Abstract
Proanthocyanidins are colorless flavonoid polymers condensed from flavan-3-ol units. They are essential secondary plant metabolites that contribute to the nutritional value and sensory quality of many fruits and the related processed products. Mounting evidence has shown that the accumulation of proanthocyanidins is associated with the resistance of plants against a broad spectrum of abiotic and biotic stress conditions. The biosynthesis of proanthocyanidins has been examined extensively, allowing for identifying and characterizing the key regulators controlling the biosynthetic pathway in many plants. New findings revealed that these specific regulators were involved in the proanthocyanidins biosynthetic network in response to various environmental conditions. This paper reviews the current knowledge regarding the control of key regulators in the underlying proanthocyanidins biosynthetic and molecular mechanisms in response to environmental stress. Furthermore, it discusses the directions for future research on the metabolic engineering of proanthocyanidins production to improve food and fruit crop quality.
Keywords: abiotic stress, biotic stress, proanthocyanidins, regulatory mechanism
1. Introduction
Horticultural crops, including vegetables and fruits, are a crucial food source for human nutrition as they contain carbohydrates, proteins, vitamins, organic acids, and minerals. Proanthocyanidins (PAs), also known as condensed tannins, are among the most abundant polyphenols in plants. They are naturally present in woody plants and herbaceous species. PAs are present in the leaves, fruits, seeds, roots, and other parts of plants [1], and play essential roles in the growth and development of leaves and fruits, as well as, the modulation of seed dormancy and germination [2,3,4,5,6,7]. In addition, PAs also influence mouthfeel by enhancing the astringency of fruits and in their processed products, e.g., wines and beverages [8,9]. Moreover, as potential dietary antioxidants, PAs are considered beneficial to human health. It has been widely accepted that the PAs from food plants and medicinal plants are associated with various bioactivities, displaying immunomodulatory, anti-inflammatory, anti-cancer, anti-microbial, and hypolipidemic properties while reducing the risk of cardiovascular disease and ameliorating obesity [10,11,12,13,14,15,16,17,18,19,20,21,22].
Developmental regulation is considered the driving factor for PAs biosynthesis, which can also be influenced by environmental fluctuations. In grape berry, the PAs accumulate in the skin prior to véraison, after which the total PAs content decreases due to intermediate metabolites deviation [4]. Many crops have shown a similar decreasing trend in PAs levels during the fruit ripening process, including bilberries, persimmons, and blueberries [23,24,25]. PAs contribute to the astringency and bitterness of the young fruits, preventing them from being eaten before they are ripe [26]. The accumulation of PAs is associated with the resistance of plants against various biotic and abiotic stimuli, such as low temperature [27,28], drought [29], wounding [30,31], UV radiation [30], and fungal pathogens [32,33,34]. Therefore, increasing attention has been focused on the metabolic engineering and regulatory mechanism of PAs in horticultural plants. The structural genes and key factors that regulate PAs biosynthesis were identified and characterized in many plants, particularly in horticultural crops rich in PAs, such as grapevines, poplars, and apples. Studies involving PAs biosynthesis regulation in response to environmental stresses revealed that the regulatory network was based on the regulators belonging to the MYB, MYC-like basic helix-loop-helix (bHLH), WD40-repeat proteins, and the MYB-bHLH-WD40 (MBW) complexes. However, research regarding more specific regulators in this complicated network is required to understand the interaction between these vital regulators during PAs biosynthesis to ultimately improve the quality of crops.
2. Structure and Biosynthesis of Proanthocyanidins
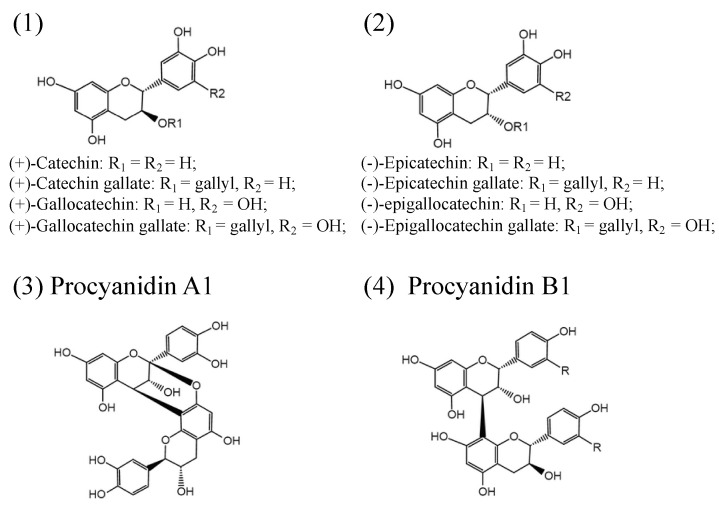
PAs consist of a mixture of flavan-3-ol units and flavan-3,4-diols (leucoanthocyanidins) in complicated ways [35]. Flavan-3-ol monomeric units include (+)-catechins, (−)-epicatechins, and their derivatives: (+)-gallocatechin, (+)-catechin gallate, (+)-gallocatechin gallate (−)-epicatechin gallate, (−)-epigallocatechin, and (−)-epigallocatechin gallate (Figure 1). Both (+)-catechins and (−)-epicatechins are believed to act as the starter units for PAs polymerization, and flavan-3-ols and flavan-3,4-diols serve as extension units for further PAs extension [1,36,37]. Different numbers of subunits polymerized to form oligomeric or polymeric PAs [1,38]. The degree of polymerization of PAs varies among species and tissues. Based on the interflavanic linkages, the subunits linked by C4-C8 and/or C4-C6 bonds are known as B-type PAs, and those with additional C2-O-C7 or C2-O-C5 bond in the structure are classified into A-type PAs (Figure 1) [1]. In addition, esterification, glycosylation, and other modifications have also been found in PAs with more complex structures. B-type PAs are widely distributed in plants and foods, such as senna alata leaves, pine trees, black wattle, beans, barley, sorghum, seeds, fruits, berries, nuts, cinnamon, chocolate, and wine [39,40,41,42,43,44,45]. A-type PAs are mainly found in bilberry, peanuts, plums, cranberries, curry, and cinnamon [46,47,48,49].
Figure 1.
Structures of the flavan-3-ols (1,2); simple A1-type proanthocyanidins (3); simple B1-type proanthocyanidins (4).
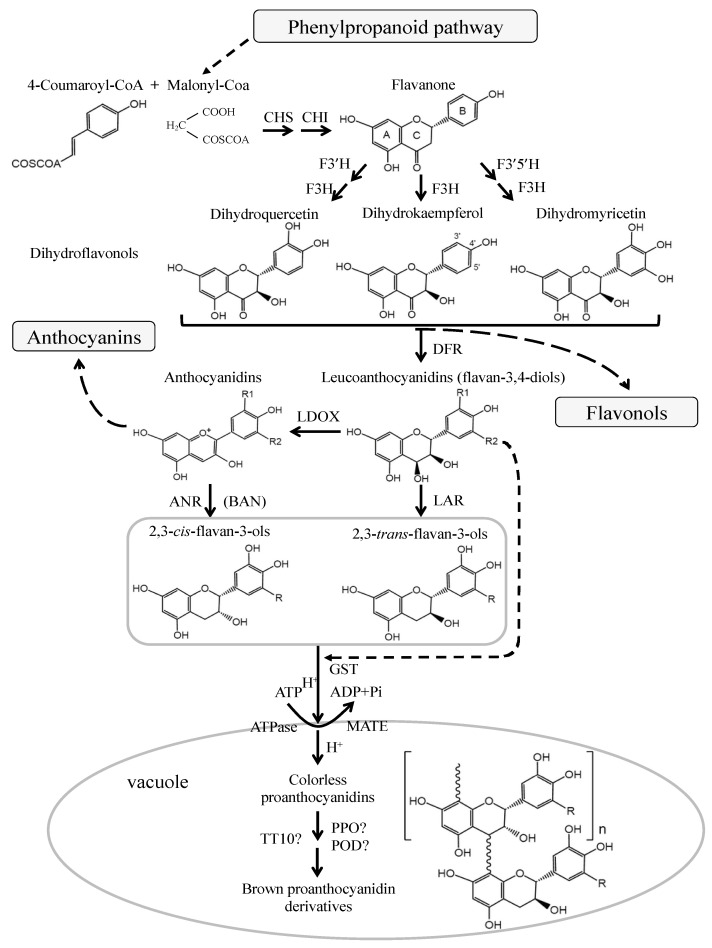
The pathway of flavonoid biosynthesis has been well studied in various plants (Figure 2). Flavonols, anthocyanins, and PAs are the main products of the flavonoid biosynthetic pathway, which belongs to the plant secondary metabolism [50,51,52]. They share the common upstream steps in the biosynthetic pathway of flavonoids, and their precursor is dihydroflavonols which can be converted to leucoanthocyanidins by dihydroflavonol-4-reductase (DFR) and to flavonols by flavonol synthase (FLS). Leucoanthocyanidins can be further catalyzed to produce anthocyanidins by the oxygenation reaction of anthocyanidin synthase (ANS) or leucoanthocyanidin dioxygenase (LDOX). Leucoanthocyanidins and anthocyanidins can be catalyzed by leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) to produce 2,3-trans-flavan-3-ols (such as (+)-catechin) and 2,3-cis-flavan-3-ols (such as (−)-epicatechin) which are the key subunits involved in the last steps of PAs biosynthesis.
Figure 2.
Biosynthetic pathway of flavonoid (adapt from [1,38]). The enzymes are: CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol-4-reductase; LAR, leucoanthocyanidin reductase; ANS, anthocyanidin synthase; LDOX, leucoanthocyanidin dioxygenase; GST, glutathione S-transferase; MATE, multidrug and toxic compound extrusion; PPO, polyphenol oxidase; POD, peroxidase; TT10, transparent testa 10.
To date, LAR and ANR genes have been cloned from several plant species, and their functions have been demonstrated both genetically and biochemically [3,36,53,54,55,56,57,58,59,60]. As is well known, leucoanthocyanidins are converted to (+)-catechins through the catalysis of LAR. Interestingly, ectopic expression of tea CsLAR, and cacao TcLAR in tobacco showed that the production of (−)-epicatechin was higher than that of catechins [57,61]. More recent studies also found that LAR from Medicago truncatula and grapevine can convert 4β-(S-cysteinyl)-epicatechin into (−)-epicatechins, thereby regulating the degree of PAs polymerization [59,62]. In addition to catalyzing the synthesis of (−)-epicatechins from anthocyanidins, ANR is proven to convert anthocyanidins to a mixture of (−)-epicatechins and (+)-catechins [61,63]. This suggests that LAR and ANR may possess more features to be explored [61,63,64].
Most of the enzymes for flavonoid biosynthesis are located on the endoplasmic reticulum and tonoplast of cells. PAs are synthesized in the cytoplasm, and then transferred and accumulated into the vacuole and apoplast [65,66,67]. In seed, PAs are mostly accumulated in the inner endothelial layer of seed testa [35,68,69], and the brown color of the seed coat may result from the oxidation of PAs [70]. The seeds without PAs content are called transparent testa (tt) phenotype and tannin deficient seeds, which exhibit yellow or pale brown in color [54,71,72]. The PA-deficient mutants have become an ideal material for investigating the biosynthesis and transportation of PAs. MATE (multidrug and toxic compound extrusion) and GST (glutathione S-transferase) are proteins responsible for flavonoids transportation. AtTT12, a member of the MATE family, localizes the PAs in the vacuoles of the testa of Arabidopsis seeds [73]. AtTT13/AtAHA10 encodes a protein belonging to the ATPase family, which is required for the deposition of PA precursors in vacuole and functions as a proton pump, providing the driving force for TT12-mediated transportation of PA precursors [74]. VvMATE1 and VvMATE2 are transport proteins located in the tonoplast and Golgi complex, respectively, and involved in the transport and accumulation of PAs through different ways in grapevine [67]. GST proteins were thought to deliver anthocyanins into vacuoles, e.g., ZmBz2 in maize, PhAN9 in petunia, AtTT19 in Arabidopsis, and VviGSTs in grape [37,75,76]. After heterologous overexpression of PhAN9 in Arabidopsis tt19 mutant, the anthocyanin pigment in seedlings was complemented, but no complementation was observed in the pale brown seeds of the tt19 mutant [37]. Moreover, PAs precursors were dispersed in the cytoplasm in tt12 mutant [73], but accumulated as membrane-like structures in endothelium cells of tt19, indicating that TT19 may function as a subsequent transporter to deliver the vesicles containing PAs precursors to the central vacuole [37,77]. VviGST3, a homolog to AtTT19, was highly expressed in the seed during grape berry development, and the complementation in seed color was observed in VviGST3 overexpressed tt19 mutant, suggesting that VviGST3 may be associated with the transport of PAs [78]. In addition, heterologous expression of pear PcGSTF12 in the Arabidopsis tt19 mutant showed that PcGSTF12 did not complement the mutant seed color to the normal brown, but it promoted the procyanidin A3 accumulation in the mutant and affected the transcription of PAs- and anthocyanin-related genes. The results of this study indicated that PcGSTF12 may be involved in the transport and accumulation of PAs and anthocyanins [79].
The vacuole is the final destination for the transportation of PAs, and the polymerization of PAs probably takes place here. Although little is known about the polymerization mechanisms of PAs, the enzymatic and nonenzymatic polymerization reactions are widely discussed [1,80,81]. Plant polyphenol oxidases, TT10 and plant peroxidases may take part in PAs polymerization and oxidation [1,65,82,83]. For instance, AtTT10 encodes a putative laccase that oxidizes epicatechin into yellow or brown oligomers, which may involve in PAs oxidative browning in the seed testa [65].
3. Transcriptional Activator of Proanthocyanidins Biosynthesis
The biosynthesis rate of PAs is mainly controlled by the key enzymes within the pathway, and these enzymes are regulated by specific transcriptional regulatory factors (TFs) and regulatory proteins. In the past few years, several TFs and proteins that either directly or indirectly regulate PAs biosynthesis have been identified from model plant and horticultural crops. The regulation involves different MYBs, bHLHs, and WD40 proteins, and many other key factors. The MYB and bHLH TFs are found in all eukaryotes, representing one of the largest plant transcription factor families according to the number of members. They play a pivotal role in growth and development, hormone signal transduction, and plants’ stress responses [84,85,86,87]. Depending on the structure of the DNA binding domain, the MYB TFs are classified into four subfamilies: 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB. The R2R3 MYBs, belonging to 2R-MYB subfamily, are most widely involved in the regulation of the flavonoid pathway, followed by the R3 MYB of 1R-MYB subfamily [85,88].
MYB with [(D/E)LX2(R-K)X3LX6LX3R] motif could be able to combine with the MYB-interacting region (MIR) at the N-terminal of bHLH [87], and MYB and bHLH can also bind to the downstream target gene promoters, respectively. It has been confirmed that MYB family members can recognize the MBSI (CNGTTR3) region and AC-rich ([A/C]CC[A/T]A[A/C]) region, while bHLH proteins specifically bind to E-box (CANNTG) or G-box (CACTG) cis-elements [89]. The MYB protein is often considered to be as a core of MBW complexes in determining DNA binding specificity. The bHLH participates in histone modification and affects the specificity of DNA binding [90], and functions as the bridge between MYB and WD40 protein to form a stable ternary protein complex. WD40 is a type of protein composed of multiple WD motifs in tandem. It often combines with other members of the family or other proteins to modulate target genes to regulate the flavonoids synthesis, signal transduction, and cell processes, such as cell division, vesicle formation and transport, packaging and processing [91,92]. The activity of the MYB-bHLH complex depends on the expression of TTG1 (WD40) in Arabidopsis protoplasts [93], indicating the WD40 protein is crucial for the stability of the MBW complexes. The complexes control the synthesis of flavonoids, including PAs, through the activation of structural gene expressions by binding with cis-elements of their promoters [87,88,90,93].
The MBW complex in Arabidopsis, composed of AtMYB123 (TT2), AtbHLH42 (TT8), and TTG1 proteins, was reported to activate the expression of DFR, LDOX, and ANR (BAN) genes, which lead to the accumulation of PAs in the seed coat [93,94]. A large number of homologs of TT2, TT8, and TTG1 have been identified in various plants, such as FaMYB9/11, FabHLH3, and FaTTG1 in strawberry [95], LjTT2a/2b/2c in Lotus japonicus [96], VvMYBPA2/PAR in Vitis vinifera [97], TaMYB14 in Trifolium arvense [98], TaMYB10-A1/-B1/-D1 in wheat [99], HvMYB10 in Hordeum vulgare [100], Tc-MYBPA in Theobroma cacao [101], MtMYB5/14 in M. truncatula [102], MdMYB9/11/12 in apple [27], PtMYB115 in poplar [34], CsMYB60 in cucumber [103,104], RrMYB10 in Rosa rugosa [31], AabHLH1 in Anthurium andraeanum [105], DkMYB2/4, DkMYC1, and DkWDR1 in persimmon [24,106,107], MtWD40-1 in M. truncatula [108], FhTTG1 in Freesia hybrida [109], and SlAN11 in tomato [110]. These factors are transcriptional activators of PAs biosynthetic pathways, suggesting that the MBW complexes are well conserved in higher plants [87,94]. The transcription of AtTT8 and CsNoemi (bHLH) was driven by an overexpressed MYB protein (AtTT2 and CsPH4) in the transgenic plants [94,111]. These results suggested that certain MYBs not only combine with bHLH to regulate the downstream of structural genes but also act as a regulator of bHLH to form a positive feedback loop [77]. In addition to TT2-MYB, there is a specific TFs, PA1-type MYB, to regulate PAs biosynthesis. MYBPA1 was first reported in grape, of which homologs were subsequently identified in persimmon [24], nectarine [112], poplar [113], and apple [27]. Recently, a new MYB family member, PpMYB7, was identified in peach. It can bind to the promoter of PpLAR, but not PpANR, to regulate PAs synthesis [114]. MtPAR is a novel MYB TF found in the seed coat of M. truncatula, which enhanced the PAs accumulation by regulating another positive regulator MtWD40-1, but not the ANR [115]. It has been reported that many MYBs require a bHLH partner to activate the promoter of downstream genes [4]. For instance, VvMYB5a and VvMYB5b were able to activate the promoters of flavonoid-related genes (e.g., VvCHI and VvLAR1) at the presence of AtEGL3, a bHLH protein involved in flavonoid pathway regulation, and thus elevated accumulation of PAs in the ectopically overexpressed tobacco [116,117]. VvMYC1, a bHLH transcription factor of grapevine, can interact with VvMYBPA1 to form a complex which regulates the transcription of two PAs structural genes (CHI and ANR) and the accumulation of PAs in skins and seeds of berries, but VvMYC1 alone is not able to promote the expression of these two PAs structural genes [118].
MBW is not the only regulator that regulate flavonoid biosynthesis. There are several other types/families of TFs which can regulate PAs biosynthetic pathway in plants. A recent study reported that the apple NAC52 can bind to the promoters of MdMYB9, MdMYB11, and MdLAR, and promote PAs accumulation in the MdNAC52-overexpressed calli [119]. AtTTG2 and VvWRKY26 belong to the WRKY family. AtTTG2 was shown to be responsible for PAs formation in seed coat by directly regulating TT13 and TT12 [120]. VvWRKY26 from V. vinifera was identified as a key regulator that controls the vacuolar transportation and the accumulation of flavonoids, especially the PAs in the berries [121]. Arabidopsis TT16, an ARABIDOPSIS BSISTER (ABS) MADS domain protein, was required for BANYULS (ANR) transcript and the deposition of PAs in the endothelium of the seed coat [122]. The Brassica napus TT16 homologs, BnTT16, regulates the transcriptional abundance of PA-related genes (CHS, CHI, F3H, LDOX, and TT10). Furthermore, a reduction was evident in the PAs accumulation in the Bntt16 RNAi seeds [123]. The PAs transcriptional regulatory networks are complex but important to investigate the PAs metabolism in plants.
4. Transcriptional Repressors Regulate Proanthocyanidins Synthesis
Feedback regulation mechanisms also play an important role in the regulation of PAs biosynthesis in plants. Despite the crucial role of transcriptional activator in PAs biosynthesis, some proteins interfered with the accumulation of PAs have been studied as well. Previous reports have documented that strawberry FaMYB1 not only negatively regulated anthocyanin and flavanol biosynthesis in Fragaria and tobacco [124], but also specifically inhibited the accumulation of PAs in the leaves of Lotus corniculatus [125]. Three negative regulators of PAs were identified in grapevine, namely, VvMYBC2-L1, VvMYBC2-L2, and VvMYBC2-L3. They have high homology with FaMYB1 and anthocyanin repressor PhMYB27 in petunia. It has been shown that VvMYBC2-L1 interacted with VvMYC1 and PhAN1 (a bHLH protein involved in anthocyanin biosynthesis in petunia), and VvMYBC2-L2 and VvMYBC2-L3 had similar expression profiles in grape tissues [126], indicating that they may possess similar functions. Overexpression of VvMYBC2-L1 and VvMYBC2-L3 in grapevine hairy roots lead to a significant reduction in PAs, and VvMYBC2-L1 and VvMYBC2-L3 also decrease both anthocyanin levels in flower petals and the PAs in the seeds of the transgenic petunia lines [126,127]. In previous reports, a few of MYB TFs were upregulated in PtMYB134- and PtMYB115- overexpressed poplar seedlings [113]. Surprisingly, among them, PtMYB182, PtMYB165, and PtMYB194 were characterized as R2R3 MYB repressors in further studies. These three negative regulators reduced the synthesis of PAs and anthocyanins in transgenic seedlings/tissues, and suppressed the transcription of several flavonoid-/phenylpropanoid-related genes. Moreover, the repressors competed with MYB activators for binding to bHLH co-activator and disrupted the transcriptional activation of MBW complex [128,129]. Similar R2R3 MYB repressors were detected in M. trunculata and peaches. M. trunculata MtMYB2 and peach PpMYB18, which can be induced by the PAs activators, inhibit the biosynthesis of PAs and anthocyanin in transgenic plant seeds and tissues [130,131]. In a dosage-response repression assay, a small dosage of PpMYB18 was enough to reduce the activity of activator (PpMYB10.1), indicating that PpMYB18 acts as a passive repressor and no titration effect was observed in the transient assays when the dosage ratio of PpMYB10.1 to PpMYB18 increased above 1:1, suggesting that PpMYB18 functions as a passive repressor by interacting with bHLH cofactor, which inhibits the transcriptional activity of the MBW complex [131]. Several studies have reported that the JAZ protein negatively regulates the flavonoid pathway by interacting with the MBW complex [132,133]. More recently, tartary buckwheat FtMYB18 has also been proposed as a negative regulator of PAs and anthocyanin pathway. Evidence from transgenic plants/hairy roots has shown that the FtMYB18 suppressed the accumulation of PAs and anthocyanin by inhibiting the expression of genes related to flavonoids. The inhibition of FtMYB18 was achieved by combining with FtTT8 and FtTTG1, or forming a MYB-JAZ complex with FtJAZ1/-3/-4/-7 protein [134]. As described above, the MYB inhibitor regulated the metabolism of multiple flavonoids including PAs. Therefore, MYB inhibitors seemed to have a less specificity than that of MYB activators, which may be determined by its binding properties to bHLH [135]. In addition, AtMYBL2, a repressor belonging to R3-MYB family in Arabidopsis, was identified to function as a negative regulator of flavonoid pathway. It inhibited the activity of MBW complex by binding with TT8 and GL3 to control the flavonoid structural gene expression, thus reducing the accumulation of PAs in seed [136].
The inhibitor from the other TFs family has also been identified. The expression of LcbHLH92 in sheepgrass appeared to be negatively correlated with the anthocyanin/PAs-related genes. Overexpression of LcbHLH92 in Arabidopsis, the transcript of DFR and ANS were downregulated, and the synthesis of anthocyanin and PAs were inhibited. Electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation and quantitative real-time (ChIP-qPCR) assay have shown that the LcbHLH92 can bind to the promoter of JAZ and JAZ inhibits the expression of TT8, resulting in a decrease in the biosynthesis of flavonoid [7]. The bric-à-brac/tramtrack/broad complex (BTB) protein is a component of the CUL3-RBX1-BTB protein E3 ubiquitin ligases complex, which mediated the degradation of diverse protein [137]. The BTB protein has been found to take part in plant growth and development and responding to various stresses [138,139]. Recently, MdMYB9 protein was shown to be degraded by MdBT2 via the 26S proteasome system, resulting in a substantial reduction of anthocyanin and PAs in apple [140].
The same as PAs activators, the repressors are an important part of transcription regulation as well. They are often regulated by other transcription factors and signals to avoid excessive accumulation of PAs in plants [125,127,135,136]. Therefore, greater insights into the mechanisms of negative regulators will help us better understand the PAs biosynthesis in response to environmental stresses.
5. Proanthocyanidins in Response to Abiotic Stresses
Plants frequently suffer from a range of environmental stresses, such as low temperature, high light intensity, drought, wounding, and other abiotic stressors. Extreme environmental conditions could disrupt the natural defense system, and the severe stresses will provoke the production and accumulation of reactive oxygen species (ROS) and the induction of oxidative stress responses in plant cells, thereby hindering metabolic activities and affecting the integrity of organelles of plants, which limits the productivity of crops [141]. In response to unfavorable environmental conditions, plants have evolved excellent defense mechanisms to perceive and adapt to these external stresses. Flavonoids, a group of protective phenolic substances of plants, can be induced significantly by the stressful conditions, and participate in response to adversity as antioxidants or as signal molecules [142,143,144,145].
The biosynthesis of PAs in response to multiple abiotic stress and the underlying mechanism has been elucidated in numerous studies. Previous research shows that, compared with the control, cucumber seedlings pretreated with PAs had less oxidative damage induced by high irradiance, artificial drought, and cold stress [146]. In tartary buckwheat, RNA-sequencing and GC-TOF MS analysis found that low temperature upregulated relevant genes of flavonoids metabolism and triggered the accumulation of PAs monomers (catechin and epicatechin), implying that PAs might be associated with enhanced tolerance to cold stress [147]. Nevertheless, how the PAs in plants respond to high temperatures is elusive. Some studies reported that high temperature did not affect PA content in grape skins during the growing season [148,149], but other studies noticed that high temperature reduced the PAs production in grape berries [150,151], and decreased in VvANR and VvLAR1 mRNA levels [151]. Researchers noticed that water deficit could promote the secondary metabolism in grape berries. However, a study showed that both regulated deficit irrigation and non-irrigation upregulated the expression of PAs-related genes in grape seeds, but did not lead to changes in PAs concentration, which may be due to other factors such as oxidation and/or degradation of PAs in the last stage of ripening [152]. As grape berry skin is exposed to the external surroundings directly, the biosynthesis of PAs in grape skin seems to be more susceptible to temperature than those in the seed [153]. Water deficit could induce the expression of VvLAR2 and VvMYBPA1, and the concentration and polymerization of PAs in Cabernet Sauvignon grape skins. High sunlight exposure has been shown to boost the PAs synthesis in Cistus clusii [154], poplar [30,155], larch [156], and apple [157]. The excess sunlight exposure increased the concentration of PAs in Cistus clusii, which was more obvious in older plants because they suffered more from UV light stress [154]. Transcriptome sequencing analysis showed that genes related to flavonoid biosynthesis were up-regulated in Pohlia nutans under high salt stress. Moreover, pre-treatment with 1% PAs significantly improved the survival rate of moss [158].
It has been reported that certain TFs can regulate the PAs biosynthesis in different manners in response to abiotic stresses. The transcriptional level of LoMYB29 in Larix olgensis was upregulated by wounding, high light intensity, NaCl, PEG6000, methyl jasmonate (MeJA), and abscisic acid (ABA) treatments, and ectopic overexpression of LoMYB29 enhanced the PAs to accumulate in Arabidopsis leaves [159]. This is in agreement that wounding, oxidative stress and salicylic acid (SA) can trigger the upregulated expression of RrMYB5, RrMYB10 (Rosa rugosa), and RcMYBPA2 (Rosa chinensis), which can then activate the promoter of PAs structural genes and result in accumulation of PAs in roses [31,160]. PAs are known as a powerful antioxidant that has been proved to increase the antioxidant capacity of plants [6]. Overexpression of RrMYB5 and RrMYB10 in rose somatic embryos and seedlings, not only increased PAs content, but also enhanced the activity of catalase, peroxidase and superoxide dismutase of plants/tissues to low accumulation MDA and scavenge ROS, which improved tolerance to the stresses [31,160,161]. This means that PAs improve plant tolerance to environmental stresses by regulating the antioxidant system to facilitate ROS scavenging. In addition, the production of endogenous ABA was promoted in the rose with over-production of PAs, and the ABA signaling pathway was closely related to the response to stress in plants [31,161]. Wounding, high sunlight exposure, and nitrogen deficiency caused oxidative stress in poplars, inducing the accumulation of PAs in the leaves and significantly upregulating the expression of PtMYB134 and PtMYB115 [30,34,155,162]. These MYB factors overexpressor in poplar seedlings exhibited dramatically higher PAs concentration, which reduced the level of H2O2, and showed the potential function in reducing leaf necrosis and photosystem II by the ROS damage [155]. MdMYB9/11/12/23 and MdMYBPA1, which can bind to the promoters of PAs structural genes, were positive regulatory factors of PAs synthesis in apple, and could also be triggered in apple by low temperature. When constitutively expressed in apple calli, the factors induced the transcription of PAs synthesis, elevated levels of the PAs to reduce the oxidant damage caused by cold stress [27,28,157]. Furthermore, a recent study found that MdMYB23 protein was induced by cold stress, but degraded via the ubiquitin-proteasome proteolytic pathway in normal conditions. MdBT2 decreased PAs accumulation by promoting the degradation of MdMYB23 [28], which can balance the levels of PAs in apple.
In general, uncomfortable temperature, high light exposure, drought, wounding, nitrogen deficiency, and other stressful conditions would trigger the generation of ROS and cause oxidative damage to plants. Plants have developed an antioxidant defense system to manage oxidative damage. Like other flavonoids, PAs possess an ortho-hydroxylated B-ring, which is a key function of phenolic antioxidants [163,164]. PAs exhibit a strong antioxidant capacity in vitro. However, the ROS are mainly generated in chloroplasts and mitochondria and are short-lived. By contrast, H2O2 is more stable and can cross membranes and diffuse into the vacuole [165,166]. Therefore, PAs may have an important role in the H2O2 scavenging system. However, the specific process and mechanism are still unclear. External stimulation promotes the PAs accumulation by activating plant hormones and MYB TFs (TT2- and PA1-type). The synthesis of PAs in plant cells is considered to be an adaptive response to oxidative stress [155]. As outlined above, plants containing high PAs levels display excellent antioxidant properties that can remove excess ROS and alleviate further damage to plants, improving their ability to respond to various stimuli [31,33].
6. Proanthocyanidins in Response to Biotic Stresses
In addition to abiotic stresses, plants often encounter biotic stresses, such as fungi, bacteria, viruses, and insects, which are highly contagious. These biotic stresses can cause the death and decay of plants, and consequently affect the quality and yield of crops. Chemical pesticides provide a broad-spectrum of resistance to pests and diseases, and it is the most effective and commonly used method. However, the long-term and improper use of chemical pesticides can cause the resistance evolution of pathogens and pests to the chemicals, negatively influencing the environment and food safety. Previous studies have shown that PAs are substantially involved in plant resistance against biotic stress, such as fungal infection and insect herbivores [1,32,33]. PAs can be considered to be potential biosafety and eco-friendly strategy to protect plants against biotic stresses.
As a kind of pre-formed protective barrier, PAs are distributed in the endothelial layer of the seed coat in many plants to protect the seed from biotic stresses [1]. PAs are also found to localize in the epidermis and vascular bundles of poplar leaves. After inoculation with rust fungus (Melampsora larici-populina), more PAs accumulated on the site of fungal infection in the epidermis of leaves, indicating that the location of PAs provided a defense barrier for leaves to resist early colonization of the fungus [33]. Using transcriptome sequencing analysis, it was found that, after fungus (Melampsora medusae) inoculation, the PAs-related genes and the accumulation of PAs in poplar leaves were strongly induced [167]. At least 2–3 times higher levels of catechin and PAs were observed in the leaves of poplar genotype with moderate resistance against rust infection compared to the ones sensitive to rust infection, and there was an increase in the concentration of flavan-3-ols in response to fungal infection. In addition, there was a negative correlation between the degree of disease occurrence and the PAs concentration of leaves [33]. Furthermore, PAs possessed toxic and growth inhibitory effects on microbial pathogens [168]. Merlot grapevine treated with benzothiadiazole (BTH) resulted in a 36% of the increase in PAs and reduced the incidence and severity of gray mold caused by Botrytis cinereal [169]. In bilberry the biosynthesis and accumulation of PAs were significantly increased when the berry was infected by endophyte (Paraphaeosphaeria sp.) and Botrytis cinereal, and the synthesis of epigallocatechin was greatly induced due to the infection of Botrytis cinereal [170]. When inoculating fungus on strawberries with different maturity levels, the riper strawberries were much more susceptible to fungus than the less ripe ones as fungal development in unripe fruits was inconspicuous and the expression levels of LAR and ANR were higher in unripe fruits [171,172]. Meanwhile, the concentrations of flavan-3-ols and PAs have been found to decrease during the ripening stages [173,174], indicating that flavan-3-ols and PAs derivatives were considered to be pre-formed defense mechanisms of fruit by inhibiting the fungal invasion. Constitutive expression of PtLAR3 in poplar results in a boost to the level of PAs and enhanced the ability to resistance to the fungus Marssonina brunnea. Compared with wild-type ones, the crude polyphenol extract from transgenic leaves inhibited the growth of fungus more effectively and has a toxic effect on mycelium which showed abnormal growth [32]. Previous research indicated that PtMYB115 and PtMYB134 interacted with PtbHLH131 to modulate PAs synthesis in poplars. In greenhouse-grown transgenic of PtMYB115 poplar seedlings, the transcriptions of PAs biosynthesis gene were activated, which increased PAs concentration and fungus resistance. However, the CRISPR/Cas9-generated myb115 mutant, which has lower PAs levels and decreased expressions of PAs-related genes, exhibited high sensitivity to fungus [34]. Similarly, overexpression and silencing of PtMYB134 also affected the content of PAs in poplar, which in turn affected resistance to rust fungus [33]. These findings further demonstrate that PAs are effective antifungal materials and play a critical role in the resistance of plants to fungus.
It has been proven that insect herbivores often induce PA accumulation in plants. For example, under natural conditions, in the leaves of old-growth black poplar, the low molecular weight flavan-3-ols and PAs concentration were not affected by gypsy moth (Lymantria dispar) larva feeding. However, in the single tree experiment, after caterpillar infestation, the PAs in the leaves increased by 10–20% and the production of low molecular weight flavan-3-ols decreased by 10% in the leaves but increased by 10% in the bark [175]. The forest tent caterpillar (Malacosoma disstria) and satin moth (Leucoma salicis) larva could strongly increase the expression level of the DFR gene, resulting in higher production of PAs in the poplar leaves [162]. Heterologous expression of tea CsDFR and CsANR in tobacco increased the generation of flavan-3-ols (catechin, epicatechin, and epigallocatechin) and protective abilities of plant anti-herbivores [176]. PAs have a deterrent and/or toxic effect on herbivorous insects, which may be one of the pre-formed protective barriers in plants [177,178]. However, there is also evidence that PAs did not possess broad-spectrum antiherbivorous activity. Researchers found that, compared with other low doses of plant metabolites, the PAs in high dose had little success against the insects [179]. Therefore, PAs have been shown to contribute to plant defense against a wide range of fungal and bacterial pathogens by its physical and biochemical properties. This can be considered one of the best methods of comprehensively guiding agricultural production, and preventing pathogen and insect invasion.
7. Hormones Regulate Proanthocyanidins Synthesis
A growing body of evidences illustrated that the plant hormones are closely involved in PAs synthesis. The phytohormone can interact with the MBW complex related to flavonoid synthesis at transcriptional or post-transcriptional levels [180]. Pre-treatment of grape berry with ABA can reduce the level of PAs accumulation by inhibiting the activity of LAR and ANR, and the expression of PAs-related genes [181]. EMSA and transient reporter assay showed that DkbZIP5, a TF involved in ABA signaling pathway, is bound to ABA-responsive elements in the DkMYB4 promoter, and thus ABA can signal the regulation of PAs biosynthesis in persimmon by regulating the expression of DkMYB4 through DkbZIP5 [182]. Exogenous application of MeJA has been shown to increase PAs content in grape berries [183]. A study on apple suggested that jasmonic acid (JA) stimulated PAs accumulation by up-regulated MdMYB9 and MdMYB11 [184]. Detailed study showed that MdSnRK1.1 can phosphorylate MdJAZ18, which acts as a repressor in the jasmonic acid (JA) signaling pathway. This interaction ultimately mediates the degradation of MdJAZ18 via the 26S proteasome, releasing MdbHLH3, the co-activator of MdMYB9 and MdMYB11, while promoting the PAs and anthocyanin production [185]. Two transcription factors belonging to the ethylene response factors (ERF) family of Malus Crabapple, RAP2-4, and RAV1, specifically bound to the promoter of McLAR1 and McANR2, respectively, and acted as positive- (RAP2-4) and negative- (RAV1) regulators that control PAs biosynthesis. Furthermore, plant hormones are involved in plant resistance to biotic and abiotic stresses [186].
Plant hormones have an important function in plant-pathogen interaction [187]. Generally, SA is mainly involved in plant resistance to biotrophic pathogens and piercing–sucking insects, and JA is mainly involved in resistance to necrotrophs and chewing insects [188]. Foliar rust fungi promoted the increase of SA, JA, and ABA in infected poplar leaves, but only the rising trend of SA was coordinated with that of flavan-3-ol. Exogenous application of benzothiadiazole, SA analogue, increases the expression of PAs-related MBW complex and the concentration of flavan-3-ol, and reduces the rust fungus invasion [189]. Likewise, another study in strawberry reported the same result. When Podosphaera aphanis infected strawberry leaves, SA-related and phenylpropanoid pathway-related genes were activated, while JA pathway-related genes were down-regulated. Moreover, SA can induce the transcription of PAs-related MBW complex and PAs accumulation in leaves, and consequently inhibits the pathogen growth [190]. In Norway spruce, SA supplement induces the biosynthesis of PAs, thereby reducing the damage from bark beetle [191]. The black poplar stems infected with Plectosphaerella populi showed increased content of flavan-3-ols, SA, and cytokinins (CKs), but the levels of JA and JA-isoleucine (JA-Ile) were only raised at the early stage of infection. Exogenous application of CKs resulted in the decrease in SA and flavan-3-ols concentrations. These results suggested that SA is the primary hormone that control antifungal flavan-3-ols and PAs synthesis [192].
The plant hormones as signals may link the changing environmental conditions and development cues and PAs biosynthesis. Although many studies illustrated the links between plant hormones and PAs synthesis, the relationship between plant hormones and the TFs regulating PAs biosynthetic pathways requires further investigation.
8. Conclusions and Further Prospects
PAs have attracted attention not only because they possess beneficial pharmacological properties but also because of their special role in the regulation of fruit quality and plant defense. The principal focus of research in the biosynthetic pathway and regulatory mechanism of PAs has been explored for years, and some encouraging progress has been made. However, there are still many issues that need to be further explored. The external environment in which plants live is constantly changing, there is a diversity of species, and the complex genetic background of woody plants, results in different regulatory mechanisms of PAs in various species in response to developmental cues and various stresses (Figure 3). In all species analyzed to date, a large number of MYBs regulate the synthesis of PAs that are involved in plant response to stress and signals. Nevertheless, the bHLH and WD40 cofactors of the MBW complex are also involved in the regulation of other pathways, so their specificity for PAs-regulation is less than that of MYB TFs, and few studies have focused on bHLH TFs, WD40 protein, and the proteins from other families that also participate in the biosynthesis of PAs. The highly induced transcription and accumulation of PAs are considered to be a systemic response of plant to adverse conditions [162]. Further work needs to reveal how the biotic and abiotic stresses trigger the transcription of these TFs, how the transcriptional and post-transcriptional regulation modifies these TFs, and how the crosstalk between these TFs and various signals and hormones is conducted.
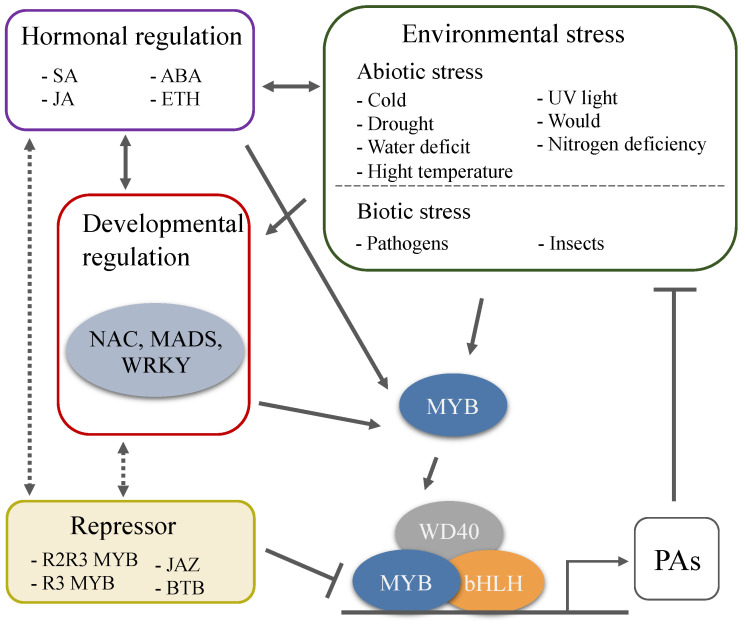
Figure 3.
Environmental and developmental regulation of PAs biosynthesis through the MBW complexes. A large number of MYBs regulate the synthesis of PAs that are involved in plant response to stress and signals. Plant hormones are response to the environmental and developmental cues, and are the key factors involved in PAs biosynthesis. Environmental stresses affect the accumulation of PAs, and the high levels of PAs further enhance plant tolerance to extreme environmental challenges.
Metabolic engineering and molecular biology methods can be used to produce PA-rich crops that can enhance the tolerance of crops to various environmental stress, and improve the quality and nutritional value of crops. PAs have a great impact on the sensory properties of fruits by contributing to the astringency and bitterness, and may also influence the digestive system in humans and animals [1,193]. For example, the accumulation of PAs in persimmon fruit leads to astringency [194], which brings unpleasant tactile sensation. Understanding the regulation of PAs biosynthesis network can provide further insights into more targeted gene modifications, and are of great importance for breeding. However, it should be noted that one factor might affect the biosynthesis of many other metabolites. For example, the overexpression of VvMYBPA1 in tobacco upregulates PAs metabolism while downregulating anthocyanin biosynthesis [195]. Therefore, the comprehensive and in-depth analysis of the molecular regulation mechanism of plant resistance mediated by PAs has a positive significance for improving our knowledge of regulatory network controlling PAs synthesis and provides a theoretical foundation for genetic improvement and breeding of the plants with moderate levels of PAs.
Author Contributions
Prepared and wrote the manuscript: D.Y.; revising the words and phrasing: T.H.; revised and edited the manuscript: B.T. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 31572078 and no. 31471835).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dixon R.A., Xie D.Y., Sharma S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005;165:9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy J.A., Hayasaka Y., Vidal S., Waters E.J., Jones G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001;49:5348–5355. doi: 10.1021/jf010758h. [DOI] [PubMed] [Google Scholar]
- 3.Bogs J., Downey M.O., Harvey J.S., Ashton A.R., Tanner G.J., Robinson S.P. Proanthocyanidin Synthesis and Expression of Genes Encoding Leucoanthocyanidin Reductase and Anthocyanidin Reductase in Developing Grape Berries and Grapevine Leaves. Plant Physiol. 2005;139:652–663. doi: 10.1104/pp.105.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogs J., Jaffé F.W., Takos A.M., Walker A.R., Robinson S.P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007;143:1347–1361. doi: 10.1104/pp.106.093203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debeaujon I., Léon-Kloosterziel K.M., Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000;122:403–413. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia L.G., Sheng Z.W., Xu W.F., Li Y.X., Liu Y.G., Xia Y.J., Zhang J.H. Modulation of anti-oxidation ability by proanthocyanidins during germination of arabidopsis thaliana seeds. Mol. Plant. 2012;5:472–481. doi: 10.1093/mp/ssr089. [DOI] [PubMed] [Google Scholar]
- 7.Zhao P., Li X., Jia J., Yuan G., Chen S., Qi D., Cheng L., Liu G. bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J. Exp. Bot. 2019;70:269–284. doi: 10.1093/jxb/ery335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesschaeve I., Noble A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005;81:330–335. doi: 10.1093/ajcn/81.1.330S. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalo-Diago A., Dizy M., Fernández-Zurbano P. Taste and mouthfeel properties of red wines proanthocyanidins and their relation to the chemical composition. J. Agric. Food Chem. 2013;61:8861–8870. doi: 10.1021/jf401041q. [DOI] [PubMed] [Google Scholar]
- 10.Nile S.H., Park S.W. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Serafini M., Bugianesi R., Maiani G., Valtuena S., De Santis S., Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 12.Liu W., Zhao S., Wang J., Shi J., Sun Y., Wang W., Ning G., Hong J., Liu R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017;61:1601082. doi: 10.1002/mnfr.201601082. [DOI] [PubMed] [Google Scholar]
- 13.Erasto P., Majinda R. Bioactive proanthocyanidins from the root bark of Cassia abbreviata. Int. J. Biol. Chem. Sci. 2012;5:2170–2179. doi: 10.4314/ijbcs.v5i5.36. [DOI] [Google Scholar]
- 14.Sobeh M., Mahmoud M.F., Abdelfattah M.A.O., Cheng H., El-Shazly A.M., Wink M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018;213:38–47. doi: 10.1016/j.jep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Natella F., Belelli F., Gentili V., Ursini F., Scaccini C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002;50:7720–7725. doi: 10.1021/jf020346o. [DOI] [PubMed] [Google Scholar]
- 16.Cos P., Bruyne T., Hermans N., Apers S., Berghe D., Vlietinck A. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2012;11:1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 17.Goufo P., Trindade H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014;2:75–104. doi: 10.1002/fsn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruger M.J., Davies N., Myburgh K.H., Lecour S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014;59:41–52. doi: 10.1016/j.foodres.2014.01.046. [DOI] [Google Scholar]
- 19.Toden S., Ravindranathan P., Gu J., Cardenas J., Yuchang M., Goel A. Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-21478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieniawska E. Activities of tannins-From in vitro studies to clinical trials. Nat. Prod. Commun. 2015;10:1877–1884. [PubMed] [Google Scholar]
- 21.Smeriglio A., Barreca D., Bellocco E., Trombetta D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017;174:1244–1262. doi: 10.1111/bph.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bladé C., Arola L., Salvadó M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010;54:37–59. doi: 10.1002/mnfr.200900476. [DOI] [PubMed] [Google Scholar]
- 23.Jaakola L., Määttä K., Pirttilä A.M., Törrönen R., Kärenlampi S., Hohtola A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002;130:729–739. doi: 10.1104/pp.006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akagi T., Ikegami A., Tsujimoto T., Kobayashi S., Sato A., Kono A., Yonemori K. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2009;151:2028–2045. doi: 10.1104/pp.109.146985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zifkin M., Jin A., Ozga J.A., Irina Zaharia L., Schernthaner J.P., Gesell A., Abrams S.R., Kennedy J.A., Peter Constabel C. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012;158:200–224. doi: 10.1104/pp.111.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanoner P., Guyot S., Marnet N., Molle D., Drilleau J.F. Polyphenol profiles of french cider apple varieties (Malus domestica sp.) J. Agric. Food Chem. 1999;47:4847–4853. doi: 10.1021/jf990563y. [DOI] [PubMed] [Google Scholar]
- 27.Wang N., Qu C., Jiang S., Chen Z., Xu H., Fang H., Su M., Zhang J., Wang Y., Liu W., et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018;96:39–55. doi: 10.1111/tpj.14013. [DOI] [PubMed] [Google Scholar]
- 28.An J.P., Li R., Qu F.J., You C.X., Wang X.F., Hao Y.J. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018;96:562–577. doi: 10.1111/tpj.14050. [DOI] [PubMed] [Google Scholar]
- 29.Malisch C.S., Salminen J.P., Kölliker R., Engström M.T., Suter D., Studer B., Lüscher A. Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J. Agric. Food Chem. 2016;64:9307–9316. doi: 10.1021/acs.jafc.6b02342. [DOI] [PubMed] [Google Scholar]
- 30.Mellway R.D., Tran L.T., Prouse M.B., Campbell M.M., Peter Constabel C. The wound-, pathogen-, and ultraviolet B-Responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol. 2009;150:924–941. doi: 10.1104/pp.109.139071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y., Sun T., Pan Q., Anupol N., Chen H., Shi J., Liu F., Deqiang D., Wang C., Zhao J., et al. RrMYB5- and RrMYB10-regulated flavonoid biosynthesis plays a pivotal role in feedback loop responding to wounding and oxidation in Rosa rugosa. Plant Biotechnol. J. 2019;17:2078–2095. doi: 10.1111/pbi.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan L., Wang L., Han Z., Jiang Y., Zhao L., Liu H., Yang L., Luo K. Molecular cloning and characterization of PtrLAR3, a gene encoding leucoanthocyanidin reductase from Populus trichocarpa, and its constitutive expression enhances fungal resistance in transgenic plants. J. Exp. Bot. 2012;63:2513–2524. doi: 10.1093/jxb/err425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ullah C., Unsicker S.B., Fellenberg C., Constabel C.P., Schmidt A., Gershenzon J., Hammerbacher A. Flavan-3-ols are an effective chemical defense against rust infection. Plant Physiol. 2017;175:1560–1578. doi: 10.1104/pp.17.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L., Ran L., Hou Y., Tian Q., Li C., Liu R., Fan D., Luo K. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytol. 2017;215:351–367. doi: 10.1111/nph.14569. [DOI] [PubMed] [Google Scholar]
- 35.Abrahams S., Tanner G.J., Larkin P.J., Ashton A.R. Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol. 2002;130:561–576. doi: 10.1104/pp.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie D.Y., Sharma S.B., Paiva N.L., Ferreira D., Dixon R.A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science. 2003;299:396–399. doi: 10.1126/science.1078540. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura S., Shikazono N., Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–114. doi: 10.1046/j.1365-313X.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- 38.Constabel C.P. Molecular controls of proanthocyanidin synthesis and structure: Prospects for genetic engineering in crop plants. J. Agric. Food Chem. 2018;66:9882–9888. doi: 10.1021/acs.jafc.8b02950. [DOI] [PubMed] [Google Scholar]
- 39.Ramsay A., Mueller-Harvey I. Senna alata leaves are a good source of propelargonidins. Nat. Prod. Res. 2016;30:1548–1551. doi: 10.1080/14786419.2015.1108976. [DOI] [PubMed] [Google Scholar]
- 40.Gu L., Kelm M., Hammerstone J.F., Beecher G., Cunningham D., Vannozzi S., Prior R.L. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem. 2002;50:4852–4860. doi: 10.1021/jf020214v. [DOI] [PubMed] [Google Scholar]
- 41.Gabetta B., Fuzzati N., Griffini A., Lolla E., Pace R., Ruffilli T., Peterlongo F. Characterization of proanthocyanidins from grape seeds. Fitoterapia. 2000;71:162–175. doi: 10.1016/S0367-326X(99)00161-6. [DOI] [PubMed] [Google Scholar]
- 42.Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Prior R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 43.Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Gebhardt S., Prior R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 44.Karonen M., Loponen J., Ossipov V., Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta. 2004;522:105–112. doi: 10.1016/j.aca.2004.06.041. [DOI] [Google Scholar]
- 45.Chen X., Xiong J., Huang S., Li X., Zhang Y., Zhang L., Wang F. Analytical profiling of proanthocyanidins from Acacia mearnsii bark and in vitro assessment of antioxidant and antidiabetic potential. Molecules. 2018;23:2891. doi: 10.3390/molecules23112891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suvanto J., Karppinen K., Riihinen K., Jaakola L., Salminen J.P. Changes in the proanthocyanidin composition and related gene expression in bilberry (Vaccinium myrtillus L.) Tissues. J. Agric. Food Chem. 2020;68:7378–7386. doi: 10.1021/acs.jafc.0c02158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foo L.Y., Lu Y., Howell A.B., Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–181. doi: 10.1016/S0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 48.Yu J., Ahmedna M., Goktepe I., Dai J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Compos. Anal. 2006;19:364–371. doi: 10.1016/j.jfca.2005.08.003. [DOI] [Google Scholar]
- 49.Appeldoorn M.M., Sanders M., Vincken J.P., Cheynier V., Le Guernevé C., Hollman P.C.H., Gruppen H. Efficient isolation of major procyanidin A-type dimers from peanut skins and B-type dimers from grape seeds. Food Chem. 2009;117:713–720. doi: 10.1016/j.foodchem.2009.04.047. [DOI] [Google Scholar]
- 50.Lepiniec L., Debeaujon I., Routaboul J.-M., Baudry A., Pourcel L., Nesi N., Caboche M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006;57:405–430. doi: 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- 51.Routaboul J.M., Dubos C., Beck G., Marquis C., Bidzinski P., Loudet O., Lepiniec L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012;63:3749–3764. doi: 10.1093/jxb/ers067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito K., Yonekura-Sakakibara K., Nakabayashi R., Higashi Y., Yamazaki M., Tohge T., Fernie A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013;72:21–34. doi: 10.1016/j.plaphy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Tanner G.J., Francki K.T., Abrahams S., Watson J.M., Larkin P.J., Ashton A.R. Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003;278:31647–31656. doi: 10.1074/jbc.M302783200. [DOI] [PubMed] [Google Scholar]
- 54.Abrahams S., Lee E., Walker A.R., Tanner G.J., Larkin P.J., Ashton A.R. The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 2003;35:624–636. doi: 10.1046/j.1365-313X.2003.01834.x. [DOI] [PubMed] [Google Scholar]
- 55.Paolocci F., Robbins M.P., Madeo L., Arcioni S., Martens S., Damiani F. Ectopic expression of a basic helix-loop-helix gene transactivates parallel pathways of proanthocyanidin biosynthesis. Structure, expression analysis, and genetic control of leucoanthocyanidin 4-reductase and anthocyanidin reductase genes in Lotus cornicu. Plant Physiol. 2007;143:504–516. doi: 10.1104/pp.106.090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pang Y., Peel G.J., Wright E., Wang Z., Dixon R.A. Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol. 2007;145:601–615. doi: 10.1104/pp.107.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Shi Z., Maximova S., Payne M.J., Guiltinan M.J. Proanthocyanidin synthesis in Theobroma cacao: Genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol. 2013;13:202. doi: 10.1186/1471-2229-13-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsui K., Hisano T., Yasui Y., Mori M., Walker A.R., Morishita T., Katsu K. Isolation and characterization of genes encoding leucoanthocyanidin reductase (FeLAR) and anthocyanidin reductase (FeANR) in buckwheat (Fagopyrum esculentum) J. Plant Physiol. 2016;205:41–47. doi: 10.1016/j.jplph.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Liu C., Wang X., Shulaev V., Dixon R.A. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nat. Plants. 2016;2:16182. doi: 10.1038/nplants.2016.182. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Tian J., Yao Y.Y., Zhang J., Song T.T., Li K.T., Yao Y.C. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol. Biochem. 2019;139:141–151. doi: 10.1016/j.plaphy.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Pang Y., Abeysinghe I.S.B., He J., He X., Huhman D., Mudith Mewan K., Sumner L.W., Yun J., Dixon R.A. Functional characterization of proanthocyanidin pathway enzymes from tea and their application for metabolic engineering. Plant Physiol. 2013;161:1103–1116. doi: 10.1104/pp.112.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu K., Jun J.H., Duan C., Dixon R.A. VvLAR1 and VvLAR2 are bifunctional enzymes for proanthocyanidin biosynthesis in grapevine. Plant Physiol. 2019;180:1362–1374. doi: 10.1104/pp.19.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gargouri M., Chaudiére J., Manigand C., Maugé C., Bathany K., Schmitter J.M., Gallois B. The epimerase activity of anthocyanidin reductase from Vitis vinifera and its regiospecific hydride transfers. Biol. Chem. 2010;391:219–227. doi: 10.1515/bc.2010.015. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y., Wang H., Peng Q., Tang Y., Xia G., Wu J., Xie D.Y. Functional characterization of an anthocyanidin reductase gene from the fibers of upland cotton (Gossypium hirsutum) Planta. 2015;241:1075–1089. doi: 10.1007/s00425-014-2238-4. [DOI] [PubMed] [Google Scholar]
- 65.Pourcel L., Routaboul J.M., Kerhoas L., Caboche M., Lepiniec L., Debeaujon I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell. 2005;17:2966–2980. doi: 10.1105/tpc.105.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitamura S., Matsuda F., Tohge T., Yonekura-Sakakibara K., Yamazaki M., Saito K., Narumi I. Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants. Plant J. 2010;62:549–559. doi: 10.1111/j.1365-313X.2010.04174.x. [DOI] [PubMed] [Google Scholar]
- 67.Pérez-Díaz R., Ryngajllo M., Pérez-Díaz J., Peña-Cortés H., Casaretto J.A., González-Villanueva E., Ruiz-Lara S. VvMATE1 and VvMATE2 encode putative proanthocyanidin transporters expressed during berry development in Vitis vinifera L. Plant Cell Rep. 2014;33:1147–1159. doi: 10.1007/s00299-014-1604-9. [DOI] [PubMed] [Google Scholar]
- 68.Devic M., Guilleminot J., Debeaujon I., Bechtold N., Bensaude E., Koornneef M., Pelletier G., Delseny M. The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 1999;19:387–398. doi: 10.1046/j.1365-313X.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 69.Debeaujon I., Nesi N., Perez P., Devic M., Grandjean O., Caboche M., Lepiniec L. Proanthocyanidin-accumulating cells in Arabidopsis Testa: Regulation of differentiation and role in seed development. Plant Cell. 2003;15:2514–2531. doi: 10.1105/tpc.014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapple C.C.S., Shirley B.W., Zook M., Hammerschmidt R., Somerville S.C. Secondary metabolism in Arabidopsis. In: Meyerowitz E.M., Somerville C.R., editors. Arabidopsis. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1994. pp. 989–1030. [Google Scholar]
- 71.Koornneef M. Mutations affecting the testa colour in Arabidopsis. Arab. Inf. Serv. 1990;27:1–4. [Google Scholar]
- 72.Shirley B.W., Kubasek W.L., Storz G., Bruggemann E., Koornneef M., Ausubel F.M., Goodman H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313X.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- 73.Debeaujon I., Peeters A.J.M., Léon-Kloosterziel K.M., Koornneef M. The transparent testa 12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–871. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Appelhagen I., Nordholt N., Seidel T., Spelt K., Koes R., Quattrochio F., Sagasser M., Weisshaar B. TRANSPARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. Plant J. 2015;82:840–849. doi: 10.1111/tpj.12854. [DOI] [PubMed] [Google Scholar]
- 75.Marrs K.A., Alfenito M.R., Lloyd A.M., Walbot V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene bronze-2. Nature. 1995;375:397–400. doi: 10.1038/375397a0. [DOI] [PubMed] [Google Scholar]
- 76.Alfenito M.R., Souer E., Goodman C.D., Buell R., Mol J., Koes R., Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10:1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koes R., Verweij W., Quattrocchio F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Pérez-Díaz R., Madrid-Espinoza J., Salinas-Cornejo J., González-Villanueva E., Ruiz-Lara S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in vitis vinífera. Front. Plant Sci. 2016;7:1166. doi: 10.3389/fpls.2016.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Z., Tian C., Zhang Y., Li C., Li X., Yu Q., Wang S., Wang X., Chen X., Feng S. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020;20:1–14. doi: 10.1186/s12870-020-02344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stafford H.A. Chemistry and Significance of Condensed Tannins. Springer; Berlin/Heidelberg, Germany: 1989. The Enzymology of proanthocyanidin biosynthesis; pp. 47–70. [Google Scholar]
- 81.Xie D.Y., Dixon R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry. 2005;66:2127–2144. doi: 10.1016/j.phytochem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Hosny M., Rosazza J.P.N. Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J. Agric. Food Chem. 2002;50:5539–5545. doi: 10.1021/jf020503j. [DOI] [PubMed] [Google Scholar]
- 83.López-Serrano M., Ros Barceló A. Comparative study of the products of the peroxidase-catalyzed and the polyphenoloxidase-catalyzed (+)-catechin oxidation. Their possible implications in strawberry (Fragaria × ananassa) browning reactions. J. Agric. Food Chem. 2002;50:1218–1224. doi: 10.1021/jf010902z. [DOI] [PubMed] [Google Scholar]
- 84.Heim M.A., Jakoby M., Werber M., Martin C., Weisshaar B., Bailey P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003;20:735–747. doi: 10.1093/molbev/msg088. [DOI] [PubMed] [Google Scholar]
- 85.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Feller A., MacHemer K., Braun E.L., Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66:94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 87.Xu W., Dubos C., Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015;20:176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Liu J., Osbourn A., Ma P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant. 2015;8:689–708. doi: 10.1016/j.molp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 89.Xu W., Grain D., Bobet S., Le Gourrierec J., Thévenin J., Kelemen Z., Lepiniec L., Dubos C. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 2014;202:132–144. doi: 10.1111/nph.12620. [DOI] [PubMed] [Google Scholar]
- 90.Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011;62:2465–2483. doi: 10.1093/jxb/erq442. [DOI] [PubMed] [Google Scholar]
- 91.Li Q., Zhao P., Li J., Zhang C., Wang L., Ren Z. Genome-wide analysis of the WD-repeat protein family in cucumber and Arabidopsis. Mol. Genet. 2014;289:103–124. doi: 10.1007/s00438-013-0789-x. [DOI] [PubMed] [Google Scholar]
- 92.He S., Tong X., Han M., Hu H., Dai F. Genome-wide identification and characterization of WD40 protein genes in the silkworm, bombyx mori. Int. J. Mol. Sci. 2018;19:527. doi: 10.3390/ijms19020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baudry A., Heim M.A., Dubreucq B., Caboche M., Weisshaar B., Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–380. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 94.Nesi N., Jond C., Debeaujon I., Caboche M., Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–2114. doi: 10.1105/TPC.010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schaart J.G., Dubos C., Romero De La Fuente I., van Houwelingen A.M.M.L., de Vos R.C.H., Jonker H.H., Xu W., Routaboul J.M., Lepiniec L., Bovy A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013;197:454–467. doi: 10.1111/nph.12017. [DOI] [PubMed] [Google Scholar]
- 96.Yoshida K., Iwasaka R., Kaneko T., Sato S., Tabata S., Sakuta M. Functional differentiation of Lotus japonicus TT2s, R2R3-MYB transcription factors comprising a multigene family. Plant Cell Physiol. 2008;49:157–169. doi: 10.1093/pcp/pcn009. [DOI] [PubMed] [Google Scholar]
- 97.Terrier N., Torregrosa L., Ageorges A., Vialet S., Verriès C., Cheynier V., Romieu C. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol. 2009;149:1028–1041. doi: 10.1104/pp.108.131862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hancock K.R., Collette V., Fraser K., Greig M., Xue H., Richardson K., Jones C., Rasmussen S. Expression of the R2R3-MYB transcription factor TaMYB14 from Trifolium arvense activates proanthocyanidin biosynthesis in the legumes Trifolium repens and Medicago sativa. Plant Physiol. 2012;159:1204–1220. doi: 10.1104/pp.112.195420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Himi E., Maekawa M., Miura H., Noda K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor. Appl. Genet. 2011;122:1561–1576. doi: 10.1007/s00122-011-1555-2. [DOI] [PubMed] [Google Scholar]
- 100.Himi E., Yamashita Y., Haruyama N., Yanagisawa T., Maekawa M., Taketa S. Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica. 2012;188:141–151. doi: 10.1007/s10681-011-0552-5. [DOI] [Google Scholar]
- 101.Liu Y., Shi Z., Maximova S.N., Payne M.J., Guiltinan M.J. Tc-MYBPA is an Arabidopsis TT2-like transcription factor and functions in the regulation of proanthocyanidin synthesis in Theobroma cacao. BMC Plant Biol. 2015;15:1–16. doi: 10.1186/s12870-015-0529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu C., Jun J.H., Dixon R.A. MYB5 and MYB14 play pivotal roles in seed coat polymer biosynthesis in Medicago truncatula. Plant Physiol. 2014;165:1424–1439. doi: 10.1104/pp.114.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu M., Zhang C., Duan L., Luan Q., Li J., Yang A., Qi X., Ren Z. CsMYB60 is a key regulator of flavonols and proanthocyanidans that determine the colour of fruit spines in cucumber. J. Exp. Bot. 2019;70:69–84. doi: 10.1093/jxb/ery336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J., Luan Q., Han J., Zhang C., Liu M., Ren Z. CsMYB60 directly and indirectly activates structural genes to promote the biosynthesis of flavonols and proanthocyanidins in cucumber. Hortic. Res. 2020;7:1–15. doi: 10.1038/s41438-020-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li C., Qiu J., Huang S., Yin J., Yang G. AaMYB3 interacts with AabHLH1 to regulate proanthocyanidin accumulation in Anthurium andraeanum (Hort.)—Another strategy to modulate pigmentation. Hortic. Res. 2019;6:14. doi: 10.1038/s41438-018-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akagi T., Ikegami A., Yonemori K. DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta. 2010;232:1045–1059. doi: 10.1007/s00425-010-1241-7. [DOI] [PubMed] [Google Scholar]
- 107.Del Mar Naval M., Gil-Muñoz F., Lloret A., Besada C., Salvador A., Badenes M.L., Ríos G. A WD40-repeat protein from persimmon interacts with the regulators of proanthocyanidin biosynthesis DkMYB2 and DkMYB4. Tree Genet. Genomes. 2016;12:1–11. [Google Scholar]
- 108.Pang Y., Wenger J.P., Saathoff K., Peel G.J., Wen J., Huhman D., Allen S.N., Tang Y., Cheng X., Tadege M., et al. A WD40 repeat protein from Medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant Physiol. 2009;151:1114–1129. doi: 10.1104/pp.109.144022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shan X., Li Y., Yang S., Gao R., Zhou L., Bao T., Han T., Wang S., Gao X., Wang L. A functional homologue of Arabidopsis TTG1 from Freesia interacts with bHLH proteins to regulate anthocyanin and proanthocyanidin biosynthesis in both Freesia hybrida and Arabidopsis thaliana. Plant Physiol. Biochem. 2019;141:60–72. doi: 10.1016/j.plaphy.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Gao Y., Liu J., Chen Y., Tang H., Wang Y., He Y., Ou Y., Sun X., Wang S., Yao Y. Tomato SlAN11 regulates flavonoid biosynthesis and seed dormancy by interaction with bHLH proteins but not with MYB proteins. Hortic. Res. 2018;5:1–18. doi: 10.1038/s41438-018-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y., Ye J., Liu C., Xu Q., Long L., Deng X. Citrus PH4-Noemi regulatory complex is involved in proanthocyanidin biosynthesis via a positive feedback loop. J. Exp. Bot. 2020;71:1306–1321. doi: 10.1093/jxb/erz506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ravaglia D., Espley R.V., Henry-Kirk R.A., Andreotti C., Ziosi V., Hellens R.P., Costa G., Allan A.C. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013;13:68. doi: 10.1186/1471-2229-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.James A.M., Ma D., Mellway R., Gesell A., Yoshida K., Walker V., Tran L., Stewart D., Reichelt M., Suvanto J., et al. Poplar MYB115 and MYB134 transcription factors regulate proanthocyanidin synthesis and structure. Plant Physiol. 2017;174:154–171. doi: 10.1104/pp.16.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou H., Lin-Wang K., Liao L., Gu C., Lu Z., Allan A.C., Han Y. Peach MYB7 activates transcription of the proanthocyanidin pathway gene encoding leucoanthocyanidin reductase, but not anthocyanidin reductase. Front. Plant Sci. 2015;6:908. doi: 10.3389/fpls.2015.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verdier J., Zhao J., Torres-Jerez I., Ge S., Liu C., He X., Mysore K.S., Dixon R.A., Udvardi M.K. MtPAR MYB transcription factor acts as an on switch for proanthocyanidin biosynthesis in Medicago truncatula. Proc. Natl. Acad. Sci. USA. 2012;109:1766–1771. doi: 10.1073/pnas.1120916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deluc L., Barrieu F., Marchive C., Lauvergeat V., Decendit A., Richard T., Carde J.P., Mérillon J.M., Hamdi S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006;140:499–511. doi: 10.1104/pp.105.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deluc L., Bogs J., Walker A.R., Ferrier T., Decendit A., Merillon J.M., Robinson S.P., Barrieu F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008;147:2041–2053. doi: 10.1104/pp.108.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hichri I., Heppel S.C., Pillet J., Léon C., Czemmel S., Delrot S., Lauvergeat V., Bogs J. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant. 2010;3:509–523. doi: 10.1093/mp/ssp118. [DOI] [PubMed] [Google Scholar]
- 119.Sun Q., Jiang S., Zhang T., Xu H., Fang H., Zhang J., Su M., Wang Y., Zhang Z., Wang N., et al. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019;289:110286. doi: 10.1016/j.plantsci.2019.110286. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez A., Brown M., Hatlestad G., Akhavan N., Smith T., Hembd A., Moore J., Montes D., Mosley T., Resendez J., et al. TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Dev. Biol. 2016;419:54–63. doi: 10.1016/j.ydbio.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 121.Amato A., Cavallini E., Zenoni S., Finezzo L., Begheldo M., Ruperti B., Tornielli G.B. A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis. Front. Plant Sci. 2017;7:1979. doi: 10.3389/fpls.2016.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nesi N., Debeaujon I., Jond C., Stewart A.J., Jenkins G.I., Caboche M., Lepiniec L. The transparent testa 16 locus encodes the arabidopsis bsister mads domain protein and is required for proper development and pigmentation of the seed coat. Plant Cell. 2002;14:2463–2479. doi: 10.1105/tpc.004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen G., Deng W., Peng F., Truksa M., Singer S., Snyder C.L., Mietkiewska E., Weselake R.J. Brassica napus TT16 homologs with different genomic origins and expression levels encode proteins that regulate a broad range of endothelium-associated genes at the transcriptional level. Plant J. 2013;74:663–677. doi: 10.1111/tpj.12151. [DOI] [PubMed] [Google Scholar]
- 124.Aharoni A., De Vos C.H.R., Wein M., Sun Z., Greco R., Kroon A., Mol J.N.M., O’Connell A.P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 2001;28:319–332. doi: 10.1046/j.1365-313X.2001.01154.x. [DOI] [PubMed] [Google Scholar]
- 125.Paolocci F., Robbins M.P., Passeri V., Hauck B., Morris P., Rubini A., Arcioni S., Damiani F. The strawberry transcription factor FaMYB1 inhibits the biosynthesis of proanthocyanidins in Lotus corniculatus leaves. J. Exp. Bot. 2011;62:1189–1200. doi: 10.1093/jxb/erq344. [DOI] [PubMed] [Google Scholar]
- 126.Cavallini E., Matus J.T., Finezzo L., Zenoni S., Loyola R., Guzzo F., Schlechter R., Ageorges A., Arce-Johnson P., Tornielli G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015;167:1448–1470. doi: 10.1104/pp.114.256172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang Y.F., Vialet S., Guiraud J.L., Torregrosa L., Bertrand Y., Cheynier V., This P., Terrier N. A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytol. 2014;201:795–809. doi: 10.1111/nph.12557. [DOI] [PubMed] [Google Scholar]
- 128.Yoshida K., Ma D., Constabel C.P. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol. 2015;167:693–710. doi: 10.1104/pp.114.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma D., Reichelt M., Yoshida K., Gershenzon J., Constabel C.P. Two R2R3-MYB proteins are broad repressors of flavonoid and phenylpropanoid metabolism in poplar. Plant J. 2018;96:949–965. doi: 10.1111/tpj.14081. [DOI] [PubMed] [Google Scholar]
- 130.Jun J.H., Liu C., Xiao X., Dixon R.A. The transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyandin and anthocyanin pigmentation in Medicago truncatula. Plant Cell. 2015;27:2860–2879. doi: 10.1105/tpc.15.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou H., Lin-Wang K., Wang F., Espley R.V., Ren F., Zhao J., Ogutu C., He H., Jiang Q., Allan A.C., et al. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytol. 2019;221:1919–1934. doi: 10.1111/nph.15486. [DOI] [PubMed] [Google Scholar]
- 132.Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xiea D. The jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell. 2011;23:1795–1814. doi: 10.1105/tpc.111.083261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie Y., Tan H., Ma Z., Huang J. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Mol. Plant. 2016;9:711–721. doi: 10.1016/j.molp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 134.Dong Q., Zhao H., Huang Y., Chen Y., Wan M., Zeng Z., Yao P., Li C., Wang X., Chen H., et al. FtMYB18 acts as a negative regulator of anthocyanin/proanthocyanidin biosynthesis in Tartary buckwheat. Plant Mol. Biol. 2020;104:309–325. doi: 10.1007/s11103-020-01044-5. [DOI] [PubMed] [Google Scholar]
- 135.Ma D., Constabel C.P. MYB Repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci. 2019;24:275–289. doi: 10.1016/j.tplants.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 136.Dubos C., Le Gourrierec J., Baudry A., Huep G., Lanet E., Debeaujon I., Routaboul J.M., Alboresi A., Weisshaar B., Lepiniec L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008;55:940–953. doi: 10.1111/j.1365-313X.2008.03564.x. [DOI] [PubMed] [Google Scholar]
- 137.Figueroa P., Gusmaroli G., Serino G., Habashi J., Ma L., Shen Y., Feng S., Bostick M., Callis J., Hellmann H., et al. Arabidopsis has two redundant cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell. 2005;17:1180–1195. doi: 10.1105/tpc.105.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stogios P.J., Downs G.S., Jauhal J.J.S., Nandra S.K., Privé G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005;6:R82. doi: 10.1186/gb-2005-6-10-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mandadi K.K., Misra A., Ren S., Mcknight T.D. BT2, a BTB protein, mediates multiple responses to nutrients, stresses, and hormones in Arabidopsis. Plant Physiol. 2009;150:1930–1939. doi: 10.1104/pp.109.139220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.An J.P., An X.H., Yao J.F., Wang X.N., You C.X., Wang X.F., Hao Y.J. BTB protein MdBT2 inhibits anthocyanin and proanthocyanidin biosynthesis by triggering MdMYB9 degradation in apple. Tree Physiol. 2018;38:1578–1587. doi: 10.1093/treephys/tpy063. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki N., Koussevitzky S., Mittler R., Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 142.Dixon R.A., Paiva N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.2307/3870059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002;5:218–223. doi: 10.1016/S1369-5266(02)00256-X. [DOI] [PubMed] [Google Scholar]
- 144.Pollastri S., Tattini M. Flavonols: Old compounds for old roles. Ann. Bot. 2011;108:1225–1233. doi: 10.1093/aob/mcr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petrussa E., Braidot E., Zancani M., Peresson C., Bertolini A., Patui S., Vianello A. Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu L.J., Deng X.G., Zou L.J., Zhang D.W., Lin H.H. Enhancement of stress tolerance in cucumber seedlings by proanthocyanidins. Biol. Plant. 2017;61:323–332. doi: 10.1007/s10535-016-0663-x. [DOI] [Google Scholar]
- 147.Jeon J., Kim J.K., Wu Q., Park S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum) Environ. Exp. Bot. 2018;155:488–496. doi: 10.1016/j.envexpbot.2018.07.027. [DOI] [Google Scholar]
- 148.Mori K., Sugaya S., Gemma H. Regulatory mechanism of anthocyanin biosynthesis in “Kyoho” grape berries grown under different temperature conditions. Environ. Control Biol. 2004;42:21–30. doi: 10.2525/ecb1963.42.21. [DOI] [Google Scholar]
- 149.Pastore C., Santo S.D., Zenoni S., Movahed N., Allegro G., Valentini G., Filippetti I., Tornielli G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017;8:929. doi: 10.3389/fpls.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bonada M., Jeffery D.W., Petrie P.R., Moran M.A., Sadras V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015;21:240–253. doi: 10.1111/ajgw.12142. [DOI] [Google Scholar]
- 151.Poudel P.R., Koyama K., Goto-Yamamoto N. Evaluating the influence of temperature on proanthocyanidin biosynthesis in developing grape berries (Vitis vinifera L.) Mol. Biol. Rep. 2020;47:3501–3510. doi: 10.1007/s11033-020-05440-4. [DOI] [PubMed] [Google Scholar]
- 152.Genebra T., Santos R.R., Francisco R., Pinto-Marijuan M., Brossa R., Serra A.T., Duarte C.M.M., Chaves M.M., Zarrouk O. Proanthocyanidin accumulation and biosynthesis are modulated by the irrigation regime in tempranillo seeds. Int. J. Mol. Sci. 2014;15:11862–11877. doi: 10.3390/ijms150711862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gouot J.C., Smith J.P., Holzapfel B.P., Walker A.R., Barril C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019;70:397–423. doi: 10.1093/jxb/ery392. [DOI] [PubMed] [Google Scholar]
- 154.Hernández I., Alegre L., Munné-Bosch S. Plant aging and excess light enhance flavan-3-ol content in Cistus clusii. J. Plant Physiol. 2011;168:96–102. doi: 10.1016/j.jplph.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 155.Gourlay G., Constabel C.P. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress. Tree Physiol. 2019;39:345–355. doi: 10.1093/treephys/tpy143. [DOI] [PubMed] [Google Scholar]
- 156.Yan J.X., Lu Y.F., Yan S.C. The effects of irradiance on the production of phenolic compounds and condensed tannins in Larix gmelinii needles. Biol. Plant. 2014;58:159–163. doi: 10.1007/s10535-013-0367-4. [DOI] [Google Scholar]
- 157.Gesell A., Yoshida K., Tran L.T., Constabel C.P. Characterization of an apple TT2-type R2R3 MYB transcription factor functionally similar to the poplar proanthocyanidin regulator PtMYB134. Planta. 2014;240:497–511. doi: 10.1007/s00425-014-2098-y. [DOI] [PubMed] [Google Scholar]
- 158.Zhang W., Liu S., Li C., Zhang P., Zhang P. Transcriptome sequencing of Antarctic moss under salt stress emphasizes the important roles of the ROS-scavenging system. Gene. 2019;696:122–134. doi: 10.1016/j.gene.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 159.Li D., Hu X., Li C. Overexpression of the LoMYB29 gene of Larix olgensis contributes to the regulation of proanthocyanidin biosynthesis in Arabidopsis thaliana. J. For. Res. 2019;30:1793–1804. doi: 10.1007/s11676-018-0709-3. [DOI] [Google Scholar]
- 160.Li Z., Chen W., Zhang C., Du C., Shao G., Cui Y., Luo P. RcMYBPA2 of Rosa chinensis functions in proanthocyanidin biosynthesis and enhances abiotic stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2019;137:441–454. doi: 10.1007/s11240-019-01580-z. [DOI] [Google Scholar]
- 161.Luo P., Shen Y., Jin S., Huang S., Cheng X., Wang Z., Li P., Zhao J., Bao M., Ning G. Overexpression of Rosa rugosa anthocyanidin reductase enhances tobacco tolerance to abiotic stress through increased ROS scavenging and modulation of ABA signaling. Plant Sci. 2016;245:35–49. doi: 10.1016/j.plantsci.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 162.Peters D.J., Constabel C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides) Plant J. 2002;32:701–712. doi: 10.1046/j.1365-313X.2002.01458.x. [DOI] [PubMed] [Google Scholar]
- 163.Hagerman A.E., Riedl K.M., Jones G.A., Sovik K.N., Ritchard N.T., Hartzfeld P.W., Riechel T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 164.Quideau S., Deffieux D., Douat-Casassus C., Pouységu L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 165.Dat J., Vandenabeele S., Vranová E., Van Montagu M., Inzé D., Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 167.Miranda M., Ralph S.G., Mellway R., White R., Heath M.C., Bohlmann J., Constabel C.P. The transcriptional response of hybrid poplar (Populus trichocarpa x P. deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol. Plant-Microbe Interact. 2007;20:816–831. doi: 10.1094/MPMI-20-7-0816. [DOI] [PubMed] [Google Scholar]
- 168.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- 169.Iriti M., Rossoni M., Borgo M., Ferrara L., Faoro F. Induction of resistance to gray mold with benzothiadiazole modifies amino acid profile and increases proanthocyanidins in grape: Primary versus secondary metabolism. J. Agric. Food Chem. 2005;53:9133–9139. doi: 10.1021/jf050853g. [DOI] [PubMed] [Google Scholar]
- 170.Koskimäki J.J., Hokkanen J., Jaakola L., Suorsa M., Tolonen A., Mattila S., Pirttilä A.M., Hohtola A. Flavonoid biosynthesis and degradation play a role in early defence responses of bilberry (Vaccinium myrtillus) against biotic stress. Eur. J. Plant Pathol. 2009;125:629–640. doi: 10.1007/s10658-009-9511-6. [DOI] [Google Scholar]
- 171.Guidarelli M., Carbone F., Mourgues F., Perrotta G., Rosati C., Bertolini P., Baraldi E. Colletotrichum acutatum interactions with unripe and ripe strawberry fruits and differential responses at histological and transcriptional levels. Plant Pathol. 2011;60:685–697. doi: 10.1111/j.1365-3059.2010.02423.x. [DOI] [Google Scholar]
- 172.Haile Z.M., Nagpala-De Guzman E.G., Moretto M., Sonego P., Engelen K., Zoli L., Moser C., Baraldi E. Transcriptome profiles of strawberry (Fragaria vesca) fruit interacting with Botrytis cinerea at different ripening stages. Front. Plant Sci. 2019;10:1131. doi: 10.3389/fpls.2019.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carbone F., Preuss A., De Vos R.C.H., D’Amico E., Perrotta G., Bovy A.G., Martens S., Rosati C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant Cell Environ. 2009;32:1117–1131. doi: 10.1111/j.1365-3040.2009.01994.x. [DOI] [PubMed] [Google Scholar]
- 174.Nagpala E.G., Guidarelli M., Gasperotti M., Masuero D., Bertolini P., Vrhovsek U., Baraldi E. Polyphenols variation in fruits of the susceptible strawberry cultivar alba during ripening and upon fungal pathogen interaction and possible involvement in unripe fruit tolerance. J. Agric. Food Chem. 2016;64:1869–1878. doi: 10.1021/acs.jafc.5b06005. [DOI] [PubMed] [Google Scholar]
- 175.Boeckler G.A., Gershenzon J., Unsicker S.B. Gypsy moth caterpillar feeding has Only a marginal impact on phenolic compounds in old-growth black poplar. J. Chem. Ecol. 2013;39:1301–1312. doi: 10.1007/s10886-013-0350-8. [DOI] [PubMed] [Google Scholar]
- 176.Kumar V., Nadda G., Kumar S., Yadav S.K. Transgenic tobacco overexpressing tea cDNA encoding dihydroflavonol 4-reductase and anthocyanidin reductase induces early flowering and provides biotic stress tolerance. PLoS ONE. 2013;8:e65535. doi: 10.1371/journal.pone.0065535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hagerman A.E., Butler L.G. Tannins and Lignins. In: Rosenthal G.A., Berenbaum M.R., editors. Herbivores: Their Interactions with Secondary Plant Metabolites. Volume 1. Academic Press, Inc., Harcourt Brace Jovanovich; New York, NY, USA: 1991. pp. 355–388. [Google Scholar]
- 178.Barbehenn R.V., Peter Constabel C. Tannins in plant-herbivore interactions. Phytochemistry. 2011;72:1551–1565. doi: 10.1016/j.phytochem.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 179.Ayres M.P., Clausen T.P., MacLean S.F., Redman A.M., Relchardt P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology. 1997;78:1696–1712. doi: 10.1890/0012-9658(1997)078[1696:DOSAAA]2.0.CO;2. [DOI] [Google Scholar]
- 180.Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013;18:477–483. doi: 10.1016/j.tplants.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 181.Lacampagne S., Gagné S., Gény L. Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: New elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. J. Plant Growth Regul. 2010;29:81–90. [Google Scholar]
- 182.Akagi T., Katayama-Ikegami A., Kobayashi S., Sato A., Kono A., Yonemori K. Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2012;158:1089–1102. doi: 10.1104/pp.111.191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gil-Muñoz R., Fernández-Fernández J.I., Portu J., Garde-Cerdán T. Methyl jasmonate: Effect on proanthocyanidin content in Monastrell and Tempranillo grapes and wines. Eur. Food Res. Technol. 2018;244:611–621. doi: 10.1007/s00217-017-2981-4. [DOI] [Google Scholar]
- 184.An X.H., Tian Y., Chen K.Q., Liu X.J., Liu D.D., Xie X.B., Cheng C.G., Cong P.H., Hao Y.J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015;56:650–662. doi: 10.1093/pcp/pcu205. [DOI] [PubMed] [Google Scholar]
- 185.Liu X.J., An X.H., Liu X., Hu D.G., Wang X.F., You C.X., Hao Y.J. MdSnRK1.1 interacts with MdJAZ18 to regulate sucrose-induced anthocyanin and proanthocyanidin accumulation in apple. J. Exp. Bot. 2017;68:2977–2990. doi: 10.1093/jxb/erx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li H., Han M., Yu L., Wang S., Zhang J., Tian J., Yao Y. Transcriptome analysis identifies two ethylene response factors that regulate proanthocyanidin biosynthesis during Malus Crabapple fruit development. Front. Plant Sci. 2020;11:76. doi: 10.3389/fpls.2020.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Robert-Seilaniantz A., Grant M., Jones J.D.G. Hormone crosstalk in plant disease and defense: More than just JASMONATE-SALICYLATE antagonism. Annu. Rev. Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 188.Thomma B.P.H.J., Eggermont K., Penninckx I.A.M.A., Mauch-Mani B., Vogelsang R., Cammue B.P.A., Broekaert W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ullah C., Tsai C.J., Unsicker S.B., Xue L., Reichelt M., Gershenzon J., Hammerbacher A. Salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 2019;221:960–975. doi: 10.1111/nph.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Feng J., Zhang M., Yang K.N., Zheng C.X. Salicylic acid-primed defence response in octoploid strawberry “Benihoppe” leaves induces resistance against Podosphaera aphanis through enhanced accumulation of proanthocyanidins and upregulation of pathogenesis-related genes. BMC Plant Biol. 2020;20:1–18. doi: 10.1186/s12870-020-02353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Felicijan M., Kristl J., Krajnc A.U. Pre-treatment with salicylic acid induces phenolic responses of norway spruce (Picea abies) bark to bark beetle (Ips typographus) attack. Trees Struct Funct. 2016;30:2117–2129. doi: 10.1007/s00468-016-1438-x. [DOI] [Google Scholar]
- 192.Ullah C., Unsicker S.B., Reichelt M., Gershenzon J., Hammerbacher A. Accumulation of catechin and proanthocyanidins in black poplar stems after infection by plectosphaerella populi: Hormonal regulation, biosynthesis and antifungal activity. Front. Plant Sci. 2019;10:1441. doi: 10.3389/fpls.2019.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smulikowska S., Pastuszewska B., Świȩch E., Ochtabińska A., Mieczkowska A., Nguyen V.C., Buraczewska L. Tannin content affects negatively nutritive value of pea for monogastrics. J. Anim. Feed Sci. 2001;10:511–523. doi: 10.22358/jafs/68004/2001. [DOI] [Google Scholar]
- 194.Ikegami A., Kitajima A., Yonemori K. Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination-constant, non-astringent (PCNA)-type persimmon fruit. J. Hortic. Sci. Biotechnol. 2005;80:225–228. doi: 10.1080/14620316.2005.11511921. [DOI] [Google Scholar]
- 195.Passeri V., Martens S., Carvalho E., Bianchet C., Damiani F., Paolocci F. The R2R3MYB VvMYBPA1 from grape reprograms the phenylpropanoid pathway in tobacco flowers. Planta. 2017;246:185–199. doi: 10.1007/s00425-017-2667-y. [DOI] [PubMed] [Google Scholar]