Abstract
Dactylorhiza hatagirea (Orchidaceae) is a perennial herb inhabiting sub-alpine to alpine regions, ranging at elevations between 2500 and 5000 m.a.s.l. With palmately lobed rhizome and lanceolate leaves having a sheathing leaf base, it bears pink flowers with purple-colored notches and a curved spur. It finds wide use in ayurveda, siddha, unani, and folk medicine in curing disorders of the circulatory, respiratory, nervous, digestive, skeletal, and reproductive systems, besides boosting the immune system to fight infectious diseases. Secondary metabolites such as dactylorhins A–E, dactyloses A–B, and others exhibit a wide spectrum of pharmacological activities (antioxidant, antimicrobial, antiseptic, anticancer, and immune enhancing activities). Its use as a dietary supplement was found to be beneficial in increasing testosterone levels, resulting in improved sexual desire and arousal. Incessant overexploitation of this medicinally important herb has resulted in the dwindling of its populations in the wild, which has resulted in its classification as a critically endangered plant species. Efforts involving mass reproduction through in vitro (through tissue culture) and in vivo (by vegetative propagation) means are currently being made to maintain the germplasm of this critically endangered orchid. Holding immense significance in clinical research and drug discovery, work on the genomic front (transcriptomics) has recently been carried out to discover the wealth of unexplored genetic information for this perennial herb. The present study is aimed at reviewing different aspects of the orchid to present collective (summarized) information on this medicinally important herb in the present, particularly its botany, ethnobotanical uses, phytochemistry, and pharmacognosy, along with the strategies that need to be adopted to prevent its overexploitation in natural habitats.
Keywords: antibiotic resistance, Dactylorhiza hatagirea, germplasm conservation, natural compounds, overexploitation
1. Introduction
The Himalayas, extending into the Indian sub-continent, are considered to be a hotspot of biodiversity. Harboring 17,500 species of plants, modern and traditional medical practices (ayurveda, unani, and siddha) make use of almost 6000 of them [1,2]. They represent a remarkable contribution to the pharmaceutical field. Their trade in India is projected to be an estimated USD 1 billion per year [3,4]. Contributing indispensable raw material (flavonoids, alkaloids, saponins, etc.) for use in the formulation of different drugs, the demand for plants with ethnomedicinal importance has lately shown a surge, with wider employment in pharmaceutical practices. Aimed at maintaining individuals’ health, plant-derived substances (PDSs) such as taxanes, taxol, and cepholomannine from Taxus brevifolia Nutt. [5], diphyllin from Diphylleia grayi F. Schmidt [6], jatrophane from Euphorbia semiperfoliata Viv. [7], thymo-quinone and dithymoquinone from Nigella sativa l. [8] vinblastine from Catharanthus roseus (L.) G. Don, and others play a pivotal role in curing different diseases. The use of PDSs in disorders such as memory loss, osteoporosis, and age-related problems along with their function in boosting the immune system has broadened their potential in terms of use in modern healthcare systems [9].
As a preferable alternative to semi-synthetic drugs, natural products of plant origin are promoted as potential options for safer medicines and other life-saving drugs. The pharmaceutical use of medicinal plants with negligible side effects presents the possibility of their widespread application in mitigating the large fatalities associated with different deadly diseases [10]. Presently, plant species such as Dactylorhiza hatagirea provide an exemplary model for studying orchids as part of their ethnopharmacological properties and therapeutic applications. Owing to its immense significance in clinical research and drug discovery, the plant (tubers, leaves) has been used to study its potential to induce anti-inflammatory [11], anti-pyretic [12], anti-cancerous [13], neuropharmacological [14], and other effects. In the context of global health concerns, this medicinally important herb has broadly been used in the evaluation of its ethnopharmacological applications towards validating its efficacy and efficiency for use in combatting different diseases. The present study puts forth a summary of information on the botany, taxonomy, chemistry, and ethnopharmacology of D. hatagirea. As overexploitation of the plant for use in pharmaceutics has drastically reduced its populations and has brought it under threat, a section of the manuscript is dedicated to approaches adopted for its conservation and long-term survival in its natural habitat.
Methodology
The article covers the literature available from 1984 to 2020. The information was located, selected, and extracted from scientific journals, books, thesis, and reports via library and electronic search (PubMed and other search engines). Documentation of the available information from the literature helped in drafting different sections of the manuscript, such as morphology, taxonomy, and others, along with ethnopharmacological uses that depict its importance in the present day.
2. Orchids in Medicine: Special Reference to D. hatagirea
Orchidaceae is a family of angiosperms with around 25,000–35,000 species and 800 genera [15]. Orchids received their recognition through herbal writings from Japan and China [16]. The Chinese were the first to cultivate and describe their use in the healthcare system [17]. Orchids such as Anoectochilus formosanus Hayata from Taiwan [18], Bletilla formosana (Hayata) Schltr. from China [19], Bulbophyllum kwangtungense Schltr. from Japan [20], Bulbophyllum odoratissimum (J.E. Sm.) Lindl. from Thailand [21], Calanthe discolour Lindl. from Korea and Malaysia [22], Catasetum barbatum Lindl. from Guianas, Japan and Paraguay [23], Coeloglossum viride (L.) Hartm. from Tibet [24], Cypripedium macranthos Sw. from Mexico, Guatemala, and Colombia [25], Dendrobrium sp. from China, Japan, Taiwan, and Australia [26], Listera ovata (L.) R. Br. from Spain [27], Maxillaria densa Lindl. from Mexico [28], Nidema boothii (Lindl.) Schltr. from Malaysia [29], Spiranthes australis (R. Br.) Lindl. from Trinidad and Tobago, and Vanda tessellate (Roxb.) ex G. Don from India, Sri Lanka, and Burma [30] have made a significant impact, as their derivates (crude extracts as well as secondary metabolites) are used to treat various diseases.
Orchids are recognized throughout the world for the production of compounds used in the treatment of different diseases. In India, 1141 species belonging to 166 genera of orchids have been reported [31]. Here, the use of orchids in medicinal practices dates back to Vedic times. In addition, some members of Orchidaceae family are used to treat nerve disorders, fever, bone fractures, general weakness, tuberculosis, and other dermal problems [32,33]. D. hatagirea finds its use in a wide array of medicinal practices [34]. As per the published records, D. hatagirea is used for the treatment of amala pitta (gastritis), madhya bhangaasthi (bone fracture), jvara (fever), vajikarana (erectile dysfunction), haima (cold), bhishajyati (wound healing), and ayurdamah (nerve tonic). With the growing research in the biopharmaceutical and drug industry, extraction of the secondary metabolites and antioxidants from this plant has increased its demand [35].
3. Morphological and Anatomical Features of D. hatagirea
D. hatagirea, an important medicinal herb belonging to the Orchidaceae family, is commonly known as Himalayan marsh orchid [36]. It is a perennial herb confined to alpine regions at an elevation of 2500–5000 m.a.s.l. [37,38]. D. hatagirea bears slightly flattened, 3–7-fingered, palmately lobed, creamish-colored, tuberous roots measuring 5–12 ± 3.3 cm in length. The peduncle is generally 27–41 ± 6.7 cm tall. Leaves are acuminate or apex obtuse, linear-lanceolate to oblong clustered and sub-opposite towards base. Flowers grow in dense spike inflorescences and are zygomorphic, having fused male and female reproductive organs (a condition referred to as gynostemium, column, or gynostegium) (Figure 1). Flowers are pink, bearing purple-colored notches with six free perianth segments. The innermost segment forms an enlarged, pink-colored and sculptured labellum, which acts a resting pad for the pollinators, while the rest of the segments are similar in shape and form. Flowers show resupination at 180°, which brings the labellum perpendicular to the ovary. Flowers bear two dark green-colored pollinaria covered inside the anther cap. Each pollinarium bears a central sterile axis which combines with the pale yellow-colored caudicle (stalk). Caudicles at the upper side are separated by rostellum; however, at their base, they are joined by a sticky structure known as retinacula or viscidia. The ovary is tricarpellate, inferior, twisted and consists of one chamber with parietal placentation, having a large mass of ovules. Fruits are loculicidal capsules and minute seeds are generally liberated as immature embryos at the globular stage.
Figure 1.
Progressive developmental stages of D. hatagirea: (A) inflorescence showing acropetal arrangement of flowers, (B) floral structure, (C) pollinarium bearing large number of pollen grain tetrads (captured on Nikon Eclipse 80 Fluorescence microscope at 10× magnification), (D) vertical section of trilocular ovary (captured on Nikon-C-FLED2 Stereo zoom at 10 × 1.7× magnification), (E) ovules (captured on Nikon-C-FLED2 Stereo zoom at 10 × 3.9× magnification), (F) fruit formation, (G) immature seeds (captured on Nikon-C-FLED2 Stereo zoom at 10 × 2.8× magnification), (H) tubers.
4. Distribution, Trade, and Consumption
D. hatagirea is regarded as an Asian species of the genus Dactylorhiza [39]. It is distributed across India, China, Pakistan, Iran, Afghanistan, Tibet, Bhutan, Europe, North Africa, Temperate Asia, Mongolia, and Nepal [37,40,41]. In India, the plant is found in Jammu and Kashmir, including Ladakh, Uttarakhand, Himachal Pradesh, Arunachal Pradesh, and Sikkim [42,43,44,45,46]. Over the years, the market value of crude drugs obtained from the plant has shown an increasing trend which has led to the expansion of its market across different Indian states [13,47,48,49]. The huge demand in the pharmaceutical sector has driven a flourishing trade of around USD 71,583 [50]. Kala [51] reported that the annual demand for D. hatagirea is approximately 5000 tons, due its use in both traditional as well as modern medicine. Around ~7.38 tons of salep (processed tubers) obtained from D. hatageria are consumed annually to cure different ailments [41]. Overexploitation of the plant has drastically reduced its populations and has brought it under threat. Based on the number of existing populations and the area under its cover, Conservation Assessment and Management Plan (CAMP), Convention on International Trade in Endangered Species of wild flora and fauna (CITES), and International Union for Conservation of Nature (IUCN) have placed this plant under different categories of threat (Table 1).
Table 1.
Status of D. hatagirea as for different categories of threat.
| Status | Category | Reference(s) |
|---|---|---|
| Endangered | CAMP and CITES | [52] |
| Critically Endangered | CAMP and CITES | [53,54] |
| Vulnerable | CAMP | [53] |
| Listed among Appendix II | CITES | [55] |
| Critically rare | IUCN | [53,55] |
| Threatened | IUCN | [53] |
| NPSMHCC | MFSC, Kathmandu | [56] |
Abbreviations: CAMP: Conservation Assessment and Management Plan, CITES: Convention on International Trade in Endangered Species of wild flora and fauna, IUCN: International Union for Conservation of Nature, NPSMHCC: National Priority Species of Medicinal herbs for Cultivation and Conservation, MFSC: Ministry of Forest and Soil Conservation.
5. Ethnopharmacological Importance of D. hatagirea
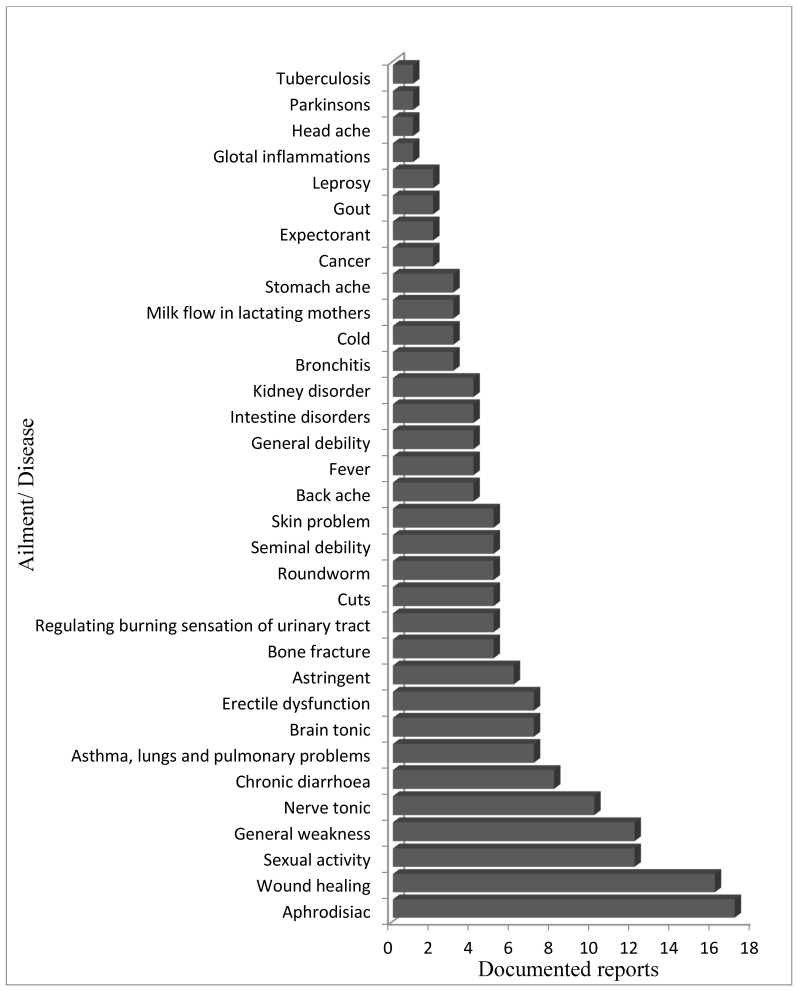
Salep obtained from tubers (77%) and leaves (23%) is used in curing ailments like dysentery, chronic diarrhoea, etc. [57], besides being used as a nerve tonic, emollient, demulcent, astringent, and aphrodisiac [58]. It is also useful in treating general debility, emaciation, seminal weakness, neurasthenia, and cerebropathy [59] (Figure 2). A decoction of the tubers is helpful to relieve colic pain and fever, besides for speckling over cuts, burns, and wounds to stop bleeding. Testosterone levels were found increased in adult male rats after giving lyophilized extract of the plant, which increased their sexual behavior. [35,60]. Thakur and Dixit [35] reported that processed tubers of D. hatagirea significantly increase the functioning of sex organs by increasing the genesis of steroids (testosterone) hormones. Table 2 shows comprehensive information of the pharmacological uses of D. hatagirea.
Figure 2.
Use of D. hatagirea against different ailments as per available literature from 1964 to 2020.
Table 2.
Traditional uses, area, and mode of application of D. hatagirea.
| S. No | Ailment/Use | Plant Part | Place/Country | Mode of Application | References |
|---|---|---|---|---|---|
| 1 | Respiratory (asthma, bronchitis, lungs, and other pulmonary problems) | leaves and tubers | India (Ladakh, Gharwal Himalaya) Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
|
[35,61,62,63,64,65,66] |
| 2 | Neurological (brain tonic, nerve tonic) | leaves and tubers | India (Gharwal Himalaya), Nepal |
|
[35,63,65,67] |
| 3 | Digestive (stomachache, chronic diarrhea, intestinal disorders) | tubers | India (Gharwal Himalaya, Arunachal Pradesh), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
|
[35,37,58,65,67,68] |
| 4 | Urinary (kidney disorders, burning sensation, and urine discharge) | tubers | India (Gharwal Himalaya) |
|
[53,61,69] |
| 5 | Sexual (sexual activity, seminal debility, erectile dysfunction) | tubers | India (Gharwal Himalaya), Pakistan (Gilgit), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
|
[70,71] |
| 6 | External uses (headache, wound healing, skin problems) | tubers | India (Gharwal Himalaya, Kuman Himalayas, Arunachal Pradesh), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
|
[65,72,73,74,75] |
| 7 | Others (backache, bone fracture, fever, weakness, general debility, milk flow in lactating mothers) | tubers and leaves | India (Gharwal Himalaya, Western Himalaya, Manali), Pakistan (Gilgit and Bugrot valley), Nepal (Rasuwa district) |
|
[13,54,57,58,62,65,66,76,77,78,79,80] |
6. Bioactive Compounds of D. hatagirea
Extract of tubers yields albumin, butanedioic acid, hydroquinone, lesoglossin, militarrin, pyranoside, pyrocatechol, and volatile oil. The antioxidant property of compounds obtained from the plant finds its use in the treatment of different human diseases. Indole alkaloids, phenolics (stilbene, e.g., resveratrol), and saponins along with ascorbic acid, phyllo- and naphthloquinones, glucomannan, and carotenoids [50] form the active constituents of D. hatagirea. Dactylorhins A-E (glycosidic compounds: dactylorhin A (C40H56O22), dactylorhin B (C40H57O23), dactylorhin C (C14H24O10), dactylorhin D (C27H40O17), dactylorhin E (C27H40O17)) and dactyloses A-B (glycosidic compounds: dactylose A, dactylose B, C12H16O6) obtained from D. hatagirea exhibit a wide range of pharmacological activities [80] (Table 3).
Table 3.
Structural aspects of secondary metabolites extracted from D. hatagirea.
| Name | Synonym | Structure | References |
|---|---|---|---|
| 1-deoxy-1-4 hydroxyphenyl-L-sorbose | Dactylose A |
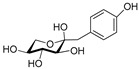
|
[80] |
| 1-deoxy-1-4 hydroxyphenyl-L-tagatose | Dactylose B |
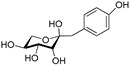
|
[80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid bis (4- β-D-glucopuranosyloxybenzyl) ester | Dactylorhin A |
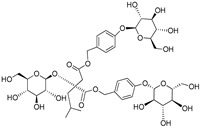
|
[80] |
| (2R-3S)-2- β-D-glucopyranosyloxy-3-hydroxy-2(2-methylpropyl) butanedioic acid bis (4 β-D—glucopyranosyloxybenzyl) ester | Dactylorhin B |
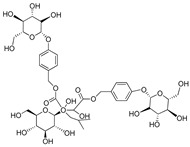
|
[80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid | Dactylorhin C |
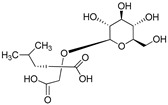
|
[80] |
| (2R-3S)-2- β-D-glucopyranosyloxy-3-hydroxy-2(2-methylpropyl) butanedioic acid 1-(4- β-D- glucopyranosyloxybenzyl) ester | Dactylorhin D |
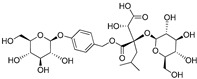
|
[80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid 1-(4- β-D-glucopuranosyloxybenzyl) ester | Dactylorhin E |
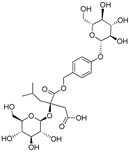
|
[80] |
| (E)-5-(4-hydroxystyryl) benzene-1,3-diol | Resveratrol |
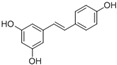
|
[50] |
| 1H- Indole | Indole alkaloids |
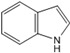
|
[50] |
| Napthalane -1,4- dione | Napthoquinone |
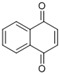
|
[50] |
| (R)- 5- ((S)- 1,2- dihydroxyethyl)- 3,4 dihydroxy furan-2 (5H)-one | Ascorbic acid |
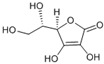
|
[50] |
| 2- methyl- 3 ((7R, 11R,E), 3,7,11,15- tetra methylhexadec 2- en-1-yl) naphthalene-1,4-dione | Phylloquinone |
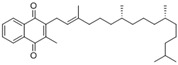
|
[50] |
| Militarrin |
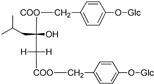
|
[80] | |
| Lesoglossin |
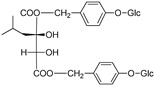
|
[80] | |
| Pyrocatechol |
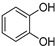
|
[80] | |
| Glucomannan |
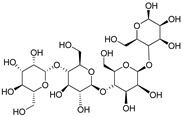
|
[50] | |
| Saponin |
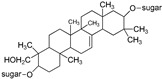
|
[50] |
7. Pharmacological Importance of D. hatagirea
D. hatagirea is a high-value orchid with a broad range of phytochemicals which exert a wide range of beneficial effects. Information on the critical medicinal benefits of D. hatagirea use is listed below.
7.1. Antibacterial Activity
The root and shoot extracts are effective in treating a broad range of diseases caused by Gram-positive and Gram-negative bacteria [81]. Salep was found to be highly effective against Escherichia coli and Shigella flexinerai, which often show resistance to synthetic medicines [82]. Different extracts of D. hatagirea prepared with petrol, ether, chloroform, methanol, and water were tested against five different bacteria for the determination of zone of inhibition (ZOI) and minimum inhibitory concentration (MIC) [83]. Petroleum ether extract and chloroform extract of the aerial part and methanolic extract of the rhizome of D. hatagirea showed significant activity against Escherichia coli. The chloroform extract showed the best inhibitory action against E. coli, while these extracts show similar effects for Shigella flexinerai. The results indicate that the rhizome part is more effective than the aerial parts against all tested organisms. Besides this, D. hatagirea extract exhibits greater ZOI than ciprofloxacin against S. aureus. D. hatagirea exerts equal effect to that of norfloxacin for S. aureus. For E. coli, the ZOI of the aerial part of D. hatagirea was found to be almost equal to ciprofloxacin. For S. flexinerai, P. aeruginosa, and B. subtilis, the ZOI is equal for tuber extract and ciprofloxacin [81]. The antibacterial activity of root and shoot ethanol and methanolic extracts of D. hatagirea against B. subtilis, S. aureus, E. coli, and P. aeruginosa showed MIC at lower values [84]. Its ability to exert an effect that is comparable to the available regimes of medicines reflects its potential for use as effective antibacterial agent.
Bacterial drug resistance could be overshadowed by resistance provided by efflux pump inhibitors [85]. The efflux pumps are specific and possibly remove either a single class or several classes of antimicrobial compounds [86]. Pumps from the major facilitator superfamily (MFS) of Gram-positive bacteria play a key role in the efflux [87]. Plants being important sources of phytoconstituents raises the prospects of acting as a source of novel chemotherapeutic compounds, especially efflux pump inhibitors. Aqueous ethyl acetate and methanolic extracts of D. hatagirea exhibited efflux pump inhibitory activity against S. aureus strains. Efflux of EtBr and uptake of berberine results in a synergistic effect of each extract with ciprofloxacin and norfloxacin [88]. The use of plant extracts to inhibit multiple-resistance bacteria (MDR) [89] and prevent oral bacterial growth of Cymbopogon [90,91] and respiratory tract (RTIs) [92,93], urinary tract [94,95], cutaneous [96], and digestive infections [97] has been studied extensively. The results clearly show the applications of plant-based products as a substitute for antibiotics in overcoming various bacteria-related complications. The capability of a plant to exert a significant effect on efflux pumps expands the potential for its use as a potent efflux pump inhibitor. Besides this, its effectiveness against MDR bacterial isolates open avenues for its use in the design of drugs that can be employed to overcome the problem of drug resistance.
7.2. Anti-Inflamatory Activity
Alkaloids, flavonoids, glycosides, steroids, diterpenes, saponins, and tannins present in the tubers of D. hatagirea show potent anti-inflammatory activity. The activity was assessed in a rat paw oedema model induced by carrageenan and a cotton pellet granuloma model for acute/chronic inflammation. Adult Wistar rats of either sex responded better to hydroalcoholic extracts of D. hatagirea as compared to standard (aspirin 100 mg/kg and Indomethacin 10mg/kg). The extract showed significant anti-inflammatory effects in both acute and chronic inflammatory conditions [98]. Hydroethanolic extract of D. hatagirea tubers showed dose-dependent anti-inflammatory responses in the carrageenan-induced oedema among Wistar rats [11]. Their anti-inflammatory activity needs further exploration in terms of understanding the mode of operation for its utilization in the design of potent anti-inflammatory drugs for use in clinics.
7.3. Neuropharmacological Activity
Soporific drugs, commonly known as sleeping pills or hypnotic drugs, are a class of psychoactive drugs whose primary function is to induce sleep for the treatment of insomnia (sleeplessness) or as surgical anesthesia. The average percentage yield of hydroalcoholic extract of D. hatagirea revealed that its extract was safe at all doses when administered orally to mice, with no mortality. Besides this, the dose-dependency prolonged the duration of sleeping time among the tested animals compared to normal [14]. However, to gain in-depth insights into the mechanisms involved, further studies on animal models are needed before being employed as effective molecules in the treatment of neurological problems in humans.
7.4. Anti-Cancerous Activity
The extract obtained from D. hatagirea shows a considerable effect on cancerous cell lines. The population of Michigan Cancer Foundation-7 (MCF-7) and breast cancer (MDA-MB-231) cell lines grown in Dulbecco’s modified Eagle medium (DMEM) and 1% antibiotics with fetal bovine serum (FBS), respectively, showed a considerable decrease in their population [13]. However, human embryonic kidney (HEK-293) cell lines (normal cell line) grown in Leibovitz (L-15) medium show negligible effect. Root extracts of D. hatagirea show higher anti-cancerous potential than shoot extracts [13]. MDA-MB-231 cells treated with 1000 µg/mL tuber extract and shoot extract showed 82.38% and 83.81% viable cells, respectively [13]. Similarly, in the case of the MCF-7 cell line, 84.24% viability was found in the cells which were treated with 1000 µg/mL of tuber extract as compared to 87.09% of viable cells obtained from shoot extract treatment [13]. However, further study needs to be conducted using different animal models, under stress conditions, to understand its effects on different signaling pathways. Factors underlying the occurrence of the diseases should be explored in order to elucidate the mechanisms for devising effective treatment regimes.
7.5. Anti-Diabetic Activity
Antihyperglycemic agents extracted from the leaves and tubers of orchids make the plant ideal for compounds with anti-diabetic properties [99]. Anti-diabetic properties of D. hatagirea methanolic leaf extract using 3T3-L1 cell line showed no cytotoxic effect. The extract exhibited anti-diabetic properties, manifested by the inhibitory effect on α-amylase and α-glucosidase enzymes, enhancing the cellular uptake of glucose by inducing the expression of glucose transporter type 4 (GLUT4) on the cell surface. Inhibition of α-amylase activity occurs at 31.25 µg/mL and 74.53 ± 0.5% inhibition at 500 µg/mL concentration with respect to the standard drug acarbose, which shows inhibition between 31.25 µg/mL and 85.27 ± 1.2%. Inhibition of α-glucosidase activity occurs between 30.16 ± 0.16% and 72.13 ± 0.78% with respect to standard drug acarbose, the positive control drug, which showed inhibition of 43.20 ± 0.09% and 94.41 ± 0.49% at 500 µg/mL. An analysis of the expression of GLUT4 using anti-mouse Glut4-FITC antibody (#NBPI-49533F, Novus Biologicals) with metformin (control) revealed that D. hatagirea extract-treated cells showed elevated levels of GLUT4 as compared to untreated cells [100]. Root extract shows a dose-dependent decrease in the blood sugar, total cholesterol, and total triglycerides and an increase in the total protein content [101].
Solvent-based extraction (chloroform, methanol, water, petroleum ether, ethanol) and photochemical screening of the secondary metabolites from D. hatagirea reveal antimicrobial and other lifesaving pharmaceutical applications. Effects of azithromycin and amikacin-resistant bacteria (E. coli) are highly neutralized by root and shoot extracts of D. hatagirea [81,84]. Root extracts show significantly greater potential than glibenclamide against increased levels of certain blood ingredients in Wistar rats [101]. Detailed applications of different extracts of D. hatagirea are shown in Table 4.
Table 4.
Different activities observed for root and shoot-derived extracts of Dactylorhiza hatagirea.
| Plant Extract/Antibiotics | Resistance Against | Dosage | Effect | Reference |
|---|---|---|---|---|
| Antibacterial | ||||
| Shoot extract | SA, EC, SF, PA, BS | 500 mg/mL | Best inhibition for EC, better for SA, and good for SF, SA, PA, and BS | [81] |
| Root extract | 500 mg/mL | Best inhibition for SF, better for SA, EC, BS, and good for PA | [81,84] | |
| Antioxidant | ||||
| Root extract | 3 μg/mL | Best antioxidant activity | [84] | |
| Plant extract | FRAP | 3% | Antioxidant activity | [102] |
| Root extract | NO, H2O2 | NA | Better antioxidant activity | [103] |
| Anti-inflammatory | ||||
| Root extract | Carrageenan-induced paw oedema | 100, 200, 300 mg/kg | Shows decrease in the volume of paw with increase in dosage | [11,98] |
| Root extract | Cotton pellet granuloma | 100, 200, 300 mg/kg | Reduced granuloma formation with increase in dosage | [98] |
| Neuropharmacological | ||||
| Root extract | Hypnosis | 100, 200, 300 mg/kg | Shows prolonged hypnosis with increase in dosage | [14] |
| Anti-pyretic | ||||
| Root extract | Brewer’s yeast induced pyrexia | 100, 200, 300 mg/kg | The influence of pathogenic fever was decreased with dose-dependent concentrations | [12] |
| Anti-diabetic Blood biochemical parameters and 3T3-L1 diabetic cell line | ||||
| Root extract | Blood glucose | 100, 200 mg/kg | Shows dose-dependent decrease in blood glucose with increase in time | [101] |
| Root extract | TC, TG and TP | 100, 200 mg/kg | TC and TG show dose-dependent decrease while TP shows increase with increase in dosage concentration | |
| Leaf extract | α amylase activity | 31.25 and 500 μg/mL | α amylase activity decreased with increased dosage | [100] |
| Leaf extract | 3T3-L1 diabetic cell line | 25–400 μg/mL | 3T3-L1 cell line viability decreased with increase in dosage | |
| Leaf extract | GLUT 4 expression and NBDG uptake | 100 μg/mL | Increased expression | |
| Anti-cancerous potential | ||||
| Shoot extract | HEK- 239, MDA, MB- 231, MCF 7 cell lines | 250–1000 μg/mL | Cell viability decreases with increased dosage | [13] |
| Root extract | 250–1000 μg/mL | Cell viability decreases with increased dosage | ||
Abbreviations: SA, Staphylococcus aureus; EC, Escherichia coli; SF, Shigella flexneria; PA, Pseudomonas aeruginosa; BS, Bacillus subtilis; TC, total cholesterol; TG, total triglycerides; TP, total proteins; GLUT4, glucose transporter type 4; NBDG, 2-(N-(7-nitroben-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose; HEK, human embryonic kidney; MDA-MD, breast cancer cell line; MCF-7, Michigan Cancer Foundation; FRAP, ferric reducing antioxidant power; NO, nitric oxide; H2O2, hydrogen peroxide.
7.6. Other Applications
The tubers of D. hatagirea show wide utilization in silk industries for sizing material [102]. The plants are grown in gardens for decorative purposes. The aesthetically appealing appearance of the flowers makes them suitable for ornamental purposes (placed in flower vases, twisted within hair ponies, making bracelets and necklaces). Grounded stem and leaves are used as insect repellant. Leaves and stem of the plant are used as fodder for livestock. D. hatagirea helps in improving the flavor and taste, color and appearance, body and texture, and melting quality of frozen milk products. Moreover, young leaves and shoots are also used as vegetables [103]. Extract of the flowers is used in perfume industries to increase fragrance. Tubers of the plants are used for witchcraft [77].
8. Conservation Approaches
The medicinal property of D. hatagirea plant has led to exploitation of its populations in nature [104]. Owing to the dwindling populations and inability of the seeds to germinate without mycorrhizal association [105], its efficient conservation is a challenging task to accomplish. However, the successful introduction of the plant by symbiotic seed germination under in vitro conditions was achieved by Aggarwal and Zettler [106]. Novotna et al. [107] established seedling development of D. hatagirea using 10 g dm-3 sucrose and glucose treatment. Further, treatment with N6-benzyladenine or cytokinins N6-(2-isopentenyl) adenine and their amalgamation amid auxin (IBA) helped in significant development of shoots. Several researchers carried out in vitro culturing of immature seeds for large-scale production of D. hatagirea [13,105]. The protocorms so formed were then cultured on rejuvenation medium with MS and BM-2 media enriched with various plant growth hormones (KN-3mg/l IBA-3 mg). For adaptation, seedlings were transferred to a blend of cocopeat, perlite, and vermiculite in the ratio of 1:1:1. The saplings formed were finally moved to a greenhouse. Plant multiplication and conservation of the plant by spliced tuber plantation were performed by Shrestha and Shrestha [108]. Green pod culture of D. hatagirea was performed by Giri and Tamta [109] in different growing media such as Vejsadova (VJ), Vacin and Went (VW), Murashige and Skoog (MS), and Knudson C (KC). Better results for seed germination were obtained in MS medium enriched with activated charcoal, morpholino ethane sulphonic acid, and peptone. Due to the threat of extinction, D. hatagirea is protected in different countries, including Luxembourg, Belgium, the United Kingdom, and Nepal [110]. As per the Forest Act 1993, Forest Regulation Act 1995, and its amendment in 2005, the collection and trade of tubers of D. hatagirea is banned in Nepal. However, collection must follow the regulations for the trade of D. hatagirea as per Forest Regulation 1995 and its amendment in 2005 [13].
9. Conclusions and Future Perspectives
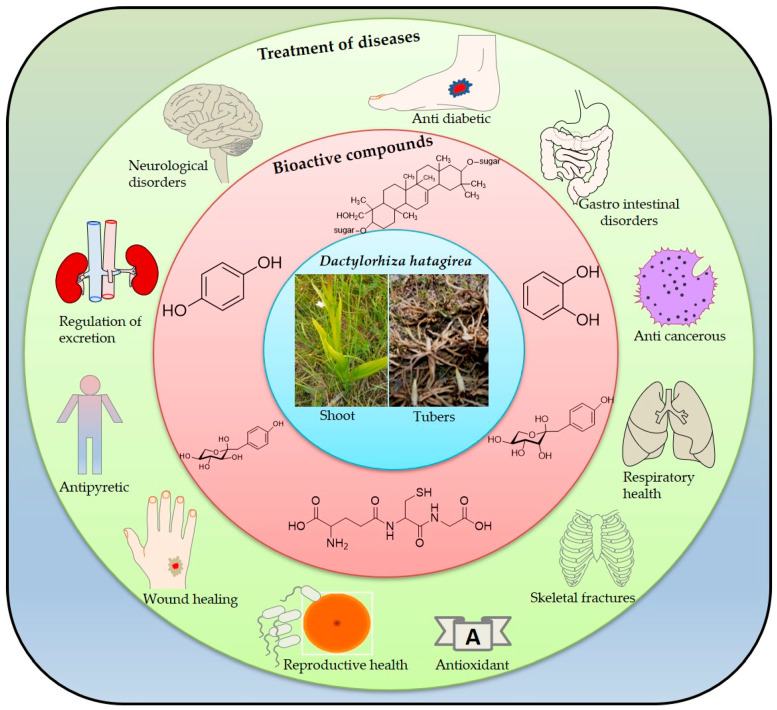
The ethnomedicinal properties of the plant reveal its widespread utilization in traditional medicine systems. The use of plants to cure ailments such as chronic diarrhea, fractured bones, seminal debility, erectile dysfunction, gout, Parkinson’s disease, tuberculosis, and stomachache is worth mentioning (Figure 3). Pharmacological studies on the secondary metabolites have confirmed its antibacterial, anti-cancerous, and testosterone-increasing potential. In the healthcare system, D. hatagirea acts as adjuvant therapy for the treatment of diseases and in maintaining good health. The current research should aim at analyzing the active constituents of the plant for their therapeutic potential by strengthening animal studies and performing clinical trials that could help in the subsequent formulation of the plant for use in modern medicine.
Figure 3.
Representation of bioactive compound-mediated pharmacological effects of D. hatagirea in humans. Note: The figure simply depicts the compounds obtained from D. hatagirea and the effects observed, without correlating them to each other in the figure.
Further studies are needed for exploring the potential of the plant for unknown bioactive constituents, studying the underlying mechanisms of bioactive components, and in performing analyses of their efficacy and possible use in assisting the exploration of new therapeutic molecules. Additionally, new possibilities also need to be explored for using the natural constituents of the plant in complementing standard medicines as a possible solution to diminish the occurrence of diseases and in combatting the infections caused by multidrug-resistant microorganisms. The natural remedies of D. hatagirea and its essential ingredients may play a vital role in the development of novel drugs for the treatment of different human diseases. There is no doubt that the extraction of secondary metabolites and their use in the drug industry is gaining pace; however, more research is needed on this plant to validate its effectiveness in humans. In addition, studies on frontier disciplines of modern aspects of biology such as genomics (transcriptomics, metabolomics, etc.) are needed in order to gain a detailed insight into this medicinally important plant. Considering the vulnerability of this orchid, effective measures through mass multiplication under in vitro and in vivo conditions should be carried out to maintain the germplasm of this critically endangered orchid above its threshold level. Additionally, the areas with natural populations of D. hatagirea should be recommended as medicinal plant conservation areas (MPCA).
Author Contributions
Conceptualization, S.V., A.T.J.; writing—original draft preparation, I.A.W., S.V., V.K.; writing—editing, I.A.W., S.V., A.T.J., I.A.R; supervision, S.V.; funding acquisition, I.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Biotechnology (DBT), India under grant number BT/Env/BC/01/2010 and Department of Science and Technology, India under Science and Engineering Research Board (DST-SERB) grant number CRG/2019/004106.
Conflicts of Interest
The authors declare that they do not have conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schipmann U., Leaman D.J., Cunningham A.B. Biodiversity and the Ecosystem Approach in Agriculture. FAO; Rome, Italy: 2002. Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues; pp. 1–21. [Google Scholar]
- 2.Prajapati N.D., Purohit S.S., Sharma A.K., Kumar T. A Handbook of Medicinal Plants. Agrobios Publisher; Jodhpur, India: 2003. [Google Scholar]
- 3.Malik A.R., Siddique M.A.A., Sofi P.A., Butola J.S. Ethnomedicnal practices and conservation status of medicinal plants of North Kashmir Himalayas. Res. J. Med. Plant. 2011;5:515–530. [Google Scholar]
- 4.Joshi K., Chavan P., Warude D. Molecular markers in herbal drug technology. Curr. Sci. 2009;87:159–165. [Google Scholar]
- 5.Prota A.E., Bargsten K., Zurwerra D., Field J.J., Díaz J.F., Altmann K.-H., Steinmetz M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587–590. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 6.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Chavan S.S., Damale M.G., Shamkuwar P.B., Pawar D. Traditional medicinal plants for anticancer activity. Int. J. Curr. Pharm. Res. 2013;5:4. [Google Scholar]
- 8.Govind P. Some important anticancer herbs: A review. Int. Res. J. Pharm. 2011;2:45–52. [Google Scholar]
- 9.Bashir A., Singh C., Chauhan N., Rani A. A review: Ethnobotanical studiy on medicinal plants of Kargil district, Ladakh, India. J. Emerg. Technol. Innov. Res. 2018;5:181–196. [Google Scholar]
- 10.Dar R.A., Shahnawaz M., Qazi P.H. General overview of medicinal plants: A review. J. Pharmacol. 2017;6:349–351. [Google Scholar]
- 11.Sharma S., Jain P.K., Parkhe G. Extraction, phytochemical screening and anti-inflammatory activity of hydro ethanolic extracts of roots of D. hatagirea. J. Drug. Discov. Ther. 2020;19:86–90. doi: 10.22270/jddt.v10i3-s.4092. [DOI] [Google Scholar]
- 12.Sirohi B., Sagar R. Antipyretic activity of hydroalcholic extract of D. hatagirea roots and Lavandula stoechas flowers on Brewers yeast induced Pyrexia in Wistar rats. J. Drug. Discov. Ther. 2019;9:701–704. [Google Scholar]
- 13.Popli D., Sood H. Optimization of Liquid Media for Increasing The Biomass of Dactylorhiza Hatagirea. JUIT; Waknaghat, India: 2016. [Google Scholar]
- 14.Sirohi B., Sagar R. Effect of Hydroalcoholic Extract of D. hatagirea Roots & Lavandula Stoechas Flower on Thiopental Sodium Induced Hypnosis in Mice. J. Drug Deliv. Ther. 2019;9:414–417. [Google Scholar]
- 15.Hossain M.M. Therapeutic orchids: Traditional uses and recent advances-An overview. Fitoterapia. 2011;82:102–140. doi: 10.1016/j.fitote.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Reinikka M.A. A History of the Orchid. Volume 23 Portland Timber Press; Portland, OR, USA: 1995. [Google Scholar]
- 17.Jalal J.S., Kumar P., Pangtey Y. Ethnomedicinal Orchids of Uttarakhand, Western Himalaya. Ethnobot. Leafl. 2008;12:1–5. [Google Scholar]
- 18.Satish M.N., Abhay P.S., Chen-Yue L., Chao-Lin K., Hsin-Sheng T. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003;44:79–98. [Google Scholar]
- 19.Lin Y.L., Chen W.P., Macabalang A.D. Dihydrophenanthrenes from Bletilla formosana. Chem. Pharm. Bull. 2005;53:1111–1113. doi: 10.1248/cpb.53.1111. [DOI] [PubMed] [Google Scholar]
- 20.Wu B., He S., Pan Y.J. New dihydrodibenzoxepins from Bulbophyllum kwangtungense. Planta Med. 2006;72:1244–1247. doi: 10.1055/s-2006-947200. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Xu J., Yut H., Qin C.W., Zhangt Y., Liu Y., Wang J. Bulbophyllum Odoratissimum 3,7-Dihydroxy-2,4,6-trimethoxyphenanthrene. J. Korean Chem. Soc. 2007;51:352–355. [Google Scholar]
- 22.Yoshikawa M., Murakami T., Kishi A., Sakurama T., Matsuda H., Nomura M., Matsuda H., Kubo M. Novel indole S,O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promoting effects, from two Calanthe species (Orchidaceae) Chem. Pharm. Bull. 1998;46:886–888. doi: 10.1248/cpb.46.886. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M., Shogawa H., Hayashi T., Arisawa M., Suzuki S., Yoshizaki M., Morita N., Ferro E., Basualdo I., Berganza L.H. Anti-inflammatory constituents of topically applied crude drugs. III. Constituents and anti-inflammatory effect of Paraguayan crude drug “Tamandá cuná” (Catasetum barbatum LINDLE) Chem. Pharm. Bull. 1988;36:4447–4452. doi: 10.1248/cpb.36.4447. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D., Zhang Y., Liu G., Zhang J. Dactylorhin B reduces toxic effects of beta-amyloid fragment (25–35) on neuron cells and isolated rat brain mitochondria. Naunyn Schmiedebergs Arch. Pharmacol. 2006;374:117–125. doi: 10.1007/s00210-006-0095-9. [DOI] [PubMed] [Google Scholar]
- 25.Shimura H., Matsuura M., Takada N., Koda Y. An antifungal compound involved in symbiotic germination of Cypripedium macranthos var. rebunense (Orchidaceae) Phytochemistry. 2007;68:1442–1447. doi: 10.1016/j.phytochem.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Ho C.K., Chen C.C. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Investig. 2003;21:729–736. doi: 10.1081/CNV-120023771. [DOI] [PubMed] [Google Scholar]
- 27.Olof T.C. Survival and flowering of some perennial herbs II. The behaviour of some orchids on permanent plots. Oikos. 1972;23:23–28. [Google Scholar]
- 28.Deciga-Campos M., Palacios-Espinosa J.F., Reyes-Ramirez A., Mata R. Antinociceptive and anti-inflammatory effects of compounds isolated from Scaphyglottis livida and Maxillaria densa. J. Ethnopharmacol. 2007;5:7–13. doi: 10.1016/j.jep.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Romero Y., Rojas J.I., Castillo R., Rojas A., Mata R. Spasmolytic effects, mode of action, and structure-activity relationships of stilbenoids from Nidema boothii. J. Nat. Prod. 2004;67:160–167. doi: 10.1021/np030303h. [DOI] [PubMed] [Google Scholar]
- 30.Suresh P.K., Subramoniam A., Pushpangadan P. Aphodisiac activity of Vanda tessellata. Indian J. Pharmacol. 2000;32:300–304. [Google Scholar]
- 31.Chang Y.Y., Chiu Y.F., Wu J.W., Yang C.H. Four orchid (Oncidium Gower Ramsey) AP1/AGL9-like MADS box genes show novel expression patterns and cause different effects on floral transition and formation in Arabidopsis thaliana. Plant Cell Physiol. 2009;50:1425–1438. doi: 10.1093/pcp/pcp087. [DOI] [PubMed] [Google Scholar]
- 32.Kant R., Verma J., Thakur K. Distribution pattern, survival threats and conservation of ‘Astavarga’ Orchids in Himachal Pradesh, North-West Himalaya. Plant Arch. 2012;1:165–168. [Google Scholar]
- 33.Sut S., Maggi F., Acqua S.D. Bioactive secondary metabolites from orchids (Orchidaceae) Chem. Biodivers. 2017;14:e1700172. doi: 10.1002/cbdv.201700172. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan N.S. Medicinal orchids of Himachal Pradesh. J. Orchid Soc. India. 1990;4:99–105. [Google Scholar]
- 35.Thakur M., Dixit V.K. Evaluation of Antioxidant Activity and Ameliorative Effect Dactylorhiza Hatagirea on Sexual Dysfunction in Hyperglycemic Male Rats Department of Pharmaceutical Sciences. Dr. H.S. Gour University; Sagar, India: 2007. [Google Scholar]
- 36.Polunin O., Stainton A. Flowers of the Himalaya New Delhi. Oxford University Press; Oxford, UK: 1984. pp. 37–41. [Google Scholar]
- 37.Bhatt A., Joshi S.K., Gairola S. Dactylorhiza hatagirea (D.Don) Soo-a west Himalayan Orchid in peril. Curr. Sci. 2005;89:610–612. [Google Scholar]
- 38.Bhakta Bahadur Raskoti . Bhakta Bahadur Raskoti and Rita Ale. Bhakta Bahadur Raskoti; Beijing, China: 2009. The Orchid of Nepal. [Google Scholar]
- 39.Gale S.W., Cribb P.J. Dactylorhiza Necker ex Nevski, Fl. URSS 4: 697, 713. 1935, nom. Cons. Flora China. 2009;25:114–117. [Google Scholar]
- 40.Samant S.S., Dhar U., Palni L.M.S. Diversity Distribution Potential Values. Gyanodaya Prakashan; Nanital, India: 1998. Medicinal Plants of Indian Himalaya. [Google Scholar]
- 41.Badola H.K., Pal M. Endangered Medicinal plant in Himachal Pradesh. Curr. Sci. 2002;7:797–798. [Google Scholar]
- 42.Dhar U., Kachroo P. Alpine flora of Kashmir Himalaya. Scientific Publishers; Jodhpur, India: 1983. [Google Scholar]
- 43.Aswal B.S., Mehrotra B.N. Flora of Lahaul-Spiti. Bishan Singh Mahendra Pal Singh; Dehra Dun, India: 1994. [Google Scholar]
- 44.Hajra P.K., Balodi B. Plant Wealth of Nanda Devi Biosphere Reserve. Botanical Survey of India; Calcutta, India: 1995. [Google Scholar]
- 45.Samant S.S., Dhar U., Rawal R.S. In: Himalayan Medicinal Plants-Potential and Prospects. Palni L.M.S., Samant S.S., editors. Gyanodaya Prakashan; Nainital, India: 2001. pp. 166–184. [Google Scholar]
- 46.Warghat A.R., Bajpai P.K., Sood H., Chaurasia O.P., Srivastava R.B. Morphometric analysis of Dactylorhiza hatagirea (D. Don), a critically endangered orchid in cold desert Ladakh region of India. Afr. J. Biotechnol. 2012;11:11943–11951. [Google Scholar]
- 47.Nautiyal S., Rajan K.S., Shibasaki R. Interaction of Biodiversity and Economic Welfare—A Case Study from the Himalayas of India. J. Environ. Inform. 2005;2:111–119. doi: 10.3808/jei.200500061. [DOI] [Google Scholar]
- 48.Selvam A.B.D. Pharmacognosy of Negative Listed Plants. Botanical Survey; Kolkata, India: 2012. pp. 59–68. [Google Scholar]
- 49.Tripathi I.P. Chemistry, Biochemistry and Ayurveda of Indian Medicinal Plants. Volume 34 International E—Publication 427; Indore, India: 2013. [Google Scholar]
- 50.Dhiman N., Sharma N.K., Thapa P., Sharma I., Swarnkar M.K., Chawla A., Shankar R., Bhattacharya A. De novo transcriptome provides insights into the growth behavior and resveratrol and trans-stilbenes biosynthesis in Dactylorhiza hatagirea—An endangered alpine terrestrial orchid of Western Himalayas. Sci. Rep. 2019;9:13133. doi: 10.1038/s41598-019-49446-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kala C.P. Assessment of species rarity. Curr. Sci. 2004;86:1058–1059. [Google Scholar]
- 52.Badola H.K., Aitken S. The Himalayas of India: A treasury of Medicinal plant under siege. Biodiversity. 2003;4:3–13. doi: 10.1080/14888386.2003.9712694. [DOI] [Google Scholar]
- 53.Kunwar R.M., Nepal B.K., Kshherti H.B., Rai S.K., Bussmann R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang Districts of Nepal. J. Ethnobiol. Ethnomed. 2006;2:1–6. doi: 10.1186/1746-4269-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Man R., Samant S.S. Diversity, indigenous uses and conservation status of medicinal plants in Manali wildlife sanctuary, North West Himalaya. Indian J. Tradit. Knowl. 2011;3:439–459. [Google Scholar]
- 55.Uniyal S.K., Awasthi A., Rawat G.S. Current status and distribution of commercially exploited medicinal and aromatic plants in upper Gori Valley, Kumaon Himalaya, Uttaranchal. Curr. Sci. 2002;82:1246–1252. [Google Scholar]
- 56.Popli D. Elicitation of Dactylorhin-E and Studying Anti-Cancerous Potential of Dactylorhiza Hatagirea D. Don. Dissertation submitted to Jay Pee University of Information and Technology; Waknaghat, India: 2017. pp. 35–39. [Google Scholar]
- 57.Giri D., Tamata S. A general account on medicinal uses of D. hatagirea. N. Y. Sci. J. 2010;2:78–79. [Google Scholar]
- 58.Rana C.S., Tiwari J.K., Dangwal L.R., Sundriyal R.C. Herbal remedies for sexual capability. Indian J. Tradit. Knowl. 2012;4:646–651. [Google Scholar]
- 59.Chauhan N.S. Master’s Thesis. HPKVV Palampur; Palampur, India: 1984. Medicinal Wealth of Pabbar Valley in Himachal Pradesh; pp. 68–69. [Google Scholar]
- 60.Bancroft J. The endocrinology of sexual arousal. J. Endocrinol. 2005;186:411–427. doi: 10.1677/joe.1.06233. [DOI] [PubMed] [Google Scholar]
- 61.Pal M.M., Raj X.J., Kumar G.P., Sunil G., Singh S.B. Phytofoods of Nubra valley, Ladakh—The cold desert. Indian J. Tradit. Knowl. 2010;2:303–308. [Google Scholar]
- 62.Balkrishan A., Srivastava A., Mishra R.K., Patel S.P., Vashistha R.K., Singh A. Astavarga plants—Threatened medicinal herbs of the North-West Himalaya. Int. J. Med. Aromat. Plants. 2012;2:661–667. [Google Scholar]
- 63.Khajuria A.K., Kumar G., Bisht N.S. Diversity with ethnomedicinal notes on Orchids: A case study of Nagdev forest range, PauriGarhwal, Uttarakhand, India. J. Med. Plants Stud. 2017;1:171–174. [Google Scholar]
- 64.Pant S., Rinchen T. Dactylorhiza hatagirea: A high value medicinal orchid. J. Med. Plants Res. 2012;6:3522–3524. [Google Scholar]
- 65.Uprety Y., Asselin H., Emmanuel B.K., Yadav S., Shrestha K.K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed. 2010;3:187–195. doi: 10.1186/1746-4269-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rawat V.S., Jalal J.S. Sustainable Utilization of Medicinal Plants by Local Community of Uttarkashi District of Garhwal, Himalaya, India. Eur. J. Med. Plants. 2011;1:18–25. doi: 10.9734/EJMP/2011/141. [DOI] [Google Scholar]
- 67.Arora M., Mahajan A., Sembi J.K. A Review on Phytochemical and Pharmacological Potential of Family Orchidaceae. Int. Res. J. Pharm. 2017;8:9–24. doi: 10.7897/2230-8407.0810176. [DOI] [Google Scholar]
- 68.Tsering J., Tam N., Tag H., Gogoi B.J., Apang O. Medicinal Orchids of Arunachal Pradesh: A review Bulletin of Arunachal. For. Res. 2017;32:1–16. [Google Scholar]
- 69.Ballabh B., Chaurasia O., Ahmed Z., Singh S.B. Traditional medicinal plants of cold desert Ladakh Used against kidney and urinary disorders. J. Ethnopharmacol. 2008;2:331–339. doi: 10.1016/j.jep.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 70.Chauhan N.S. Medicinal and Aromatic Plants of Himachal Pradesh. Indus Publication Company; New Delhi, India: 1999. Dactylorhiza hatagirea D Don Soo; pp. 180–182. [Google Scholar]
- 71.Watanabe T., Rajbhandari K.R., Malla K.J., Yahara S. A Handbook of Medicinal Plants of Nepal. Kobfai Publishing Project Foundation for Democracy and Development Studies; Bangkok, Thailand: 2005. [Google Scholar]
- 72.Bhatt D., Sharma P., Sharma L., Joshi G.C. Folk herbal remedies for skin in Kamaun Himalaya. J. Non Timber For. Prod. 2012;4:309–312. [Google Scholar]
- 73.Vij S.P., Srivastav R.C., Mainra A.K. On the occurrence of Dactylorhiza hatagirea (D.Don) Soo in Sikkim. Orchard News. 1992;9:14–15. [Google Scholar]
- 74.Sharma P.V., Charaka S. Chaukhambha orientalis. Varanasi India. 2001;2:7–14. [Google Scholar]
- 75.Hamilton A.C., Radford E.A. Identification and Conservation of Important Plant Areas for Medicinal Plants in the Himalaya. Plant life International; Salisbury, UK: Ethnobotanical Society of Nepal; Kathmandu, Nepal: 2007. pp. 45–51. [Google Scholar]
- 76.Arditti J. Factors affecting the germination of orchid seeds. Bot. Rev. 1967;33:1–97. doi: 10.1007/BF02858656. [DOI] [Google Scholar]
- 77.Khan S.W., Khatoon S. Ethnobotanical Studies on Some Useful Herbs of Haramosh and Bugrote Valleys in Gilgit, Northern Areas of Pakistan. Pak. J. Bot. 2008;1:43–58. [Google Scholar]
- 78.Ballabh B., Chaurasia O.P. Medicinal plants of cold desert Ladakh used in the treatment of stomach disorders. Indian J. Tradit. Knowl. 2009;8:185–190. [Google Scholar]
- 79.Dorji K.L. Ecological status of high-altitude medicinal plants and their sustainability: Lingshi, Bhutan. BMC Ecol. 2016;16:45. doi: 10.1186/s12898-016-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kizu H., Kaneko E.I., Tomimore T. Studies on Nepalese Crude Drugs. XXVI.1) Chemical Constituents of PanchAunle, the Roots of Dactylorhiza hatagirea D. DON. Chem. Pharm. Bull. 1999;11:1618–1625. doi: 10.1248/cpb.47.1618. [DOI] [Google Scholar]
- 81.Ranapal S. Bachelor’s Thesis. Institute of Forestry, Tribhuvan University; Pokhara, Nepal: 2009. An Assessment of Status and Antibacterial Properties of Dactylorhiza hatagirea in Annapurna Conservation Area (A Case Study of Paplekharka, Lete VDC, Mustang) [Google Scholar]
- 82.Vij S.P., Pathak P., Mahant K.C. Green pod culture of a therapeutically important species D. hatagirea (D.Don) Soo. J. Orchid Soc. India. 1995;9:7–12. [Google Scholar]
- 83.Charpinella M.C., Herrero G.G., Alonso R.A., Palacios S.M. Antifungal Activity of Melia azedarch Fruit Extract. Fitterapia. 1999;70:296–298. doi: 10.1016/S0367-326X(99)00009-X. [DOI] [Google Scholar]
- 84.Kumar P., Kumar R., Badere R., Singh S.B. Antibacterial and antioxidant activities of ethanol extracts from trans Himalayan medicinal plants. Pharmacogn. J. 2010;2:62–69. doi: 10.1016/S0975-3575(10)80013-6. [DOI] [Google Scholar]
- 85.Lomovskaya O., Warren M.S., Lee A., Galazzo J., Fronko R., Lee M., Blais J., Cho D., Chamberland S., Renau T., et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001;1:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stavri M., Piddock L.J., Gibbons S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007;6:1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 87.Neyfakh A.A., Bidnenko V.E., Chen L.B. Efflux-mediated multidrug resistance in Bacillus subtilis: Similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urmila, Jandaik S., Mehta J., Mohan M. Synergistic and efflux pump inhibitory activity of plant extracts and antibiotics on Staphylococcus aureus strains. Asian J. Pharm. Clin. Res. 2016;2:277–282. [Google Scholar]
- 89.Farooqui A., Khan A., Borghetto I., Kazmi S.U., Rubino S., Paglietti B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS ONE. 2015;10:0118431. doi: 10.1371/journal.pone.0118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guiotti A.M., Cunha B.G., Paulini M.B., Goiato M.C., Dos Santos D.M., Duque C., Caiaffa K.S., Brandini D.A., De Oliveira D.T.N., Brizzotti N.S., et al. Antimicrobial activity of conventional and plant-extract disinfectant solutions on microbial biofilms on a maxillofacial polymer surface. J. Prosthet. Dent. 2016;116:136–143. doi: 10.1016/j.prosdent.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 91.Widen C., Renvert S., Persson G.R. Antibacterial activity of berry juices, an in vitro study. Acta Odontol. Scand. 2015;73:539–543. doi: 10.3109/00016357.2014.887773. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y., Huang Z., Zou X., Zhong X., Liang X., Zhou J. The antibacterial effect of Urena lobata L. from Guangxi on mice with Staphylococcus aureus pneumonia. Afr. J. Tradit. Complementary Altern. Med. 2017;14:73–88. doi: 10.21010/ajtcam.v14i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brochot A., Guilbot A., Haddioui L., Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiology. 2017;6:e00459. doi: 10.1002/mbo3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flower A., Harman K., Lewith G., Moore M., Bishop F.L., Stuart B., Lampert N. Standardised Chinese herbal treatment delivered by GPs compared with individualised treatment administered by practitioners of Chinese herbal medicine for women with recurrent urinary tract infections (RUTI): Study protocol for a randomised controlled trial. Trials. 2016;17:358. doi: 10.1186/s13063-016-1471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wojnicz D., Kucharska A.Z., Sokol-Lętowska A., Kicia M., Tichaczek-Goska D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012;40:683–697. doi: 10.1007/s00240-012-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Owen L., Grootveld M., Arroo R., Ruiz-Rodado V., Price P., Laird K. A multifactorial comparison of ternary combinations of essential oils in topical preparations to current antibiotic prescription therapies for the control of acne vulgaris-associated bacteria. Phytother. Res. 2017;31:410–417. doi: 10.1002/ptr.5762. [DOI] [PubMed] [Google Scholar]
- 97.Hajimahmoodi M., Shams-Ardakani M., Saniee P., Siavoshi F., Mehrabani M., Hosseinzadeh H., Foroumadi P., Safavi M., Khanavi M., Akbarzadeh T., et al. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat. Prod. Res. 2011;25:1059–1066. doi: 10.1080/14786419.2010.501763. [DOI] [PubMed] [Google Scholar]
- 98.Sirohi B., Sagar R., Jain P. Evaluation of the Anti-Inflammatory Activity of Hydroalcoholic Extract of Dactylorhiza hatagirea Roots and Lavandula stoechas Flower in Rats EC Pharmacol. Toxicology. 2019;7:110–118. [Google Scholar]
- 99.Kesari A.N., Kesari S., Singh S.K., Gupta R.K., Watal G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J. Ethnopharmacol. 2007;112:305–311. doi: 10.1016/j.jep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 100.Mohan S., Alsawalha M., Al-Subaei A., Al-Jindan R., Bolla S., Sen D., Balakrishna J., Ravi P., Gollapalli S.R., Veeraraghavan V., et al. Anti-diabetic activities of Dactylorhiza hatagirea leaf extract in 3T3-L1 cell line model. Pharmacogn. Mag. 2019;15:212. doi: 10.4103/pm.pm_8_19. [DOI] [Google Scholar]
- 101.Choukarya R., Choursia A., Rathi J. In vivo and in vitro anti-diabetic activity of hydroalcholic extract of D. hatagirea roots: An evaluation of possible phytoconstituents. J. Drug. Discov. Ther. 2020;9:76–81. [Google Scholar]
- 102.Anon . The Wealth of India. Volume 10. CSIR; New Delhi, India: 1976. pp. 77–81. [Google Scholar]
- 103.Singh B., Gupta V., Bansal P., Singh R., Kumar D. Pharmacological potential of plant used as aphrodisiacs. Int. J. Pharm. Sci. Rev. Res. 2010;1:104–113. [Google Scholar]
- 104.Bulpitt C.J. The Uses and Misuses of Orchids in Medicine. QJM Int. J. Med. 2005;98:625–631. doi: 10.1093/qjmed/hci094. [DOI] [PubMed] [Google Scholar]
- 105.Warghat A.R., Bajpai P.K., Srivastava B.R., Chaurasia O.P., Chauhan R.S., Sood H. In vitro protocorm development and mass multiplication of an endangered orchid, Dactylorhiza hatagirea. Turk. J. Bot. 2014;38:737–746. doi: 10.3906/bot-1308-48. [DOI] [Google Scholar]
- 106.Aggarwal S., Zettler L.W. Reintroduction of an endangered terrestrial orchid, Dactylorhiza hatagirea (D. Don) Soo, assisted by symbiotic seed germination: First report from the Indian subcontinent. Nat. Sci. 2010;8:139–145. [Google Scholar]
- 107.Novotna K.W., Vejsadova H., Kindlmann P. Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol. Plant. 2007;51:198–200. doi: 10.1007/s10535-007-0040-x. [DOI] [Google Scholar]
- 108.Shrestha N., Shrestha K.K. Vulnerability assessment of high-valued medicinal plants in Langtang National Park, Central Nepal. Biodiversity. 2012;1:24–36. doi: 10.1080/14888386.2012.666715. [DOI] [Google Scholar]
- 109.Giri D., Tamta S. Propagation and conservation of Dactylorhiza hatagirea (D.Don) Soo, an endangered alpine orchid. Afr. J. Biotechnol. 2012;11:12586–12594. [Google Scholar]
- 110.Pillon Y., Fay M.F., Shipunov A.B., Chase M.W. Species diversity versus phylogenetic diversity: A practical study in the taxonomically difficult genus Dactylorhiza (Orchidaceae) Biol. Conserv. 2006;4:129. doi: 10.1016/j.biocon.2005.06.036. [DOI] [Google Scholar]