Abstract
The emergence and spread of insecticide resistance among the main malaria vectors is threatening the effectiveness of vector control interventions in Senegal. The main drivers of this resistance in the Anopheles gambiae complex (e.g., An. gambiae and Anopheles coluzzii) remains poorly characterized in Senegal. Here we characterized the main target site and metabolic resistances mechanisms among the An. gambiae and An. coluzzii populations from their sympatric and allopatric or predominance area in Senegal. Larvae and pupae of An. gambiae s.l. were collected, reared to adulthood, and then used for insecticides susceptibility and synergist assays using the WHO (World Health Organisation) test kits for adult mosquitoes. The TaqMan method was used for the molecular characterization of the main target site insecticide resistance mechanisms (Vgsc-1014F, Vgsc-1014S, N1575Y and G119S). A RT-qPCR (Reverse Transcriptase-quantitative Polymerase Chaine Reaction) was performed to estimate the level of genes expression belonging to the CYP450 (Cytochrome P450) family. Plasmodium infection rate was investigated using TaqMan method. High levels of resistance to pyrethroids and DDT and full susceptibility to organophosphates and carbamates where observed in all three sites, excepted a probable resistance to bendiocarb in Kedougou. The L1014F, L1014S, and N1575Y mutations were found in both species. Pre-exposure to the PBO (Piperonyl butoxide) synergist induced a partial recovery of susceptibility to permethrin and full recovery to deltamethrin. Subsequent analysis of the level of genes expression, revealed that the CYP6Z1 and CYP6Z2 genes were over-expressed in wild-resistant mosquitoes compared to the reference susceptible strain (Kisumu), suggesting that both the metabolic resistance and target site mutation involving kdr mutations are likely implicated in this pyrethroid resistance. The presence of both target-site and metabolic resistance mechanisms in highly pyrethroid-resistant populations of An. gambiae s.l. from Senegal threatens the effectiveness and the sustainability of the pyrethroid-based tools and interventions currently deployed in the country. The Kdr-west mutation is widely widespread in An. coluzzii sympatric population. PBO or Duo nets and IRS (Indoor Residual Spraying) with organophosphates could be used as an alternative measure to sustain malaria control in the study area.
Keywords: malaria, An. coluzzii, An. gambiae, pyrethroid, kdr, N1575Y, metabolic resistance, Senegal
1. Introduction
The control of malaria vector in Africa relies mainly on the two core insecticide-based interventions: Long-Lasting Insecticide Nets (LLINs) and Indoor Residual Spraying (IRS) [1]. Four main classes of insecticide are available for use in the public health (pyrethroids, organochlorines, organophosphates and carbamates), with pyrethroids being the main class approved for the impregnation of nets [2]. Given the heavy reliance on pyrethroid-based strategies for malaria vector control, the spread across sub-Saharan Africa of the resistance to this class of insecticide threatens the sustainability of current and future vector control interventions. Thus, providing accurate and timely information about the evolution of the main insecticide resistance mechanisms is vital for the implementation of targeted and cost-effective control measures.
Two main types of mechanisms are involved in the resistance of An. gambiae to the principal classes of insecticide use in the public health sector: the target-site insensitivity [3,4] and the metabolic activity of detoxification enzyme families such as cytochrome P450s, glutathione S-transferases and esterases [5]. Target site insensitivity to DDT and pyrethroid in An. gambiae is associated to single-point mutation at the 1014 position in the voltage-gated sodium channel gene (Vgsc) known as knock-down resistance (kdr). The Vgsc-1014F and Vgsc-1014S mutations, respectively known as kdr-west and kdr-east [6,7], are among the most widespread target-site insecticide resistance mechanisms found among the natural population of An. coluzzii and An. gambiae [8] across the Western and Eastern Africa [4,9,10,11,12]. Previous studies in West Africa reported the absence of the kdr mutation in An. coluzzii even in the sympatric population [6,7,13]. However, subsequently, this mutation was found in both species with higher frequency of L1014F mutation in An. coluzzii [14] suggesting a introgression from An. gambiae to An. coluzzii [15]. The G119S-Ace-1 mutation is involved in bendiocarb resistance in An. gambiae s.l. in West Africa [16,17] and the N1575Y confer resistance to DDT and pyrethroid in west Africa [18,19].
In Senegal, four members of the An. gambiae complex were described so far, including the two incipient species An. coluzzii and An. gambiae, the two main malaria vectors across the continent. It have been reported in 2016 the presence and wide distribution of the Vgsc-1014F mutation among the wild populations of An. gambiae and An. coluzzii from a sympatric area in the south-eastern part of the country [20]. However, few data are available on the frequency and distribution of the Vgsc-1014F and Vgsc-1014S mutations in areas where one of the two species is predominant (allopatric area). The Moreover, few if any study has taken a holistic approach to characterize altogether the main target sites (Vgsc-1014F, Vgsc-1014S, N1575Y and G119S) as well as the putative metabolic mechanism. This infer the evolutionary processes underlying the emergence and spread of insecticide resistance among the natural populations of the two incipient species of the An. gambiae complex (e.g., An. gambiae and An. coluzzii) across their different range of distribution.
Here, we characterized the main target site and metabolic resistance mechanisms among the natural populations of An. gambiae s.s. and An. coluzzii in two ecogeographical regions of Senegal. The introgression of the kdr-west mutation from An. gambiae to An. coluzzii in the sympatric area was also assessed.
2. Materials and Methods
2.1. Study Areas
This study was conducted during the 2017 and 2018 raining seasons in three health districts located in two different eco-geographical zones of Senegal.
The districts of Tambacounda (13°46′14″ N; 13°40′02″ W) and Kedougou (12°33′28″ N; 12°10′27″ W) are both located in the southern region of the country and belong to the Sudanese eco-geographical zone. The health district of Fatick (14°21′29″ N; 16°35′08″ W is in the Sudan-Sahelian ecozone in the centre of Senegal. Tambacounda is located along the Gouloumbou River and was chosen as a sympatric area of the two incipient species. The main activity is agriculture of banana and rice involving high pesticide use. Kedougou is characterized by an important raining season with temporary breeding sites and was chosen as an allopatric area (or area of predominance) for An. gambiae whereas Fatick characterized by a low and irregular rainfall with a permanent river (Nema) was retained as an allopatric area (or area of predominance) for An. coluzzii. Subsistence crops are practiced in both areas with insecticide used.
2.2. Samples Collection
Larvae and pupae of An. gambiae s.l. were collected from breeding sites, during the two successive rainy seasons (August–October) in 2017 and (October–November) in 2018 and reared until emergence then exposed to insecticides. In addition, resting adult mosquitoes were collected indoor using the pyrethrum spray collection method, early on the morning (6:00 to 8:00 am), once every surveyed month during the all the study period (August–November).
2.3. Plasmodium spp. Infection Rate
Taqman method described by Bass [21] was used to screen samples for the presence of the Plasmodium spp. on the real-time PCR MX 3005 machine (Agilant, Santa Clara, CA, USA). The PlasF (5′-GCT TAG TTA CGA TTA ATA GGA GTA GCT TG-3′) and PlasR (5′-GAA AAT CTA AGA ATT TCA CCT CTG ACA-3′) primers set were used together with two probes labeled with the FAM fluorophore (Falcip+ 5′-TCT GAA TAC GAA TGT C-3′) to detect Plasmodium falciparum, and the HEX fluorophore (OVM+ 5′-CTG AAT ACA AAT GCC-3′) to detect Plasmodium ovale, Plasmodium vivax and Pplasmodium malariae. All positives samples were confirmed by nested PCR [22].
2.4. WHO Insecticide Susceptibility and Synergist Tests
Non-blood-fed females of An. gambiae s.l. aged of 3–5 days were exposed to DDT (4%), deltamethrin (0.05%), permethrin (0.75%), alphacypermethrin (0.1%), lambda-cyhalothrin (0.05%), bendiocarb (0.1%) and pirimiphos methyl (1%) using the standard WHO-susceptibility test procedures for adult mosquitoes at a temperature of 25 ± 2 °C and at 80 ± 10% relative humidity [23].
To investigate the putative role of detoxification enzyme in the pyrethroid resistance among highly resistant populations of An. gambiae s.l. from Kedougou, 3–5 days non-blood-fed females were tested against permethrin and deltamethrin as described above, after 1-h pre-exposure to 4% of Piperonal butoxide (PBO). For each insecticide molecules a batch of at least 50 specimens of 3–5 days non-blood-fed females were exposed to untreated papers as control.
Knock-downed specimens were recorded at 10, 15, 20, 30, 40, 50- and 60-min post exposure, and mortality was measured after a period of observation 24 h post-exposure.
2.5. Estimation of Resistance Intensity
To establish the intensity of pyrethroid resistance in Kedougou and Tambacounda, additional bioassays were conducted with 1×, 5× and 10× of the discriminating concentration of deltamethrin (0.05, 0.25, and 0.5%) and permethrin (0.75, 3.75, and 7.5%) as described by the standard protocol of WHO-susceptibility test procedures for adult mosquitoes [19].
2.6. Morphological and Molecular Identification of An. gambiae s.l. Species
All specimens collected indoor and those exposed to insecticides were identified using the Afrotropical Anopheline morphological keys of Gillies & de Meillon [24]. A sub-sample of indoors resting adult females, with together dead and alive specimens from insecticide susceptibility tests were randomly selected by area for subsequent analyses.
The genomic DNA was extracted from single mosquito’s wings and legs using the Livak method [25], then the members of the An. gambiae complex were identified by the PCR [26,27]
2.7. Molecular Genotyping of the Vgsc-1014F, Vgsc-1014S, N1575Y and G119S Mutations
TaqMan assays were performed on the Agilent MX3005P qPCR (quantitative Polymerase Chain Reaction) system (Agilent, Santa Clara, CA, USA) to characterize the putative target site insecticide resistance mechanisms, including the Vgsc-1014F (West) and Vgsc-1014S (East) Kdr mutations [28], the N1575Y mutation [18] and the G119S Ace-1 mutation [29].
2.8. Analysis of the Polymorphism of the Voltage-Gated Sodium Channel
To assess the genetic diversity and detect putative mutations associated with the knockdown resistance (kdr), a fragment of 1014 of the voltage-gated sodium channel gene spanning the 1014 coding was analysed. This fragment which includes a portion of intron 19 and the entire exon 20 in the domain II of the segment 6 was amplified, purified, and sequenced in wild An. gambiae s.l. populations sampled in 2017 in Kedougou (12 An. gambiae, 6 An. coluzzii, and 4 hybrids), and Tambacounda (12 An. gambiae, 11 An. coluzzii and 4 hybrids).
The genomic DNA was extracted from legs and wings as described by Livak [25] then amplified using the kdr-CL primers set (kdr-CL-F: 5′-AAATGTCTCGCCCAAATCAG-3′ and kdr-CL-R: 5′-GCA CCTGCAAAACAATGTCA-3′) as described by Pinto [30]. PCR products were purified using the exonuclease Ι (Exo Ι)/Shrimp Alkaline Phosphate (Exo-SAP) purification Kit (New England Biolabs, MA, USA) according to the manufacturer’s instructions, and sequenced using the ABI automated sequencer (Applied Biosystems, Foster City, CA, USA).
The amplified sequences were corrected using BioEdit v.7.2.1 [31] then aligned using ClustalW [32]. Phylogenetic analysis and haplotype reconstruction were done using the DnaSP v.5.10 [33]. Sequences were compared with reference sequences retrieved from Genbank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the maximum likelihood phylogenetic tree was constructed using MEGA v.7.0 [34].
2.9. Metabolic Resistance Genes Expression
The expression level of the CYP450 genes family (CYP6M2, CYP6P3, CYP4G16, CYP4G16, CYP9K1, CYP6Z1, and CYP6Z2), and GSTe2 was assessed from three biological replicates of surviving An. gambiae after exposure to Permethrin (Kedougou and Tambacounda) and Deltamethrin (Kedougou). RNA (Rubonucleic acid) was extracted and purified using the picopure RNA isolation Kit (Life Technologies, Camarillo, CA, USA) according to the manufacturer’s instructions. cDNA (complementary Deoxyribonucleic acid) was synthesized from the purified RNA by quantitative RT-PCR using the SuperScript III (Invitrogen, Waltham, MA, USA) and the oligo-dT20 and RNAse H (New England Biolabs, Ipswich, MA, USA) kit in a total reactional volume of 20 μL including of 19 μL PCR mix (10 μL of SyBr Green, 7.8 μL of dH2O, 0.6 μL of forward and reverse primers at the concentration of 10 mM for each gene of interest), and 1 μL of cDNA (or dH2O water for controls). Amplification was performed with an initial step of denaturation at 95 °C for 3 min followed by 40 cycles of 10 s at 95 °C, 10 s at 60 °C, then one cycle of 1 min at 95 °C, 30 s at 55 °C and 30 s at 95 °C. The cDNA extract from the An. gambiae Kisumu susceptible strain was used as a susceptible biological control.
2.10. Data Analysis
The 24 h post-exposure mortality for bioassay was estimated for each insecticide tested by dividing the number of dead mosquitoes per replicate by the total number of mosquitoes exposed. Odds ratios, Chi-square and Fisher’s exact tests were used for statistical comparisons. The relative expression for each metabolic gene was calculated according to the 2−ΔΔCT method [35] and the statistical significance between gene expression estimates was performed using unpaired Student t test. The 5% significance level was considered for all the statistical tests. All analyses were conducted using GraphPad Prism version 7.00 and R version 3.5.2 software version.
3. Results
3.1. Species Composition
3.1.1. Indoor Collection
A total of 1474 specimens were collected, 657 and 817 in 2017 and 2018, respectively. In the An. gambiae complex, An. arabiensis was predominant in Fatick (81.68 vs. 65.17%) and was present in Kedougou (16.36 vs. 7.46%) and Tambacounda (28.94 vs. 15.64%). An. melas was found only in Fatick (Table S1). Compared to An. gambiae, An. coluzzii was most abundant in Fatick (67.85% vs. 88.52%) but less abundant in Kedougou (93.99% vs. 90.32%) in 2017 and 2018 respectively (Table S1). In Tambacounda, considered as the sympatric area of An. coluzzii and An. gambiae, the latter was found predominant. Hybrids An. coluzzii/An. gambiae were found in all areas in 2018 with frequencies ranging from 1.64 to 3.57% except in Kedougou (Table S1).
3.1.2. Larval Collection
A total of 1091 specimens were identified, An. arabiensis was the predominant species in Fatick and Tambacounda (Table S2). When considering the two incipient species An. coluzzii and An. gambiae, the latter was predominant in Kedougou (86.64%). In Tambacounda An. coluzzii and An. gambiae were found almost at the same proportion (50% and 47.03% respectively). In Fatick, An. gambiae was found predominant (84.85%) (Table S2).
3.2. Plasmodium spp. Infection Rate
DNA was extracted from 314 mosquitoes (head-thorax) collected in 2017 and 2018 and analyzed using TaqMan assay for Plasmodium infection. In Kedougou, 3.16% (3/95) mosquitoes were found infected with Plasmodium ovale, vivax or malariae and 1.05% (1/95) infected with Plasmodium falciparum. All the mosquitoes infected were An. gambiae. In Tambacounda, 2.53% (4/158) of mosquitoes were found infected with P. falciparum and 0.63% (1/158) were co-infected with P. falciparum and P. ovale or vivax or malariae (OVM+). Among these infected mosquitoes, 1.27% (2/158) were An. gambiae, 0.63% (1/158) were An. coluzzii and 0.63% (1/158) was hybrid An. coluzzii/An. gambiae. The co-infected mosquitoes were identified as An. coluzzii. In Fatick, no mosquito was found infected. The nested PCR confirmed all Plasmodium falciparum positive mosquitoes, but failed to confirm the OVM+ from TaqMan probably because of the low sensitivity of this method [21].
3.3. Insecticide Resistance Profile
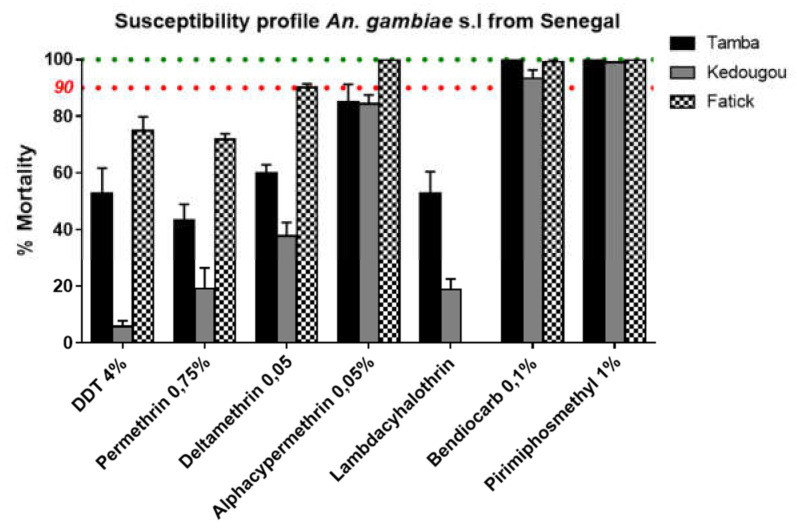
A total of 2141 mosquitoes from the 2017 collection were tested for the conventional WHO bioassay including 655 from Fatick, 262 from Kedougou, and 724 from Tambacounda. Mosquitoes tested were fully susceptible to bendiocarb and pirimiphos methyl. However, in all Kedougou probable resistance to bendiocarb was noted with 93.3 ± 3% (SEM) mortality (Figure 1). High level of resistance to DDT (5.8 ± 2%; 52.9 ± 8%), permethrin (19.1 ± 7.4%; 43.3 ± 5.6%), deltamethrin (37.7 ± 4.8%; 60 ± 2.8%), lambda-cyhalothrin (18.9 ± 3.6%; 52.9 ± 7.5%) and alphacypermethrin (84.3 ± 3.2%; 85.1 ± 6.2%) was recorded in Kedougou and Tambacounda respectively (Figure 1). However, in Fatick, full susceptibility to alphacypermethrin and probable resistance to deltamethrin (90.2 ± 1.2%) were observed whereas moderate resistance was noted for DDT (74.8 ± 5%) and permethrin (71.8 ± 2%) (Figure 1).
Figure 1.
Susceptibility profile of Anopheles gambiae s.l. to insecticides. Recorded mortalities following 60-min exposure of Anopheles gambiae s.l. from Fatick, Tambacounda and Kedougou to different insecticides. Data are shown as mean ± standard error of the mean (SEM).
3.4. Estimation of Resistance Intensity
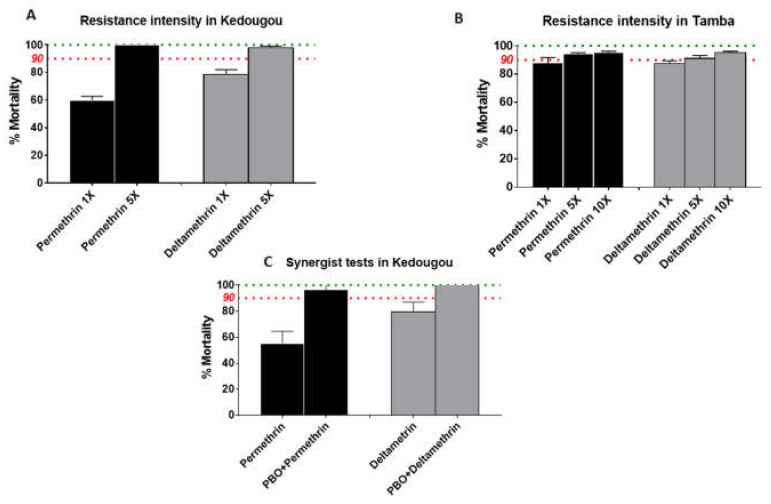
To assess the strength of the phenotype resistance to permethrin and deltamethrin, the resistant population collected in 2018 from Kedougou and Tambacounda were exposed to 5× and 10× of discriminating concentration of permethrin and deltamethrin. Results showed a low intensity of resistance to permethrin (5×: 100%) and deltamethrin (5×: 98 ± 1.1%) in Kedougou (Figure 2A) whereas in Tambacounda a higher intensity of resistance to permethrin (5×: 94.3 ± 0.9%; 10×: 95.3 ± 0.9%) and deltamethrin (5×: 91.2 ± 2%; 10×: 95.3 ± 0.9%) were found (Figure 2B).
Figure 2.
Results of resistance intensity and synergist tests. Resistance intensity in Tambacounda (A) and Kedougou (B); activities of PBO combined to permethrin, and deltamethrin on An. gambiae s.l. from Kedougou (C). Data are shown as mean ± standard error of the mean.
3.5. Synergist Bioassay with PBO
To assess the implication of the cytochrome P450s in the resistance observed to permethrin and deltamethrin, mosquitoes collected in 2018 from Kedougou were pre-exposed to PBO then to permethrin or deltamethrin. Compared to the result of the permethrin alone (mortality: 55.42 ± 9.19%) a nearly full recovery of the susceptibility was observed after exposure to permethrin + PBO (mortality: 96.47 ± 9.19%). For deltamethrin, a total recovery of the susceptibility was observed after pre-exposure to the PBO (mortality: 100%) compared to the result of deltamethrin alone (mortality: 79.74 ± 7.16%) (Figure 2C).
3.6. Distribution of Resistance Markers in the Adult Mosquitoes Collected
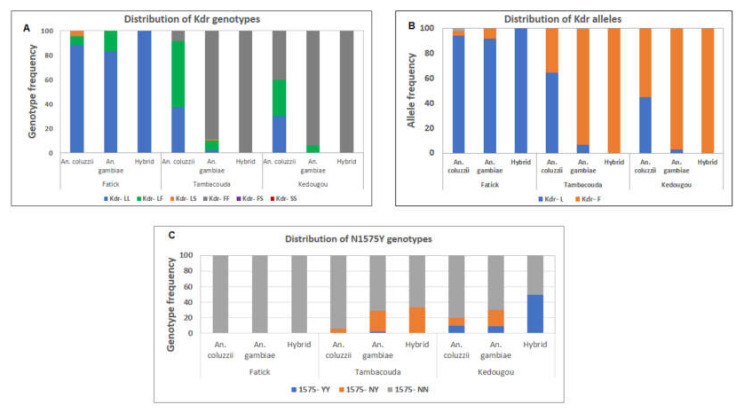
In all the three sites, the L1014F mutation was found in both species. In Kedougou, the predominance area of An. gambiae, all An. gambiae mosquitoes (64/64) harboured the mutation whereas only 70% (7/10) of the An. coluzzii harboured it (Figure 3A). The frequency of the L1014F resistant allele was higher in An. gambiae (96.88%) compared to An. coluzzii (55%) (χ2 = 18.9; p < 0.0001) (Figure 3B). No difference was found between the frequency of the L1014F resistant allele in An. gambiae from this site compared to Tambacounda (χ2 = 0.79, df = 1, p = 0.37).
Figure 3.
Genotyping of resistance markers in An. coluzzii, An. gambiae s.s. and their hybrids. Distribution of Kdr(s) genotypes (A) and alleles (B) and N1575Y genotypes (C) in the different species. Kdr − LL = Kdrw susceptible homozygous; Kdr − LF = Kdrw heterozygous; Kdr − FF = Kdrw resistant homozygous; Kdr − LS = Kdre resistant heterozygous; Kdr − SS = Kdre resistant heterozygous; Kdr − FS = Kdrw and Kdre resistant; 1575 − YY = Resistant homozygous; 1575−NY = Resistant heterozygous; 1575 − NN = Susceptible homozygous.
The N1575Y mutation was also found in both species with a frequency of 29.69% (19/64) in An. gambiae and 20% (2/10) in An. coluzzii (Figure 3C), The L1014S mutation was absent in this area (Figure 3A).
As observed in Kedougou, the frequency L1014F mutation was higher in An. gambiae 96.63% (86/89) from Tambacounda compared to An. coluzzii 62.5% (30/48) (χ2 = 28.7; p < 0.0001) (Figure 3A,B). Moreover, a significant difference was found also when comparing the distribution of this mutation in An. coluzzii from Fatick compared to Tambacounda (χ2 = 28.57, df = 1, p < 0.001).
The N1575Y mutation was found at 29.21% (26/89) in An. gambiae and at 6.25% (3/48) in An. coluzzii. Only the heterozygote (N1575Y) was detected in An. coluzzii (Figure 3C). The L1014S mutation was found in only An. gambiae at the heterozygote form as well (Figure 3A).
In Fatick, the predominance area of An. coluzzii, the L1014F mutation was at 6.82% (3/44) in this species and at 16.67% (1/6) in An. gambiae (Figure 3A). The N1575Y mutation was not found in this area (Figure 3C) and only two An. coluzzii were found carrying the L1014S mutation (Figure 3A).
All the hybrids genotyped in Kedougou (n = 2) and Tambacounda (n = 6) harboured the L1014F mutation (Figure 3A). In Kedougou, 50% of them carried the N1575Y mutation whereas only 33.33% in Tambacounda carried this mutation (Figure 3C).
3.7. Correlation between the 1014F Mutation and Resistance to Pyrethroid
To assess the implication of kdr-w mutation in the pyrethroid resistance observed in An. gambiae, an allelic and genotypic association analysis was performed on 110 individuals, including 71 alive and 39 dead after exposition to pyrethroids in Kedougou. Pearson correlation test showed no significant association between pyrethroid resistance and the presence of L1014F resistant allele (Odds Ratio = 3.7 (95% CI: 0.7–18.2, p = 0.08)). This was confirmed when comparing the likelihood of surviving of females with RR genotypes to survive compared to RS (Odds Ratio 1.3 (95% CI: 0.3–5.1, p = 0.2)), and SS (Odds Ratio 5.3 (95% CI: 0.6–46.5, p = 0.1)). The same pattern was observed between RS and SS (Odds Ratio 4.0 (95% CI: 0.3–49.6, p = 0.3)) (Table 1). The association between pyrethroid resistance and L1014F mutation was not assessed in the other locality due to the low number of dead An. coluzzii and An. gambiae.
Table 1.
Association between L1014F-kdrw mutation and resistance to pyrethroids in Anopheles gambiae from Kedougou.
| Combination of Genotypes at the L1014F-kdr Locus |
An. gambiae | |
|---|---|---|
| Odds Ratio | p-Value | |
| RR vs. RS | 1.3 | 0.2 |
| (0.3–5.1) | ||
| RR vs. SS | 5.3 | 0.1 |
| (0.6–46.5) | ||
| RS vs. SS | 4.0 | 0.3 |
| (0.3–49.6) | ||
| R vs. S | 3.7 | 0.08 |
| (0.7–18.2) | ||
3.8. Genetic Diversity in the kdr Locus of the Voltage-Gated Sodium Channel
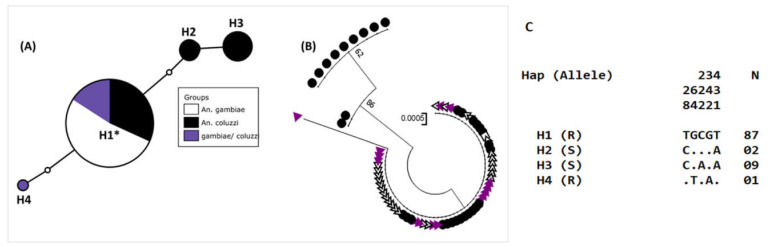
A total of 484-bp fragments of the VGSC spanning the 1014 codon were successfully sequenced in 24 An. gambiae, 17 An. coluzzii and 8 An. coluzzii/An. gambiae from Kedougou and Tambacounda. The genetic diversity parameters of this fragment is provided in the Table S3. Overall, five polymorphic sites defining 4 haplotypes were detected with a haplotypic diversity of 0.219. The overall nucleotide diversity was 0.001. At the species level, low haplotypic and genetic diversity were found in An. coluzzii and the hybrids. Analysis of the haplotype Network showed that the major and ancestral haplotype H1 (87/99) was shared between An. coluzzii, An. gambiae and their hybrids and was specific to the L1014F resistant allele. The two following haplotypes H3 (9/99) and H2 (2/99) were specific to L1014F susceptible allele and carried by An. coluzzii only. The lowest H4 (1/99) belonged to the L1014F resistant allele and was specific to the hybrid (Figure 4A,C).
Figure 4.
Genetic diversity parameters of Vgsc in An. coluzzii, An. gambiae and their hybrids from Senegal in relation to the species. (A) Haplotype network in relation to the species composition; (B) phylogenetic trees (using a maximum likelihood method) and the nucleotide diversity of the L1014F mutation in Senegal (C).
The analysis of the maximum likelihood phylogenetic tree between mosquitoes from different localities showed two main clades: the major with the two species and their hybrids and the second made up only by An. coluzzii (Figure 4B).
3.9. Implication of the G119S Mutation in the Observed Bendiocarb Resistance
To assess the implication of the G119S mutation in the moderate resistance to bendiocarb observed in Kedougou, 7 mosquitoes alive to bendiocarb and 16 dead after exposition were genotyped. All dead samples were homozygous susceptible (G/G119) and among the 7 alive the only one which was amplified was homozygous resistant (119S/S). This low sample size did not allow to draw a conclusion on the role of this mutation in the resistance to bendiocarb in this area. However, genotyping of the G119S-Ace1 marker in 62 field-collected mosquitoes revealed the presence of resistant allele with the frequency of 22.58%. This frequency of the resistant allele suggest that this mutation could be involved in this resistance to carbamates.
3.10. Expression Profiling of Metabolic Genes
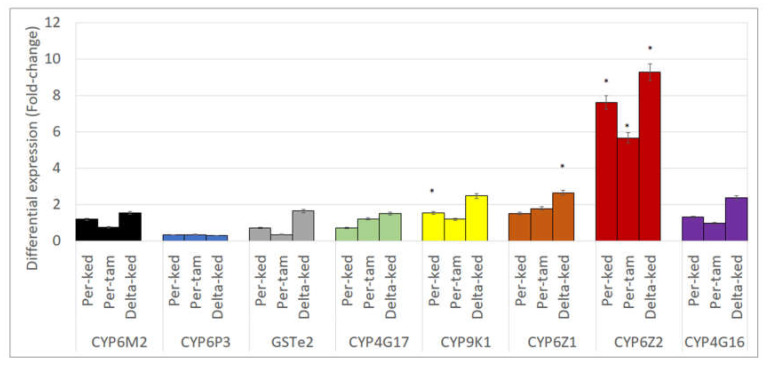
The expression level of CYP6M2, CYP6P3, CYP4G16, CYP4G17, CYP9K1, CYP6Z1, CYP6Z2 and GSTe2 was evaluated in An. gambiae from Kedougou and Tambacounda using the susceptible An. gambiae laboratory strain (Kisumu) as a control. The results showed no difference in the expression level of CYP6M2, CYP6P3, CYP4G16, CYP4G17, and GSTe2 between Kisumu and the field-collected An. gambiae. Only CYP6Z2 was found highly overexpressed, in Deltamethrin (Fold-change (FC) = 9.26 ± 4.99) (t = 2.4; df = 4; p = 0.03) and Permethrin (FC = 7.62 ± 5.26) (t = 3.2; df = 4; p = 0.01) resistant mosquitoes from Kedougou compared to Kisumu. This gene tended also to be overexpressed (FC = 5.66 ± 0.14) in Permethrin resistant mosquitoes from Tambacounda compared to Kisumu (t = 1.9; df = 4; p = 0.05) like the CYP9K1 gene in Permethrin resistant mosquitoes from Kedougou (t = 2.09; df = 4; p = 0.05). Furthermore, CYP6Z1 was significantly overexpressed in Deltamethrin resistant mosquitoes from Kedougou (FC: 2.64 ± 0.29) (t = 2.7; df = 4; p = 0.02) (Figure 5).
Figure 5.
Differential expression by quantitative reverse-transcription polymerase chain reaction of the major insecticide resistance genes in An. gambiae in Senegal compared with the susceptible Kisumu. Error bars represent standard error of the mean. *—statistically significant p ≤ 0.05.
4. Discussion
In this study the main aim was to determine the insecticide resistance profile and the distribution of kdr mutation in An. coluzzii and An. gambiae population from their sympatric and allopatric or predominance area in Senegal in the 2017 and 2018 raining season.
An. gambiae was predominant in rural and most humid areas (Kedougou and Tambacounda), while An. coluzzii was the most abundant in the arid area (Fatick). An. coluzzii and An. gambiae differ in their ecological preference both at the larval or adult stages [36] thus explaining their spatial and temporal distribution [37,38]. An. gambiae larvae are found in rain-dependent surface water bodies/puddles while those of An. coluzzii are more adapted to more permanent anthropogenic breeding sites such as irrigated rice fields [6,11,39,40,41]. Furthermore, An. coluzzii larvae displayed a greater tolerance to aridity and even organic pollution [42].
The low Plasmodium infection rate found in An. coluzzii and An. gambiae from Fatick and Tambacounda and Kedougou corroborate with other findings [43,44]. It could be due by the use of LLINs which reduce the human vector contact or cause a behavioural change of the vector. This was demonstrated in An. gambiae which was highly anthropophilic before the widespread use of nets showed a trophic deviation towards cattle [45].
Globally, the results of bioassays showed that the populations tested were resistant to DDT and Pyrethroids and susceptible to organophosphate and carbamate except in Kedougou where bendiocarb resistance was suspected. Previous studies in Senegal showed the same status of resistance in Tambacounda, Kedougou and other location [20,46,47]. However, in Fatick, as our findings, it was reported a susceptibility to bendiocarb and suspected resistance to deltamethrin but, they found a suspected resistance to pirimiphos methyl with what we have found a susceptibility [48]. In contrast to our findings, recently it was found a high resistance to bendiocarb and Pirimiphos methyl in an urban area in Senegal due to the large use of this molecule in the crops protection [9].
The resistance to DDT and pyrethroids is common in most African countries [6,40,47,49]. DDT resistance has often been linked to its historical use for vector-borne diseases and crop pest control. Despite the fact that this insecticide have been abandoned, DDT could persist in the environment due to its widespread use in public health and agriculture in the past decades [40,50]. Furthermore, the cross-resistance between pyrethroids and DDT through kdr could further explain the high DDT resistance. Resistance to pyrethroids could be due to the fact that they are the main molecules recommended for bed nets impregnation which is largely distributed across several African countries [2] as noted in Senegal [51,52,53,54]. In Kedougou food crops were practiced during the raining season which could involve the use of commercial pesticides comprising carbamate and pyrethroids. This could explain the suspected resistance to bendiocarb in this area.
The resistance level varied between the three sites. It was low in Fatick compared to Tambacounda and Kedougou. The resistance assay showed a high intensity of resistance in Tambacounda and moderate intensity in Kedougou. This could be explained by the fact that in addition to the LLNs use in Tambacounda, this site is an area with intense agriculture activity (banana, rice) with recurrent use of pesticides on crops throughout the year compared to Kedougou where subsistence crops are practicing only during the raining season. This finding corroborates with previous studies showing a significant correlation between agriculture intensity and phenotypic resistance in Tanzania [55]. Previous studies showed that in west Africa, pyrethroid resistance is high and predominant in An. gambiae compared to An. arabiensis [20,56] this could explain the decrease of pyrethroid resistance in 2018 compared to 2017. In this latter, bioassay was conducted in September–October when An. gambiae was predominant and in 2018 in October–November where An. arabiensis proportion became important.
The predominance of the L1014F mutation has been highlighted in An. gambiae from west Africa [6,12,57]. Previous studies have reported the presence of this mutation in An. gambiae only and not in An. coluzzii even in the sympatric areas [57,58]. This situation was found in 2009 in Tambacounda (Senegal) where no An. coluzzii carried the mutation [13]. However, subsequent study in this same area in 2016 by the same authors [20] reported the presence of the L1014F mutation in both species. In the present study, the findings corroborate with those of Niang [20] with the presence of the 1014F mutation in both species in the same area, but the frequency of the mutation was higher in our study. This support the results in Benin [10] and Mali [59] with a high frequency of the mutation in both species.
In Kedougou the An. gambiae predominant area, the L1014F mutation was higher and tended to fixation in this population. However, in Fatick the predominance area of An. coluzzii 89% of this latter were susceptible. A very low frequency of the mutation in a predominant area of An. coluzzii was also found in previous studies in west Africa [6,60]. The presence of the L1014F mutation in An. coluzzii has been attributed to introgression from An. gambiae [15,61] and the results found here in the sympatric can support this hypothesis.
The L1014S mutation was detected only in An. arabiensis and An. coluzzii [9,48] in Senegal and only in An. arabiensis in Benin [10]. However, in this study, in addition to An. coluzzii (two) in Fatick we have also found one An. gambiae carrying the mutation but all at the heterozygote state. Similar result was also found in central Africa (in Equatorial Guinea [62] and in Cameroun [63,64])
The presence of the N1575Y mutation has been reported in West Africa [18,19,65]. This study was the first showing the presence of this mutation in Senegal It was found to be present in both An. coluzzii and An. gambiae in Tambacounda the sympatric area and Kedougou the predominance zone of An. gambiae where the frequency of the L1014F mutation was high. However, it was absent in Fatick, the predominance area of An. coluzzii where the frequency of the L1014F mutation was very low. This corroborates the results of Jones and collaborators reporting no N1575Y mutation in the samples from areas of low frequency of the L1014F mutation. In this study, the N1575Y mutation in both species were found exclusively in mosquitoes harbouring the L1014F mutation as found in Burkina Faso [19]. This finding supports the hypothesis that N1575Y mutation was linked to the L1014F mutation suggesting that the N1575Y mutation compensates for deleterious fitness effects of L1014F and/or confers additional resistance to insecticides [18].
The G119S-Ace-1 mutation is found to be involved in bendiocarb resistance in the An. gambiae s.l. in West Africa [16,17]. However, in this study the mutation is well present in the adult population, but further studies are needed to confirm its implication in the bendiocarb resistance in this area.
As found in previous studies [66,67], the absence of correlation between the kdrw mutation and resistance to pyrethroid in the An. gambiae population from Kedougou is probably due to the fact that this resistance allele is already fixed in this location masking it’s role. Experiments performed here also suggest that metabolic resistance is playing an important role in this resistance. This was most evident based on the results of synergist bioassay with PBO showing a nearly or full recovery of the susceptibility to permethrin and deltamethrin respectively. Overexpression of P450 enzymes has been demonstrated to play a major role in pyrethroid resistance in insects [63] including in other malaria vectors such as An. funestus in Senegal [68]. Likewise, high GSTs activity was reported to be associated with insect resistance to DDT and pyrethroids [59,69]. The following candidate genes used in this study (CYP6M2, CYP6P3 [70,71,72], CYP6Z2 [73], CYP4G16, CYP4G17 [19,74,75] and CYP9K1) have been reported to be involved in pyrethroid resistance in An. gambiae in Africa [76].
In this study only CYP6Z1 and CYP6Z2 have been differentially expressed between field-resistant mosquitoes and the susceptible strain suggesting a potential implication of these two genes in the pyrethroid resistance observed.
5. Conclusions
This findings of high pyrethroid and DDT resistance in An. gambiae and An. coluzzii from Senegal is a major obstacle to malaria control using pyrethroid or DDT-based tools. PBO or Duo nets and IRS with organophosphates could be used as an alternative measure to sustain malaria control in the study area as metabolic resistance was found implicated. Full susceptibility was noticed with organophosphate and carbamates. Our findings showed that the L1014F mutation is widespread in the sympatric An. coluzzii population and that the L1014S is present at very low frequency in both species. This study reveals for the first time the presence of the N1575Y mutation in An. coluzzii and An. gambiae in Senegal. Further studies are needed to better understand the evolution of this mutation and its implication to the resistance.
Acknowledgments
We would like to thank the team of the entomology laboratory of Institut Pasteur de Dakar where the molecular identification of the species was carried out. Thanks to The CRID team and also the population from the different study areas for their support and for making possible this work.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/12/1403/s1, Table S1. Species composition of the An. gambiae s.l collected indoor. Table S2. Species composition of adult from Larvae collection. Table S3. Genetic diversity parameter of voltage gate sodium channel gene.
Author Contributions
O.K.G., M.B.F. and A.A.A. undertook the field work. O.K.G., M.J.W., L.M.J.M. carried the lab work. O.K.G., M.T., A.K.D. and D.N.N. analysed the results. O.K.G., M.T., E.H.A.N. and C.S.W. drafted the manuscript. F.T., C.S.W., A.D. and E.H.A.N. designed the study. F.T., I.D., C.S.W., A.D., L.K., O.G., O.F. and E.H.A.N. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the DELTAS Africa Initiative [DEL-15-010]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Welcome Trust [grant:107741/A/15/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Conflicts of Interest
The authors declare that they have no competing interest.
Availability of Data and Material
The datasets generated and/or analysed during the current study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics committee of University Cheikh Anta DIOP of Dakar, Senegal with the number 0233/2017/CER/UCAD.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Okumu F.O., Chipwaza B., Madumla E.P., Mbeyela E., Lingamba G., Moore J., Ntamatungro A.J., Kavishe D.R., Moore S.J. Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and long-lasting insecticide nets are combined for malaria prevention. Malar. J. 2012;11:378. doi: 10.1186/1475-2875-11-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation . World Malaria Report. World Health Organisation; Geneva, Switzerland: 2019. p. 232. [Google Scholar]
- 3.Martinez-Torres D., Chandre F., Williamson M.S., Darriet F., Berge J.B., Devonshire A.L., Guillet P., Pasteur N., Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 4.Ranson H., Jensen B., Vulule J.M., Wang X., Hemingway J., Collins F.H. Identification BlackwellScience, Ltd of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000:7. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Ranson H., N’Guessan R., Lines J., Moiroux N., Nkuni Z., Corbel V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Diabate A., Thierry B., Chandre C., Roch D.K., Pierre K., Robert G.T., Frederic S., Pierre G., Janet H., Marc H.J. KDR Mutation, a Genetic Marker to Assess Events of Introgression Between the Molecular M and S Forms of Anopheles gambia (Diptera: Culicidae) in the Tropical Savannah Area of West Africa. J. Med. Entomol. 2003;40:195–198. doi: 10.1603/0022-2585-40.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Della Torre A., Fanello C., Akogbeto M., Dossou-yovo J., Favia G., Petrarca V., Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol. Biol. 2001;10:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 8.Coetzee M., Hunt R.H., Wilkerson R., Torre A.D., Coulibaly M.B., Besansky N.J. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013:3619. doi: 10.11646/zootaxa.3619.3.2. [DOI] [PubMed] [Google Scholar]
- 9.Dia A.K., Guèye O.K., Niang E.A., Diédhiou S.M., Sy M.D., Konaté A., Samb B., Diop A., Konaté L., Faye O. Insecticide resistance in Anopheles arabiensis populations from Dakar and its suburbs: Role of target site and metabolic resistance mechanisms. Malar. J. 2018;17:116. doi: 10.1186/s12936-018-2269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djègbè I., Boussari O., Sidick A., Martin T., Ranson H., Chandre F., Akogbéto M., Corbel V. Dynamics of insecticide resistance in malaria vectors in Benin: First evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar. J. 2011;10:261. doi: 10.1186/1475-2875-10-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djogbénou L., Pasteur N., Akogbéto M., Weill M., Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: A nationwide survey. Med. Vet. Entomol. 2011;25:256–267. doi: 10.1111/j.1365-2915.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- 12.Santolamazza F., Calzetta M., Etang J., Barrese E., Dia I., Caccone A., Donnelly M.J., Petrarca V., Simard F., Pinto J., et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar. J. 2008;7:74. doi: 10.1186/1475-2875-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niang E.A. Université Cheikh Anta Diop de Dakar, Dakar, Senegal, Distribution des Formes Moléculaires d’Anopheles Gambiae s.s. et du Gène de la Résistance au DDT et aux Pyréthrinoides (kdr) chez les Vecteurs du Paludisme au Sénégal. Dakar. Sénégal, UCAD; DEA de Biologie Animale: 97–2009. 2009. Unpublished work.
- 14.Koukpo C.Z., Fassinou A.J.Y.H., Ossè R.A., Agossa F.R., Sovi A., Sewadé W.T., Aboubakar S., Assogba B.S., Akogbeto M.C., Sezonlin M. The current distribution and characterization of the L1014F resistance allele of the kdr gene in three malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis) in Benin (West Africa) Malar. J. 2019;18:175. doi: 10.1186/s12936-019-2808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weill M., Chandre F., Brengues C., Manguin S., Akogbeto M., Pasteur N., Guillet P., Raymond M. The kdr mutation occurs in the Mopti form of Anopheles gambiaes.s. through introgression. Insect Mol. Biol. 2000;9:451–455. doi: 10.1046/j.1365-2583.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- 16.Djogbénou L., Dabiré R., Diabaté A., Kengne P., Akogbéto M., Hougard J.M., Chandre F. Identification and Geographic Distribution of the ACE-1 R Mutation in the Malaria Vector Anopheles gambiae in South-Western Burkina Faso, West Africa. Am. J. Trop. Med. Hyg. 2008;78:298–302. doi: 10.4269/ajtmh.2008.78.298. [DOI] [PubMed] [Google Scholar]
- 17.Djogbénou L. Vector Control Method against Malaria and Vector Resistance to Insecticides in Africa. Med. Trop. 2009;69:160–164. [PubMed] [Google Scholar]
- 18.Jones C.M., Liyanapathirana M., Agossa F.R., Weetman D., Ranson H., Donnelly M.J., Wilding C.S. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2012;109:6614–6619. doi: 10.1073/pnas.1201475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toé K.H., N’Falé S., Dabiré R.K., Ranson H., Jones C.M. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015;16:146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niang E.H.A., Konaté L., Diallo M., Faye O., Dia I. Patterns of insecticide resistance and knock down resistance (kdr) in malaria vectors An. arabiensis, An. coluzzii and An. gambiae from sympatric areas in Senegal. Parasites Vectors. 2016;9 doi: 10.1186/s13071-016-1354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass C., Nikou D., Blagborough A.M., Vontas J., Sinden R.E., Williamson M.S., Field L.M. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 2008;7:177. doi: 10.1186/1475-2875-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snounou G., Viriyakosol S., Jarra W., Thaithong S., Brown K.N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 24.Gillies M.T., De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) Publ. S. Afr. Inst. Med. Res. 1968;54:1–343. [Google Scholar]
- 25.Livak K.J. Organization and Mapping of a Sequence on the DROSOPHILA MELANOGASTER X and Y Chromosomes That is Transcribed during Spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santolamazza F., Mancini E., Simard F., Qi Y., Tu Z., della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkins E.E., Marcet P.L., Sutcliffe A.C., Howell P.I. Authentication scheme for routine verification of genetically similar laboratory colonies: A trial with Anopheles gambiae. BMC Biotechnol. 2009;9:91. doi: 10.1186/1472-6750-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bass C., Nikou D., Donnelly M.J., Williamson M.S., Ranson H., Ball A., Vontas J., Field L.M. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 2007;6:111. doi: 10.1186/1475-2875-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bass C., Nikou D., Vontas J., Williamson M.S., Field L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010;96:80–85. doi: 10.1016/j.pestbp.2009.09.004. [DOI] [Google Scholar]
- 30.Pinto J., Lynd A., Vicente J.L., Santolamazza F., Randle N.P., Gentile G., Moreno M., Simard F., Charlwood J.D., do Rosário V.E., et al. Multiple Origins of Knockdown Resistance Mutations in the Afrotropical Mosquito Vector Anopheles gambiae. PLoS ONE. 2007;2:e1243. doi: 10.1371/journal.pone.0001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall T.B. A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acid Symp. 1999;41:95–98. [Google Scholar]
- 32.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Gimonneau G., Pombi M., Choisy M., Morand S., Dabiré R.K., Simard F. Larval habitat segregation between the molecular forms of the mosquito Anopheles gambiae in a rice field area of Burkina Faso, West Africa. Med. Vet. Entomol. 2012;26:9–17. doi: 10.1111/j.1365-2915.2011.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niang A., Epopa P.S., Sawadogo S.P., Maïga H., Konaté L., Faye O., Dabiré R.K., Tripet F., Diabaté A. Does extreme asymmetric dominance promote hybridization between Anopheles coluzzii and Anopheles gambiae s.s. in seasonal malaria mosquito communities of West Africa? Parasites Vectors. 2015;8:586. doi: 10.1186/s13071-015-1190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touré Y.T., Petrarca V., Traoré S.F., Coulibaly A., Maiga H.M., Sankaré O., Sow M., Di M.D., Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- 39.Diabate A., Dabire R.K., Kim E.H., Dalton R., Millogo N., Baldet T., Simard F., Gimnig J.E., Hawley W.A., Lehmann T. Larval Development of the Molecular Forms of Anopheles gambiae (Diptera: Culicidae) in Different Habitats: A Transplantation Experiment. J. Med. Entomol. 2005;42:6. doi: 10.1093/jmedent/42.4.548. [DOI] [PubMed] [Google Scholar]
- 40.Innocent D., Abel M.A., Rousseau D., Martin A. Surveillance Entomologique: Dynamique de la population et de la résistance aux insecticides chez Anopheles gambiae s.l en milieu de riziculture irriguée au Sud Bénin. J. Appl. Biosci. 2017;11:10943. [Google Scholar]
- 41.Torre A.D., Tu Z., Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem. Mol. Biol. 2005;35:755–769. doi: 10.1016/j.ibmb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Kamdem C., Tene Fossog B., Simard F., Etouna J., Ndo C., Kengne P., Boussès P., Etoa F.-X., Awono-Ambene P., Fontenille D., et al. Anthropogenic Habitat Disturbance and Ecological Divergence between Incipient Species of the Malaria Mosquito Anopheles gambiae. PLoS ONE. 2012;7:e39453. doi: 10.1371/journal.pone.0039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wotodjo A.N., Richard V., Boyer S., Doucoure S., Diagne N., Touré-Baldé A., Tall A., Faye N., Gaudart J., Trape J.-F., et al. The implication of long-lasting insecticide-treated net use in the resurgence of malaria morbidity in a Senegal malaria endemic village in 2010–2011. Parasites Vectors. 2015;8:267. doi: 10.1186/s13071-015-0871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niang E.H.A., Touré A., Ngom E.H.M., Konaté L., Faye O., Diallo M., Dia I. Malaria Transmission Pattern in an Area Selected for Clinical Trials in the Sudanian Area of Senegal (West Africa) J. Trop. Med. 2013;2013:907375. doi: 10.1155/2013/907375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefèvre T., Gouagna L.-C., Dabire K.R., Elguero E., Fontenille D., Costantini C., Thomas F. Evolutionary lability of odour-mediated host preference by the malaria vector Anopheles gambiae. Trop. Med. Int. Health. 2009;14:228–236. doi: 10.1111/j.1365-3156.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 46.Fall Evaluation de la Sensibilité Des Vecteurs du Paludisme Aux Insecticides Dans la Région de Kédougou et Recherche de Mécanismes de Résistance. [(accessed on 20 September 2020)];2016 Available online: www.memoironline.com.
- 47.Faye O., Konate L., Diop A. Profil Entomologique du Sénégal. Senegal National Malaria Control Program; Dakar, Senegal: 2011. p. 47. [Google Scholar]
- 48.Thiaw O., Doucouré S., Sougoufara S., Bouganali C., Konaté L., Diagne N., Faye O., Sokhna C. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar. J. 2018;17:123. doi: 10.1186/s12936-018-2276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandre F., Darrier F., Manga L., Akogbeto M., Faye O., Mouchet J. Situation de la resistance aux pyre thrinoıdes chez Anopheles gambiae sensu lato. Bull. l’Organ. Mond. Santé. 1999;1:32–36. [PMC free article] [PubMed] [Google Scholar]
- 50.Reid M.C., McKenzie F.E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 2016;15:107. doi: 10.1186/s12936-016-1162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diouf M., Diouf E., Niang E., Diagne C., Konaté L., Faye O. Evaluation de l’état physique et de l’efficacité biologique de deux types de moustiquaires imprégnées à longue durée d’action utilisés depuis 5 à 36 mois et collectés dans 11 districts du Sénégal. Bull. Soc. Pathol. Exot. 2018;111:126–131. doi: 10.3166/bspe-2018-0015. [DOI] [PubMed] [Google Scholar]
- 52.PNLP Bulletin Epidemiologique Annuel du Paludisme au Senegal 2015. [(accessed on 20 September 2020)]; Available online: www.pnlp.sn.
- 53.PNLP Bulletin Epidemiologique Annuel du Paludisme au Senegal 2017. [(accessed on 20 September 2020)]; Available online: www.pnlp.sn.
- 54.Thwing J.I., Perry R.T., Townes D.A., Diouf M.B., Ndiaye S., Thior M. Success of Senegal’s first nationwide distribution of long-lasting insecticide-treated nets to children under five-contribution toward universal coverage. Malar. J. 2011;10:86. doi: 10.1186/1475-2875-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nkya T.E., Akhouayri I., Poupardin R., Batengana B., Mosha F., Magesa S., Kisinza W., David J.-P. Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: A case study in Tanzania. Malar. J. 2014;13:28. doi: 10.1186/1475-2875-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbel V., N’Guessan R. Distribution, mechanisms, impact and management of insecticide resistance in Malaria vectors: A pragmatic review. In: Manguin S., editor. Anopheles Mosquitoes–New Insights into Malaria Vectors. InTech; London, UK: 2013. [Google Scholar]
- 57.Awolola T.S., Oyewole I.O., Amajoh C.N., Idowu E.T., Ajayi M.B., Oduola A., Manafa O.U., Ibrahim K., Koekemoer L.L., Coetzee M. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop. 2005;95:204–209. doi: 10.1016/j.actatropica.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Awolola T.S., Oduola O.A., Strode C., Koekemoer L.L., Brooke B., Ranson H. Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2009;103:1139–1145. doi: 10.1016/j.trstmh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 59.Cisse M.B.M., Keita C., Dicko A., Dengela D., Coleman J., Lucas B., Mihigo J., Sadou A., Belemvire A., George K., et al. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar. J. 2015;14:327. doi: 10.1186/s12936-015-0847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czeher C., Labbo R., Arzika I., Duchemin J.-B. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar. J. 2008;7:189. doi: 10.1186/1475-2875-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gentile G., Santolamazza F., Fanello C., Petrarca V., Caccone A., Della Torre A. Variation in an intron sequence of the voltage-gated sodium channel gene correlates with genetic differentiation between Anopheles gambiae s.s. molecular forms: Genetic differentiation in A. gambiae molecular forms. Insect Mol. Biol. 2004;13:371–377. doi: 10.1111/j.0962-1075.2004.00494.x. [DOI] [PubMed] [Google Scholar]
- 62.Ridl F.C., Bass C., Torrez M., Govender D., Ramdeen V., Yellot L., Edu A.E., Schwabe C., Mohloai P., Maharaj R., et al. A pre-intervention study of malaria vector abundance in Rio Muni, Equatorial Guinea: Their role in malaria transmission and the incidence of insecticide resistance alleles. Malar. J. 2008;7:194. doi: 10.1186/1475-2875-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nwane P., Etang J., Chouaїbou M., Toto J.C., Koffi A., Mimpfoundi R., Simard F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites Vectors. 2013;6:41. doi: 10.1186/1756-3305-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reimer L., Fondjo E., Patchoke S., Diallo B., Lee Y., Ng A., Ndjemai H.M., Atangana J., Traore S.F., Lanzaro G., et al. Relationship Between kdr Mutation and Resistance to Pyrethroid and DDT Insecticides in Natural Populations of Anopheles gambiae. J. Med. Entomol. 2008;45:7. doi: 10.1093/jmedent/45.2.260. [DOI] [PubMed] [Google Scholar]
- 65.Edi A.V.C., N’Dri B.P., Chouaibou M., Kouadio F.B., Pignatelli P., Raso G., Weetman D., Bonfoh B. First detection of N1575Y mutation in pyrethroid resistant Anopheles gambiae in Southern Côte d’Ivoire. Wellcome Open. Res. 2017;2:71. doi: 10.12688/wellcomeopenres.12246.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donnelly M.J., Corbel V., Weetman D., Wilding C.S., Williamson M.S., Black W.C. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Okorie P.N., Ademowo O.G., Irving H., Kelly-Hope L.A., Wondji C.S. Insecticide susceptibility of Anopheles coluzzii and Anopheles gambiae mosquitoes in Ibadan, Southwest Nigeria: Susceptibility to insecticide of Anopheles. Med. Vet. Entomol. 2015;29:44–50. doi: 10.1111/mve.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samb B., Konate L., Irving H., Riveron J.M., Dia I., Faye O., Wondji C.S. Investigating molecular basis of lambda-cyhalothrin resistance in an Anopheles funestus population from Senegal. Parasites Vectors. 2016;9:449. doi: 10.1186/s13071-016-1735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding Y., Ortelli F., Rossiter L.C., Hemingway J., Ranson H. The Anopheles gambiae glutathione transferase supergene family: Annotation, phylogeny and expression profiles. BMC Genom. 2003;4:35. doi: 10.1186/1471-2164-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adolfi A., Poulton B., Anthousi A., Macilwee S., Ranson H., Lycett G.J. Functional genetic validation of key genes conferring insecticide resistance in the major African malaria vector, Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2019;116:25764–25772. doi: 10.1073/pnas.1914633116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Djouaka R.F., Bakare A.A., Coulibaly O.N., Akogbeto M.C., Ranson H., Hemingway J., Strode C. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genom. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stevenson B.J., Bibby J., Pignatelli P., Muangnoicharoen S., O’Neill P.M., Lian L.-Y., Müller P., Nikou D., Steven A., Hemingway J., et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011;41:492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Donnelly M.J., Isaacs A.T., Weetman D. Identification, Validation, and Application of Molecular Diagnostics for Insecticide Resistance in Malaria Vectors. Trends Parasitol. 2016;32:197–206. doi: 10.1016/j.pt.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balabanidou V., Kampouraki A., MacLean M., Blomquist G.J., Tittiger C., Juárez M.P., Mijailovsky S.J., Chalepakis G., Anthousi A., Lynd A., et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2016;113:9268–9273. doi: 10.1073/pnas.1608295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ingham V.A., Jones C.M., Pignatelli P., Balabanidou V., Vontas J., Wagstaff S.C., Moore J.D., Ranson H. Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: Known and novel candidate genes. BMC Genom. 2014;15:1018. doi: 10.1186/1471-2164-15-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vontas J., Grigoraki L., Morgan J., Tsakireli D., Fuseini G., Segura L., Niemczura de Carvalho J., Nguema R., Weetman D., Slotman M.A., et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA. 2018;115:4619–4624. doi: 10.1073/pnas.1719663115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon request.