Abstract
Simple Summary
The Asian tiger mosquito, Aedes albopictus, is widely considered to be one of the most dangerous vectors transmitting human diseases. Meanwhile, entomopathogenic nematodes (EPNs) have been applied for controlling insect pests of agricultural and public health importance for years. However, infection of mosquitoes with the infective juveniles (IJs) of terrestrial-living EPNs when released to aquatic habitats needs further investigated. In this study, we observed that a Taiwanese isolate of EPN, Steinernema abbasi, could invade through oral route and then puncture the wall of gastric caecum to enter the body cavity of Ae. albopictus larvae. The nematode could complete infection of mosquitoes by inserting directly into trumpet, the intersegmental membrane of the cuticle, and the basement of the paddle of pupae. After inoculation of mosquito larvae with nematode suspensions, the invading IJs in the hemocoel were melanized and encapsulated and only a few larvae were able to survive to adult emergence. The mosquitocidal effect of S. abbasi could be due to its overload or destruction of the mosquito defense systems. Eventually, the mosquito larvae failed to recover and died a few days after infection. Our results suggest that the terrestrial EPN, S. abbasi, is effective against the aquatic living mosquitoes.
Abstract
The Asian tiger mosquito, Aedes albopictus, is of crucial concern to the public and veterinary health because of its vector role in transmission of several mosquito-borne diseases. Over the past decades, entomopathogenic nematodes (EPNs) have been used to control important agricultural insect pests and are considered to be effective against mosquitoes as well. The objectives of this study were to investigate the mosquitocidal effects of Steinernema abbasi to Ae. albopictus and the encapsulation processes of invading nematodes in the mosquito host. In this study, we found that S. abbasi was pathogenic to 3rd and 4th instar larvae of Ae. albopictus by entering the hemocoel of the 3rd and 4th instar larvae mainly through mouth and gastric caecum or by penetrating pupae through the intersegmental membrane or trumpet. The mosquito larvae infected with a single nematode caused a high mortality. Although EPNs in the hemocoel of mosquitoes were melanized and encapsulated, most Ae. albopictus larvae failed to survive after infection with S. abbasi. Overall, we demonstrated that S. abbasi is pathogenic to Ae. albopictus larvae, suggesting that this S. abbasi isolate has potential as a biocontrol agent for managing this vector mosquito.
Keywords: Aedes albopictus, Steinernema abbasi, defense reaction, encapsulation
1. Introduction
In the past few decades, the emergence and re-emergence of several vector-borne viral diseases have threatened human health [1,2]. The Asian tiger mosquito, Aedes albopictus, is a daytime biting mosquito [3] and is known to transmit several arboviruses including dengue virus, chikungunya viruses, West Nile virus, eastern equine encephalitis virus, and Japanese encephalitis virus in nature [4,5,6,7]. In addition, Ae. albopictus is also an important vector of dog heartworm parasites, Dirofilaria immitis and D. repens [8]. To date, there is no effective therapeutics or vaccine available for the treatment of these mosquito-borne diseases. Conventional chemical insecticides are commonly applied to suppress the epidemics of mosquito-transmitted diseases [9,10,11]. Nevertheless, there has been progressive development of insecticide resistance in the vector populations over the years [12]. Inherited resistance to chemical insecticides in mosquitoes usually arises through one of two mechanisms, i.e., target-site resistance mutations and/or metabolic-based resistance [13]. Moreover, application of chemical insecticides could cause hazard to non-target organisms, contaminate the surrounding environment, and bioaccumulate through biomagnified food chain [14]. Biological control is thus considered as an environmentally friendly alternative to chemical insecticides.
Since 1980s, the entomopathogenic nematodes (EPNs) have been reported to be effective biocontrol agents for managing a diverse number of the insect pests of crops including those on foliar, soil surface and cryptic or subterranean habitats [15,16,17,18,19]. Recently, EPN-based products have already been available in many countries [20,21]. The majority of EPNs belong to two genera Steinernema and Heterorhabditis, which can kill insects rapidly by symbiotic bacteria of the genera, Xenorhabdus and Photorhabdus, respectively [22,23]. In the process of infection, the infective juveniles (IJs) of EPNs penetrate through natural body openings of insects such as mouth, spiracles, and anus [24], while some heterorhabditids can also penetrate through the intersegmental membranes of the insect using their dorsal tooth [25,26,27]. After penetration, the symbiotic bacteria are released from the nematode gut causing septicemia [28] and the death of insects within approximately 48 h [17,29].
Since EPNs are able to infect, develop, and complete their life cycle in about 250 insect species belonging to 75 families of 11 orders [30,31], they can be a potential biological control agent against insect pests. Importantly, EPNs can be easily mass-produced and applied using the available spray equipment and being safe to the environment [23,30]. For management strategies of Ae. albopictus, it is necessary to know the breeding habitats of Aedes species. Their females usually prefer to oviposit either in areas of relatively clean water or small containers placed in home yards such as uncovered barrels, plastic buckets, water storage drums, jars, discarded tires, flower vases, and trash cans [32,33,34,35]. Although both steinernematids and heterorhabditids are terrestrial nematodes, the aquatic habitat is also best for the nematode survival [36,37,38,39,40,41]. In aquatic habitat, IJs of terrestrial nematodes sink to the bottom of the containers where mosquito larvae frequently feed on the organic detritus and may swim actively in search of hosts resulting in successful infection of mosquitoes [40,42]. EPNs can infect a wide range of susceptible mosquito species [36,37,39,40,41,43]. Therefore, we investigated the mosquitocidal effects of S. abbasi, which is an indigenous nematode isolated from Taiwan by Liao et al. (2001) [44], against Ae. albopictus in aquatic habitats, and observations on routes of entry of S. abbasi into Ae. albopictus, and encapsulation of the invading nematodes in the host mosquitoes.
2. Materials and Methods
2.1. Mosquito Rearing
Colonies of Ae. albopictus were originally collected from Kaoshiung, Taiwan, in 1998. This mosquito strain has been maintained in the laboratory since then. Mosquitoes were reared at 28 ± 1 °C and 80% ± 10% relative humidity (RH) on a photoperiod of 12:12 h (L:D) under standard laboratory conditions. Adults were provided with a 10% sugar solution on soaked cotton rolls, and females were blood-fed biweekly using anaesthetized mice. Larvae were fed daily on a 1:1 mixture of goose liver powder and yeast powder.
All mice that were used frequently as a source of blood for mosquitoes were treated in accordance with the institutional Animal Care and Use Committee (IACUC) of NCHU, Taiwan. The study protocols were reviewed and approved by the Committee on Animal Research and Care in NCHU (No. 102-76, 23 October 2013 to 17 October 2018). All efforts were made to minimize suffering of mice.
2.2. Nematode Propagation
Two Steinernema nematodes were studied in the laboratory: S. abbasi was the stock colony maintained at Insect Pathology Laboratory of our department, whereas S. carpocapsae (Weiser; All strain) was obtained from BioSys Inc. (Columbia, MD, USA) given by Frank F. N. Chang, Temple University, USA in 1990. Nematode propagation was conducted by the methods described by Liao et al. (2001) [44]. Both nematodes were produced by passing through larvae of Spodoptera litura (Fabricius). The IJs were collected with the White trap and then were surface sterilized three times with 0.1% formalin solution. After sterilization, the nematodes were washed three times with sterilized distilled water. The IJs were transferred to a 5.5-cm petri dish containing a sponge pieces soaked with sterilized distilled water. Cultures of S. carpocapsae was maintained at 26 ± 2 °C and 90% ± 10% RH, while S. abbasi was reared at 20 ± 1 °C and 90% ± 10% RH in the dark.
2.3. Infection Assay of Ae. albopictus
The infection assay was carried out as described by Beresky and Hall (1977) [45] with some modifications. Briefly, each group of thirty 1st, 2nd, 3rd, or 4th instar larvae or pupae of Ae. albopictus was rinsed in 30 mL of sterilized water and then placed in a 250-mL glass beaker (6 cm in diameter) with a water level of ca. 1.5 cm from the bottom. Five concentrations of nematodes were tested, i.e., 1, 10, 100, 1000, and 10,000 IJs/mL. Distilled water was used for controls. Mortality rates of Ae. albopictus were recorded every 4 h for 72 h after inoculation, and were compared using Tukey’s honestly significant difference (HSD) test. A probit analysis was performed to calculate the lethal time values (LT50 and LT90) of Ae. albopictus. Steinernema carpocapsae could reduce larval density and adult emergence of Aedes spp. [46] and is also a commercialized entomopathogenic nematode. We thus used it as a control for comparing the infectivity of S. abbasi to Ae. albopictus in this study. There were four replicates with each treatment containing thirty larvae or pupae of Ae. albopictus in graded concentrations of nematode.
2.4. Observations on Routes of Entry of S. abbasi Into Ae. albopictus
Optical microscopy was performed for observations on entry of EPNs into mosquitoes. The 4th instar larvae of Ae. albopictus were rinsed in 30 mL of sterilized water (1 × 104 IJs/mL) and placed in a glass beaker for 30 min. After the incubation period, the mosquitoes were washed three times with sterilized distilled water, and immediately the entire intestinal tract of the mosquito was dissected and photographed under a microscope (MZ75; Leica microsystems, Wetzlar, Hesse, Germany) in cold phosphate buffered saline (150 mM NaCl, 1 mM CaCl2, 2 mM KCl, and 1 mM NaHCO3, pH 7.0; Merck KGaA, Darmstadt, Hesse, Germany).
For entry through cuticle or natural body openings of larvae and pupae, fifty larvae or pupae were placed on filter paper and were incubated at 0.5 mL of sterilized water (1 × 103 IJs/mL) for 30 min. After the incubation period, the larvae or pupae were fixed with 2.5% glutaraldehyde (Merck KGaA, Darmstadt, Hesse, Germany) in 0.1 M sodium cacodylate buffer (pH 7.4; Merck KGaA, Darmstadt, Hesse, Germany) for 3 h at 4 °C. The fixed samples were washed three times in 0.33 M cold sucrose (Merck KGaA, Darmstadt, Hesse, Germany) in 0.1 M sodium cacodylate buffer for 15 min each, and were post-fixed in 1% osmium tetroxide (Acros Organics, Morris Plains, NJ, USA) in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at 4 °C. Subsequently, the fixed samples were washed three times in sterilized distilled water for 15 min followed by dehydration in graded acetone series (50–100%), were critical point-dried using a critical-point dryer (Hitachi, Tokyo, Japan), and then visualized and photographed using a stereomicroscope (Olympus, Tokyo, Japan).
2.5. Observations on Encapsulation of Invading Nematodes in Ae. albopictus
Encapsulation of invading S. abbasi was observed using an optical microscopy as described above. The number of the penetrating nematodes and the encapsulated nematodes were counted and photographed under a microscope (MZ75; Leica microsystems, Wetzlar, Hesse, Germany).
Transmission electron microscopy (TEM) was performed for ultrastructural observations on encapsulation of invading S. abbasi. In brief, at 10 min, 30 min, 1 h, 2 h, 24 h, or 48 h after inoculation, body fragments of the EPN-infected 4th instar larvae were excised and fixed with 2.5% glutaraldehyde (Merck KGaA, Darmstadt, Hesse, Germany) in 0.1 M sodium cacodylate buffer (pH 7.4; Merck KGaA, Darmstadt, Hesse, Germany) for 12 h at 4 °C. The fixed samples were washed three times in 0.33 M cold sucrose (Merck KGaA, Darmstadt, Hesse, Germany) in 0.1 M sodium cacodylate buffer for 15 min each, and were post-fixed in 1% osmium tetroxide (Acros Organics, Morris Plains, NJ, USA) in 0.1 M sodium cacodylate buffer (pH 7.4) for 2 h at 4 °C. The resulting samples were washed three times in sterilized distilled water for 15 min each, dehydrated in a series of ethanol solutions, and then embedded in Epon 812 resin (Electron Microscopy Science, Hatfield, PA, USA). The blocks were sectioned using an ultramicrotome (Ultracut; Leica Microsystems, Wetzlar, Hesse, Germany). The ultrathin sections were placed on formvar-coated copper grids, post-stained with 2.5% uranyl acetate (Merck KGaA, Darmstadt, Hesse, Germany) and 1% lead citrate (Merck KGaA, Darmstadt, Hesse, Germany), and then visualized and photographed using a transmission electron microscope (JEM 2000EX II; Japanese Electron Optic Laboratory, Tokyo, Japan).
3. Results
3.1. Virulence of S. abbasi and S. carpocapsae to Ae. albopictus Larvae
S. carpocapsae is a commercial product of EPNs and is one of the most commonly applied species for controlling a variety of insects in agricultural pest control [15]. Therefore, we selected S. carpocapsae for comparing the mosquitocidal effect of S. abbasi. We first examined mosquitocidal effects of S. abbasi isolated from Taiwan on each developmental stage of mosquito. At 72 h after inoculation with S. abbasi and S. carpocapsae, no mortalities of the 1st and 2nd instar larvae and pupae of Ae. albopictus were observed, while those of the 3rd and 4th instar larvae were increased as elevated EPN concentrations (Figure S1). Subsequently, both LT50 and LT90 values were determined with treatments of 30 mosquito larvae. Most of LT50 and LT90 values of S. abbasi to Ae. albopictus were similar and no significant difference at a concentration of 1 × 104 IJs/mL although slightly lower than those of S. carpocapsae. However, at a concentration of 1 × 103 IJs/mL, LT50 and LT90 values of S. abbasi to 3rd instar larvae of Ae. albopictus was significantly lower than those of S. carpocapsae. In addition, comparing with the development time of larval stages of untreated larvae, the next molt and pupation of Ae. albopictus larvae seem to be prolonged after inoculation (Table 1 and Table S1). The mortality rate did not reach 90% within 72 h at a concentration of 1 × 103 IJs/mL (Table 1). Collectively, the inoculation of S. abbasi caused effects similar to S. carpocapsae infection.
Table 1.
The time-mortality response of two entomopathogenic nematodes, Steinernema abbasi and S. carpocapsae, to 3rd- and 4th-instars of Ae. albopictus assayed with thirty larvae.
| Nematode Species | Mosquito Instars | Lethal Time (LT50 and LT90) Values (h) | |||
|---|---|---|---|---|---|
| 1 × 103 (IJs/mL) a | 1 × 104 (IJs/mL) | ||||
| LT50 | LT90 | LT50 | LT90 | ||
| S. abbasi | 3rd | 37.9 (35.7–40.1) b |
69.1 (67.2–70.9) |
31.2 (30.2–32.2) |
60.6 (59.7–61.6) |
| 4th | 47.1 (44.6–49.6) |
>72 c | 34.9 (33.3–36.5) |
57.1 (56.1–58.1) |
|
| S. carpocapsae | 3rd | 46.4 (45.3–47.4) |
>72 | 31.6 (29.8–33.5) |
62.9 (59.8–66) |
| 4th | 47.8 (45.6–49.9) |
>72 | 26.9 (25.7–28.1) |
53.1 (51.4–54.8) |
|
a Concentration of nematode suspension. b The 95% confidence intervals are given in parentheses. c The mortality rate did not achieve 90% within 72 h.
3.2. Routes of Entry of S. abbasi Into Ae. albopictus
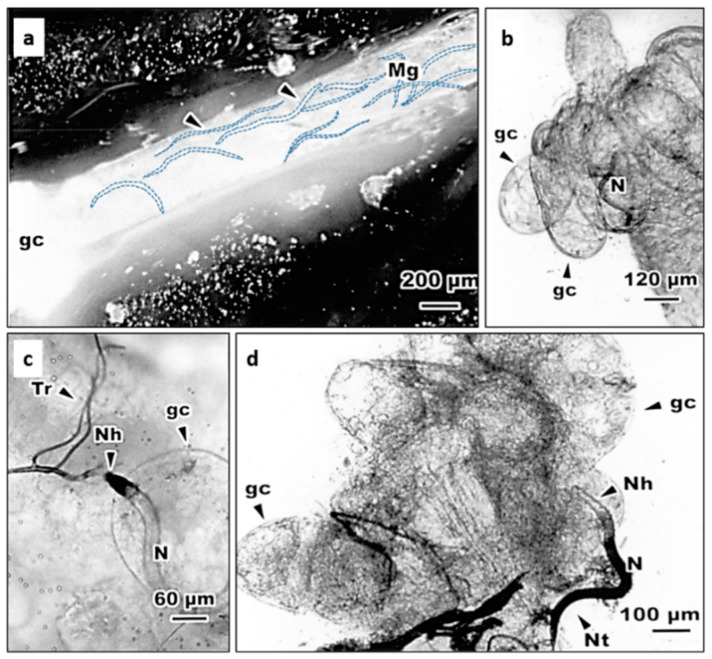
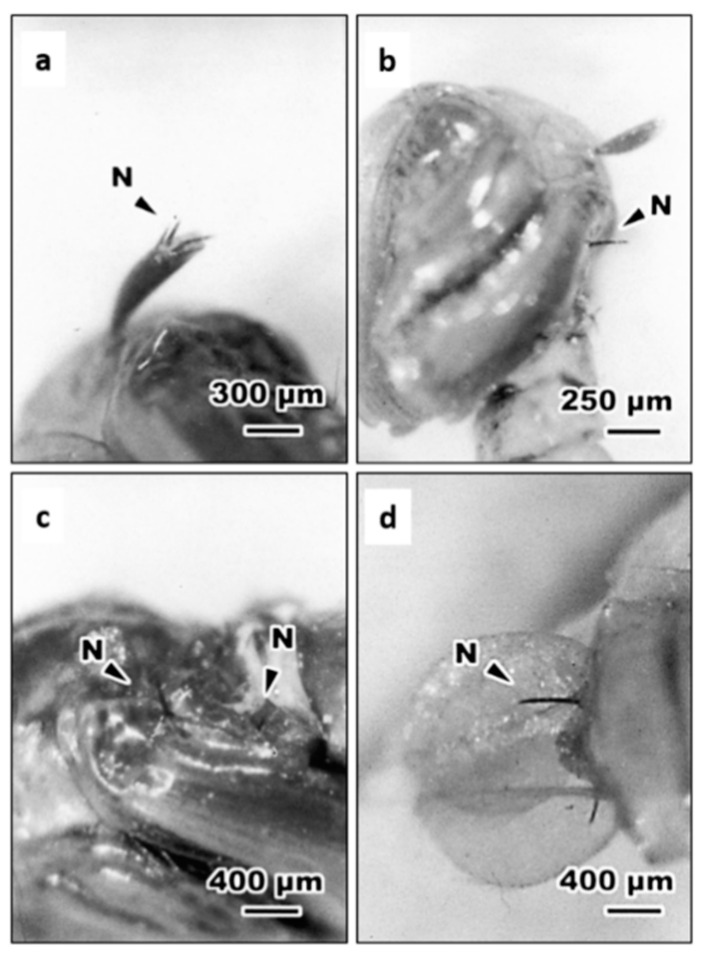
It was indicated that Steinernema nematodes are only able to enter their insect body via natural openings [25,27]. In this study, we further examined histologically the entry of S. abbasi into mosquitoes after incubation. Optical microscopic observations showed that, at 10 min after inoculation, IJs of S. abbasi aggregated within the midgut of 4th instar larva (Figure 1a). Some IJs were also found in the gastric caecum (Figure 1b) and penetrated through the gastric caecum into the hemocoel (Figure 1c). As penetrated into the hemocoel, IJs within the hemocoel were found melanized, while those remaining in the lumen of gastric caecum were non-melanized (Figure 2c,d). We also observed that the IJs inserted parts of their body into trumpet (Figure 2a), the intersegmental membrane of the cuticle and the basement of the paddle of pupae (Figure 2b–d).
Figure 1.
The penetration of S. abbasi to the hemocoel of the 4th instar larva of Ae. albopictus at 10 min after inoculation. (a) Nematode in the midgut of the 4th instar larva of Ae. albopictus. gc: gastric caecum, Mg: midgut. Arrow head: S. abbasi. (b) Nematode in the gastric caecum of the 4th instar larva of Ae. albopictus. (c) Nematode penetrated by its head from gastric caecum to the hemocoel of the 4th instar larva of Ae. albopictus. (d) Nematode penetrated by its tail from gastric caecum to the hemocoel of the 4th instar larva of Ae. albopictus. N: S. abbasi, Nh: nematode head, Nt: nematode tail, gc: gastric caecum, Tr: tracheae.
Figure 2.
The penetration of S. abbasi into the pupa of Ae. albopictus. (a) Nematode penetrated through the trumpet of Ae. albopictus pupa. (b) Penetrated through the intersegmental membrane of the cephalothorax of Ae. albopictus pupa. (c) Penetrated through the intersegmental membrane of the abdomen of Ae. albopictus pupa. (d) Penetrated through the basement membrane of paddle of of Ae. albopictus pupa. N = S. abbasi.
3.3. The Encapsulation of S. abbasi in the 4th Instar Larvae of Ae. albopictus
In order to find out whether the nematodes are able to survive within a mosquito larva and to utilize mosquito hemocoel for the mass production of S. abbasi, and additionally, whether this S. abbasi isolate is capable of producing massive offspring in mosquitoes as a synergistic control agent. The larvae were dissected at several time intervals after inoculation with nematodes, we observed that the invading nematodes were encapsulated after entering the hemocoel of mosquito larvae, indicating that the nematodes are unable to use mosquito body for propagation. Additionally, invasions by a large number of nematodes may overburden the immune system of mosquito larva. Observations from dissecting larvae infected with EPNs showed that as many as 31 melanized nematodes were found in one S. abbasi-infected larva, in contrast, the maximal number of melanized EPNs, was only 15 found in one S. carpocapsae-infected larva (Table 2 and Table S2). The melanotic capsules were distributed mainly in thorax (76%), followed by the abdomen (20%), and few in head (4%) in S. abbasi-infected Ae. albopictus, while there were 86% in the thorax, 9% in the abdomen and only 5% in the head in S. carpocapsae-infected larvae.
Table 2.
The number of encapsulated Steinernema abbasi in an Aedes albopictus larva and dead/survived mosquito larvae after inoculation.
| Number of Encapsulated Nematode in a Mosquito Larva | Number of Mosquito Larvae | |
|---|---|---|
| Dead | Survived | |
| 0 | 0 | 7 |
| 1 | 25 | 6 |
| 2 | 47 | 4 |
| 3 | 28 | 0 |
| 4 | 25 | 1 |
| 5 | 10 | 0 |
| 6 | 9 | 0 |
| 7 | 7 | 0 |
| 8 | 3 | 0 |
| 9 | 2 | 0 |
| 10 | 1 | 0 |
| 11 | 1 | 0 |
| 13 | 1 | 0 |
| 14 | 1 | 0 |
| 16 | 1 | 0 |
| 17 | 1 | 0 |
| 20 | 1 | 0 |
| 31 | 1 | 0 |
| Total | 164 | 18 |
| Percentage (%) | 90.11 | 9.89 |
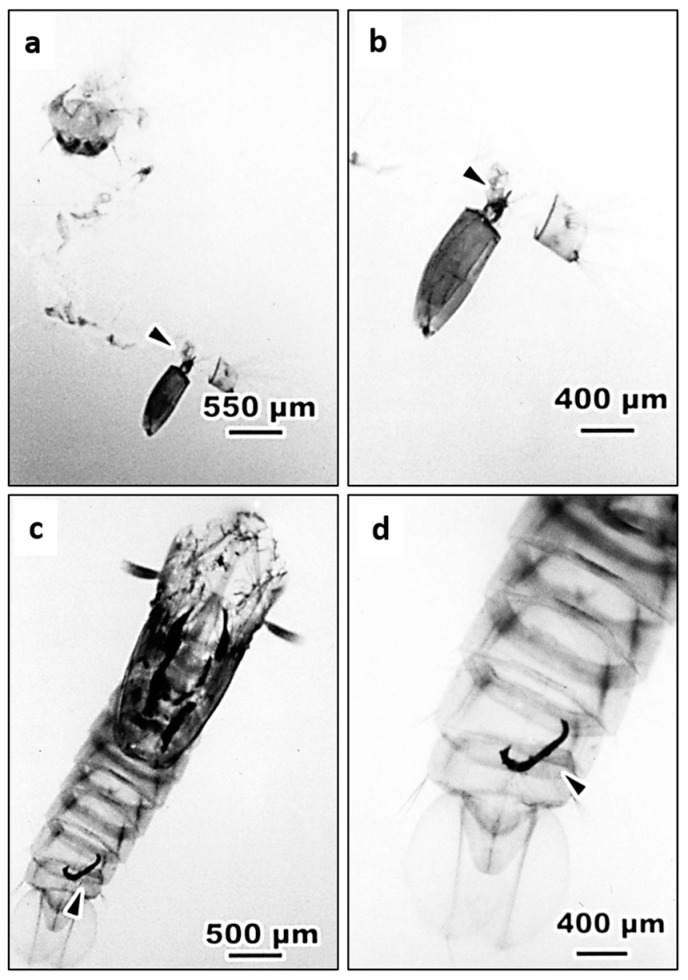
Among 183 of 4th instar larvae inoculated with S. abbasi, only 18 of them were able to survive, pupate, and emerge to adults in which 11 larvae harbored melanized EPNs in their body including 6 with one capsule, 4 with two capsules and 1 with four capsules. Some melanized capsules were extruded from the end of exuvia in larval abdomen (Figure 3a,b) or pupal abdomen (Figure 3c,d). Similarly, in 177 larvae inoculated with S. carpocapsae, only 6 of them developed to an adult including 4 without a capsule and 2 with two capsules (Table S2).
Figure 3.
The encapsulated S. abbasi extruded from larva or pupa of Ae. albopictus. (a) The capsule (arrow head) excluded from the end of exuvia in larval abdomen of Ae. albopictus. (b) The higher magnification of (a). (c) The capsule (arrow head) extruded from the end of the exuvia in pupal abdomen of Ae. albopictus. (d) The higher magnification of (c).
3.4. The Processes of Encapsulation of S. abbasi in Ae. albopictus
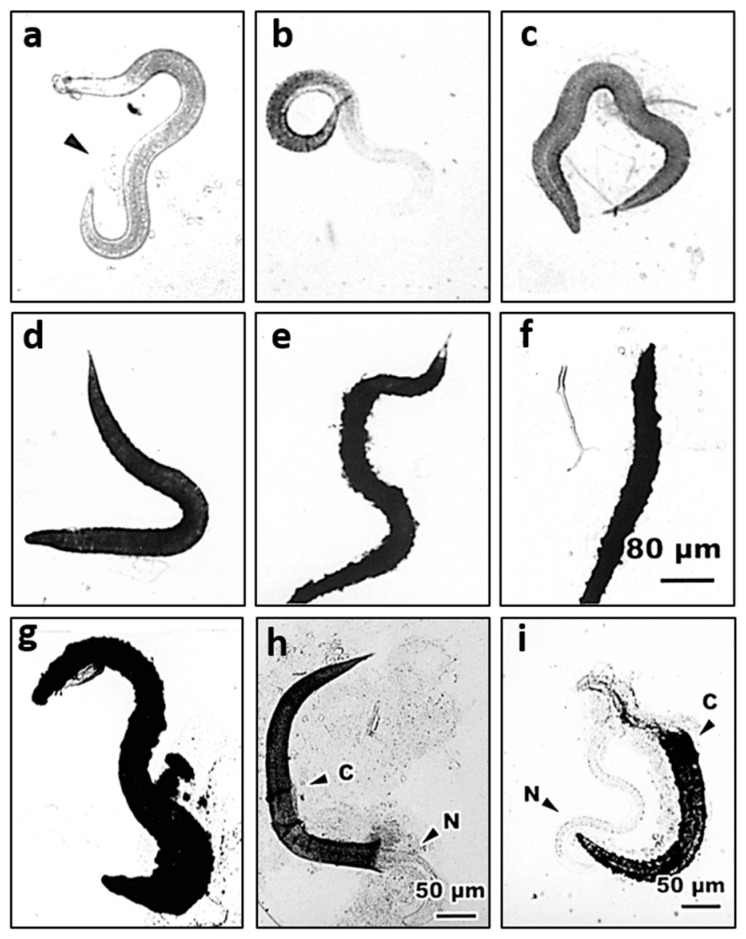
The above results showed that although S. abbasi IJs infection caused high mortality of Ae. albopictus larvae, they were encapsulated effectively suppressing their development after entering the hemocoel of mosquito larvae (Table 2). Further studies are necessary to clarify whether the encapsulation process or internal structures interfere with the nematode infection. Optical micrographs showed that transparent granules were found to be deposited on the surface of S. abbasi at 5 min after inoculation (Figure 4a). Subsequently, the transparent capsule was slightly melanized at 10 min after inoculation (Figure 4b). The invading nematode was completely encapsulated and was slightly melanized showing a smooth surface after 30 min (Figure 4c). The transparent capsule enclosing S. abbasi was completely melanized after 1 h (Figure 4d). The capsule was gradually thickened around the nematode and the surface of capsule was fiberizing after 2 h (Figure 4e). The invading nematode was covered with roughly melanized capsule after 4 h (Figure 4f). At 8 h after inoculation, the capsules became heavily melanized around the nematode (Figure 4g). Occasionally, we observed that a few nematodes were partially encapsulated (Figure 4h) and an unencapsulated nematode might form an empty capsule (Figure 4i).
Figure 4.
Encapsulation of S. abbasi in the hemocoel of 4th instar Ae. albopictus larvae. (a) At 5 min after inoculation, transparent granules (arrow head) deposited on the surface. (b) At 10 min after inoculation, partially encapsulated and slightly melanized. (c) At 30 min after inoculation, completely encapsulated and slightly melanized. (d) At 1 h after inoculation, melanized and completely covered by capsule materials. (e) At 2 h after inoculation, the surface of capsule fiberized. (f) At 4 h after inoculation, S. abbasi covering with roughly melanized capsule. (g) At 8 h after inoculation, S. abbasi and the capsules heavily melanized. (h) S. abbasi (N) partially covered by capsule materials (C). (i) An unencapsulated nematode (N) forming an empty capsule (C).
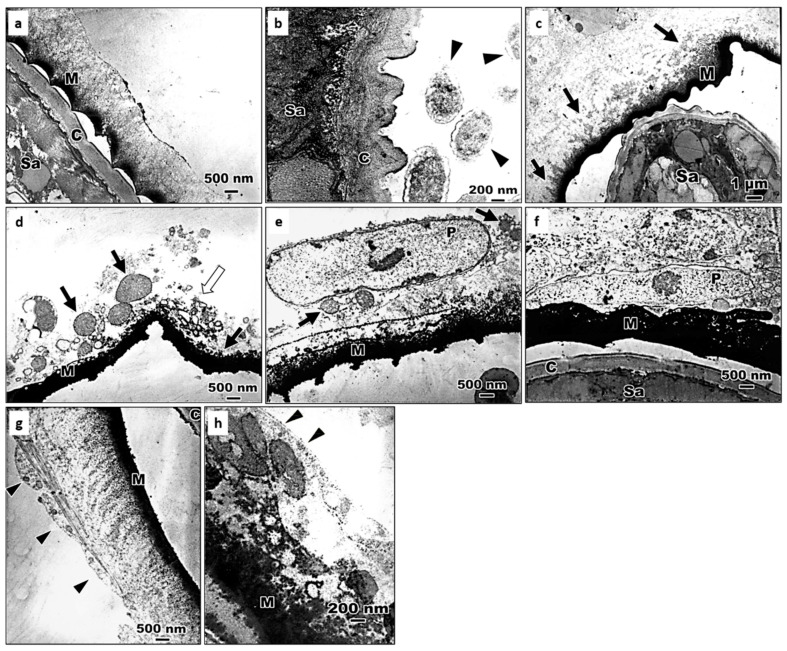
Electron micrographs further showed that at 10 min after inoculation of the 4th instar larvae with S. abbasi, the homogeneous materials deposited onto the cuticle of S. abbasi and electron-dense materials, which appear to be melanin surrounding the surface of S. abbasi (Figure 5a) and some symbiotic bacterium-like structures were visualized around S. abbasi (Figure 5b). At 30 min after inoculation, the inner electron-dense materials gradually became thickened (Figure 5c). Some cell debris, mitochondria, vacuoles, and intact plasmatocytes attached to the outer surface of the melanotic capsules at 1 h after inoculation (Figure 5d,e). Interdigitated plasmatocytes enclosed the melanotic capsule at 2 h after inoculation (Figure 5f). One layer of plasmatocytes attached to the melanized capsule after 24 h (Figure 5g). Finally, the encapsulation process was complete and the outermost surface of the cellular capsule was surrounded by basement membrane-like materials at 48 h after inoculation (Figure 5h).
Figure 5.
Electron micrographs showing the process of encapsulation of S. abbasi in the 4th instar larvae of Ae. albopictus. (a) At 10 min after inoculation, melanotic capsule deposited on the surface. C: the cuticle of S. abbasi; M: melanotic capsule; Sa: S. abbasi. (b) At 10 min after inoculation, the electron-condense homogeneous material attached to the cuticle of S. abbasi and symbiotic bacteria-like structures around S. abbasi in the hemocoel of 4th instar larvae. Arrow head (▲): bacteria-like structures; Sa: S. abbasi. C: the cuticle of S. abbasi. (c) At 30 min after inoculation, the inner electron-dense material thickened. Arrows (↑): the electron-condense homogeneous materials; M: melanotic capsule; Sa: S. abbasi. (d) At 1 h after inoculation, cell debris appeared on the out layer of humoral capsule. Black arrows (↑): mitochondria; White arrows (↑): cell debris; M: melanotic capsule. (e) At 1 h after inoculation. M: melanotic capsule; P: plasmatocyte; Arrows (↑): mitochondria. (f) At 2 h after inoculation, plasmatocyte on the melanotic capsule. C: the cuticle of S. abbasi; M: melanotic capsule; P: plasmatocyte; Sa: S. abbasi. (g) At 24 h after inoculation, intact hemocytes appeared on the humoral capsule. Arrow head: a hemocyte. (h) At 48 h after inoculation, the basement membrane-like structures between cellular capsule and hemocoel. Arrow head: the basement membrane-like structures.
4. Discussion
Unlike a free-living organism, the IJs of EPNs rely on their host behaviors, body size of different host developmental stages, and host immunity to maximize their chances of a successful infection [36,39,47]. In this study, we demonstrated that this S. abbasi isolate exhibited a significant larvicidal activity to 3rd and 4th instar larvae of Ae. albopictus in aquatic habitats.
EPNs are often applied as short-term inundative biological control agents in large numbers to bring about a rapid and severe decline in pest numbers [17,23,48], but only a fraction of these successes was found in a host [17]. In addition, the susceptibility of target insects varies depending on nematode species and strains [17,30]. Welch and Bronskill (1962) first reported that S. carpocapsae could kill more than 82% of Ae. aegypti larvae before pupating although the nematode was shortly encapsulated after penetrating through the gut wall into the hemocoel [46]. They also observed that some EPNs-infected larvae pupated 1 or 2 weeks behind the normal ones, and some failed to pupate and then died after 3 or 4 weeks. Zohdy et al. (2013) indicated that both S. carpocapsae and S. feltiae failed to establish in larvae of Culex quinquefasciatus [38]. This S. abbasi isolate could cause a delay in the next molt and pupation (Table 1 and Table S1), but all the nematodes were melanized and encapsulated (Table 2). More recently, steinernematids have been reported to be highly virulent to larvae of Aedes spp. [49,50]. Steinernematids are symbiotically associated with Xenorhabdus bacteria, which are lethal to many insect species [22]. Tsai et al. (2008) identified the symbiotic bacterium of this S. abbasi isolate to be Xenorhabdus indica [51]. The mortality rate of Ae. albopictus was between 82% and 96% when exposed to X. indica for 96 h [52], indicating that the host insects are chiefly killed by symbiotic bacteria. We found that at a concentration of 1000 IJs/mL, IJs of S. abbasi and S. carpocapsae caused a high mortality of Ae. albopictus 3rd and 4th instar larvae, but not the 1st and 2nd instar larvae and pupae. This difference might be due to two reasons: (1) the nematodes are difficult to penetrate directly through the cuticle or per os in the 1st and 2nd instar larvae with a tiny body size [36,37,43,47,53]. (2) The pupal stage does not have natural openings for nematode entry. Edmunds et al. (2017) indicated that, in the aquatic environment, EPNs could survive long enough to parasitize and to kill Chironomus plumosus larvae [42]. Steinernematids invade through natural openings and then puncture the gut wall to enter the body cavity in caterpillars and mosquitoes [43,54,55,56]. We also found similar results in Ae. albopictus after inoculation with S. abbasi. Peters and Ehler (1994) pointed out that an encapsulated Steinernema feltiae stuck in the integument of Tipula oleracea larva but its posterior end remained outside of the insect body [57]. Steinernema glaseri is able to release proteolytic enzymes to assist its penetration through the cuticle [58]. In addition to entry through natural body openings, S. abbasi could insert directly into the trumpet, the intersegmental membrane of the cuticle, and the basement of the paddle of Ae. albopictus pupae. Although steinernematid species do not have a dorsal tooth or hook on the tip of the head, their invasion can easily accomplish being possibly due to a weak larval cuticle and a lack of exocuticle similar to those of T. oleracea [54,57].
Encapsulation is a cellular defense of the insect host against invading parasites that are too large to be phagocytosed. Cellular encapsulation occurs mainly in insects with a large number of hemocytes, for example, lepidopteran species [59,60]. Schmit and Ratcliffe (1977) described cellular encapsulation in Galleria mellonella that is formed by the involvement of granular hemocyte and plasmatocytes [61]. However, a cell-free (humoral) encapsulation was induced in Chinonomus larvae to combat mermithid nematodes because of a small number of blood cells in Diptera [62,63]. In contrast, Chen and Laurence (1985) reported that the encapsulation of microfilariae in the hemocoel of Anopheles quadrimaculatus combined both humoral and cellular encapsulation in which microfilaria was first enclosed in an acellular melanized capsule and then plasmatocytes spread around the humoral capsule to form an outer cellular capsule with one layer of cells [59]. Similarly, the encapsulation of S. abbasi in the hemocoel of Ae. albopictus larvae combined both humoral and cellular encapsulation in this study (Figure 4 and Figure 5). At 48 h after infection of An. quadrimaculatus with a nematode, Brugia malayi, the outer surface of the cellular capsule is completely enclosed by basement membrane-like structures, suggesting that these structures laid down to prevent further attachment of any additional hemocytes [64]. We also found that the basement membrane-like structures formed an outer surface of the capsule enclosing S. abbasi in the hemocoel of Ae. albopictus larvae at 48 h after inoculation (Figure 5h) and the encapsulation structures seem effective in suppressing the development of S. abbasi.
Our study showed that at 10 min after inoculation, IJs of S. abbasi were observed in the midgut and the gastric caecum of 4th instar larvae, revealing that the IJs could be rapidly ingested by mosquito larvae and effectively killed larvae of Ae. albopictus. Notably, after inoculation with EPNs, only few larvae were able to survive to adult emergence (Table 2 and Table S2). It may be due to that the immune systems of mosquitoes overloaded after being exposed to nematode infection, and then the larvae failed to recover and eventually died [65] despite the toxicity of insecticidal proteins produced from the symbiotic bacteria of IJs.
In the present study, high mortalities occurred in the 3rd and 4th instar larvae of Ae. albopictus, but not in the 1st and 2nd instar larvae and pupae, indicating that 3rd and 4th instar larvae were more susceptible to Steinernema nematodes. Moreover, inoculation of 30 mosquito larvae with S. abbasi (1000 IJs/mL) resulted in a higher mortality against 3rd instar larvae than with S. carpocapsae.
It was reported that S. abbasi is able to kill and produce more IJs in host insects in a temperature ranging from 20 to 30 °C [66,67,68,69,70]. The survival rate of S. abbasi was not affected when stored in distilled water up to 6 weeks at 8 °C [71], and even up to 90 days at 30 °C resulting in 70.22% survival [72]. Temperature ranging from 25 to 32 °C was suitable for infectivity and virulence of S. abbasi [68]. In our study, approximately 90% larval mortality of Ae. albopictus were caused by the infection of this S. abbasi isolate within 72 h and were similar to that by S. carpocapsae in an aquatic environment. Therefore, this S. abbasi isolate when applied to larvae of Ae. albopictus can be a potential biocontrol agent for managing this vector mosquito in water.
5. Conclusions
In this study, we observed that a S. abbasi IJ invaded through the oral route and then punctured the wall of gastric caecum to enter the body cavity of Ae. albopictus larvae, and also inserted directly into trumpet, the intersegmental membrane of the cuticle, and the basement of the paddle of pupae. After invading larval hemocoel, the nematodes induced both humoral and cellular encapsulations of Ae. albopictus larvae. Whilst this S. abbasi isolate could possibly avoid the host cellular defense and cause appreciable mortality of mosquitoes. Therefore, this S. abbasi isolate exhibited a significant mosquitocidal effect on the 3rd and 4th instar larvae of Ae. albopictus and could thus be considered to be a potential biocontrol agent as an alternative for managing this vector mosquito.
Acknowledgments
Special thanks are due to Li-Cheng Tang, Department of Entomology, National Chung Hsing University (NCHU) for technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/12/832/s1. Figure S1: The mortality of Aedes albopictus larvae inoculated with Steinernema abbasi or S. carpocapsae at concentrations of 0, 1, 10, 100, 1000, and 10,000 IJs/mL at 72 h after inoculation; Table S1: The development time (mean ± SD) of immature stages of Aedes albopictus at 28 ± 1 °C; Table S2: The number of encapsulated Steinernema carpocapsae and degree of encapsulation in Aedes albopictus larvae.
Author Contributions
T.-L.C. designed and carried out various experiments. T.-L.C. and W.-T.L. produced the images of mosquitoes and transmission electron microscopy and analyzed the data. W.-T.L. and C.-C.C. wrote the paper. R.F.H. supervised the experiments and edited the manuscript. W.-C.T. designed and supervised the experiments. All authors contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kraemer M.U., Sinka M.E., Duda K.A., Mylne A.Q., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leta S., Beyene T.J., De Clercq E.M., Amenu K., Kraemer M.U.G., Revie C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonizzoni M., Gasperi G., Chen X., James A.A. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott T.W., Lorenz L.H., Weaver S.C. Susceptibility of Aedes albopictus to infection with eastern equine encephalomyelitis virus. J. Am. Mosq. Control. Assoc. 1990;6:274–278. [PubMed] [Google Scholar]
- 5.Mitchell C.J., Niebylski M.L., Smith G.C., Karabatsos N., Martin D., Mutebi J.P., Craig G.B., Jr., Mahler M.J. Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science. 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- 6.Sardelis M.R., Turell M.J., O’Guinn M.L., Andre R.G., Roberts D.R. Vector competence of three north American strains of Aedes albopictus for West Nile virus. J. Am. Mosq. Control. Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- 7.De Wispelaere M., Desprès P., Choumet V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl. Trop. Dis. 2017;11:e0005294. doi: 10.1371/journal.pntd.0005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morchón R., Carretón E., González-Miguel J., Mellado-Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe—New distribution trends. Front. Physiol. 2012;3:196. doi: 10.3389/fphys.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelakanta G., Sultana H. Transmission-blocking vaccines: Focus on anti-vector vaccines against tick-borne diseases. Arch. Immunol. Ther. Exp. 2015;63:169–179. doi: 10.1007/s00005-014-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yooyangket T., Muangpat P., Polseela R., Tandhavanant S., Thanwisai A., Vitta A. Identification of entomopathogenic nematodes and symbiotic bacteria from Nam Nao National Park in Thailand and larvicidal activity of symbiotic bacteria against Aedes aegypti and Aedes albopictus. PLoS ONE. 2018;13:e0195681. doi: 10.1371/journal.pone.0195681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agumba S., Gimnig J.E., Ogonda L., Ombok M., Kosgei J., Munga S., Guyah B., Omondi S., Ochomo E. Diagnostic dose determination and efficacy of chlorfenapyr and clothianidin insecticides against Anopheles malaria vector populations of western Kenya. Malar. J. 2019;18:243. doi: 10.1186/s12936-019-2858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chareonviriyaphap T., Bangs M.J., Suwonkerd W., Kongmee M., Corbel V., Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors. 2013;6:280. doi: 10.1186/1756-3305-6-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David J.P., Ismail H.M., Chandor-Proust A., Paine M.J. Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120429. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lushchak V.I., Matviishyn T.M., Husak V.V., Storey J.M., Storey K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018;17:1101–1136. doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacey L.A., Georgis R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012;44:218–225. [PMC free article] [PubMed] [Google Scholar]
- 16.Abate B.A., Wingfield M.J., Slippers B., Hurley B.P. Commercialisation of entomopathogenic nematodes: Should import regulations be revised? Biocontrol. Sci. Technol. 2017;27:149–168. doi: 10.1080/09583157.2016.1278200. [DOI] [Google Scholar]
- 17.Labaude S., Griffin C.T. Transmission success of entomopathogenic nematodes used in pest control. Insects. 2018;9:72. doi: 10.3390/insects9020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abd-Elgawad M.M.M. Towards optimization of entomopathogenic nematodes for more service in the biological control of insect pests. Egypt. J. Biol. Pest. Control. 2019;29:77. doi: 10.1186/s41938-019-0181-1. [DOI] [Google Scholar]
- 19.San-Blas E., Campos-Herrera R., Dolinski C., Monteiro C., Andaló V., Leite L.G., Rodríguez M.G., Morales-Montero P., Sáenz-Aponte A., Cedano C., et al. Entomopathogenic nematology in Latin America: A brief history, current research and future prospects. J. Invertebr. Pathol. 2019;165:22–45. doi: 10.1016/j.jip.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Askary T.H., Nermut J., Ahmad M.J., Ganai M.A. Future thrusts in expanding the use of entomopathogenic and slug parasitic nematodes in agriculture. In: Abd-Elgawad M.M.M., Askary T.H., Coupland J., editors. Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes. CAB International; Wallingford, UK: 2017. pp. 620–627. [Google Scholar]
- 21.Caoili B.L., Latina R.A., Sandoval R.F.C., Orajay J.I. Molecular identification of entomopathogenic nematode isolates from the philippines and their biological control potential against lepidopteran pests of corn. J. Nematol. 2018;50:99–110. doi: 10.21307/jofnem-2018-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazir S., Kaya H.K., Stock S.P., Keskin N. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk. J. Biol. 2003;27:181–202. [Google Scholar]
- 23.Shapiro-Ilan D.I., Han R., Dolinksi C. Entomopathogenic nematode production and application technology. J. Nematol. 2012;44:206–217. [PMC free article] [PubMed] [Google Scholar]
- 24.Bedding R.A., Molyneux A.S., Akhurst R.J. Heterorhabditis spp., Neoaplectana spp., and Steinernema kraussei: Interspecific and intraspecific differences in infectivity for insects. Exp. Parasitol. 1983;55:249–257. doi: 10.1016/0014-4894(83)90019-X. [DOI] [PubMed] [Google Scholar]
- 25.Bedding R.A., Molyneux A.S. Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda) Nematologica. 1982;28:354–359. doi: 10.1163/187529282X00402. [DOI] [Google Scholar]
- 26.Poinar G.O., Jr., Georgis R. Characterization and field application of Heterorhabditis bacteriophora strain HP88 (Heterorhabditidae: Rhabditida) Revue Nématol. 1990;13:387–393. [Google Scholar]
- 27.Burnell A.M., Stock S.P. Heterorhabditis, Steinernema and their bacterial symbionts-lethal pathogens of insects. Nematology. 2000;2:31–42. doi: 10.1163/156854100508872. [DOI] [Google Scholar]
- 28.Dziedziech A., Shivankar S., Theopold U. High-resolution infection kinetics of entomopathogenic nematodes entering Drosophila melanogaster. Insects. 2020;11:60. doi: 10.3390/insects11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillman A.R., Chaston J.M., Adams B.J., Ciche T.A., Goodrich-Blair H., Stock S.P., Sternberg P.W. An entomopathogenic nematode by any other name. PLoS Pathog. 2012;8:e1002527. doi: 10.1371/journal.ppat.1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grewal P.S., Nardo E.A.B.D., Aguillera M.M. Entomopathogenic nematodes: Potential for exploration and use in south America. Neotrop. Entomol. 2001;30:191–205. doi: 10.1590/S1519-566X2001000200001. [DOI] [Google Scholar]
- 31.Sharmila R., Subramanian S., Poornima K. Host range of entomopathogenic nematodes. J. Entomol. Zool. Stud. 2018;6:1310–1312. [Google Scholar]
- 32.Wong J., Stoddard S.T., Astete H., Morrison A.C., Scott T.W. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl. Trop. Dis. 2011;5:e1015. doi: 10.1371/journal.pntd.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kampen H., Werner D. Out of the bush: The asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasites Vectors. 2014;7:59. doi: 10.1186/1756-3305-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getachew D., Tekie H., Gebre-Michael T., Balkew M., Mesfin A. Breeding sites of Aedes aegypti: Potential dengue vectors in Dire Dawa, east Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015;2015:706276. doi: 10.1155/2015/706276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dom N.C., Madzlan M.F., Nur S., Hasnan A., Misran N. Water quality characteristics of dengue vectors breeding containers. Int. J. Mosq. Res. 2016;3:2529. [Google Scholar]
- 36.Poinar G.O., Jr., Kaul H.N. Parasitism of the mosquito Culex pipiens by the nematode Heterorhabditis bacteriophora. J. Invertebr. Pathol. 1982;39:382–387. doi: 10.1016/0022-2011(82)90063-5. [DOI] [Google Scholar]
- 37.Molta N.B., Hominick W.M. Dose- and time-response assessments of Heterorhabditis heliothidis and Steinernema feltiae [Nem.: Rhabitida] against Aedes aegypti larvae. Entomophaga. 1989;34:485–493. doi: 10.1007/BF02374386. [DOI] [Google Scholar]
- 38.Zohdy Z., Shamseldean M., El-Samiee E.A., Hamama H.M. Efficacy of the Steinernematid and Heterorhabditid nematodes for controlling the mosquito, Culex quinquefasciatus Say (Diptera: Culicidae) J. Mosq. Res. 2013;3:33–44. doi: 10.5376/jmr.2013.03.0005. [DOI] [Google Scholar]
- 39.Peschiutta M., Cagnolo S.R., Almirón W.R. Susceptibilidad de larvas de Aedes aegypti (Linnaeus) (Diptera: Culicidae) al nematodo entomopatógeno Heterorhabditis bacteriophora (Poinar) (Rhabditida: Heterorhabditidae) Rev. Soc. Entomol. Argent. 2014;73:99–108. [Google Scholar]
- 40.Cagnolo S.R., Almirón W.R. Capacity of the terrestrial entomopathogenic nematode Steinernema rarum (Rhabditida: Steinernematidae) to parasite Culex apicinus larvae (Diptera: Culicidae) Rev. Soc. Entomol. Argent. 2010;69:141–145. [Google Scholar]
- 41.Silva B., Almeida A.M., Dolinski C., Souza R.M. Efficacy of Heterorhabdits indica LPP35 against Aedes aegypti in domiciliary oviposition sites. J. Nematol. 2019;51:1–7. doi: 10.21307/jofnem-2019-050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmunds C.V., Wilding C.S., Rae R. Susceptibility of Chironomus plumosus larvae (Diptera: Chironomidae) to entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae): Potential for control. Eur. J. Entomol. 2017;114:526–532. doi: 10.14411/eje.2017.067. [DOI] [Google Scholar]
- 43.Ulvedal C., Bertolotti M.A., Cagnolo S.R., Almirón W.R. Ensayos de sensibilidad de larvas de Aedes aegypti y Culex quinquefasciatus frente al nematodo Heterorhabditis bacteriophora en condiciones de laboratorio. Biomédica. 2017;37:67–76. doi: 10.7705/biomedica.v37i0.3470. [DOI] [PubMed] [Google Scholar]
- 44.Liao C.Y., Tang L.C., Pai C.F., Hsiao W.F., Briscoe B.R., Hou R.F. A new isolate of the entomopathogenic nematode, Steinernema abbasi (Nematoda: Steinernematidae), from Taiwan. J. Invertebr. Pathol. 2001;77:78–80. doi: 10.1006/jipa.2000.4997. [DOI] [PubMed] [Google Scholar]
- 45.Beresky M.A., Hall D.W. The influence of phenylthiourea on encapsulation, melanization, and survival in larvae of the mosquito Aedes aegypti parasitized by the nematode Neoaplectana carpocapsae. J. Invertebr. Pathol. 1977;29:74–80. doi: 10.1016/0022-2011(77)90175-6. [DOI] [PubMed] [Google Scholar]
- 46.Welch H.E., Bronskill J.F. Parasitism of mosquito larvae by the nematode, DD136 (Nematoda: Neoaplectanidae) Can. J. Zool. 1962;40:1263–1268. doi: 10.1139/z62-102. [DOI] [Google Scholar]
- 47.Dadd R.H. Size limitations on the infectibility of mosquito larvae by nematodes during filter-feed. J. Invertebr. Pathol. 1971;18:246–251. doi: 10.1016/0022-2011(71)90152-2. [DOI] [PubMed] [Google Scholar]
- 48.Fenton A., Norman R., Fairbairn J.P., Hudson P.J. Evaluating the efficacy of entomopathogenic nematodes for the biological control of crop pests: A nonequilibrium approach. Am. Nat. 2001;158:408–425. doi: 10.1086/321993. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhary M.Z., Majeed S., Tayyib M., Javed N., Farzand A., Moosa A., Shehzad M., Mushtaq F. Antagonistic potential of Steinernema kraussei and Heterorhabditis bacteriophora against dengue fever mosquito Aedes aegypti. J. Entomol. Zool. Stud. 2017;5:865–869. [Google Scholar]
- 50.Dilipkumar A., Raja Ramalingam K., Chinnaperumal K., Govindasamy B., Paramasivam D., Dhayalan A., Pachiappan P. Isolation and growth inhibition potential of entomopathogenic nematodes against three public health important mosquito vectors. Exp. Parasitol. 2019;197:76–84. doi: 10.1016/j.exppara.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Tsai M.H., Tang L.C., Hou R.F. The bacterium associated with the entomopathogenic nematode Steinernema abbasi (Nematoda: Steinernematidae) isolated from Taiwan. J. Invertebr. Pathol. 2008;99:242–245. doi: 10.1016/j.jip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Vitta A., Thimpoo P., Meesil W., Yimthin T., Fukruksa C., Polseela R., Mangkit B., Tandhavanant S., Thanwisai A. Larvicidal activity of Xenorhabdus and Photorhabdus bacteria against Aedes aegypti and Aedes albopictus. Asian Pac. J. Trop. Biomed. 2018;8:31–36. doi: 10.4103/2221-1691.221134. [DOI] [Google Scholar]
- 53.Gaugler R., Kaplan B., Alvarado C., Montoya J., Ortega M. Assessment of Bacillus thuringiensis serotype 14 and Steinernema feltiae [Nematoda: Steinernematidae] for control of the Simulium vectors of onchocerciasis in Mexico. Entomophaga. 1983;28:309–315. doi: 10.1007/BF02372182. [DOI] [Google Scholar]
- 54.Poinar G.O., Jr. Entomopathogenic Nematodes in Biological Control. CRC Press; Boca Raton, FL, USA: 1990. Taxonomy and biology of Steinernematidae and Heterorhabdititidae; pp. 23–61. [Google Scholar]
- 55.Walsh K.T., Webster J.M. Interaction of microbial populations in Steinernema (Steinernematidae, Nematoda) infected Galleria mellonella larvae. J. Invertebr. Pathol. 2003;83:118–126. doi: 10.1016/S0022-2011(03)00079-X. [DOI] [PubMed] [Google Scholar]
- 56.Poinar G.O., Jr., Grewal P.S. History of entomopathogenic nematology. J. Nematol. 2012;44:153–161. [PMC free article] [PubMed] [Google Scholar]
- 57.Peters A., Ehlers R.U. Susceptibility of leather jackets (Tipula paludosa and T. oleracea, Tipulidae: Nematocera) to the entomopathogenic nematode Steinernema feltiae. J. Invertebr. Pathol. 1994;69:163–171. doi: 10.1006/jipa.1994.1031. [DOI] [PubMed] [Google Scholar]
- 58.Abu Hatab M., Selvan S., Gaugler R. Role of proteases in penetration of insect gut by the entomopathogenic nematode Steinernema glaseri (Nematoda: Steinernematidae) J. Invertebr. Pathol. 1995;66:125–130. doi: 10.1006/jipa.1995.1074. [DOI] [Google Scholar]
- 59.Chen C.C., Laurence B.R. An ultrastructural study on the encapsultion of microfilaria of Brugia pahangi in the haemocoel of Anopheles quadrimaculatus. Int. J. Parasitol. 1985;15:421–428. doi: 10.1016/0020-7519(85)90028-1. [DOI] [PubMed] [Google Scholar]
- 60.Hillyer J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016;58:102–118. doi: 10.1016/j.dci.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmit A.R., Ratcliffe N.A. The encapsulation of foreign tissue implants in Galleria mellonella larvae. J. Insect. Physiol. 1977;23:175–184. doi: 10.1016/0022-1910(77)90027-0. [DOI] [PubMed] [Google Scholar]
- 62.Götz P., Vey A. Humoral encapsulation in Diptera (Insecta): Defense reactions of Chironomus larvae against fungi. Parasitology. 1974;68:193–205. doi: 10.1017/S003118200004573X. [DOI] [PubMed] [Google Scholar]
- 63.Browne N., Heelan M., Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence. 2013;4:597–603. doi: 10.4161/viru.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C.T., Hou R.F., Chen C.C. Formation of basement membrane-like structure terminates the cellular encapsulation of microfilariae in the haemocoel of Anopheles quadrimaculatus. Parasitology. 1998;116:511–518. doi: 10.1017/S0031182098002595. [DOI] [PubMed] [Google Scholar]
- 65.Barreaux A.M., Barreaux P., Koella J.C. Overloading the immunity of the mosquito Anopheles gambiae with multiple immune challenges. Parasites Vectors. 2016;9:210. doi: 10.1186/s13071-016-1491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elawad A.S., Ahmad W., Reid A.P. Steinernema abbasi sp. n. (Nematoda: Steinernematidae) from the Sultanate of Oman. Fundam. Appl. Nematol. 1997;20:435–442. [Google Scholar]
- 67.Aasha R., Chaubey A.K., Bhat A.H. Notes on Steinernema abbasi (Rhabditida: Steinernematidae) strains and virulence tests against lepidopteran and coleopterans pests. J. Entomol. Zool. Stud. 2019;7:954–964. [Google Scholar]
- 68.Sunanda B.S. Effect of temperature on the life cycle of entomopathogenic nematodes, Steinernema abbasi and Heterorhabditis indica. J. Biol. Control. 2009;23:185–189. doi: 10.18311/jbc/2009/3645. [DOI] [Google Scholar]
- 69.Yoshida M. A new distribution record of Steinernema abbasi Elawad, Ahmad and Reid, 1997 (Rhabditida: Steinernematidae) in Japan with a note on its pathogenicity against the turnip moth larvae, Agrotis segetum (Lepidoptera: Noctuidae) Jpn. J. Nematol. 2007;37:51–61. doi: 10.3725/jjn.37.51. [DOI] [Google Scholar]
- 70.Nagesh M., Balachander M., Shivalingaswamy T.M., Patil J., Shylesha A.N., Raghavendra A. Variability in foraging behaviour, thermal requirement and virulence of entomopathogenic nematodes against sod webworm, Herpetogramma phaeopteralis Gueneè (Lepidoptera: Crambidae) J. Biol. Control. 2019;33:36–47. doi: 10.18311/jbc/2019/23512. [DOI] [Google Scholar]
- 71.Hussaini S.S., Singh S.P., Parthasarathy R., Shakeela V. Storage effects on activity of native Steinernema and Heterorhabditis spp. Indian J. Nematol. 2000;30:231–232. [Google Scholar]
- 72.Sunanda B.S., Siddiqui A.U., Sharma S. Effect of temperature on longevity of entomopathogenic nematodes, Steinernema abbasi and Heterorhabditis indica. Indian J. Nematol. 2012;42:17–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.