Abstract
A 69-year-old man with stage III lung squamous cell carcinoma developed immune-related hepatitis following treatment with durvalumab, and was given high-dose corticosteroids and immunosuppressive drugs (mycophenolate mofetil, azathioprine, tacrolimus) but without demonstrating any improvement. Two cycles of infliximab (5 mg/kg) were then administered and thereafter the hepatitis improved. At the time of writing (9 months after the initiation of first course of durvalumab), the patient is alive without either any hepatitis symptoms nor any lung cancer progression.
Infliximab may be effective for treating non-small cell lung cancer (NSCLC) patients who develop immunosuppressive drug-resistant immune-related hepatitis caused by durvalumab.
Keywords: non-small-cell lung cancer, immune checkpoint inhibitor, durvalumab, immune-related adverse event, hepatitis, infliximab
Introduction
Immune checkpoint inhibitors (ICIs) have become the treatment options for several types of cancers (1,2), including non-small cell lung cancer (NSCLC). Durvalumab, a human IgG1 monoclonal antibody that blocks programed death 1 (PD1), is an ICI given to patients with stage III NSCLC following chemoradiotherapy (3,4). However, ICIs can cause immune-related adverse events (irAEs), including hepatitis. The American Society of Clinical Oncology (ASCO) recommends that a corticosteroid at 1 to 2 mg/kg should be administered to patients with severe hepatitis, while mycophenolate mofetil (MMF) or azathioprine (AZA) should be used for those resistant to corticosteroid therapy (5), while tacrolimus has also been recommended (6).
However, effective treatments for patients with immune-related hepatitis who are resistant to those immunosuppressive drugs remain unclear. While several reports (7,8) have shown the efficacy of infliximab for melanoma patients with immune-related hepatitis due to combination therapy with nivolmab and ipilimumab, no such report has been presented in regard to those with NSCLC.
To the best of our knowledge, this is the first case report showing the efficacy of infliximab for immunosuppressive drug-resistant immune-related hepatitis due to durvalumab administration. Our findings suggest that infliximab may be an effective treatment option for such cases.
Case Report
The present patient provided his informed consent to publish the pertinent details regarding this case, including images.
A 69-year-old man with a 50-year history of smoking was referred to our hospital for an evaluation of abnormal chest radiograph findings. He was a regular drinker, approximately 20 grams of alcohol daily, but had no medical history of liver disease including viral hepatitis. The examination results led to a diagnosis of squamous cell lung cancer (cT4N0M0: Stage IIIA), with a PD-L1 tumor proportion score of less than 1%. Chemoradiotherapy with carboplatin and paclitaxel was administered, and a partial response was achieved.
Within 2 days after the end of chemoradiotherapy, radiation pneumonitis (grade 2) developed and prednisolone (PSL) treatment (1 mg/kg) was started. After tapering PSL to 10 mg, chemotherapy with durvalumab was initiated. At a follow-up examination, the prolongation of a slight fever and a high level of C-reactive protein (CRP) (4-5 mg/dL) were noted, thus the next course of durvalumab was not administered. At 38 days following the initiation of the first course of durvalumab, liver dysfunction appeared (Table 1). There was no evidence of liver disease, such as viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis in blood test (Table 1). Contrast enhanced computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP) and abdominal ultrasound also showed no abnormality in the liver, gall bladder or biliary tract. According to these results, immune-related hepatitis due to durvalumab was diagnosed. Liver biopsy was not performed because the patient was receiving antithrombotic agents for internal carotid artery stenosis and arteriosclerosis obliterans with an artificial blood vessel.
Table 1.
Laboratory Findings.
| [Hematology] | [Biochemistry] | Onset (38th days) | Peak value | ||||||||
| RBC | 327×104 | /μL | T-Bil | 7.5 | 10.2 (44th days) | mg/dL | |||||
| Hgb | 12.9 | g/dL | D-Bil | 6.1 | 8.8 (44th days) | mg/dL | |||||
| WBC | 9,000 | /mm³ | AST | 260 | 385 (64th days) | IU/L | |||||
| Neu | 88 | % | ALT | 337 | 615 (64th days) | IU/L | |||||
| Lym | 3 | % | ALP | 3,196 | 4,780 (48th days) | IU/L | |||||
| Mono | 8 | % | γ-GTP | 1,462 | 1,888 (62nd days) | IU/L | |||||
| Eo | 1 | % | LDH | 298 | IU/L | ||||||
| PLT | 28.8×104 | /μL | Amy | 74 | IU/L | ||||||
| BUN | 16 | mg/dL | |||||||||
| [Coagulation] | Cre | 0.61 | mg/dL | ||||||||
| PT | 110 | % | ChE | 193 | IU/L | ||||||
| APTT | 85 | % | TP | 5.6 | g/dL | ||||||
| Fibrinogen | 400 | mg/dL | Alb | 2.6 | g/dL | ||||||
| FDP | 3.1 | μg/dL | |||||||||
| D-Dimer | 1.3 | μg/dL | [Viral marker] | ||||||||
| IgM-HA | Negative | ||||||||||
| [Serology] | HBsAg | Negative | |||||||||
| CRP | 4.3 | mg/dL | HBcAb | Negative | |||||||
| ANA | Negative | HBsAb | Negative | ||||||||
| AMA | Negative | HCV-Ab | Negative | ||||||||
| AMA-M2 | Negative | IgM-EB-VCA | Negative | ||||||||
| ASMA | Negative | IgM-CMV | Negative | ||||||||
| IgG | 635 | mg/dL | IgM-HSV | Negative | |||||||
| IgA | 161 | mg/dL | |||||||||
| IgM | 50 | mg/dL | |||||||||
| IgE | 37 | mg/dL | |||||||||
| βD-glucan | <6 | pg/mL | |||||||||
PLT: platelet, PT: prothrombin time, APTT: activated partial thromboplastin time, ANA: anti-nuclear antibody, AMA: anti-mitochondrial antibody, ASMA: anti-smooth mascle antibody, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, Cre: creatine, ChE: cholinesterase, Alb: albumin, IgM-HA: immunoglobulin M hepatitis A, HBsAg: hepatitis B virus antigen, HBcAb: hepatitis B virus antibody, HCV: hepatitis C virus, IgM-EB-VCA: immunoglobulin M Epstein-Barr virus-viral capsid antigen antibody, IgM-CMV: immunoglobulin M cytomegalovirus, IgM-HSV: immunoglobulin M herpes simplex virus
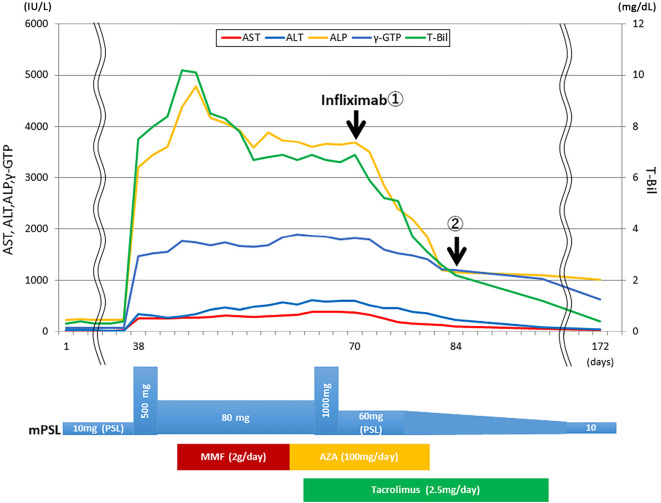
Treatments with methylpredonosolone (mPSL) at 80 mg/day + MMF at 2 g/day, mPSL at 80 mg/day + AZA at 2 mg/kg/day, mPSL at 80 mg/day + AZA at 2 mg/kg/day + tacrolimus at 2.5 mg/day (blood trough level 5 ng/mL) were given to treat the severe hepatitis. Furthermore, we also performed steroid half-pulse therapy on days 38 to 40 and steroid pulse therapy on days 66 to 68 during the course. However, despite these strong immunosuppressive treatments, liver dysfunction remained at Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 grade 3. The peak values related to liver dysfunction during the course were as follows: aspartate aminotransferase (AST) 385 IU/L (grade 3) (day 64), alanine aminotransferase (ALT) 615 IU/L (grade 3) (day 64), alkaline phosphatase (ALP) 4,780 IU/L (grade 3) (day 48), T-Bil 10.2 mg/dL (grade 3) (day 44), γ-glutamyl transpeptidase (GTP) 1,888 IU/L (day 62). Therefore, 2 cycles of infliximab (5 mg/kg) were administered 2 weeks apart, on days 70 and 84, following informed consent and ethics committee approval. At the start of infliximab therapy, the blood test results were as follows: AST 375 IU/L (grade 3), ALT 602 UI/L (grade 3), ALP 3,692 IU/L (grade 3), T-Bil 6.9 mg/dL (grade 3), γ-GTP 1,823 IU/L. Following the administration of infliximab, the hepatitis amerliorated and all related results improved to CTCAE grade 2 within 14 days. On day 172, normal levels of AST, ALT, and T-Bil were noted, whereas those of ALP and γ-GTP remained abnormal, as follows: AST 28 IU/L, ALT 37 UI/L, ALP 1,155 IU/L (grade 2), T-Bil 0.4 mg/dL, γ-GTP 1,189 IU/L. The clinical course is shown in Figure.
Figure.
Patient clinical course. MMF: mycophenolate mofetil, AZA: azathioprine
At the time of writing (9 months after initiation of first course of durvalumab), the patient is alive without hepatitis relapse nor lung cancer progression. The treatment with tacrolimus was discontinued at day 172. Treatment with PSL at 10 mg/day is continuing.
Discussion
In the PACIFIC study (3), a clinical study of durvalumab, grade 3 or higher hepatitis was seen in only 1.9% of the enrolled patients. Furthermore, there were no cases of immunosuppressive drugs-resistant immune-related hepatitis due to durvalumab administration. For severe immune-related hepatitis, a corticosteroid at 1 to 2 mg/kg, as well as mycophenolate mofetil, azathioprine and tacrolimus are recommended, while effective treatments for affected patients who are resistant to those drugs are unknown (5,6), though needed.
It is generally considered that most irAE including hepatitis are caused by cytotoxic T lymphocytes activated by treatment with an ICI (9,10), and some studies have reported liver biopsy findings of immune-related hepatitis due to ICI treatment showing infiltrating cells mainly comprised of CD8-positive lymphocytes (8,11-13). Thus, immunosuppressive drugs, including corticosteroids, mycophenolate mofetil, azathioprine, and tacrolimus, are usually effective for immune-related hepatitis (14,15).
Some reports (7,8) of immune-related hepatitis cases resistant to corticosteroid and mycophenolate mofetil therapy among melanoma patients treated with a combination of nivolumab and ipilimumab have been presented. Those findings indicated the presence of other mechanisms of irAE in patients with hepatitis who are resistant to those drugs. Other studies (16,17) have reported a high concentration of mucosal tumor necrosis factor (TNF)-α observed in cases of ICI-related colitis. In addition, Kim et al. (18) noted the presence of irAE subtypes, such as those related to CD8, Th17, Treg, and TNF. These findings indicated that TNF was one of the factors associated with irAEs, although the mechanisms have yet to be elucidated. Immunosuppressive drugs, including corticosteroids, MMF, AZA, and tacrolimus, may be resistant to TNF-related irAEs, while a TNF-α blockade may be effective. Notably, some reports (7,8) have shown the efficacy of infliximab for melanoma patients with immunosuppressive drug-resistant hepatitis caused by combination therapy with nivolumab and ipilimumab (Table 2). In present case, persistent liver dysfunction did not improve with corticosteroids, MMF, AZA, and tacrolimus, although each drug was continued for at least 7 days. In contrast, liver dysfunction improved immediately after the initiation of infliximab. This clinical course was similar to other reports which indicated the efficacy of infliximab for immune-related hepatitis (7,8). Therefore, we considered that the effect which led to an improvement of his hepatitis was not due to corticosteroids, MMF, AZA, and tacrolimus, but instead was due to infliximab in the present case. We performed maintenance therapy with PSL and tacrolimus referring to the case presented by Corrigan. et al (8), although the efficacy remains unknown. At present, tacrolimus was discontinued and PSL 10 mg/day is continuing without any hepatitis relapse. We are going to continue to carry out a careful follow-up.
Table 2.
Clinical Data of Patients Who Received Infliximab for Liver Dysfunction Due to ICIs.
| No. | Sex | Age | Cancer | Drugs (cycles) |
Days until onset | Liver biopsy | Peak AST (IU/L) | Peak ALT (IU/L) | Peak T-Bil (mg/dL) | Peak ALP (IU/L) | Peak γ-GTP (IU/L) | Other treatments | Effect of infliximab | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 46 | melanoma | Ipi+Niv (1) | 30 days | No | - | 2,854 | - | - | - | mPLS, PSL, MMF | improve | 7 |
| 2 | F | 53 | melanoma | Ipi+Niv (3) | 81 days | Yes | - | 1,200< | 29< | 857 | - | mPSL, PLS, MMF | improve | 8 |
| present case | M | 69 | NSCLC | Durvalumab (1) | 38 days | No | 385 | 615 | 10.2 | 4,780 | 1,888 | mPSL, PSL, MMF, AZA, tacrolimus | improve |
AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, Ipi: ipilimumab, Niv: nivolumab, NSCLC: non-small cell lung cancer, PSL: prednisolone, MMF: mycophenolate mofetil, AZA: azathioprine
Several reports (19,20) presented immune-related cholangitis with non-obstructive dilation of the bile ducts. In the present case, a blood test demonstrated the presence of cholestatic liver dysfunction. However, imaging findings showed no abnormality of bile duct such as non-obstructive dilation of bile ducts was in the present case. Therefore, we diagnosed immune-related hepatitis due to durvalumab, although a pathological evaluation with liver biopsy could not be performed due to various comorbidities. Immunohistochemistry with anti TNF-α antibody may promote an understanding of immunosuppressive drug-resistant hepatitis. Furthermore, measurement of the serum ferritin level may be helpful to understand the association between TNF-α and immunosuppressive drug-resistant hepatitis, although we did not measure it. Further accumulation of the number of cases of immunosuppressive drug-resistant hepatitis due to ICIs is necessary to confirm these findings.
To the best of our knowledge, this is the first report to present findings indicating the efficacy of infliximab for immunosuppressive drug-resistant immune-related hepatitis due to durvalumab. Infliximab may be considered to be an effective treatment option for such cases. Further studies are needed to elucidate the mechanism of immunosuppressive drug-resistant immune-related hepatitis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 359: 1350-1355, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8: 1069-1086, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Antonia SJ, Villegas A, Daniel D, et al. . Durvalmab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377: 1919-1929, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Antonia SJ, Villegas A, Daniel D, et al. . Overall survival with Durvalmab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379: 2342-2350, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer JR, Lacchetti C, Schneider BJ, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist 23: 991-997, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung V, Gupta T, Payne M, et al. . Immunotherapy-related hepatitis: real-world experience from a tertiary centre. Frontline Gastroenterol 10: 364-371, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrigan M, Haydon G, Thompson F, et al. . Infliximab for the treatment of refractory immune-related hepatitis secondary to checkpoint inhibitors: a case report. JHEP Rep 1: 66-69, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shivaji UN, Jeffery L, Gui X, et al. . Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their management. Ther Adv Gastroenterol 12: 1-15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michot JM, Bigenwald C, Champiat S, et al. . Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54: 139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Kleiner D, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 57: 2233-2240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sue M, Ueno M, Takabata H, et al. . A case report of pembrolizumab-induced liver injury: the implication of CD8-positive lymphocytes in the immune-related adverse event. Kanzo 59: 571-577, 2018(in Japanese, Abstract in English). [Google Scholar]
- 13. Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol 36: 434-440, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Mathew TV, Bindal P, Ann AS, et al. . Nivolmab-induced hepatitis: a rare side effect of an immune check point inhibitor. J Oncol Pharm Pract 26: 459-461, 2020. [DOI] [PubMed] [Google Scholar]
- 15. Imoto K, Kohjima M, Hioki T, et al. . Clinical features of liver injury induced by immune checkpoint inhibitors in Japanese patiets. Can J Gastroenterol Hepatol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coutzac C, Adam J, Soularue E, et al. . Colon immune-related adverse events: anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entitis. J Crohns Colitis 11: 1238-1246, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Perez-Ruiz E, Minute L, Otano I, et al. . Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569: 428-432, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Kim KH, Hur JY, Cho J, et al. . Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. Oncoimmunology 9: 1722023, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizuno K, Ito T, Ishigami M, et al. . Real world data of liver injury induced by immune checkpoint inhibitors in Japanese patients with advanced malignancies. J Gastroenterol 55: 653-661, 2020. [DOI] [PubMed] [Google Scholar]
- 20. Kawakami H, Tanizaki J, Tanaka K, et al. . Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 35: 529-536, 2017. [DOI] [PubMed] [Google Scholar]