Abstract
Introduction
Personalised cancer screening aims to improve benefits, reduce harms and being more cost-effective than age-based screening. The objective of the DECIDO study is to assess the acceptability and feasibility of offering risk-based personalised breast cancer screening and its integration in regular clinical practice in a National Health System setting.
Methods and analysis
The study is designed as a single-arm proof-of-concept trial. The study sample will include 385 women aged 40–50 years resident in a primary care health area in Spain. The study intervention consists of (1) a baseline visit; (2) breast cancer risk estimation; (3) a second visit for risk communication and screening recommendations based on breast cancer risk and (4) a follow-up to obtain the study outcomes.
A polygenic risk score (PRS) will be constructed as a composite likelihood ratio of 83 single nucleotide polymorphisms. The Breast Cancer Surveillance Consortium risk model, including age, race/ethnicity, family history of breast cancer, benign breast disease and breast density will be used to estimate a preliminary 5-year absolute risk of breast cancer. A Bayesian approach will be used to update this risk with the PRS value.
The primary outcome measures will be attitude towards, intention to participate in and satisfaction with personalised breast cancer screening. Secondary outcomes will include the proportions of women who accept to participate and who complete the different phases of the study. The exact binomial and the Student’s t-test will be used to obtain 95% CIs.
Ethics and dissemination
The study protocol was approved by the Drug Research Ethics Committee of the University Hospital Arnau de Vilanova. The trial will be conducted in compliance with this study protocol, the Declaration of Helsinki and Good Clinical Practice.
The results will be published in peer-reviewed scientific journals and disseminated in scientific conferences and media.
Trial registration number
Keywords: public health, epidemiology, breast tumours, health policy
Strengths and limitations of this study.
This proof-of-concept study will provide evidence on feasibility and acceptance of personalised breast cancer screening, by the target population, under a publicly funded health system.
Breast cancer risk will be estimated using known risk factors and a polygenic risk score.
Tailored recommendations on breast cancer screening will be provided to women by primary care doctors in their health centre.
Women’s attitudes towards, participating intentions in and satisfaction with personalised breast screening will be assessed as primary outcomes.
The design of a single arm has some limitations, such as having no outcome comparisons between groups.
Introduction
The Horizon 2020 Advisory Group defines personalised medicine as ‘a medical model using characterisation of individuals’ phenotypes and genotypes for tailoring the right therapeutic or preventive strategy for the right person at the right time’.1 Personalised cancer screening pursues improving age-based screening by detecting malignant tumours in younger subjects at higher risk, reducing harms through reduction of false-positive results and overdiagnosis in subjects at lower risk and being more cost-effective.2 3
Personalised screening for breast cancer consists of estimating the individual risk of developing breast cancer in a specific time horizon and providing tailored recommendations for early detection that combine (1) frequency of the screening exams (eg, annual, biennial, triennial); (2) age at the start and end of screening and (3) screening modality (mammogram, ultrasound, MRI).
Personalised screening requires an accurate measure of individual risk. Tice et al 4 developed and validated the Breast Cancer Surveillance Consortium (BCSC) model including age, race or ethnicity, breast density, family history of breast cancer and previous biopsy. An updated version of the BCSC model replaced previous biopsy by type of benign breast disease.5 Recently, genome studies have identified >90 genetic variants called single-nucleotide polymorphisms (SNPs) that would explain between 15% and 20% of the inherited variance in breast cancer risk.6 Michailidou et al 7 identified 65 new breast cancer risk loci and estimated that, in total, common susceptibility variants explain 18% of the familial relative risk. Mavaddat et al 8 proposed a polygenic risk score (PRS) that grouped the individual effects and the interactions of 77 SNPs to estimate the risk of breast cancer. Vachon et al 9 10 observed independence between a PRS similar to the previous one and breast density in case-control studies.
In a critical review of clinical applications of polygenic breast cancer risk, Yanes et al 11 highlight that there is considerable debate about the clinical utility of polygenic information for assessing breast cancer risk. Whereas, the sceptics argue that there is not enough evidence for their implementation in clinical practice, the supporters consider that PRS have the potential of (1) providing risk information to women with uninformative genetic testing results; (2) being risk modifiers for those with pathogenic variants in high and moderate risk genes and (3) providing personalised risk assessments and risk management strategies in population screening programmes. In fact, the Yanes et al review11 showed that, in European populations, the addition of a PRS to the existing risk models has improved their accuracy. The highest area under the curve, 0.72, was reported in a model that combined BCSC, circulating oestradiol levels and PRS, for oestrogen receptor-positive breast cancer.12
Few studies have assessed the implementation of risk-based breast cancer screening. Román et al 13 performed the first systematic review of studies assessing personalised breast cancer screening strategies and evaluated the quality of the evidence. Thirteen studies were included, three randomised controlled trials in the recruitment phase and no reported results yet, nine mathematical modelling studies and one observational pilot study. In all models and in the observational study, personalised screening strategies were shown to be effective and efficient. However, as the authors indicate, evidence is lacking on feasibility, acceptability and the legal and ethical aspects of personalised screening strategies.
Evans et al 14 assessed the feasibility of determining breast cancer risk and communicating it in the context of a population-based mammographic screening programme in England. In the Predicting Risk of Cancer At Screening (PROCAS) study, they used the Tyrer-Cuzick model15 for risk assessment and investigated risk perception, the proportion wishing to know their 10-year risk and whether subsequent screening attendance was affected. Women at high or at low risk were invited for risk feedback and counselling. The authors concluded that (a) the majority of women wished to receive risk information; (b) perception of breast cancer risk in the general population is poor and (c) high-risk women increased their attendance to the subsequent screening. In a later analysis, Evans et al 16 added a PRS and mammographic density to the Tyrer-Cuzick model, with the objective of improving breast cancer risk stratification and enabling more targeted early detection/prevention strategies in population screening programmes. The combined risk tool improved the Tyrer-Cuzick model and defined a low-risk group of women that was approximately 30% of the total, such that cancers identified in this group were significantly more likely to have an extremely good prognosis. According to the authors, in a risk-stratified approach with an assessment at first mammogram around age 45–50 years, the extra screening in the high-risk group could be offset by reducing or eliminating screening in the low-risk group, where the benefits of screening may be outweighed by false-positive results and the potential for overdiagnosis and overtreatment.16
On the basis of the PROCAS project, and considering the National Institute for Health and Care Excellence clinical guidelines for women with familial breast cancer, French et al 17 developed an automated system (BC-Predict) for offering an assessment of breast cancer risk to women when they receive their National Health Service Breast Screening Programme (NHSBSP) invitation, and generating feedback letters to communicate this risk to women and health professionals. The recently published protocol describes that the study aims at identifying and resolving key uncertainties regarding the feasibility of integrating BC-Predict into the NHSBSP.17 In addition to including an explicit quantitative and qualitative analysis of the effects of implementing the BC-Predict system in the NHSBSP, the research will assess the feasibility of a definitive study to evaluate whether the intervention translates into measurable effects on breast cancer incidence and stage, and is a cost-effective use of the National Health Service resources.
In Spain, most of population-based screening programmes target women aged 50–69 years and perform biennial mammograms. Extending the programme to older or younger women has been a matter of interest for health policy-makers. On the one hand, opportunistic screening in women younger than 50 years is widely used. The InforMa study found that around 80% of the participants reported previous use of screening mammograms before age 50 years, outside the organised screening programme,18 with potential harms of screening such as overdiagnosis and false-positive results in low-risk women. On the other hand, in Catalonia, the incidence of ductal carcinoma in situ and invasive breast cancer in young women increased steadily during the 1990s and the first decade of the 2000s, in parallel with an increase in mammography use over time in women in their late 30s or early 40s.19 20 Including women younger than 50 years in risk-based screening, so low-risk women are recommended to wait and high-risk women are screened, may improve the balance of benefits and harms of the intervention, as mathematical models have shown.3
There will be several challenges to the implementation of personalised screening. In women, barriers related to their culture, socioeconomic level, personal experience, the trust in the infallibility and innocuousness of screening and the social alarm that cancer generates, among others.21 22 In healthcare professionals, some barriers are common with those in women and others are specific, such as the inertia to adapt to new evidence and the social pressure to avoid clinical error or delayed diagnosis.23–25 Two systematic reviews showed that both women and healthcare professionals overestimate benefits and underestimate harms of treatments, screening and tests.26 27 In addition, women overestimate the risk of breast cancer and most of them have not been informed of the adverse effects of screening. In clinical practice, personalised screening probably will require more time than the current ‘one-size-fits-all’ approach, at least at the beginning. But new ways of organising, integrating and analysing data will allow for better identification of individual features that could indicate the optimal intervention, at any time, while saving time and increasing effectiveness and efficiency. When the ongoing clinical trials finish, if they show that risk-based screening is effective and efficient with respect to the current practice, country-specific proof-of-concept studies on implementing the risk-based screening approach will be of great value.
Study objective
The objective of the DECIDO project is to assess the acceptability and feasibility of offering personalised breast cancer screening and its integration in the usual clinical practice. This proof-of-concept study will assess the women’s attitudes, participating intentions and satisfaction with personalised breast screening.
Methods and analysis
Study design
The study is designed as a single-arm proof-of-concept trial. A pilot study was carried out with 20 women to test the suitability of the recruitment and data collection processes and the coordination of the involved healthcare professionals.
Participants
From January 2019 onwards, 385 women aged 40–50 years will be enrolled in the study. Potential participants are the 2038 women living in the ‘Primer de Maig’ Basic Health Area in Lleida, Catalonia, on 31 December 2018, that would turn 40–50 years during the following 1.5 years, according to the primary care information system.
All the women that will turn 50 during the study period will be invited to participate. If they decline to participate in the study, they will be invited to participate in the population-based Early Detection Programme for Breast Cancer, since women resident in Spain, aged 50–69 years, are invited biennially for a breast screening mammogram.
From women that will turn 40–49 years during the study period, a random sample of 20–50 women will be selected, on a monthly basis, and invited to participate until the accrual goal of 385 women is achieved.
Women with a previous diagnosis of breast cancer or a breast study in process, or those fulfilling clinical criteria for cancer genetic counselling will be excluded. Women not understanding or speaking Catalan or Spanish, or with a physical or cognitive disability that prevents breast screening or the main outcomes assessment will be also excluded.
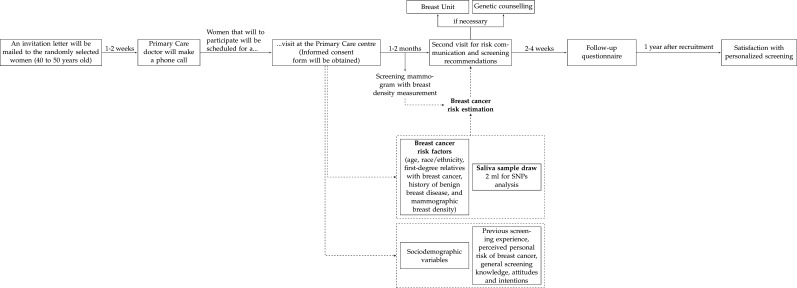
An invitation letter with a brief summary of the study objectives will be mailed to the selected women. After 1–2 weeks, a primary care doctor of the study team will make a phone call, provide a brief information about the study, determine eligibility and ask if they are interested in participating. Women that will to participate will be scheduled for a visit at the primary care centre. An informed consent form will be obtained at the beginning of the first visit.
Intervention
The study intervention consists of a baseline visit, the breast cancer risk estimation, a visit for risk communication and screening recommendations and the administration of a follow-up questionnaire. Figure 1 shows the timeline of the intervention.
Figure 1.
Timeline of the study intervention. SNP, single nucleotide polymorphism.
First visit at the primary care centre
The study intervention consists of: (1) providing detailed information about the study objectives by a primary care doctor, member of the study team; (2) providing an informative brochure about the benefits and adverse effects of breast cancer screening28; (3) obtaining information on sociodemographic variables, breast cancer risk factors, previous screening experience, perceived personal risk of breast cancer and general screening knowledge, attitudes and intentions; (4) obtaining a saliva sample to determine the genomic profile and (5) scheduling a screening mammogram with breast density measurement. For women that had a mammogram during the year before the first visit, breast density and presence/absence of benign lesions will be obtained from that mammogram and the radiologist report.
Breast density measurement and breast findings
A senior radiologist (MR-R) expert in screening will evaluate the mammograms of all participating women. Mammographic breast density will be classified according to the Breast Imaging Reporting and Data System (BI-RADS) scoring system29: almost entirely fatty (a), scattered areas of fibroglandular density (b), heterogeneously dense (c) and extremely dense (d). Mammographic findings will be coded from 0 (incomplete—additional imaging needed) to 6 (known biopsy-proven malignancy). Additional tests will be requested in case of abnormal results in coordination with the hospital breast unit.
Although automated breast density methods have some advantages and are common practice in research, they were not available to us. We used the clinical BI-RADS system, which is the standard of care in our healthcare centres. Even though the BI-RADS system has a non-negligible degree of inter-reader and intra-reader variability in the categorisation of breast density, Kerlikowske et al 30 found that automated and clinical BI-RADS density similarly predict interval and screen-detected cancer risk, suggesting that either measure may be used to inform women of their breast density. And regarding automated methods, which have shown high reproducibility and robustness, Conant et al 31 suggest that more research and collaborative effort is needed to develop affordable, well validated and broadly available automated software.
SNP determination and polygenic risk score estimation
Collection, conservation and delivery of saliva samples will be done following the saliva collection protocol provided by the University of Lleida’s Proteomics and Genomics Service (PGS) that details the procedures and indicates its storage conditions until it is delivered to the PGS. The shipment of samples from the primary care centre will be done once a week. The SNP analysis will be carried out from genomic DNA (gDNA) extracted using a suitable commercial kit. If the obtained gDNA does not meet the quality and quantity requirements necessary for the genotyping, another sample of saliva will be requested.
Genotyping of the 83 SNPs will be performed using the matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry technique based on the primer extension procedure where a DNA polymerase extends a primer upstream of the SNP with a set of dideoxynucleotide triphosphates (ddNTPs) on a PCR amplicon, resulting in allele-specific products for MALDI detection.32
The PRS will be obtained using the 85 SNPs associated with breast cancer that were listed in studies by Shieh et al 33 or Mavaddat et al.8 Both studies used data from the Breast Cancer Association Consortium as part of the Collaborative Oncological Gene-Environment Study.
Breast cancer risk estimation
First, the BCSC V.2.0 risk model5 and the Catalan breast cancer incidence and mortality by all causes of death will be used to estimate a preliminary 5-year absolute risk of breast cancer. The BCSC V.2.0 model includes age, race/ethnicity, first-degree relatives with breast cancer, history of benign breast disease and mammographic breast density. The incidence data from the Girona and Tarragona cancer registries (2011 Catalan Official census, IDESCAT) will be averaged and the locally weighted smoothing (LOESS) regression will be used to smooth them by age groups. The baseline risk for the model will be obtained using the smoothed breast cancer incidence rates risk factors distribution of the BCSC dataset as described by Gail et al.34 The combination of the BCSC V.2.0 hazard ratios of the risk factors’ distributions will provide the preliminary 5-year absolute risks of breast cancer.
To obtain the 5-year absolute risk that includes the PRS, the Shieh et al approach33 using the allele frequencies and odds ratios from Caucasian populations will be used. The PRS will be constructed as a composite likelihood ratio representing the individual effects of each SNP. It will be assumed that all the SNPs are inherited independently, and that there are no interactions between them. Once the PRS is obtained, a Bayesian approach will be used to update preliminary 5-year risk obtained with the BCSC V.2.0 model with the PRS value.
Risk communication and screening recommendations
Within 1–2 months after the first visit and once the breast cancer risk is obtained, a second visit will be scheduled for risk communication and screening recommendations. The visit will be performed by the same primary care doctor and team member that performed the first visit. Women will be given a report including their status for family history, benign lesions, breast density, PRS and two pictograms with the 5-year absolute risk of breast cancer, one with their risk and the other with the risk of a same age woman of the general population. The screening recommendations are presented in table 1. Women with any anomalous or suspicious finding in mammography or with a very high risk of breast cancer after PRS assessment will be referred to the public hospital breast unit and/or genetic counselling.
Table 1.
Breast cancer screening recommendations according to the absolute risk of breast cancer at 5 years
| Age group (years) | Absolute risk of breast cancer at 5 years | Screening recommendations |
| 40–44 | <0.99% 0.99%–1.16% >1.16% |
Watch and wait Biennial Annual |
| 45–48 | <0.99% 0.99%–1.19% >1.19% |
Watch and wait Biennial Annual |
| 49–50 | <0.8% 0.8%–1.19% >1.19% |
Triennial Biennial Annual |
| 40–50 | >6% | Referral to the hospital breast unit and/or genetic counseling |
Absolute risk of breast cancer at 5 years for average women in Catalonia (Spain) aged 45 years: 0.8%; 50 years: 0.99%; 60 years:1.16%; 65 years: 1.19%.
It is important to remark that women aged 50 years of age and older are invited to breast cancer screening by the public system, biennially. Therefore, there is no watch and wait recommendation for women older than 49 years.
Women who receive recommendations to be screened annually or biennially and do not fulfil the age or periodicity criteria of the public screening programme are advised to inform their primary care doctors, who eventually may refer them to the radiology unit for screening mammograms at the corresponding time points.
Outcomes questionnaire at follow-up
At the end of the risk communication visit a follow-up questionnaire will be given to women. They will be asked to fill it at home and return it within 2–4 weeks. A phone call will remind women to return the questionnaire or to provide the answers by phone. The following primary and secondary outcomes will be obtained from the follow-up questionnaire.
After the study is completed, there is no plan to follow-up the participants. The study sample is too small for estimating cancer detection rates or interval cancer rates with accuracy.
Sample size calculation
Most of the primary and secondary outcomes can be expressed as proportions that will facilitate the interpretation as positive or neutral-negative outcomes. With a sample size of 385 women, proportions will be estimated with a confidence of 95% and a minimum accuracy of 0.05. Given that the target population consists of 2038 women, a 20% participation acceptance would provide the needed sample size.
Participant timeline
The study started to include participants on 11 December 2018. On 13 March 2019 participant number 252 was included and the next day accrual was suspended because of the COVID-19 pandemic. On 21 October 2020, the study enrolment resumed. We estimate that three additional months of accrual are needed. The participant timeline is detailed in figure 1.
Outcomes
Primary outcomes
The primary outcome measures will be attitude towards, intention to participate in and satisfaction with personalised breast cancer screening by participant women.
Attitude towards personalised breast cancer screening
It will be measured with a scale with three items adapted from Hersch et al.35 Each item ranges from 1 to 5, with a total score ranging from 3 to 15. One of the items asks participants their opinion on varying the frequency of screening exams depending on breast cancer risk. The other two items ask if participants would be satisfied to be invited more/less frequently in case that they had a higher/lower risk of breast cancer than the average women. Higher scores indicate more positive attitudes. A ‘positive attitude’ is defined as a total score ≥12.
Intention to participate in personalised breast cancer screening
It will be measured with a 5-point Likert scale from definitely will (1) to definitely will not (5), adapted from Hersch et al.35 The variable will be dichotomised as intending to participate (definitely or likely) or not.
Satisfaction with personalised screening
It will be measured in a 5-point Likert scale from not at all satisfied (1) to extremely satisfied (5).5 36 This variable will be measured after 1 year of recruitment.
Secondary outcomes
Attitudes towards screening mammography
It will be measured using five items adapted from Hersch et al.35 Each item ranges from 1 to 5. Total scores can range from 5 to 25. A positive attitude is defined as a total score ≥20. Higher scores indicate more positive attitudes.
Attitude towards measuring breast cancer risk
One categorical variable with four categories. It asks if the measure of breast cancer risk will do: more harm than good, more good than harm, it depends, do not know. The absolute and relative frequencies of the four categories will be obtained.36
Emotional impact of the measure of breast cancer risk
Three categorical variables with five categories, ranging from strongly disagree (1) to strongly agree (5). First variable: the information about the individual risk of breast cancer provides calmness. Second variable: receiving information about risks produces anxiety. Third variable: the information about the individual risk of breast cancer makes me worry. The scores of the three items will not be added as a scale, they will be reported separately.35
Preference with regard to the current screening
Categorical variable with three categories. It asks what type of screening the participants would choose, personalised risk-based or ‘one-size-fits-all,’ which is biennial between 50 and 69 years in Spain.
Knowledge of the benefits and harms of breast cancer screening
Eleven conceptual knowledge questions (yes/no) and four numerical knowledge questions with categories on the effect of screening. A total of 22 marks could be obtained, 11 coming from the questions on conceptual knowledge and 11 coming from the questions on numerical knowledge that measured absolute and relative values of the screening outcomes. The threshold to define adequate knowledge is to score at least 50% of the available marks, including at least one numerical mark, on all the three screening outcome subscales that refer to mortality reduction, overdiagnosis and false positives.35 37
Decisional conflict
O’Connor Decisional Conflict Scale, 10-item low literacy version, on a scale from 0 (no decisional conflict) to 100 (extreme decisional conflict).38 Scores <25 are associated with implementing decisions; scores exceeding 37.5 are associated with decision delay or feeling unsure about implementation.
Confidence in the decision
Three Likert scale statements rated from 1 (not at all confident) to 5 (very confident). A total score is obtained summing the scores of the three items and dividing by three.39
Anxiety about screening participation
Six-item short form of the Spielberger State Trait Anxiety Inventory (STAI) on a scale from 20 to 80, with higher scores indicating greater levels of anxiety.40 To calculate the total STAI score, each item is rated from 1 (not at all) to 4 (very much so), scoring of the positive items (calm, relaxed, content) is reversed, all six scores are summed and the total score is multiplied by 20/6.
Perceived significance of the benefits and the adverse effects of screening
Women will be asked how important it is for them to consider the chances of (1) avoiding breast cancer death, (2) being diagnosed and treated for a cancer that is not harmful and (3) having a false positive. The response options range from very important (1) to not at all important (4). The scores of the three items will not be added as a scale, they will be reported separately.35
Self-efficacy
Four items ranging from strongly disagree (1) to strongly agree (5). Total scores can range from 4 to 20. Higher scores indicate higher self-efficacy.36
Experience assessment
It will be measured using five items. Each item ranges from 1 to 5. Total scores can range from 5 to 25. A positive assessment is defined as a total score ≥20. Higher scores indicate more positive experience assessment.36
Confidence in personalised screening
Confidence Likert scale with one item ranging from very low confidence (1) to very high confidence (5).36
Understanding of the individual risk and the screening recommendations
Two categorical variables with five categories each, ranging from strongly disagree (1) to strongly agree (5). First variable: I understood the information I received about my risk of breast cancer in relation to women of my age. Second variable: I have understood the recommendations given to me about the screening of breast cancer in the coming years, based on my risk of breast cancer. The scores of the two items will be reported separately.36
Time spent on risk communication
Continuous variable, number of minutes. Recorded by the participant doctors.
Proportion of women who accept to participate in the study
Number of women that accept to participate divided by number of women contacted.
Proportion of participating women who complete the different phases of the study
Number of women that complete the different phases divided by number of participating women.
Data management
The data will be collected and analysed by the DECIDO research team. The data will be stored securely in password-protected computer files and in locked cabinets at the Lleida Institute for Biomedical Research (IRBLleida). Access to these files will be granted only to the research team.
Data analysis
Primary and secondary outcomes will be described with means and SD or medians and quartiles. For scale variables, individual items, subscales and overall scores will be presented. Proportions of positive responses, such as positive attitude or adequate knowledge, will be obtained as stated in the outcomes definitions.
The 95% CIs for proportions will be obtained using the exact binomial distribution. For means, the 95% CIs will be obtained assuming that the sample mean distribution follows the Student’s t-test distribution with n−1 df.
As a descriptive analysis, primary and secondary outcomes by level of knowledge of the benefits and harms of screening and by the age groups and screening recommendations shown in table 1, will be presented.
The R programming language41 and the RStudio environment42 will be used for the data analysis.
Patient and public involvement
Participants were not and will not be involved in the research process.
Ethics and dissemination
The study protocol was approved by the Drug Research Ethics Committee of the University Hospital Arnau de Vilanova. The trial will be conducted in compliance with this study protocol, the Declaration of Helsinki and Good Clinical Practice.
The results will be published in peer-reviewed scientific journals, and locally and internationally disseminated in scientific conferences and media.
Supplementary Material
Acknowledgments
The authors would like to thank the Oncolliga Lleida organisation for their commitment to the DECIDO project. The authors would also like to thank JP Glutting for review and editing.
Footnotes
MM-A and MR contributed equally.
Correction notice: This article has been corrected since it was first published. The table 1 has been updated. The provenance and peer review statement has been included.
Collaborators: The DECIDO Group: Pau Balaguer-Llaquet; Iván-David Benítez; Alexandra Bertran; Àngels Cardona; Misericòrdia Carles-Lavila; Cristina Cazorla-Sánchez; Núria Codern; Inés Cruz-Esteve; Carles Forné-Izquierdo; Maria José Hernández-Andreu; Edelmir Iglesias; Gisela Galindo-Ortego; Marta Hernández; Celmira Laza-Vásquez; Montserrat Llorens-Gabandé; Montserrat Martínez-Alonso; Maria José Pérez-Lacasta; Hèctor Perpiñán; Anna Pons-Rodríguez; Montserrat Rué; Isabel Sánchez-López; Jordi Vilaplana-Mayoral; Mercè Reñé-Reñé.
Contributors: Conception and design of the study: MR, CFI, MM-A. Clinical visits, early detection exams and acquisition of the data: IC-E, MR-R, CC, MH-A, GG-O, MLG, CL-V, AP-R. Genomics analyses: IS-E. Information system design and elaboration: JV-M, PB-L. Risk models adaptation and statistical analysis plan: CFI, MM-A, MR. Initial draft of the manuscript: AP-R, MM-A, MR. Critical revision: CFI, IS-L, IC-E, GG-O, MH-A, CC, CL-V. All authors critically revised the manuscript for intellectual content and approved the final version.
Funding: This study was supported by the research grant 'Personalized breast cancer screening: assessment of its feasibility and acceptability in the National Health System' (PI17/00834) from the Instituto de Salud Carlos III and cofunded by Fondo Europeo de Desarrollo Regional (FEDER) 'Una manera de hacer Europa'. Celmira Laza Vásquez received a grant from Santander Program scholarship 2020 as a predoctoral fellow at the University of Lleida. The non-profit organisation Oncolliga Lleida (grant number N/A) will fund part of the genomic analyses for breast cancer risk assessment.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
The DECIDO Group:
Pau Balaguer-Llaquet, Iván-David Benítez, Alexandra Bertran, Àngels Cardona, Misericòrdia Carles-Lavila, Cristina Cazorla-Sánchez, Núria Codern, Inés Cruz-Esteve, Carles Forné-Izquierdo, Maria José Hernández-Andreu, Edelmir Iglesias, Gisela Galindo-Ortego, Marta Hernández, Celmira Laza-Vásquez, Montserrat Llorens-Gabandé, Montserrat Martínez-Alonso, Maria José Pérez-Lacasta, Hèctor Perpiñán, Anna Pons-Rodríguez, Montserrat Rué, Isabel Sánchez-López, Jordi Vilaplana-Mayoral, and Mercè Reñé-Reñé
Ethics statements
Patient consent for publication
Not required.
References
- 1.European Commission . European Commission 2015/C421/01 – Non-opposition to a notified concentration. Official Journal of the European Union 2015. [Google Scholar]
- 2.Chowdhury S, Dent T, Pashayan N, et al. Incorporating genomics into breast and prostate cancer screening: assessing the implications. Genet Med 2013;15:423–32. 10.1038/gim.2012.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilaprinyo E, Forné C, Carles M, et al. Cost-Effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PLoS One 2014;9:e86858. 10.1371/journal.pone.0086858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 2008;148:337–47. 10.7326/0003-4819-148-5-200803040-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tice JA, Miglioretti DL, Li C-S, et al. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol 2015;33:3137–43. 10.1200/JCO.2015.60.8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shieh Y, Eklund M, Madlensky L, et al. Breast cancer screening in the precision medicine era: risk-based screening in a population-based trial. J Natl Cancer Inst 2017;109:djw290. 10.1093/jnci/djw290 [DOI] [PubMed] [Google Scholar]
- 7.Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551:92–4. 10.1038/nature24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavaddat N, Pharoah PDP, Michailidou K, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 2015;107:djv036. 10.1093/jnci/djv036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 2015;107:1–4. 10.1093/jnci/dju397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vachon CM, Scott CG, Tamimi RM, et al. Joint association of mammographic density adjusted for age and body mass index and polygenic risk score with breast cancer risk. Breast Cancer Res 2019;21:1–10. 10.1186/s13058-019-1138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanes T, Young M-A, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 2020;22:1–10. 10.1186/s13058-020-01260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shieh Y, Hu D, Ma L, et al. Joint relative risks for estrogen receptor-positive breast cancer from a clinical model, polygenic risk score, and sex hormones. Breast Cancer Res Treat 2017;166:603–12. 10.1007/s10549-017-4430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Román M, Sala M, Domingo L, et al. Personalized breast cancer screening strategies: a systematic review and quality assessment. PLoS One 2019;14:e0226352. 10.1371/journal.pone.0226352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DGR, Donnelly LS, Harkness EF, et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br J Cancer 2016;114:1045–52. 10.1038/bjc.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004;23:1111–30. 10.1002/sim.1668 [DOI] [PubMed] [Google Scholar]
- 16.Evans DGR, Harkness EF, Brentnall AR, et al. Breast cancer pathology and stage are better predicted by risk stratification models that include mammographic density and common genetic variants. Breast Cancer Res Treat 2019;176:141–8. 10.1007/s10549-019-05210-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French DP, Astley S, Brentnall AR, et al. What are the benefits and harms of risk stratified screening as part of the NHS breast screening programme? study protocol for a multi-site non-randomised comparison of BC-predict versus usual screening (NCT04359420). BMC Cancer 2020;20:1–14. 10.1186/s12885-020-07054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Lacasta MJ, Sala M, Perestelo-Pérez L, et al. Effect of information about the benefits and harms of mammography on women ’ s decision making : The InforMa randomised controlled trial. PLoS One 2019:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puig-Vives M, Pollan M, Rue M, et al. Rapid increase in incidence of breast ductal carcinoma in situ in Girona, Spain 1983-2007. Breast 2012;21:646–51. 10.1016/j.breast.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Alonso M, Vilaprinyo E, Marcos-Gragera R, et al. Breast cancer incidence and overdiagnosis in Catalonia (Spain). Breast Cancer Res 2010;12:R58. 10.1186/bcr2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainey L, Jervaeus A, Donnelly LS, et al. Women’s perceptions of personalized risk‐based breast cancer screening and prevention: An international focus group study. Psychooncology 2019;28:1056–62. 10.1002/pon.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koitsalu M, Sprangers MAG, Eklund M, et al. Public interest in and acceptability of the prospect of risk-stratified screening for breast and prostate cancer. Acta Oncol 2016;55:45–51. 10.3109/0284186X.2015.1043024 [DOI] [PubMed] [Google Scholar]
- 23.Rainey L, van der Waal D, Donnelly LS, et al. Women's decision-making regarding risk-stratified breast cancer screening and prevention from the perspective of international healthcare professionals. PLoS One 2018;13:e0197772. 10.1371/journal.pone.0197772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puzhko S, Gagnon J, Simard J, et al. Health professionals’ perspectives on breast cancer risk stratification: understanding evaluation of risk versus screening for disease. Public Health Rev 2019;40:1–19. 10.1186/s40985-019-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esquivel-Sada D, Lévesque E, Hagan J, et al. Envisioning implementation of a personalized approach in breast cancer screening programs: stakeholder perspectives. Healthc Policy 2019;15:39–54. 10.12927/hcpol.2019.26072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann TC, Del Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2015;175:274. 10.1001/jamainternmed.2014.6016 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann TC, Del Mar C. Clinicians' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2017;177:407–19. 10.1001/jamainternmed.2016.8254 [DOI] [PubMed] [Google Scholar]
- 28.Toledo-Chávarri A, Rué M, Codern-Bové N, et al. A qualitative study on a decision aid for breast cancer screening: views from women and health professionals. Eur J Cancer Care 2017;26:e12660–11. 10.1111/ecc.12660 [DOI] [PubMed] [Google Scholar]
- 29.American College of Radiology . Breast imaging reporting and data system (BI-RADS. 5th ed, 2013. [Google Scholar]
- 30.Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med 2018;168:757–65. 10.7326/M17-3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conant EF, Sprague BL, Kontos D. Beyond BI-RADS density: a call for quantification in the breast imaging clinic. Radiology 2018;286:401–4. 10.1148/radiol.2017170644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer S, Gut IG. Genotyping single-nucleotide polymorphisms by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002;782:73–87. 10.1016/S1570-0232(02)00692-X [DOI] [PubMed] [Google Scholar]
- 33.Shieh Y, Hu D, Ma L, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 2016;159:513–25. 10.1007/s10549-016-3953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879–86. 10.1093/jnci/81.24.1879 [DOI] [PubMed] [Google Scholar]
- 35.Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet 2015;385:1642–52. 10.1016/S0140-6736(15)60123-4 [DOI] [PubMed] [Google Scholar]
- 36.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:1–13. 10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carles M, Martínez-Alonso M, Pons A, et al. The effect of information about the benefits and harms of mammography on women’s decision-making: study protocol for a randomized controlled trial. Trials 2017;18:1–8. 10.1186/s13063-017-2161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor AM. Decisional conflict Scale—user manual 1993. decision aid evaluation measures, 2010. Available: http://decisionaid.ohri.ca/eval_dcs.html;
- 39.O’Connor AM. Decision self-efficacy Scale—user manual 1995. decision aid evaluation measures, 2002. Available: http://decisionaid.ohri.ca/eval_self.html;
- 40.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait anxiety inventory (STAI). Br J Clin Psychol 1992;31:301–6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 41.R Core Team . R: a language and environment for statistical computing, 2020. Available: https://www.R-project.org/
- 42.RStudio Team . RStudio: integrated development environment for R, 2020. Available: http://www.rstudio.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.