Abstract
BACKGROUND
Coats disease is an idiopathic exudative outer retinopathy caused by abnormal retinal vascular development.
AIM
To evaluate the long-term outcomes of intravitreal conbercept injection with laser photocoagulation as a treatment for Coats disease in adults.
METHODS
This retrospective case series study included patients diagnosed with Coats disease and treated with intravitreal conbercept injection and 532-nm laser photocoagulation at the Ophthalmology Department of Shenzhen People’s Hospital between January 2016 and January 2017. Best-corrected visual acuity (BCVA) measurements, noncontact tonometry, ophthalmoscopy, fundus photography, fundus fluorescein angiography and optical coherence tomography were performed before treatment and at 1 wk, 1 mo, 3 mo, 6 mo, 9 mo, 12 mo, 24 mo and 36 mo after therapy. Best-corrected visual acuity was measured using the early treatment of diabetic retinopathy study chart.
RESULTS
The study included eight eyes of 8 patients (7 men) aged 36.10 ± 6.65 years. The average BCVA of the affected eye before treatment was 51.17 ± 15.15 letters (range, 28–70 letters), and the average central macular thickness was 303.30 ± 107.87 µm (range, 221–673 µm). Four eyes were injected once, three were injected twice, and one was injected three times. Average follow-up duration was 37.33 ± 2.26 mo. Average BCVA of the affected eye was 51.17 ± 15.15 letters before treatment and was increased by 13.50 ± 3.20, 16.25 ± 7.73, 18.25 ± 8.96, 18.03 ± 5.27, 18.63 ± 3.35, 19.75 ± 6.96, 18.05 ± 5.36 and 17.88 ± 3.45 letters at 1 wk, 1 mo, 3 mo, 6 mo, 9 mo, 12 mo, 24 mo and 36 mo after treatment, respectively (P < 0.01). The patients showed varying degrees of subretinal fluid resorption after treatment. None of the patients had serious complications such as increased intraocular pressure, development/progression of cataracts, endophthalmitis or retinal detachment.
CONCLUSION
Intravitreal injection of conbercept combined with 532-nm laser photocoagulation may be a feasible treatment for Coats disease in adult patients.
Keywords: Coats disease, Adults, Retinal telangiectasis, Conbercept, Laser therapy, Retrospective study
Core Tip: Coats disease is an idiopathic exudative outer retinopathy caused by abnormal retinal vascular development. Intravitreal injection of conbercept combined with 532-nm laser photocoagulation may be a feasible treatment for Coats disease in adult patients.
INTRODUCTION
Coats disease is an idiopathic exudative outer retinopathy caused by abnormal retinal vascular development[1,2]. The main clinical features of Coats disease are dilated retinal capillaries, miliary or small aneurysms and retinal and subretinal exudation[1,2]. The estimated incidence of Coats disease in the population is 0.09 per 100000 persons, and males are more commonly affected than females[3]. The disorder usually affects adolescents with a mean age at presentation of around 12 years[3]. However, a recent study reported that 7% of cases occur in adults (mainly males) with an onset age of 43–50 years, and the incidence of monocularity was 93.3%-100.0%[4]. Standard treatments for Coats disease include laser therapy, cryotherapy and surgical techniques such as subretinal fluid drainage and vitrectomy[5].
Recent studies have shown that patients with Coats disease have elevated levels of vascular endothelial growth factor (VEGF) in the aqueous humor, vitreous and subretinal fluid, suggesting that a disorder of VEGF-mediated vascular regeneration may be involved in the pathogenesis of Coats disease[6-9]. It has been suggested that ischemia around a region of unperfused retinal capillaries may lead to VEGF upregulation that increases the permeability of dilated capillaries and causes exudation of lipoproteins below the retina, resulting in edema and exudative retinal detachment[6-9]. There has been increasing interest in the use of anti-VEGF agents as an adjunct treatment for Coats disease[1,2,10]. Several clinical studies have reported that the intravitreal injection of a VEGF-binding antibody (such as bevacizumab or ranibizumab) or aptamer (pegaptanib) exerts beneficial effects in patients with Coats disease, including a reduction in exudation and edema and an improvement in visual acuity[11-15].
Conbercept is a recombinant fusion protein comprising extracellular domain 2 of VEGF receptor-1, extracellular domains 3 and 4 of VEGF receptor-2 and the Fc region of human IgG1[16]. This fusion protein binds VEGF with high affinity, has a long half-life in the vitreous and blocks all isoforms of VEGF-A, VEGF-B, VEGF-C and placental growth factor[16]. Conbercept has been shown to be an effective treatment for retinal diseases such as diabetic retinopathy-induced macular edema[17,18] and age-related macular degeneration[19-22]. However, to the best of our knowledge, only one previous study has investigated the use of conbercept as an adjunct treatment to laser therapy in the management of Coats disease[23].
The aim of this retrospective case series study was to summarize the clinical outcomes of adult patients who were diagnosed with Coats disease at our ophthalmology department and treated with intravitreal injection of conbercept in combination with laser photocoagulation.
MATERIALS AND METHODS
Patients
This retrospective case series study included patients diagnosed with Coats disease and treated with intravitreal injection of conbercept and laser photocoagulation at the Department of Ophthalmology of Shenzhen People’s Hospital between January 2016 and January 2017. The inclusion criteria were: (1) Age > 18 years; (2) Newly diagnosed with Coats disease; and (3) Treated with intravitreal injection of conbercept and laser photocoagulation. Coats disease was diagnosed according to the results of the fundus examination (the presence of abnormal dilated retinal blood vessels and retinal interstitial or subretinal exudation with or without retinal detachment), fluorescein fundus angiography (FFA; typical dilatation of retinal capillaries and small blood vessels) and optical coherence tomography (OCT; characteristic features such as retinal thickening, exudation and subretinal fluid)[24]. The exclusion criteria were: (1) Other eye diseases such as age-related macular degeneration; and (2) Complications such as severe refractive interstitial opacity or vitreous hemorrhage that impaired observation of the fundus.
The severity of Coats disease in each patient was classified as stage 1 (retinal capillary dilation only), stage 2A (retinal capillary dilation and subretinal exudation outside the central fovea), stage 2B (retinal capillary dilation and exudation inside the central fovea), stage 3A (incomplete retinal detachment), stage 3B (complete retinal detachment), stage 4 (total retinal detachment with secondary glaucoma) or stage 5 (the terminal stage of eyeball atrophy)[24].
The study was approved by the ethics committee of Shenzhen People’s Hospital, No. LL-KY-2020238.
Treatment
Written consent was obtained from each patient after they and their families had been fully informed about the treatment strategy (intravitreal injection of conbercept combined with laser photocoagulation) and potential adverse effects. A standard method was used for intravitreal injection of conbercept into the affected eye. Before treatment, 0.5% levofloxacin eye drops were administered as a local anti-inflammatory therapy. The same doctor, who had a third-level surgery qualification, performed all the procedures. The patient was placed in a supine position in the operating room. Tropicamide was used for mydriasis, and oxybuprocaine hydrochloride was applied for surface anesthesia. Disinfection and surgical draping were performed routinely according to the requirements of internal eye surgery. A protective film was pasted, an eyelid opener was used to open the eyelids, and the conjunctival sac was washed with diluted povidone iodine. A 30-gauge needle was inserted into the flat part of the ciliary body at a distance of 3.5–4 mm from the limbus. The intravitreal cavity was injected with 0.5 mg conbercept (trade name, Langmu; Chengdu Kanghong Biological Co., Ltd, China) administered as 0.05 mL of a 10 mg/mL solution. Gentle pressure was applied with a cotton swab for 10 s to prevent reflux. At the end of the procedure, light sensation was checked, tobramycin-dexamethasone eye ointment was applied, and the eye was covered with a blindfold. Postoperatively, 0.5% levofloxacin eye drops were given for 3 d as prophylaxis against bacterial infection. Subsequently, the anterior segment of the eye was examined for signs of an inflammatory reaction, intraocular pressure was measured, and fundus examination and FFA were performed. Based on the FFA results, 532-nm laser photocoagulation was carried out after conbercept injection to block retinal aneurysms, abnormally dilated capillaries or capillaries without perfusion. The laser equipment used in this study was Lumenis Vision One. Retinal laser photocoagulation was operated routinely with a 532-nm light. The size of the spot was 200 µm, the energy was 150 MW, and the duration was 200 ms.
Follow-up
Periodic reviews were conducted postoperatively at 1 wk, 1 mo, 3 mo, 6 mo, 9 mo, 12 mo and annually thereafter. Changes in the condition of the eye were used to determine whether the treatment needed to be repeated.
Data collection
Clinical data were extracted from the medical records. Best-corrected visual acuity (BCVA) was measured using the early treatment of diabetic retinopathy study chart. Intraocular pressure was measured with a noncontact tonometer (NT-530; Nidek Co., Ltd, Japan). Fundus color photography and FFA examinations were performed using a TRC-50DX retinal camera (Topcon Medical Systems Inc., Japan). Spectral-domain OCT was carried out with a Spectralis system (Heidelberg Engineering GmbH, Germany). The central macular thickness was measured in high-definition scanning mode. The change in vision at each follow-up was evaluated as follows: an increase of ≥ 15 in the number of letters in the chart was considered a substantial improvement in vision; an increase of 5–14 was considered an improvement in vision; an increase or decrease of < 5 was considered stable vision; a decrease of 5–14 was considered a worsening of vision; and a decrease of ≥ 15 was considered a substantial worsening of vision[25].
Statistical analysis
SPSS 23.0 (IBM Corp., Armonk, NY, United States) was used for the statistical analyses. Measurement data are expressed as mean ± standard deviation values. BCVA was compared between time points using repeated-measures analysis of variance and Fisher’s LSD post-hoc test. P < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics of the study participants
The study included eight eyes of 8 patients (7 men, 87.5%) aged 36.10 ± 6.65 years (range, 27-51 years). The clinical characteristics of the study participants are summarized in Table 1. The average BCVA of the affected eye before treatment was 51.17 ± 15.15 letters (range, 28-70 letters), and the average central macular thickness was 303.30 ± 107.87 µm (range, 221–673 µm). Intraocular pressure was normal in all affected eyes. The average number of injections in the affected eyes was 1.37 ± 0.92: four eyes were injected once, three eyes were injected twice, and one eye was injected thrice. The average follow-up duration was 37.33 ± 2.26 mo (range, 34–39 mo).
Table 1.
Clinical characteristics of the study participants
|
Case no.
|
Sex/age in yr/eye
|
Stage
|
Comorbidity
|
Alcohol/smoking status
|
Quadrants of telangiectasia
|
No. of injections
|
No. of laser therapies
|
BCVA baseline
|
BCVA 1 wk
|
BCVA 1 mo
|
BCVA 3 mo
|
BCVA 6 mo
|
BCVA 9 mo
|
BCVA 12 mo
|
BCVA 24 mo
|
BCVA 36 mo
|
Termination
|
| 1 | M/31/os | 2A | None | Smoker | 3 | 2 | 1 | 60 | 70 | 68 | 75 | 75 | 74 | 72 | 66 | 65 | 65 |
| 2 | M/35/os | 2A | None | Drinker | 2 | 1 | 1 | 55 | 70 | 75 | 70 | 70 | 70 | 75 | 80 | 76 | 76 |
| 3 | M/45/od | 2A | HTN. | Drinker | 2 | 1 | 1 | 28 | 40 | 45 | 50 | 53 | 55 | 55 | 53 | 55 | 55 |
| 4 | M/29/os | 2A | None | None | 3 | 1 | 2 | 39 | 55 | 65 | 60 | 58 | 60 | 60 | 58 | 62 | 62 |
| 5 | M/33/od | 2B | None | None | 3 | 2 | 1 | 55 | 70 | 70 | 75 | 75 | 75 | 77 | 75 | 75 | 75 |
| 6 | F/32/od | 2B | None | None | 3 | 2 | 2 | 70 | 78 | 75 | 78 | 78 | 80 | 80 | 77 | 75 | 75 |
| 7 | M/35/os | 2B | None | Drinker | 3 | 3 | 2 | 56 | 70 | 75 | 78 | 75 | 75 | 78 | 75 | 75 | 75 |
| 8 | M/37/od | 2A | Cervical spondylosis | Smoker | 2 | 1 | 1 | 54 | 72 | 74 | 77 | 77 | 77 | 78 | 77 | 77 | 77 |
BCVA: Best-corrected visual acuity; HTN.: Hypertension; os: Oculus sinister; od: Oculus dexter.
Comparison of BCVA before and after treatment
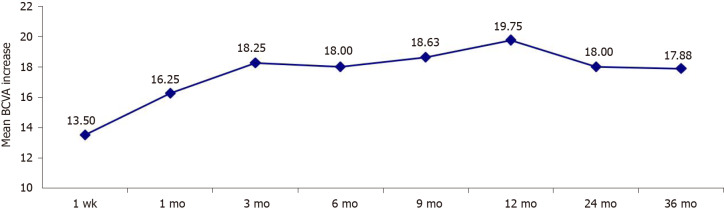
There was a substantial improvement in vision in four eyes (50.0%), an improvement in vision in three eyes (37.5%) and stable vision in one eye (12.5%). Table 2 presents the BCVA before treatment and at 1 wk, 1 mo, 3 mo, 6 mo, 9 mo, 1 year, 2 years and 3 years after treatment. There was a significant upward trend in the BCVA (P-trendlinear < 0.001). Compared to the pretreatment value, the average BCVA at 1 wk, 1 mo, 3 mo, 6 mo, 9 mo, 1 year, 2 years and 3 years after treatment increased by 13.50 ± 3.20, 16.25 ± 7.73, 18.25 ± 8.96, 18.03 ± 5.27, 18.63 ± 3.35, 19.75 ± 6.96, 18.05 ± 5.36 and 17.88 ± 3.45 letters, respectively (P < 0.01 for all pairwise comparisons; Figure 1). The number of letters by which the average BCVA increased at 1 mo after the treatment showed no significant difference compared to that at 3 mo, 12 mo and 24 mo post-treatment and at the final follow-up (P > 0.05), suggesting that 1 mo after treatment was the critical period for recovery of visual acuity.
Table 2.
Best-corrected visual acuity
|
Time of measurement
|
Best-corrected visual acuity
|
P
(vs pretreatment value)
|
| Pretreatment | 52.12 ± 12.93 | |
| 1 wk after treatment | 65.63 ± 12.19 | < 0.001 |
| 1 mo after treatment | 68.38 ± 10.17 | < 0.001 |
| 3 mo after treatment | 70.38 ± 10.18 | < 0.001 |
| 6 mo after treatment | 70.13 ± 9.42 | < 0.001 |
| 9 mo after treatment | 70.75 ± 8.75 | < 0.001 |
| 12 mo after treatment | 71.88 ± 9.28 | < 0.001 |
| 24 mo after treatment | 70.13 ± 9.98 | < 0.001 |
| 36 mo after treatment | 70.01 ± 8.23 | < 0.001 |
| P-trendlinear < 0.001 |
Data are presented as mean ± standard deviation.
Figure 1.
Mean increase in the best-corrected visual acuity from the pretreatment value. BCVA: Best-corrected visual acuity.
Comparison of subretinal edema before and after treatment
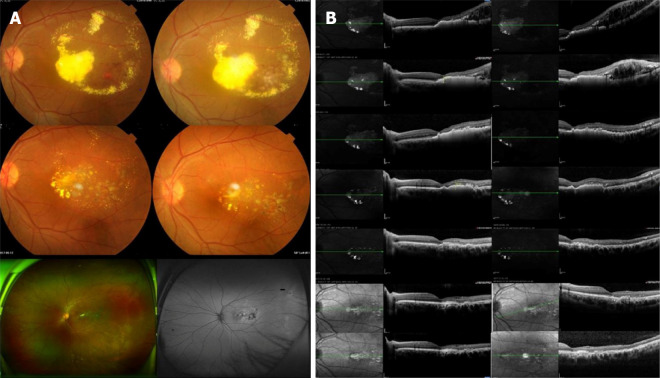
Only one of the patients in this study had macular lesions and developed macular edema. The central macular thickness in this patient was 673 µm before treatment but decreased to 587 µm, 372 µm, 322 µm, 267 µm, 221 µm and 208 µm at 1 wk, 1 mo, 3 mo, 1 year, 2 years and 3 years after treatment, respectively. Macular edema was substantially improved at the final follow-up. In the other patients, treatment led to varying degrees of subretinal fluid resorption and a decrease in retinal thickness (a representative case is shown in Figure 2).
Figure 2.
Coats disease of the left eye treated with conbercept combined with 532-laser photocoagulation. A: Fundus photographs of the patient’s eye before treatment and at 1 mo, 1 yr and 2 yrs after treatment as well as at the last follow-up; B: Changes in central retinal thickness and subretinal fluid before treatment and at 1 wk, 1 mo, 3 mo, 1 yr and 2 yrs after treatment as well as at the final follow-up. The retinal interstitial layer and subretinal regions showed gradual reductions in edema and exudation.
Complications
No patients had serious complications such as increased intraocular pressure, development/progression of cataracts, endophthalmitis or retinal detachment.
DISCUSSION
An important finding of this study was that adult patients with Coats disease exhibited significant improvements in BCVA that were maintained for at least 3 years after treatment with intravitreal injection of conbercept and laser photocoagulation. Furthermore, the treatment resulted in the resorption of subretinal fluid. Notably, no patients had serious complications such as increased intraocular pressure, development/progression of cataracts, endophthalmitis or retinal detachment. Our findings suggest that intravitreal injection of conbercept combined with 532-nm laser photocoagulation may be a feasible treatment for Coats disease in adult patients.
Coats disease is more common in men and is usually monocular. The average age of onset is 8-16 years[26]; hence, Coats disease is rarely diagnosed in adults. It can be challenging to distinguish Coats disease from other ocular diseases such as exudative age-related macular degeneration, diabetic retinopathy and retinal vasculitis. We observed that Coats disease in adulthood has similar fundal manifestations to Coats disease of childhood onset, although FFA showed that the abnormal expansion of the retinal capillaries was limited and mostly confined to the temporal retina. Only one patient in this study had an affected macula, which resulted in local superficial retinal detachment. Our observations are similar to those reported previously[27], suggesting that adult patients with Coats disease have a limited disease type with slow progression, mild macular damage and a good visual prognosis. Otani et al[28] followed two adult patients with Coats disease for more than 10 years: 1 patient showed a reduction in subretinal exudation within 1 year after retinal laser photocoagulation and an improvement in vision that was maintained for 11 years; the other patient exhibited increased exudation at 1 mo after photocoagulation and retinal detachment at 2 mo, but these subsequently resolved to result in visual improvement that was maintained for 10 years.
There are various management strategies for Coats disease, and the decision whether to use a single therapy or combination of treatments is made according to the disease stage. The pathogenesis of Coats disease involves abnormal dilatation and enhanced permeability of retinal vessels leading to vascular leakage. It is thought that VEGF may play an important role in these pathogenetic mechanisms, and several studies have reported that VEGF concentration is significantly increased in the affected eyes of patients with Coats disease[6-9]. Intravitreal injection of anti-VEGF drugs can reduce VEGF concentration, stabilize abnormal blood vessels and promote the resorption of subretinal effusion and exudation. Numerous clinical investigations have found that the intravitreal injection of a VEGF-binding agent (such as bevacizumab, ranibizumab or pegaptanib) can reduce exudation and edema and improve visual acuity in patients with Coats disease[29-31]. However, there is no current consensus on the choice, dosage and timing of anti-VEGF drug in the treatment of Coats disease, and research in this area is still in the exploratory stage.
Only one previous study has described the use of conbercept in the management of Coats disease[23]: The combination of intravitreal injection of conbercept with laser therapy resulted in a significant improvement in BCVA after 2 years with no serious adverse effects. The above observations are consistent with our findings, which showed a reduction in subretinal fluid and an improvement in BCVA that was sustained for 3 years without any major adverse effects.
This study has some limitations. First, the results may be prone to information bias or selection bias due to the retrospective study design. Second, the generalizability of the findings is not known because this was a single center study with a small sample size. Third, a comparator group was not included. Prospective randomized clinical trials are needed to confirm our findings and establish whether conbercept is a useful adjunct therapy for Coats disease.
CONCLUSION
In conclusion, intravitreal injection of conbercept combined with 532-nm laser photocoagulation may be a feasible treatment for Coats disease in adult patients.
ARTICLE HIGHLIGHTS
Research background
Coats disease is an idiopathic exudative outer retinopathy caused by abnormal retinal vascular development
Research motivation
The disorder usually affects adolescents. However, a recent study reported that 7% of cases occur in adults.
Research objectives
We investigated the long-term outcomes of intravitreal conbercept injection with laser photocoagulation to treat Coats disease in adults.
Research methods
This is a retrospective case series on 8 adult patients (eight eyes) diagnosed with Coats disease. Patients were treated with intravitreal conbercept injection and 532-nm laser photocoagulation.
Research results
There was a significant upward trend in the best-corrected visual acuity after treatment, which was maintained for at least 3 years.
Research conclusions
Intravitreal conbercept injection plus 532-nm laser photocoagulation may be a feasible treatment for Coats disease in adult patients.
Research perspectives
Prospective randomized clinical trials are needed to confirm our findings and establish whether conbercept is a useful adjunct therapy for Coats disease.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of Shenzhen People’s Hospital (No. LL-KY-2020238).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Unsolicited manuscript
Peer-review started: August 4, 2020
First decision: August 21, 2020
Article in press: November 2, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buchaim RL, Mehdipour P S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Li Jiang, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Bo Qin, Department of Ophthalmology, Shenzhen Aier Eye Hospital Affiliated to Jinan University, Shenzhen 518032, Guangdong Province, China. jiang.li@szhospital.com.
Xiao-Ling Luo, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
He Cao, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Ting-Ming Deng, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Ming-Ming Yang, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Ting Meng, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Hui-Qin Yang, Department of Ophthalmology, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen 518020, Guangdong Province, China.
Data sharing statement
Not applicable.
References
- 1.Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: An overview of classification, management and outcomes. Indian J Ophthalmol. 2019;67:763–771. doi: 10.4103/ijo.IJO_841_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Wang C, Su G. Recent advances in the diagnosis and treatment of Coats' disease. Int Ophthalmol. 2019;39:957–970. doi: 10.1007/s10792-019-01095-8. [DOI] [PubMed] [Google Scholar]
- 3.Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24:1797–1801. doi: 10.1038/eye.2010.126. [DOI] [PubMed] [Google Scholar]
- 4.Rishi E, Rishi P, Appukuttan B, Uparkar M, Sharma T, Gopal L. Coats' disease of adult-onset in 48 eyes. Indian J Ophthalmol. 2016;64:518–523. doi: 10.4103/0301-4738.190141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusaka S. Surgical Management of Coats Disease. Asia Pac J Ophthalmol (Phila) 2018;7:156–159. doi: 10.22608/APO.201867. [DOI] [PubMed] [Google Scholar]
- 6.Kase S, Rao NA, Yoshikawa H, Fukuhara J, Noda K, Kanda A, Ishida S. Expression of vascular endothelial growth factor in eyes with Coats' disease. Invest Ophthalmol Vis Sci. 2013;54:57–62. doi: 10.1167/iovs.12-10613. [DOI] [PubMed] [Google Scholar]
- 7.He YG, Wang H, Zhao B, Lee J, Bahl D, McCluskey J. Elevated vascular endothelial growth factor level in Coats' disease and possible therapeutic role of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2010;248:1519–1521. doi: 10.1007/s00417-010-1366-1. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Jain A, Moshfeghi DM. Elevated vascular endothelial growth factor levels in Coats disease: rapid response to pegaptanib sodium. Graefes Arch Clin Exp Ophthalmol. 2007;245:1387–1388. doi: 10.1007/s00417-007-0559-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Peng XY, Chen FH, Zhang YP, Wang L, You QS, Jonas JB. Vascular endothelial growth factor in Coats' disease. Acta Ophthalmol. 2014;92:e225–e228. doi: 10.1111/aos.12158. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Udyaver S, Dalvin LA, Lim LS, Atalay HT, L Khoo CT, Mazloumi M, Shields JA. Coats disease in 351 eyes: Analysis of features and outcomes over 45 years (by decade) at a single center. Indian J Ophthalmol. 2019;67:772–783. doi: 10.4103/ijo.IJO_449_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng XX, Jiang YR. The effect of intravitreal bevacizumab injection as the initial treatment for Coats' disease. Graefes Arch Clin Exp Ophthalmol. 2014;252:35–42. doi: 10.1007/s00417-013-2409-1. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Deng G, Liu J, Ma Y, Lu H. The effects of a treatment combination of anti-VEGF injections, laser coagulation and cryotherapy on patients with type 3 Coat's disease. BMC Ophthalmol. 2017;17:76. doi: 10.1186/s12886-017-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard MC, Mataftsi A, Balmer A, Houghton S, Munier FL. ranibizumab in the management of advanced Coats disease Stages 3B and 4: long-term outcomes. Retina. 2014;34:2275–2281. doi: 10.1097/IAE.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 14.Raoof N, Quhill F. Successful use of intravitreal bevacizumab and pascal laser photocoagulation in the management of adult Coats' disease. Middle East Afr J Ophthalmol. 2013;20:256–258. doi: 10.4103/0974-9233.114805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villegas VM, Gold AS, Berrocal AM, Murray TG. Advanced Coats' disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin Ophthalmol. 2014;8:973–976. doi: 10.2147/OPTH.S62816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira Dias JR, de Andrade GC, Novais EA, Farah ME, Rodrigues EB. Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept. Int J Retina Vitreous. 2016;2:3. doi: 10.1186/s40942-016-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Guo C, You A, Wang D, Wang W, Zhang X. One-year outcomes of novel VEGF decoy receptor therapy with intravitreal conbercept in diabetic retinopathy-induced macular edema. Mol Vis. 2019;25:636–644. [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Zhang L, Wang Y, Xu W, Jiao W, Ma A, Zhao B. One-Year Outcome of Conbercept Therapy for Diabetic Macular Edema. Curr Eye Res. 2018;43:218–223. doi: 10.1080/02713683.2017.1379542. [DOI] [PubMed] [Google Scholar]
- 19.Wu BH, Wang B, Wu HQ, Chang Q, Lu HQ. Intravitreal conbercept injection for neovascular age-related macular degeneration. Int J Ophthalmol. 2019;12:252–257. doi: 10.18240/ijo.2019.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, Song Y, Xu G, Ye J, Wu Z, Liu X, Dong X, Zhang M, Xing Y, Zhu S, Chen X, Shen Y, Huang H, Yu L, Ke Z, Rosenfeld PJ, Kaiser PK, Ying G, Sun X, Xu X PHOENIX Study Group. Conbercept for Treatment of Neovascular Age-related Macular Degeneration: Results of the Randomized Phase 3 PHOENIX Study. Am J Ophthalmol. 2019;197:156–167. doi: 10.1016/j.ajo.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Cui C, Lu H. Clinical observations on the use of new anti-VEGF drug, conbercept, in age-related macular degeneration therapy: a meta-analysis. Clin Interv Aging. 2018;13:51–62. doi: 10.2147/CIA.S151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, Sun D, Lu H, Dai R, Xing L, Dong H, Wang L, Wei D, Jiang B, Jiao Y, Jablonski MM, Charles S, Gu W, Chen H. Comparison of effectiveness and safety between conbercept and ranibizumab for treatment of neovascular age-related macular degeneration. A retrospective case-controlled non-inferiority multiple center study. Eye (Lond) 2018;32:391–399. doi: 10.1038/eye.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Ke Y, Wang W, Shi X, Hei K, Li X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats' disease. Graefes Arch Clin Exp Ophthalmol. 2018;256:1339–1346. doi: 10.1007/s00417-018-3949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131:561–571. doi: 10.1016/s0002-9394(00)00883-7. [DOI] [PubMed] [Google Scholar]
- 25.Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, Weichselberger A, Staurenghi G SUSTAIN Study Group. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–671. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Wang KY, Cheng CK. A combination of intravitreal bevacizumab injection with tunable argon yellow laser photocoagulation as a treatment for adult-onset Coats' disease. J Ocul Pharmacol Ther. 2011;27:525–530. doi: 10.1089/jop.2011.0088. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Dong F, Wang R. Clinical characteristics and diagnosis and treatment of Coats disease in adults. Zhonghua Yandibing Zazhi . 2008;24:279–282. [Google Scholar]
- 28.Otani T, Yasuda K, Aizawa N, Sakai F, Nakazawa T, Shimura M. Over 10 years follow-up of Coats' disease in adulthood. Clin Ophthalmol. 2011;5:1729–1732. doi: 10.2147/OPTH.S27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Surv Ophthalmol. 2014;59:30–46. doi: 10.1016/j.survophthal.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Kaul S, Uparkar M, Mody K, Walinjkar J, Kothari M, Natarajan S. Intravitreal anti-vascular endothelial growth factor agents as an adjunct in the management of Coats' disease in children. Indian J Ophthalmol. 2010;58:76–78. doi: 10.4103/0301-4738.58480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina. 2010;30:617–622. doi: 10.1097/IAE.0b013e3181c2e0b7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.