Abstract
Ingesting protein-containing supplements and foods provides essential amino acids (EAA) necessary to increase muscle and whole-body protein synthesis (WBPS). Large variations exist in the EAA composition of supplements and foods, ranging from free-form amino acids to whole protein foods. We sought to investigate how changes in peripheral EAA after ingesting various protein and free amino acid formats altered muscle and whole-body protein synthesis. Data were compiled from four previous studies that used primed, constant infusions of L-(ring-2H5)-phenylalanine and L-(3,3-2H2)-tyrosine to determine fractional synthetic rate of muscle protein (FSR), WBPS, and circulating EAA concentrations. Stepwise regression indicated that max EAA concentration (EAACmax; R2 = 0.524, p < 0.001), EAACmax (R2 = 0.341, p < 0.001), and change in EAA concentration (ΔEAA; R = 0.345, p < 0.001) were the strongest predictors for postprandial FSR, Δ (change from post absorptive to postprandial) FSR, and ΔWBPS, respectively. Within our dataset, the stepwise regression equation indicated that a 100% increase in peripheral EAA concentrations increases FSR by ~34%. Further, we observed significant (p < 0.05) positive (R = 0.420–0.724) correlations between the plasma EAA area under the curve above baseline, EAACmax, ΔEAA, and rate to EAACmax to postprandial FSR, ΔFSR, and ΔWBPS. Taken together our results indicate that across a large variety of EAA/protein-containing formats and food, large increases in peripheral EAA concentrations are required to drive a robust increase in muscle and whole-body protein synthesis.
Keywords: protein quality, essential amino acids, protein, muscle protein synthesis, whole body protein synthesis, amino acid kinetics, nutrition, aging, anabolism
1. Introduction
Amino acids are the fundamental constituents of body proteins and serve as substrates for protein synthesis. Nine amino acids are considered essential amino acids (EAA), meaning they cannot be synthesized de novo, or the synthesis rate does not adequately meet the body’s demand. Therefore, EAA must be obtained through dietary protein. Dietary protein formats have a wide variance of EAA content, digestion, and absorption kinetics that can be used to meet EAA requirements [1].
The net balance between muscle protein synthesis (MPS) and breakdown (MPB) distinguishes the anabolic (synthesis exceeds breakdown) and the catabolic (breakdown exceeds synthesis) states. Since nonessential amino acids are normally readily available in muscle, the intracellular appearance of EAA derived from MPB or inward influx from plasma govern the anabolic response [2]. The possible fates of intracellular EAA are protein synthesis via charging the appropriate transfer ribonucleic acid (tRNA), oxidation, or efflux back to plasma. In the post-absorptive state, the primary source of intracellular EAA appearance is the accelerated rate of MPB, which is the principal determinant of the amount of intracellular EAA available as precursors for MPS [3]. Since intracellular amino acid recycling is not 100% efficient, MPB will always exceed MPS in the post-absorptive state, resulting in a net loss of muscle protein. In order to replace the lost muscle protein, exogenous EAA are required to increase circulating concentrations to induce a stimulation in MPS [4], while simultaneously reducing MPB [5,6,7]. Thus, dietary EAA are the primary stimuli for an increase in MPS and subsequent expansion of the skeletal muscle protein pool [5,6]. In addition to its role in functionality, skeletal muscle serves as a reservoir of EAA for splanchnic organs and tissue during periods of stress or insufficient dietary intake [8].
Skeletal muscle accounts for a significant portion of protein in the body, but other tissues may account for more than half the total protein turnover in the body. Recent investigations have demonstrated multiple tissues with higher turnover rates than skeletal muscle [9,10,11]. Estimates of the contribution of skeletal muscle to whole-body protein turnover range considerably (25–50%), and likely dependent upon metabolic status [12,13], and in part the tracer methodology used to quantify whole-body and MPS rates. Considering the important role of the body protein pool during catabolic stress and anabolic resistance, it is important to understand the effects of dietary EAA intakes on muscle and whole-body protein synthesis. It is also important to note that the measurement of muscle and whole-body protein synthesis are indications of protein turnover, not anabolism. Anabolism cannot be ascertained unless both protein synthesis and breakdown rates are measured. Measurement of MPB following a meal is only possible using arterial and venous catheterization, and available data using this invasive approach are limited. MPB rate can only be measured in the post-absorptive state [14,15,16,17]. Whole-body protein breakdown can be ascertained, but requires certain assumptions that are controversial [18,19,20,21]. Therefore, we chose to focus on the relationship between EAA availability and the measurement of protein synthesis according to established isotope methodology [22].
2. Materials and Methods
2.1. Data Selection and Extraction
The present study includes data from previously published studies from our lab [23,24,25,26]. Participant demographics and treatments are outlined in Table 1. Written informed consent was obtained from all subjects, and studies were approved by the Institutional Review Board (#89220, 205336, 206579, 206814, and 217658) at the University of Arkansas for Medical Sciences. Participants were healthy young or older males who had refrained from physical activity for at least 72-h. Studies were conducted after an overnight fast, and isotope infusion was utilized to determine mixed MPS and whole-body protein synthesis.
Table 1.
Participant demographics from compiled studies 1.
| Study | Group | N (M/F) | Age (y) | BM (kg) | BMI (kg/m2) | LBM (kg) | BF (%) |
|---|---|---|---|---|---|---|---|
| Park 2020 European Journal of Nutrition [25] | 3.5 oz Ground Beef and 2 green kiwis | 11 (5/5) | 72.5 ± 1.9 | 82.5 ± 2.2 | 28.7 ± 0.8 | 48.5 ± 2.5 | 36.4 ± 2.1 |
| 3.5 oz Ground Beef and 2 gold kiwis | |||||||
| Park 2020 Journal of International Society of Sports Nutrition [23] | 2.4 g Whey + 3.2 g Free Form EAA | 8 (3/5) | 21.4 ± 0.5 | 73.8 ± 4.8 | 24.6 ± 0.8 | 51.6 ± 4.9 | 21.1 ± 2.2 |
| 4.8 g Whey + 6.4 g Free Form EAA | |||||||
| 12.6 g Whey | 8 (4/4) | 26.9 ± 2.0 | 76.2 ± 3.1 | 25.7 ± 1.6 | 49.5 ± 2.6 | 24.8 ± 4.1 | |
| Park 2020 Journal of Nutrition [26] | 2 oz Ground Beef | 8 (4/4) | 21.8 ± 2.2 | 76.3 ± 4.6 | 24.9 ± 1.0 | 49.2 ± 4.1 | 30.1 ± 3.1 |
| 2 oz Beef Sirloin | 8 (4/4) | 23.9 ± 1.6 | 68.0 ± 4.0 | 23.5 ± 1.0 | 43.5 ± 3.3 | 31.0 ± 2.4 | |
| 2 Cooked Eggs | 8 (4/4) | 23.9 ± 1.9 | 74.2 ± 5.4 | 24.4 ± 1.3 | 49.1 ± 3.4 | 27.5 ± 2.5 | |
| 2 oz Pork Loin | 8 (4/4) | 22.1 ± 1.0 | 74.3 ± 3.5 | 24.5 ± 0.9 | 51.8 ± 4.6 | 29.9 ± 3.9 | |
| 1/2C Kidney Beans | 8 (4/4) | 23.8 ± 1.9 | 69.8 ± 5.7 | 24.1 ± 1.7 | 44.8 ± 3.1 | 30.1 ± 2.5 | |
| 2T Peanut Butter | 8 (4/4) | 20.3 ± 1.5 | 70.4 ± 3.8 | 24.1 ± 1.1 | 48.1 ± 3.8 | 27.1 ± 2.3 | |
| 4 oz Tofu | 8 (4/4) | 25.9 ± 2.2 | 75.9 ± 2.2 | 25.9 ± 1.0 | 49.7 ± 4.2 | 33.0 ± 3.0 | |
| 1 oz Mixed Nuts | 8 (4/4) | 24.3 ± 2.1 | 74.3 ± 5.3 | 24.9 ± 1.2 | 49.3 ± 3.9 | 32.2 ± 2.2 | |
| Church 2020 Journal of International Society of Sports Nutrition [24] | 3.6 g Free Form EAA | 11 (5/6) | 68.8 ± 1.8 | 81.4 ± 5.69 | 31.8 ± 5.7 | 49.7 ± 3.6 | 35.7 ± 2.2 |
| 10.8 g Free Form EAA | 12 (8/4) | 67.4 ± 1.5 | 83.4 ± 5.5 | 27.5 ± 1.3 | 53.1 ± 3.9 | 35.4 ± 3.2 | |
| Sample Means | - | 39.9 ± 2.0 | 76.1 ± 1.2 | 26.1 ± 0.6 | 49.5 ± 0.9 | 32.2 ± 0.8 | |
1 Data reported as mean ± SEM. BM = body mass; BMI= body mass index; LBM = lean body mass; BF = body fat; y = years; kg = kilograms; m = meters; oz. = ounces; g = grams; EAA = essential amino acids; C = cup; T = tablespoon. Data are means ± SEM.
2.2. Infusion Trials
Studies were conducted after an overnight fast, and isotope infusion was utilized to determine mixed MPS and whole-body protein synthesis. Two intravenous catheters were placed; one in the antecubital space for the continuous isotope infusions, and the second in the contralateral dorsal hand vein for serial blood draws. The dorsal hand vein was kept warm using heating pads to reflect arterialized blood [27]. After collecting the baseline blood sample, primed, constant infusions of L-(ring-2H5)-phenylalanine and L-(3,3-2H2)-tyrosine were started and maintained. A priming dose of L-(ring-2H4)-tyrosine was also administered at the same time to achieve isotopic equilibrium of L-(ring-2H4) tyrosine enrichment derived from L-(ring-2H5)-phenylalanine for the measurement of phenylalanine hydroxylation. All isotopes were purchased from Cambridge Isotope Laboratories (Andover, MA, USA) and the preparations were constituted by the research pharmacist at UAMS.
2.3. Analytic Procedures
Plasma was precipitated with 125 µL of 10% sulfosalicylic acid (SSA), centrifuged, and the supernatant was used to determine EAA concentrations using the internal standard technique [28]. Phenylalanine and tyrosine enrichments were measured using the tert-butyldimethylsilyl derivative and gas chromatography-mass spectrometry. Ions of mass-to-charge ratio of 234, 235, and 239 for phenylalanine and of 466, 467, 468, and 470 for tyrosine were monitored with electron impact ionization and selective ion monitoring. Serum insulin concentrations were measured using a Siemens Immulite 2000 XPi (Siemens Medical Solutions USA, Inc., Malvern, PA, USA).
Muscle samples were weighed, and tissue proteins were precipitated with 0.5 mL of 4% SSA. Samples were then homogenized, centrifuged, and the muscle pellet (bound protein) was washed, dried, and hydrolyzed in 0.5 mL of 6 N HCl at 105 °C for 24-h. Mixed muscle-bound protein enrichments were determined as described above for plasma enrichments.
2.4. Muscle Fractional Synthetic Rate
The precursor-product model was used to determine mixed MPS (i.e., fractional synthetic rate) [29]:
| Mixed MPS (%/h) = ((EBP2 − EBP1)/(Ep)) × 60 × 100, | (1) |
where EBP1 and EBP2 are the enrichments of bound L-(ring-2H5)-phenylalanine in muscle collected in the postabsorptive and postprandial states. In the postprandial state, the precursor enrichment (Ep) is the calculated area under the curve (AUC) for L-(ring-2H5)-phenylalanine enrichment in the plasma since it more accurately reflects the blood perturbations consistent with our and others’ previous work [23,24,25,30,31]. Factors 60 and 100 were used to express mixed MPS as percent per hour. Both the postprandial FSR and change in FSR (ΔFSR) were used for statistical analysis.
2.5. Whole-Body Protein Synthesis
Whole-body protein synthesis rates were calculated based on the determinations of the rate of appearance (Ra) into the plasma of phenylalanine and tyrosine and the fractional Ra of endogenous tyrosine converted from phenylalanine [22]. The phenylalanine (Phe) and tyrosine (Tyr) plasma enrichment mean and AUC were calculated for the postabsorptive and postprandial states, respectively. Whole-body protein synthesis was calculated by dividing kinetic values of phenylalanine by its fractional contribution to protein. The following equations were used to calculate whole-body protein synthesis [23,25,30]:
| Total plasma RaPhe = F/E, | (2) |
| Fractional Ra of Tyr from Phe = ETyr M+4/EPhe M+5, | (3) |
| Phe hydroxylation = fractional Ra of Tyr from Phe × Ra Tyr, | (4) |
| PS = ((Ra Phe − Phe hydroxylation) × 25), | (5) |
where E is enrichment of respective tracers at plateau and expressed as mole percent excess (MPE), calculated as TTR/(TTR + 1). F is the respective tracer infusion rate into a venous side. ETyr M+4 and EPhe M+5 are plasma enrichments of tyrosine and phenylalanine tracers at M + 4 and M + 5 relative to M + 0, respectively. In the fed state, fractional Ra of Tyr from Phe was divided by 0.8 to account for hepatic dilution [32]. The correction factor of 25 is for conversion of phenylalanine values to total protein based on the assumption that the contribution of phenylalanine to skeletal muscle protein is 4% (100/4 = 25) [3]. Phe hydroxylation is the Ra of tyrosine derived via endogenous hydroxylation of phenylalanine. Post-absorptive whole-body protein synthesis was subtracted from the postprandial whole-body protein synthesis and expressed as grams per hour.
2.6. Amino Acid Pharmacokinetics
The temporal response of the EAA curve was used to calculate the following variables for statistical analysis: Area under the curve above baseline (AUCi), max EAA concentration (EAACmax), EAA concentration change from baseline to EAACmax (ΔEAA), and the rate to EAACmax.
2.7. Statistical Analysis
To identify the best EAA predictor of ΔFSR, postprandial FSR, and ΔWBPS a stepwise linear regression model was used to assess the contribution of independent (AUCi, EAACmax, ΔEAA, rate to EAACMax, age, lean body mass, total kilocalories, total carbohydrates, total protein, total fat, and protein quality (EAA:total protein)) variables. Relationships between variables were assessed with Pearson’s product moment correlation. A priori α < 0.05 was used and data are expressed as means ± SEMs. Statistical analyses were performed with IBM SPSS software (version 26; IBM Corp. Armonk, NY, USA).
3. Results
3.1. Stepwise Regression
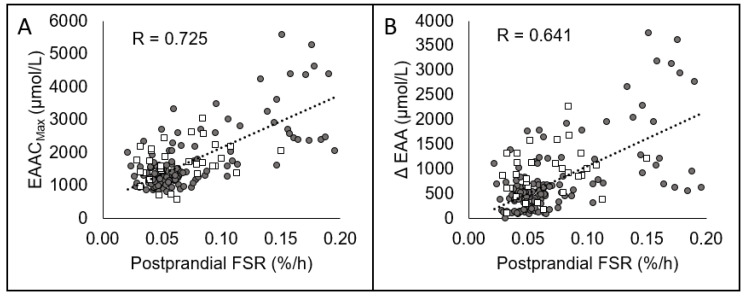
The best predictors for ΔFSR, postprandial FSR, and ΔWBPS are presented in Table 2. Stepwise regression indicated that EAACmax (R2 = 0.341, p < 0.001), EAACmax (R2 = 0.524, p < 0.001), and ΔEAA (R2 = 0.345, p < 0.001) were the strongest predictors for ΔFSR, postprandial FSR, and ΔWBPS, respectively.
Table 2.
Stepwise regression results 1.
| Best Predictor | R2 | p | Formula |
|---|---|---|---|
| ΔFSR | |||
| EAACMax | 0.341 | ≤0.001 | (0.00001933 × EAACMax) − 0.017 |
| Postprandial FSR | |||
| EAACMax | 0.524 | ≤0.001 | (0.00003307 × EAACMax) + 0.016 |
| ΔWBPS | |||
| ΔEAA | 0.345 | ≤0.001 | (0.001 × ΔEAA) + 0.242 |
1 ΔFSR = postprandial fractional synthetic rate minus postabsorptive FSR; ΔWBPS = postprandial whole-body protein synthesis minus postabsorptive whole-body protein synthesis; EAA = essential amino acid; Cmax = max concentration; Δ = change in concentration from baseline to CMax.
3.2. Correlations
The relationships between postprandial FSR and EAACmax and ΔEAA are highlighted in Figure 1A,B. Correlations between macronutrients and protein kinetics are outlined in Table 3. Correlations between AUCi, EAACmax, ΔEAA, and rate to EAACmax and postprandial FSR, ΔFSR, and ΔWBPS are outlined in Table 4.
Figure 1.
Relationships between postprandial fractional synthetic rate (FSR) to maximum essential amino acid (EAA) concentration (EAACmax; Panel A) and the change in EAA concentrations from baseline to EAACmax (ΔEAA; Panel B). Individual participant data from our lab in filled circles (●), means gathered from post-feeding data from other studies from the literature [6,27,29,30,31,32,33,34,35,36,37,38,39] in open boxes (□). Means were not used in statistical analysis, they are used to represent consistency in data relationships between labs.
Table 3.
Correlations between macronutrients and physiological variables 1.
| kcals | Protein (g) | EAA (g) | CHO (g) | Fat (g) | EAA:Protein | ||
|---|---|---|---|---|---|---|---|
| Postprandial FSR (%/h) | R | −0.334 * | −0.189 * | 0.230 * | −0.210 * | −0.402 * | 0.644 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 | |
| ΔFSR (%/h) | R | −0.346 * | −0.205 * | 0.227 * | −0.255 * | −0.383 * | 0.585 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 | |
| ΔWBPS (g/h) | R | −0.227 * | 0.033 | 0.386 * | −0.269 * | −0.279 * | 0.409 * |
| N | 112 | 112 | 112 | 112 | 112 | 112 | |
| EAA AUCi (umol/L/min) | R | 0.096 | 0.400 * | 0.822 * | 0.063 | −0.121 | 0.379 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 | |
| EAACmax (umol/L) | R | −0.429 * | −0.169 | 0.510 * | −0.342 * | −0.524 * | 0.781 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 | |
| Δ[EAA] (umol/L) | R | −0.383 * | −0.094 | 0.604 * | −0.323 * | −0.495 * | 0.733 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 | |
| Rate to Peak EAACmax (umol/L/min) | R | −0.546 * | −0.303 * | 0.418 * | −0.430 * | −0.611 * | 0.841 * |
| N | 134 | 134 | 134 | 134 | 134 | 134 |
1 kcal = kilocalories; EAA = essential amino acid; CHO = carbohydrate; EAA: protein = EAA to total protein ratio; g = grams; h = hours; FSR = fractional synthetic rate; ΔFSR = postprandial FSR minus postabsorptive FSR; WBPS = whole-body protein synthesis; ΔWBPS = postprandial WBPS minus postabsorptive WBPS; EAA AUCi = EAA area under the curve above basal; EAACmax = max EAA concentration; ΔEAA = EAACmax minus basal EAA concentration. * = significant correlation.
Table 4.
Correlations between protein kinetics and essential amino acid (EAA) pharmacokinetics 1.
| Postprandial FSR (%/h) | ΔFSR (%/h) | ΔWBPS (g/h) | ||
|---|---|---|---|---|
| EAA AUCi (umol/L/min) | R | 0.475 * | 0.420 * | 0.438 * |
| N | 130 | 130 | 112 | |
| EAACmax (umol/L) | R | 0.724 * | 0.584 * | 0.559 * |
| N | 130 | 130 | 112 | |
| Δ[EAA] (umol/L) | R | 0.641 * | 0.562 * | 0.587 * |
| N | 130 | 130 | 112 | |
| Rate to Peak EAACmax (umol/L/min) | R | 0.626 * | 0.577 * | 0.444 * |
| N | 130 | 130 | 112 |
1 FSR = fractional synthetic rate; h = hours; ΔFSR = postprandial FSR minus postabsorptive FSR; WBPS = whole-body protein synthesis; ΔWBPS = postprandial WBPS minus postabsorptive WBPS; EAA AUCi = EAA area under the curve above basal; EAACmax = max EAA concentration; ΔEAA = EAACmax minus basal EAA concentration. * = significant correlation.
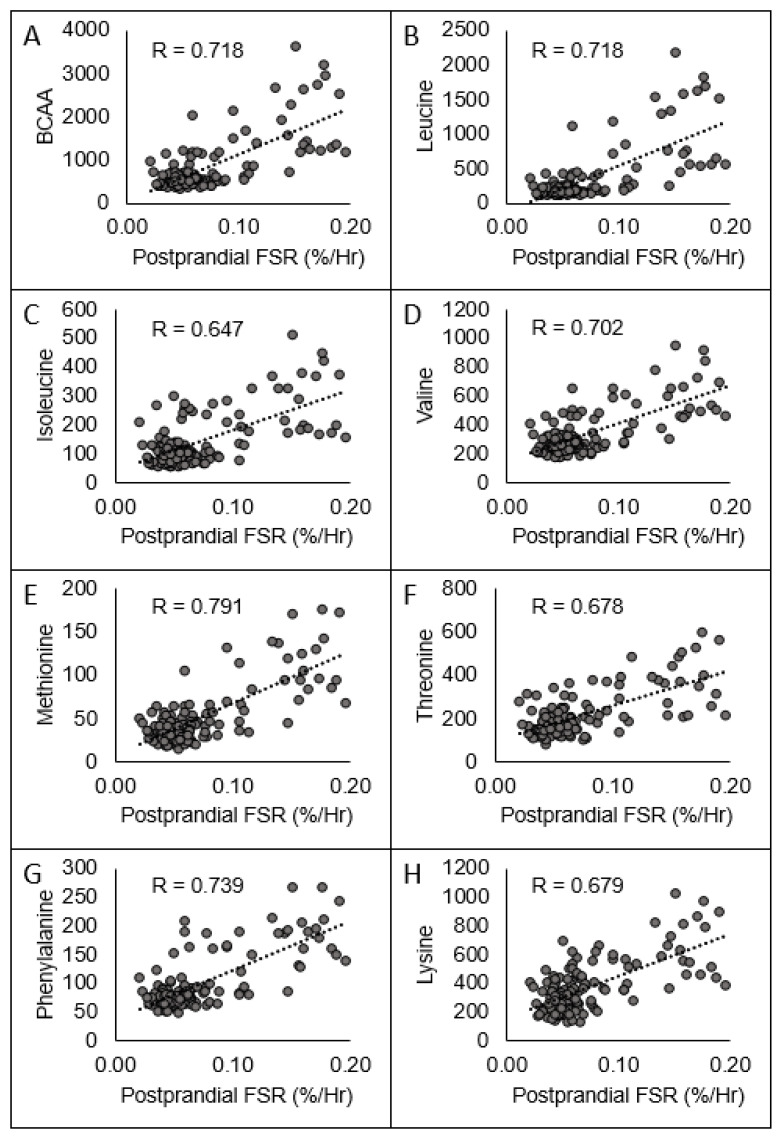
The Cmax values for the sum of the branched chain amino acids (BCAA) and all individual EAA, highlighted in Figure 2A–H, were significantly (p ≤ 0.05) correlated to the postprandial FSR (r = 0.647–0.761), with the exception of tryptophan and histidine; ΔFSR (r = 0.545–0.621), again with the exception of tryptophan and histidine; and ΔWBPS (r = 0.439–0.541), again with the exception of tryptophan and histidine. The AUCi for the sum of the BCAA and all individual EAA were significantly (p ≤ 0.05) correlated to the postprandial FSR (r = 0.322–0.690), except for tryptophan and histidine; ΔFSR (r = 0.331–0.528), except tryptophan and histidine, and ΔWBPS (r = 0.307–0.590) except tryptophan and histidine. The Δ for the sum of the BCAA and all individual EAA were all significantly (p ≤ 0.05) correlated to the postprandial FSR (r = 0.197–0.701), except for histidine, ΔFSR (r = 0.481–0.567) except tryptophan and histidine, and ΔWBPS (r = 0.218–0.462) except tryptophan.
Figure 2.
Relationships between CMax (umol/L) of the sum of the branched chain amino acids (A; BCAAs) and individual amino acids (B–H) to postprandial FSR. Not pictured: non-significant relationships between tryptophan and histidine.
4. Discussion
Our results indicate that greater peripheral EAA concentrations are related to the stimulation of MPS and WBPS and in response to feeding. Our stepwise regression results indicated that the strongest predictors for postprandial FSR, ΔFSR, and ΔWBPS were measures of EAA concentrations; explaining approximately 30–50% of the variance in protein synthesis measures. The results support the requirement for increased peripheral EAA concentrations to stimulate MPS and WBPS. Expressed differently, a larger EAA gradient between the extracellular (peripheral) and intracellular compartments enables greater inward transport and subsequent charging of tRNA and stimulation of synthesis [3,25].
Based upon the average basal EAA concentrations (961 µmol/L) in the current dataset, the regression equation indicates that a 100% increase in EAA concentrations (EAACMax = 1922 µmol/L) would result in a ΔFSR of 0.020, or a ~34% increase (based upon the average post-absorptive FSR; 0.059). Our results are nearly identical to those of Pennings and colleagues [33] who demonstrated a significant correlation (r = 0.55, p < 0.01) between ΔEAA concentrations and postprandial FSR following the ingestion of whey, casein, or casein hydrolysate protein in older men. Considering the large variance of FSR measures [34], we feel the similar correlation coefficients (r = 0.64 and r = 0.55) obtained by separate laboratories provides consistent evidence for an established relationship between peripheral EAA concentrations and FSR within the range of protein consumed. Further, study means collected from the literature [7,33,35,36,37,38,39,40,41,42,43,44,45] fall in line with measured values from participants in our laboratory (Figure 2). Previous reports have demonstrated that the stimulation of the inward amino acid transport is the mechanism by which MPS is stimulated [46]. For example, Bohé and colleagues [4] demonstrated MPS was related to extracellular, not intracellular, EAA concentrations during infusion of mixed amino acids at four different rates. This seminal work provides the mechanistic foundation of the control of MPS by peripheral EAA. However, infusion of mixed amino acids directly into blood bypasses first pass splanchnic uptake and does not accurately represent a real-life feeding situation. Nonetheless, studies have routinely demonstrated a dose-response relationship between total protein intake and FSR throughout a wide range of EAA sources such as; free-form EAA, whey, egg, soy, beef [31,37,47,48,49,50,51,52]. Although EAA concentrations were not always measured, a reasonable assumption can be made that increasing the dose of EAA source results in higher peripheral EAA concentrations [23,30,37,39,53]. The oral ingestion studies indicate a point where increasing doses of complete protein, and thus EAA concentrations, no longer further stimulate FSR. We did not observe this in our data since the maximal EAA dose was 11.2 g and most likely below a dose (~15 g of EAA), which achieves a maximal FSR response [5,7,37,47,52,54,55]. Altogether, these data demonstrate large increases in peripheral/extracellular EAA concentrations are an important factor in driving the postprandial rise in FSR.
In addition to a robust sample size, a strength of the present analysis is the measurement of all EAA concentrations, allowing for correlations between the sum of the EAA, BCAA, and each individual EAA on its own. Many studies only measure leucine or the BCAAs. Leucine has been shown to be an important amino acid within a protein source and to serve as a “trigger” to stimulate MPS via mammalian target or rapamycin complex 1 signaling [56]. Our data are consistent with these findings in that we observed significant relationships between leucine concentrations and both postprandial FSR and ΔFSR. More importantly, we observed significant correlations between all the individual EAA, with the exception of tryptophan and histidine, and FSR measures. Previous work has demonstrated the requirement for all the combined EAA to stimulate MPS [57]. Numerous studies have demonstrated the inability of leucine and BCAAs alone to induce a rise in MPS or improvements in muscle hypertrophy or strength with chronic use [57,58,59,60,61]. Partial EAA administration (BCAA or leucine) produces a transient anabolic response, at best, due to the activation of translational molecular pathways [62]. Unless an adequate supply of EAA are available, MPB must increase to compensate for the lack of precursors. For this reason, the correlation of nearly all the EAA with FSR denotes the adequacy of precursor provision.
Our results demonstrate that ΔWBPS was also significantly correlated with EAA AUCi, EAACmax, ΔEAA, and rate to EAACmax, with ΔEAA being the strongest predictor of ΔWBPS (Table 2). To our knowledge, we are the first to demonstrate significant relationships between peripheral EAA concentrations and ΔWBPS. The lack of investigation in this area makes direct comparisons difficult and indicates an important gap in the literature. Measurement of WBPS provides additional insight beyond MPS. Though skeletal muscle constitutes the majority of the body protein pool, the labile splanchnic tissue contributes substantially to whole-body protein turnover [9,10,11,63]. Recent work provides evidence of disparate muscle and whole-body anabolism at different protein intakes [30,52,64,65]. Thus, nutritional recommendations based upon muscle response alone fails to consider the beneficial whole-body effects of elevated EAA concentrations. Similar to results concerning FSR, studies demonstrating higher WBPS in response to increased amounts of free-form EAA, intact protein, and food sources are in agreement with our results [30,39,52,64,65]. Furthermore, the absorption and digestion of an EAA source are important in determining EAA availability and whole-body protein metabolism [66]. Our data are consistent with these findings, as total energy, and protein within an EAA source was negatively correlated, while the ratio of EAA:total protein was positively correlated with postprandial FSR, ΔFSR, and ΔWBPS. The large digestive requirement of whole food protein sources, as compared to simpler EAA matrices, results in a slower liberation and subsequent entry of EAA into circulation.
Our results provide evidence for peripheral EAA concentrations dictating protein synthesis at both the muscle and whole-body level. An attenuated rate of EAA liberation from digestion results in lower peripheral EAA concentrations at the membrane receptor, reducing the EAA concentration gradient from plasma to intracellular space. Transmembrane transport of amino acids is the determinant of the availability of circulating EAA for protein synthesis [67], and maximal transport rates occur when EAA gradients between intra- and extracellular concentrations are large. By maximizing the EAA gradient, the rate limiting step is no longer the supply of substrate (i.e., precursor amino acids) but rather the quantity of translational machinery (i.e., tRNA and ribosome content). This notion is supported by our previous work in rabbits demonstrating liver, with protein synthesis rates ~4-fold greater than muscle, corresponded with 4-fold greater tRNA content than muscle [68]. These data indicate that achieving a large EAA gradient is an important determinate of the protein synthetic response to feeding.
The current analysis is not without limitations. First, the analysis was accomplished by combining data from multiple studies, rather than a single study systematically altering protein formats and amounts. While performing a single study would be ideal, the present analysis is arguably only plausible by leveraging multiple studies. Further, the variety of interventions resulted in varied postprandial EAA responses in a large group of human subjects. As a result, our analysis clearly demonstrates the importance elevated EAA concentrations have on FSR and WBPS measurements. Another limitation is that we only measured one side of total protein status at both the muscle and whole-body level via protein synthesis. Thus, our results should be interpreted as indications of protein turnover, not protein anabolism. Measurement of only one variable in the protein balance equation potentially explains the low(er) variance in muscle and WBPS related to increased peripheral EAA concentrations. Our dataset has many cases where the modest rise in peripheral EAA would require a concomitant increase in MPB to supply EAA precursors for muscle and WBPS. At the muscle level it is often asserted that rises in MPS dictate the anabolic response, and subsequent muscle hypertrophy. However, this assertion can lead to incorrect conclusions. For example, burn injury patients display a 2-fold increase in FSR values; however, MPB increases by 3-fold, such that muscle protein balance is dramatically negative [69]. This also indicates that EAA availability, whether from ingestion or endogenous sources is the primary determinate of protein synthesis. While not included in the present analysis, data from our lab indicated that whole-body protein breakdown is strongly correlated with WBPS in the postprandial period. Taken together it is likely that the measurement of circulating EAA concentrations would be more predictive of anabolism if protein breakdown was also determined.
5. Conclusions
This work extends previous findings denoting the relationship between peripheral EAA concentrations and FSR by outlining responses to various formats of EAA intake. Our regression equations indicate that a 100% increase in peripheral EAA concentrations results in approximately a 34% increase in FSR from post-absorptive conditions. The present analysis expands upon previous findings by demonstrating that this relationship also extends to whole-body protein synthesis over a wide range of EAA sources. These findings also demonstrate that ingestion formats with a high proportion of EAA relative to total protein content represent a viable means of improving muscle and whole-body protein synthetic responses. Taken together, EAA sources that produce a large and rapid increase in peripheral EAA concentrations are recommended to improve muscle and whole-body protein synthesis.
Acknowledgments
We would like to thank the research volunteers for their willingness to participate in these studies. We thank the research staff and associates for their assistance in conducting tracer infusion protocols: Cosby J. Lasley for coordinating study subjects; Rick Williams for gas- and liquid-chromatography mass-spectrometry analysis; and we thank Deb Viane for her support and administrative assistance. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations.
Author Contributions
Conceptualization, D.D.C. and A.A.F.; methodology, D.D.C. and S.P.; data curation, D.D.C., K.R.H., and S.P.; writing—original draft preparation, D.D.C.; writing—review and editing, D.D.C., K.R.H., I.-Y.K., S.P., J.A.G., S.M.P., R.R.W., and A.A.F.; funding acquisition, R.R.W. and A.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Pork Board (A.A.F.), Egg Nutrition Center (A.A.F.), Adesso LLC (A.A.F.), Beef Checkoff (NCBA; R.R.W.), and Zespri Group Limited through Massey University (R.R.W.).
Conflicts of Interest
D.D.C., K.R.H., S.P., I.-Y.K., J.A.G., and S.M.P. have no conflict of interest to declare. R.R.W. is an inventor of United States patent 16; 382,984 entitled “Composition for Stimulating Muscle Growth, Repair, and Maintenance,” US Patent (16; 382,984). AAF and RRW are listed as inventors on United States patent 9364463 B2 entitled “Use of amino acid supplementation for improved muscle recovery,” and United States patent application 20200253908 entitled “Use of amino acid supplementation for improved muscle protein synthesis.” RRW is a shareholder in Essential Blends, LLC, and The Amino Company, Inc. R.R.W. has received research grants, travel expenses, and honoraria from the National Cattleman’s Beef Checkoff Program. A.A.F. has received research grants from the National Pork Board and Egg Nutrition Center. All funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gwin J.A., Church D.D., Wolfe R.R., Ferrando A.A., Pasiakos S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients. 2020;12:2457. doi: 10.3390/nu12082457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutz N.E.P., Wolfe R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2012;32:309–313. doi: 10.1016/j.clnu.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G., Fleming R.Y., Maggi S.P., Wolfe R.R. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am. J. Physiol. Metab. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 4.Bohé J., Low A., Wolfe R.R., Rennie M.J. Human Muscle Protein Synthesis is Modulated by Extracellular, Not Intramuscular Amino Acid Availability: A Dose-Response Study. J. Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tipton K.D., Ferrando A.A., Phillips S.M., Doyle D., Wolfe R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. Metab. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 6.Volpi E., Ferrando A.A., Yeckel C.W., Tipton K.D., Wolfe R.R. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J. Clin. Investig. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpi E., Kobayashi H., Sheffield-Moore M., Mittendorfer B., Wolfe R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church D.D., Gwin J.A., Wolfe R.R., Pasiakos S.M., Ferrando A.A. Mitigation of Muscle Loss in Stressed Physiology: Military Relevance. Nutrients. 2019;11:1703. doi: 10.3390/nu11081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smeets J.S.J., Horstman A.M.H., Vles G.F., Emans P.J., Goessens J.P.B., Gijsen A.P., Van Kranenburg J.M.X., Van Loon L.J.C. Protein synthesis rates of muscle, tendon, ligament, cartilage, and bone tissue in vivo in humans. PLoS ONE. 2019;14:e0224745. doi: 10.1371/journal.pone.0224745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeets J.S.J., Horstman A.M.H., Schijns O.E.M.G., Dings J.T.A., Hoogland G., Gijsen A.P., Goessens J.P.B., Bouwman F.G., Wodzig W.K.W.H., Mariman E.C., et al. Brain tissue plasticity: Protein synthesis rates of the human brain. Brain. 2018;141:1122–1129. doi: 10.1093/brain/awy015. [DOI] [PubMed] [Google Scholar]
- 11.Nakshabendi I.M., McKee R., Downie S., Russell R.I., Rennie M.J. Rates of small intestinal mucosal protein synthesis in human jejunum and ileum. Am. J. Physiol. 1999;277:E1028–E1031. doi: 10.1152/ajpendo.1999.277.6.E1028. [DOI] [PubMed] [Google Scholar]
- 12.Phillips S.M., Paddon-Jones D., Layman D.K. Optimizing Adult Protein Intake during Catabolic Health Conditions. Adv. Nutr. 2020;11:S1058–S1069. doi: 10.1093/advances/nmaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennie M.J., Tipton K.D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. 2000;20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X.J., Chinkes D.L., Sakurai Y., Wolfe R.R. An isotopic method for measurement of muscle protein fractional breakdown rate in vivo. Am. J. Physiol. 1996;270:E759–E767. doi: 10.1152/ajpendo.1996.270.5.E759. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X.-J., Chinkes D.L., Wolfe R.R. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am. J. Physiol. Endocrinol. Metab. 2002;283:E753–E764. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X.-J., Chinkes D.L., Herndon D.N., Wolfe R.R. Measurement of protein fractional synthesis and breakdown rates in the skin of rabbits using a subflooding dose method. Metab. Clin. Exp. 2009;58:1239–1247. doi: 10.1016/j.metabol.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Crossland H., Smith K., Atherton P.J., Wilkinson D.J. A novel stable isotope tracer method to simultaneously quantify skeletal muscle protein synthesis and breakdown. Metabol. Open. 2020;5:100022. doi: 10.1016/j.metop.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe R.R., Park S., Kim I.-Y., Starck C., Marquis B.J., Ferrando A.A., Moughan P.J. Quantifying the contribution of dietary protein to whole body protein kinetics: Examination of the intrinsically labeled proteins method. Am. J. Physiol. Endocrinol. Metab. 2019;317:E74–E84. doi: 10.1152/ajpendo.00294.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trommelen J., Holwerda A.M., Nyakayiru J., Gorissen S.H.M., Rooyackers O., Burd N.A., Boirie Y., Van Loon L.J.C. The intrinsically labeled protein approach is the preferred method to quantify the release of dietary protein-derived amino acids into the circulation. Am. J. Physiol. Endocrinol. Metab. 2019;317:E433–E434. doi: 10.1152/ajpendo.00155.2019. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe R.R., Park S., Kim I.-Y., Starck C., Marquis B.J., Ferrando A.A., Moughan P.J. Reply to Letter to the Editor: “The intrinsically labeled protein approach is the preferred method to quantify the release of dietary protein-derived amino acids into the circulation. ” Am. J. Physiol. Endocrinol. Metab. 2019;317:E435. doi: 10.1152/ajpendo.00186.2019. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe R.R., Park S., Kim I.-Y., Moughan P.J., Ferrando A.A. Advances in stable isotope tracer methodology part 2: New thoughts about an “old” method-measurement of whole body protein synthesis and breakdown in the fed state. J. Investig. Med. 2020;68:11–15. doi: 10.1136/jim-2019-001108. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe R.R., Chinkes D.L. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. John Wiley & Sons; Hoboken, NJ, USA: 2004. [Google Scholar]
- 23.Park S., Church D.D., Azhar G., Schutzler S.E., Ferrando A.A., Wolfe R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020;17:9. doi: 10.1186/s12970-020-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Church D.D., Ferrando A.A., Wolfe R.R. Improved Muscle Protein Synthesis is Achieved with 3.6 g of Free Form Essential Amino Acid Ingestion in Elderly. J. Int. Soc. Sports Nutr. 2019;17:13. [Google Scholar]
- 25.Park S., Church D.D., Starck C., Schutzler S.E., Azhar G., Kim I.-Y., Ferrando A.A., Moughan P.J., Wolfe R.R. The impact of Hayward green kiwifruit on dietary protein digestion and protein metabolism. Eur. J. Nutr. 2020 doi: 10.1007/s00394-020-02363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S., Church D.D., Schutzler S.E., Azhar G., Kim I.Y., Ferrando A.A., Wolfe R.R. Metabolic evaluation of the Dietary Guideline’s ounce equivalents of protein food sources in young adults: A randomized controlled trial. J. Nutr. 2020 doi: 10.1093/jn/nxaa401. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abumrad N.N., Rabin D., Diamond M.P., Lacy W.W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 28.De Betue C.T.I., Joosten K.F.M., Deutz N.E.P., Vreugdenhil A.C.E., Van Waardenburg D.A. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am. J. Clin. Nutr. 2013;98:907–916. doi: 10.3945/ajcn.112.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann P.Q., Stirewalt W.S., O’Rourke B.D., Howard D., Nair K.S. Precursor pools of protein synthesis: A stable isotope study in a swine model. Am. J. Physiol. 1994;267:E203–E209. doi: 10.1152/ajpendo.1994.267.2.E203. [DOI] [PubMed] [Google Scholar]
- 30.Gwin J.A., Church D.D., Hatch-McChesney A., Howard E.E., Carrigan C.T., Murphy N.E., Wilson M.A., Margolis L.M., Carbone J.W., Wolfe R.R., et al. Effects of high versus standard essential amino acid intakes on whole-body protein turnover and mixed muscle protein synthesis during energy deficit: A randomized, crossover study. Clin. Nutr. 2020 doi: 10.1016/j.clnu.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Witard O.C., Jackman S.R., Breen L., Smith K., Selby A., Tipton K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014;99:86–95. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 32.Reeds P.J., Hachey D.L., Patterson B.W., Motil K.J., Klein P.D. VLDL apolipoprotein B-100, a potential indicator of the isotopic labeling of the hepatic protein synthetic precursor pool in humans: Studies with multiple stable isotopically labeled amino acids. J. Nutr. 1992;122:457–466. doi: 10.1093/jn/122.3.457. [DOI] [PubMed] [Google Scholar]
- 33.Pennings B., Boirie Y., Senden J.M.G., Gijsen A.P., Kuipers H., Van Loon L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 34.Smith G.I., Patterson B.W., Mittendorfer B. Human muscle protein turnover—Why is it so variable? J. Appl. Physiol. 2011;110:480–491. doi: 10.1152/japplphysiol.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpi E., Mittendorfer B., Wolf S.E., Wolfe R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell W.K., Phillips B.E., Williams J.P., Rankin D., Lund J.N., Wilkinson D.J., Smith K., Atherton P.J. The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: Clinical efficacy of pulse vs. bolus supply. Am. J. Physiol. Endocrinol. Metab. 2015;309:E450–E457. doi: 10.1152/ajpendo.00112.2015. [DOI] [PubMed] [Google Scholar]
- 37.Pennings B., Groen B., de Lange A., Gijsen A.P., Zorenc A.H., Senden J.M.G., Van Loon L.J.C. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 38.Kramer I.F., Verdijk L.B., Hamer H.M., Verlaan S., Luiking Y.C., Kouw I.W.K., Senden J.M., Van Kranenburg J., Gijsen A.P., Bierau J., et al. Both basal and post-prandial muscle protein synthesis rates, following the ingestion of a leucine-enriched whey protein supplement, are not impaired in sarcopenic older males. Clin. Nutr. 2017;36:1440–1449. doi: 10.1016/j.clnu.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Gorissen S.H., Horstman A.M., Franssen R., Crombag J.J., Langer H., Bierau J., Respondek F., Van Loon L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016;146:1651–1659. doi: 10.3945/jn.116.231340. [DOI] [PubMed] [Google Scholar]
- 40.Symons T.B., Schutzler S.E., Cocke T.L., Chinkes D.L., Wolfe R.R., Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs C.J., Hermans W.J.H., Holwerda A.M., Smeets J.S.J., Senden J.M., Van Kranenburg J., Gijsen A.P., Wodzig W.K.H.W., Schierbeek H., Verdijk L.B., et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: A double-blind, randomized trial. Am. J. Clin. Nutr. 2019;110:862–872. doi: 10.1093/ajcn/nqz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendtsen L.Q., Thorning T.K., Reitelseder S., Ritz C., Hansen E.T., Van Hall G., Astrup A., Sjödin A., Holm L. Human Muscle Protein Synthesis Rates after Intake of Hydrolyzed Porcine-Derived and Cows’ Milk Whey Proteins-A Randomized Controlled Trial. Nutrients. 2019;11:989. doi: 10.3390/nu11050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glynn E.L., Fry C.S., Drummond M.J., Timmerman K.L., Dhanani S., Volpi E., Rasmussen B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010;140:1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wall B.T., Hamer H.M., de Lange A., Kiskini A., Groen B.B.L., Senden J.M.G., Gijsen A.P., Verdijk L.B., Van Loon L.J.C. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013;32:412–419. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Chanet A., Verlaan S., Salles J., Giraudet C., Patrac V., Pidou V., Pouyet C., Hafnaoui N., Blot A., Cano N., et al. Supplementing Breakfast with a Vitamin D and Leucine-Enriched Whey Protein Medical Nutrition Drink Enhances Postprandial Muscle Protein Synthesis and Muscle Mass in Healthy Older Men. J. Nutr. 2017;147:2262–2271. doi: 10.3945/jn.117.252510. [DOI] [PubMed] [Google Scholar]
- 46.Sheffield-Moore M., Wolfe R.R., Gore D.C., Wolf S.E., Ferrer D.M., Ferrando A.A. Combined effects of hyperaminoacidemia and oxandrolone on skeletal muscle protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2000;278:E273–E279. doi: 10.1152/ajpendo.2000.278.2.E273. [DOI] [PubMed] [Google Scholar]
- 47.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P.M., Rennie M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 48.Moore D.R., Robinson M.J., Fry J.L., Tang J.E., Glover E.I., Wilkinson S.B., Prior T., Tarnopolsky M.A., Phillips S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Breen L., Burd N.A., Hector A.J., Churchward-Venne T.A., Josse A.R., Tarnopolsky M.A., Phillips S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012;108:1780–1788. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Churchward-Venne T.A., Burd N.A., Breen L., Tarnopolsky M.A., Phillips S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012;9:57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson M.J., Burd N.A., Breen L., Rerecich T., Yang Y., Hector A.J., Baker S.K., Phillips S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2013;38:120–125. doi: 10.1139/apnm-2012-0092. [DOI] [PubMed] [Google Scholar]
- 52.Holwerda A.M., Paulussen K.J.M., Overkamp M., Goessens J.P.B., Kramer I.F., Wodzig W.K.W.H., Verdijk L.B., Van Loon L.J.C. Dose-Dependent Increases in Whole-Body Net Protein Balance and Dietary Protein-Derived Amino Acid Incorporation into Myofibrillar Protein During Recovery from Resistance Exercise in Older Men. J. Nutr. 2019;149:221–230. doi: 10.1093/jn/nxy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunlop M.V., Kilroe S.P., Bowtell J.L., Finnigan T.J.A., Salmon D.L., Wall B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: A dose-response study. Br. J. Nutr. 2017;118:673–685. doi: 10.1017/S0007114517002409. [DOI] [PubMed] [Google Scholar]
- 54.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 55.Miller S.L., Tipton K.D., Chinkes D.L., Wolf S.E., Wolfe R.R. Independent and combined effects of amino acids and glucose after resistance exercise. Med. Sci. Sports Exerc. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- 56.Gordon B.S., Kazi A.A., Coleman C.S., Dennis M.D., Chau V., Jefferson L.S., Kimball S.R. RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells. Cell. Signal. 2014;26:461–467. doi: 10.1016/j.cellsig.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verhoeven S., Vanschoonbeek K., Verdijk L.B., Koopman R., Wodzig W.K.W.H., Dendale P., Van Loon L.J.C. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 58.Church D.D., Schwarz N.A., Spillane M.B., McKinley-Barnard S.K., Andre T.L., Ramirez A.J., Willoughby D.S. l-Leucine Increases Skeletal Muscle IGF-1 but Does Not Differentially Increase Akt/mTORC1 Signaling and Serum IGF-1 Compared to Ursolic Acid in Response to Resistance Exercise in Resistance-Trained Men. J. Am. Coll. Nutr. 2016;35:627–638. doi: 10.1080/07315724.2015.1132019. [DOI] [PubMed] [Google Scholar]
- 59.DE Andrade I.T., Gualano B., Hevia-LarraÍn V., Neves-Junior J., Cajueiro M., Jardim F., Gomes R.L., Artioli G.G., Phillips S.M., Campos-Ferraz P., et al. Leucine Supplementation Has No Further Effect on Training-induced Muscle Adaptations. Med. Sci. Sports Exerc. 2020;52:1809–1814. doi: 10.1249/MSS.0000000000002307. [DOI] [PubMed] [Google Scholar]
- 60.Backx E.M.P., Horstman A.M.H., Marzuca-Nassr G.N., Van Kranenburg J., Smeets J.S., Fuchs C.J., Janssen A.A.W., de Groot L.C.P.G.M., Snijders T., Verdijk L.B., et al. Leucine Supplementation Does Not Attenuate Skeletal Muscle Loss during Leg Immobilization in Healthy, Young Men. Nutrients. 2018;10:635. doi: 10.3390/nu10050635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrando A.A., Williams B.D., Stuart C.A., Lane H.W., Wolfe R.R. Oral Branched-Chain Amino Acids Decrease Whole-Body Proteolysis. J. Parenter. Enter. Nutr. 1995;19:47–54. doi: 10.1177/014860719501900147. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez A.M., Church D.D., Townsend J.R., Bagheri R. Emerging Nutritional Supplements for Strength and Hypertrophy. Strength Cond. J. 2020;42:57–70. doi: 10.1519/SSC.0000000000000552. [DOI] [Google Scholar]
- 63.Nair K.S., Schwartz R.G., Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am. J. Physiol. 1992;263:E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- 64.Kim I.-Y., Schutzler S., Schrader A., Spencer H.J., Azhar G., Ferrando A.A., Wolfe R.R. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. Endocrinol. Metab. 2016;310:E73–E80. doi: 10.1152/ajpendo.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Churchward-Venne T.A., Pinckaers P.J.M., Smeets J.S.J., Betz M.W., Senden J.M., Goessens J.P.B., Gijsen A.P., Rollo I., Verdijk L.B., Van Loon L.J. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: A double-blind randomized trial. Am. J. Clin. Nutr. 2020;112:303–317. doi: 10.1093/ajcn/nqaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rémond D., Machebeuf M., Yven C., Buffière C., Mioche L., Mosoni L., Patureau Mirand P. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. Am. J. Clin. Nutr. 2007;85:1286–1292. doi: 10.1093/ajcn/85.5.1286. [DOI] [PubMed] [Google Scholar]
- 67.Miller S., Chinkes D., MacLean D.A., Gore D., Wolfe R.R. In vivo muscle amino acid transport involves two distinct processes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe R.R., Song J., Sun J., Zhang X. Total aminoacyl-transfer RNA pool is greater in liver than muscle in rabbits. J. Nutr. 2007;137:2333–2338. doi: 10.1093/jn/137.11.2333. [DOI] [PubMed] [Google Scholar]
- 69.Wolfe R.R. The 2017 Sir David P Cuthbertson lecture. Amino acids and muscle protein metabolism in critical care. Clin. Nutr. 2018;37:1093–1100. doi: 10.1016/j.clnu.2017.12.010. [DOI] [PubMed] [Google Scholar]