression model. The regression models assumptions were checked using a residual versus fitted values plot. An alpha of 0.05 (two-tailed, equal variances not assumed) was considered statistically significant.
RESULTS
Lesion characteristics at 7T
Demographics and clinical data of study subjects are reported in Table 1.Thirty-eight out of 90 patients (42%) showed thalamic lesions (53% in RRMS and 47% in SPMS). Overall, SPMS patients had a higher thalamic lesion count and volume relative to RRMS patients, though there were large individual differences (mean±SD; count: 2.6±1.9 versus 1.3±0.7, p = 0.01; volume: 162.5±247.6 versus 42.3±45.8, p = 0.01 by Mann-Whitney U-test).
Table 1.
Demographic, clinical, and MRI characteristics of study subjects with thalamic lesions.
| All | RRMS | SPMS | |
|---|---|---|---|
| N | 90 | 61 | 29 |
| Gender (M/F) | 23/67 | 13/48 | 10/19 |
| Age, years | 42.3 (9.2) | 40 (8.6) | 47.1 (8.7) |
| Disease duration, years | 9.1 (9.2) | 5.2 (5.7) | 17.6 (9.6) |
| EDSS score, median (range) | 2.5 (8) | 2.1 (1.1) | 4.5 (6) |
| SDMT, Z-score | −0.04 (1.5) | 0.5 (1.1) | −1.3 (1.4) |
| Education | 15.9 (2.7) | 16.2 (2.8) | 15.2 (2.5) |
| Caucasian: African American: Asian | 87:2:1 | 59:2:0 | 28:0:1 |
| Therapy | |||
| Dimethyl Fumarate | 18 | 14 | 4 |
| Natalizumab | 16 | 12 | 4 |
| Glatiramer Acetate | 18 | 12 | 6 |
| Interferon Beta-1a | 16 | 10 | 6 |
| Cyclophosphamide | 1 | 0 | 1 |
| Monoclonal Antibody | 2 | 0 | 2 |
| Immunosuppressant | 1 | 1 | 0 |
| No Treatment | 18 | 12 | 6 |
Abbreviations: RRMS = relapsing remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis; EDSS = Expanded Disability Status Scale; All values are reported as mean (SD) if not as otherwise noted
Seventy-two thalamic lesions were identified in the MS cohort. Ovoid lesion were more prevalent than the periventricular lesions (Figure 2) both in the entire cohort (mean±SD; 1.6±1.4 versus 0.3±0.5, p < 0.001, by related samples Wilcoxon signed rank test) and in each disease phenotype (mean±SD; RRMS: 1±0.6 versus 0.3±0.6, p < 0.001; SPMS: 2.3±1.7 versus 0.2±0.4, p < 0.001, by related samples Wilcoxon signed rank test). The mean volume of ovoid lesions was smaller than periventricular lesions (mean±SD; 73.7 ± 99.4 mm3 versus 133.2 ± 186.5 mm3, p = 0.295, by related samples Wilcoxon signed rank test, Table 2).
Figure 2. Examples of thalamic multiple sclerosis lesions on 7 Tesla T2*-weighted MRI images.
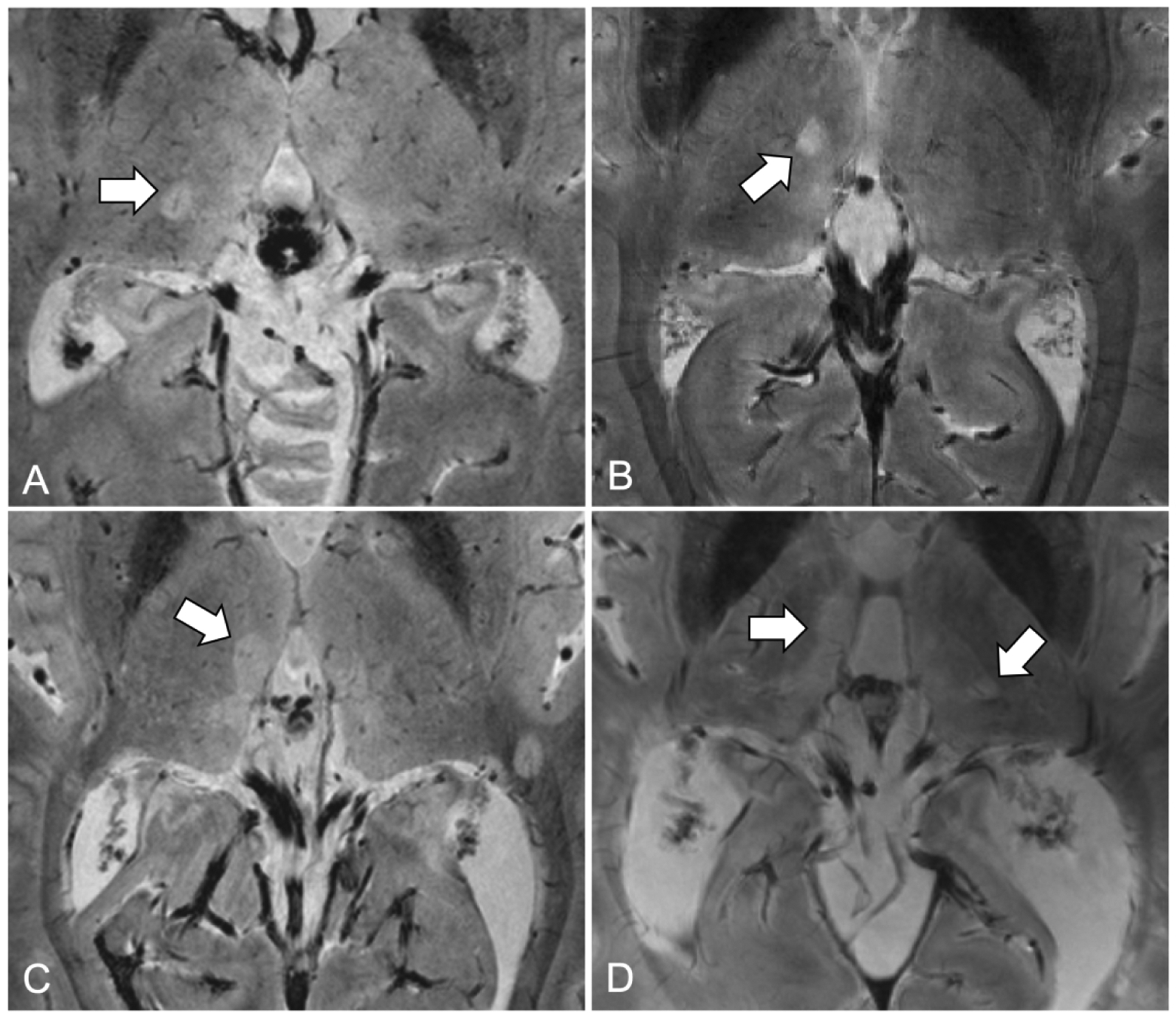
(A) Ovoid lesion with central venule on right thalamus; (B) Ovoid lesion without central venule on right thalamus; (C) Periventricular lesion on third ventricle; (D) Multiple thalamic lesions including periventricular and ovoid lesions.
Table 2.
Counts and volumes of lesions in thalamus.
| All (N = 38) | RRMS (N = 20) | SPMS (N = 18) | p-value* | |
|---|---|---|---|---|
| Total Thalamic Lesions | ||||
| Count | 1.9 ± 1.5 | 1.3 ± 0.7 | 2.6 ± 1.9 | 0.006 |
| Volume, mm3 | 99 ± 182 | 42 ± 46 | 163 ± 248 | 0.01 |
| Ovoid Lesions | ||||
| Count | 1.6 ± 1.4 | 1 ± 0.6 | 2.3 ± 1.7 | 0.002 |
| Volume, mm3 | 74 ± 99 | 32 ± 28 | 113 ± 125 | 0.003 |
| Number of subjects | 35 | 17 | 18 | |
| Periventricular Lesions | ||||
| Count | 0.3 ± 0.5 | 0.3 ± 0.6 | 0.2 ± 0.4 | 0.797 |
| Volume, mm3 | 133 ± 187 | 62 ± 50 | 223 ± 265 | 0.678 |
| Number of subjects | 9 | 5 | 4 | |
| Venular Lesions | ||||
| Count | 1.5 ± 1.4 | 1 ± 0.6 | 2.1 ± 1.7 | 0.021 |
| Volume, mm3 | 63 ± 77 | 28 ± 24 | 97 ± 96 | 0.006 |
| Number of subjects | 32 | 16 | 16 | |
| Non-venular Lesions | ||||
| Count | 0.4 ± 0.9 | 0.4 ± 0.9 | 0.5 ± 0.8 | 0.343 |
| Volume, mm3 | 177 ± 301 | 100 ± 69 | 229 ± 391 | 0.208 |
| Number of subjects | 10 | 4 | 6 | |
Abbreviations: RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis;
for comparisons between RRMS and SPMS subjects; all values are reported as mean ± SD
Amongst MS patients with thalamic lesions, 28/38 (74%) of them displayed only lesions with a central venule, 6/38 (16%) of patients only lesions without a central venule, 4/38 (10%) both lesions with and without a central venule. In each disease phenotype, lesions harboring a central venule predominated (RRMS: 16/20 [80%]; SPMS: 16/18 [89%]).
Cortical lesions were identified in 88/90 (98%) of patients. Fifty-nine out of 61 (97%) RRMS patients had at least one cortical lesion while all SPMS patients presented with cortical lesions (Table 3). Thirty-six out of 38 (95%) patients with thalamic lesions also displayed cortical lesions. Interestingly, patients with thalamic lesions had on average, a higher cortical lesion load both in terms of counts and volume (cortical lesion counts: 31.7 vs. 9.8, p < 0.001; cortical lesion volume: 2361.5 mm3 vs. 590.2 mm3, p < 0.001, by Mann-Whitney U Test) than patients with no evidence of thalamic lesions. However, in the same group comparison, only WM lesion count was higher in patients with thalamic lesions (71.3 vs. 41.8, p = 0.011) whereas WM lesion volume did not differ. No lesions were identified in healthy controls.
Table 3.
Counts and volumes of lesions in cortex and white matter.
| All (N = 90) | RRMS (N = 61) | SPMS (N = 29) | p-value* | |
|---|---|---|---|---|
| Total Cortical Lesions | ||||
| Count | 20.7 ± 31.3 | 10.3 ± 10.9 | 42.2 ± 46.3 | <0.001 |
| Volume, mm3 | 1405 ± 2406 | 608 ± 871 | 2775 ± 3626 | <0.001 |
| Intracortical Lesions | ||||
| Count | 9.4 ± 11.7 | 6.3 ± 7.9 | 14.9 ± 13.9 | 0.003 |
| Volume, mm3 | 477 ± 723 | 311 ± 514 | 824 ± 955 | 0.008 |
| Type II Cortical Lesions | ||||
| Count | 0.5 ± 1.0 | 0.5 ± 0.8 | 0.5 ± 1.2 | 0.522 |
| Volume, mm3 | 5.4 ± 11.8 | 5.2 ± 9.7 | 5.7 ± 15.5 | 0.610 |
| Subpial Cortical Lesions | ||||
| Count | 8.9 ± 11.5 | 5.9 ± 7.6 | 14.4 ± 13.6 | 0.002 |
| Volume, mm3 | 471 ± 722 | 306 ± 513 | 819 ± 954 | 0.007 |
| Leukocortical Lesions | ||||
| Count | 11.5 ± 24.8 | 4.0 ± 5.9 | 27.3 ± 38.4 | <0.001 |
| Volume, mm3 | 705 ± 1489 | 276 ± 548 | 1606 ± 2271 | <0.001 |
| White Matter Lesions | ||||
| Count | 54.2 ± 56.2 | 36.3 ± 34.9 | 90.4 ± 72.9 | <0.001 |
| Volume, mm3 | 4884 ± 7925 | 2268 ± 2964 | 10386 ± 11600 | <0.001 |
Abbreviations: RRMS = relapsing remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis;
for comparisons between RRMS and SPMS subjects; all values are reported as mean ± SD
Correlates of thalamic lesion volume
We were not able to find any correlations between individual thalamic (total, ovoid, periventricular, venular, non-venular) and cortical (total, intracortical, leukocortical) lesion subtypes (P-values ranged from 0.14 to 0.97).
Also, no correlations were found between overall thalamic lesion volume and WM lesion volume. Interestingly, a mild correlation was found between thalamic venular lesion volume and WM lesion volume (rho = 0.4, p = 0.02).
Thalamic atrophy relates with white matter lesion volume
MS patients had a lower mean normalized thalamic volume of compared to healthy controls (6.1·10−3 ± 1.1·10−3 vs 6.8·10 −3 ± 1·10 −3 mm3, p = 0.001 by linear regression, adjusting for age and gender). This finding was confirmed in the MS subgroups including RRMS (6.5·10 −3 ± 7.4·10 −4 mm3; p = 0.003) and SPMS (5.4·10 −3 ± 9.8·10 −4 mm3; p < 0.001) (Table 5).
Table 5.
Multivariate regression estimates for thalamic atrophy
| Estimate | p-value | 95% CI | |
|---|---|---|---|
| Intercept | 3.2·10−3 | 0.18 | −1.5·10−3, 7.8·10−3 |
| Age | −3.0·10−6 | 0.59 | −1.4·10−5, 8.1·10−6 |
| Gender | 5.0·10−4 | 0.65 | −1.7·10−4, 2.7·10−4 |
| Thalamic Lesion Volume | −6.5·10−5 | 0.24 | −1.7·10−4, 4.4·10−5 |
| Cortical Lesion Volume | −3.6·10−5 | 0.63 | −1.8·10−4, 1.1·10−4 |
| White Matter Lesion Volume | −2.8·10−4 | 0.003 | −4.6·10−4, −1·10−4 |
| Cortical Thickness | 1.2·10−3 | 0.77 | −6.8·10−3, 9.2·10−3 |
We were not able to find any differences in thalamic volume between groups of patients with or without thalamic lesions (p = 0.198) (Table 4).
Table 4.
Comparative MRI metrics of patients with or without thalamic lesions
| With thalamic lesions | Without thalamic lesions | p-value* | |
|---|---|---|---|
| N | 38 | 52 | |
| Gender (M/F) | 11/27 | 11/41 | 0.396 |
| Age, years | 44.4 (9.4) | 41.2 (8.7) | 0.107 |
| Disease duration, years | 10 (8.9) | 8.8 (9.4) | 0.312 |
| EDSS score, median (range) | 3.5 (8) | 2 (7) | 0.021 |
| SDMT, Z-score | −0.4 (1.6) | 0.2 (1.4) | 0.064 |
| RRMS:SPMS | 20:18 | 41:11 | 0.009 |
| Leukocortical lesion volume | 1512 ± 2214 | 318 ± 605 | 0.054 |
| Intracortical lesion volume | 6.9 ± 15.3 | 300 ± 542 | 0.002 |
| Subpial lesion volume | 711 ± 864 | 296 ± 542 | 0.002 |
| White matter lesion volume | 6228 ± 10325 | 3848 ± 5487 | 0.191 |
| Thalamus volume | 4349 ± 792 | 4583 ± 931 | 0.198 |
All values are reported as mean (SD);
for comparisons between subjects with and without thalamic lesions
Univariate correlations showed that normalized thalamic volume was inversely associated with thalamic (rho = −0.2, p = 0.02), WM (rho = −0.6, p < 0.001), and cortical lesion volume (rho = −0.4, p < 0.00), and was related to cortical thinning (rho = 0.4, p < 0.001).
Multivariate regression analysis results demonstrated that thalamic atrophy in MS patients had a strong association with WM lesion volume (p = 0.002, R2 = 0.24), unrelated to cortical thickness (p = 0.77) and both thalamic (p = 0.24) and cortical lesion volume (p = 0.63) (Figure 3, Table 5). Age and gender were included as adjustment variables. Model residuals had a normal distribution.
Figure 3. Relationship between normalized mean thalamic volume and MRI metrics at 7 Tesla and 3 Tesla.
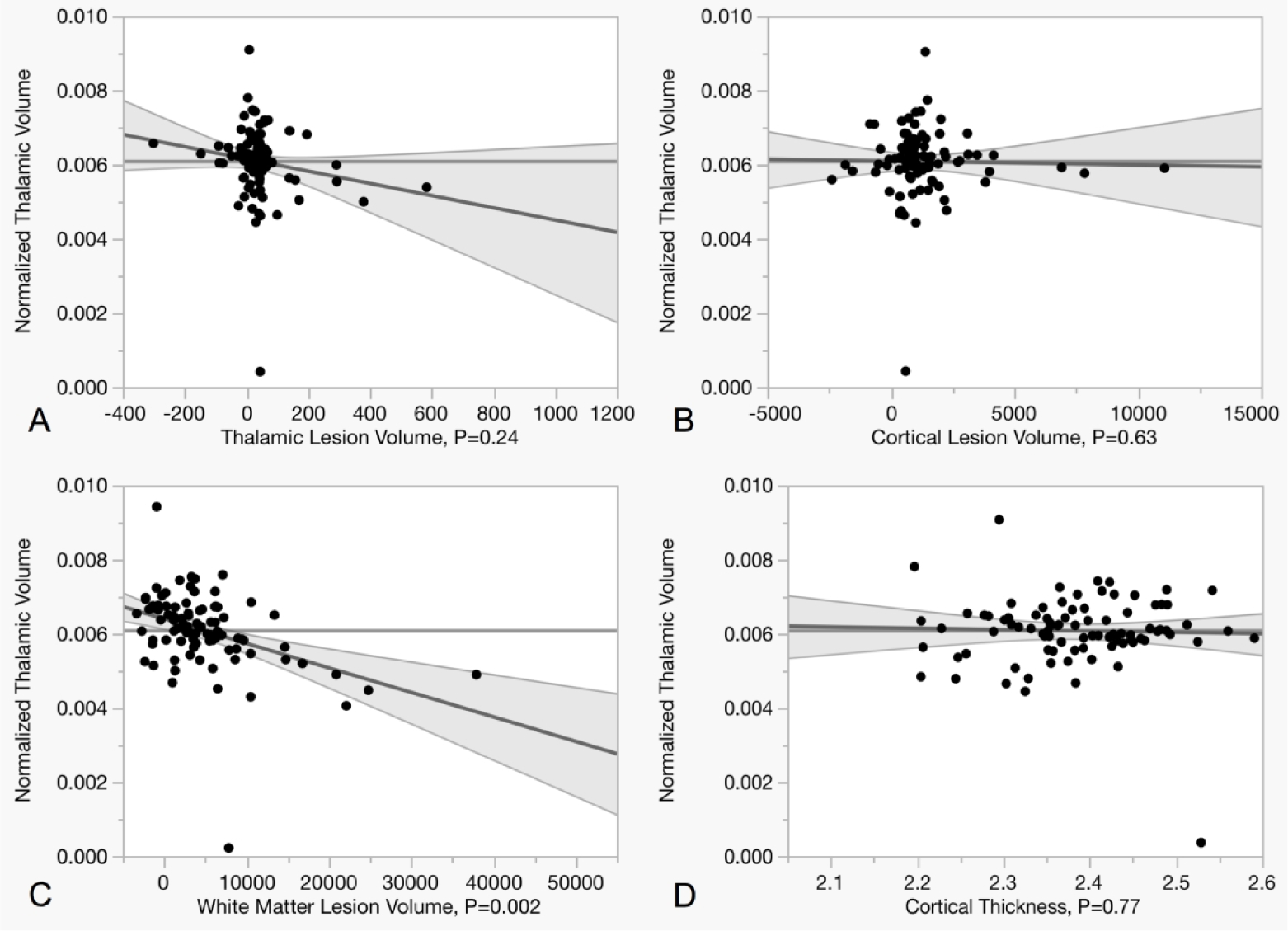
Scatter plots summarizing the relationship between thalamic volume and thalamic (A), cortical (B), and white matter (C) lesion load at 7T as well as with cortical thickness (D). The multivariate regression shows how thalamic volume correlates with white matter lesion load, but not with cortical thickness and thalamic or cortical lesion load.
Outcomes of clinical metric associations
In MS subjects, the independent contribution of imaging metrics to EDSS and SDMT was assessed by stepwise regression models. We found that after adjusting for age, gender, and cortical thickness, WM lesion volume (B = 17.2 [95% CI: 10.4, 24.1]; p < 0.001) and subpial cortical lesion volume (B = 5.2 [95% CI: 1.0, 9.4]; p = 0.016, R2=0.36) were associated with a higher EDSS score. Impairment in SDMT was significantly associated with WM (B = −1 [95% CI: −1.4, −0.6]; p < 0.001) and thalamic lesion volume (B = −0.4 [95% CI: −0.7, −0.05]; p = 0.03, R2=0.29).
DISCUSSION
In this study, using 7T MRI in a large cohort of MS patients, we found that WM lesion volume was associated with thalamic atrophy. Furthermore, our findings demonstrate that thalamic atrophy in MS patients is not related with either thalamic or cortical lesion volume. We also observed that the prevalence of thalamic lesions (46%) is lower compared to cortical lesions (98%). However, no association was detected between thalamic and cortical lesion volume. In the thalamus, ovoid morphology was more prominent than the periventricular type and frequently overlapped with the presence of a central venule. Our results also show that EDSS relates to WM and subpial cortical lesion volume independently from thalamic damage and cortical atrophy. Instead, lower SDMT z-scores were associated with an increase in both thalamic and WM lesion burden.
With 7T T2* susceptibility imaging, there is a greater ability to characterize distinct thalamic lesion patterns that could be used in future studies for better understanding thalamic pathology in MS. The presence of a central venule could underlie a distinct pathological process, where lesion morphology is influenced by location and orientation of the veins23. This could provide further evidence that demyelination in focal thalamic lesions is perivascular and that venular anatomy may influence lesion morphology24. “Dawson’s finger” has been used as a model to represent the direction of demyelination in WM because it points in the direction of the vessel which correlates with the shape of the lesion25. It could also indicate the presence of inflammatory activity within the perivenular space of the lesion26. These findings suggest that ovoid lesions are determined by vascularity since there were abundantly more central veins in that type of lesion compared to periventricular. Furthermore, we found a correlation between thalamic venular lesion volume and WM lesion volume, cluing towards common pathologic source.
However, despite the high sensitivity for central vein detection demonstrated by 7T MRI27, in our study, not all ovoid lesions were centered by a venule. This could be related to weak visualization of the internal lesion morphology due to a reduced lesion/venule size. Alternatively, it can also indicate a type of lesion that is linked more to Wallerian degeneration/axonal loss11 from distal WM lesions than to local inflammation.
In contrast, periventricular thalamic pathology may be more susceptible to demyelination and interaction with factors produced by adjacent CSF due to the proximity of the thalamus to the ventricles17. Studies provide also evidence that subpial cortical pathology is influenced by cytotoxic factors in meningeal inflammation28, 29. Despite this, we were not able to identify a relationship between periventricular thalamic lesions and subpial lesions, but however, we are underpowered for such an analysis since we identified periventricular thalamic lesions only in a reduced number of patients (8).
Contrary to previous 7T imaging findings16, we did not find a correlation between thalamic lesions and cortical lesions, although patients with thalamic lesions had overall increased WM and cortical lesion burden. Our study included a larger MS cohort, and differences in the type of sequence and resolution used for cortical lesion detection may also account for the disparities with previous 7T data. Given the high prevalence of cortical lesions as opposed to thalamic lesions and the fact that the thalamus is a smaller GM structure compared to the cortex, a strong association between the two measures might not necessarily be expected.
We also investigated the relationships between thalamic, cortical, and WM lesions to determine the role of local and distant pathological processes in inducing thalamic atrophy. Using ultra-high field MRI, which shows increased sensitivity to thalamic and cortical lesion pathology, we found that thalamic lesions are not as frequent as cortical lesions. While cortical lesions were detected in almost all subjects (98%), thalamic lesions were identified in less than half of the cohort. All but two patients with thalamic lesions also presented with cortical lesions.
Consistent with previous studies, we found that thalamic atrophy was greater in all disease stages relative to healthy controls1,7, 8, 10, 12, 14, 15, 17, 18, 30, 31. However, pathological processes underlying neurodegeneration in the thalamus are still uncertain. Our data indicated that thalamic atrophy in MS is not associated with local T2* lesion formation or distant cortical lesion formation. The lack of association between thalamic focal lesions and thalamic volume is consistent with prior findings31. Instead, we found a strong association between WM lesion volume and thalamic atrophy, in line with previous reports32. Taken together, these results suggest that Wallerian degeneration from distant WM lesions and disconnection mechanisms, might play a greater role than local thalamic lesions in inducing thalamic atrophy in MS. Due to the thalamus’ interconnectivity with cortical areas and other brain structures, and evidence that thalamic atrophy develops from the earliest disease stages, a better understanding of the substrates of thalamic demyelination and neurodegeneration is critical to improving our understanding on the pathogenesis of MS.
Future longitudinal studies are needed for better understanding the processes leading to thalamic atrophy and how these can be used as predictors of disease burden, or even if they vary according to age or disease phenotype. Also, considering the fact that WM lesions harboring a paramagnetic rim have been associated with a poor prognosis33, 34 it will be valuable to determine if the paramagnetic rim is present also at the thalamic level and how it relates with overall lesion load, stage or disease aggressiveness.
Finally, we used stepwise regression models to quantify the association between thalamic lesion volume, thalamic atrophy together with heterogeneous MRI metrics and clinical outcome measures. Our findings are consistent with previous reports in highlighting the contribution of cortical subpial lesion type to physical disability28, 35, 36 since the retained predictors of EDSS increase were only cortical subpial and WM lesion volume. Our data also suggest that WM and thalamic lesion volume account for the 29% of the variance in cognitive performance. Given that the thalamic axons transmit information between subcortical and specific cortical areas it is not surprisingly that the thalamic nuclei/connections damage are clinically translate into cognitive disability37, 38.
CONCLUSIONS
Our study showed that ovoid lesions were more prevalent than periventricular lesions in the thalamus and venular lesions were more common than non-venular lesions. The data demonstrated that thalamic lesion volume is not related to overall cortical lesion volume including intracortical and leukocortical lesions, thereby suggesting either that thalamic and cortical lesion pathology may not share common pathogenic mechanisms or that may develop at different rates. Furthermore, thalamic atrophy is independent from thalamic and cortical lesion volume. Instead, WM lesion volume serves as better predictor for thalamic neurodegeneration. The combined metrics of WM, subpial and thalamic lesion volumes acquired at 7T explain disease severity.
Funding
This work was supported by the Clafin Award; the National Institute of Health [NIH R01NS07832201 A1]; the National Multiple Sclerosis Society [NMSS; RG 4729A2/1]; and the US Army, Department of Defense [DoD; W81XWH13-10112]. Elena Herranz has received research support by the NMSS fellowship [FG150705459].
Footnotes
Declaration of Conflicting Interests
All authors declare no potential conflicts of interest.
References
- 1.Vercellino M, Masera S, Lorenzatti M, et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol. 2009; 68: 489–502. [DOI] [PubMed] [Google Scholar]
- 2.Fisher E, Lee JC, Nakamura K and Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008; 64: 255–65. [DOI] [PubMed] [Google Scholar]
- 3.Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008; 64: 247–54. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese M, Rinaldi F, Mattisi I, et al. The predictive value of gray matter atrophy in clinically isolated syndromes. Neurology. 2011; 77: 257–63. [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology. 2013; 81: 1759–67. [DOI] [PubMed] [Google Scholar]
- 6.Parra Corral MA, Govindarajan ST, Stefancin P, Bangiyev L, Coyle PK and Duong TQ. Characterization of gray-matter multiple sclerosis lesions using double inversion recovery, diffusion, contrast-enhanced, and volumetric MRI. Mult Scler Relat Disord. 2019; 31: 74–81. [DOI] [PubMed] [Google Scholar]
- 7.Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G and Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010; 257: 463–9. [DOI] [PubMed] [Google Scholar]
- 8.Zivadinov R, Havrdova E, Bergsland N, et al. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology. 2013; 268: 831–41. [DOI] [PubMed] [Google Scholar]
- 9.Hanninen K, Viitala M, Paavilainen T, et al. Thalamic Atrophy Without Whole Brain Atrophy Is Associated With Absence of 2-Year NEDA in Multiple Sclerosis. Front Neurol. 2019; 10: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007; 69: 1213–23. [DOI] [PubMed] [Google Scholar]
- 11.Kipp M, Wagenknecht N, Beyer C, Samer S, Wuerfel J and Nikoubashman O. Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell Mol Life Sci. 2015; 72: 1127–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J and Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002; 52: 650–3. [DOI] [PubMed] [Google Scholar]
- 13.Wagenknecht N, Becker B, Scheld M, et al. Thalamus Degeneration and Inflammation in Two Distinct Multiple Sclerosis Animal Models. J Mol Neurosci. 2016; 60: 102–14. [DOI] [PubMed] [Google Scholar]
- 14.Zivadinov R, Bergsland N, Dolezal O, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. AJNR Am J Neuroradiol. 2013; 34: 1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M and Matthews PM. Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology. 2003; 60: 1949–54. [DOI] [PubMed] [Google Scholar]
- 16.Harrison DM, Oh J, Roy S, et al. Thalamic lesions in multiple sclerosis by 7T MRI: Clinical implications and relationship to cortical pathology. Mult Scler. 2015; 21: 1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louapre C, Govindarajan ST, Gianni C, et al. Heterogeneous pathological processes account for thalamic degeneration in multiple sclerosis: Insights from 7 T imaging. Mult Scler. 2018; 24: 1433–44. [DOI] [PubMed] [Google Scholar]
- 18.Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology. 2013; 80: 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippi M, Evangelou N, Kangarlu A, et al. Ultra-high-field MR imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014; 85: 60–6. [DOI] [PubMed] [Google Scholar]
- 20.Kilsdonk ID, Jonkman LE, Klaver R, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016; 139: 1472–81. [DOI] [PubMed] [Google Scholar]
- 21.Beck ES, Sati P, Sethi V, et al. Improved Visualization of Cortical Lesions in Multiple Sclerosis Using 7T MP2RAGE. AJNR Am J Neuroradiol. 2018; 39: 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016; 12: 714–22. [DOI] [PubMed] [Google Scholar]
- 23.Tan IL, van Schijndel RA, Pouwels PJ, et al. MR venography of multiple sclerosis. AJNR Am J Neuroradiol. 2000; 21: 1039–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Oztoprak B, Oztoprak I and Yildiz OK. The effect of venous anatomy on the morphology of multiple sclerosis lesions: a susceptibility-weighted imaging study. Clin Radiol. 2016; 71: 418–26. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz AL, Kaplan RD, Grewe G, White RT and Salberg LM. The ovoid lesion: a new MR observation in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1989; 10: 303–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Ge Y, Law M, Herbert J and Grossman RI. Prominent perivenular spaces in multiple sclerosis as a sign of perivascular inflammation in primary demyelination. AJNR Am J Neuroradiol. 2005; 26: 2316–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseini Z, Matusinec J, Rudko DA, et al. Morphology-Specific Discrimination between MS White Matter Lesions and Benign White Matter Hyperintensities Using Ultra-High-Field MRI. AJNR Am J Neuroradiol. 2018; 39: 1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007; 130: 1089–104. [DOI] [PubMed] [Google Scholar]
- 29.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010; 68: 477–93. [DOI] [PubMed] [Google Scholar]
- 30.Henry RG, Shieh M, Amirbekian B, Chung S, Okuda DT and Pelletier D. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci. 2009; 282: 61–6. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018; 83: 223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B and Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. 2016; 22: 1327–36. [DOI] [PubMed] [Google Scholar]
- 33.Absinta M, Sati P, Gaitan MI, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol. 2013; 74: 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009; 73: 941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen AS, Kinkel RP, Madigan N, Tinelli E, Benner T and Mainero C. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology. 2013; 81: 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler. 2013; 19: 1478–84. [DOI] [PubMed] [Google Scholar]
- 38.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013; 80: 1025–32. [DOI] [PubMed] [Google Scholar]


