Abstract
Metal–organic frameworks (MOFs) are a class of promising sorbents for effective sequestration of radioactive 99TcO4– anions. However, their poor stability and slow sorption kinetics in the industrial condition pose a great challenge. Herein, we demonstrate an optimizing strategy via in situ polymerization of ionic liquids (ILs) encapsulated in the pores of MOFs, forming polyILs@MOFs composites with greatly enhanced TcO4– sequestration compared with the pristine MOFs. Notably, the cross-linked polymerization of ILs facilitates the formation of both the inside ionic filler as the active sites and outside coating as the protective layers of MOFs, which is significantly beneficial to obtain the optimized sorption materials of exceptional stability under extreme conditions (e.g., in 6 M HNO3). The final optimized composite shows fast sorption kinetics (<30 s), good regeneration (>30 cycles), and superior uptake performance for TcO4– in highly acidic conditions and simulated recycle stream. This strategy opens up a new opportunity to construct the highly stable MOF-based composites and extend their applicability in different fields.
Short abstract
An optimizing strategy to enhance the stability and pertechnetate sequestration ability of poly(ILs)@MOFs composites was developed via in situ cross-linked polymerization of ILs encapsulated in MOFs.
Introduction
Radioactive waste pollution, which is formed as the byproducts of the nuclear fuel industry and nuclear weapons, is one of the most severe threats to the international community.1−4 The current proposed managing strategy of radioactive waste is very complicated by the inventory of long-living radionuclides, such as 99Tc, because of its long half-life (t1/2 = 2.1 × 105 years), high water solubility (11.3 M at 20 °C in the form of sodium salt), and serious perniciousness as low activity waste (LAW) under aerobic conditions.5−8 Disposition of 99Tc during the vitrification process of nuclear waste remains challenging due to the generation of volatile Tc2O7.9 Therefore, 99Tc removal from high-level waste steam prior to vitrification is greatly essential. The main form of 99Tc in industry is pertechnetate (99TcO4–), which can easily spread into the environment and enter into the food chain after accidental release. Notably, the large size and low charge density of TcO4– result in its low binding constant and the small complexing enthalpy in water, which makes it very difficult to be selectively recognized.10−13 Removal of 99Tc in groundwater is rather hard, because of the requirement to meet the low detection limit below the standard of drinking water (5.2 × 10–10 M or 900 pCi L–1) defined by the U.S. Environmental Protection Agency.14 Now, great success has been achieved on 99Tc sequestration from alkaline solutions, especially the high-level nuclear waste stored in underground tanks at the Savannah River.15−18 Nevertheless, it is still a great challenge to manage TcO4– species in highly concentrated HNO3 medium, prior to the plutonium uranium redox extraction (PUREX) process.19,20
For the established technologies for TcO4– remediation, the utilization of solid sorbents holds great promise, considering their simple, safe, and low-cost processes and superior performances for removal of anions at a low concentration level and point-of-use applications.21−27 Although the pioneering endeavor of this topic was explored long ago, it is definitely a long-term battle for 99Tc decontamination. As a matter of fact, the traditional commercialized polymeric anion-change resins (e.g., superLig-639 or Purolite-A-520E) have slow anion-exchange kinetics and poor radiation resistance.28,29 The inorganic cationic materials, such as the layered double hydroxides (LDHs) or layered rare-earth hydroxides (LRHs), possess a limited sorption capability and poor selectivity.30,31 As an emerging candidate to sequester TcO4– from nuclear waste, metal–organic frameworks (MOFs) are endowed with the unique advantages of tailored porous structures and facile functionalization, relying on their modular building blocks.32−35 Some cationic MOFs exhibit good sorption capability and selectivity to pertechnetate, but slow kinetics, low stability, and poor recyclability under highly acidic conditions.36−38
In fact, most MOF materials are constructed by carboxylate- or azolate-involved ligands and thus have the neutral porous frameworks. Considering the available database of numerous MOFs, it can be envisioned that the incorporation of cationic polymers into neutral MOFs will afford diverse composites, which can serve as the anion receptors with high density of easily accessible anion-exchange sites. This strategy may also endow the porous composites with fast sorption kinetics and high stability.39,40 Nevertheless, a simple incorporation of cationic polymers into MOFs will suffer from the size incompatibility, owing to the limited open windows of MOFs. On the other hand, several cationic monomers have been used to bind to the organic ligands in MOFs, either prior to or after the synthesis of MOFs, to obtain polymers@MOFs composites.41−44 However, either the complex synthetic procedure or the need of a special active group largely limits their universal applications. As a result, a more feasible approach has been developed to fabricate polymers@MOFs composites, by encapsulating the small monomers into MOFs in advance and then implementing polymerization in situ. In this way, the intrinsic properties of both polymers and MOFs could be reserved in the resultant composites.45−48
To demonstrate the above proof-of-concept for the design of anion sorbents, in this work, imidazolium-based ionic liquids (ILs) were used as the monomers given their cationic nature, tunable sizes, and easy-to-obtain properties.49 Besides these, ILs could be easily impregnated to the pores of MOFs, even under the vacuum condition,50 due to their liquid nature and extremely low vapor pressure. Moreover, the interactions between ILs and pore walls of MOFs allow the immobilization of ILs into MOFs.51 Up to now, some ILs@MOFs or polyILs@MOFs composites have been developed for catalysis, gas adsorption/separation, and ion conduction.47,52,53 In the reported examples, ILs or polyILs are normally considered to be entrapped within the MOFs due to size confinement.54,55 Thus, the leaching of ILs or polyILs under industrial conditions is a significant problem to the practicability of composites. Also, the performances of such composites will be greatly affected by their stability toward water and acidic or alkaline solutions.
With the above points in mind, cross-linked polyILs@MOFs composites can be constructed and applied in pertechnetate sequestration, considering the following features: (i) The high density of anion-exchange sites of polyILs and hierarchical porosity of composites could facilitate a rapid anion transportation. (ii) The in situ generated cross-linked polymers may improve the stability of composites. (iii) The highly cross-linked polymers and hydrophobic pores of MOFs afford a high affinity to the less hydrated anion (e.g., TcO4–) to enhance the selectivity. To establish the structure–property relationship and optimize the sorption performances, a variety of ILs (linear or cross-linked monomers) and MOFs (MIL-101, UiO-66, and ZIF-8) were employed for de novo synthesis. Notably, the polyILs@MOFs composites integrate the advantages of each individual component, with highly enhanced sorption kinetics, uptake capacity, stability, and recyclability under extreme conditions, toward TcO4– or ReO4– (a nonradioactive surrogate for TcO4–) remediation. Further, an optimizing strategy to enhance the stability and reusability for MOF-based composites was proposed, which can be generally applied in different fields.
Results and Discussion
Synthesis and Characterization
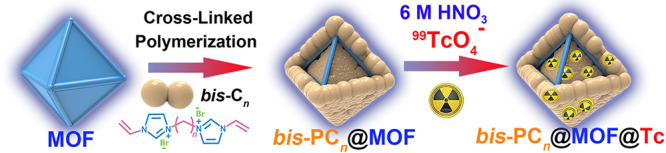
The targeted polyILs@MOFs sorbents were obtained by impregnation of ILs into MOFs and then in situ polymerization (see Figure 1). By this approach, the polyILs@MOFs composites with different structural features and adsorption performances could be easily achieved, through changing the IL monomers and/or MOF precursors. In this work, we chose two series of imidazolium-based monomers, including the linear species bearing one vinyl group (Cn, n = 2, 6, and 12) and the cross-linked species bearing two vinyl terminals (bis-Cn, n = 2 and 6) with the following considerations (Figure 1): (i) Such polyILs in hierarchical pores of polyILs@MOFs have the rich positive charges, therefore enhancing the sorption kinetics of anions. (ii) The cross-linked polymers may coat on the surface of MOFs, thus protecting MOFs in extreme conditions. (iii) The ILs modified with different substituents in polyILs@MOFs composites may affect their sorption ability, thus providing the opportunities to optimize these sorbents. As a result, two series of PCn@ MOFs and bis-PCn@MOFs composites (here, MOFs = MIL-101, UiO-66, or ZIF-8) can be achieved. Prior to the anion-exchange, the toxic Br– anion in composites was replaced with the Cl– ion, via treating with the saturated NaCl solution. Herein, PC2(Cl)@MIL-101 and bis-PC2(Cl)@MIL-101 were selected as the representatives for a thorough illustration, owing to their superior sorption capabilities. The enhanced sorption capacities were also observed in polyILs@UiO-66 and polyILs@ZIF-8, compared with the corresponding pristine MOFs, which thus were enumerated to confirm the universality of the proposed strategy.
Figure 1.
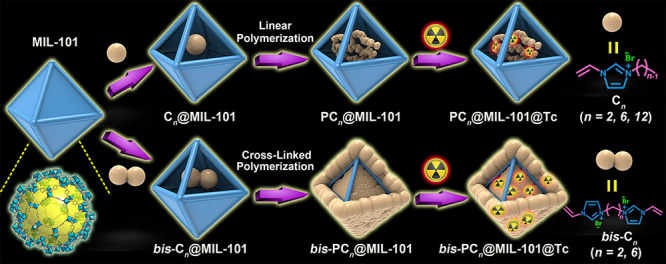
Designing strategy of polyILs@MOFs composites for radionuclide sequestration, by in situ polymerization of the encapsulated imidazolium-based ILs in the pores of MOFs (MIL-101 was chosen as the representative MOF here).
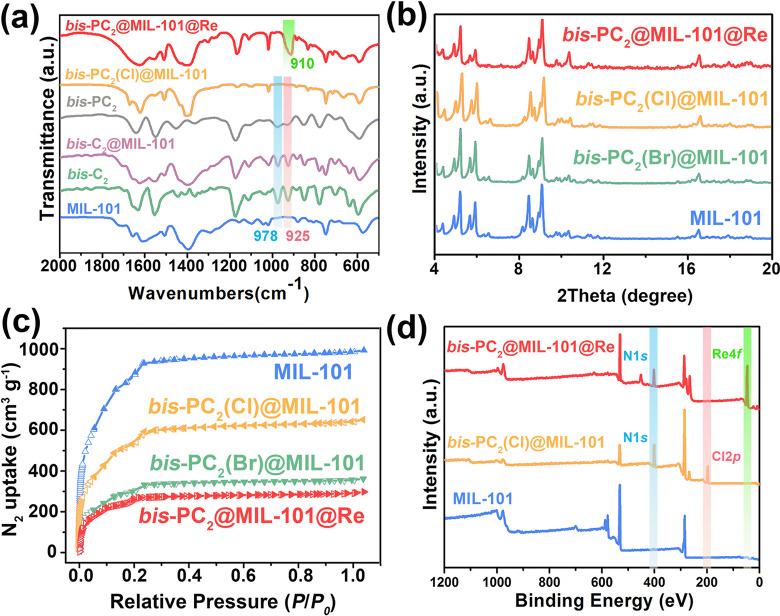
FT-IR spectra were taken to illustrate the polymerization of monomers in MIL-101. All characteristic peaks of bis-C2 and MIL-101 can be observed in the FT-IR spectrum of bis-C2@MIL-101, where the peaks at 925 and 978 cm–1 reveal the bending vibrations of =CH2 and =C—H groups, respectively (Figure 2a). Upon polymerization, the two peaks are clearly weakened in bis-PC2(Cl)@MIL-101 and bis-PC2. With regard to bis-PC2@MIL-101@Re, the peak at 910 cm–1 corresponds to the adsorbed ReO4– anions. Similar results were also observed in the FT-IR spectra of PCn@MIL-101 (n = 2, 6, and 12) and bis-PC6@MIL-101 composites (Figure S1). Additionally, PXRD patterns illustrate the framework integrity of composites (Figure 2b and Figure S2). Their porosity was evaluated by N2 adsorption isotherms at 77 K (Figure 2c). The Brunauer–Emmett–Teller (BET) surface areas are significantly reduced for bis-PC2(Br)@MIL-101 (1055 m2 g–1) and bis-PC2(Cl)@MIL-101 (1891 m2 g–1), compared with that of MIL-101 (2926 m2 g–1), due to the introduction of polyILs. The larger Br– anions result in a smaller BET surface area of bis-PC2(Br)@MIL-101, compared with bis-PC2(Cl)@MIL-101. Moreover, compared with MIL-101, the pore volumes of bis-PC2(Br)@MIL-101 and bis-PC2(Cl)@MIL-101 show a shrinkage due to the occupation of voids in MIL-101 by polyILs (Figure S3). Such hierarchical pores in polyILs@MIL-101 facilitate the anion transportation and/or sorption. The BET surface area of bis-PC2@MIL-101@Re is further decreased to 875 m2 g–1, and a continuous shrinking of pore volume is found after anion uptake. This can be attributed to the void consumption and mass increment after ReO4– sorption. XPS was employed to investigate the chemical compositions of MIL-101, bis-PC2(Cl)@MIL-101, and bis-PC2(Cl)@MIL-101@Re (Figure 2d). Compared to MIL-101, the XPS survey for bis-PC2(Cl)@MIL-101 shows the additional peaks of N 1s (401.3 eV) and Cl 2p (197.1 eV), revealing the existence of imidazolium and Cl–. Moreover, the appearance of Re 4f peaks and disappearance of Cl 2p peak in XPS of bis-PC2(Cl)@MIL-101@Re indicate the complete anion-exchange of Cl– by ReO4–. For free ReO4–, the XPS signals of Re 4f5/2 and Re 4f7/2 are observed at the binding energies of 48.3 and 45.9 eV,56 which shift to 48.4 and 46.0 eV, respectively, for bis-PC2@MIL-101@Re (Figure S4). This result clearly reveals a decrease of electron density of ReO4– in bis-PC2@MIL-101@Re, caused by the interactions between ReO4– and bis-PC2@MIL-101 in the composite.
Figure 2.
(a) FT-IR spectra of MIL-101, bis-C2, bis-C2@MIL-101, bis-PC2, bis-PC2(Cl)@ MIL-101, and bis-PC2@MIL-101@Re. (b) PXRD patterns and (c) N2 sorption isotherms of MIL-101, bis-PC2(Cl)@MIL-101, bis-PC2(Br)@MIL-101, and bis-PC2@MIL-101@Re. (d) XPS survey spectra of MIL-101, bis-PC2(Cl)@MIL-101, and bis-PC2@MIL-101@Re.
To gain a better understanding of ILs’ entrapment within MIL-101, STEM-EDS images of MIL-101 and polyILs@MIL-101 were further performed (Figures S5–S7). The in situ linear polymerization of C2–ILs occurs in the inner void of MIL-101, and the formed PC2 polymer is not observed on the crystal surface (Figure S5). By introducing a mixture of C2 and bis-C2 IL monomers, most of the formed polyILs are located within the pores of MIL-101, whereas a few polyILs cover on the surface of MOF particles (Figure S6). By contrast, the use of cross-linked bis-C2 monomers affords the bis-PC2@MIL-101 composite, where the surface of MIL-101 crystals is coated by a mass of polyILs (Figure S7). Notably, the toxic Br– in polyILs@MIL-101 could be fully replaced by Cl–, which motivates us to explore its application in radionuclide polluted water treatment. The TGA curve (Figure S8) also confirms the presence of ILs in the pores of bis-PC2(Cl)@MIL-101, showing additional weight loss of 13.2% at ca. 300 °C that is not observed for MIL-101.
Sorption Kinetics
The sorption experiments of TcO4– ions were initially performed by mixing 5 mg of bis-PC2(Cl)@MIL-101 with 5 mL of aqueous solution containing 26 ppm TcO4–. The concentration of TcO4– in solution was traced by the UV–vis characteristic peak at 290 nm and liquid scintillation counting (LSC) measurements. As depicted in Figure 3a, bis-PC2(Cl)@MIL-101 shows an extremely high removal rate for the TcO4– anion and the UV–vis characteristic peak at 290 nm fully disappears within 30 s, which is the fastest limitation for the manual experimental operation. This result is comparable to that of SCU-CPN-1,57 an anion-exchange polymer with the fastest TcO4– sorption kinetics and shortest equilibrium time thus far. For bis-PC2(Cl)@MIL-101, the cationic polyILs coated on the outside of the MOF particles and easily attract TcO4– in solution via electrostatic interactions. Then, the attracted TcO4– anions enter into the channels of the composite, which are trapped by the inner network. Thus, the polymeric components and the hierarchical pores in bis-PC2(Cl)@MIL-101 should account for the rapid anion transportation. For the similarity in both magnitude and trend in solubility of TcO4– and ReO4– anions, bis-PC2(Cl)@MIL-101 shows similar sorption kinetics for two such anions (Figure S9). The experiment was initially performed by mixing 2 mg of bis-PC2(Cl)@MIL-101 with a 5 mL water solution of 50 ppm ReO4–. The ReO4– sorption kinetics data can be fitted by using the pseudo-second-order model, with a high correlation coefficient of >0.9999 (Figure S10 and Table S1). The rate constant k2 of 22.03 g mg–1 min–1 shows an ultrafast sorption rate. The distribution coefficient Kd of bis-PC2(Cl)@MIL-101 is 3.3 × 106 mL g–1, which is comparable to PQA-pN(Me)2Py-Cl (1.0 × 107 mL g–1) that represents the highest performance now.58 To evaluate its real application for the instant radionuclide sequestration, a chromatographic column was fabricated with bis-PC2(Cl)@MIL-101 as the stationary phase. Notably, the water solution of ReO4– (188 ppm) could be successfully purified (95.4% removal), via simply passing it through the column under ambient pressure (Figure S11). In contrast, most known sorbents require lengthy contacting time and vigorous stirring to this aim, which are clearly unfavorable for the fast treatment of slightly contaminated water.
Figure 3.
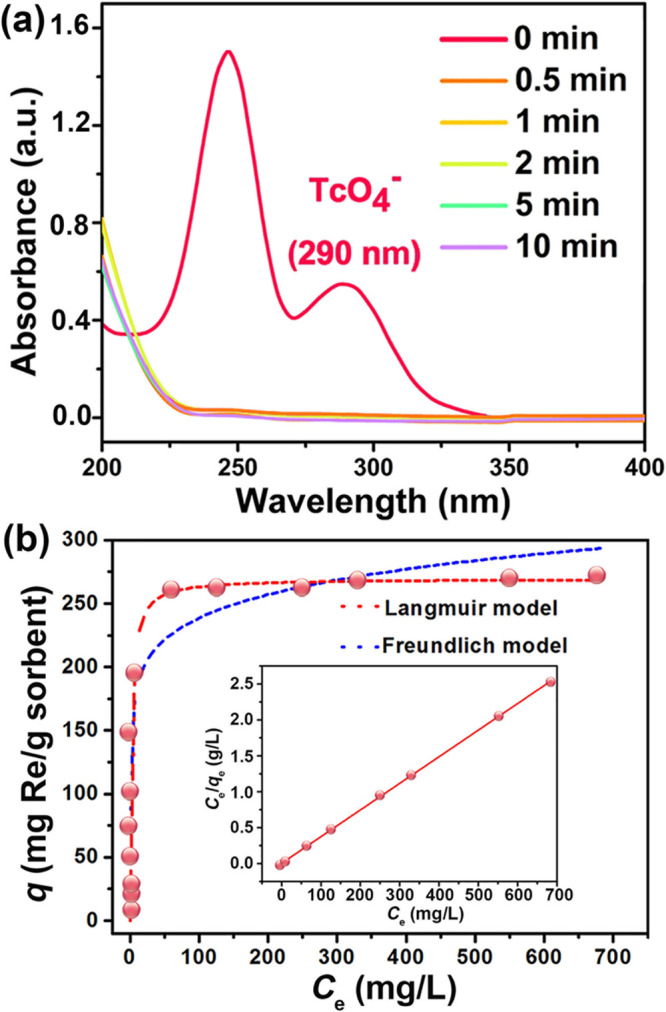
(a) UV–vis spectra of the TcO4– solution during anion-exchange by bis-PC2(Cl)@MIL-101. (b) Sorption isotherm of bis-PC2(Cl)@MIL-101 for ReO4–. Inset: linear regression by fitting the equilibrium data with the Langmuir sorption model.
Due to the limited availability and high radioactivity of 99TcO4–, ReO4– was used in the experiment of the sorption isotherm. It was initially taken by mixing bis-PC2(Cl)@MIL-101 (5 mg) with a water solution of TcO4– (5 mL) at a certain concentration (0–1000 ppm). The sorption of ReO4– by bis-PC2(Cl)@MIL-101 follows the Langmuir isotherm model (Figure 3b), with a high correlation coefficient of >0.99 (Table S2). The sorption capacity is 270 mg of Re per gram of sorbent, which corresponds to 362 mg of ReO4– per gram of sorbent. This value amounts to ca. 5 times that found in MIL-101 (55 mg of Re per gram of sorbent), indicating the great advantages of the composite optimization strategy. Notably, bis-PC2 has a good water solubility, which thus cannot be used for ReO4– sorption in water.
Sorption Mechanism
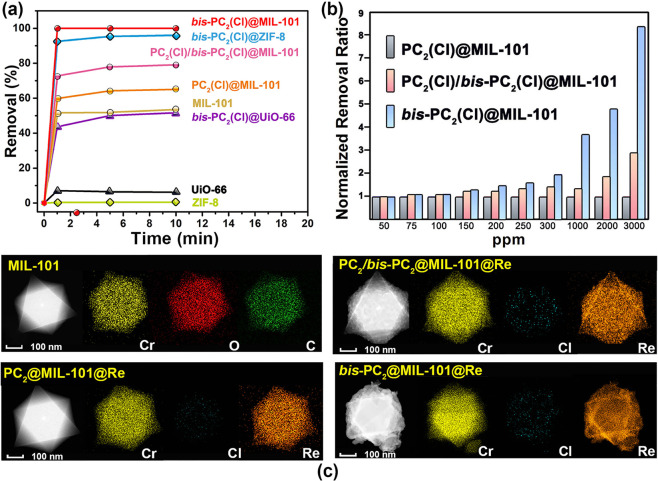
To reveal the sorption mechanism, a comprehensive comparative study on ReO4– sorption of MIL-101, PC2(Cl)@MIL-101, PC2(Cl)/bis-PC2(Cl)@MIL-101, and bis-PC2(Cl)@MIL-101 (Figure 4a) was taken under similar conditions (5 mg of sorbents; 5 mL aqueous solution of 200 ppm ReO4–). The removal percentages for the ReO4– ion follow the order bis-PC2(Cl)@MIL-101 (99.86%) > PC2(Cl)/bis-PC2(Cl)@MIL-101 (79.03%) > PC2(Cl)@MIL-101 (64.98%) > MIL-101 (53.49%), as shown in Table S3. Accordingly, the values of distribution coefficient (Kd) and rate constant (k2) of bis-PC2(Cl)@MIL-101 are 642 and 87 times more than those of MIL-101. The remarkable increments in ReO4– uptake capacity and rate can be well ascribed to the presence of cross-linked polymers in bis-PC2(Cl)@MIL-101. To confirm this assumption, a full study on serial polyILs@MIL-101 composites was taken. As a result, both the ReO4– removal percentages and Kd values follow the order (Table S3) bis-PC2(Cl)@MIL-101 > PC2(Cl)/bis-PC2(Cl)@MIL-101 > bis-PC6(Cl)@MIL-101 > PC2(Cl)@MIL-101 > PC6(Cl)@MIL-101 > PC12(Cl)@MIL-101. Based on these results, the composites of bis-PCn@MIL-101 produced from ILs with two vinyl groups show better sorption performances due to the cross-linked polymerization. In comparison, those single-chain polymers in PCn@MIL-101 may partially pass through the pores and transfer to the water solution during anion-exchange. On the other hand, with the increment of the spacers in ILs of Cn (n = 2, 6, and 12) and bis-Cn (n = 2 and 6), the resultant polyILs have the lower charge density, thus reducing the sorption efficiency of composites. To demonstrate the universality of this optimizing strategy, two other well-known MOFs (UiO-66 and ZIF-8) and their composites bis-PC2(Cl)@UiO-66 and bis-PC2(Cl)@ZIF-8 were explored by sorption tests (Figure 4a and Table S3). The ReO4– removal percentages by bis-PC2(Cl)@UiO-66 and bis-PC2(Cl)@ZIF-8 are 7 and 181 times, respectively, more than those by UiO-66 and ZIF-8. After composition, the distribution coefficient (Kd) and rate constant (k2) show a 13 and 2 times increment to UiO-66, and 4469 and 10 times increment to ZIF-8. These results clearly highlight the great potential of this optimizing strategy on MOF-based composite materials for radionuclide sequestration.
Figure 4.
(a) Sorption kinetics of ReO4– by polyILs@MOFs and the corresponding MOFs. (b) Comparison of the sorption capacities of the ReO4– anion by PC2(Cl)@MIL-101, PC2(Cl)/bis-PC2(Cl)@MIL-101, and bis-PC2(Cl)@MIL-101 in ReO4– water solutions (50–3000 ppm). (c) STEM and EDS mapping images of MIL-101, PC2@MIL-101@Re, PC2/bis-PC2@MIL-101@Re, and bis-PC2@MIL-101@Re.
To further elucidate the effect of in situ polymerization on the sorption efficiency, ReO4– sorption experiments were performed by PC2(Cl)@MIL-101, PC2(Cl)/bis-PC2(Cl)@MIL-101, and bis-PC2(Cl)@MIL-101 at diverse concentrations within 50–3000 ppm (Table S4). As the removal percentage by PC2(Cl)@MIL-101 is normalized to 1.0 in each case (Figure 4b), with the increase of concentration for ReO4–, bis-PC2(Cl)@MIL-101 shows a significant superiority in removal percentage. In a ReO4– solution at 3000 ppm, the removal percentage of bis-PC2(Cl)@MIL-101 is 8 times more than that of PC2(Cl)@MIL-101. Herein, the difference between the removal percentages of the three materials is obvious, which is due to the fact that bis-PC2(Cl)@MIL-101 has a larger portion of anionic components and, thus, is more stable in solution with high ionic strength. Therefore, it is convincing that the rational fabrication of polyILs@MOFs composites can greatly enhance their working capacities toward TcO4–/ReO4– sequestration, in which both the small monomers and cross-linking polymerization are beneficial for sorption improvement.
Considering the greatly enhanced performances after polymerization, it is presumed that the high density of anion-exchange sites and the coating of cross-linked polyILs on MOFs are the fundamental contributors to the high ReO4– sorption efficiency of polyILs@MOFs. As shown in the STEM-EDS images of MIL-101 and polyILs@MIL-101@Re (Figure 4c), the distribution of Re is significantly different along with the change of IL monomers. In the mapping image of PC2@MIL-101@Re, the coating polymer is not found outside MIL-101, and all the Re species are inside the MIL-101 particles. For PC2/bis-PC2@MIL-101@Re, with adding the cross-linked monomers, the MIL-101 particles are partly coated by polyILs, in which the Re species are dotted. Utilization of cross-linked ILs monomers affords bis-PC2@MIL-101@Re, where cross-linked polyILs, both inside and outside MIL-101 particles, could capture large amounts of ReO4–. In this case, it could be hypothesized that in situ cross-linked polymerization of suitable IL monomers across the voids of MOFs will lead to the interknitting of polyILs through the MOF networks. Moreover, the cross-linked polyILs in the composite will be sufficiently intertangled with MOFs to serve as the tough external shield, thus greatly improving the stability. Notably, it is of great importance that polyILs with cross-linked IL monomers can serve as both the filler and coating of MOF particles, which not only provide the high density of active sites but also boost the stability of polyILs@MOFs. Therefore, such composites can be generally extended to wide-ranging applications, especially under extreme conditions. Considering the superior ReO4– sorption capacity of bis-PC2(Cl)@MIL-101, it is reasonably chosen for further investigation.
Stability and Recyclability
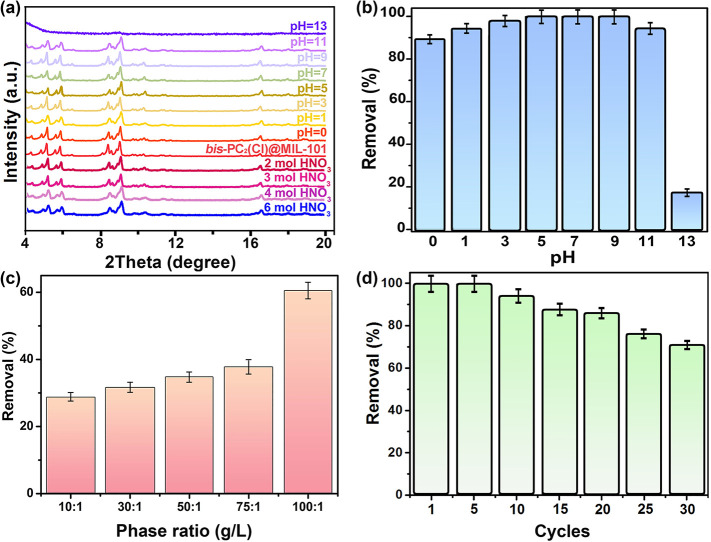
The stability and reusability of sorbents in extreme conditions represent the substantial prerequisite to their actual application in nuclear fuel reprocessing and waste management. As depicted in Figure 5a, the framework structure of bis-PC2(Cl)@MIL-101 remains unchanged after being soaked in highly acidic (6 M HNO3) or alkaline (pH = 11) water solution for 1 week. Accordingly, their removal percentages for ReO4– are >90% under pH = 0–11 (Figure 5b). In the highly alkaline solution, MIL-101 is not stable, and the digestion of composite occurs.45 At pH = 13, bis-PC2(Cl)@MIL-101 shows almost no PXRD peak, and its sorption performance is poor (ca. 20% of ReO4– removal) due to framework collapse. The result also reveals that the survived polyILs still show some anion-exchange ability. Although bis-PC2 is known as water-soluble, the cross-linked polymerization of bis-PC2 in the pores of MIL-101 can largely affect its polymerization degree and thus the solubility in water. In order to verify this hypothesis, the digestion of bis-PC2@MIL-101@Re was taken, and the resultant porous floccus bis-PC2@Re solids were insoluble in the solution. As shown in Figure S12, almost no Cr, Br, and Cl element can be observed in the EDS mapping, where C, N, O, and Re elements are evenly distributed. More impressively, bis-PC2(Cl)@MIL-101 can maintain ca. 62% of ReO4– removal from 3 M HNO3 (NO3–/ReO4– molar ratio = 1619) at a solid/liquid phase ratio of 100 g L–1 (Figure 5c). This ultrahigh selectivity for ReO4– over NO3– anions can be ascribed to the hydrophobic pores in bis-PC2(Cl)@MIL-101 with a strong affinity to the anion of lower charge density, which is similar to MOF and polymer systems.57−60
Figure 5.
(a) PXRD patterns of bis-PC2(Cl)@MIL-101 after immersing in the water solutions with pH values varied from 0 to 13, and in the 2, 3, 4, and 6 M HNO3 solutions. (b) Effect of pH on the ReO4– sorption performances of bis-PC2(Cl)@MIL-101. (c) Sorption of ReO4– by bis-PC2(Cl)@MIL-101 as a function of solid/liquid ratio in 3 M HNO3. (d) Recyclability of bis-PC2(Cl)@MIL-101 in different sorption cycles of ReO4–.
Encouraged by the above results, the recyclability of bis-PC2(Cl)@MIL-101 was further evaluated, which will afford great advantage as the cost-effective sorbents (the current cost of bis-PC2(Cl)@MIL-101 is ∼$300 per kilogram). Notably, almost 100% ReO4– could be removed after five cycles, and even after 30 cycles, above 70% removal percentage is still retained (Figure 5d and Figure S13). These features show that this material is highly recyclable and thus has a great priority toward radionuclide sequestration in the real fuel repossessing conditions.
ReO4– Removal from Simulated Nuclear Waste
The superior sorption performance of bis-PC2(Cl)@MIL-101 inspires us to study its practicability in a simulated nuclear waste solution. In the simulated Hanford LAW melter recycle stream, the amounts of NO3–, Cl–, and NO2– ions are over 300 times more than that of the ReO4– ion (Table S5), which thus poses a great challenge for TcO4– sequestration. By adding bis-PC2(Cl)@MIL-101 (20 mg) into the simulated solution (5 mL), 74% of ReO4– ions therein can be removed at a solid/liquid phase ratio of 4 g L–1, which further reveals its great potential in practical applications.
Conclusion
This work establishes an optimizing strategy to greatly enhance the stability and pertechnetate sequestration for MOF-based sorbents. By in situ polymerization of encapsulated ILs in the pores of MOFs, the polyILs@MOF composites exhibit superior TcO4– removal performances compared with their MOF parents. Notably, both the smaller monomer and cross-linked polymerization are beneficial to achieve an optimal sorbent with exceptional stability under highly acidic conditions, by virtue of cross-linked polyILs as both the filler and coating of the MOF particles, the universality of which has also been testified by different MOFs. Considering the excellent stability, recyclability, sorption capacity, uptake kinetics, and the high performances in the simulated Hanford LAW melter recycle stream, the optimal bis-PC2(Cl)@MIL-101 can act as a promising scavenger for 99TcO4– decontamination. The result will expand the applicability of MOF-based materials for radionuclide sequestration and, further, provide a universal approach to design multifunctional composites with high activity and stability for diverse applications such as catalysis, sorption, and ion conduction.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21771139), Tianjin Natural Science Foundation (17JCYBJC22800), and the Key Research Project of University of Henan Province (19zx004).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c01342.
Additional experimental details; figures including FT-IR spectra, PXRD patterns, pore size distribution, XPS spectra, STEM and EDS mapping images, TGA curves, sorption kinetic curves, column-chromatographic sorption images, NMR spectra; and tables including sorption results and composition of the simulated Hanford LAW melter recycle stream (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Attix F. H.Introduction to Radiological Physics and Radiation Dosimetry; Wiley-VCH: Weinheim, Germany, 1986. [Google Scholar]
- Dresselhaus M. S.; Thomas I. L. Alternative Energy Technologies. Nature 2001, 414, 332–337. 10.1038/35104599. [DOI] [PubMed] [Google Scholar]
- Abney C. W.; Mayes R. T.; Saito T.; Dai S. Materials for the Recovery of Uranium from Seawater. Chem. Rev. 2017, 117, 13935–14013. 10.1021/acs.chemrev.7b00355. [DOI] [PubMed] [Google Scholar]
- Ion S. Reaction: Recycling and Generation IV Systems. Chem. 2016, 1, 663–665. 10.1016/j.chempr.2016.10.008. [DOI] [Google Scholar]
- Schulz W. W.; Lombardo N. J.. Science and Technology for Disposal of Radioactive Tank Wastes; Plenum Press: New York, 1998. [Google Scholar]
- Ojovan M. I.; Lee W. E.. An Introduction to Nuclear Waste Immobilization; Elsevier: Amsterdam, 2005. [Google Scholar]
- Lee M.-S.; Um W.; Wang G. H.; Kruger A. A.; Lukens W. W.; Rousseau R.; Glezakou V.-A. Impeding 99Tc(IV) Mobility in Novel Waste Forms. Nat. Commun. 2016, 7, 12067. 10.1038/ncomms12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. Reaction: A Role for Actinide Chemists. Chem 2016, 1, 662–665. 10.1016/j.chempr.2016.10.004. [DOI] [Google Scholar]
- Popova N. N.; Tananaev I. G.; Rovnyi S. I.; Myasoedov B. F. Technetium: Behaviour During Reprocessing of Spent Nuclear Fuel and in Environmental Objects. Russ. Chem. Rev. 2003, 72, 101–121. 10.1070/RC2003v072n02ABEH000785. [DOI] [Google Scholar]
- Banerjee D.; Kim D.; Schweiger M. J.; Kruger A. A.; Thallapally P. K. Removal of TcO4– Ions from Solution: Materials and Future Outlook. Chem. Soc. Rev. 2016, 45, 2724–2739. 10.1039/C5CS00330J. [DOI] [PubMed] [Google Scholar]
- Katayev E. A.; Kolesnikov G. V.; Sessler J. L. Molecular Recognition of Pertechnetate and Perrhenate. Chem. Soc. Rev. 2009, 38, 1572–1586. 10.1039/b806468g. [DOI] [PubMed] [Google Scholar]
- zur Loye H. C.; Besmann T.; Amoroso J.; Brinkman K.; Grandjean A.; Henager C. H.; Hu S. Y.; Misture S. T.; Phillpot S. R.; Shustova N. B.; Wang H.; Koch R. J.; Morrison G.; Dolgopollova E. Hierarchical Materials as Tailored Nuclear Waste Forms: A Perspective. Chem. Mater. 2018, 30, 4475–4488. 10.1021/acs.chemmater.8b00766. [DOI] [Google Scholar]
- Burgeson I. E.; Deschane J. R.; Blanchard D. L. Removal of Technetium from Hanford Tank Waste Supernates. Sep. Sci. Technol. 2005, 40, 201–223. 10.1081/SS-200041916. [DOI] [Google Scholar]
- EPA Facts about Technetium-99; U.S. EPA, 2002.
- King W. D.; Hassan N. M.; McCabe D. J.; Hamm L. L.; Johnson M. E. Technetium Removal from Hanford and Savannah River Site Actual Tank Waste Supernates with Superlig® 639 Resin. Sep. Sci. Technol. 2003, 38, 3093–3114. 10.1081/SS-120022588. [DOI] [Google Scholar]
- Xiao C.; Khayambashi A.; Wang S. Separation and Remediation of 99TcO4– from Aqueous Solutions. Chem. Mater. 2019, 31, 3863–3877. 10.1021/acs.chemmater.9b00329. [DOI] [Google Scholar]
- Li D.; Seaman J. C.; Kaplan D. I.; Heald S. M.; Sun C. J. Pertechnetate (TcO4–) Sequestration from Groundwater by Cost-Effective Organoclays and Granular Activated Carbon under Oxic Environmental Conditions. Chem. Eng. J. 2019, 360, 1–9. 10.1016/j.cej.2018.11.146. [DOI] [Google Scholar]
- Li D. E.; Seaman J. C.; Murph S. E. H.; Kaplan D. I.; Taylor-Pashow K.; Feng R. F.; Chang H.; Tandukar M. Porous Iron Material for TcO4– and ReO4– Sequestration from Groundwater under Ambient Oxic Conditions. J. Hazard. Mater. 2019, 374, 177–185. 10.1016/j.jhazmat.2019.04.030. [DOI] [PubMed] [Google Scholar]
- Luo W. S.; Kelly S. D.; Kemner K. M.; Watson D.; Zhou J. Z.; Jardine P. M.; Gu B. H. Sequestering Uranium and Technetium through Co-Precipitation with Aluminum in a Contaminated Acidic Environment. Environ. Sci. Technol. 2009, 43, 7516–7522. 10.1021/es900731a. [DOI] [PubMed] [Google Scholar]
- He L.; Liu S.; Chen L.; Dai X.; Li J.; Zhang M.; Ma F.; Zhang C.; Yang Z.; Zhou R.; Chai Z.; Wang S. Mechanism Unravelling for Ultrafast and Selective 99TcO4– Uptake by a Radiation-Resistant Cationic Covalent Organic Framework: A Combined Radiological Experiment and Molecular Dynamics Simulation Study. Chem. Sci. 2019, 10, 4293–4305. 10.1039/C9SC00172G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.; Aguila B.; Ma S. Opportunities of Porous Organic Polymers for Radionuclide Sequestration. Trends Chem. 2019, 1, 292–303. 10.1016/j.trechm.2019.02.010. [DOI] [Google Scholar]
- Oliver S. R. J. Cationic Inorganic Materials for Anionic Pollutant Trapping and Catalysis. Chem. Soc. Rev. 2009, 38, 1868–1881. 10.1039/b710339p. [DOI] [PubMed] [Google Scholar]
- Ravi A.; Oshchepkov A. S.; German K. E.; Kirakosyan G. A.; Safonov A. V.; Khrustalev V. N.; Kataev E. A. Finding a Receptor Design for Selective Recognition of Perrhenate and Pertechnetate: Hydrogen vs. Halogen Bonding. Chem. Commun. 2018, 54, 4826–4829. 10.1039/C8CC02048E. [DOI] [PubMed] [Google Scholar]
- Alberto R.; Bergamaschi G.; Braband H.; Fox T.; Amendola V. 99TcO4–: Selective Recognition and Trapping in Aqueous Solution. Angew. Chem., Int. Ed. 2012, 51, 9772–9776. 10.1002/anie.201205313. [DOI] [PubMed] [Google Scholar]
- Wang S.; Alekseev E. V.; Juan D. W.; Casey W. H.; Phillips B. L.; Depmeier W.; Albrecht-Schmitt T. E. NDTB-1: A Supertetrahedral Cationic Framework That Removes TcO4– from Solution. Angew. Chem., Int. Ed. 2010, 49, 1057–1060. 10.1002/anie.200906397. [DOI] [PubMed] [Google Scholar]
- Wang S.; Yu P.; Purse B. A.; Orta M. J.; Diwu J.; Casey W. H.; Phillips B. L.; Alekseev E. V.; Depmeier W.; Hobbs D. T.; Albrecht-Schmitt T. E. Selectivity, Kinetics, and Efficiency of Reversible Anion Exchange with TcO4– in a Supertetrahedral Cationic Framework. Adv. Funct. Mater. 2012, 22, 2241–2250. 10.1002/adfm.201103081. [DOI] [Google Scholar]
- Amendola V.; Bergamaschi G.; Boiocchi M.; Alberto R.; Braband H. Fluorescent Sensing of 99Tc Pertechnetate in Water. Chem. Sci. 2014, 5, 1820–1826. 10.1039/C3SC53504E. [DOI] [Google Scholar]
- Long K. M.; Goff G. S.; Ware S. D.; Jarvinen G. D.; Runde W. H. Anion Exchange Resins for the Selective Separation of Technetium from Uranium in Carbonate Solutions. Ind. Eng. Chem. Res. 2012, 51, 10445–10450. 10.1021/ie300534e. [DOI] [Google Scholar]
- Wilmarth W. R.; Lumetta G. J.; Johnson M. E.; Poirier M. R.; Thompson M. C.; Suggs P. C.; Machara N. P. Review: Waste-Pretreatment Technologies for Remediation of Legacy Defense Nuclear Wastes. Solvent Extr. Ion Exch. 2011, 29, 1–48. 10.1080/07366299.2011.539134. [DOI] [Google Scholar]
- Fei H.; Bresler M. R.; Oliver S. R. J. A New Paradigm for Anion Trapping in High Capacity and Selectivity: Crystal-to-Crystal Transformation of Cationic Materials. J. Am. Chem. Soc. 2011, 133, 11110–11113. 10.1021/ja204577p. [DOI] [PubMed] [Google Scholar]
- Gándara F.; Perles J.; Snejko N.; Iglesias M.; Gómez-Lor B.; Gutiérrez-Puebla E.; Monge M. Á. Layered Rare-Earth Hydroxides: A Class of Pillared Crystalline Compounds for Intercalation Chemistry. Angew. Chem., Int. Ed. 2006, 45, 7998–8001. 10.1002/anie.200602502. [DOI] [PubMed] [Google Scholar]
- Desai A. V.; Manna B.; Karmakar A.; Sahu A.; Ghosh S. K. A Water-Stable Cationic Metal–Organic Framework as a Dual Adsorbent of Oxoanion Pollutants. Angew. Chem., Int. Ed. 2016, 55, 7811–7815. 10.1002/anie.201600185. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Sheng D.; Xu C.; Dai X.; Silver M. A.; Li J.; Li P.; Wang Y.; Wang Y.; Chen L.; Xiao C.; Chen J.; Zhou R.; Zhang C.; Farha O. K.; Chai Z.; Albrecht-Schmitt T. E.; Wang S. Identifying the Recognition Site for Selective Trapping of 99TcO4– in a Hydrolytically Stable and Radiation Resistant Cationic Metal–Organic Framework. J. Am. Chem. Soc. 2017, 139, 14873–14876. 10.1021/jacs.7b08632. [DOI] [PubMed] [Google Scholar]
- Sheng D.; Zhu L.; Dai X.; Xu C.; Li P.; Pearce C. I.; Xiao C.; Chen J.; Zhou R.; Duan T.; Farha O. K.; Chai Z.; Wang S. Successful Decontamination of 99TcO4– in Groundwater at Legacy Nuclear Sites by a Cationic Metal–Organic Framework with Hydrophobic Pockets. Angew. Chem., Int. Ed. 2019, 58, 4968–4972. 10.1002/anie.201814640. [DOI] [PubMed] [Google Scholar]
- Mei L.; Li F.; Lan J.; Wang C.; Xu C.; Deng H.; Wu Q.; Hu K.; Wang L.; Chai Z.; Chen J.; Gibson J. K.; Shi W. Anion-Adaptive Crystalline Cationic Material for 99TcO4– Trapping. Nat. Commun. 2019, 10, 1532. 10.1038/s41467-019-09504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang X.; Zhao G.; Chen C.; Chai Z.; Alsaedi A.; Hayat T.; Wang X. Metal–Organic Framework-based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions. Chem. Soc. Rev. 2018, 47, 2322–2356. 10.1039/C7CS00543A. [DOI] [PubMed] [Google Scholar]
- Li C. P.; Zhou H.; Chen J.; Wang J. J.; Du M.; Zhou W. A Highly Efficient Coordination Polymer for Selective Trapping and Sensing of Perrhenate/Pertechnetate. ACS Appl. Mater. Interfaces 2020, 12, 15246–15254. 10.1021/acsami.0c00775. [DOI] [PubMed] [Google Scholar]
- Li C. P.; Zhou H.; Wang J. J.; Liu B. L.; Wang S.; Yang X.; Wang Z. L.; Liu C. S.; Du M.; Zhou W. Z. Mechanism-Property Correlation in Coordination Polymer Crystals toward Design of a Superior Sorbent. ACS Appl. Mater. Interfaces 2019, 11, 42375–42384. 10.1021/acsami.9b16386. [DOI] [PubMed] [Google Scholar]
- Gao L.; Li C.-Y. V.; Chan K.-Y.; Chen Z.-N. Metal–Organic Framework Threaded with Aminated Polymer Formed in Situ for Fast and Reversible Ion Exchange. J. Am. Chem. Soc. 2014, 136, 7209–7212. 10.1021/ja501958u. [DOI] [PubMed] [Google Scholar]
- Gao L.; Li C.-Y. V.; Chan K.-Y. Polystyrenesulfonate Threaded in MIL-101Cr(III): A Cationic Polyelectrolyte Synthesized Directly into a Metal–Organic Framework. Chem. Mater. 2015, 27, 3601–3608. 10.1021/cm504623r. [DOI] [Google Scholar]
- Banerjee D.; Elsaidi S. K.; Aguila B.; Li B.; Kim D.; Schweiger M. J.; Kruger A. A.; Doonan C. J.; Ma S.; Thallapally P. K. Removal of Pertechnetate-Related Oxyanions from Solution Using Functionalized Hierarchical Porous Frameworks. Chem. - Eur. J. 2016, 22, 17581–17584. 10.1002/chem.201603908. [DOI] [PubMed] [Google Scholar]
- Liang J.; Chen R. P.; Wang X. Y.; Liu T. T.; Wang X. S.; Huang Y. B.; Cao R. Postsynthetic Ionization of An Imidazole-Containing Metal–Organic Framework for the Cycloaddition of Carbon Dioxide and Epoxides. Chem. Sci. 2017, 8, 1570–1575. 10.1039/C6SC04357G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A.; Hasan Z.; Jhung S. H. Ionic liquid@MIL-101 Prepared via the Ship-in-Bottle Technique: Remarkable Adsorbents for the Removal of Benzothiophene from Liquid Fuel. Chem. Commun. 2016, 52, 2561–2564. 10.1039/C5CC08896H. [DOI] [PubMed] [Google Scholar]
- Tharun J.; Bhin K.-M.; Roshan R.; Kim D. W.; Kathalikkattil A. C.; Babu R.; Ahn H. Y.; Won Y. S.; Park D.-W. Ionic Liquid Tethered Post Functionalized ZIF-90 Framework for the Cycloaddition of Propylene Oxide and CO2. Green Chem. 2016, 18, 2479–2487. 10.1039/C5GC02153G. [DOI] [Google Scholar]
- Ding M.; Jiang H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. 10.1021/acscatal.7b03404. [DOI] [Google Scholar]
- Aguila B.; Sun Q.; Wang X.; O’Rourke E.; Al-Enizi A. M.; Nafady A.; Ma S. Lower Activation Energy for Catalytic Reactions through Host-Guest Cooperation within Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2018, 57, 10107–10111. 10.1002/anie.201803081. [DOI] [PubMed] [Google Scholar]
- Kinik F. P.; Uzun A.; Keskin S. Ionic Liquid/Metal–Organic Framework Composites: From Synthesis to Applications. ChemSusChem 2017, 10, 2842–2863. 10.1002/cssc.201700716. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Huang H.; Vardhan H.; Aguila B.; Zhong C.; Perman J. A.; Al-Enizi A. M.; Nafady A.; Ma S. Facile Approach to Graft Ionic Liquid into MOF for Improving the Efficiency of CO2 Chemical Fixation. ACS Appl. Mater. Interfaces 2018, 10, 27124–27130. 10.1021/acsami.8b08914. [DOI] [PubMed] [Google Scholar]
- Rogers R. D.; Seddon K. R. Ionic Liquids-Solvents of the Future?. Science 2003, 302, 792–793. 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Zhang J.; Zhang Y.; Deng Y. Nanoconfined Ionic Liquids. Chem. Rev. 2017, 117, 6755–6833. 10.1021/acs.chemrev.6b00509. [DOI] [PubMed] [Google Scholar]
- Khan N. A.; Hasan Z.; Jhung S. H. Ionic Liquids Supported on Metal–Organic Frameworks: Remarkable Adsorbents for Adsorptive Desulfurization. Chem. - Eur. J. 2014, 20, 376–380. 10.1002/chem.201304291. [DOI] [PubMed] [Google Scholar]
- Fujie K.; Kitagawa H. Ionic Liquid Transported into Metal–Organic Frameworks. Coord. Chem. Rev. 2016, 307, 382–390. 10.1016/j.ccr.2015.09.003. [DOI] [Google Scholar]
- Yoshida Y.; Kitagawa H. Ionic Conduction in Metal–Organic Frameworks with Incorporated Ionic Liquids. ACS Sustainable Chem. Eng. 2019, 7, 70–81. 10.1021/acssuschemeng.8b05552. [DOI] [Google Scholar]
- Li Z.; Wang W.; Chen Y.; Xiong C.; He G.; Cao Y.; Wu H.; Guiver M. D.; Jiang Z. Constructing Efficient Ion Nanochannels in Alkaline Anion Exchange Membranes by the in situ Assembly of a Poly(ionic liquid) in Metal–Organic Frameworks. J. Mater. Chem. A 2016, 4, 2340–2348. 10.1039/C5TA10452A. [DOI] [Google Scholar]
- Sun Y.; Jia X.; Huang H.; Guo X.; Qiao Z.; Zhong C. Solvent-Free Mechanochemical Route for the Construction of Ionic Liquid and Mixed-Metal MOF Composites for Synergistic CO2 Fixation. J. Mater. Chem. A 2020, 8, 3180–3185. 10.1039/C9TA10409G. [DOI] [Google Scholar]
- Liu Z. W.; Han B. H. Evaluation of an Imidazolium-Based Porous Organic Polymer as Radioactive Waste Scavenger. Environ. Sci. Technol. 2020, 54, 216–224. 10.1021/acs.est.9b05308. [DOI] [PubMed] [Google Scholar]
- Li J.; Dai X.; Zhu L.; Xu C.; Zhang D.; Silver M. A.; Li P.; Chen L.; Li Y.; Zuo D.; Zhang H.; Xiao C.; Chen J.; Diwu J.; Farha O. K.; Albrecht-Schmitt T. E.; Chai Z.; Wang S. 99TcO4– Remediation by a Cationic Polymeric Network. Nat. Commun. 2018, 9, 3007. 10.1038/s41467-018-05380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.; Zhu L.; Aguila B.; Thallapally P. K.; Xu C.; Chen J.; Wang S.; Rogers D.; Ma S. Optimizing Radionuclide Sequestration in Anion Nanotraps with Record Pertechnetate Sorption. Nat. Commun. 2019, 10, 1646. 10.1038/s41467-019-09630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Xiao C.; Dai X.; Li J.; Gui D.; Sheng D.; Chen L.; Zhou R.; Chai Z.; Albrecht-Schmitt T. E.; Wang S. Exceptional Perrhenate/Pertechnetate Uptake and Subsequent Immobilization by a Low-Dimensional Cationic Coordination Polymer: Overcoming the Hofmeister Bias Selectivity. Environ. Sci. Technol. Lett. 2017, 4, 316–322. 10.1021/acs.estlett.7b00165. [DOI] [Google Scholar]
- Banerjee D.; Xu W. Q.; Nie Z. M.; Johnson L. E. V.; Coghlan C.; Sushko M. L.; Kim D.; Schweiger M. J.; Kruger A. A.; Doonan C. J.; Thallapally P. K. Zirconium-Based Metal–Organic Framework for Removal of Perrhenate from Water. Inorg. Chem. 2016, 55, 8241–8243. 10.1021/acs.inorgchem.6b01004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.