Abstract
Background
Studies have shown unwarranted variation in test ordering among GP practices and regions, which may lead to patient harm and increased health care costs. There is currently no robust evidence base to inform guidelines on monitoring long-term conditions.
Objectives
To map the extent and nature of research that provides evidence on the use of laboratory tests to monitor long-term conditions in primary care, and to identify gaps in existing research.
Methods
We performed a scoping review—a relatively new approach for mapping research evidence across broad topics—using data abstraction forms and charting data according to a scoping framework. We searched CINAHL, EMBASE and MEDLINE to April 2019. We included studies that aimed to optimize the use of laboratory tests and determine costs, patient harm or variation related to testing in a primary care population with long-term conditions.
Results
Ninety-four studies were included. Forty percent aimed to describe variation in test ordering and 36% to investigate test performance. Renal function tests (35%), HbA1c (23%) and lipids (17%) were the most studied laboratory tests. Most studies applied a cohort design using routinely collected health care data (49%). We found gaps in research on strategies to optimize test use to improve patient outcomes, optimal testing intervals and patient harms caused by over-testing.
Conclusions
Future research needs to address these gaps in evidence. High-level evidence is missing, i.e. randomized controlled trials comparing one monitoring strategy to another or quasi-experimental designs such as interrupted time series analysis if trials are not feasible.
Keywords: Chronic disease, common illnesses, continuity of care, diagnostic tests, primary care, scoping review
Key Messages.
• Optimal testing for chronic diseases is an area of uncertainty in primary care.
• The uncertainty causes unwarranted variation in test ordering among GP practices.
• We identified gaps in research on determining the optimal frequency of testing.
• Optimal testing strategies improve patient outcomes and reduce patient harms.
• To optimize testing strategies, high-level evidence is needed.
• Ideally, by running randomized controlled trials or quasi-experimental designs.
Introduction
In primary care, ~50% of laboratory tests are used for monitoring long-term conditions (1). Appropriate monitoring ensures early detection of disease progression and the development of complications, potentially enabling GPs to intervene at an early stage, e.g. by adjusting treatment. The use of laboratory tests among GP practices and regions varies substantially (2–9), suggesting that chronic disease monitoring is not optimal in many places. Both over- and under-testing can cause patient harm and increase health care costs (10,11).
To avoid under- and over-testing, robust evidence is needed on what optimal monitoring looks like. Our recent review of UK guidelines on monitoring patients with hypertension, type 2 diabetes and chronic kidney disease found that most recommendations were solely based on expert opinion and none had a strong evidence base (12).
Guidance on how to generate this evidence base or a framework to standardize evaluations of testing strategies are lacking. The aim of this scoping exercise is 2-fold. The primary aim is to map the nature, extent and range of research on the use of laboratory tests in monitoring long-term conditions in primary care, e.g. which tests should be used or not used, how frequently and how can this be evaluated. In contrast to a systematic review, we aim to scope the methodology used by these studies, i.e. how have researchers tried to answer these questions, instead of extracting their research findings. The secondary aim is to identify gaps in existing research, i.e. identifying questions that are not being addressed. Because we are addressing a broad topic from different angles, where study design is an outcome rather than an inclusion criterion, we considered a scoping review approach to be most suitable.
Methods
A scoping review is a relatively new but increasingly used method for mapping research evidence across broad topics. This scoping review is reported according to the PRISMA Extension for Scoping Reviews reporting guidelines (13). The review protocol is available online (14).
Sources of evidence
We searched CINAHL, EMBASE and MEDLINE to April 2019. The search included terms for specific long-term conditions (e.g. hypertension, diabetes, chronic kidney disease), monitoring tests (e.g. glucose, lipids, creatinine, HbA1c) and primary care (see protocol appendix for detailed search strategy) (14). Search terms were adapted for each database. We did not apply any language restrictions.
Study selection
Studies that fulfilled the following criteria were eligible for inclusion: primary care setting, adult patients (>18 years of age) with non-communicable long-term conditions (e.g. cardiovascular disease, chronic kidney disease and type 2 diabetes), laboratory tests (e.g. urine and blood tests) and aiming to address any of the following questions (Fig. 1):
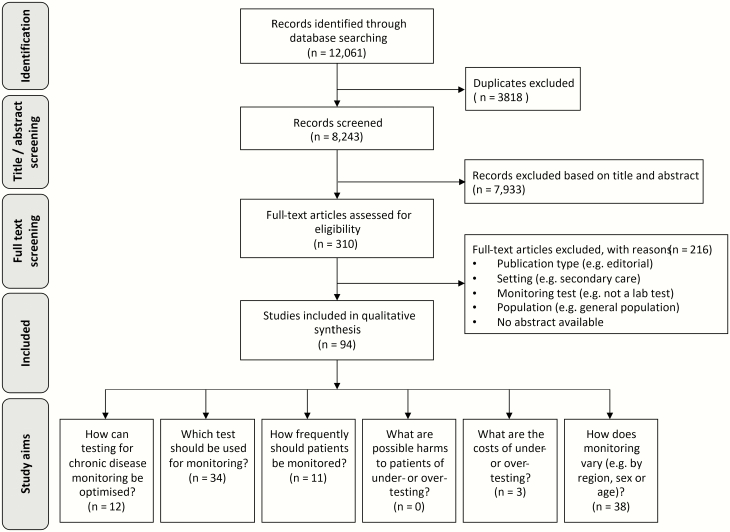
Figure 1.
PRISMA flow diagram. Some studies had more than one aim and appear more than once in under ‘study aims’ in this diagram.
-
•
How can testing for chronic disease monitoring be optimized? (i.e. studies that present strategies or methods to define or evaluate optimal testing)
-
•
Which test should be used for monitoring? (i.e. studies on test performance or appropriateness)
-
•
How frequently should patients be monitored?
-
•
What are possible harms to patients of under- or over-testing?
-
•
What are the costs of under- or over-testing?
-
•
How does monitoring vary (e.g. by region, sex or age)?
We did not apply any restrictions on study design or outcome measures. Studies investigating a general population, patients with infectious diseases or patients with mental health disorders were excluded. Publications that did not use a methodology (e.g. editorial) were also excluded, whereas papers that reported on methodology but not on research findings were included (e.g. protocols).
Identified papers were screened independently by at least two reviewers (ME, LS and KA) using Rayyan (15). Discrepancies were resolved through consensus or a third reviewer (PW). Foreign language records were translated and assessed by the review team.
Data abstraction
Data were abstracted using standardized forms developed in Google Forms. Forms were piloted on a small sample of studies and adapted as necessary. The form included study characteristics (first author, year of publication, geographical location of study population), study aims, population details (which chronic disease(s) and sample size), methodology (study design and statistical methods), monitoring test and outcome measures. In order to minimize bias and errors, data abstraction was performed by one reviewer and checked by a second reviewer (ME and LS). Disagreements were resolved through discussion or referral to a third reviewer where necessary. If full texts were unavailable, data were abstracted from the abstract where possible.
Synthesis
The results are described and charted according to the scoping framework published by Arksey and O’Malley (16) and guidance by Peters et al. (17). An overview of the volume and nature of available evidence is represented graphically using tables and charts. Several simplifications were made in order to categorize variables and visualize them in charts. For instance, ‘hepatic injury’ and ‘liver cirrhosis’ were categorized as ‘liver disease’, and ‘glomerular filtration rate’ and ‘microalbuminuria tests’ were categorized as ‘renal function tests’. Most categories were predefined in the data abstraction form (14), although some extra categories were added based on the data. For example, ‘response to test results’ (e.g. whether the clinician adjusted the medication in response to the test results) was included as an outcome category because it was identified during data abstraction and did not fit into any existing category. Sparse or unclear data were put in an ‘other’ category.
Results
The database search yielded 12 061 citations; of which, 8243 were unique. Seven thousand nine hundred and thirty-three citations were excluded based on title and abstract. The remaining 310 citations underwent full-text review. At the full-text screening stage, 216 papers were excluded based on publication type (no methods reported, e.g. editorial), setting, monitoring test, population or no abstract available (Fig. 1). Ninety-four studies were included in the scoping review (Fig. 1) (8,18–109), including seven studies for which full texts could not be retrieved, so data were abstracted from the abstract (101,102,104,106–109). Six included non-English papers were in French, German, Hebrew, Hungarian, Polish and Portuguese, which were translated and assessed by the review team (34,40,110–113).
Studies were labelled with one or more of the six prespecified study aims (Fig. 1). The key questions ‘How can testing for chronic disease monitoring be optimized?’ (i.e. studies that presented strategies to optimize testing) and ‘how frequently should patients be monitored?’ were addressed by only 12 studies (18–20,22,35,37,50,51,63,64,72,93) and 11 studies (22,33,46,47,49,52,56,72,82,84,99), respectively. Studies labelled as ‘which test should be used for monitoring’ (n = 34) focussed on the diagnostic performance of specific monitoring tests instead of test appropriateness (e.g. whether performing the test improves patient outcomes). The most common aim was ‘how does monitoring vary’ (n = 38), in which studies described the variation in monitoring between regions, patient subgroups or over time. Three studies investigated the costs of over-testing, but none of the studies reported on the possible harms of over-testing to patients (34,78,105).
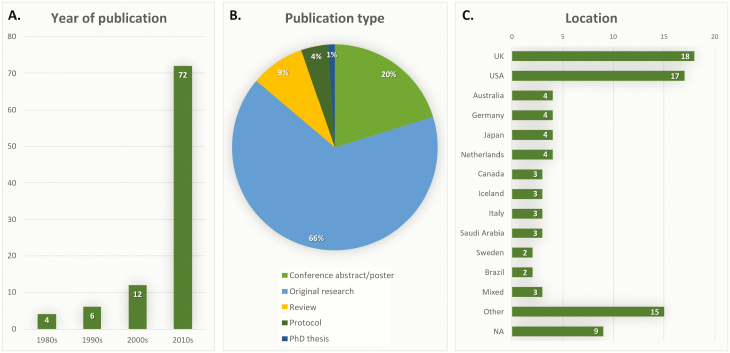
Seventy studies (74%) were published within the last 10 years (Fig. 2A, Supplementary Table 1). The selected studies included 62 (66%) original research reports, 19 (20%) conference abstracts or posters, 8 (9%) reviews (including 5 systematic reviews (8,31,47,52,80) and 3 narrative reviews (33,50,63)), 4 protocols (21,27,71,90) and 1 PhD thesis (38) (Fig. 2B). More studies were based in the UK (18%) and the USA (17%) than elsewhere (Fig. 2C, Supplementary Table 1).
Figure 2.
Characteristics of included studies: year of publication (A), publication type (B) and location (i.e. origin of study population) (C). Not applicable (NA), if there were no patients included in the study, such as in reviews or studies using simulated data.
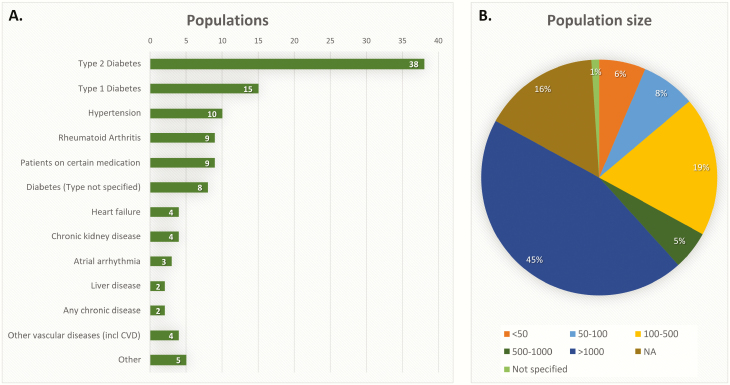
The majority of studies focussed on diabetes (51%), especially type 2 diabetes (40%), followed by hypertension (11%) and rheumatoid arthritis (10%) (Fig. 3A). In nine studies, the study population consisted of patients on a certain type of medication, e.g. patients on statins (23,46,51), warfarin (90,104,108,109) or ‘high risk medication’ (55). Population sizes varied from 6 (89) to 2,395,340 (23) patients (Fig. 3B).
Figure 3.
Population characteristics included disease groups (A) and size of study population (B). Not applicable (NA), if there were no patients included in the study, such as in reviews or studies using simulated data.
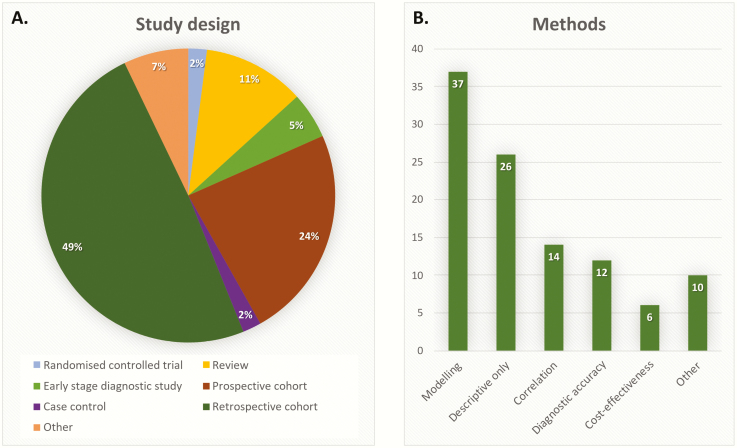
Forty-eight studies (49%) employed a retrospective cohort design, using routinely collected data and large sample sizes (>1000). Other study designs included prospective cohort design (23%), reviews (11%), early-stage diagnostic studies (5%) (45,61,89,98,114), randomized controlled trials (2%) (22,108) and case–control design (2%) (44,84) (Fig. 4A). Many studies solely used descriptive statistics to explore the data (28%). These were mainly the studies investigating variation in monitoring. Many studies (39%) used some form of modelling, e.g. regression analysis. Studies looking at test performance, either used correlations (i.e. measuring the correlation between an old and a new test) or formal diagnostic accuracy measures (i.e. estimating sensitivity and specificity of a new test against a reference standard) (Fig. 4B).
Figure 4.
Methodology of included studies: study design (A) and statistical methods (B).
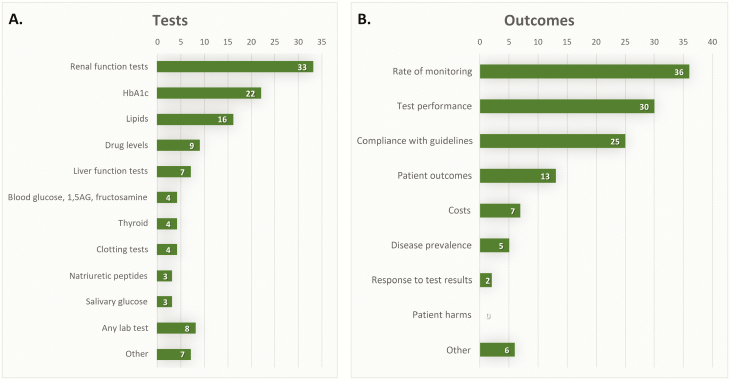
The most frequently studied laboratory tests were renal function tests (35%), including estimated glomerular filtration rate, creatinine, urea, potassium and proteinuria, followed by HbA1c (23%) and lipids (17%). Other tests that were less common, were liver function tests (including liver enzymes, 99mTc-Hepida plasma clearance and bilirubin), blood glucose, clotting tests, thyroid and natriuretic peptides (Fig. 5A).
Figure 5.
Tests (A) and reported outcome measures (B) of included studies.
Thirty-six studies (38%) reported ‘rate of monitoring’ as the primary outcome. These were studies aiming to show the variation in monitoring and often compared the actual rate of monitoring to what is recommended in current guidelines (‘compliance with guidelines’) (Fig. 5B). Thirteen studies (14%) reported patient outcomes (18,19,22,30,60,70,71,82,84,90,93,103,115), such as disease progression or incidence of complications. Seven studies (7%) reported costs as the outcome (34,52,76,78,92,99,105), such as cost-effectiveness of a screening program for certain patient subgroups. Two studies investigated the response to test results (51,72), i.e. whether to the clinician acted on the abnormal test results. Although one study reported patient harms associated with not-testing, i.e. incidence of adverse outcomes in patients who were not monitored regularly (77), none of the studies investigated patient harms due to over-testing.
Discussion
Most research on laboratory test monitoring of long-term conditions either describes the variation in monitoring or reports the test performance of specific tests. This does not address the fundamental question of whether the test is necessary or beneficial.
The most important reason for monitoring is to improve patient outcomes, especially due to early intervention. Nevertheless, only a few studies reported on this outcome. Another important outcome is whether GPs responded to an abnormal test results because monitoring tests are only useful if the result informs patient treatment. However, only two studies investigated this outcome. Finally, over-testing can cause harms to patients (116), but none of the included studies investigated this.
Most research on optimal testing focusses on patients with type 2 diabetes, testing for renal function and HbA1c. The most common study design was retrospective cohort design using routinely collected data, followed by prospective cohort design. Outcomes were often summaries of current practice compared to guideline recommendation or test performance, i.e. whether a new test works just as well as the old test.
This scoping review has identified several gaps in the literature. More evidence is needed on establishing the best testing interval, and what the harms of under- and over-testing are, as well as what strategies or methods can successfully optimize testing to improve patient outcomes. We believe that the best study design to obtain the highest level of evidence on optimal testing is a randomized or cluster randomized controlled trial including an economic evaluation of cost-effectiveness. For instance, patients or GP practices can be randomized to different testing strategies based on comparing different testing strategies comprising different testing sets and different testing intervals. Important outcomes to consider would be (i) testing rates, (ii) adverse outcomes specific for the disease (such as disease progression), (iii) harms to patients due to testing (such as patient anxiety and unnecessary follow-up testing) and (iv) costs (such as health care usage, GP workload). While some of these outcomes would be expected to show changes within short time period (<12 months), for example testing rates, other outcome such as disease progression will require longer follow-up periods.
The second-best approach is to use a quasi-experimental design, comparing the effect of an already implemented policy change (such as the publication of new testing guidelines). Routinely collected primary and secondary care data could then be used to perform an interrupted time series analysis. However, this is problematic because GP practices do not strictly follow the guidelines on testing and the guidelines often do not give clear recommendations (12). Alternatively, a new testing strategy could be implemented in one area and compared to a demographically similar region. These approaches may be cheaper but can still be time consuming because a long follow-up will be needed and are more prone to bias.
Finally, a retrospective comparative cohort study using routinely collected health care data may give some insight on the benefits of using ‘a lot of tests’ versus using ‘a minimal number of tests’ and ‘frequent testing’ versus ‘applying longer testing intervals’. Because of the large variation in testing between GP practices, irrespective of the demographics of the population that they serve, these practices could be divided into practices that tend to test often and practices that tend to test less. Patients from ‘high’ and ‘low’ testing practices could be matched, i.e. on demographic factors, co-morbidities and disease severity. The differences in adverse outcomes specific for the disease and health care usage between both groups could be investigated. Although this approach would be much cheaper and time-saving, it will be difficult to investigate patient harms due to over-testing (especially patient anxiety or unnecessary follow-up testing) and it will be impossible to control for all possible biases.
The main strengths of this scoping review are that we have used a very broad search and inclusion criteria and did not restrict by language or publication date. To our knowledge, this is the first scoping review on methods used to create an evidence base underlying chronic disease monitoring in primary care.
The purpose of this scoping review was to map the main sources and types of evidence available; therefore, the findings of the individual studies were not extracted or analysed. Studies on secondary care populations were excluded, which is a limitation of this review. Gaps identified in the primary care literature may have been addressed in secondary care, although their results may not necessarily be applicable to the primary care setting. Another limitation is that we excluded studies that included a general population, i.e. those investigating how to optimize screening, and focussed on studies investigating populations with long-term conditions. These studies may have used methods that are also relevant to monitoring chronic disease populations.
Future research in this area needs to address these gaps in evidence. High-level evidence is missing, i.e. randomized controlled trials comparing one monitoring strategy to another or quasi-experimental designs such as interrupted time series analysis if trials are not feasible. Methods and reporting guidelines should be developed for studies evaluating optimal testing for long-term conditions. This will improve the quality of evidence, as well as reproducibility and transparency of future research and could serve as a framework to judge the trustworthiness of research findings. For clinicians, appropriate tests and testing frequencies can only be identified if we know whether testing improves patient outcomes and whether the benefits outweigh patient harms. Until better evidence is available, decisions around testing should be a collaborative process between patients and clinicians. These decisions should be informed by the patients’ personal preferences and views in combination with current guidelines.
Supplementary Material
Acknowledgements
We would like to thank Alison Richard for developing and performing the searches.
Declaration
Funding: National Institute for Health Research Applied Research Collaboration West; Bristol, North Somerset and South Gloucestershire Clinical Commissioning Group (to KA); National Institute for Health Research (NIHR-2016-09-034 to JW).
Ethical approval: none.
Conflict of interest: none.
References
- 1. NHS RightCare. The 2nd Atlas of variation in risk factors and healthcare for respiratory disease in England. Public Health England 2019. http://fingertips.phe.org.uk/profile/atlas-of-variation.
- 2. Verstappen WH, ter Riet G, Dubois WI et al. Variation in test ordering behaviour of GPs: professional or context-related factors? Fam Pract 2004; 21(4): 387–95. [DOI] [PubMed] [Google Scholar]
- 3. Davis P, Gribben B, Lay-Yee R, Scott A. How much variation in clinical activity is there between general practitioners? A multi-level analysis of decision-making in primary care. J Health Serv Res Policy 2002; 7(4): 202–8. [DOI] [PubMed] [Google Scholar]
- 4. Chami N, Simons JE, Sweetman A, Don-Wauchope AC. Rates of inappropriate laboratory test utilization in Ontario. Clin Biochem 2017; 50(15): 822–7. [DOI] [PubMed] [Google Scholar]
- 5. Lin DC, Straseski JA, Schmidt RL; The Thyroid Benchmarking Group. Multi-center benchmark study reveals significant variation in thyroid testing in United States. Thyroid 2017; 27(10). doi: 10.1089/thy.2017.0190. [DOI] [PubMed] [Google Scholar]
- 6. The 2nd Atlas of Variation in NHS Diagnostic Services. England Reducing Unwarranted Variation to Improve Health Outcomes and Value. London, UK: Public Health England, 2017. [Google Scholar]
- 7. Sá L, Teixeira ASC, Tavares F et al. Diagnostic and laboratory test ordering in Northern Portuguese Primary Health Care: a cross-sectional study. BMJ Open 2017; 7(11): e018509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Sullivan JW, Albasri A, Nicholson BD et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open 2018; 8(2): e018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Busby J, Schroeder K, Woltersdorf W et al. Temporal growth and geographic variation in the use of laboratory tests by NHS general practices: using routine data to identify research priorities. Br J Gen Pract 2013; 63(609): e256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hobbs FDR, Bankhead C, Mukhtar T et al. ; National Institute for Health Research School for Primary Care Research Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016; 387(10035): 2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrie KJ, Sherriff R. Normal diagnostic test results do not reassure patients. Evid Based Med 2014; 19(1): 14. [DOI] [PubMed] [Google Scholar]
- 12. Elwenspoek MMC, Patel R, Watson JC, Whiting P. Are guidelines for monitoring chronic disease in primary care evidence based? BMJ 2019; 365: 12319. [DOI] [PubMed] [Google Scholar]
- 13. Tricco AC, Lillie E, Zarin W et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169(7): 467–73. [DOI] [PubMed] [Google Scholar]
- 14. Elwenspoek MMC, Scott LJ, Alsop K et al. Methods used to develop the evidence base to optimise chronic disease monitoring in primary care: a scoping review protocol. OSFHome 2019; osf.io/mctqx/. [Google Scholar]
- 15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5(1): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8(1): 19–32. [Google Scholar]
- 17. Peters MD, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015; 13(3): 141–6. [DOI] [PubMed] [Google Scholar]
- 18. Glasziou PP, Irwig L, Kirby AC, Tonkin AM, Simes RJ. Which lipid measurement should we monitor? An analysis of the LIPID study. BMJ Open 2014; 4(2): e003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bull BS, Levy WC, Westengard JC et al. Ranking of laboratory tests by consensus analysis. Lancet 1986; 2(8503): 377–80. [DOI] [PubMed] [Google Scholar]
- 20. DeLong ER, Vernon WB, Bollinger RR. Sensitivity and specificity of a monitoring test. Biometrics 1985; 41(4): 947–58. [PubMed] [Google Scholar]
- 21. Duddy C, Wong G. Explaining variations in test ordering in primary care: protocol for a realist review. BMJ Open 2018; 8(9): e023117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A; LIPID Study Investigators Monitoring cholesterol levels: measurement error or true change? Ann Intern Med 2008; 148(9): 656–61. [DOI] [PubMed] [Google Scholar]
- 23. Hajati F, Atlantis E, Bell KJL, Girosi F. Patterns and trends of potentially inappropriate high-density lipoprotein cholesterol testing in Australian adults at high risk of cardiovascular disease from 2008 to 2014: analysis of linked individual patient data from the Australian Medicare Benefits Schedule and Pharmaceutical Benefits Scheme. BMJ Open 2018; 8(3): e019041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harano Y, Kosugi K, Hyosu T et al. Ketone bodies as markers for type 1 (insulin-dependent) diabetes and their value in the monitoring of diabetic control. Diabetologia 1984; 26(5): 343–8. [DOI] [PubMed] [Google Scholar]
- 25. McCoy RG, Van Houten HK, Ross JS, Montori VM, Shah ND. HbA1c overtesting and overtreatment among US adults with controlled type 2 diabetes, 2001-13: observational population based study. BMJ 2015; 351: h6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGill JB, Cole TG, Nowatzke W et al. ; U.S. Trial of the GlycoMark Assay Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care 2004; 27(8): 1859–65. [DOI] [PubMed] [Google Scholar]
- 27. Nansseu JR, Fokom-Domgue J, Noubiap JJ et al. Fructosamine measurement for diabetes mellitus diagnosis and monitoring: a systematic review and meta-analysis protocol. BMJ Open 2015; 5(5): e007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamanouchi T, Ogata N, Tagaya T et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 1996; 347(9014): 1514–8. [DOI] [PubMed] [Google Scholar]
- 29. Afifa K, Belguith Asma S, Nabil H et al. Screening for nephropathy in diabetes mellitus: is micral-test valid among all diabetics? Int J Chronic Dis 2016; 2016: 2910627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aizpuru F, Latorre A, Ibáñez B et al. Variability in the detection and monitoring of chronic patients in primary care according to what is registered in the electronic health record. Fam Pract 2012; 29(6): 696–705. [DOI] [PubMed] [Google Scholar]
- 31. Al-Foraih N, Touma Z, Chatterley T, Keeling S. The role of laboratory tests in monitoring systemic lupus erythematosus: a systematic review. J Rheumatol 2017; 44(6): 906–7. [Google Scholar]
- 32. Allen AS, Forman JP, Orav EJ et al. Primary care management of chronic kidney disease. J Gen Intern Med 2011; 26(4): 386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Naher A, Wright D, Devonald MAJ, Pirmohamed M. Renal function monitoring in heart failure – what is the optimal frequency? A narrative review. Br J Clin Pharmacol 2018; 84(1): 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arena TR, Jericó Mde C, de Castro LC, Castilhod V, Lima AF. Spending with unnecessary complementary tests for hypertension and diabetes in health services. Rev Gaucha Enferm 2014; 35(4): 86–93. [DOI] [PubMed] [Google Scholar]
- 35. Bajwa-Dulai ST, Dulai PS, Kieffer KA. Limited provider response to abnormal microalbumin test results: implications for the utility of screening rate as a quality of care metric. J Gen Intern Med 1: S115. [Google Scholar]
- 36. Bakarman MA, Kurashi NY, Hanif M. Utilization of laboratory investigations in primary health care centers in Al-khobar, Saudi Arabia. J Fam Community Med 1997; 4(1): 37–45. [PMC free article] [PubMed] [Google Scholar]
- 37. Bell KJ, Glasziou PP, Hayen A, Irwig L. Criteria for monitoring tests were described: validity, responsiveness, detectability of long-term change, and practicality. J Clin Epidemiol 2014; 67(2): 152–9. [DOI] [PubMed] [Google Scholar]
- 38. Bialkowska-Warzecha J. Assessment of 99mTc-Hepida plasma clearance test evaluation for monitoring of acute and chronic liver diseases. Med Sci Monit 1998; 4(1): 153–7. [Google Scholar]
- 39. Božič-Mijovski M, Malmström RE, Malovrh P et al. Diluted thrombin time reliably measures low to intermediate plasma dabigatran concentrations. Ann Clin Biochem 2016; 53 (Pt 4): 446–51. [DOI] [PubMed] [Google Scholar]
- 40. Bramlage P, Wittchen HU, Pittrow D et al. Diabetes, hypertension and microalbuminuria in primary care. Fortschr Med Orig 2003; 121 (suppl 1): 33–8. [PubMed] [Google Scholar]
- 41. Cohen SS, Chamberlain AM, Killian JM et al. LDLC monitoring frequency after the diagnosis of diabetes or atherosclerotic cardiovascular disease. Circulation 2017; 136: A15727. [Google Scholar]
- 42. Coleman JJ, McDowell SE, Evans SJ, Gill PS, Ferner RE. Oversight: a retrospective study of biochemical monitoring in patients beginning antihypertensive drug treatment in primary care. Br J Clin Pharmacol 2010; 70(1): 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crippa M, Camanini S, Costa R et al. Uric acid as a possible marker of cardiovascular risk in an essential hypertensive population. High Blood Press Cardiovasc Prev 2012; 19(3): 73. [Google Scholar]
- 44. Curtis JR, John A, Baser O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2012; 64(9): 1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dauernheimer Machado J, Boff R, Aguiar Soares A et al. Accuracy of creatinine and cystatin C equations to estimate glomerular filtration rate in patients with type 2. Clin Chem Lab Med 2015; 53(1): S618. [Google Scholar]
- 46. Doganer YC, Rohrer JE, Angstman KB, Merry SP, Erickson JL. Variations in lipid screening frequency in family medicine patients with cardiovascular risk factors. J Eval Clin Pract 2015; 21(2): 215–20. [DOI] [PubMed] [Google Scholar]
- 47. Dolan G, Smith LA, Collins S, Plumb JM. Effect of setting, monitoring intensity and patient experience on anticoagulation control: a systematic review and meta-analysis of the literature. Curr Med Res Opin 2008; 24(5): 1459–72. [DOI] [PubMed] [Google Scholar]
- 48. Doll H, Shine B, Kay J, James T, Glasziou P. The rise of cholesterol testing: how much is unnecessary. Br J Gen Pract 2011; 61(583): e81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duff CJ, Solis-Trapala I, Driskell OJ et al. The frequency of testing for glycated haemoglobin, HbA1c, is linked to the probability of achieving target levels in patients with suboptimally controlled diabetes mellitus. Clin Chem Lab Med 2018; 57(2): 296–304. [DOI] [PubMed] [Google Scholar]
- 50. Dufour DR, Lott JA, Nolte FS et al. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000; 46(12): 2027–49. [PubMed] [Google Scholar]
- 51. Elhayany A, Mishaal RA, Vinker S. Is there clinical benefit to routine enzyme testing of patients on statins? Expert Opin Drug Saf 2012; 11(2): 185–90. [DOI] [PubMed] [Google Scholar]
- 52. Farmer AJ, Stevens R, Hirst J et al. Optimal strategies for identifying kidney disease in diabetes: properties of screening tests, progression of renal dysfunction and impact of treatment – systematic review and modelling of progression and cost-effectiveness. Health Technol Assess 2014; 18(14): 1–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fazlalizadeh H, Pellicori P, Zhang J et al. Screening with natriuretic peptides to detect micro- and macro-vascular complications amongst older patients with type-2 diabetes mellitus. A report from sica-diabetes study. Eur J Heart Fail 2014; 16: 312. [Google Scholar]
- 54. Fazlalizadeh H, Pellicori P, Zhang J et al. Natriuretic peptides or glycosolated haemogolbin as markers of micro and macro-vascular complications amongst older patients with type-2 diabetes mellitus. A report from sica-diabetes study. Eur J Heart Fail 2014; 16(suppl 2): 312. [Google Scholar]
- 55. Fischer SH, Tjia J, Reed G et al. Factors associated with ordering laboratory monitoring of high-risk medications. J Gen Intern Med 2014; 29(12): 1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Garg D, Naugler C, Bhella V, Yeasmin F. Chronic kidney disease in type 2 diabetes: does an abnormal urine albumin-to-creatinine ratio need to be retested? Can Fam Physician 2018; 64(10): e446–52. [PMC free article] [PubMed] [Google Scholar]
- 57. Griger DR, Higgs JB, Roane DW. Azathioprine hepatotoxicity is uncommon in patients with rheumatic diseases. J Clin Rheumatol 1999; 5(2): 60–4. [DOI] [PubMed] [Google Scholar]
- 58. Heyman C, Gunnarsson R. Normal S-cholesterol indicates unchanged S-LDL, S-HDL and S-triglycerides in patients with previously acceptable blood lipids. Scand J Clin Lab Invest 2007; 67(5): 498–506. [DOI] [PubMed] [Google Scholar]
- 59. Hinton W, McGovern A, Arrowsmith B et al. Disparities in the monitoring and complication screening of people with type 2 diabetes. J Diabetic Med 34(suppl S1): 36–194. [Google Scholar]
- 60. Hirst JA, Stevens RJ, Shine B, James T, Farmer AJ. Frequency of HbA1c test requests in primary care: an analysis of hospital laboratory data. Diabetic Med 2015; 32: 169–70. [Google Scholar]
- 61. Holden WE, Bartos F, Theime T, Green TR, Fitchen JH. Theophylline in oral mucosal transudate. A practical method for monitoring outpatient therapy. Am Rev Respir Dis 1993; 147(3): 739–43. [DOI] [PubMed] [Google Scholar]
- 62. Jacob L, Seitz F, Kostev K. Frequency of blood pressure and estimated glomerular filtration rate monitoring in patients affected by hypertension: a retrospective study with 176 565 patients in Germany. Blood Press Monit 2018; 23(2): 85–90. [DOI] [PubMed] [Google Scholar]
- 63. Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract 2010; 64(8): 1130–9. [DOI] [PubMed] [Google Scholar]
- 64. Kent PD, Luthra HS, Michet C Jr. Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J Rheumatol 2004; 31(9): 1727–31. [PubMed] [Google Scholar]
- 65. Khan FA, Al Jameil N, Arjumand S et al. Comparative study of serum copper, iron, magnesium, and zinc in type 2 diabetes-associated proteinuria. Biol Trace Elem Res 2015; 168(2): 321–9. [DOI] [PubMed] [Google Scholar]
- 66. Kneepkens EL, Pouw MF, Wolbink GJ et al. Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol 2017; 83(11): 2474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Knudsen ST, Mosbech TH, Hansen B et al. Screening for microalbuminuria in patients with type 2 diabetes is incomplete in general practice. Dan Med J 2012; 59(9): A4502. [PubMed] [Google Scholar]
- 68. Kostev K, Grunow S, Rockel T. Hba1c testing frequency in primary care diabetes patients in Germany and in the UK. Value Health 2012; 15(7): A519. [Google Scholar]
- 69. Kostev K, Jacob L, Lucas A, Rathmann W. Low annual frequency of HbA1c testing in people with Type 2 diabetes in primary care practices in Germany. Diabet Med 2018; 35(2): 249–54. [DOI] [PubMed] [Google Scholar]
- 70. Kostev K, Lucas A, Jacob L. Frequency of blood pressure and estimated glomerular filtration rate testing in type 2 diabetes mellitus: a retrospective study with 43,509 patients. Exp Clin Endocrinol Diabetes 2019; 127(7): 455–60. [DOI] [PubMed] [Google Scholar]
- 71. Lamb EJ, Brettell EA, Cockwell P et al. The eGFR-C study: accuracy of glomerular filtration rate (GFR) estimation using creatinine and cystatin C and albuminuria for monitoring disease progression in patients with stage 3 chronic kidney disease – prospective longitudinal study in a multiethnic population. BMC Nephrol 2014; 15(13): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lau CC, Sayeed SM, Kennedy A, Skelly J, Cooper S. Are we over-testing for liver enzyme abnormalities in rheumatoid arthritis patients prescribed methotrexate? Arthritis Rheumatol 2017; 69(suppl 10). [Google Scholar]
- 73. Lenzi S, Giampietro O, Giovannitti G et al. The clinical usefulness of glycated hemoglobin in monitoring diabetes mellitus: a long-term study. Clin Chem 1987; 33(1): 55–6. [PubMed] [Google Scholar]
- 74. Liang H, Kennedy C, Manne S, Lin JH, Dolin P. Monitoring for proteinuria in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care 2015; 3(1): e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Manski-Nankervis JE, Thuraisingam S, Lau P et al. Screening and diagnosis of chronic kidney disease in people with type 2 diabetes attending Australian general practice. Aust J Prim Health 2018; 24(3): 280–6. [DOI] [PubMed] [Google Scholar]
- 76. McAlister FA, Tu K, Majumdar SR et al. Laboratory testing in newly treated elderly hypertensive patients without co-morbidities: a population-based cohort study. Open Med 2007; 1(2): e60–7. [PMC free article] [PubMed] [Google Scholar]
- 77. McGovern AP, Rusholme B, Jones S et al. Association of chronic kidney disease (CKD) and failure to monitor renal function with adverse outcomes in people with diabetes: a primary care cohort study. BMC Nephrol 2013; 14: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mohiuddin S, Hollingworth W, Maishman R et al. A model-based cost-effectiveness analysis of B-type natriuretic peptide monitoring in patients with heart failure. Value Health 2016; 19(7): A867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moroni G, Radice A, Giammarresi G et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis 2009; 68(2): 234–7. [DOI] [PubMed] [Google Scholar]
- 80. Naing C, Mak JW. Salivary glucose in monitoring glycaemia in patients with type 1 diabetes mellitus: a systematic review. J Diabetes Metab Disord 2017; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nilsson E, De Deco P, Trevisan M et al. A real-world cohort study on the quality of potassium and creatinine monitoring during initiation of mineralocorticoid receptor antagonists in patients with heart failure. Eur Heart J Qual Care Clin Outcomes 2018; 4(4): 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oke JL, Stevens RJ, Gaitskell K, Farmer AJ. Establishing an evidence base for frequency of monitoring glycated haemoglobin levels in patients with Type 2 diabetes: projections of effectiveness from a regression model. Diabet Med 2012; 29(2): 266–71. [DOI] [PubMed] [Google Scholar]
- 83. Ormseth M, Solus J, Vickers K et al. Utility of select plasma miRNAs for diagnosis of rheumatoid arthritis and monitoring of disease activity and cardiovascular risk. Ann Rheum Dis 2015; 74: 696. [Google Scholar]
- 84. Parcero AF, Yaeger T, Bienkowski RS. Frequency of monitoring hemoglobin A1C and achieving diabetes control. J Prim Care Community Health 2011; 2(3): 205–8. [DOI] [PubMed] [Google Scholar]
- 85. Pereira M, Cachapuz I. Inappropriate requesting of glycated hemoglobin (HBA1C). Clin Chem Lab Med 2018; 56(2): eA8. [Google Scholar]
- 86. Perrotta PL, Jones R, Souers RJ, Darcy TP, Howanitz PJ. Frequency monitoring of hemoglobin A1c, low-density lipoprotein, and urine protein laboratory testing: a College of American Pathologists Q-Probes study. Arch Pathol Lab Med 2014; 138(8): 1009–14. [DOI] [PubMed] [Google Scholar]
- 87. Razi F, Nasli Esfahani E, Rahnamaye Farzami M, et al. Effect of the different assays of HbA1c on diabetic patients monitoring. J Diabetes Metab Disord 2015; 14: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rickard JP, Negrelli J, Olson JL, Dick T. Frequency of Adverse event monitoring in ambulatory patients on amiodarone or dofetilide. J Pharm Pract 2018; 31(5): 457–61. [DOI] [PubMed] [Google Scholar]
- 89. Satoh K, Fukasawa I, Kanemaru K, et al. Platelet aggregometry in the presence of PGE(1) provides a reliable method for cilostazol monitoring. Thromb Res 2012; 130(4): 616–21. [DOI] [PubMed] [Google Scholar]
- 90. Sezgin G, Georgiou A, Hardie RA et al. Compliance with pathology testing guidelines in Australian general practice: protocol for a secondary analysis of electronic health record data. BMJ Open 2018; 8(11): e024223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shah KB, Gottlieb SS. Laboratory monitoring for spironolactone in congestive heart failure. HCPLive 2008. https://www.mdmag.com/journals/cardiology-review-online/2006/june2006/june-2006-shah. [DOI] [PubMed] [Google Scholar]
- 92. Shardlow A, McIntyre NJ, Fraser SDS et al. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: a primary care cohort study. PLoS Med 2017; 14(10): e1002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stevens RJ, Oke J, Perera R. Statistical models for the control phase of clinical monitoring. Stat Methods Med Res 2010; 19(4): 394–414. [DOI] [PubMed] [Google Scholar]
- 94. Tanaka H, Tomio J, Sugiyama T, Kobayashi Y. Process quality of diabetes care under favorable access to healthcare: a 2-year longitudinal study using claims data in Japan. BMJ Open Diabetes Res Care 2016; 4(1): e000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Thorpe CT, Thorpe JM, Kind AJ et al. Receipt of monitoring of diabetes mellitus in older adults with comorbid dementia. J Am Geriatr Soc 2012; 60(4): 644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Bruggen S, Rauh SP, Kasteleyn MJ et al. Association between full monitoring of biomedical and lifestyle target indicators and HbA1c level in primary type 2 diabetes care: an observational cohort study (ELZHA-cohort 1). BMJ Open 2019; 9(3): e027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van de Ree MA, Christiaan G, Huisman MV, van der Vijver JC, Meinders AE. Monitoring renal function in obese patients with type 2 diabetes mellitus in daily practice. Diabetes Nutr Metab 2001; 14(2): 66–70. [PubMed] [Google Scholar]
- 98. Wang B, Du J, Zhu Z et al. Evaluation of parotid salivary glucose level for clinical diagnosis and monitoring type 2 diabetes mellitus patients. Biomed Res Int 2017; 2017: 2569707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wermeling PR, Gorter KJ, Stellato RK et al. Effectiveness and cost-effectiveness of 3-monthly versus 6-monthly monitoring of well-controlled type 2 diabetes patients: a pragmatic randomised controlled patient-preference equivalence trial in primary care (EFFIMODI study). Diabetes Obes Metab 2014; 16(9): 841–9. [DOI] [PubMed] [Google Scholar]
- 100. Yoo KH, Shin DW, Cho MH et al. Regional variations in frequency of glycosylated hemoglobin (HbA1c) monitoring in Korea: a multilevel analysis of nationwide data. Diabetes Res Clin Pract 2017; 131: 61–9. [DOI] [PubMed] [Google Scholar]
- 101. Aziz KM. Correlation of urine biomarkers: microalbuminuria and spot urine protein among diabetic patients. Application of spot urine protein in diabetic kidney disease, nephropathy, proteinuria estimation, diagnosing and monitoring. Recent Pat Endocr Metab Immune Drug Discov 2015; 9(2): 121–33. [DOI] [PubMed] [Google Scholar]
- 102. Flynn D, Murphy P. Audit of new oral anticoagulant monitoring in a general practice: are patients having their renal function checked? Ir J Med Sci 2015; 184: S251–2. [Google Scholar]
- 103. Garg D, Thind A, Maddocks H. Screening for chronic kidney disease in primary care patients with type 2 diabetes. Longitudinal study. 64: S68 https://gomainpro.ca/wp-content/uploads/2017/05/ScreeningforCKDinFamilyMedicinePractice_Poster.pdf. [Google Scholar]
- 104. Gudmundsdottir BR, Onundarson PT. Warfarin anticoagulation variability, testing, and dose-adjustment frequency is reduced in clinical practice after switch from prothrombin time based international normalized ratio (Inr) to Fiix-prothrombin time based normalized ratio (Fiix-Nr). Am J Hematol 2018; 93(9): E23. [Google Scholar]
- 105. Kessler R, Keusch G, Szucs TD et al. Health economic modelling of the cost-effectiveness of microalbuminuria screening in Switzerland. Swiss Med Wkly 2012; 142: w13508. [DOI] [PubMed] [Google Scholar]
- 106. Lloyd LA, Breslin A, Jones J. An audit of methotrexate monitoring in primary care as part of a shared care agreement. Rheumatology 2009; 48: I61. [Google Scholar]
- 107. Luqmani R, Sheeran T, Robinson M et al. Systemic cytokine measurements: their role in monitoring the response to therapy in patients with rheumatoid arthritis. Clin Exp Rheumatol 1994; 12(5): 503–8. [PubMed] [Google Scholar]
- 108. Onundarson PT, Francis CW, Bjornsson ES et al. Monitoring warfarin with the Fiix-prothrombin time improves anticoagulation stability and long-term clinical outcome: the Fiix-trial. Blood 2014; 124(21): 347. [Google Scholar]
- 109. Oskarsdottir AR, Gudmundsdottir BR, Onundarson PT. Replacing PT-INR monitoring of warfarin with Fiix-NR in clinical practice reduces thromboembolism without increasing bleeding despite reduced number of dose adjustments. Blood 2018; 132: 1239. [Google Scholar]
- 110. Mogyorósy Z, Mogyorósy G. Practice pattern and geographic variation in test ordering. A literature review. Orv Hetil 2006; 147(1): 25–31. [PubMed] [Google Scholar]
- 111. Atsmon J, Priel IE, Dolev E. Is routine monitoring of theophylline blood levels really effective? Harefuah 1995; 129(9): 304–8, 68. [PubMed] [Google Scholar]
- 112. Gerhardt MF, Guéchot J, Imbert-Bismut F et al. Biological examination in the diagnosis and monitoring of liver disease. Algorithms to aid the decision. Pathol Biol (Paris) 1999; 47(9): 1016–32. [PubMed] [Google Scholar]
- 113. Orzechowska-Juzwenko K, Jaźwińska-Tarnawska E, Hurkacz ML, Loboz-Grudzień K. Monitoring kidney function in patients with essential hypertension treated with enalapril. Pol Arch Med Wewn 1997; 97(1): 15–21. [PubMed] [Google Scholar]
- 114. Harish S, Shantaram M. A comparative and correlative study between blood and salivary glucose with blood HbA1c IN type 2 diabetes. Int J Pharm Sci Res 2019; 10(1): 401–6. [Google Scholar]
- 115. Shah KB, Rao K, Sawyer R, Gottlieb SS. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol 2005; 46(5): 845–9. [DOI] [PubMed] [Google Scholar]
- 116. Hofmann B, Welch HG. New diagnostic tests: more harm than good. BMJ 2017; 358: j3314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.