Abstract
Thioamides quench tryptophan and tyrosine fluorescence in a distance-dependent manner and thus can be used to monitor the binding of thioamide-containing peptides to proteins. Since thioamide analogs of the natural amino acids can be synthetically incorporated into peptides, they can function as minimally-perturbing probes of protein/peptide interactions.
Intrinsic protein fluorescence, which chiefly arises from excitation of tryptophan (Trp) or tyrosine (Tyr) residues, has been used extensively to probe protein folding, conformational rearrangement, and ligand binding.1 Many of these studies employ distance-dependent quenching of Trp and Tyr through energy transfer to an extrinsic probe, but data interpretation in these experiments is limited by the size of the acceptor chromophore.2 If a chromophore were sufficiently compact to be incorporable at any position in a protein without severely perturbing native structure, the structural resolution of these experiments could be greatly improved. Here we show that a thioamide – a single atom substitution of the peptide bond – is such a probe and can quench Trp and Tyr fluorescence in a distance-dependent fashion. We also show that this technique can be used to monitor protein/protein interactions in vitro.
We have previously demonstrated that thioamide quenching of p-cyanophenylalanine (Cnf) can be used to study protein dynamics.3 Cnf was chosen for these initial experiments because of its large extinction coefficient (ε = 13,000 M−1 cm−1 at 240 nm) and substantial spectral overlap with thioamides.4 However, the Cnf/thioamide pair is not generally compatible with Trp- and Tyr-containing proteins because Förster resonant energy transfer (FRET) between the residues can complicate data interpretation.5 Since global replacement of these aromatic residues is undesirable, we chose to explore thioamide quenching of Trp and Tyr fluorescence in order to understand the effects of thioamide incorporation on the near-UV fluorescence of typical proteins.
Although electron-transfer-induced quenching of Trp and Tyr fluorescence by many functional groups is well documented, there are only a few reports of the effect of a thioamide on protein fluorescence.6 Wiczk et al. examined FRET quenching of Trp in neat propylene glycol, but since thioamide absorbance is red-shifted and Trp emission is blueshifted under these conditions, it is not clear that their calculations are appropriate for aqueous solutions.7 Also, we note a report by Równicka et al. in which thiouracil, which contains a thioamide, is shown to quench intrinsic fluorescence of bovine serum albumin upon binding through an energy transfer mechanism.8
We began our investigation by considering a possible FRET mechanism for quenching.9 According to Förster theory, the efficiency of energy transfer between a fluorophore and quencher depends on the spectral overlap of donor emission and acceptor absorbance. Although the Tyr emission spectrum has moderate overlap with the absorbance of a thioamide, the Trp emission spectrum has almost no overlap (Fig. 1). These observations are borne out in the theoretical calculations of R0, the distance at which energy transfer is 50% efficient. Using the previously reported quantum yields for Tyr (Φ = 0.14) and Trp (Φ = 0.13), and assuming the transition moment dipoles to be randomly oriented during the course of energy transfer (κ2 = 2/3), we calculated R0 to be 13.4 Å for the Tyr/thioamide FRET pair, and 4.0 Å for the Trp/thioamide pair in aqueous solutions (see the Supplementary Information for calculations).10 For small values of R0, such as those that we calculated for Trp, Förster theory may not provide an adequate prediction of quenching, since other short-range mechanisms will contribute significantly.11
Fig. 1.
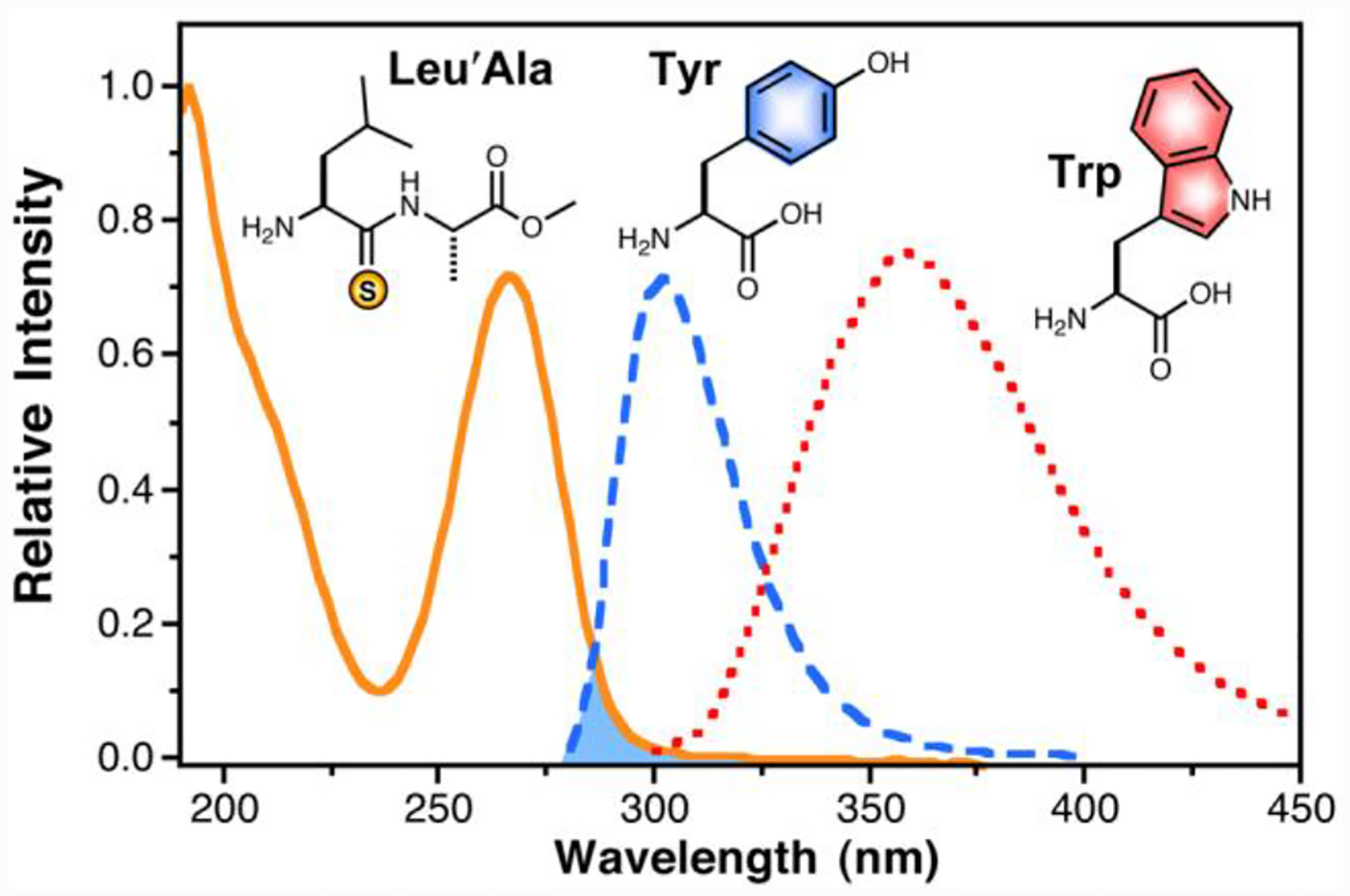
Thioleucylalanine ester (Leu’Ala), Tyrosine (Tyr) and Tryptophan (Trp) Spectra. Absorption spectrum of Leu’Ala (solid orange line) shown with relative absorption intensity normalized to extinction coefficient (12,400 M−1cm−1 at 266 nm). The fluorescence spectra of Tyr and Trp (dashed blue and dotted red lines, respectively) are arbitrarily normalized to emission maxima. The shaded area indicates the spectral overlap that contributes to FRET between a thioamide and Tyr.
To verify the distance dependence predicted by Förster theory, we synthesized groups of peptides that contained either Trp or Tyr at the C-terminus and thioleucine (Leu’) at the N-terminus, separated by an increasing number of proline residues.12 Fluorescence spectra of dilute samples of each peptide were taken in phosphate buffer at pH 7.00. In both cases, the fluorescence intensity showed a strong dependence on distance, at length scales much greater than that predicted by Förster theory (Fig. 2). This is particularly noticeable for Trp, where a half-maximal change in fluorescence is observed at 17 Å, far exceeding the predicted R0 of 4.0 Å. The distance dependence of both Tyr and Trp are shown fit to a sigmoidal expression, which provides a reasonable description of the distance dependence without assigning a mechanistic interpretation. Fits of the data to other mechanism-based equations, including Forster, Dexter, and electron transfer are provided in Supplementary Information, with accompanying discussion. The sigmoidal fit in Fig. 2 serves as a working, empirical “ruler” based on quenching efficiency to be used while we investigate the mechanism of quenching further.
Fig. 2.
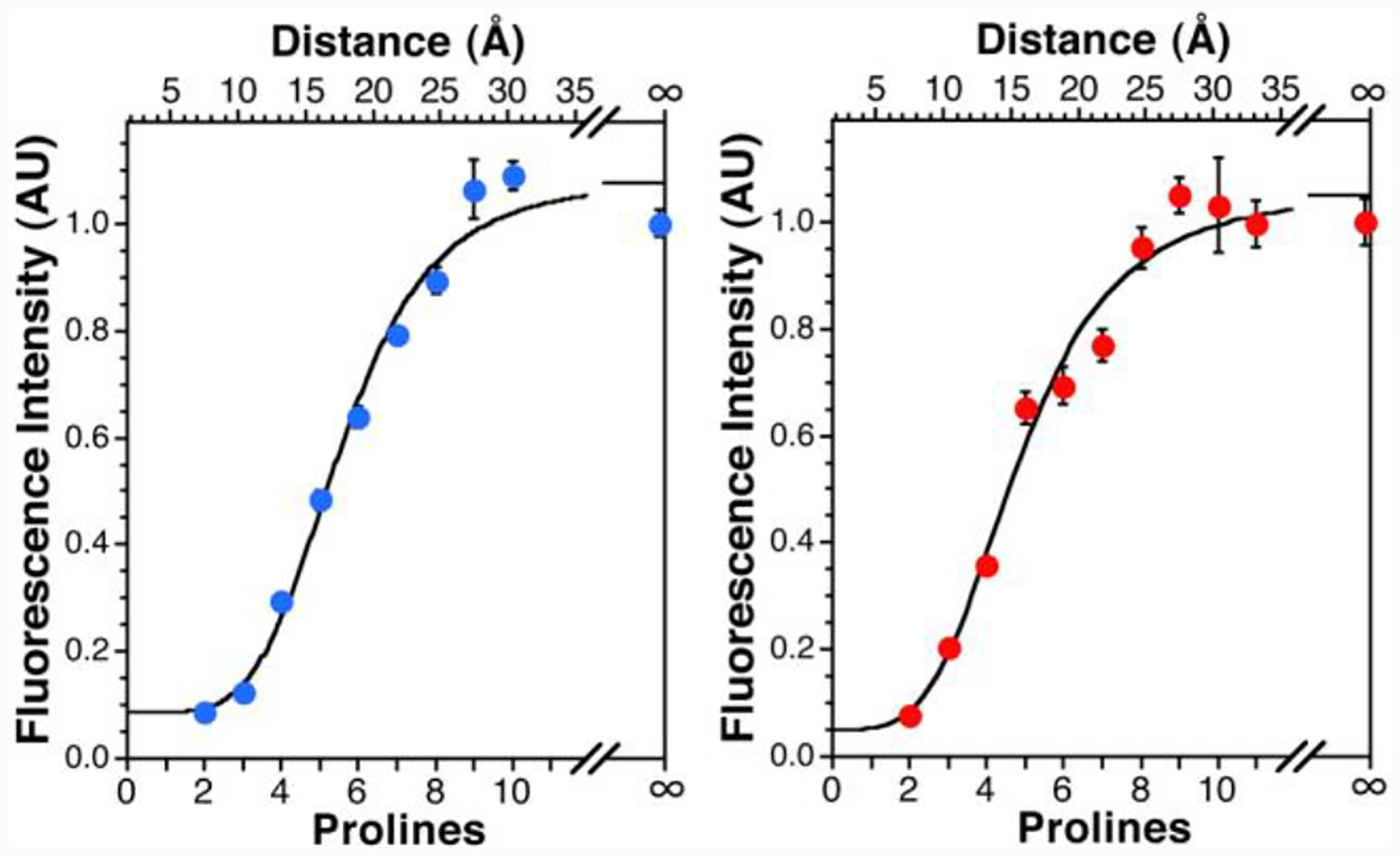
Fluorescence Intensity as a Function of Chromophore Separation. Left: The fluorescence emission at 305 nm of Leu’- Pron-Tyr (n = 2–10) is shown. Right: Fluorescent emission at 355 nm of Leu’-Pron-Trp (n = 2–11). In both plots, the “∞” data point indicates the fluorescence of Leu-Pro2-Tyr or Leu-Pro2-Trp. Sigmoidal fits to the data shown (3 or more trials per peptide, bars represent standard error). Distances determined from molecular dynamics simulations.
To test the utility of our spectroscopic ruler and verify that quenching takes place in a molecular context other than the Pro series, we conducted protein-binding studies in which the thioamide was incorporated into peptides that bind to the protein calmodulin (CaM). Since the peptides bind with a well-defined geometry, this experiment allowed us to examine quenching in an intermolecular context and to evaluate the ability of our model to predict observed quenching efficiencies in proteins with multiple fluorescent residues.
Chicken CaM contains two tyrosine residues, Y100 and Y139, and, in the presence of Ca2+, binds helical peptides with diverse sequences.13 We prepared derivatives of bOCNCp, a fragment of an olfactory cyclic nucleotide-gated channel, in which thiophenylalanine (Phe’) was incorporated at the N-terminus of the peptide (pOCNC-F1’).14 As a control for any changes in fluorescence due to factors other than thioamide quenching, we prepared an oxoamide version of the peptide (pOCNC) with phenylalanine at the N-terminus. From NMR structural data, we estimated the distance between the thiocarbonyl carbon and the center of the phenol ring to be 19 Å for Y100 and 16 Å for Y139.14 Titrations of each peptide into CaM solutions are shown in Fig. 3.
Fig. 3.
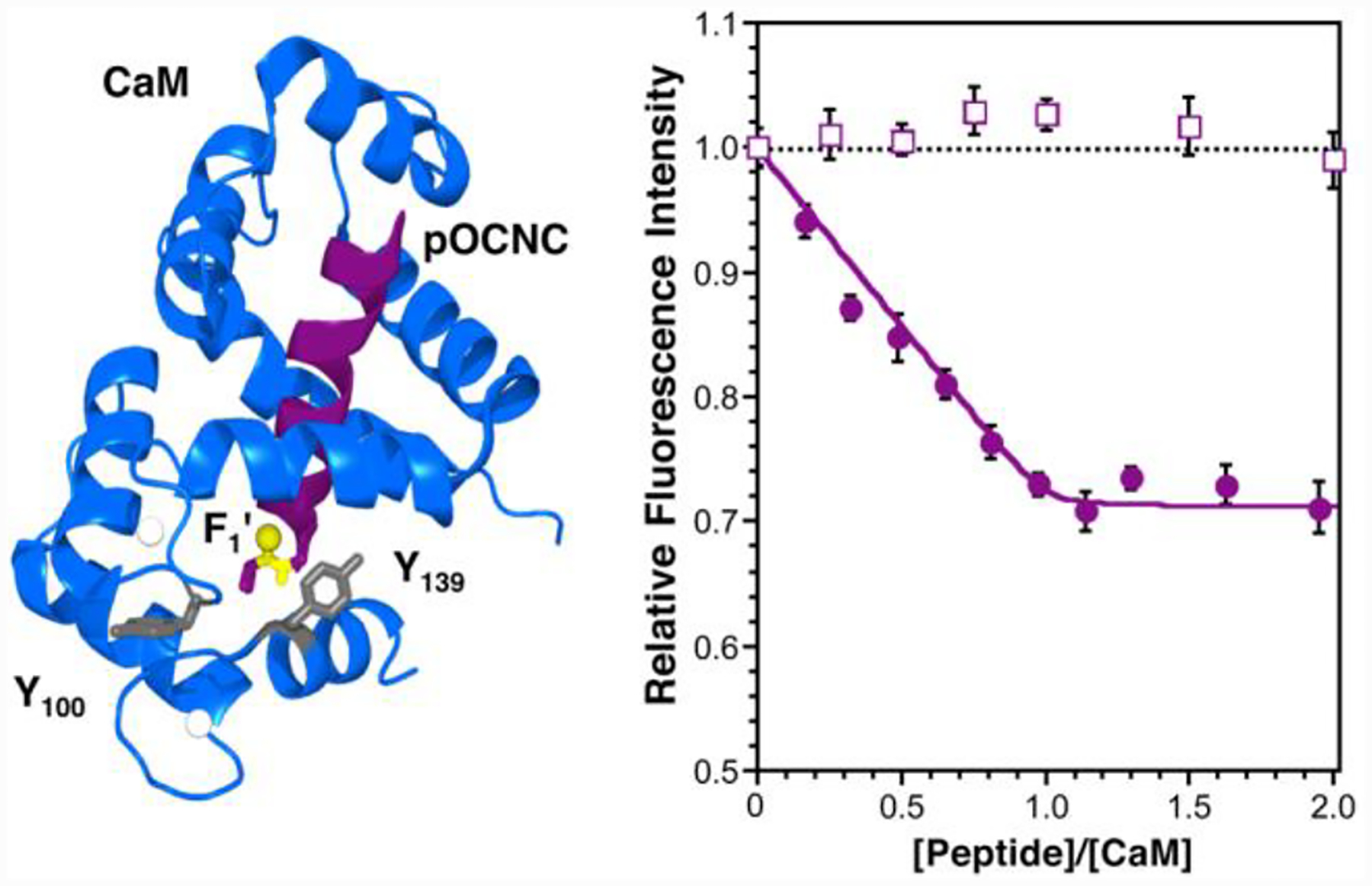
CaM Binding pOCNC. Left: CaM structure taken from PDB 1SY9.14 Image created in PyMOL. (Delano Scientific, LLC; South San Francisco, CA) Y100 and Y139 are rendered in grey; the thioamide bond is highlighted in yellow. Right: Titration of pOCNC (filled circles) and pOCNC-F1’ (open squares) into a solution of 10 μM CaM in 15 mM HEPES buffer, 140 mM KCl, and 6 mM CaCl2, pH 6.70, monitored by fluorescence spectroscopy. Fluorescence data are normalized to CaM in the absence of peptide and corrected for peptide fluorescence.
A 28% decrease in fluorescence is seen at saturating concentrations of pOCNC-F1’, whereas essentially no change in fluorescence is seen with the oxoamide control pOCNC. Since these peptides differ only in the O-to-S substitution in the F1/R2 amide bond, we assign the quenching to the Tyr/thioamide interaction. Both Trp and Tyr fluorescence are sensitive to their local environment with respect to solvent accessibility, pH, neighboring residues, and other chromophores.15 CaM undergoes a substantial conformational rearrangement upon peptide binding, and we were concerned that these factors might alter Tyr fluorescence independent of thioamide quenching.16, 17 Therefore, the inclusion of an oxoamide control is essential to assign quenching specifically to the Tyr/thioamide interaction. Corrected pOCNC-F1’ fluorescence values can be fit to a 1:1 binding model to obtain a KD of 280 nM.‡
Prediction of the efficiency of quenching (EQ) from Förster theory is complicated by the fact that the two donor Tyr have different extinction coefficients (ε) and quantum yields (Φ), both of which contribute to FRET (i.e. quenching) efficiency.16, 18 Using reported ε and Φ values for each Tyr, we calculated EQ values from Förster theory and an empirical calibration based on Fig. 2. (See Supplementary Information for calculations) Since Phe is not noticeably fluorescent at the excitation wavelength of Tyr, we generated Y100F and Y139F mutants to verify the effect of thioamide quenching on each Tyr residue independently. Table 1 summarizes the observed quenching of each mutant in a stoichiometric ratio with pOCNC-F1’, as well as theoretical predictions from Förster theory (EQ Förster) and our empirical spectroscopic ruler (EQ Calculated) using distances from the NMR structure. There is reasonable agreement between our empirical EQ and the observed EQ for the single Tyr mutants, but the WT values deviate, perhaps because we are not weighting the fluorophore contributions or orientation effects (κ2) properly.
Table 1.
Quenching of CaM Mutant Fluorescence by Thioamide Peptide
| CaM Mutant (Donor, DQ Distance)a |
EQ (%) Experimentalb |
EQ (%) Calculatedb,c |
EQ (%) Försterb,c |
|---|---|---|---|
| WT | 28 ± 1 | 42 | 10 |
| Y100F (Y139, 16 Å) | 50 ± 2 | 56 | 14 |
| Y100W (W100, 19 Å) | 27 ± 3 | 30 | 0 |
| Y139F (Y100, 19 Å) | 22 ± 2 | 34 | 8 |
| Y139W (W139, 17 Å) | 58 ± 2 | 40 | 0 |
Donor/quencher (DQ) distance measured in PyMol from aromatic ring of Tyr or Trp to thiocarbonyl carbon.
Calculations described in the Supplementary Information.
We estimate the error to be ± 15% for Tyr predictions and ± 20% for Trp predictions.
To further examine quenching of Trp, we mutated either Y100 or Y139 to Trp. Stimulation at 295 nm allowed us to selectively excite the Trp residue. The observed EQ of the Trp mutants at stoichiometric pOCNC-F1’ are reported in Table 1 along with predicted quenching efficiencies.
Several observations are worth noting from these data. First, given the predicted and observed distance dependencies, a FRET mechanism is clearly not responsible for Trp quenching, and is at best partially responsible for Tyr quenching. Second, the exponential distance-dependence one might expect for through-bond electron transfer is seen both in the Pro series and the CaM-binding experiments, which shows that the rigidity of the Pro σ-bond framework is not necessary for efficient transfer over 15–20 Å distances. Third, the observed quenching efficiency in the CaM binding experiments is in moderate agreement with the quenching level predicted from our polyproline rulers, indicating that they may be useful as empirical standards. Further investigation in other systems will be required to gain a greater understanding of local environment and orientation effects.
In conclusion, we have demonstrated that thioamides quench Trp and Tyr fluorescence in a distance-dependent fashion that can be used to monitor biological interactions, such as macromolecular binding events. It is well established that Trp and Tyr are also quenched by many other functional groups. However, since we can compare our synthetic thioamide proteins to all-oxoamide equivalents, we should be able to separate thioamide-quenching from quenching by protein sidechains or backbone in interpreting our data. We are currently uncertain of the mechanism of quenching, but recent data collected in our laboratory suggest that thioamides are capable of quenching red-shifted fluorophores with no spectral overlap such as fluorescein, implicating an electron transfer process in quenching. Given the substantial difference in the oxidation potentials between oxoamides (3.25 eV) and thioamides (1.21 eV), it is not surprising that thioamide-specific quenching can occur.19 We are investigating this phenomenon further in the context of Tyr and Trp as well as other fluorophores. We are also developing methods for the semi-synthesis of large thioamide-containing proteins so that the findings here may be extended to mapping protein/protein interactions.
Supplementary Material
Acknowledgments
This work was supported by funding from the University of Pennsylvania, the National Science Foundation (CHE-1020205 to EJP), and the Searle Scholars Program (10-SSP-214 to EJP). RFW thanks the NIH for funding through the Chemistry-Biology Interface Training Program (T32 GM07133). AMK thanks the NSF for a Summer REU Fellowship (DMR05–20020). We thank Jeff Saven for use of the fluorometer, Feng Gai for assistance with the CD spectrometer (supported by NSF DMR05–20020), Rakesh Kohli for assistance with MALDI-MS (supported by NSF MRI-0820996), and Joshua Wand for the calmodulin plasmid.
Footnotes
Electronic Supplementary Information (ESI) available: Descriptions of peptide and protein preparation, purification, and characterization; fluorescence and UV/Vis spectroscopy; molecular dynamics simulations; and calculations. See DOI: 10.1039/b000000x/
Notes and references
- ‡.We note that the concentrations of peptide and protein used here (low μM) are not optimal for determining the KD for such a high affinity interaction. This may be a limitation in the use of thioamide quenching to monitor protein binding: typical “benchtop” fluorimeters have a sensitivity such that a protein containing a single Trp or Tyr would need to be used at μM concentrations.
- 1.(a) Beechem JM and Brand L, Ann. Rev. Biochem, 1985, 54, 43–71; [DOI] [PubMed] [Google Scholar]; (b) Brown MP and Royer C, Curr. Opin. Biotechnol, 1997, 8, 45–49; [DOI] [PubMed] [Google Scholar]; (c) Eftink MR, Methods Biochem. Anal, 1991, 35, 127–205. [DOI] [PubMed] [Google Scholar]
- 2.(a) Chapman ER, Alexander K, Vorherr T, Carafoli E and Storm DR, Biochemistry, 1992, 31, 12819–12825; [DOI] [PubMed] [Google Scholar]; (b) Miyake-Stoner SJ, Miller AM, Hammill JT, Peeler JC, Hess KR, Mehl RA and Brewer SH, Biochemistry, 2009, 48, 5953–5962; [DOI] [PubMed] [Google Scholar]; (c) Wu P and Brand L, Anal. Biochem, 1994, 218, 1–13; [DOI] [PubMed] [Google Scholar]; (d) Xie Y, Maxson T and Tor Y, J. Am. Chem. Soc, 2010, 132, 11896–11897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg JM, Batjargal S and Petersson EJ, J. Am. Chem. Soc, 2010, 132, 14718–14720. [DOI] [PubMed] [Google Scholar]
- 4.Tucker MJ, Oyola R and Gai F, Biopolymers, 2006, 83, 571–576. [DOI] [PubMed] [Google Scholar]
- 5.(a) Glasscock JM, Zhu YJ, Chowdhury P, Tang J and Gai F, Biochemistry, 2008, 47, 11070–11076; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Taskent-Sezgin H, Chung J, Patsalo V, Miyake-Stoner SJ, Miller AM, Brewer SH, Mehl RA, Green DF, Raleigh DP and Carrico I, Biochemistry, 2009, 48, 9040–9046; [DOI] [PubMed] [Google Scholar]; (d) Taskent-Sezgin H, Marek P, Thomas R, Goldberg D, Chung J, Carrico I and Raleigh DP, Biochemistry, 2010, 49, 6290–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Doose S, Neuweiler H, Barsch H and Sauer M, Proc. Natl. Acad. Sci. USA, 2007, 104, 17400–17405; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Steiner RF and Kirby EP, J. Phys. Chem, 1969, 73, 4130–4135. [DOI] [PubMed] [Google Scholar]
- 7.Wiczk WM, Gryczynski I, Szmacinski H, Johnson ML, Kruszynski M and Zboinska J, Biophys. Chem, 1988, 32, 43–49. [DOI] [PubMed] [Google Scholar]
- 8.Rownicka-Zubik J, Sulkowska A, Bojko B, Maciazek-Jurczyk M, Pozycka J, Pentak D and Sulkowski WW, J. Photochem. Photobiol. B, 2009, 97, 54–59. [DOI] [PubMed] [Google Scholar]
- 9.(a) Forster T, Discuss. Faraday Soc, 1959, No. 27, 7–17; [Google Scholar]; (b) Speiser S, Chem. Rev, 1996, 96, 1953–1976. [DOI] [PubMed] [Google Scholar]
- 10.Lakowicz JR, Principles of fluorescence spectroscopy, Third edn., Springer, New York, NY, 2006. [Google Scholar]
- 11.Braslavsky SE, Fron E, Rodriguez HB, Roman ES, Scholes GD, Schweitzer G, Valeur B and Wirz J, Photochem. Photobiol. Sci, 2008, 7, 1444–1448. [DOI] [PubMed] [Google Scholar]
- 12.Stryer L and Haugland RP, Proc. Natl. Acad. Sci. USA, 1967, 58, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbauer JL, Short JH, Dow LK and Wand AJ, Biochemistry, 1995, 34, 8099–8109. [DOI] [PubMed] [Google Scholar]
- 14.Contessa GM, Orsale M, Melino S, Torre V, Paci M, Desideri A and Cicero DO, J. Biomol. NMR, 2005, 31, 185–199. [DOI] [PubMed] [Google Scholar]
- 15.(a) Chen Y and Barkley MD, Biochemistry, 1998, 37, 9976–9982; [DOI] [PubMed] [Google Scholar]; (b) Chen Y, Liu B, Yu HT and Barkley MD, J. Am. Chem. Soc, 1996, 118, 9271–9278; [Google Scholar]; (c) Qiu WH, Li TP, Zhang LY, Yang Y, Kao YT, Wang LJ and Zhong DP, Chem. Phys, 2008, 350, 154–164. [Google Scholar]
- 16.Kilhoffer MC, Demaille JG and Gerard D, Biochemistry, 1981, 20, 4407–4414. [DOI] [PubMed] [Google Scholar]
- 17.Pundak S and Roche RS, Biochemistry, 1984, 23, 1549–1555. [DOI] [PubMed] [Google Scholar]
- 18.(a) Haiech J and Kilhoffer M-C, Top. Fluoresc. Spectrosc, 2000, 6, 175–209; [Google Scholar]; (b) Kilhoffer MC, Roberts DM, Adibi A, Watterson DM and Haiech J, Biochemistry, 1989, 28, 6086–6092; [DOI] [PubMed] [Google Scholar]; (c) Klee CB, Biochemistry, 1977, 16, 1017–1024. [DOI] [PubMed] [Google Scholar]
- 19.Bordwell FG, Algrim DJ and Harrelson JA, J. Am. Chem. Soc, 1988, 110, 5903–5904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


