Abstract
Although the invention of right heart catheterisation in the 1950s enabled accurate clinical diagnosis of pulmonary arterial hypertension (PAH), it was not until 2000 when the landmark discovery of the causative role of bone morphogenetic protein receptor type II (BMPR2) mutations shed new light on the pathogenesis of PAH. Since then several genes have been discovered, which now account for around 25% of cases with the clinical diagnosis of idiopathic PAH. Despite the ongoing efforts, in the majority of patients the cause of the disease remains elusive, a phenomenon often referred to as “missing heritability”. In this review, we discuss research approaches to uncover the genetic architecture of PAH starting with forward phenotyping, which in a research setting should focus on stable intermediate phenotypes, forward and reverse genetics, and finally reverse phenotyping. We then discuss potential sources of “missing heritability” and how functional genomics and multi-omics methods are employed to tackle this problem.
Keywords: forward phenotyping, forward genetics, reverse genetics, reverse phenotyping, pulmonary arterial hypertension, intermediate phenotypes, whole-genome sequencing, epigenetic inheritance, genetic heterogeneity, phenotypic heterogeneity
1. Introduction
Rare diseases, such as pulmonary arterial hypertension (PAH), are enriched with underlying genetic causes and are defined as life-threatening or chronically debilitating disorders with a prevalence of less than 1 in 2000 [1]. Although individually characterised by low prevalence, in total, rare diseases pose a significant burden to health care systems and a diagnostic challenge. Over 6000 rare diseases have been reported to date (ORPHANET [2]) and new genotype-phenotype associations are discovered every month [3]. Despite the several-fold increase in genetic diagnoses in the area of rare diseases [4], the cause of the disease remains elusive in a significant proportion of cases.
Since the 2008 World Symposium on Pulmonary Hypertension (WSPH), the term “heritable PAH” (HPAH) has been used to describe both familial PAH and sporadic PAH with an identified underlying pathogenic variant [5] (Table 1). Family history is a vital disease component directly linked to the proportion of variation attributable to genetic factors, known as heritability [6,7]. Familial studies have been used historically as a tool for gene mapping, with the classical example of twin studies commonly used to disentangle the relative contribution of genes and environment to complex human traits [2].
Table 1.
Definitions and changes to the classification of Group 1 PAH. Abbreviations: WSPH—World Symposium on Pulmonary Hypertension; ERS/ESC—European Respiratory Society and European Society of Cardiology; mPAP—mean pulmonary artery pressure; (R)—resting; (E)—exercise; PAWP—pulmonary artery wedge pressure; PVR—pulmonary vascular resistance; LV—left ventricle; PPH—primary pulmonary hypertension; BMPR2—Bone morphogenetic protein receptor, type II; ACVRL1—Activin A receptor like type 1; SMAD9—SMAD Family Member 9; CAV1—Caveolin 1; KCNK3—Potassium two pore domain channel subfamily K member 3; PVOD/PCH—pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis; PAH—pulmonary arterial hypertension; PH—pulmonary hypertension; PPHN—persistent pulmonary hypertension of the newborn; RHC—right heart catheterization.
| WSPH Proceedings and ERS/ESC Guidelines | Definition of Group 1 | Comments | Changes to the Classification |
|---|---|---|---|
| 1st WSPH, Geneva, 1973 [12] | No haemodynamic definition mentioned | ||
| 2nd WSPH, Evian, 1998 [13] | No haemodynamic definition mentioned, but RHC recommended for diagnosis | Introduction of the terms primary (PPH) and secondary (related to other conditions) pulmonary hypertension, recognition of familial forms of PH | |
| 3rd WSPH, Venice, 2003 [14] | mPAP(R) > 25 mmHg; mPAP(E) > 30 mmHg; PAWP < 15 mmHg; PVR > 3 WU | Abandonment of the term primary pulmonary hypertension, the introduction of terms idiopathic and familial PAH as well as associated PAH, BMPR2 and ACVRL1 implicated in the pathogenesis of PAH | |
| 4th WSPH, Dana Point, 2008 [5] | mPAP(R) ≥ 25 mmHg; PAWP ≤ 15 mmHg | Exercise-induced PH removed from the definition as although (R) mPAP has been shown to be stable across age groups, (E) mPAP increases with age hence based on the available data it was not possible to define a cutoff | Introduction of the terms idiopathic (no family history, no precipitating risk factor) and hereditary (encompassing familial cases with or without identified germline mutations and PAH). Inclusion of PH associated with Schistosomiasis and PH associated with chronic hemolytic anaemia to Group 1 |
| ERS/ESC Guidelines, 2009 [15] | mPAP(R) ≥ 25 mmHg; PAWP ≤ 15 mmHg; CO normal or reduced | No definition of PH on exercise | |
| 5th WSPH, Nice, 2013 [16] | mPAP(R) ≥ 25 mmHg; PAWP ≤ 15 mmHg; PVR > 3 WU | Introduction of PVR to the definition, a recommendation to report PVR in WU; fluid challenge may be helpful to unmask occult LV diastolic dysfunction | SMAD9, CAV1 and KCNK3 included as risk genes for HPAH |
| ERS/ESC Guidelines, 2015 [17] | mPAP(R) ≥ 25 mmHg; PAWP ≤ 15 mmHg | The clinical significance of a mPAP between 21 and 24 mmHg is unclear | Group 1′ PVOD/PCH has been expanded and includes idiopathic, heritable, drug-, toxin- and radiation-induced and associated forms; PPHN includes a heterogeneous group of conditions that may differ from classical PAH. As a consequence, PPHN has been sub-categorised as group I′′. |
| 6th WSPH, Nice, 2018 [18] | mPAP(R) ≥ 20 mmHg; PAWP ≤ 15 mmHg; PVR ≥ 3 WU | PVR ≥ 3WU should be used as a diagnostic criterion for all forms of PH | PAH long-term responses to calcium channel blockers established as a subtype of Group 1; PAH with overt features of venous/capillaries (PVOD/PCH) involvement established as a subtype of Group 1 |
In statistical terms, heritability is defined as a proportion of the phenotypic variance that can be attributed to the variance of genotypic values:
| (1) |
H2 estimates are specific to the population, disease and circumstances on which they are estimated [8]. In theory, total genotypic variance () can be divided into multiple components: total additive variance (breeding values); —dominance variance (interactions between alleles at the same locus); —epistatic variance (interactions between alleles at different loci); —variation arising from interactions between genes and the environment.
| (2) |
In practice, total genotypic variation is difficult to measure, although estimates can be made, and the components of genotypic variation are nearly unattainable. Hence genetic studies usually refer to heritability in its narrow sense, which is the proportion of the phenotypic variance that can be attributed to favourable or unfavourable alleles.
| (3) |
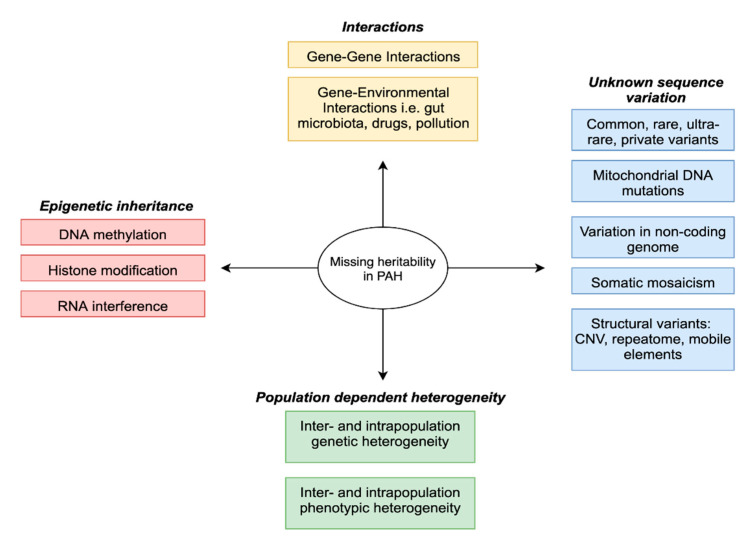
Recently, genome-wide association studies (GWAS) have enabled the estimation of additive heritability attributed to common genetic variation (single nucleotide polymorphisms—SNPs), albeit with a typically small effect size [9]. This has led to the issue of “missing heritability” [10], whereby SNP-based estimates were not sufficient to explain prior heritability predictions arising from twin or familial recurrence studies. To that effect, several hypotheses have been proposed (Figure 1 and Figure 2) including the complex interplay between genes and environment and the often overlooked potential contribution of structural variation [11].
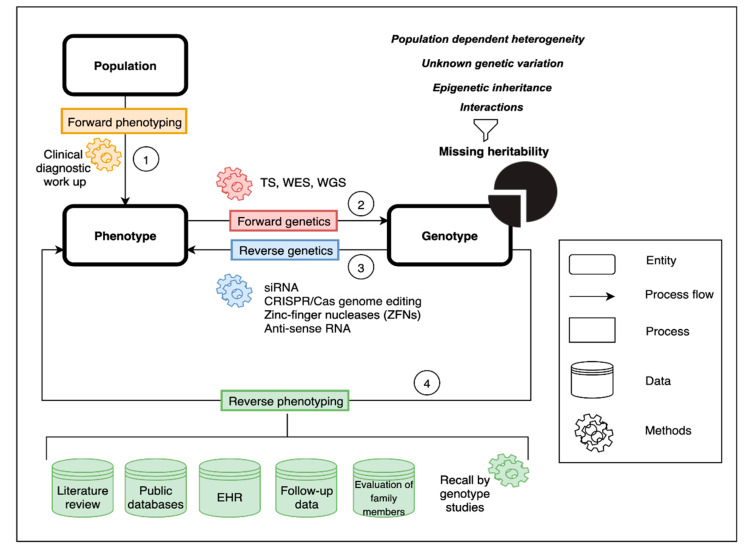
Figure 1.
Graphical abstract. Schematic representation of the concepts of forward phenotyping (1) and genetics (2) and reverse genetics (3) and phenotyping (4). Public databases include, but are not limited to, Online Mendelian Inheritance in Man (OMIM), Human Phenotype Ontology (HPO), DatabasE of genomiC varIation and Phenotype in Humans using Ensembl Resources (DECIPHER), ClinVar databases. Forward genetics—‘genotype to phenotype’ approach; reverse genetics—analysis of the impact of induced variation within a specific gene on gene function; reverse phenotyping—clinical assessment directed by genetic results. Abbreviations: TS—targeted sequencing; WES—whole-exome sequencing; WGS—whole-genome sequencing; siRNA—small interfering RNA; EHR—Electronic Healthcare Records.
Figure 2.
Potential factors contributing to missing heritability in PAH.
To date, about 75% of patients with a clinical diagnosis of idiopathic pulmonary arterial hypertension (IPAH) have no defined genetic cause of the disease. This review outlines the role of forward and reverse genomic and phenomic approaches as well as other omic technologies in the search for missing heritability of PAH (Figure 1). Understanding the genetic architecture of PAH and its dynamic interplay with the environment is a prerequisite to predict personalized patient risk, decipher interaction pathways underlying the disease and develop strategies for therapy and prevention.
2. Genetic and Phenotypic Heterogeneity
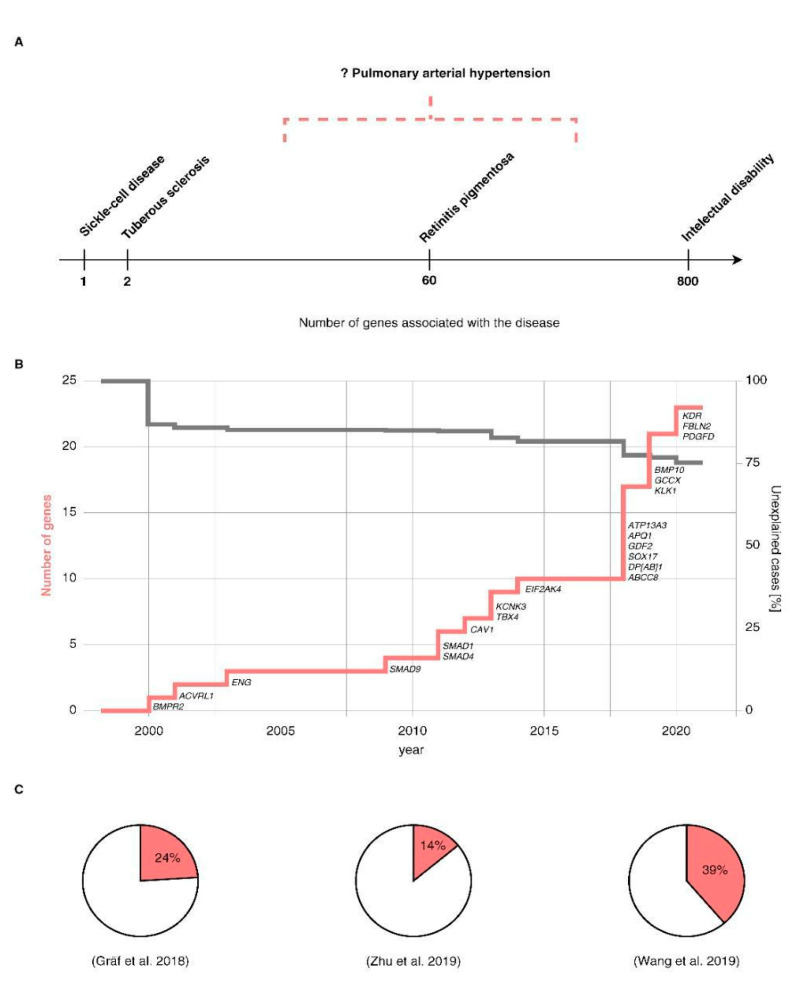
Discoveries in the field of rare diseases have been hampered by genetic and phenotypic heterogeneity of these entities. Genetic heterogeneity is a situation in which sequence variation in two or more genes results in the same or very similar phenotype. The degree of genetic heterogeneity varies between different diseases (Figure 3A). For instance, sickle cell anaemia has only been associated with mutations in one gene [19], Haemoglobin subunit β (HBB) and tuberous sclerosis [20] in two genes, retinitis pigmentosa [21] with over 60 and intellectual disability with over 800 [22]. PAH also shows high genetic heterogeneity (Figure 3B), with so far around ~20 risk genes reported [23] and more expected to be found.
Figure 3.
(A). Genetic heterogeneity in various genetic disorders, (B). Genetic discoveries in PAH, (C). Proportion of explained cases by cohort (Gräf et al., 2018—I/HPAH [24]; Zhu et al., 2019—Group 1 PAH [25]; Wang et al., 2019—IPAH [26]). ‘?’ denotes uncertainty around number of genes involved in the pathogenesis of PAH.
Genotypic heterogeneity is further complicated by phenotypic heterogeneity. Pulmonary hypertension (PH) is a highly heterogeneous condition, defined as an elevation of mean pulmonary artery pressure (mPAP) equal to or greater than 25 mmHg measured by right heart catheterisation (RHC) in the supine position at rest [17]. This somewhat arbitrary threshold for defining PH was proposed at the 1st WSPH in Geneva in 1973 and has now been challenged by a new body of evidence showing that normal mPAP is 14 ± 3.3 mmHg, which suggests an upper limit of normal at 20 mmHg (14 mmHg + 2SD). This new threshold has been endorsed by the 6th WSPH along with the inclusion of pulmonary vascular resistance (PVR) and pulmonary artery wedge pressure (PAWP) cut-offs into the new haemodynamic definition. This new definition categorises PH into three groups based on haemodynamic criteria: pre-capillary PH, isolated post-capillary PH (IpcPH) and combined pre- and post-capillary PH (CpcPH) [18] (Table 1).
Haemodynamic definitions of PH encompass multiple cardiopulmonary entities that have been classified into five groups: PAH, PH secondary to left heart disease, PH due to lung disease and/or hypoxia, chronic thromboembolic pulmonary hypertension (CTEPH) or other pulmonary artery obstructions, and PH with unclear and/or multifactorial mechanisms.
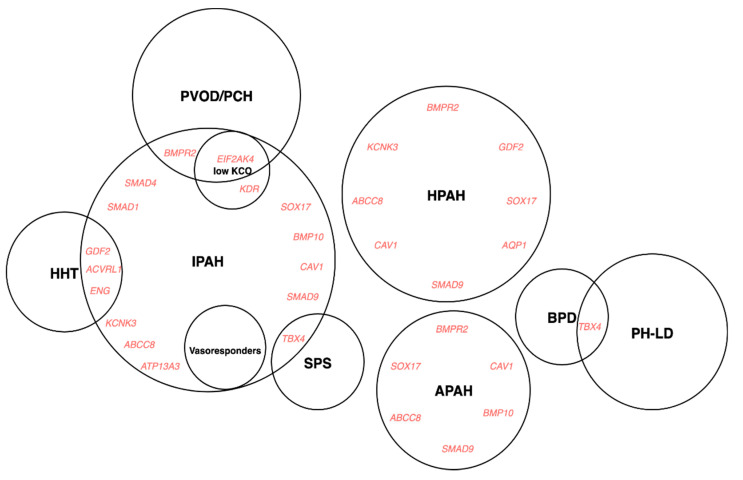
The clinical classification aims to categorise patients into groups according to pathophysiological mechanisms, haemodynamics and therapeutic management. Despite this, there is persistent heterogeneity within groups and subgroups, and not all patients fit easily into a single category, which can be a reflection of genetic pleiotropy (Figure 4).
Figure 4.
Genotype-phenotype associations in PAH. Abbreviations: I/H/APAH—idiopathic/hereditary/associated pulmonary arterial hypertension; PVOD/PCH—pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis; SPS—small patella syndrome; BPD—bronchopulmonary dysplasia; HHT—hereditary hemorrhagic telangiectasia.
Based on the recent advances in mechanistic understanding of the disease, the 6th WSPH proposed new changes to the classification of PH, including two previously recognised phenotypes as the subgroups of Group 1, namely “PAH long-term responders to calcium channel blockers” (Group 1.5) and “PAH with overt features of venous/capillaries (Pulmonary veno-occlusive disease(PVOD)/Pulmonary capillary haemangiomatosis (PCH)) involvement” (Group 1.6) [18]. Other phenotypes previously reported in the literature and summarised in An Official American Society Statement: Pulmonary Hypertension Phenotypes [27] such as “severe” PH in respiratory disease, maladaptive right ventricular (RV) hypertrophy, PH in elderly individuals, PAH in children and PAH with metabolic syndrome [28] are awaiting clinical validation and confirmation of utility both in clinical practice and research settings.
3. Forward Phenotyping for Genetic Studies
A precise definition of the phenotype of interest is a cornerstone of any genetic study (Figure 1, process 1). As described above, clinical diagnosis relies on clustering patients based on observable and measurable traits, signs and symptoms, which are the product of genetic, epigenetic and environmental factors. As a consequence, clinical phenotypes can be dynamic and reactive, which is useful and desirable in the clinical setting but unsuitable for genetic studies. A distinction must be made between clinical and research diagnosis, particularly diagnosis for genetic analysis. The former is usually spread over time and acquired in several stages: history taking, physical examination, differential diagnosis and confirmation, the latter usually needs to be ascertained during a single encounter. To make this feasible and reliable, standardised checklist and operating procedures need to be in place, diagnostic criteria should follow simple inclusion/exclusion rules and phenotypes need to be described using controlled vocabulary to avoid ambiguity. Additionally, the data must be in a format amenable to computational analysis. Finally, the validity of phenotypes is confirmed in test cohorts, through functional studies and ultimately via reverse phenotyping (see below). The accuracy and precision of phenotype measurements are of paramount importance. In genetic studies, the diagnosis misclassification or admixture of phenocopies can significantly affect power to detect an association [29]. Equally, categorising biologically continuous phenotypes (i.e., age, mPAP, diffusing capacity of the lungs for carbon monoxide (DLCO)) is prone to errors due to flaws in quantification methods and arbitrary thresholds.
Phenotype optimisation for genetic studies aims at finding homogenous groups of patients that likely share the same genetic architecture. This can be approached through various strategies. For example, an extreme phenotype strategy aims at identifying rare variants with large effect sizes through recruitment of patients with traits at either end of the phenotypic spectrum. These phenotypes can be based on family history, age of onset, outcome, severity scores, biomarker levels, disease trajectory or response to treatment [30,31,32]. Such stratification was proven to increase the power to detect novel disease risk genes [30,33,34] and to be cost-effective [35]. Other strategies include covariate-based methods which jointly estimate the effect of multiple variables and data reduction techniques. Alternatively, intermediate phenotypes can be used. Intermediate phenotypes are features closer to underlying biology that are at least as heritable as the phenotype itself, stable over time, and are associated with the disease of interest [36].
Although clinical phenotyping remains the most widely used method of patient stratification both in clinical practice and research, it requires substantial domain knowledge and is time-consuming. Computational phenotyping based on clinical and/or “omics” datasets using machine learning might be an alternative due to unparalleled diagnostic precision, accuracy and speed. Two recent publications exemplify the power of computational tools in identifying disease phenotypes. Based on blood cytokine profiles, a prospective observational study of Group 1 PAH discovered and validated four immune phenotypes; importantly, these phenotypes differed in clinical outcomes despite the fact that demographics, PAH aetiologies, comorbidities, and treatments were similar across clusters [37]. Likewise, clinical data mining using the Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) again revealed four clusters with differing survival and response to therapy [38]. A viable alternative approach for clinical data mining is utilising phenotype ontologies, such as Human Phenotype Ontology (HPO), which allow standardised, highly granular and precise phenotyping across different disease domains [39]. Use of ontologies to define phenotypes has already proven useful in identifying novel candidate genes for rare disorders [40]. Ontology-based analysis of phenotypes has been further facilitated by the implementation of methods for manipulation, visualisation and computation of semantic similarity between ontological terms and sets of terms [41].
4. Forward Genetics
4.1. Concepts
Forward genetics is a classic molecular genetics approach used to elucidate the genetic underpinnings of a mutant phenotype of interest [42]. Forward genetics is typically considered a ‘phenotype to genotype’ approach as mutant phenotypes are first observed before their corresponding genes are identified (Figure 1, process 2). In humans, forward genetics approaches most commonly include family-based linkage studies and/or genome-wide association studies (GWAS) and, more recently, rare variant association studies (RVAS).
4.2. Methodology
4.2.1. Study Design
The two main approaches for studying the underlying genetics of PAH are family-based studies and case-control studies. The former is based on studying inheritance patterns of genetic polymorphisms, the second involves comparing genotype frequencies between cases and controls. Family-based studies are effective when parental samples along with phenotype information are available and the disease in question has a high penetrance; they are particularly useful for studying dichotomous traits and are robust to population stratification [43]. Case-control studies are a viable alternative if the above criteria are not met, although they have their own challenges which need to be addressed. To name the most important:
Selection of cases (recognition of selection bias, incident vs. prevalent cases recruitment)
Case definition (precise definition of the phenotype that can be ascertained in a research setting)
Selection of controls (healthy vs. disease controls, matched in respect to age, sex and ethnicity, having a comparable evaluation of presence or absence of the phenotype in question)
Power calculations in genetic studies are an absolute necessity as ignoring this basic step can lead to both underpowered (risking false rejection of null hypothesis and characterised by wide sampling distributions for sample estimates) and overpowered (wasteful and often unethical) experiments. Factors limiting power to detect new genotype-phenotype associations which need to be accounted for are phenotypic variance, phenocopies, the effect size of risk alleles and minor allele frequency (MAF), with the last two factors driving the difference of sample sizes between GWAS and RVAS.
4.2.2. Statistical Methods
Prior to the widespread use of GWAS, the most important tool in genetics were linkage studies in families, these were particularly useful in single-gene disorders in which implicated genes have large effect sizes. GWAS on the other hand compares the frequency of common SNPs between unrelated cases and controls. The associated SNPs are then considered markers of relevant regions that influence the risk of the phenotype. In fact, power calculations provided evidence that GWAS are better than linkage studies at detecting variation with small effect sizes [44]. Multiple statistical methods can be applied in GWAS, for example, Pearson X2 test, normal approximation to Fisher’s Exact Test, logistic regression, categorical model tests, Cochran–Armitage Trend test, and allele tests. The best method depends on the mode of inheritance and trait frequency. Importantly, the assumptions used in various tests may differ; these assumptions directly impact the results, as tests that assume the same mode of inheritance should yield the same results (i.e., Cochran–Armitage Trend Additive test, Logistic Regression Additive test and Allele test). Due to a large number of comparisons, adjustment for multiple testing is necessary; therefore, the p-value threshold is Bonferroni corrected (which encourages high type II error). Additionally, genotype and phenotype misclassification errors impact on power in GWAS. Epistatic scenarios and modelling gene–environment interactions require yet another set of methods and are computationally challenging although feasible [45].
GWAS is unsuitable for single-variant testing, due to the potentially low prevalence of mutation carriers and small effect size, which would both require unfeasibly large sample sizes. Instead, gene and region-based aggregation approaches have been developed which compare mutation frequencies between cases and controls within the boundaries of the gene. These techniques are appropriate when different variants exhibit an equal risk of disease and thus have the same phenotypic impact. For instance, several variants may result in LoF (e.g., nonsense, frameshift, essential splice site), and thus analysis would determine the association by counting the presence of LoF variants between cases and controls. Prior to association testing, quality control and filtering methods are utilised, namely sequencing quality scores, MAF filters [46] (usually MAF of 1:10,000 for autosomal dominant disorders and MAF of 1:1000 for autosomal recessive disorders) and in silico predictions. Predictions include deleteriousness scores for missense variants such as PolyPhen-2 [47], Sorting Intolerant From Tolerant (SIFT) [47,48], and rare exome variant ensemble learner (REVEL) [49], conservation scores such as Genomic Evolutionary Rate Profiling (GERP) [50], PhyloP [51] or PhastCons [52], or the Combined Annotation Dependent Depletion (CADD) score [53], which combines several metrics in one score. Analysis of the protein-coding region, consisting of ~20,000 genes, requires adjustment for multiple-testing. This can be done using the Bonferroni correction, where α = (0.05/20,000) ≈ 2.5 × 10−6). Where several models are applied, this adjustment must be made more stringent by dividing by the number of models tested. Region-based collapsing approaches hinge on the notion that different regions within genes may vary in their tolerance to missense variation. An alternative approach, particularly useful in smaller studies, is collapsing variants that belong to the same gene set (i.e., genes that belong to the same pathway). Candidate gene testing is a powerful approach to avoid overcorrection and, therefore, false-negative results. This proved useful when investigating members of the transforming growth factor-β (TGF-β) pathway, such as SMAD9 [54], which did not reach statistical significance in the exome-wide analysis [24]. More recently, the same approach revealed an association between TET2 and PAH, which was further supported by experimental evidence [55] but did not reach exome-wide significance [25].
Complex genetic models such as recessive inheritance pose additional challenges. In the recessive mode of inheritance, the MAF threshold must be more lenient as heterozygotes are unaffected (higher MAF in reference populations); also, variants in cis configuration (affecting the same allele) might be wrongly counted (as a pose to trans variants, which are those present on opposing alleles). Similarly, testing for digenic inheritance is particularly problematic due to the large number of possible combinations requiring testing and adjusting for [56].
A number of statistical methods have been developed to test for rare variant associations. Burden tests [57,58,59] aggregate the information found within a predefined genetic region into a summary dose variable. In weighted burden tests [60], variants are weighted according to their frequency or functional significance. Adaptive burden tests [61] aim to account for bidirectional effects by selecting appropriate weights. Variance component (kernel) tests such as (Sequence) Kernel Association Test (SKAT) [62] allow to test risk and protective variants simultaneously but are underpowered when most variants are causal, and effects are unidirectional. Omnibus tests such as SKAT-O [63], which combines burden tests with the variance-component test, might be particularly useful when there is little knowledge of the underlying disease architecture. In addition to frequentist approaches, a Bayesian statistical framework offers a robust alternative. Bayesian model comparison methods such as BeviMed [64] allow for the testing of associations between rare Mendelian disease and a genomic locus by comparing support for a model where disease risks depend on genotypes at rare variant sites in the locus and a genotype-independent “null” model. The prior probability in such models can vary across variants (reflective of external biological information, i.e., depending on MAF, conservation scores, gene ontologies, expression in the tissue of interest) or be constant for all genes/variants reflecting the prior belief of the overall proportion of variants that are associated with a given phenotype.
Last but not least, an essential step in rare variant discovery is to ascertain the pathogenicity of a given variant and its causative role in the disease. Not all damaging variants are pathogenic and in silico approaches alone are not enough to predict if the variant is disease-causing [65]. Viability and phenotyping inferred from knockout mice screens, as well as essentiality screens on human cell lines, may further help predict variant impact [66]. To aid both research and clinical decision making, the American College of Medical Genetics and the Association for Molecular Pathology (ACMG) issued recommendations that combine and weigh the computational, functional, population and clinical evidence to determine pathogenicity [67]. Other initiatives such as ClinGen and ClinVar aim to define the clinical relevance of genes and variants reported in the literature for use in precision medicine and research [68].
4.2.3. Molecular Genetic Techniques
Molecular genetics techniques used for genetic diagnosis, including the detection of specific gene mutations and copy number variants, have been recently summarized [69]. Traditional methods used to identify candidate genes involved in the pathogenesis of PAH include linkage analysis, but more recently next-generation sequencing (NGS) has taken centre stage. The advent of NGS technologies has opened a plethora of opportunities both for clinical diagnostics and research. Such technologies that fall under the umbrella of NGS include targeted panel sequencing, whole-exome sequencing (WES) and whole-genome sequencing (WGS). Nevertheless, the need for more conventional methods such as Sanger sequencing and multiplex ligand-dependent probe amplification (MLPA) remains. Disease-specific panels include a set of genes or regions of genes that are known to be causative of a specific phenotype. This is particularly beneficial in the clinical context when assessing highly heterogeneous traits, such as intellectual disability. Although these panels are not always consistent across laboratories, efforts are being made to produce guidance around their design and development [70]. Targeted panel testing has been introduced in PAH including known and candidate disease genes [71].
WES includes < 2% of the genome i.e., the coding regions only. This method is clinically useful given that 85% of all described disease-causing sequence variants are in this region [72]. For diseases that are more genetically heterogeneous, WES has proven to be a fruitful method, especially when incorporating segregation analysis, which increases the diagnostic yield from 23.6% in probands to 31% in child–parent trios [73]. WES has been used for both the identification and discovery of candidate genes in PAH and has been applied to family-based [74] and case-control studies [75]. Limitations of WES include poor coverage of some exons such as GC rich regions and low confidence to identify structural variation [76].
WGS is a high-throughput sequencing technology predominantly used in the research setting [77]. WGS is massively parallel, DNA fragments are aligned to form a contiguous sequence. The cost of this technology is halving approximately every two years [78]; however, the $1000 genome often referred to is still some way off unless sequencing is performed at scale [79]; as an example, WGS is currently being introduced to the UK National Health Service in collaboration with Genomics England.
European Respiratory Society and European Society of Cardiology (ERS/ESC) guidelines published in 2015 recommended sequential testing starting with bone morphogenetic protein receptor type-2 (BMPR2) sequencing and MLPA in patients with sporadic/familial PAH and EIF2AK4 sequencing in sporadic/familial PVOD/PCH [17], and this approach has been successfully used in many clinical and research settings across the world [80]. With the decreasing cost of WES/WGS, a common practice is now that of virtual panel testing whereby a selected number of genes are chosen for bioinformatics analysis based on the individual’s phenotype. This also allows for data reanalysis when a novel disease-gene is identified and/or another condition is suspected (emerging phenotype over time). In the UK, this is coordinated at a national level via the Genomics England PanelApp tool; virtual disease gene panels applied to WGS data are continuously curated and include a PAH panel [81]. As more patients with PAH are being tested via NGS methods, a diagnostic benefit is starting to emerge (Figure 3C).
Despite the benefits of NGS technologies, there are some challenges that require attention and systematic solutions, among these, storage and handling of big data remains a significant consideration, also management of incidental findings as well as the reporting of variants of unknown significance (VUS).
Along with the growing number of research projects using NGS to uncover the genetic basis of various diseases, there has been an ongoing effort to aggregate and harmonise WES and WGS data from large-scale disease and population projects and to make them publicly available as a reference variome. This started in 2012 with a funder project called Exome Aggregation Consortium (ExAC) which harvested WES data from over 60,000 individuals; this was followed by The Genome Aggregation Database (gnomAD), of which three versions have been released so far, covering 71,702 genomes from unrelated individuals aligned against GRCh38 (v3). Also, in 2012, came the announcement of the 100,000 genomes project by the UK Government. The project sequenced the genomes of 100,000 NHS patients with particular focus on those with rare disease(s) and cancer. Another useful resource is the Trans-Omics for Precision Medicine (TopMed) program, which aims to sequence over 120,000 well-phenotyped individuals as well as collect other omics datasets. The assertion of ethnic diversity is an important consideration, and several initiatives such as KoVariome [82], Genes and Health [83] and BioBank Japan [84] are addressing this issue. Furthermore, new disease cohort genomic databases are being established. Examples include: NIHR BioResource Rare Disease Study (NBR) [77], Inflammatory Bowel Disease BioResource [85], Genetic Links to Anxiety and Depression (GLAD) [86] and Eating Disorders Genetics Initiative (EDGI) [87]. The advantages of these datasets are numerous; first, they provide allele frequencies for diverse populations, second, they help to address the overestimation of disease penetrance arising from the historical focus on multiplex pedigrees [88], and third, through acknowledging variable penetrance, they help to identify genetic and environmental disease modifiers [89].
4.2.4. Reference Genome
The Human Genome Project was completed in 2003 [90,91] and since then, successive iterations of the human reference genome have been published, updated, and refined by the Genome Reference Consortium (GRC). Recent versions include GRCh37 (hg19) and GCRh38 (hg38) released in 2009 and 2013, respectively. These are both composite genomes, i.e., derived from the sequence of several anonymous donors; the make-up of these two assemblies is largely similar, with approximately 93% of the primary assembly composed of sequences from 11 genomic clone libraries.
To date, most large-scale PAH studies have aligned their data to the GRCh37 reference genome (Table 2), with only a couple of recent studies aligning their data to GRCh38 [25,55]. Even though GRCh38 was released seven years ago, the transition from GRCh37 to GRCh38 has been a long process and recent analyses have sought to compare the two reference panels. Guo et al. [92] demonstrated that GRCh38 provides a more accurate analysis of human sequencing data due to the improved annotation of the exome and the additional reads aligned to GRCh38, findings which indicate better structural and sequence representation. In addition, Pan et al. [93] noted that GRCh38 had better genome coverage, with a 5% increase in the number of SNVs identified. In comparison to GRCh37, GRCh38 altered 8000 nucleotides, corrected several misassembled regions, filled in gaps, and increased the number of genes and protein-coding transcripts [92]; additionally, GRCh38 is the first human reference genome to contain sequence-based representations for the centromeres [94]. Whilst GRCh37 is a single representation of multiple genomes, with only three regions containing alternative sequences (UDP-glucuronosyltransferases 2B subfamily (UGT2B) on chromosome 4, the major histocompatibility complex (MHC) region on chromosome 6, and the MAPT gene on chromosome 17) [95], GRCh38 includes 261 alternate loci across 178 genomic regions, providing a more robust representation of human population variation [94]. The increased level of alternative sequence representation requires new analysis methods to support their inclusion yet at present, most tools and pipelines do not make use of these [95]. Despite the advantages, GRCh38 still contains gaps and errors at repetitive and structurally diverse regions [96]. Additionally, as it is a mosaic haploid representation of the human genome [94], in which poor alignment can affect the detection of alleles in regions of high variation, such as the MHC locus and KIR, it is unlikely to truly represent human diversity [96]. It does, however, provide the starting point for a more inclusive population-based reference genome, or pan-genome [97] and as such, will play an evolving role in the generation of individual diploid genome assemblies and graph-based representations of genome-wide population variation [98,99,100], thereby providing unique opportunities for data analysis.
Table 2.
Landmark forward genetics studies in Group 1 PAH. Abbreviations: BMPR2—Bone morphogenic protein receptor type 2; ENG—Endoglin; ACVRL1—Activin A Receptor Like Type 1; SMAD—SMAD Family Member; CAV1—Caveolin 1; KCNK3—Potassium Two Pore Domain Channel Subfamily K Member 3; TBX4—T-Box Transcription Factor 4; EIF2AK4—Eukaryotic Translation Initiation Factor 2 α Kinase 4; GDF2—Growth Differentiation Factor 2; SOX17—SRY-Box Transcription Factor 17; ATP13A3—ATPase 13A3; AQP1—Aquaporin 1; ABCC8—ATP Binding Cassette Subfamily C Member 8; BMP10—Bone Morphogenetic Protein 10; KLK1—Kallikrein 1; GCCX—γ-Glutamyl Carboxylase; KDR—Kinase insert domain receptor; TET2—Tet Methylcytosine Dioxygenase 2; FBLN2—Fibulin 2; PDGFD—Platelet-Derived Growth Factor D.
| Study (Reference) | Genes | Study Design | Sample | Ethnicity | Method | Reference Genome |
|---|---|---|---|---|---|---|
| Lane et al. 2000, [101] | BMPR2 | Case-level data | Cases: n = 8 PPH kindreds for candidate gene mutational analysis | Not stated | TS | H.sapiens mRNA for BMPR-II: Genbank Z48923 |
| Thomson 2000, [102] | BMPR2 | Case-level data | Cases: n = 50 PPH | Not stated | TS | Not stated |
| Trembath et al. 2001, [103] | ACVRL1 | Case-level data | Cases: 5 kindreds plus 1 individual patient with HHT, including n = 10 cases with PH | Not stated | TS | Not stated |
| Chaouat 2004, [104] | ENG | Case-level data | Case: n=1 HHT, PPH with history of anorexigen use | Not stated | TS | Not stated |
| Harrison et al. 2005, [105] | ACVRL1, ENG | Case-level data | Cases: n = 18 I/APAH | Not stated | TS | Not stated |
| Shintani et al. 2009, [106] | SMAD9 (SMAD8) | Case-level data | Cases: n = 23 IPAH | Japanese | TS | Not stated |
| Nasim et al. 2011, [54] | SMAD1, SMAD4, SMAD9 | Case-level data | Cases: n = 324 IPAH/APAH/CTEPH; Controls: n = 1584 | European & Japanese | TS | Not stated |
| Austin et al. 2012, [74] | CAV1 | Case-level data | Cases: 3-generation family, 6 with PAH; Additional cohort: n = 260 unrelated I/HPAH cases; Controls: n = 1000 | European | WES | GRCh37 |
| Ma et al. 2013, [107] | KCNK3 | Case-level data | Cases: Family in which multiple members had PAH | Not stated | WES | GRCh37 |
| Kerstjens-Frederikse et al. 2013, [108] | TBX4 | Case-level data | Cases: n = 20 childhood-onset I/HPAH; n = 49 adult-onset I/HPAH; n = 23 SPS | Not stated | TS | Not stated |
| Eyries et al. 2014, [109] | EIF2AK4 | Case-level data | Cases: n = 13 PVOD families | Not stated | WES | GRCh37 |
| Best et al. 2017, [110] | EIF2AK4 | Case-level data | Cases: n = 81 I/HPAH | Not stated | TS | Not stated |
| Hadinnapola et al. 2017, [111] | EIF2AK4 | Case-control data | Cases: n = 880 I/FPAH, PVOD/PCH; Controls: n = 7134 non-PAH controls and their relatives recruited to NBR | European: 84.6% | WGS | GRCh37 |
| Gräf et al. 2018, [24] | GDF2, SOX17, ATP13A3, AQP1 | Case-control data | Cases: n = 1048 I/F/PAH, PVOD/PCH; Controls: n = 7979 non-PAH controls and their relatives recruited to NBR | European: 84.6% | WGS | GRCh37 |
| Zhu et al. 2018, [75] | SOX17 | Case-control data | Cases: n = 256 I/FPAH-CHD; Additional cohort: n = 413 I/FPAH screened for rare variants in SOX17; Controls: n = 7509 gnomAD | Not stated | WES | GRCh37 |
| Hiraide et al. 2018, [112] | SOX17 | Case-level data | Cases: n = 12 IPAH and 12 family members; Additional cohort: n = 128 I/HPAH screened for SOX17 mutations | Japanese: 100% | WES | Not stated |
| Bohnen et al. 2018, [113] | ABCC8 | Case-control data | Cases: n = 913; Controls: n = 33,369 European adults from ExAC & n = 49,630 Europeans from the Regeneron-Geisinger DiscovEHR study | Not stated | WES, WGS | GRCh37 |
| Wang et al. 2019, [26] | GDF2 | Case-control data | Cases: n = 331 IPAH; Controls: n = 10,508 from available reference data sets | East Asian: 100% | WES, WGS | GRCh37 |
| Eyries et al. 2019, [114] | BMP10 | Case-level data | Cases: n = 268 I/HPAH, PVOD/PCH | European: > 90% | TS | GRCh37 |
| Hodgson et al. 2020, [115] | BMP10 | Case-level data | Cases: n = 1048 I/FPAH, PVOD/PCH | European: 84.6% | WGS | GRCh37 |
| Zhu et al. 2019, [25] | KLK1, GCCX | Case-control data | Cases: n = 2572 Group 1 PAH; Controls: n = 12,771 | European: 72% | WES | GRCh38 |
| Rhodes et al. 2019, [116] | HLA-DPA1/DPB1, SOX17 enhancer | Case-control data | Cases: n = 2085 cases; Controls: n = 9659 | European: 100% | WGS | GRCh37 |
| Swietlik et al. 2019, [30] | KDR | Case-control data | Cases: n = 1122 PAH; Controls: n = 11,889 non-PAH NBR | European: 84% | WGS | GRCh37 |
| Eyries et al. 2020, [117] | KDR | Case-level data | Cases: n = 311 unrelated PAH | Not stated | TS | Not stated |
| Potus et al. 2020, [55] | TET2 | Case-control data | Cases: n = 2572; Controls: n = 7509 non-Finnish European subjects from gnomAD | European: 72% | WES | GRCh38 |
| Zhu et al. 2020, [118] | FBLN2, PDGFD | Case-control data | Cases: n = 4175; Controls: n = 18,819 from SPARK and gnomAD cohorts | European: 54.5% | WES | GRCh38 |
4.3. Studies
4.3.1. Rare Genetic Variation
Over the last two decades, forward genetics approaches have associated PAH with numerous genes (Table 2); the level of evidence supporting the causal role of these genes, however, is variable and depends on multiple factors (Table 3). PAH is considered to be a monogenic condition transmitted in autosomal dominant fashion with incomplete penetrance. Heterozygous germline mutations in BMPR2, a member of the TGF-β superfamily, are the most common genetic cause of PAH [101,119], accounting for over 80% of familial PAH, and approximately 25% of idiopathic PAH [24]. Additional mutations within the TGF-β/BMP signalling pathway, such as activin A like type 1 (ACVRL1), endoglin (ENG), SMAD family members (SMAD1, SMAD4, SMAD9), caveolin 1 (CAV1), growth differentiation factor 2 (GDF2), loss of function variants in channelopathy genes, potassium two pore domain channel subfamily K member 3 (KCNK3), ATP binding cassette subfamily C member 8 (ABCC8), ATPase type 13A3 (ATP13A3), variants within developmental transcription factors, SRY-box transcription factor 17 (SOX17) and T-box transcription factor 4 (TBX4), and newly reported risk genes, γ-glutamyl carboxylase (GGCX), kallikrein 1 (KLK1) [25], kinase insert domain receptor (KDR) [30], fibulin 2 (FBLN2) and platelet-derived growth factor D (PDGFD) [118], have all been identified as individually rare causes of PAH. Infrequent cases of autosomal recessive transmission in KCNK3 [120] and GDF2 [121] have been associated with early disease onset and severe phenotype. A subtype of PAH, PVOD/PCH is linked to biallelic mutations in EIF2AK4 [109].
Table 3.
Supporting evidence for the role of risk genes in PAH pathogenesis. Abbreviations: (+) indicates that the paper provides information in favour of the role of the given gene in the pathogenesis of PAH, (−) indicates that the paper does not provide support for the role of the given gene in the pathogenesis of PAH.
| Forward Genetics | Reverse Genetics | |||||
|---|---|---|---|---|---|---|
| Gene | Case-Control Data | Case-Level Data | Segregation Data | Functional Aberration | Disease Model | Rescue |
| MOI: Autosomal Dominant | ||||||
| BMPR2 | (+) [24,25] | (+) [101,114] | (+) [101,122] | (+) [123,124,125,126,127,128,129,130] | Animal: (+) [123,126,128,129,130,131] Cell culture: (+) [124,129,130] | (+) [128,129,130,131] (+) |
| ACVRL1 | (+) [24] | (+) [25,103,105,114] | (+) [103] | |||
| ENG | (−) [24,25] | (+) [24,75,104] | (+) [104] | Animal: (−) [132] | ||
| SMAD9 | (−) [24,25] | (+) [24,25,75,106,114] | (+) [54,106,133] | Animal: (+) [134] Cell culture: (+) [54,106,133] |
(+) [133,135] | |
| SMAD1 | (−) [24,25] | (+) [54], (−) [24,25] | (+) [54,136] | Animal: (+) [136] Cell culture: (+) [54] | ||
| SMAD4 | (−) [24,25] | (+) [25,54], (−) [24] | (+) [54], (−) [54] | Cell culture: (+) [54] | ||
| CAV1 | (+) [74] | (+) [25,75], (−) [24] | (+) [137] | Animal: (+) [138,139] Cell culture: (+) [137] | (+) [137] | |
| TBX4 | (+) [24,140] | (+) [75,108,114,141,142] | (+) [143,144] | |||
| KCNK3 | (+) [24,25,107] | (+) [107] | (+) [107,145] | Animal: (+) [146] Cell culture: (+) [107,145] | (+) [107] | |
| ATP13A3 | (+) [24] | (+) [147,148] | Animal: (+) [147,148] | |||
| AQP1 | (+) [24] | (+) [149,150] (−) [149] | Animal: (+) [149,151] Cell culture: (+) [150] | (+) [149] | ||
| GDF2 | (+) [24,25,26] | (+) [114] | (+) [115] | (+) [26,115] | Animal: (−) [152] Cell culture: (+) [26,115] | |
| SOX17 | (+) [24,75] | (+) [112] | (+) [153,154] | Animal: (+) [153] Cell culture: (+) [154] | ||
| ABCC8 | (+) [113] | (+) [113] | (+) [113] | Cell culture: (+) [113] | (+) [113] | |
| BMP10 | (+) [114,115] | |||||
| GGCX | (+) [25] | |||||
| KLK1 | (+) [25] | |||||
| KDR | (+) [30] | (+) [30,117] | (+) [155] | Animal: (+) [155] | ||
| FBLN2 | (+) [118] | |||||
| PDGFD | (+) [118] | |||||
| TET2 | (+) [55] | (+) [55] | Animal: (+) [55] | (+) [55] | ||
| BMPR1A | (+) [25,75] | (+) [156,157,158,159] | Animal: (+) [156,157,159] | (+) [156] | ||
| BMPR1B | (+) [24,25,75] | (+) [160] | Cell culture: (+) [160] | |||
| TOPBP1 | (+) [24] | |||||
| THBS1 | (+) [161] | Cell culture: (+) [161] | (+) [161] | |||
| KCNA5 | (+) [162] | (+) [163] | Cell culture: (+) [163] | (+) [163] | ||
| MOI: Autosomal Recessive | ||||||
| EIF2AK4 | (+) [111] | (+) [114] | (+) [109,110] | (+) [109] | Animal: (+) [164] | |
| Common Variation | ||||||
| enhancer near SOX17 | (+) [116] | (+) [116] | (+) [116] | |||
| locus within HLA-DPA1/DPB1 | (+) [116] | |||||
| CBLN2 | (+) [165] (−) [116] |
|||||
| SIRT3 | (+) [166] | (+) [166] | (+) [166] | Animal: (+) [166,167] | ||
| UCP2 | (+) [168] | Animal: (+) [167,169] | ||||
| EDN1 | (+) [170] | (+) [171] | ||||
| AGTR1 | (+) [34] | |||||
| TOPBP1 | (+) [172] | (+) [172] | Cell culture: (+) [172] | |||
| Endostatin | (+) [173] | |||||
| TRPC6 | (+) [174] | Cell culture: (+) [174] | ||||
Autosomal Dominant Mode of Inheritance
TGF-ß Pathway
In 2000, the International Primary Pulmonary Hypertension (PPH) Consortium demonstrated that familial PAH (FPAH) is caused by mutations in BMPR2, located on chromosome 2, encoding a TGF-β type II receptor [101]. They established a panel of eight kindreds, in which at least two members had the typical manifestations of PAH. Sequence variants were detected in seven probands; these variants, including two frameshift, two nonsense and three missense mutations, were distributed across the gene and each of the amino acid substitutions occurred at a highly conserved and functionally important site of the BMPR2 protein. They observed segregation of the mutations with the disease phenotype in seven of the eight families studied. As control subjects, they screened 150 normal chromosomes from the same population and 64 normal chromosomes from ethnically diverse subjects and observed no BMPR2 mutations [101]. The predicted functional impact of these mutations, their segregation with the phenotype, and the absence of these variants in healthy controls provided strong support for the role of BMPR2 and the TGF-β signalling pathway in the pathobiology of PAH. The role of BMPR2 mutations has been subsequently reported in IPAH. Thomson et al. [102] investigated BMPR2 gene mutations in 50 unrelated IPAH patients with no family history of the disease. In 13 patients (26%), 11 novel heterozygous mutations in BMPR2 were identified, these included three missense, three nonsense and five frameshift. They also sequenced both parents for five of the 13 probands; paternal transmission was observed for three families, whereas the remaining two mutations arose spontaneously. BMPR2 mutations were not observed in 150 normal chromosomes [102]. Screening of other disease subtypes revealed BMPR2 mutations among patients with PAH associated with congenital heart disease (PAH-CHD) [175] and PVOD [176].
Large cohort studies have proved useful in defining the relative contribution of BMPR2 mutations in various PAH subtypes. Gräf et al. [24] reported rare heterozygous BMPR2 mutations in 160 of 1048 PAH cases (15.3%); the frequency of BMPR2 mutations in FPAH, IPAH and anorexigen-exposed PAH were 75.9%, 12.2% and 8.3%, respectively. Fourteen percent of BMPR2 mutations resulted in the deletion of larger protein-coding regions, ranging from 5 kb to 3.8 Mb in size. Additionally, 52% of the observed BMPR2 mutations were newly identified in their study [24], suggesting that nearly two decades after the first BMPR2 mutation was identified, the use of WGS has allowed for closer study of BMPR2, including large deletions around the BMPR2 locus, and the TGF-β pathway. Another large-scale study in a more heterogeneous group of patients (Group 1 PAH) [25] reported BMPR2 mutations in 180 of 2572 cases (7%); the frequency of BMPR2 mutations in FPAH and IPAH patients were 62.4% and 9.3%, respectively. Taken together, over 600 distinct mutations in BMPR2 have been identified in PAH patients [24,25,177,178,179] of which around 70–80% are identified in FPAH and 10–20% in IPAH [180].
Importantly, impaired BMPR2 signalling was shown to be a universal feature of PAH and pointed towards other key members of the canonical BMPR2 signalling pathway as potential culprits for the disease [181,182,183].
Mutations in ACVRL1 and ENG have been reported in PAH patients and in patients with PH in association with hereditary hemorrhagic telangiectasia (HHT). HHT is a rare autosomal dominant genetic disorder characterised by arteriovenous malformations and multiple telangiectasias [184]; it is frequently linked to defects in ACVRL1 and ENG and as HHT and PAH may co-present in families, suggests a common molecular aetiology [103,104,105]. Of note, PH secondary to high cardiac output from arteriovenous fistulas is much more common in HHT, and such phenocopies, if unrecognised, may introduce significant bias to the studies [185]. Conversely, I/HPAH associated with ACVRL1 and ENG can occur without clinical features of HHT [105,185,186], as the latter shows age-related penetrance. In a large case-control study, which employed deep phenotyping prior to association analysis, ACVRL1 was associated with HPAH [30] but fell just below the cut-off for significance when studied in unselected patients with Group 1 PAH [25].
Two studies using targeted sequencing of BMPR2 signalling intermediates provided further evidence supporting the role of this pathway in the pathogenesis of PAH. Shintani et al. [106] screened 23 patients with IPAH for mutations in ENG, SMAD1, SMAD2, SMAD3, SMAD4, SMAD5, SMAD6 and SMAD9 (SMAD8) and identified a nonsense mutation in SMAD9 in a child who was diagnosed at eight years of age and his unaffected father. The results of immunoblotting and co-immunoprecipitation assays indicated that the SMAD9 mutant disturbs the downstream signalling of TGF-β/BMP. In a later study, SMAD1, SMAD4, SMAD5 and SMAD9 were screened by direct sequencing in a cohort of 324 PAH cases (188 IPAH and 136 anorexigen-induced PAH) [54]. Four gene defects in three genes were observed. A novel missense variant in SMAD1 was observed in an IPAH patient, a predicted splice-site mutation and a missense variant in SMAD4 were observed in two IPAH patients and a novel missense variant in SMAD9 was observed in a patient of Japanese origin. These four variants were absent in the 960 European and 284 French control samples and the SMAD9 variant was excluded from the panel of 340 Japanese controls. A case-control study using WGS detected two cases harbouring protein-truncating variants in SMAD1, of which one co-existed with a protein-truncating variant in BMPR2, and eight SMAD9 variants, two of which co-occurred with protein-truncating variants in BMPR2 and GDF2; statistical analysis did not reveal significant association with studied phenotypes [30]. In another large cohort study (n = 2572 cases, 72% European), deleterious variants were observed in SMAD1 (two cases), SMAD4 (two cases) and SMAD9 (13 cases) but were not statistically significant [25]. Taken together, these findings demonstrate that variations within the SMAD family have a small effect size, suggesting that a second genetic or environmental hit is needed, or that they perturb other non-investigated pathways.
Besides BMPR2 mutations, CAV1 mutations are a rare cause of PAH. Variants in CAV1 were initially implicated in PAH pathogenesis by exome sequencing of a three-generation family with autosomal dominant HPAH who were negative for established variants in the TGF-β family [74]. They identified a frameshift mutation in CAV1; all PAH patients and several unaffected family members carried the CAV1 mutation, suggesting incomplete penetrance. Subsequent evaluation of an additional 62 unrelated HPAH and 198 IPAH patients identified an independent de novo CAV1 mutation in a child with IPAH. Two separate studies have since identified CAV1 mutations in PAH patients; the first identified a novel heterozygous frameshift mutation in an adult PAH patient with a paediatric-onset daughter who died at nine years old [140], and the second identified deleterious variants in 10 patients with I/F/APAH, with three related cases carrying the same likely gene damaging mutation [25]. WGS in an I/HPAH cohort did not detect deleterious variants in CAV1 [24]. Whilst these findings highlight the importance of caveolae in the homeostasis of the pulmonary vasculature, the link between CAV1 mutations and PAH requires further study.
Bone morphogenetic protein (BMP)-9 (encoded by GDF2) and BMP10 are ligands involved in TGF-β signalling pathway. Wang et al. [121] identified a novel homozygous nonsense mutation in the GDF2 gene in a five-year-old Hispanic child with severe PAH. Genetic testing revealed that both parents were heterozygous for the same mutation, indicating that the child inherited the GDF2 mutant allele from each parent. This study was the first to report a novel homozygous nonsense mutation in GDF2 in an IPAH patient, suggestive of the causative role of GDF2 mutations in PAH. Further evidence came from the NBR study which identified associations between rare heterozygous missense (n = 7) and frameshift variants (n = 1) in adult-onset IPAH (88% European) [24]; additionally, Hodgson et al. [115] identified two patients with large deletions encompassing the GDF2 locus and several neighbouring genes.
The identification of GDF2 mutations has since been independently replicated in a Chinese cohort [26]. Wang et al. [26] performed an exome-wide gene-based burden analysis on two independent case-control studies. The discovery analysis, containing 251 IPAH patients, identified rare heterozygous mutations in BMPR2 (49 cases), ACVRL1 (15 cases), TBX4 (10 cases), SMAD1 (two cases), BMPR1B (one case), KCNK3 (one case) and SMAD9 (one case). In a gene-based burden analysis (cases: n = 251; controls: n = 1884), only three genes (BMPR2, GDF2 and ACVRL1) had an exome-wide significant enrichment of mutations in IPAH cases when compared to healthy controls. GDF2 mutations were identified in 17 cases (6.8%) and ranked second to BMPR2 (56 cases, 22.3%). To validate the risk effects of GDF2, they performed WES in an independent replication cohort of 80 IPAH cases and in a second gene-based burden analysis (cases: n = 80; controls: n = 8624), BMPR2, GDF2 and ACVRL1 were again identified as the top three disease-associated genes. Within this analysis, five additional GDF2 heterozygous mutations were identified. Among the 331 IPAH patients, they identified 22 cases carrying 21 distinct rare heterozygous mutations in GDF2, only two of which had been reported previously [24], accounting for 6.7% of IPAH cases. An independent cohort confirmed the genome-wide association of GDF2 among 1832 PAH and 812 IPAH cases of European ancestry [25]; twenty-four GDF2 variants were observed in 28 cases, only two of which had been reported previously, and 75% of these occurred in IPAH cases.
Additionally, gene panel sequencing of 263 PAH patients (180 IPAH, 11 FPAH, 13 drug and toxin-induced PAH and 59 sporadic PVOD) revealed two (1.2%) BMP9 mutations in adult PAH cases [114]; due to the close similarity of BMP9 and BMP10 (a close paralogue of BMP9 that encodes an activating ligand for ACVRL1), the BMP10 gene was also included in the capture design. Two mutations were identified in BMP10, a truncating mutation and a predicted loss of function variant were identified in two severely affected IPAH patients. Two rare missense variants in BMP10 were identified in patients with IPAH in an independent cohort [115]. These results emphasise the role of GDF2 in the pathobiology of PAH and suggest BMP10 might act as a predisposing risk factor.
Channelopathies
Channelopathies are a group of diseases caused by dysfunction of ion channels localised in cellular membranes and organelles. These diseases include, but are not limited to, cardiac, respiratory, neurological and endocrine disorders. LoF variants in channelopathy genes have also been reported in PAH.
The first channelopathy described in PAH was caused by a genetic defect in KCNK3 in patients with familial and sporadic PAH [107]. KCNK3 belongs to a family of mammalian potassium channels and encodes for a two-pore potassium channel which is expressed in pulmonary artery smooth muscle cells (PASMCs); this channel plays a role in the regulation of resting membrane potential and pulmonary vascular tone and vascular remodelling [120]. In studying a family in which multiple members had PAH, Ma et al. [107] identified a novel heterozygous missense variant in KCNK3 as a disease-causing candidate gene within the family. WES was used to study an additional 10 probands with FPAH and two novel heterozygous KCNK3 variants were identified and segregated with the disease. In addition, three novel KCNK3 variants were identified in 230 patients with IPAH. These five variants were predicted to be damaging. In summary, KCNK3 mutations were identified in three of 93 unrelated patients (3.2%) with FPAH and in three of 230 patients (1.3%) with IPAH [107].
In another study using targeted sequencing, two KCNK3 mutations were observed in three patients from two families. One of these mutations, a homozygous missense variant in KCNK3, was identified in a patient belonging to a consanguineous Romani family; his affected mother and asymptomatic father were carriers of the same KCNK3 mutation. This is the first report of a young patient with severe PAH carrying a homozygous mutation in KCNK3 [120]. Of note, in the Ma et al. [107] study, as the pedigree suggested an autosomal dominant mode of inheritance, homozygous variants were excluded from the analysis. The two biggest case-control studies, reported by Gräf et al. [24] and Zhu et al. [25], identified heterozygous KCNK3 mutations in only four (0.4%) and three (0.1%) cases, respectively, and did not show statistically significant associations.
Conversely, Gräf et al. [24] detected statistically significant enrichment of rare deleterious variants in two new channel genes: ATP13A3 and AQP1. Utilising a rigorous case-control comparison using a tiered search for variants, they searched for high-impact protein-truncating variants (PTVs) overrepresented in cases and identified a higher frequency of PTVs in ATP13A3 (six cases). ATP13A3 is a poorly characterised member of the P-type ATPase family of proteins that transport a variety of cations across membranes [187]; ATP13A3 is thought to play a role in polyamine transport [188]. Within PAH cases, Gräf et al. [24] identified three heterozygous frameshift variants, two stop gain, two splice region variants and four heterozygous likely pathogenic missense variants in ATP13A3. These variants were predicted to lead to loss of ATPase catalytic activity, and to destabilise the conformation of the catalytic domain; six variants were predicted to cause protein truncation, suggesting that loss of function of ATP13A3 contributes to PAH pathogenesis.
Within the same study, SKAT-O analyses revealed a significant association with rare variants in AQP1 [24]; AQP1 ranked second in their combined rare PTV and missense variant case-control analysis. Along with statistical evidence, familial segregation of AQP1 variants with the phenotype was shown in three families [24]. AQP1 belongs to the aquaporins family, a family of water-specific membrane channel proteins that facilitate water transport in response to osmotic gradients [189]. Zhu et al. [25] identified seven cases with ATP13A3 rare deleterious variants. However, ATP13A3 and AQP1 failed to reach genome-wide significance in their study and additionally, AQP1 was not among the expanded list of genes with p≤ 0.001 for either the whole cohort or the IPAH subset.
Interestingly, a mutation in ATPase Na+/K+ transporting subunit α 2 (ATP1A2), previously associated with familial hemiplegic migraine (FHM), was reported in a 24-year old male with IPAH and history of FHM [190]. Genetic analysis of the proband and two siblings (one with FHM) revealed a nucleotide substitution in the coding sequence of the ATP1A2 gene for both the proband and the affected sibling [190]. A co-occurrence of PAH and FHM supports the hypothesis of a potential common pathophysiological link; several studies have reported the presence and activity of the α2-subunit of the Na⁺/K⁺-ATPase in pulmonary vascular smooth muscle cells [191,192], and the decrease in expression and/or activity of different types of K⁺ channels in PASMCs of IPAH patients [193,194], and Montani et al. [190] suggested that mutations in ATP1A2 may contribute to pulmonary arterial remodelling through the disturbance of intracellular Ca2⁺ and K⁺ concentrations.
Mutations in ABCC8 have recently been identified as a potential second potassium channelopathy in PAH [113]. ABCC8 encodes SUR1 (sulfonylurea receptor 1), a regulatory subunit of the ATP-sensitive potassium channel; ABCC8 is highly expressed in the human brain and endocrine pancreas and moderately expressed in human lungs [195]. Mutations in ABCC8 have previously been related to type II diabetes mellitus and congenital hyperinsulinism [196]. In exome-sequencing a cohort of 99 paediatric- and 134 adult-onset Group 1 PAH patients (182 IPAH and 52 HPAH), Bohnen et al. [113] identified a de novo heterozygous predicted deleterious missense variant in ABCC8 in a child with IPAH. All individuals within this cohort and the second cohort of 680 adult-onset PAH patients (NBR study) were screened for rare or novel variants in ABCC8. Eleven heterozygous predicted damaging ABCC8 variants were identified, seven of these were observed in the original cohort, including one familial case. In a study of 2572 PAH cases, Zhu et al. [25] identified rare deleterious variants in newly reported risk genes and nearly two-thirds of these variants were in ABCC8 (26 variants in 29 IPAH/APAH patients). In a more recent study, utilising a custom NGS targeted sequencing panel of 21 genes, Lago-Docampo et al. [197] identified 11 rare variants in ABCC8 within a cohort of 624 paediatric and adult patients from the Spanish PAH registry. To date, ABCC8 variants have been identified in patients with IPAH, FPAH, PAH-CHD and APAH and account for ~0.5–1.7% of cases.
Transcription Factors
An emerging category of HPAH is the one that can be labelled as a disorder of transcriptional regulation. Interestingly, variants within developmental transcription factors are enriched in paediatric patients.
The first transcription factor implicated in the pathogenesis of PAH was TBX4. TBX4, expressed in the atrium of the heart, limbs, and the mesenchyme of the lung and trachea, encodes a transcription factor in the T-box gene family [198]. Deletions and LoF mutations in TBX4 cause a variety of developmental lung disorders [199] and have been identified as a prominent risk factor in small patella syndrome (SPS) [200], childhood-onset PAH [108] and more recently, persistent pulmonary hypertension in neonates [201]. In a 2013 study incorporating array-comparative hybridisation and direct sequencing, three TBX4 mutations and three novel TBX4 microdeletions were detected in six out of 20 children with I/HPAH and interestingly, features of SPS were detected in all living TBX4 mutation carriers [108].
Zhu et al. [140] performed exome sequencing on a cohort of 155 paediatric- and 257 adult-onset PAH patients. Within 13 probands (12 paediatric- and one adult-onset), they identified 13 likely pathogenic/predicted highly deleterious TBX4 variants; eight of these variants were inherited from an unaffected parent, whereas one was de novo. This pattern is consistent with the incomplete penetrance observed for BMPR2 mutation carriers [122]. Similar frequencies of rare, deleterious BMPR2 mutations were observed in paediatric- and adult-onset I/FPAH patients; however, there was significant enrichment of rare, predicted deleterious TBX4 mutations in paediatric- (10 of 130 patients) compared with adult-onset (0 of 178 patients) IPAH patients. In comparison to BMPR2 mutation carriers, TBX4 carriers had a 20-year younger age of onset, with a mean age of onset of 28.2 ± 15.4 years and 7.9 ± 9.0, respectively. After BMPR2 mutations (10%), variants in TBX4 (7.7%) conferred the highest degree of genetic risk of paediatric-onset IPAH [140]. Similar estimates of BMPR2 (12.5% of I/FPAH patients) and TBX4 mutation carriers (7.5% of I/FPAH patients) were observed in a study of 66 paediatric patients [141]. Additionally, in a 2019 study, examining 263 PAH and PVOD/PCH patients (paediatric and adult cases), TBX4 mutations were the second most frequent mutations after BMPR2 in both paediatric and adult cases [114].
Indicative of bimodal age distribution, pathogenic TBX4 variants have also been reported in adult-onset PAH. Gräf et al. [24] identified deleterious heterozygous rare variants in 14 cases, Navas Tejedor et al. [120], in a Spanish cohort of 136 adult-onset PAH patients, identified three pathogenic mutations, and Kerstjens-Frederikse et al. [108], in a much smaller adult cohort (n = 49), detected a rare TBX4 mutation. In a more recent study, 448 index patients were screened for PAH predisposing genes; 20 patients (nine childhood-onset) from 17 unrelated families carried heterozygous mutations in the TBX4 gene, bringing the frequency of TBX4 mutations in France to 6% and 3% in childhood- and adult-onset PAH, respectively [142]. Within this cohort, SPS was present in 80% of cases [142] and interestingly, all patients showed decreased DLCO and 87% had parenchymal abnormalities. These findings suggest that TBX4 mutations may occur with or without skeletal abnormalities and whilst such mutations are mainly associated with childhood-onset PAH, the prevalence of PAH in adult TBX4 mutation carriers could be up to 3%, depending on the population studied.
A recent study of 1038 PAH patients observed that rare heterozygous variants in SOX17 were significantly overrepresented in the I/HPAH cohort [24]. SOX17 is a member of the conserved SOX family of transcription factors. These transcription factors play a pivotal role in cardiovascular development and figure prominently in the aetiology of human vascular disease; they are involved in the regulation of embryonic development, the determination of cell fate and participate in vasculogenesis and remodelling [202]. In the NBR study [24], deleterious variants in SOX17 were detected in less than 1% of the studied population and were characterised by younger age at diagnosis; familial segregation was shown in one patient.
To identify novel genetic causes of PAH-CHD, Zhu et al. [75] performed WES in 256 PAH-CHD patients; the cohort included 15 familial and 241 sporadic cases. Fifty-six percent of the cohort were of European ancestry and 26% were Hispanic; most cases (56%) had an age of onset < 18 years and so were categorised as paediatric-onset. The cohort was screened for 11 known risk genes for PAH and 253 candidate risk genes for CHD; PAH risk variants were identified in only 6.4% of sporadic PAH-CHD cases and four of the 15 familial cases. They performed a case-control gene-based association test of rare deleterious variants comparing European cases and controls (gnomAD: n = 7509 non-Finnish Europeans) and identified SOX17 as a novel PAH-CHD candidate risk gene [75]. They estimated that rare deleterious variants in SOX17 contributed to approximately 3% of European PAH-CHD patients. Following this discovery, they screened for SOX17 variants in non-European cases and an additional cohort of PAH patients without CHD (n = 413) and identified five additional rare variants in the PAH-CHD cohort and three additional rare variants in the I/HPAH cohort [75]. In this second cohort, rare deleterious variants in SOX17 were observed in 0.7% of cases. In total, 13 patients across the two cohorts were observed to have rare deleterious SOX17 variants; nine of these had paediatric-onset PAH, suggesting these variants may be enriched in paediatric patients.
A Japanese study also demonstrated familial segregation of SOX17 variants [112]. This study whole-exome sequenced 12 patients with PAH, 12 asymptomatic family members and 128 index cases and identified SOX17 mutations in four PAH patients (three of these had congenital heart defects, i.e., atrial septal defect or patent ductus arteriosus) and one asymptomatic family member. Interestingly, the same heterozygous missense mutation in SOX17 (c.397C) was observed in a Japanese patient [112] and in a patient with PAH from the NBR study [24], suggesting that this base position may be a pan-ethnic mutational hot spot. Taken together, these data strongly implicate SOX17 as a new risk gene for PAH-CHD and suggest that this gene has a pleiotropic effect. Replication analyses in other PAH cohorts, with specific PAH subclasses, are needed to confirm the precise role of SOX17.
New Genes
In recent years, new risk genes for PAH have emerged from large WES and WGS studies. Zhu et al. [25] performed targeted gene sequencing alongside WES in a large cohort from the National Biological Sample and Data Repository for PAH (US PAH Biobank: n = 2572). Despite screening for 11 established PAH risk genes and seven recently reported risk genes, they failed to identify rare deleterious variants in known risk genes for 86% of the PAH Biobank cases (Group 1 PAH). They performed gene-based case-control association analysis and to prevent confounding by genetic ancestry, only participants of European ancestry (cases: n = 1832; controls: n = 7509 gnomAD WGS subjects and n = 5262 unaffected parents from the Pediatric Cardiac Genomics Consortium) were included. Using a variable threshold method, they identified two genes that exceeded the Bonferroni-corrected cut-off for significance: BMPR2 and KLK1. KLK1, which encodes kallikrein 1, also known as tissue kallikrein, has not previously been associated with pulmonary hypertension.
The analysis was then repeated using 812 European IPAH cases and significant associations were observed for BMPR2, KLK1 and GGCX. GGCX encodes γ-glutamyl carboxylase and has previously been implicated in coagulation factor deficiencies and vascular calcification [203], but again, never in PAH. In a final analysis, the entire PAH Biobank cohort was screened for rare deleterious variants in KLK1 and GGCX; twelve cases carried KLK1 variants (all European), whereas 28 cases carried GGCX variants (19 European, six African, three Hispanic), accounting for ~0.4% and ~0.9% of PAH Biobank cases, respectively. Carriers of KLK1 and GGCX had a later mean age of onset and relatively moderate disease phenotype compared to BMPR2 carriers. Both KLK1 and GGCX, expressed in the lung and vascular tissues, play an important role in vascular haemodynamics and inflammation. Whilst Zhu et al. [25] identified KLK1 and GGCX as new candidate risk genes for IPAH, suggesting new pathogenic mechanisms outside of the TGF-β/BMP signalling pathway, further research needs to be conducted to better understand these findings, especially in larger cohorts of similar phenotypic characteristics.
In a recent large-scale analysis utilising the 13,037 participants enrolled in the NBR study, of which 1148 patients were recruited to the PAH domain, we discovered KDR as a novel PAH candidate gene [24,30] utilising the Bayesian model comparison method, BeviMed [64], and deep phenotype data. Under an autosomal dominant mode of inheritance, high impact variants in KDR were associated with a significantly reduced KCO (transfer coefficient for carbon monoxide) and older age at diagnosis [30]. Six ultra-rare high impact variants in KDR were identified in the study cohort; four of these were in unrelated PAH cases, one in a relative and one nonsense variant was identified in a non-PAH control subject. The latter variant appeared late in the protein sequence and hence might not impair protein structure. To seek further evidence for KDR as a new candidate gene for PAH, we analysed subjects recruited to two cohorts with similar phenotypic characteristics (US PAH Biobank: n = 2572; Columbia University Medical Center: n = 440); four additional individuals harbouring rare high impact KDR variants were identified, with one variant identified in both cohorts. A combined analysis of both cohorts confirmed the association of KDR with PAH. Further evidence came from a French cohort of 311 PAH patients prospectively analysed by targeted panel sequencing (which included KDR) [117]. Two index cases with severe PAH, from two different families, were found to carry LoF mutations in KDR, providing further genetic evidence for considering KDR as a newly identified PAH-causing gene. Across both studies [30,117], there are now three reported familial cases with a distinct phenotype in which LoF variants in KDR segregate with PAH and significantly reduced KCO.
Power to detect novel genotype–phenotype associations can be increased by merging existing datasets, aligning sequences to the most recent genome assembly, using the most up to date reference variome, and improved variant classification tools, as well as updated clinical phenotypes. Such an approach was recently taken by a large international consortium of 4241 PAH cases from three cohorts (US PAH Biobank: n = 2572; Columbia University Medical Center: n = 469; NBR: n = 1134). Of the available 4175 sequenced exomes, most cases (92.6%) were adult-onset, with 54.6% IPAH, 34.8% APAH, 5.9% FPAH and 4.6% other; 74.5% of the cohort was European [118]. Gene-based case-control association analysis in unrelated participants of European ancestry was performed, using 2789 cases and 18,819 controls taken from the Simons Foundation Powering Autism Research for Knowledge (SPARK) cohort and gnomAD, before screening the whole cohort, including non-Europeans, for rare deleterious variants in associated genes. Statistical analyses revealed that rare predicted deleterious variants in seven genes were significantly associated with IPAH, including three established PAH risk genes (BMPR2, GDF2, and TBX4), two recently identified candidate genes (SOX17 and KDR) and two new candidate genes (FBLN2 and PDGFD).
Both new candidate genes have known functions in vasculogenesis and remodelling but have not previously been implicated in PAH. In total, they identified seven cases with FBLN2 variants and ten cases with PDGFD variants, accounting for 0.26% and 0.35% of IPAH cases, respectively; most of these were of European ancestry and all were adult-onset, except for one paediatric PDGFD variant carrier. Analysis of single-cell RNAseq data showed that FBLN2 and PDGFD have similar expression patterns to well-known PAH risk genes [118]. Whilst this provides additional support and mechanistic insight for the new genes, as with the discovery of KLK1 and GGCX, variants within FBLN2 and PDGFD require independent validation.
Autosomal Recessive Mode of Inheritance
PVOD/PCH has recently been reclassified as an ultra-rare form of Group 1 PAH [18]. PVOD and PCH often show significant phenotypic overlap. Indeed, 73% of patients diagnosed with PVOD are found to have capillary proliferation and 80% of patients with PCH demonstrate typical venous and arterial changes [204], and are, therefore, referred to as PVOD/PCH. Clinically, PVOD/PCH is characterised by early-onset, significantly reduced DLCO and patchy centrilobular ground-glass opacities, septal lines and lymph node enlargement seen on high-resolution computed tomography. The disease outcome is dismal, with rapid progression and frequent pulmonary oedema in response to PAH medication. Similarly to PAH, PVOD/PCH can present as either a sporadic or familial disease [205,206]. It was the latter that triggered a family-based study into the genetic basis of this condition. Familial linkage mapping, WES, and Sanger sequencing were employed and identified biallelic EIF2AK4 mutations in affected siblings. Subsequently, biallelic EIF2AK4 mutations were also identified in 25% of sporadic PVOD cases [109], 11.1% of HPAH cases (one of nine cases) [110] and 1.04% of I/HPAH cases (nine of 864 cases) [111]. Harbouring EIF2AK4 mutations confer a poor prognosis irrespective of clinical diagnosis and importantly, radiological assessments were unable to distinguish reliably between PVOD/PCH patients and patients with IPAH [111].
4.3.2. Common Genetic Variation
Complex pathobiology, low penetrance, heterogeneous phenotype and variable disease trajectory allow for common sequence variation that contributes to PAH risk and natural history. In HPAH, several common genetic variants were shown to impact the disease. Firstly, BMPR2 mutations are a significant source of sex-related bias in disease penetrance (42% in females vs 14% in males) [122]; one explanation for this could be gene polymorphisms involved in estrogen metabolism [207]. Females harbouring deleterious variants in BMPR2 show a significant reduction in CYP1B1 gene expression and as a consequence, a lower 2-hydroxyestrone to 16α-hydroxyestrone ratio leading to activation of mitogenic pathways [207]. Moreover, direct estrogen receptor α binding to BMPR2 promoter results in reduced BMPR2 gene expression in females and may contribute to the increased prevalence of PAH [208]. Secondly, variation in BMPR2 expression in HPAH, caused by nonsense-mediated decay positive (NMD+) BMPR2 mutations, is conditional upon individual polymorphisms of the wild type (WT) allele [209]. Thirdly, a SNP in TGFβ1 modulates age at disease onset in patients harbouring BMPR2 mutations [210].
In IPAH, a SNP (rs11246020) in the Sirtuin3 (SIRT3), mitochondrial deacetylase, in either homozygote or heterozygote fashion, was associated with increased acetylation of mitochondrial proteins compared to the IPAH patients or disease comparator group with the WT genotype [166]. SIRT3, by deacetylating and thus activating multiple enzymes and electron transport chain complexes, plays a significant role in cell bioenergetics, and its polymorphism has been associated with susceptibility to metabolic syndrome [211] and IPAH, but not APAH [166]. Similarly, uncoupling protein 2 (UCP2), shown to conduct calcium from the endoplasmic reticulum (ER) to mitochondria and suppressing mitochondrial function, has been implicated in the pathogenesis of PAH [168]. These findings have important therapeutic implications as common variants in SIRT3 and UCP2 predicted response to dichloroacetate, pyruvate dehydrogenase kinase inhibitor, in a PAH phase 2 clinical trial [167]. Renin–angiotensin–aldosterone (RAA) system has been implicated in the pathogenesis of both systemic and pulmonary hypertension. For example, polymorphisms in the gene encoding angiotensin-converting enzyme (ACE) have been associated with IPAH [212] and with diaphragmatic hernia with persistent pulmonary hypertension [213]. Likewise, common variation in angiotensin II type 1 receptor (AGTR1) was associated with age at diagnosis in PAH. These findings indicate that RAA might be a therapeutic target [34].
Considering the important role of vasoactive, angiogenic and angiostatic substances in the pathogenesis and therapy of PAH, studies investigating the impact of common variation on gene expression of these substances are warranted. Recently, Villar et al. [170] found a recurrent SNP (rs397751713) in the promoter region of the endothelin-1 (END1) gene that had important regulatory consequences in both IPAH and APAH patients; rs397751713, which consists of an adenine deletion, allows transcription factors (Peroxisome proliferator-activated receptor γ (PPARG) and Kruppel Like Factor 4 (KLF4)) to bind to the promoter. Both of these transcription factors are linked to PAH pathogenesis and have been suggested as potential therapeutic targets [214,215,216,217]. In a similar fashion, a polymorphism in a potent angiostatic factor, endostatin, Collagen Type XVIII α 1 Chain (Col18a1), was studied. A SNP (rs12483377) in Col18a1 was observed at an increased frequency in PAH patients (MAF 21.6) relative to published controls (MAF 7.5), or patients with scleroderma without PAH (MAF 12.5). Rs12483377 encodes uncharged amino acid asparagine (N) at residue 104 in place of negatively charged aspartic acid (D); carriers of single minor allele A (genotype AG), had lower serum endostatin levels than those who carried WT (genotype GG) and showed better survival even after adjusting for confounders [173].
Along with studies looking at polymorphisms in a particular gene or its promoter region, two GWAS have been carried out in PAH. The first consisted of a discovery and validation cohort totalling 625 patients (sans BMPR2 mutations) and 1525 healthy individuals. A genome-wide significant association was found at Cerebellin 2 Precursor (CBLN2) locus; rs2217560-G allele was more frequent in cases than in controls in both the discovery and validation cohorts with a combined odds ratio (OR) for I/HPAH of 1.97 [95% CI 1.59;2.45] (p-value 7.47 × 10−7). Although the rs2217560 genotype did not correlate with CBLN2 mRNA expression in monocytes, higher CBLN2 mRNA levels were seen in lung tissue explanted from PAH patients than in control lung samples. Further analysis showed that increasing concentrations of CBLN2 lead to inhibition of proliferation of PASMCs [165].
To date, the largest PAH GWAS of multiple international I/HPAH cohorts, totalling 2085 cases and 9659 controls, identified two novel loci associated with PAH: an enhancer near SOX17 (rs10103692, OR 1.80 (95% CI 1.55;2.08), p = 5.13 × 10–15), and a locus within HLA-DPA1/DPB1 (rs2856830, OR 1.56 (95% CI 1.42;1.71), p = 7.65 × 10–20) [116]. CRISPR-mediated inhibition of the enhancer reduced SOX17 expression; this finding corroborates and extends the previous discovery of the association of rare variants in SOX17 with PAH [24]. The HLA-DPA1/DPB1 rs2856830 genotype was not only associated with I/HPAH but also with a beneficial effect on survival (CC genotype 13.5 vs TT genotype 6.97 years) irrespective of baseline disease severity, age and sex [116]. The latter study did not replicate previous results at genome-wide significance.
4.4. Limitations, Challenges and Future Directions
The major limitations in PAH genetic studies originate from either their study design or the genetic methods used. The former is mostly limited by sample size and population structure and features in GWAS and RVAS alike, the latter is impacted by sequencing technology, genome assembly and analysis pipelines. Additionally, the variable quality of reporting may obscure the validity of the results. Several approaches may accelerate causal variant discovery. Firstly, international efforts to set up platforms and governance, thereby reducing the barriers to genotype and phenotype data harmonisation, across studies and countries, along with regulatory standards, compatible with the international legislative landscape, aid variant discovery. Secondly, the application of computational and/or intermediate phenotypes may further increase power to detect new risk genes. Likewise, researching isolated populations resulting from recent bottlenecks (i.e., Icelanders, Ashkenazi Jews) have shown promising results in other rare diseases. As for genetic methods, long-read technologies will improve significantly and with improvements to the error rate, long-read alignment to a pan-genome graph may identify additional genetic variants. Expansion of resources for allele frequency estimation (i.e., gnomAD), encompassing diverse ethnicities and variant classes, including structural variants, will improve variant interpretation. Studying gene sets that are likely to be enriched for disease-associated loci (i.e., implicated by GWAS, or expressed in disease relevant tissues) and extending RVAS to non-coding regions (a natural step given GWAS findings) is likely to further increase discovery yield.
5. Reverse Genetics
5.1. Concepts
Reverse genetic techniques have been used extensively in the context of PAH. These experimental approaches involve targeted modifications to specific genes in order to analyse phenotypic impact (Figure 1, process 3) and are critical in validating in silico predictions. Most deleterious mutations found in PAH patients are LoF, suggesting a knockout or knockdown approach to analysing the phenotypic effect of a mutation. The main benefit of direct gene mutagenesis is the permanence and potentially ubiquitous effect of that alteration. In comparison, gene knockdown is a transient change that can be created after the developmental stage in the animal life cycle. This is particularly useful for understanding LoF in genes that are embryonically lethal [123]. Conditional and inducible systems are also excellent means of circumventing this issue, permitting temporally and spatially controlled gene disruption [55,125,128,218]. Inducible systems display higher efficiency and limited side effects compared to stably-expressed mutations and have the added benefit of reversibility [219].
5.2. Methodology
Techniques for genetic modification have permitted targeted genetic analysis using in vitro and in vivo models of PAH. Modification of a specific gene, through mutagenesis or knockout, can be carried out using a wide range of tools, including Zinc-finger nucleases (ZFNs) [220], transcription activator-like effector nucleases (TALENs) [221], and CRISPR/Cas9 [222]. Similarly, transient genetic changes can be achieved through gene expression knockdown, such as RNAi [223], or using conditional/inducible expression systems, such as Cre/LoxP and Flp/FRT sites or the Tet inducible system [224]. Generating models with specific mutations is also critical for the identification of therapeutic targets and drug testing. Specific techniques and their utility in animal models have been extensively reviewed [224,225,226].
5.2.1. In Vitro
As PAH is a disease of the pulmonary circulation, most studies seek to model genetic changes in physiologically relevant cell lines. This includes pulmonary artery endothelial cells (PAECs), PASMCs, blood outgrowth endothelial cells (BOECs) [227] and human umbilical vein endothelial cells (HUVECs). PAH is characterised by molecular and cellular deviations in pulmonary vascular function [228,229], including hyperproliferation [124,129,130,149], impaired migration [127,130,150] and aberrant apoptosis [124,230]. Traditionally, research has focused on using 2D cellular monolayers for analysis of these features; however, with rapidly evolving technologies, future analysis may utilise multi-layer, 3D models such as organoids [231] and hydrogels [232] to better replicate the pathobiology of PAH.
5.2.2. In Vivo
Understanding the pathobiology of PAH is dependent upon robust animal models for functional analysis and exploration of potential therapeutics. To achieve this aim, a wide range of animal models have been developed across species, including pig, sheep, dog and most commonly rodents [233].
The most widely accepted rat models for PAH are pharmacologically induced, namely monocrotaline (MCT) and Sugen 5416-hypoxia (SuHx) models [234]. MCT is a toxin that induces damage to PAECs, causes RV and pulmonary arterial medial hypertrophy, and alters pulmonary artery pressures [235,236]. In the SuHx rat model, SU5416, a Vascular Endothelial Growth Factor Receptor (VEGFR) antagonist that promotes endothelial cell apoptosis and smooth muscle cell (SMC) proliferation [237], is administered followed by a period of hypoxia [234]. SU5416 requires hypoxia to induce severe PAH, causing vascular changes that model human IPAH [237]. Both models induce non-PAH related symptoms, including alveolar oedema, pulmonary vein occlusion, acute lung injury, liver toxicity and emphysema [238,239].
The chronic hypoxia mouse model is also widely used in PAH research, with mice housed at 10% oxygen for a variable length of time [234,240,241]. While mice display changes in pulmonary artery pressure, they display limited vascular remodelling, which consists of muscularization of vessels and medial hypertrophy of muscular resistance vessels [242]. Alternatively, pulmonary artery banding (PAB) has been used in various species to increase pulmonary artery pressures and induce RV hypertrophy. The main benefit of this technique is the lack of pharmacologic or environmental modification of the animal [243]. In practicality, the popularity of each model varies across PAH research groups; a recent poll showed that two-thirds of groups used two or more models [244], suggesting that no one model truly replicates the disease.
In comparison, genetic models tend to be more favourable as they are free from the systemic effects of pharmacological models. Several heterozygous BMPR2 mutant models have been developed. While some models have shown elevated mPAP and PVR compared to WT littermates [123], others did not replicate these findings [245,246]. Alternate studies have shown the development of pulmonary vascular lesions [126] and often, an environment hit may be required for development of PAH [131,245,246]. More pronounced phenotypes are seen in homozygous deletions; as BMPR2 is critical for embryogenesis, conditional deletion in pulmonary endothelial cells has been used and showed incomplete penetrance of the mutation, with a subset of mice displaying elevated RVSP, RV hypertrophy and inflammation [125].
The following genetic models have also been developed: KDR (cell-specific conditional deletion of Kdr in mice) [155], AQP1 (Aqp1 null mice [151] and Aqp1 with a COOH-terminal tail deletion [149]), TET2 (conditional, haematopoietic heterozygous and homozygous deletion in mice, generated using the Vav1-Cre system) [55], BMPR1a (several conditional knockout mice models) [156,157,159], SOX17 (conditional deletion in mice, using Demo1-Cre in descendants of the splanchnic mesenchyme) [153], UCP2 (knockout mouse model created using a plasmid vector carrying modified Ucp2) [169], CAV1 (modified mouse cav-1 targeting construct) [138], EIF2AK4 (modified mouse Gcn2 targeting construct) [164], SIRT3 (heterozygous and homozygous mice, generated using targeted embryonic stem cell clones harbouring a mutated SIRT3) [166], ATP13A3 (generation of a protein truncating mutation in mouse Atp13a3 using CRISPR/Cas9) [147], SMAD9 (Disrupted Smad8 allele generated in mice using LoxP/cre and Frt sites) [134], KCNK3 (heterozygous and homozygous rats harbouring a CRISPR/Cas9-generated exonic, inframe deletion) [146] and SMAD1 (mice with Smad1 deletion in endothelial cells or SMCs, generated using L1Cre or Tagln-Cre, respectively) [136].
In humans, the severity of disease corresponds to the extent of pulmonary vascular changes and impairment of RV function. However, rodent models may display milder and isolated symptoms of PAH and, thus, require thorough characterisation to validate the model. Echocardiography is useful for the assessment of RV function but estimates of pulmonary pressures are inferior to direct measurements obtained through RHC [234]. Alternatively, cardiac magnetic resonance imaging is another noninvasive tool for assessment of RV function; it supersedes echocardiography in its ability to produce high quality, three-dimensional images from which the RV can be directly measured. However, it also has limited availability and bears higher running costs [247]. Close-chested RHC is preferred over open-chested RHC, as it is less invasive and preserves the negative intrathoracic pressure associated with breathing. Open-chested RHC is usually a terminal procedure, thus preventing longitudinal assessment of rodents; however, it also permits a more comprehensive assessment of haemodynamic characteristics and analysis using pressure–volume loops. Tissue harvesting permits further characterisation of the heart and lungs, including evaluation of RV hypertrophy [234].
It is important that measures are taken to recognise and limit bias in experimentation and phenotyping. This includes ensuring randomisation of groups when carrying out tests that require a control and treatment group, such as selecting mice to be pharmacological models or therapeutic treatment. Additionally, mice should be matched for all possible qualities, including strain, age and sex. During phenotype assessment, researchers should aim to avoid implicit bias by using blinded observers during all procedures. Furthermore, the data collected must be comprehensive and relevant for PAH, including RHC data and histological analysis [248].
5.3. Studies
5.3.1. Rare Genetic Variation
Autosomal Dominant Mode of Inheritance
TGF-β Family
BMPR2, a receptor in the TGF-β pathway, is the most commonly mutated gene in PAH. BMP ligands induce signalling through binding with BMPR2 and ALK 1, 2, 3 or 6 to induce downstream SMAD-mediated transcription [249]. This pathway plays important roles in cell proliferation, differentiation, migration, apoptosis and inflammation, which are often dysregulated in PAH. Alongside BMPR2, several pathway components have been implicated in disease; this includes the ALK and SMAD proteins, as well as coreceptors, such as endoglin [250]. Reverse genetics techniques have been used extensively on these genes to understand their role in PAH and their interaction with other members of the pathway. BMPR2 has been extensively analysed using the methods described; under normal conditions it is highly expressed in vascular ECs, with lower levels of expression in SMC; however, reduced levels have been reported in the lungs of PAH patients [181]. In the endothelium, BMP9 and BMP10 induce binding of BMPR2 and ALK1, along with co-receptor endoglin, to induce downstream signalling. Such signalling is often disrupted in HPAH patients that harbour mutations in BMPR2 [251]. However, disruption of BMP-mediated signalling is also apparent in IPAH patients, despite the absence of BMPR2 mutation [252].
Gene disruption and knockout techniques have been key to elucidating the role of BMPR2 in pathogenesis. Such techniques have revealed dysregulation of endothelial nitric oxide synthase (eNOS) in cell culture models of PAH; siRNA-mediated knockdown of BMPR2 induced endothelial dysfunction in human PAECs, caused by a reduction in BMP2/4-mediated production of the vasodilator nitric oxide by eNOS [127]. In addition, reduced BMPR2 has been associated with overactive glycolysis [130] and hyperproliferation [124].
Furthermore, a key feature of PAH is incomplete penetrance in BMPR2 mutation carriers. This has been replicated in mice carrying conditional knockout mutations of Bmpr2 in pulmonary endothelial cells, with only a proportion of mice developing haemodynamic symptoms of PAH [125]. In addition, it has been shown that BMPR2 mutations disrupt RV function, with heterozygous mice displaying impaired hypertrophy and lipid accumulation in the RV [128]. Moreover, EndoMT has been suggested to be a characteristic of PAH in both animal models and patients [253,254]. This includes a heterozygous BMPR2 mutant rat model, which displayed overexpression of two molecular markers of EndoMT. Such changes were also seen in PAH patients when analysing mRNA and protein level changes in lung and artery tissue [129]. These in vivo models have therapeutic utility, having been used to test treatments that include rapamycin [129] and metformin [128] to alleviate symptoms.
The experimental techniques discussed have been highly important in identifying other members of the TGF-β pathway that are implicated in PAH pathogenesis. This includes the Smad proteins, for which functional evidence is limited. Functional analysis of variants detected in SMAD1, SMAD4 and SMAD9, have shown impaired signalling in Smad4 and Smad9. However, variants only have a moderate impact on phenotype, suggesting further genetic or environmental hits may be required to induce PAH [54]. Furthermore, analysis of CAV1 mutations have revealed impaired protein trafficking in mouse embryonic fibroblasts carrying a disrupted copy of CAV1. Furthermore, patient fibroblast cells displayed reductions in both CAV1 and associated accessory proteins [137]. Thrombospondin 1 (THBS1) mutants, which regulate TGFβ function, have been found to have impaired ability to activate TGFβ, and permit proliferation of PASMCs. Additionally, an electrophoretic mobility shift assay (EMSA) was used to analyse the binding of biotin labelled oligonucleotides with two transcription factors, MAZ and SP1, showing a reduction in efficiency [174].
As previously discussed, GDF2, encoding the ligand BMP9, is critical in BMPR2-mediated signalling in endothelial cells. Functional studies are particularly important in validating in silico predictions regarding the pathogenicity of certain variants. Hodgson et al. [115] analysed seven missense mutations in GDF2, which had been previously associated with PAH and predicted pathogenic in silico. When analysed in vitro, all variants displayed dysfunctional processing, secretion or protein stability. In addition, functional studies found a predicted benign mutation to be deleterious. Similarly, association analysis in IPAH patients of Chinese Han ethnicity identified GDF2 mutations [26]. Six mutations were selected for functional studies, using mutant GDF2 plasmids transfected into HEK293E cells/PAECs. Mutants displayed reduced BMP9 levels, reduced BMP activity and increased apoptosis [26].
There is some evidence implicating BMPR1A, which encodes the type I receptor ALK3, in PAH. A hypoxia mouse model harbouring a mosaic deletion of Bmpr1a, generated through conditional deletion of the gene in SMCs using the TRE-Cre system, displayed proximal artery stiffening caused by excess collagen. This may lead to constricted vessels and impairment of right ventricular function [255]. Furthermore, two independent studies have implicated reduced Bmpr1a expression with EndoMT and shown that the phenotype may be rescued by attenuating expression of TGFBR2, which is upregulated in the mice models [156,157]. Additionally, functional analysis of BMPR1B mutations found in PAH patients found changes in transcriptional activity, which resulted in increased SMAD8 phosphorylation [160].
Channelopathies
Alongside TGFβ-family members, various channel proteins have been linked to pathogenesis. Functional analysis was conducted on six mutations reported in KCNK3, all of which resulted in LoF. Patch clamp variants showed a reduction in current, with rescue of channel function, in a subset of variants by administration of a phospholipase inhibitor, ONO-RS-082 [107]. In addition, Bohnen et al. [145] introduced a heterozygous, single amino acid variant into KCNK3 using site-directed mutagenesis and transfected hPASMCs with this mutant via a pcDNA3.1 plasmid. The physiological impact of this mutation was assessed using whole-patch clamp tests to observe changes to membrane potential and current, with experiments demonstrating loss of channel function in mutant cells compared to WT KCNK3. More limited evidence is available for the role of ATP13A3, a P-type ATPase, in PAH; siRNA-mediated knockdown of expression resulted in a reduction in cell proliferation and increased apoptosis, as well as the loss of endothelial integrity in hPAECs [230]. Furthermore, mutant ATP13A3 mice, carrying a protein-truncating variant introduced using CRISPR/Cas9, demonstrated haemodynamic measurements indicative of PAH. This included reduced pulmonary artery acceleration time (PAAT) and increased RVSP, with rats also exhibiting reduced polyamine levels in their lungs [147].
AQP1 has been associated with cell migration and vascularity. AQP1 is highly expressed in microvascular endothelial cells, vascular smooth muscle cells and non-vascular endothelium [256], and has recently been proposed as a novel promoter of tumour angiogenesis [257]. A recent study demonstrated that depletion of AQP1 reduced proliferation, migratory potential, and increased apoptosis of PASMCs [258]. Saadoun et al. [150] produced an AQP1 knockout mouse model using targeted gene disruption; when implanted with melanoma cells, the developing tumours displayed impaired angiogenesis. In-depth analysis of APQ1 function in endothelial cells, produced from mouse aortic cells, using cell migration, wound healing, proliferation and cord development assays, revealed critical roles of AQP1 in cell migration. AQP1 null cells showed reduced ability in all four assays. This was corroborated by the plasmid expression of AQP1 and another water channel protein, AQP4, in non-endothelial cells, resulting in increased migration. Functional analysis has confirmed the role of AQP1 in cell migration under hypoxic conditions. Yun et al. [149] used rat pulmonary-artery-derived PASMCs, infected with adenovirus vectors carrying either WT AQP1 or AQP1 with a 37 amino acid terminal deletion removing the COOH tail. Overexpression of AQP1 increased levels of β-catenin and its downstream targets, including c-Myc and cyclin D1. However, loss of the COOH tail produced no such results, suggesting COOH-mediated upregulation of β-catenin. Both AQP1 and β-catenin displayed elevated levels under hypoxic conditions. This was further corroborated by siRNA-mediated silencing of β-catenin, in cells subject to hypoxia, and consequent infection with adenoviral constructs. Cells were rescued from the migration and proliferation associated with hypoxic conditions.
Additionally, functional analysis has been conducted on ABCC8. Eight mutations were analysed in COS cells using patch-clamp tests and rubidium flux assays, as a measure of ion efflux. Loss of channel function was seen in all variants, with the rescue of function by the administration of the SUR1 activator drug, diazoxide [113]. Additionally, functional analysis of KCNA5, by overexpressing the gene in PASMCs, showed an increase in channel current that was rescued by administering either nicotine, bepridil, correolide or endothelin-1 [163]. Additional genes have been reported, but have limited functional evidence confirming their role in PAH. One such gene is UCP2, encoding uncoupling protein 2, which functions as a calcium channel in vascular mitochondria that mediates ion influx from the ER. Fluorescence resonance energy transfer imaging of mitochondria from resistance pulmonary arteries of mice harbouring a Ucp2 knockout showed a reduced calcium concentration, impairing their function, and mimicking the effects of hypoxia. Impaired mitochondrial function has been associated with the hyperproliferative and anti-apoptotic symptoms seen in PH [168]. Another channel protein is TRPC6. Using Western blot analysis, TRPC6 was found to be upregulated in PASMCs obtained from IPAH patients, compared to control cells. The endothelin receptor antagonist, Bosentan, was found to act by downregulating TRPC6 and had a more pronounced anti-proliferative effect on IPAH cells than on WT cells [259]. Alternatively, potassium channel function has been rescued using adenoviral infection of human Kv1.5, into mice exposed to chronic hypoxia for three to four weeks. Mice were alleviated of hypoxia-induced vasoconstriction, displaying reduced right ventricle and pulmonary artery medial hypertrophy, when compared to control mice subject to hypoxia [260].
Transcription Factors
TBX4 is an evolutionarily conserved transcription factor, which plays a critical role in limb development during embryogenesis [261]. Detection of a lung-specific Tbx4 enhancer, specific to the lung mesenchyme and trachea during embryogenesis, suggest an important role in lung development [262]. It has been shown to induce myofibroblast proliferation and drive invasion of the matrix by fibroblasts, promoting the development of pulmonary fibrosis, and deletion of Tbx4 has led to a reduction in fibrosis [262]. TBX4 plays a role in lung development and has been linked to pulmonary fibrosis, suggesting a possible role in PAH; nonetheless, experimental evidence is required to confirm this. Similarly, SOX17 has been shown to be critical for the development of pulmonary vasculature. A mouse model, harbouring a conditional deletion of Sox17 in splanchnic mesenchyme descendants, displayed impaired pulmonary vascular development leading to death by three weeks of age [153]. Shih et al. [154] assessed the role of SOX17 using siRNA-mediated knockdown of expression in PAECs, followed by angiogenesis assays that showed impaired vessel formation. Concurrently, a mutant SOX17 cell line, generated through CRISPR/Cas9 mutagenesis of human iPSC, showed a reduction in expression of arterial genes compared to WT cells.
New Genes
More recently, a plethora of new genes have been found to harbour mutations in PAH patients; however, functional evidence for their involvement remains limited. TET2, involved in DNA demethylation, is a key epigenetic regulator and has been found dysregulated in PAH. Potus et al. [55] developed heterozygous and homozygous Tet2 knockout mice models, by crossing floxed Tet2 parents and Vav1-cre to excise the gene. Their phenotype was ascertained through echocardiography and RHC. Patients carrying TET2 mutations were found to have a later age of onset compared to other PAH patients; this finding was replicated in mice. Genetic evidence has also implicated KDR in PAH; Winter et al. [155] developed a mouse model with a conditional deletion of the KDR gene, encoding VEGFR2, in endothelial cells. The mice developed mild PAH symptoms under normoxic conditions; however, these were exacerbated under hypoxia with mice displaying pulmonary vascular remodelling.
Other genes which have been associated with PAH through sequencing analysis have no functional evidence with respect to PAH; however, physiological functions reveal possible mechanistic roles in the disease. GGCX has also been associated with PAH using forward genetics studies but lacks any functional evidence to corroborate this. This gene encodes a protein that carboxylates glutamate residues on Vitamin-K-dependent proteins; these are vital for the activation of coagulation proteins [263], inhibiting vascular calcification and inflammation [25]. Mutations in GGCX have been associated with Vitamin-K-dependent clotting factors deficiency, a congenital bleeding disorder [264] and Pseudoxanthoma elasticum [265].
Additionally, KLK1 plays a key role in cardiac and renal function, notably regulating blood pressure. Kinins are known to affect endothelial cells, especially playing a role in vasodilation, increasing vascular permeability, nitric oxide production and inflammation. Tissue kallikrein is highly expressed in the kidney, pancreas, central nervous system and blood vessels [266]. Kinins, which include bradykinin, are regulated by inactivating enzymes known as kininases, of which the ACE is the most well known [267]; ACE-inhibitors are effectively used to reduce blood pressure [268]. Indeed, hypertension has been associated with polymorphisms and deficiencies in KLK1 expression in rat models [269,270]. While genetic variation in KLK1 has been associated with essential hypertension in a Chinese Han cohort [271], other studies have reported conflicting evidence on its role in the condition [268]. Nonetheless, this gene and pathway offer potential therapeutic targets, having major functions in areas of dysfunction seen in PAH, demonstrated by the evidence showing that KLK1 can be used for its vasodilative properties in the treatment of acute ischemic stroke [272].
Autosomal Recessive Mode of Inheritance
Anthony et al. [273] demonstrated that homozygous Eif2ak4 knockout mice displayed increased levels of protein carbonylation, a symptom of oxidative damage that was induced by dietary leucine deprivation. Additionally, these mice exhibited reduced viability compared to controls, with some dying shortly after birth. Their findings suggest that the gene may elicit protective effects against oxidative damage.
5.3.2. Common Genetic Variation
Another interesting PH animal model is the Sirt3 knockout mice, which displays higher mPAP, PVR, RV hypertrophy and reduced exercise tolerance, compared with controls. This gene has been implicated in IPAH, with reduced expression in hPASMCs obtained from patients. The severity of symptoms in the model occur in a dose dependent manner, with Sirt3 heterozygous mice displaying milder symptoms than homozygous knockout mice. This includes the extent of muscularisation and medial wall thickness of resistance PAs, which is more severe in homozygotes [166]. However, another Sirt3KO mouse model, against a C57BL/6 background [274] failed to develop PH, which may be attributed to differences between the mouse strains used. The 129/Sv strain used by Paulin et al. [166] possess a slower metabolism compared to C57BL/6 mice, which may mean loss of Sirt3 is more damaging than in the latter strain.
Mutations in vasodilatory and vasoconstrictive proteins have been implicated in PAH, including END-1. In situ hybridisation has shown END-1 upregulation in the lungs of patients with PAH, with greatest abundance in vessels subject to disease-associated remodelling [171]. Furthermore, analysis of a SNP found in the reporter region of END-1 using a luciferase assay has shown upregulated activity. Homozygous mutants display a 30% increase in promoter activity and loss of binding of KLF4 and PPAR γ, which have both been linked to PAH [170].
Common variation in the DNA Topoisomerase II Binding Protein 1 (TopBP1) has been found in IPAH patients. Immunohistochemical analysis of vascular lesions demonstrated a reduction in TopBP1 protein. Analysis of cultured pulmonary microvascular endothelial cells from IPAH lungs, using q-PCR and Western blotting, showed a reduction in TopBP1 mRNA and protein. Further analysis of cells for increased susceptibility to DNA damage, by administering hydroxyurea and staining nuclei for phosphorylated histone foci, revealed an excess of DNA strand breaks and apoptosis. However, patient cells could be rescued by transfection with a plasmid containing wild-type human TopBP1, displaying a reduction in DNA damage-induced apoptosis [172].
In addition, functional analysis has been completed in SOX17. A luciferase reporter construct was generated for each haplotype of four heterozygous SOX17 mutations and transfected into hPAECs to assess transcriptional activity [116,275]. Comparison of each haplotype revealed a haplotype-specific difference in promoter activity in a subset of variants. Two variants showed a statistically significant decrease in promoter activity in the risk allele, compared to the non-risk allele. Evidence exists for the intricate function of the various genes implicated in PAH, including regulatory roles of SOX17. VEGF has been shown to upregulate SOX17, while the Notch pathway plays critical roles in downregulating Sox17 in the endothelium. Additionally, there is a positive feedback loop between VEGR and SOX17 [275]. These findings support the evidence that a wide range of mutations across genes are capable of eliciting similar phenotypic impacts through large-scale changes to pathway regulation.
5.4. Limitations, Challenges and Future Directions
To conclude, reverse genetics techniques are vital components of PAH research, without which forward genetics’ discoveries cannot be validated. Nonetheless, it is important that such studies are interrogated for any limitations that may affect their results. As previously mentioned, it is important to have a robust experimental design and protocol, which includes power calculations for animal studies, minimising bias and reporting all measurements in publications. Cellular models are powerful in vitro tools for understanding specific processes in PAH; however, focusing on certain cell types may produce more pronounced effects than those seen in vivo. Understanding the widespread impact of PAH mutations are best captured using animal models. However, it is important to remember that all fail to fully recapitulate the disease and that any toxic side effects of pharmacological models may confound results. Studies must choose models which are most appropriate for the hypothesis, or use multiple where necessary. Furthermore, focusing specifically on rodent models, these often capture longitudinal data through the use of terminal procedures for haemodynamic measurements. The experimental techniques discussed here are well established in the field; however, several new tools have been developed which may increase the speed and depth of information derived using reverse genetics; these include high-throughput technologies for mutagenesis and spatial gene expression analysis [276,277]. Such developments offer exciting prospects for future research in PAH.
6. Reverse Phenotyping
6.1. Concepts
The concept of reverse phenotyping (Figure 1, process 4) refers to the use of genetic marker data to refine the disease subgroup definitions [36]. The method was first used in phenotyping patients with sarcoidosis [278] and has proven successful in many other diseases characterised by high heterogeneity [279].
6.2. Theoretical Basis
Heterogeneity provides a unique challenge in the diagnosis and treatment of rare diseases, and is further complicated by the accuracy of reporting. Knowledge about the genetic makeup of the individual allows targeted treatments, thereby enhancing efficacy and decreasing the risk of potential side effects [280]. Conversely, knowledge about the phenotypic characteristics of mutation carriers is indispensable to match study design and methodological approaches to disease attributes [281].
6.3. Methodology
Studying the complexity of genotype–phenotype associations is of particular interest to medical genetics. Evidence for such studies can be retrieved from published peer-review literature and publicly available databases, and facilitated by computational tools such as the R package VarformPDB (Disease-Gene-Variant Relations Mining from the Public Databases and Literature), which captures and compiles the genes and variants related to a disease, a phenotype or a clinical feature from public databases including HPO (Human Phenotype Ontology), Orphanet, OMIM (Online Mendelian Inheritance in Man), ClinVar, and UniProt (Universal Protein Resource) and PubMed abstracts. Alternatively, the development of large biobanks that link rich electronic health record (EHR) data and dense genetic information have made it possible to enhance clinical characterisation of mutation carriers in a cost-effective fashion. Besides deep clinical, often longitudinal phenotyping, multi-omic approaches can be employed thanks to the concurrent collection of patients’ biological samples. A number of efforts have been made to facilitate EHR-based genetic and genomic research, such as the establishment of the UK Biobank [282], the Electronic Medical Record and Genomics Network [283] or the Precision Medicine Initiative Cohort Program [284], which allow both forward genetics and reverse phenotyping applications. Mining EHRs for genetic research purposes possesses a number of advantages over classical population or family-based genetic association studies, large samples can be collected over a short period of time in a cost-effective fashion. Moreover, the robustness of EHRs allows for the investigation of a spectrum of phenotypes in a hypothesis-free manner. Familial relationships reported in EHRs have also been used for the estimation of disease heritability shown to be consistent with the literature [285].
Multiple tools are being used by the Clinical Genetics community to address the issue of reverse phenotyping when interpreting NGS data. This includes DECIPHER [286], a publicly-available database that houses a collection of case-level evidence for 36,801 individuals with genetic diseases, for comparison of phenotypic and genotypic data. Projects affiliated to DECIPHER can deposit and share patients, variants and phenotypes to invite collaboration and increase diagnostic yield. Another example (also accessible via DECIPHER) is that of Matchmaker Exchange [287] or Genematcher [288], for mining other databases based on matching genotype and phenotype data. VarSome is a data aggregator that encompasses platforms and bioinformatic tools with the aim of consolidating information required for variant analysis. VarSome draws information from 30 databases [289] and is also embedded into another web-based tool, VariantValidator, which enables validation, mapping and formatting of sequence variants using HGVS nomenclature [290].
Knowledge of age-dependent and/or reduced penetrance is vital in reverse-phenotyping as new clinical features may emerge in an individual over time. This is applicable to relatives of PAH patients found to carry presumed pathogenic variants in known disease-associated genes. A detailed evaluation of multiple family members alongside longitudinal follow-up of these individuals is vital to study the disease trajectory which has often higher definition than snapshot phenotyping at diagnosis.
Apart from mining existing databases and health records, recall by genotype (RbG) studies will be a relevant and valuable source of information regarding the biological mechanics of genotype–phenotype associations. By making use of the random allocation of alleles at conception (mendelian randomization—MR) these studies enhance the ability to draw causal inferences in population-based studies and minimise the problems related to confounders; additionally, the focus on phenotypic assessment of a specific carrier subgroup can enhance the understanding of pathobiology in a cost-effective manner. RbG studies can focus on a single variant or multiple variants. The former considers rare and large effect loci to understand the biological pathways of interest, and as a result, the groups are relatively small so the phenotype can be studied in detail, in the latter, genetic variation is used as a proxy of relevant exposure (proviso there is a credible association between genetic markers and exposure). To increase power for studies in which a single variant has a small effect size deployment of aggregate genetic risk scores can be beneficial [291].
6.4. Studies
The reversal of the usual hypothesis-driven paradigm for the refined diagnosis was first used by Grunewald and Eklund [278] who by deep clinical phenotyping of patients with Lofgren’s syndrome, showed that erythema nodosum predominantly occurs in women and bilateral periarticular arthritis in men, and that the acute sarcoidosis subgroup can be further characterized according to the presence of HLA-DRB1*0301/DQB1*0201. The reverse phenotyping method has been subsequently adopted in other disease domains (Table 4) and has led to new disease discovery, such as the Koolen–DeVries syndrome, due to a 17q21.31 microdeletion involving the KANSL1 gene [292], and the Potocki–Lupski Syndrome, due to a 17p11.2 microduplication [293,294]. Recently, a retrospective study of 111 patients with nephrotic syndrome, who underwent WES, showed that reverse phenotyping increased the diagnostic accuracy in patients referred with the diagnosis of steroid-resistant nephrotic syndrome [279].
Table 4.
Examples of successful studies that used reverse phenotyping. Abbreviations: BMPR2—Bone morphogenic protein receptor type 2; PAH—pulmonary arterial hypertension; WES—whole-exome sequencing, * denotes allele number.
| Gene | Condition | Study Design | Data Sources | Results | References |
|---|---|---|---|---|---|
| HLA-DRB1*0301/DQB1*0201 | Sarcoidosis | Mix of retrospective and prospective cases with acute onset sarcoidosis | Health records | Patients with acute onset sarcoidosis carrying HLA-DRB1*0301/DQB1*0201 genotype have good prognosis, manifestations of Lofgren’s syndrome differ between man and woman. | [278,302] |
| BMPR2 | PAH | Retrospective analysis of 169 PAH patients | WES & Health records | Patients with missense mutations that escape nonsense-mediated decay have more severe disease than those with truncating mutations. | [296] |
| BMPR2 | PAH | Retrospective analysis of 171 patients | Missense variants in the cytoplasmic tail appear to confer less severe phenotype than other BMPR2 variants with a later age of onset, milder haemodynamics, and more vasoreactivity. | [297] | |
| BMPR2 | PAH | A large individual participant data meta-analysis | Literature review | Patients harbouring deleterious BMPR2 mutations have earlier disease onset, worse haemodynamics, are less likely to respond to NO challenge and have lower survival when compared to those without BMPR2 mutations. | [180] |
| BMPR2 | PAH | Retrospective analysis of 44 PAH patients who underwent lung transplantation between 2005 and 2014 | Histology, immunohistochemistry, morphometry of explanted lungs; French registry database | BMPR2 mutation carriers are more prone to haemoptysis; haemoptysis is closely correlated to bronchial arterial remodelling and angiogenesis; pronounced changes in the systemic vasculature correlate with increased pulmonary venous remodelling, creating a distinctive profile in PAH patients harbouring a BMPR2 mutation. | [295] |
| multiple genes | Nephrotic syndrome | A retrospective analysis of all patients diagnosed with nephrotic syndrome between 2000 and 2018 | WES & personalised diagnostic workflow | Reverse phenotyping after WES increased the diagnostic accuracy in patients referred with the diagnosis of steroid-resistant nephrotic syndrome. | [279] |
The reverse phenotyping of BMPR2 mutation carriers has been studied in PH patients. A large individual participant data meta-analysis found that patients with BMPR2 mutations have earlier disease onset, worse haemodynamics, are less likely to respond to nitric oxide challenge and have lower survival when compared to those without BMPR2 mutations [180]. The histological analysis of lungs explanted from those patients also revealed a higher degree of bronchial artery hypertrophy/dilatation, which correlated with the frequency of haemoptysis at presentation [295]. Patients with missense mutations in BMPR2 that escape nonsense-mediated decay have more severe disease than those with truncating mutations, suggestive of a dominant-negative impact of mutated protein on downstream signalling [296]. However, missense variants in the cytoplasmic tail appear to confer less severe phenotype than other BMPR2 variants with a later age of onset, milder haemodynamics, and more vasoreactivity [297].
Other good candidates for reverse phenotyping are patients with a mutation in transcription factors, i.e., TBX4, which by virtue of gene function would be associated with a more complex and heterogeneous phenotype. Patients with mutations in TBX4 present with severe PAH associated with bronchial and parenchymal changes, low DLCO, with or without skeletal abnormalities [142], and bimodal age of onset [25]. Interestingly, the penetrance of TBX4 mutations for skeletal abnormalities is much higher than for PAH [108]. Descriptions of paediatric subjects with mutations in TBX4, and likely loci nearby including TBX2, characterised by skeletal dysplasias, developmental delay and hearing loss have been reported in CHD and PH patients [298,299]. Interestingly, the US PAH Biobank study confirmed a causative role of TBX4 not only across various age groups (12/266; 4.6%—paediatric-onset; 11/2345; 0.47%—adult-onset) but also across different PH phenotypes: 2 (one adult; one paediatric)/23; 8.7% PAH-CTD, 3 (two paediatric and one adult-onset)/23; 13% PAH-CHD [25]. Not all patients harbouring deleterious variants in TBX4 fit neatly into Group 1 PH, some individuals with heterozygous mutations in TBX4 or large deletions encompassing TBX4 were characterised by death in infancy secondary to acinar dysplasia [300], congenital alveolar dysplasia and pulmonary hypoplasia [301]. The studies dissecting phenotype by mutation type or looking for subtle features (i.e., SPS) among patients harbouring deleterious variants in TBX4 who were initially diagnosed with PAH are lacking.
Reverse phenotyping of patients with PTVs in KDR revealed mild interstitial lung disease in all index cases and one affected relative [30], which was further confirmed in an independent case report [117].
6.5. Limitations, Challenges and Future Directions
Reverse phenotyping has the potential to shed light on genotype–phenotype associations and accelerate the recruitment of homogenous, well-defined groups to clinical trials, thereby increasing the chances of effective treatment and decreasing the risk of side effects. Its success depends on the quality of forward phenotyping, and forward and reverse genetic studies accurately reporting the phenotype of interest (use of standardised vocabulary to describe phenotypic abnormalities (i.e., HPO terms), clinical measurements (i.e., Logical Observation Identifiers (LOINC) [303]) and systematic approaches to data sharing.
In the future, national and international efforts should be directed at optimising recruitment of large homogeneous cohorts for forward genetics studies and, simultaneously, preempting any potential ethical issues related to subsequent recall by genotype studies. Similarly, recall by genotype studies will face challenges related to specific study design, i.e., when recruiting newborns or children who might not express the full phenotype by the time of diagnosis/inclusion.
7. Missing Heritability in the Postgenomic Era
As already alluded to in the introduction, missing heritability is the proportion of phenotypic variance not explained by the additive effects of known variants. There are multiple sources of missing heritability beyond the rare and common sequence variation described above (Figure 2). In the following paragraphs, we will discuss studies which contribute to a better understanding of the genetic landscape in PAH, beyond germline, rare, and common sequence variation.
7.1. Unknown Genetic Variation
Recently, large case-control studies have allowed us to discover new genes involved in the pathogenesis of PAH; all these studies, however, assumed that PAH is a monogenic condition and examined only protein-coding space. Although this permitted the discovery of more than 16 genes associated with PAH (Table 3), and increased the diagnostic yield, most PAH cases remain unexplained, suggesting additional variation in non-coding regions. It is now known that the remaining 98% of non-coding space plays a crucial role in the regulation of gene expression and requires interpretation using its 3D structure that allows regulatory elements to function across long distances [304]. Regulatory elements such as enhancers, promoters and actively transcribed genes are located in open-chromatin regions so they can be accessed by transcriptional machinery. A number of methods for the identification of non-coding regulatory elements (NCRE) have been developed over the last 20 years [305]. A recent study investigated variation in non-coding regions by researching differences in chromatin marks and gene expression in PAECs from patients with I/HPAH and controls. Using ChiP-Seq profiling of the active histone marks (H3K27ac, H3K4me1, H3K4me3) and RNA-Seq, it was shown that while PAECs from PAH patients and healthy controls are similar in terms of gene expression, they undergo large-scale remodelling of the active chromatin regions, which leads to differential gene expression in response to external stimuli. Overall, this study not only validated and expanded our knowledge regarding the genes involved in the pathogenesis of PAH, but also highlighted that steady-state expression analyses are of limited value in systems where the mechanism involves an aberrant response to stimuli [306].
So far, mostly germline mutations have been implicated in the pathogenesis of PAH but the ever-increasing age of onset may suggest a role of acquired somatic mutations in disease development. While rare somatic mutations of the BMPR2 gene within pulmonary vasculature cells could cause or aggravate BMPR2 haploinsufficiency, previous work has failed to find this to be true among those with a germline BMPR2 mutation [307]. Conversely, a study by Aldred et al. [308] showed a high frequency of genetically abnormal subclones of PAEC from patients with PAH including one patient harbouring a deleterious BMPR2 variant. Similarly, Drake et al. [309] demonstrated that a somatic deletion on chromosome 13, encompassing SMAD9, in PAECs from a patient with PAH associated with CHD could have contributed to the pathogenesis of PAH, and potentially also to CHD [309]. Likewise, postzygotic mutations have been implicated in the pathogenesis of HHT [310] and HHT with concomitant PAH [311,312]. Somatic mutations in TET2 have recently been reported in PAH cases, and the phenotype was replicated in the mouse model [55].
Impaired bioenergetics is an established feature of PAH [313,314] whether its origins can be traced back to mitochondrial DNA (mtDNA) remains an open question, but a recent study revealed that mitochondrial haplogroups influence the risk of PAH and that susceptibility to PAH emerged as a result of selective enrichment of specific haplogroups upon the migration of populations out of Africa [315]. Studies into the role of mtDNA in PAH will require novel bioinformatics tools and variant prioritisation approaches to account for differences between nuclear and mitochondrial genomes [316].
7.2. Epigenetic Inheritance
Epigenetics are heritable changes in gene function that occur without a change in the sequence of DNA and include DNA methylation, histone modification and RNA interference. All three of these modifications have been shown to be involved in the development of PAH. Epigenetic changes can be heritable [317,318], de novo and may be modified by environmental factors such as diet, smoking, and drugs.
7.2.1. DNA Methylation
DNA methylation is a well-described form of epigenetic modification which involves the transfer of a methyl group to cytosine residues in dinucleotide CpG sequences of DNA resulting in gene silencing. In PAH, the best described is hypermethylation of the gene encoding superoxide dismutase 2 (SOD2), an enzyme involved in H2O2 regulation which also acts as a tumour suppressor gene. Hypermethylation of SOD2 leads to uncontrolled cell proliferation in multiple cancers; similarly reduced expression of SOD2 has been shown in the rat model of PH [319] and in IPAH [320]. Further evidence comes from a recent study which found rare germline and somatic mutations in the key regulator of DNA demethylation, TET2, in 0.39% of PAH cases [55]. The phenotype was replicated in the Tet-/- mouse and reversed with IL-1β blockade.
7.2.2. Histone Acetylation
Histone acetylation is the most common form of histone modification [321], with a proven impact on PAH development. In rat and human PH histone deacetylases, HDAC1 and HDAC5 concentrations are elevated and histone deacetylase inhibitor can reverse hyperproliferation of PASMCs and decrease mPAP and RV hypertrophy [322].
7.2.3. RNA Interference
A large number of non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs), miRNAs, circular RNAs (circRNAs), and piwi-interacting RNAs (piRNAs) are involved in the regulation of a variety of cellular processes. For example, so far, almost 27,000 lncRNAs have been identified in the human genome [323] and reported regulating gene expression via diverse mechanisms [324]. In PAH, both miRNA and lncRNAs have been studied and reported to impact cells relevant to PAH pathogenesis, including PASMCs, PAECs and fibroblasts. Regulatory effects of lncRNA in PAH range from enhanced proliferation of PASMCs (H19 [325], PAXIP-AS1 [326], MALAT1 [327], Inc-Ang362 [328], Tug1 [329], HOXA-AS3 [330], MEG3 [331], TCONS_00034812 [148], and UCA1 [332]), over suppressed proliferation and/or migration of PASMCs (MEG3 [333,334], CASC2 [335], and LnPRT [336]) to induction of EndoMT in PAECs (MALT1 [337] and GATA6-AS [338]). Significant causative links exist between multiple miRNAs and BMPR2 expression and signalling [339,340,341], as well as hypoxia [342]. Importantly, although attractive as a therapeutic target, miRNAs have not been shown to have the potential to reverse the disease, which is most likely due to the high degree of redundancy and “fine-tuning” rather than “switch on/off” mode of action of these molecules.
Although still limited, our knowledge of ncRNA in the pathobiology of cardiovascular diseases [343,344] including PAH, is rapidly growing. The major limitation of studies so far is that the focus has been mostly on identifying the role of preselected ncRNA with very limited effort to investigate ncRNA in a global manner accounting for multiple interactions.
7.3. Interactions
Although difficult to estimate, gene and environment covariation and gene-environment interactions may play a significant role in heritability, expressivity and penetration of the disease. The most commonly studied factors are drugs, diet, toxins, radiation and stress, and some of these factors have been implicated in the development of PAH. Definitive associations between PAH and drugs and toxins based on outbreaks, epidemiological case-control studies or large multicentre series have been confirmed for aminorexigenes, methamphetamines, dasatinib and toxic rapeseed oil, yet some others require further validation [18]. Interestingly, several studies reported that BMPR2 expression and degradation can be affected by viral proteins and cocaine [345,346,347]. Similarly, a number of volatile organic compounds (VOCs) identified in exhaled breath condensate in association with PAH, are exogenous [348] and may be considered pollutants due to exposure to cigarette smoke, air pollution and radiation [349]. Contribution of volatile compounds was also reported in the pathogenesis of PVOD [350].
A specific example of the environmental effects on phenotype is the foetal origins of adult diseases (FOAD) hypothesis. This hypothesis was based on the studies which reported that intrauterine exposure increased the risk of specific cardiovascular and metabolic diseases [351,352,353] as well as impacted on lung function [354,355,356] in adult life. In the area of PH, prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn (PPHN) has been a subject of multiple studies. A meta-analysis revealed that the risk for PPHN in infants exposed to SSRIs during late pregnancy is small, although significantly increased [357]. In systemic hypertension, the transmission of gut microbiota from parent to offspring was shown to influence the disease risk in adulthood [358] and similarly, bacterial translocation may contribute to PAH development [359]. Air pollution was also shown to correlate with PAH severity and outcomes [360].
With the advent of NGS, the notion of digenic or oligogenic inheritance has gained traction. HPAH has been historically considered a monogenic condition but incomplete penetrance may indicate that other germline or somatic variants are required for the disease to develop. Therefore, at least in a proportion of cases, the inheritance may be digenic or even oligogenic. In the true digenic model, both genes are required to develop the disease. Conversely, in the composite class model, a variant in one gene is sufficient to produce the phenotype, but an additional variant in a second gene impacts the disease phenotype or alters the age of onset [361]. The latter model seems to be plausible in PAH, where co-occurrence of the variants in different PAH risk genes has been reported to impact on disease onset and penetrance [362]. Patients harbouring deleterious variants in more than one PAH risk gene have been reported in case reports [162], small [363] and large cohorts of HPAH patients [24,26,113]. Finally, the phenomenon of synergistic heterozygosity, whereby the effect of susceptibility genes is enhanced by modifier genes, in which common variants may influence the disease onset and severity, has been described in PAH [210].
7.4. Population Dependent Heterogeneity
It is well recognised that although rare diseases can occur in any population, some ethnic groups are characterised by their higher incidence, i.e., sickle cell disease is more common in African, African-Americans and Mediterranean populations and similarly, Tay-Sachs disease occurs mostly in people of Ashkenazi Jewish or French Canadian ancestry. National and international PAH registries have shown differences in demographic characteristics between European and East Asian patients. East Asian IPAH cohorts resemble those of European cohorts from 40 years ago; additionally, they show more pronounced female predominance, a younger age of onset and lower comorbidity burden, and importantly, they demonstrate that significantly more cases can be explained by variation in known PAH risk genes (24% [24,25] vs. 39% [26]).
8. Summary
In this review, we have summarised how four theoretical and methodological approaches impact genetic discoveries in rare diseases, with a particular focus on PAH. These steps include: (1) forward phenotyping, which refers to clustering patients into homogenous groups likely to share genetic architecture, (2) forward genetics, which aims to identify the candidate genes responsible for the phenotype, (3) reverse genetics, which consists of experimental in vivo and in vitro targeted modifications to candidate genes in order to analyse their phenotypic impact, and (4) reverse phenotyping, which uses genetic marker data to refine phenotype definitions. Whilst these approaches have been employed in PAH research, it is important to mention that at present, the majority of patients diagnosed with PAH do not have a genetic diagnosis, a finding that indicates “missing heritability”. To date, some progress has been made in addressing this issue; however, there is still a way to go and only through the use of large-scale international cohorts will studies have the power to detect novel genetic risk loci underlying PAH pathobiology and to elucidate this missing heritability.
Author Contributions
Conceptualization, E.M.S.; writing—original draft preparation, E.M.S., M.P., J.M.M., D.P., K.A.; writing—review and editing, E.M.S., M.P., J.M.M., D.P., K.A., N.W.M., S.G.; visualization, E.M.S., S.G.; supervision, E.M.S., N.W.M., S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montserrat Moliner A., Waligóra J. The European union policy in the field of rare diseases. Public Health Genom. 2013;16:268–277. doi: 10.1159/000355930. [DOI] [PubMed] [Google Scholar]
- 2.Nguengang Wakap S., Lambert D.M., Olry A., Rodwell C., Gueydan C., Lanneau V., Murphy D., Le Cam Y., Rath A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020;28:165–173. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OMIM Entry Statistics. [(accessed on 27 August 2020)]; Available online: https://www.omim.org/statistics/entry.
- 4.Wright C.F., FitzPatrick D.R., Firth H.V. Paediatric genomics: Diagnosing rare disease in children. Nat. Rev. Genet. 2018;19:253–268. doi: 10.1038/nrg.2017.116. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G., Robbins I.M., Beghetti M., Channick R.N., Delcroix M., Denton C.P., Elliott C.G., Gaine S.P., Gladwin M.T., Jing Z.-C., et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Fisher R.A. XV.—The Correlation between Relatives on the Supposition of Mendelian Inheritance. Trans. R. Soc. Edinb. 1919;52:399–433. doi: 10.1017/S0080456800012163. [DOI] [Google Scholar]
- 7.Wright S. The Relative Importance of Heredity and Environment in Determining the Piebald Pattern of Guinea-Pigs. Proc. Natl. Acad. Sci. USA. 1920;6:320–332. doi: 10.1073/pnas.6.6.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenesa A., Haley C.S. The heritability of human disease: Estimation, uses and abuses. Nat. Rev. Genet. 2013;14:139–149. doi: 10.1038/nrg3377. [DOI] [PubMed] [Google Scholar]
- 9.Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., McCarthy M.I., Ramos E.M., Cardon L.R., Chakravarti A., et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 11.Génin E. Missing heritability of complex diseases: Case solved? Hum. Genet. 2020;139:103–113. doi: 10.1007/s00439-019-02034-4. [DOI] [PubMed] [Google Scholar]
- 12.WSPH, Geneva. [(accessed on 25 November 2020)];1973 Available online: http://www.wsphassociation.org/wp-content/uploads/2019/03/Programma-WSPH-Geneva-1973.pdf.
- 13.WSPH, Evian. [(accessed on 25 November 2020)];1998 Available online: http://www.wsphassociation.org/wp-content/uploads/2019/04/Primary-Pulmonary-Hypertension-Evian-1998.pdf.
- 14.Simonneau G., Galiè N., Rubin L.J., Langleben D., Seeger W., Domenighetti G., Gibbs S., Lebrec D., Speich R., Beghetti M., et al. Clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Galiè N., Hoeper M.M., Humbert M., Torbicki A., Vachiery J.-L., Barbera J.A., Beghetti M., Corris P., Gaine S., Gibbs J.S., et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 16.Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N., Humbert M., Vachiery J.-L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Respir. J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 18.Simonneau G., Montani D., Celermajer D.S., Denton C.P., Gatzoulis M.A., Krowka M., Williams P.G., Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53 doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato G.J., Piel F.B., Reid C.D., Gaston M.H., Ohene-Frempong K., Krishnamurti L., Smith W.R., Panepinto J.A., Weatherall D.J., Costa F.F., et al. Sickle cell disease. Nat. Rev. Dis. Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 20.Peron A., Au K.S., Northrup H. Genetics, genomics, and genotype-phenotype correlations of TSC: Insights for clinical practice. Am. J. Med. Genet. C Semin. Med. Genet. 2018;178:281–290. doi: 10.1002/ajmg.c.31651. [DOI] [PubMed] [Google Scholar]
- 21.Bravo-Gil N., González-Del Pozo M., Martín-Sánchez M., Méndez-Vidal C., Rodríguez-de la Rúa E., Borrego S., Antiñolo G. Unravelling the genetic basis of simplex Retinitis Pigmentosa cases. Sci. Rep. 2017;7:41937. doi: 10.1038/srep41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiurazzi P., Pirozzi F. Advances in understanding - genetic basis of intellectual disability. F1000Res. 2016;5 doi: 10.12688/f1000research.7134.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southgate L., Machado R.D., Gräf S., Morrell N.W. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol. 2020;17:85–95. doi: 10.1038/s41569-019-0242-x. [DOI] [PubMed] [Google Scholar]
- 24.Gräf S., Haimel M., Bleda M., Hadinnapola C., Southgate L., Li W., Hodgson J., Liu B., Salmon R.M., Southwood M., et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat. Commun. 2018;9:1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu N., Pauciulo M.W., Welch C.L., Lutz K.A., Coleman A.W., Gonzaga-Jauregui C., Wang J., Grimes J.M., Martin L.J., He H., et al. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med. 2019;11:69. doi: 10.1186/s13073-019-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X.-J., Lian T.-Y., Jiang X., Liu S.-F., Li S.-Q., Jiang R., Wu W.-H., Ye J., Cheng C.-Y., Du Y., et al. Germline BMP9 mutation causes idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2019;53:1801609. doi: 10.1183/13993003.01609-2018. [DOI] [PubMed] [Google Scholar]
- 27.Dweik R.A., Rounds S., Erzurum S.C., Archer S., Fagan K., Hassoun P.M., Hill N.S., Humbert M., Kawut S.M., Krowka M., et al. An official American Thoracic Society Statement: Pulmonary hypertension phenotypes. Am. J. Respir. Crit. Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutendra G., Michelakis E.D. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Buyske S., Yang G., Matise T.C., Gordon D. When a Case Is Not a Case: Effects of Phenotype Misclassification on Power and Sample Size Requirements for the Transmission Disequilibrium Test with Affected Child Trios. Hum. Hered. 2009;67:287–292. doi: 10.1159/000194981. [DOI] [PubMed] [Google Scholar]
- 30.Swietlik E.M., Greene D., Zhu N., Megy K., Cogliano M., Rajaram S., Pandya D., Tilly T., Lutz K.A., Welch C.C.L., et al. Reduced transfer coefficient of carbon monoxide in pulmonary arterial hypertension implicates rare protein-truncating variants in KDR. bioRxiv. 2020 doi: 10.1101/2019.12.11.871210. [DOI] [Google Scholar]
- 31.Pullabhatla V., Roberts A.L., Lewis M.J., Mauro D., Morris D.L., Odhams C.A., Tombleson P., Liljedahl U., Vyse S., Simpson M.A., et al. De novo mutations implicate novel genes in systemic lupus erythematosus. Hum. Mol. Genet. 2018;27:421–429. doi: 10.1093/hmg/ddx407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husson T., Duboc J.-B., Quenez O., Charbonnier C., Rotharmel M., Cuenca M., Jegouzo X., Richard A.-C., Frebourg T., Deleuze J.-F., et al. Identification of potential genetic risk factors for bipolar disorder by whole-exome sequencing. Transl. Psychiatry. 2018;8:268. doi: 10.1038/s41398-018-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjørnland T., Bye A., Ryeng E., Wisløff U., Langaas M. Powerful extreme phenotype sampling designs and score tests for genetic association studies. Stat. Med. 2018;37:4234–4251. doi: 10.1002/sim.7914. [DOI] [PubMed] [Google Scholar]
- 34.Chung W.K., Deng L., Carroll J.S., Mallory N., Diamond B., Rosenzweig E.B., Barst R.J., Morse J.H. Polymorphism in the angiotensin II type 1 receptor (AGTR1) is associated with age at diagnosis in pulmonary arterial hypertension. J. Heart Lung Transplant. 2009;28:373–379. doi: 10.1016/j.healun.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D., Lewinger J.P., Gauderman W.J., Murcray C.E., Conti D. Using extreme phenotype sampling to identify the rare causal variants of quantitative traits in association studies. Genet. Epidemiol. 2011;35:790–799. doi: 10.1002/gepi.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulze T.G., McMahon F.J. Defining the phenotype in human genetic studies: Forward genetics and reverse phenotyping. Hum. Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- 37.Sweatt A.J., Hedlin H.K., Balasubramanian V., Hsi A., Blum L.K., Robinson W.H., Haddad F., Hickey P.M., Condliffe R., Lawrie A., et al. Discovery of Distinct Immune Phenotypes Using Machine Learning in Pulmonary Arterial Hypertension. Circ. Res. 2019;124:904–919. doi: 10.1161/CIRCRESAHA.118.313911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeper M.M., Pausch C., Grünig E., Klose H., Staehler G., Huscher D., Pittrow D., Olsson K.M., Vizza C.D., Gall H., et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Köhler S., Carmody L., Vasilevsky N., Jacobsen J.O.B., Danis D., Gourdine J.-P., Gargano M., Harris N.L., Matentzoglu N., McMurry J.A., et al. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019;47:D1018–D1027. doi: 10.1093/nar/gky1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Köhler S., Schulz M.H., Krawitz P., Bauer S., Dölken S., Ott C.E., Mundlos C., Horn D., Mundlos S., Robinson P.N. Clinical diagnostics in human genetics with semantic similarity searches in ontologies. Am. J. Hum. Genet. 2009;85:457–464. doi: 10.1016/j.ajhg.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene D., Richardson S., Turro E. ontologyX: A suite of R packages for working with ontological data. Bioinformatics. 2017;33:1104–1106. doi: 10.1093/bioinformatics/btw763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters J.L., Cnudde F., Gerats T. Forward genetics and map-based cloning approaches. Trends Plant Sci. 2003;8:484–491. doi: 10.1016/j.tplants.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Ionita-Laza I., Lee S., Makarov V., Buxbaum J.D., Lin X. Family-based association tests for sequence data, and comparisons with population-based association tests. Eur. J. Hum. Genet. 2013;21:1158–1162. doi: 10.1038/ejhg.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Risch N., Merikangas K. The Future of Genetic Studies of Complex Human Diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 45.Marchini J., Donnelly P., Cardon L.R. Genome-wide strategies for detecting multiple loci that influence complex diseases. Nat. Genet. 2005;37:413–417. doi: 10.1038/ng1537. [DOI] [PubMed] [Google Scholar]
- 46.Petrovski S., Todd J.L., Durheim M.T., Wang Q., Chien J.W., Kelly F.L., Frankel C., Mebane C.M., Ren Z., Bridgers J., et al. An Exome Sequencing Study to Assess the Role of Rare Genetic Variation in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017;196:82–93. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sim N.-L., Kumar P., Hu J., Henikoff S., Schneider G., Ng P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ioannidis N.M., Rothstein J.H., Pejaver V., Middha S., McDonnell S.K., Baheti S., Musolf A., Li Q., Holzinger E., Karyadi D., et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016;99:877–885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper G.M., Stone E.A., Asimenos G., NISC Comparative Sequencing Program. Green E.D., Batzoglou S., Sidow A. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–913. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard K.S., Hubisz M.J., Rosenbloom K.R., Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siepel A., Bejerano G., Pedersen J.S., Hinrichs A.S., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L.W., Richards S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasim M.T., Ogo T., Ahmed M., Randall R., Chowdhury H.M., Snape K.M., Bradshaw T.Y., Southgate L., Lee G.J., Jackson I., et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum. Mutat. 2011;32:1385–1389. doi: 10.1002/humu.21605. [DOI] [PubMed] [Google Scholar]
- 55.Potus F., Pauciulo M.W., Cook E.K., Zhu N., Hsieh A., Welch C.L., Shen Y., Tian L., Lima P., Mewburn J., et al. Novel Mutations and Decreased Expression of the Epigenetic Regulator TET2 in Pulmonary Arterial Hypertension. Circulation. 2020;141:1986–2000. doi: 10.1161/CIRCULATIONAHA.119.044320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Povysil G., Petrovski S., Hostyk J., Aggarwal V., Allen A.S., Goldstein D.B. Rare-variant collapsing analyses for complex traits: Guidelines and applications. Nat. Rev. Genet. 2019;20:747–759. doi: 10.1038/s41576-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 57.Asimit J.L., Day-Williams A.G., Morris A.P., Zeggini E. ARIEL and AMELIA: Testing for an accumulation of rare variants using next-generation sequencing data. Hum. Hered. 2012;73:84–94. doi: 10.1159/000336982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris A.P., Zeggini E. An evaluation of statistical approaches to rare variant analysis in genetic association studies. Genet. Epidemiol. 2010;34:188–193. doi: 10.1002/gepi.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Leal S.M. Methods for detecting associations with rare variants for common diseases: Application to analysis of sequence data. Am. J. Hum. Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madsen B.E., Browning S.R. A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet. 2009;5:e1000384. doi: 10.1371/journal.pgen.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ionita-Laza I., Buxbaum J.D., Laird N.M., Lange C. A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease. PLoS Genet. 2011;7:e1001289. doi: 10.1371/journal.pgen.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu M.C., Lee S., Cai T., Li Y., Boehnke M., Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S., Emond M.J., Bamshad M.J., Barnes K.C., Rieder M.J., Nickerson D.A., NHLBI GO Exome Sequencing Project—ESP Lung Project Team. Christiani D.C., Wurfel M.M., Lin X. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am. J. Hum. Genet. 2012;91:224–237. doi: 10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greene D., NIHR BioResource. Richardson S., Turro E. A Fast Association Test for Identifying Pathogenic Variants Involved in Rare Diseases. Am. J. Hum. Genet. 2017;101:104–114. doi: 10.1016/j.ajhg.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eilbeck K., Quinlan A., Yandell M. Settling the score: Variant prioritization and Mendelian disease. Nat. Rev. Genet. 2017;18:599–612. doi: 10.1038/nrg.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cacheiro P., Muñoz-Fuentes V., Murray S.A., Dickinson M.E., Bucan M., Nutter L.M.J., Peterson K.A., Haselimashhadi H., Flenniken A.M., Morgan H., et al. Human and mouse essentiality screens as a resource for disease gene discovery. Nat. Commun. 2020;11:655. doi: 10.1038/s41467-020-14284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehm H.L., Berg J.S., Brooks L.D., Bustamante C.D., Evans J.P., Landrum M.J., Ledbetter D.H., Maglott D.R., Martin C.L., Nussbaum R.L., et al. ClinGen—The Clinical Genome Resource. N. Engl. J. Med. 2015;372:2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goswami R.S., Harada S. An Overview of Molecular Genetic Diagnosis Techniques. Curr. Protoc. Hum. Genet. 2020;105:e97. doi: 10.1002/cphg.97. [DOI] [PubMed] [Google Scholar]
- 70.Bean L.J.H., Funke B., Carlston C.M., Gannon J.L., Kantarci S., Krock B.L., Zhang S., Bayrak-Toydemir P. Diagnostic gene sequencing panels: From design to report—A technical standard of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2020;22:453–461. doi: 10.1038/s41436-019-0666-z. [DOI] [PubMed] [Google Scholar]
- 71.Song J., Eichstaedt C.A., Viales R.R., Benjamin N., Harutyunova S., Fischer C., Grünig E., Hinderhofer K. Identification of genetic defects in pulmonary arterial hypertension by a new gene panel diagnostic tool. Clin. Sci. 2016;130:2043–2052. doi: 10.1042/CS20160531. [DOI] [PubMed] [Google Scholar]
- 72.Rabbani B., Tekin M., Mahdieh N. The promise of whole-exome sequencing in medical genetics. J. Hum. Genet. 2014;59:5–15. doi: 10.1038/jhg.2013.114. [DOI] [PubMed] [Google Scholar]
- 73.Retterer K., Juusola J., Cho M.T., Vitazka P., Millan F., Gibellini F., Vertino-Bell A., Smaoui N., Neidich J., Monaghan K.G., et al. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 74.Austin E.D., Ma L., LeDuc C., Berman Rosenzweig E., Borczuk A., Phillips J.A., 3rd, Palomero T., Sumazin P., Kim H.R., Talati M.H., et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu N., Welch C.L., Wang J., Allen P.M., Gonzaga-Jauregui C., Ma L., King A.K., Krishnan U., Rosenzweig E.B., Ivy D.D., et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med. 2018;10:691. doi: 10.1186/s13073-018-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zare F., Dow M., Monteleone N., Hosny A., Nabavi S. An evaluation of copy number variation detection tools for cancer using whole exome sequencing data. BMC Bioinform. 2017;18:286. doi: 10.1186/s12859-017-1705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turro E., Astle W.J., Megy K., Gräf S., Greene D., Shamardina O., Allen H.L., Sanchis-Juan A., Frontini M., Thys C., et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature. 2020;583:96–102. doi: 10.1038/s41586-020-2434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Human Genome Research Institute Home. [(accessed on 17 October 2020)]; Available online: https://www.genome.gov.
- 79.Schwarze K., Buchanan J., Fermont J.M., Dreau H., Tilley M.W., Taylor J.M., Antoniou P., Knight S.J.L., Camps C., Pentony M.M., et al. The complete costs of genome sequencing: A microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet. Med. 2020;22:85–94. doi: 10.1038/s41436-019-0618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barozzi C., Galletti M., Tomasi L., De Fanti S., Palazzini M., Manes A., Sazzini M., Galiè N. A Combined Targeted and Whole Exome Sequencing Approach Identified Novel Candidate Genes Involved in Heritable Pulmonary Arterial Hypertension. Sci. Rep. 2019;9:753. doi: 10.1038/s41598-018-37277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pulmonary Arterial Hypertension (Version 2.5) [(accessed on 14 October 2020)]; Available online: https://panelapp.genomicsengland.co.uk/panels/193/
- 82.Kim J., Weber J.A., Jho S., Jang J., Jun J., Cho Y.S., Kim H.-M., Kim H., Kim Y., Chung O., et al. KoVariome: Korean National Standard Reference Variome database of whole genomes with comprehensive SNV, indel, CNV, and SV analyses. Sci. Rep. 2018;8:5677. doi: 10.1038/s41598-018-23837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finer S., Martin H.C., Khan A., Hunt K.A., MacLaughlin B., Ahmed Z., Ashcroft R., Durham C., MacArthur D.G., McCarthy M.I., et al. Cohort Profile: East London Genes & Health (ELGH), a community-based population genomics and health study in British Bangladeshi and British Pakistani people. Int. J. Epidemiol. 2020;49:20–21i. doi: 10.1093/ije/dyz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.BioBank Japan (BBJ) [(accessed on 14 October 2020)]; Available online: http://biobankjp.org.
- 85.IBD BioResource—Translating Today’s Science into Tomorrow’s Treatments. [(accessed on 14 October 2020)]; Available online: https://www.ibdbioresource.nihr.ac.uk.
- 86.Genetic Links to Anxiety and Depression Study—GLAD Study. [(accessed on 14 October 2020)]; Available online: https://gladstudy.org.uk.
- 87.Eating Disorders Genetics Initiative. [(accessed on 14 October 2020)]; Available online: https://edgiuk.org/
- 88.Flannick J., Beer N.L., Bick A.G., Agarwala V., Molnes J., Gupta N., Burtt N.P., Florez J.C., Meigs J.B., Taylor H., et al. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat. Genet. 2013;45:1380–1385. doi: 10.1038/ng.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castel S.E., Cervera A., Mohammadi P., Aguet F., Reverter F., Wolman A., Guigo R., Iossifov I., Vasileva A., Lappalainen T. Modified penetrance of coding variants by cis-regulatory variation contributes to disease risk. Nat. Genet. 2018;50:1327–1334. doi: 10.1038/s41588-018-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 91.International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 92.Guo Y., Dai Y., Yu H., Zhao S., Samuels D.C., Shyr Y. Improvements and impacts of GRCh38 human reference on high throughput sequencing data analysis. Genomics. 2017;109:83–90. doi: 10.1016/j.ygeno.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Pan B., Kusko R., Xiao W., Zheng Y., Liu Z., Xiao C., Sakkiah S., Guo W., Gong P., Zhang C., et al. Similarities and differences between variants called with human reference genome HG19 or HG38. BMC Bioinform. 2019;20:17–29. doi: 10.1186/s12859-019-2620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schneider V.A., Graves-Lindsay T., Howe K., Bouk N., Chen H.-C., Kitts P.A., Murphy T.D., Pruitt K.D., Thibaud-Nissen F., Albracht D., et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 2017;27:849–864. doi: 10.1101/gr.213611.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Church D.M., Schneider V.A., Steinberg K.M., Schatz M.C., Quinlan A.R., Chin C.-S., Kitts P.A., Aken B., Marth G.T., Hoffman M.M., et al. Extending reference assembly models. Genome Biol. 2015;16:13. doi: 10.1186/s13059-015-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lappalainen T., Scott A.J., Brandt M., Hall I.M. Genomic Analysis in the Age of Human Genome Sequencing. Cell. 2019;177:70–84. doi: 10.1016/j.cell.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li R., Li Y., Zheng H., Luo R., Zhu H., Li Q., Qian W., Ren Y., Tian G., Li J., et al. Building the sequence map of the human pan-genome. Nat. Biotechnol. 2010;28:57–63. doi: 10.1038/nbt.1596. [DOI] [PubMed] [Google Scholar]
- 98.Paten B., Novak A.M., Eizenga J.M., Garrison E. Genome graphs and the evolution of genome inference. Genome Res. 2017;27:665–676. doi: 10.1101/gr.214155.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rakocevic G., Semenyuk V., Lee W.-P., Spencer J., Browning J., Johnson I.J., Arsenijevic V., Nadj J., Ghose K., Suciu M.C., et al. Fast and accurate genomic analyses using genome graphs. Nat. Genet. 2019;51:354–362. doi: 10.1038/s41588-018-0316-4. [DOI] [PubMed] [Google Scholar]
- 100.Ballouz S., Dobin A., Gillis J.A. Is it time to change the reference genome? Genome Biol. 2019;20:159. doi: 10.1186/s13059-019-1774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.International PPH Consortium. Lane K.B., Machado R.D., Pauciulo M.W., Thomson J.R., Phillips J.A., 3rd, Loyd J.E., Nichols W.C., Trembath R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 102.Thomson J.R. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J. Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trembath R.C., Thomson J.R., Machado R.D., Morgan N.V., Atkinson C., Winship I., Simonneau G., Galie N., Loyd J.E., Humbert M., et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 104.Chaouat A. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harrison R.E., Berger R., Haworth S.G., Tulloh R., Mache C.J., Morrell N.W., Aldred M.A., Trembath R.C. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441. doi: 10.1161/01.CIR.0000153798.78540.87. [DOI] [PubMed] [Google Scholar]
- 106.Shintani M., Yagi H., Nakayama T., Saji T., Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J. Med. Genet. 2009;46:331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 107.Ma L., Roman-Campos D., Austin E.D., Eyries M., Sampson K.S., Soubrier F., Germain M., Trégouët D.-A., Borczuk A., Rosenzweig E.B., et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerstjens-Frederikse W.S., Bongers E.M.H.F., Roofthooft M.T.R., Leter E.M., Douwes J.M., Van Dijk A., Vonk-Noordegraaf A., Dijk-Bos K.K., Hoefsloot L.H., Hoendermis E.S., et al. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J. Med. Genet. 2013;50:500–506. doi: 10.1136/jmedgenet-2012-101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eyries M., Montani D., Girerd B., Perret C., Leroy A., Lonjou C., Chelghoum N., Coulet F., Bonnet D., Dorfmüller P., et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat. Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 110.Best D.H., Sumner K.L., Smith B.P., Damjanovich-Colmenares K., Nakayama I., Brown L.M., Ha Y., Paul E., Morris A., Jama M.A., et al. EIF2AK4 Mutations in Patients Diagnosed with Pulmonary Arterial Hypertension. Chest. 2017;151:821–828. doi: 10.1016/j.chest.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 111.Hadinnapola C., Bleda M., Haimel M., Screaton N., Swift A., Dorfmüller P., Preston S.D., Southwood M., Hernandez-Sanchez J., Martin J., et al. Phenotypic Characterization of Mutation Carriers in a Large Cohort of Patients Diagnosed Clinically with Pulmonary Arterial Hypertension. Circulation. 2017;136:2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hiraide T., Kataoka M., Suzuki H., Aimi Y., Chiba T., Kanekura K., Satoh T., Fukuda K., Gamou S., Kosaki K. SOX17 Mutations in Japanese Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2018;198:1231–1233. doi: 10.1164/rccm.201804-0766LE. [DOI] [PubMed] [Google Scholar]
- 113.Bohnen M.S., Ma L., Zhu N., Qi H., McClenaghan C., Gonzaga-Jauregui C., Dewey F.E., Overton J.D., Reid J.G., Shuldiner A.R., et al. Loss-of-Function ABCC8 Mutations in Pulmonary Arterial Hypertension. Circ. Genom. Precis. Med. 2018;11:e002087. doi: 10.1161/CIRCGEN.118.002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eyries M., Montani D., Nadaud S., Girerd B., Levy M., Bourdin A., Trésorier R., Chaouat A., Cottin V., Sanfiorenzo C., et al. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur. Respir. J. 2019;53 doi: 10.1183/13993003.01371-2018. [DOI] [PubMed] [Google Scholar]
- 115.Hodgson J., Swietlik E.M., Salmon R.M., Hadinnapola C., Nikolic I., Wharton J., Guo J., Liley J., Haimel M., Bleda M., et al. Characterization of Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020;201:575–585. doi: 10.1164/rccm.201906-1141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rhodes C.J., Batai K., Bleda M., Haimel M., Southgate L., Germain M., Pauciulo M.W., Hadinnapola C., Aman J., Girerd B., et al. Genetic determinants of risk in pulmonary arterial hypertension: International genome-wide association studies and meta-analysis. Lancet Respir Med. 2019;7:227–238. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eyries M., Montani D., Girerd B., Favrolt N., Riou M., Faivre L., Manaud G., Perros F., Gräf S., Morrell N.W., et al. Familial pulmonary arterial hypertension by KDR heterozygous loss of function. Eur. Respir. J. 2020;55:1902165. doi: 10.1183/13993003.02165-2019. [DOI] [PubMed] [Google Scholar]
- 118.Zhu N., Swietlik E.M., Welch C.L., Pauciulo M.W., Hagen J.J., Zhou X., Guo Y., Karten J., Pandya D., Tilly T., et al. Rare variant analysis of 4,241 pulmonary arterial hypertension cases from an international consortium implicate FBLN2, PDGFD and rare de novo variants in PAH. bioRxiv. 2020 doi: 10.1101/2020.05.29.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deng Z., Morse J.H., Slager S.L., Cuervo N., Moore K.J., Venetos G., Kalachikov S., Cayanis E., Fischer S.G., Barst R.J., et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am. J. Hum. Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Navas Tejedor P., Tenorio Castaño J., Palomino Doza J., Arias Lajara P., Gordo Trujillo G., López Meseguer M., Román Broto A., Lapunzina Abadía P., Escribano Subía P. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin. Genet. 2017;91:453–457. doi: 10.1111/cge.12869. [DOI] [PubMed] [Google Scholar]
- 121.Wang G., Fan R., Ji R., Zou W., Penny D.J., Varghese N.P., Fan Y. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: A case report. BMC Pulm. Med. 2016;16:17. doi: 10.1186/s12890-016-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larkin E.K., Newman J.H., Austin E.D., Hemnes A.R., Wheeler L., Robbins I.M., West J.D., Phillips J.A., 3rd, Hamid R., Loyd J.E. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012;186:892–896. doi: 10.1164/rccm.201205-0886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beppu H., Ichinose F., Kawai N., Jones R.C., Yu P.B., Zapol W.M., Miyazono K., Li E., Bloch K.D. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004;287:L1241–L1247. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 124.Yang X., Long L., Southwood M., Rudarakanchana N., Upton P.D., Jeffery T.K., Atkinson C., Chen H., Trembath R.C., Morrell N.W. Dysfunctional Smad Signaling Contributes to Abnormal Smooth Muscle Cell Proliferation in Familial Pulmonary Arterial Hypertension. Circ. Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 125.Hong K.-H., Lee Y.J., Lee E., Park S.O., Han C., Beppu H., Li E., Raizada M.K., Bloch K.D., Oh S.P. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.West J., Harral J., Lane K., Deng Y., Ickes B., Crona D., Albu S., Stewart D., Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L744–L755. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gangopahyay A., Oran M., Bauer E.M., Wertz J.W., Comhair S.A., Erzurum S.C., Bauer P.M. Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J. Biol. Chem. 2011;286:33134–33140. doi: 10.1074/jbc.M111.274100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hemnes A.R., Brittain E.L., Trammell A.W., Fessel J.P., Austin E.D., Penner N., Maynard K.B., Gleaves L., Talati M., Absi T., et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ranchoux B., Antigny F., Rucker-Martin C., Hautefort A., Péchoux C., Bogaard H.J., Dorfmüller P., Remy S., Lecerf F., Planté S., et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 130.Caruso P., Dunmore B.J., Schlosser K., Schoors S., Dos Santos C., Perez-Iratxeta C., Lavoie J.R., Zhang H., Long L., Flockton A.R., et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation. 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tian W., Jiang X., Sung Y.K., Shuffle E., Wu T.-H., Kao P.N., Tu A.B., Dorfmüller P., Cao A., Wang L., et al. Phenotypically Silent Bone Morphogenetic Protein Receptor 2 Mutations Predispose Rats to Inflammation-Induced Pulmonary Arterial Hypertension by Enhancing the Risk for Neointimal Transformation. Circulation. 2019;140:1409–1425. doi: 10.1161/CIRCULATIONAHA.119.040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gore B., Izikki M., Mercier O., Dewachter L., Fadel E., Humbert M., Dartevelle P., Simonneau G., Naeije R., Lebrin F., et al. Key role of the endothelial TGF-β/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS ONE. 2014;9:e100310. doi: 10.1371/journal.pone.0100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Drake K.M., Zygmunt D., Mavrakis L., Harbor P., Wang L., Comhair S.A., Erzurum S.C., Aldred M.A. Altered MicroRNA Processing in Heritable Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2011;184:1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang Z., Wang D., Ihida-Stansbury K., Jones P.L., Martin J.F. Defective pulmonary vascular remodeling in Smad8 mutant mice. Hum. Mol. Genet. 2009;18:2791–2801. doi: 10.1093/hmg/ddp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drake K.M., Dunmore B.J., McNelly L.N., Morrell N.W., Aldred M.A. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2013;49:403–409. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han C., Hong K.-H., Kim Y.H., Kim M.-J., Song C., Kim M.J., Kim S.-J., Raizada M.K., Oh S.P. SMAD1 deficiency in either endothelial or smooth muscle cells can predispose mice to pulmonary hypertension. Hypertension. 2013;61:1044–1052. doi: 10.1161/HYPERTENSIONAHA.111.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Copeland C.A., Han B., Tiwari A., Austin E.D., Loyd J.E., West J.D., Kenworthy A.K. A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal. Mol. Biol. Cell. 2017;28:3095–3111. doi: 10.1091/mbc.e17-06-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Y.-Y., Liu Y., Stan R.-V., Fan L., Gu Y., Dalton N., Chu P.-H., Peterson K., Ross J., Jr., Chien K.R. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc. Natl. Acad. Sci. USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F.C., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 140.Zhu N., Gonzaga-Jauregui C., Welch C.L., Ma L., Qi H., King A.K., Krishnan U., Rosenzweig E.B., Ivy D.D., Austin E.D., et al. Exome Sequencing in Children with Pulmonary Arterial Hypertension Demonstrates Differences Compared with Adults. Circ Genom Precis Med. 2018;11:e001887. doi: 10.1161/CIRCGEN.117.001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levy M., Eyries M., Szezepanski I., Ladouceur M., Nadaud S., Bonnet D., Soubrier F. Genetic analyses in a cohort of children with pulmonary hypertension. Eur. Respir. J. 2016;48:1118–1126. doi: 10.1183/13993003.00211-2016. [DOI] [PubMed] [Google Scholar]
- 142.Thoré P., Girerd B., Jaïs X., Savale L., Ghigna M.-R., Eyries M., Levy M., Ovaert C., Servettaz A., Guillaumot A., et al. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur. Respir. J. 2020;55:1902340. doi: 10.1183/13993003.02340-2019. [DOI] [PubMed] [Google Scholar]
- 143.Jansen S.M.A., van den Heuvel L., Meijboom L.J., Alsters S.I.M., Vonk Noordegraaf A., Houweling A., Bogaard H.J. Correspondence regarding “T-box protein 4 mutation causing pulmonary arterial hypertension and lung disease”: A single-centre case series. Eur. Respir. J. 2020;55:1902272. doi: 10.1183/13993003.02272-2019. [DOI] [PubMed] [Google Scholar]
- 144.Hernandez-Gonzalez I., Tenorio J., Palomino-Doza J., Martinez Meñaca A., Morales Ruiz R., Lago-Docampo M., Valverde Gomez M., Gomez Roman J., Enguita Valls A.B., Perez-Olivares C., et al. Clinical heterogeneity of Pulmonary Arterial Hypertension associated with variants in TBX4. PLoS ONE. 2020;15:e0232216. doi: 10.1371/journal.pone.0232216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohnen M.S., Roman-Campos D., Terrenoire C., Jnani J., Sampson K.J., Chung W.K., Kass R.S. The Impact of Heterozygous KCNK3 Mutations Associated with Pulmonary Arterial Hypertension on Channel Function and Pharmacological Recovery. J. Am. Heart Assoc. 2017;6:e006465. doi: 10.1161/JAHA.117.006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lambert M., Capuano V., Boet A., Tesson L., Bertero T., Nakhleh M.K., Remy S., Anegon I., Pechoux C., Hautefort A., et al. Characterization of Kcnk3 -Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ. Res. 2019;125:678–695. doi: 10.1161/CIRCRESAHA.119.314793. [DOI] [PubMed] [Google Scholar]
- 147.Legchenko E., Liu B., West J., Vangheluwe P., Upton P., Morrell N. Protein truncating mutations in ATP13A3 promote pulmonary arterial hypertension in mice. ERJ Open Res. 2020;6:83. [Google Scholar]
- 148.Liu Y., Sun Z., Zhu J., Xiao B., Dong J., Li X. LncRNA-TCONS_00034812 in cell proliferation and apoptosis of pulmonary artery smooth muscle cells and its mechanism. J. Cell. Physiol. 2018;233:4801–4814. doi: 10.1002/jcp.26279. [DOI] [PubMed] [Google Scholar]
- 149.Yun X., Jiang H., Lai N., Wang J., Shimoda L.A. Aquaporin 1-mediated changes in pulmonary arterial smooth muscle cell migration and proliferation involve β-catenin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L889–L898. doi: 10.1152/ajplung.00247.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saadoun S., Papadopoulos M.C., Hara-Chikuma M., Verkman A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 151.Ma T., Yang B., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 152.Tu L., Desroches-Castan A., Mallet C., Guyon L., Cumont A., Phan C., Robert F., Thuillet R., Bordenave J., Sekine A., et al. Selective BMP-9 Inhibition Partially Protects Against Experimental Pulmonary Hypertension. Circ. Res. 2019;124:846–855. doi: 10.1161/CIRCRESAHA.118.313356. [DOI] [PubMed] [Google Scholar]
- 153.Lange A.W., Haitchi H.M., LeCras T.D., Sridharan A., Xu Y., Wert S.E., James J., Udell N., Thurner P.J., Whitsett J.A. Sox17 is required for normal pulmonary vascular morphogenesis. Dev. Biol. 2014;387:109–120. doi: 10.1016/j.ydbio.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shih J., Liu B., Huang C., Haimel M., Bleda M., Gräf S., Rana A., Upton P.D., Morrell N.W. SOX17 Deficiency Impairs Tube Network Formation Through the Reduction of Arterial Identity in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020;201:A2366. [Google Scholar]
- 155.Winter M.-P., Sharma S., Altmann J., Seidl V., Panzenböck A., Alimohammadi A., Zelniker T., Redwan B., Nagel F., Santer D., et al. Interruption of vascular endothelial growth factor receptor 2 signaling induces a proliferative pulmonary vasculopathy and pulmonary hypertension. Basic Res. Cardiol. 2020;115:58. doi: 10.1007/s00395-020-0811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee H.-W., Adachi T., Park S., Kowalski P., Anderson D., Simons M., Jin S.W., Chun H.J. Bmpr1a and Its Role in Endomt in Pulmonary Arterial Hypertension. Circulation. 2018;138:A17070. [Google Scholar]
- 157.Lee H.W., Adachi T., Park S., Pak B., Kowalski P., Choi W., Clapham K., Lee A., Anderson D., Simons M., et al. Abstract 14616: Loss of Endothelial BMPR1A Results in Pulmonary Hypertension Through Endothelial to Mesenchymal Transition. Circulation. 2019;140:A14616. doi: 10.1161/circ.140.suppl_1.14616. [DOI] [Google Scholar]
- 158.Du L., Sullivan C.C., Chu D., Cho A.J., Kido M., Wolf P.L., Yuan J.X.-J., Deutsch R., Jamieson S.W., Thistlethwaite P.A. Signaling molecules in nonfamilial pulmonary hypertension. N. Engl. J. Med. 2003;348:500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 159.El-Bizri N., Wang L., Merklinger S.L., Guignabert C., Desai T., Urashima T., Sheikh A.Y., Knutsen R.H., Mecham R.P., Mishina Y., et al. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ. Res. 2008;102:380–388. doi: 10.1161/CIRCRESAHA.107.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chida A., Shintani M., Nakayama T., Furutani Y., Hayama E., Inai K., Saji T., Nonoyama S., Nakanishi T. Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ. J. 2012;76:1501–1508. doi: 10.1253/circj.CJ-11-1281. [DOI] [PubMed] [Google Scholar]
- 161.Yu Y., Keller S.H., Remillard C.V., Safrina O., Nicholson A., Zhang S.L., Jiang W., Vangala N., Landsberg J.W., Wang J.-Y., et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang G., Knight L., Ji R., Lawrence P., Kanaan U., Li L., Das A., Cui B., Zou W., Penny D.J., et al. Early onset severe pulmonary arterial hypertension with “two-hit” digenic mutations in both BMPR2 and KCNA5 genes. Int. J. Cardiol. 2014;177:e167–e169. doi: 10.1016/j.ijcard.2014.08.124. [DOI] [PubMed] [Google Scholar]
- 163.Remillard C.V., Tigno D.D., Platoshyn O., Burg E.D., Brevnova E.E., Conger D., Nicholson A., Rana B.K., Channick R.N., Rubin L.J., et al. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 2007;292:C1837–C1853. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- 164.Zhang P., McGrath B.C., Reinert J., Olsen D.S., Lei L., Gill S., Wek S.A., Vattem K.M., Wek R.C., Kimball S.R., et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Germain M., Eyries M., Montani D., Poirier O., Girerd B., Dorfmüller P., Coulet F., Nadaud S., Maugenre S., Guignabert C., et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat. Genet. 2013;45:518–521. doi: 10.1038/ng.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Paulin R., Dromparis P., Sutendra G., Gurtu V., Zervopoulos S., Bowers L., Haromy A., Webster L., Provencher S., Bonnet S., et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20:827–839. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 167.Michelakis E.D., Gurtu V., Webster L., Barnes G., Watson G., Howard L., Cupitt J., Paterson I., Thompson R.B., Chow K., et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 168.Dromparis P., Paulin R., Sutendra G., Qi A.C., Bonnet S., Michelakis E.D. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ. Res. 2013;113:126–136. doi: 10.1161/CIRCRESAHA.112.300699. [DOI] [PubMed] [Google Scholar]
- 169.Zhang C.Y., Baffy G., Perret P., Krauss S., Peroni O., Grujic D., Hagen T., Vidal-Puig A.J., Boss O., Kim Y.B., et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/S0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 170.Baloira Villar A., Valverde D., Lago M. Functional study of polymorphisms in the promoter region of the endothelin-1 gene in Pulmonary Arterial Hypertension. Eur. Respir. J. 2019;54:PA5043. [Google Scholar]
- 171.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W.P., Stewart D.J. Expression of Endothelin-1 in the Lungs of Patients with Pulmonary Hypertension. N. Engl. J. Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 172.De Jesus Perez V.A., Yuan K., Lyuksyutova M.A., Dewey F., Orcholski M.E., Shuffle E.M., Mathur M., Yancy L., Jr., Rojas V., Li C.G., et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014;189:1260–1272. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Damico R., Kolb T.M., Valera L., Wang L., Housten T., Tedford R.J., Kass D.A., Rafaels N., Gao L., Barnes K.C., et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015;191:208–218. doi: 10.1164/rccm.201409-1742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maloney J.P., Stearman R.S., Bull T.M., Calabrese D.W., Tripp-Addison M.L., Wick M.J., Broeckel U., Robbins I.M., Wheeler L.A., Cogan J.D., et al. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L541–L554. doi: 10.1152/ajplung.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pfarr N., Fischer C., Ehlken N., Becker-Grünig T., López-González V., Gorenflo M., Hager A., Hinderhofer K., Miera O., Nagel C., et al. Hemodynamic and genetic analysis in children with idiopathic, heritable, and congenital heart disease associated pulmonary arterial hypertension. Respir. Res. 2013;14:3. doi: 10.1186/1465-9921-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Runo J.R., Vnencak-Jones C.L., Prince M., Loyd J.E., Wheeler L., Robbins I.M., Lane K.B., Newman J.H., Johnson J., Nichols W.C., et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am. J. Respir. Crit. Care Med. 2003;167:889–894. doi: 10.1164/rccm.200208-861OC. [DOI] [PubMed] [Google Scholar]
- 177.Machado R.D., Southgate L., Eichstaedt C.A., Aldred M.A., Austin E.D., Best D.H., Chung W.K., Benjamin N., Elliott C.G., Eyries M., et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum. Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Machado R.D., Eickelberg O., Elliott C.G., Geraci M.W., Hanaoka M., Loyd J.E., Newman J.H., Phillips J.A., 3rd, Soubrier F., Trembath R.C., et al. Genetics and genomics of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54:S32–S42. doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Machado R.D., Aldred M.A., James V., Harrison R.E., Patel B., Schwalbe E.C., Gruenig E., Janssen B., Koehler R., Seeger W., et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum. Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 180.Evans J.D.W., Girerd B., Montani D., Wang X.-J., Galiè N., Austin E.D., Elliott G., Asano K., Grünig E., Yan Y., et al. BMPR2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir. Med. 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Atkinson C., Stewart S., Upton P.D., Machado R., Thomson J.R., Trembath R.C., Morrell N.W. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.CIR.0000012754.72951.3D. [DOI] [PubMed] [Google Scholar]
- 182.Lavoie J.R., Ormiston M.L., Perez-Iratxeta C., Courtman D.W., Jiang B., Ferrer E., Caruso P., Southwood M., Foster W.S., Morrell N.W., et al. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation. 2014;129:2125–2135. doi: 10.1161/CIRCULATIONAHA.114.008777. [DOI] [PubMed] [Google Scholar]
- 183.Chen N.-Y., D Collum S., Luo F., Weng T., Le T.-T., M Hernandez A., Philip K., Molina J.G., Garcia-Morales L.J., Cao Y., et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;311:L238–L254. doi: 10.1152/ajplung.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Donaldson J.W., McKeever T.M., Hall I.P., Hubbard R.B., Fogarty A.W. The UK prevalence of hereditary haemorrhagic telangiectasia and its association with sex, socioeconomic status and region of residence: A population-based study. Thorax. 2014;69:161–167. doi: 10.1136/thoraxjnl-2013-203720. [DOI] [PubMed] [Google Scholar]
- 185.Harrison R.E., Flanagan J.A., Sankelo M., Abdalla S.A., Rowell J., Machado R.D., Elliott C.G., Robbins I.M., Olschewski H., McLaughlin V., et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J. Med. Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fujiwara M., Yagi H., Matsuoka R., Akimoto K., Furutani M., Imamura S.-I., Uehara R., Nakayama T., Takao A., Nakazawa M., et al. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ. J. 2008;72:127–133. doi: 10.1253/circj.72.127. [DOI] [PubMed] [Google Scholar]
- 187.Kwasnickacrawford D., Carson A., Roberts W., Summers A., Rehnstrom K., Jarvela I., Scherer S. Characterization of a novel cation transporter ATPase gene (ATP13A4) interrupted by 3q25–q29 inversion in an individual with language delay. Genomics. 2005;86:182–194. doi: 10.1016/j.ygeno.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 188.Madan M., Patel A., Skruber K., Geerts D., Altomare D.A., Iv O.P. ATP13A3 and caveolin-1 as potential biomarkers for difluoromethylornithine-based therapies in pancreatic cancers. Am. J. Cancer Res. 2016;6:1231–1252. [PMC free article] [PubMed] [Google Scholar]
- 189.Sui H., Han B.G., Lee J.K., Walian P., Jap B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 190.Montani D., Girerd B., Günther S., Riant F., Tournier-Lasserve E., Magy L., Maazi N., Guignabert C., Savale L., Sitbon O., et al. Pulmonary arterial hypertension in familial hemiplegic migraine with ATP1A2 channelopathy. Eur. Respir. J. 2014;43:641–643. doi: 10.1183/09031936.00147013. [DOI] [PubMed] [Google Scholar]
- 191.Firth A.L., Remillard C.V., Platoshyn O., Fantozzi I., Ko E.A., Yuan J.X.-J. Functional ion channels in human pulmonary artery smooth muscle cells: Voltage-dependent cation channels. Pulm. Circ. 2011;1:48–71. doi: 10.4103/2045-8932.78103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Makino A., Firth A.L., Yuan J.X.-J. Endothelial and smooth muscle cell ion channels in pulmonary vasoconstriction and vascular remodeling. Compr. Physiol. 2011;1:1555–1602. doi: 10.1002/cphy.c100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burg E.D., Remillard C.V., Yuan J.X.-J. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: Pharmacotherapeutic implications. Br. J. Pharmacol. 2008;153(Suppl. 1):S99–S111. doi: 10.1038/sj.bjp.0707635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lambert M., Capuano V., Olschewski A., Sabourin J., Nagaraj C., Girerd B., Weatherald J., Humbert M., Antigny F. Ion Channels in Pulmonary Hypertension: A Therapeutic Interest? Int. J. Mol. Sci. 2018;19:3162. doi: 10.3390/ijms19103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Babenko A.P., Polak M., Cavé H., Busiah K., Czernichow P., Scharfmann R., Bryan J., Aguilar-Bryan L., Vaxillaire M., Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 196.Flanagan S.E., Clauin S., Bellanné-Chantelot C., de Lonlay P., Harries L.W., Gloyn A.L., Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell KATPchannel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 197.Lago-Docampo M., Tenorio J., Hernández-González I., Pérez-Olivares C., Escribano-Subías P., Pousada G., Baloira A., Arenas M., Lapunzina P., Valverde D. Characterization of rare ABCC8 variants identified in Spanish pulmonary arterial hypertension patients. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arora R., Metzger R.J., Papaioannou V.E. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Karolak J.A., Vincent M., Deutsch G., Gambin T., Cogné B., Pichon O., Vetrini F., Mefford H.C., Dines J.N., Golden-Grant K., et al. Complex Compound Inheritance of Lethal Lung Developmental Disorders Due to Disruption of the TBX-FGF Pathway. Am. J. Hum. Genet. 2019;104:213–228. doi: 10.1016/j.ajhg.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bongers E.M.H.F., Duijf P.H.G., van Beersum S.E.M., Schoots J., Van Kampen A., Burckhardt A., Hamel B.C.J., Losan F., Hoefsloot L.H., Yntema H.G., et al. Mutations in the human TBX4 gene cause small patella syndrome. Am. J. Hum. Genet. 2004;74:1239–1248. doi: 10.1086/421331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Galambos C., Mullen M.P., Shieh J.T., Schwerk N., Kielt M.J., Ullmann N., Boldrini R., Stucin-Gantar I., Haass C., Bansal M., et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur. Respir. J. 2019;54:1801965. doi: 10.1183/13993003.01965-2018. [DOI] [PubMed] [Google Scholar]
- 202.Francois M., Koopman P., Beltrame M. SoxF genes: Key players in the development of the cardio-vascular system. Int. J. Biochem. Cell Biol. 2010;42:445–448. doi: 10.1016/j.biocel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 203.De Vilder E.Y.G., Debacker J., Vanakker O.M. GGCX-Associated Phenotypes: An Overview in Search of Genotype-Phenotype Correlations. Int. J. Mol. Sci. 2017;18:240. doi: 10.3390/ijms18020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lantuéjoul S., Sheppard M.N., Corrin B., Burke M.M., Nicholson A.G. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: A clinicopathologic study of 35 cases. Am. J. Surg. Pathol. 2006;30:850–857. doi: 10.1097/01.pas.0000209834.69972.e5. [DOI] [PubMed] [Google Scholar]
- 205.Davies P., Reid L. Pulmonary veno-occlusive disease in siblings: Case reports and morphometric study. Hum. Pathol. 1982;13:911–915. doi: 10.1016/S0046-8177(82)80051-8. [DOI] [PubMed] [Google Scholar]
- 206.Voordes C.G., Kuipers J.R., Elema J.D. Familial pulmonary veno-occlusive disease: A case report. Thorax. 1977;32:763–766. doi: 10.1136/thx.32.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Austin E.D., Cogan J.D., West J.D., Hedges L.K., Hamid R., Dawson E.P., Wheeler L.A., Parl F.F., Loyd J.E., Phillips J.A., 3rd Alterations in oestrogen metabolism: Implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur. Respir. J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Austin E.D., Hamid R., Hemnes A.R., Loyd J.E., Blackwell T., Yu C., Phillips J.A., III, Gaddipati R., Gladson S., Gu E., et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol. Sex Differ. 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hamid R., Cogan J.D., Hedges L.K., Austin E., Phillips J.A., 3rd, Newman J.H., Loyd J.E. Penetrance of pulmonary arterial hypertension is modulated by the expression of normal BMPR2 allele. Hum. Mutat. 2009;30:649–654. doi: 10.1002/humu.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Phillips J.A., 3rd, Poling J.S., Phillips C.A., Stanton K.C., Austin E.D., Cogan J.D., Wheeler L., Yu C., Newman J.H., Dietz H.C., et al. Synergistic heterozygosity for TGFbeta1 SNPs and BMPR2 mutations modulates the age at diagnosis and penetrance of familial pulmonary arterial hypertension. Genet. Med. 2008;10:359–365. doi: 10.1097/GIM.0b013e318172dcdf. [DOI] [PubMed] [Google Scholar]
- 211.Hirschey M.D., Shimazu T., Jing E., Grueter C.A., Collins A.M., Aouizerat B., Stančáková A., Goetzman E., Lam M.M., Schwer B., et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Abraham W.T., Raynolds M.V., Badesch D.B., Wynne K.M., Groves B.M., Roden R.L., Robertson A.D., Lowes B.D., Zisman L.S., Voelkel N.F., et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: Increased frequency and association with preserved haemodynamics. J. Renin-Angiotensin-Aldosterone Syst. 2003;4:27–30. doi: 10.3317/jraas.2003.003. [DOI] [PubMed] [Google Scholar]
- 213.Solari V., Puri P. Genetic polymorphisms of angiotensin system genes in congenital diaphragmatic hernia associated with persistent pulmonary hypertension. J. Pediatr. Surg. 2004;39:302–306. doi: 10.1016/j.jpedsurg.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 214.Sutliff R.L., Kang B.-Y., Michael Hart C. PPARγ as a potential therapeutic target in pulmonary hypertension. Ther. Adv. Respir. Dis. 2010;4:143–160. doi: 10.1177/1753465809369619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Green D.E., Sutliff R.L., Michael Hart C. Is Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) a Therapeutic Target for the Treatment of Pulmonary Hypertension? Pulm. Circ. 2011;1:33–47. doi: 10.4103/2045-8932.78101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Malenfant S., Neyron A.-S., Paulin R., Potus F., Meloche J., Provencher S., Bonnet S. Signal Transduction in the Development of Pulmonary Arterial Hypertension. Pulm. Circ. 2013;3:278–293. doi: 10.4103/2045-8932.114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shatat M.A., Tian H., Zhang R., Tandon G., Hale A., Fritz J.S., Zhou G., Martínez-González J., Rodríguez C., Champion H.C., et al. Endothelial Krüppel-Like Factor 4 Modulates Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2014;50:647–653. doi: 10.1165/rcmb.2013-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Crosby A., Toshner M.R., Southwood M.R., Soon E., Dunmore B.J., Groves E., Moore S., Wright P., Ottersbach K., Bennett C., et al. Hematopoietic stem cell transplantation alters susceptibility to pulmonary hypertension in Bmpr2-deficient mice. Pulm. Circ. 2018;8 doi: 10.1177/2045894018801642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kallunki T., Barisic M., Jäättelä M., Liu B. How to Choose the Right Inducible Gene Expression System for Mammalian Studies? Cells. 2019;8:796. doi: 10.3390/cells8080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Urnov F.D., Rebar E.J., Holmes M.C., Steve Zhang H., Gregory P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 221.Joung J.K., Keith Joung J., Sander J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim D.H., Rossi J.J. RNAi mechanisms and applications. Biotech. 2008;44:613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lewandoski M. Conditional control of gene expression in the mouse. Nat. Rev. Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 225.Van der Weyden L., White J.K., Adams D.J., Logan D.W. The mouse genetics toolkit: Revealing function and mechanism. Genome Biol. 2011;12:224. doi: 10.1186/gb-2011-12-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Van Boxtel R., Cuppen E. Rat traps: Filling the toolbox for manipulating the rat genome. Genome Biol. 2010;11:217. doi: 10.1186/gb-2010-11-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Toshner M., Voswinckel R., Southwood M., Al-Lamki R., Howard L.S.G., Marchesan D., Yang J., Suntharalingam J., Soon E., Exley A., et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Morrell N.W., Adnot S., Archer S.L., Dupuis J., Jones P.L., MacLean M.R., McMurtry I.F., Stenmark K.R., Thistlethwaite P.A., Weissmann N., et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Humbert M., Guignabert C., Bonnet S., Dorfmüller P., Klinger J.R., Nicolls M.R., Olschewski A.J., Pullamsetti S.S., Schermuly R.T., Stenmark K.R., et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019;53 doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu B., Haimel M., Bleda M., Li W., Gräf S., Upton P.D., Morrell N.W. S42 Characterizing ATP13A3 loss of function in pulmonary arterial hypertension (PAH) Fundam. Mech. Pulm. Arter. Hypertens. 2018 [Google Scholar]
- 231.Tian L., Gao J., Garcia I.M., Chen H.J., Castaldi A., Chen Y.-W. Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling. Wiley Interdiscip. Rev. Dev. Biol. 2020:e399. doi: 10.1002/wdev.399. [DOI] [PubMed] [Google Scholar]
- 232.D’Amico R.W., Faley S., Shim H.-N., Prosser J.R., Agrawal V., Bellan L.M., West J.D. Pulmonary Vascular Platform Models the Effects of Flow and Pressure on Endothelial Dysfunction in BMPR2 Associated Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018;19:2561. doi: 10.3390/ijms19092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nogueira-Ferreira R., Faria-Costa G., Ferreira R., Henriques-Coelho T. Animal models for the study of pulmonary hypertension: Potential and limitations. Cardiol. Cardiovasc. Med. 2016;1:1–22. doi: 10.26502/fccm.9292001. [DOI] [Google Scholar]
- 234.Ma Z., Mao L., Rajagopal S. Hemodynamic Characterization of Rodent Models of Pulmonary Arterial Hypertension. J. Vis. Exp. 2016 doi: 10.3791/53335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gomez-Arroyo J.G., Farkas L., Alhussaini A.A., Farkas D., Kraskauskas D., Voelkel N.F., Bogaard H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 236.Akhavein F., St-Michel E.J., Seifert E., Rohlicek C.V. Decreased left ventricular function, myocarditis, and coronary arteriolar medial thickening following monocrotaline administration in adult rats. J. Appl. Physiol. 2007;103:287–295. doi: 10.1152/japplphysiol.01509.2005. [DOI] [PubMed] [Google Scholar]
- 237.Taraseviciene-stewart L., Kasahara Y., Alger L., Hirth P., Mc Mahon G., Waltenberger J., Voelkel N.F., Tuder R.M. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 238.Kojonazarov B., Hadzic S., Ghofrani H.A., Grimminger F., Seeger W., Weissmann N., Schermuly R.T. Severe Emphysema in the SU5416/Hypoxia Rat Model of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2019;200:515–518. doi: 10.1164/rccm.201902-0390LE. [DOI] [PubMed] [Google Scholar]
- 239.Mancuso M.R., Davis R., Norberg S.M., O’Brien S., Sennino B., Nakahara T., Yao V.J., Inai T., Brooks P., Freimark B., et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tabima D.M., Hacker T.A., Chesler N.C. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H2069–H2075. doi: 10.1152/ajpheart.00805.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luo F., Wang X., Luo X., Li B., Zhu D., Sun H., Tang Y. Invasive Hemodynamic Assessment for the Right Ventricular System and Hypoxia-Induced Pulmonary Arterial Hypertension in Mice. J. Vis. Exp. 2019 doi: 10.3791/60090. [DOI] [PubMed] [Google Scholar]
- 242.Stenmark K.R., Meyrick B., Galie N., Mooi W.J., McMurtry I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 243.Akazawa Y., Okumura K., Ishii R., Slorach C., Hui W., Ide H., Honjo O., Sun M., Kabir G., Connelly K., et al. Pulmonary artery banding is a relevant model to study the right ventricular remodeling and dysfunction that occurs in pulmonary arterial hypertension. J. Appl. Physiol. 2020;129:238–246. doi: 10.1152/japplphysiol.00148.2020. [DOI] [PubMed] [Google Scholar]
- 244.Lawrie A. A report on the use of animal models and phenotyping methods in pulmonary hypertension research. Pulm. Circ. 2014;4:2–9. doi: 10.1086/674886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Song Y., Jones J.E., Beppu H., Keaney J.F., Jr., Loscalzo J., Zhang Y.-Y. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Long L., MacLean M.R., Jeffery T.K., Morecroft I., Yang X., Rudarakanchana N., Southwood M., James V., Trembath R.C., Morrell N.W. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ. Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 247.Peacock A.J., Vonk Noordegraaf A. Cardiac magnetic resonance imaging in pulmonary arterial hypertension. Eur. Respir. Rev. 2013;22:526–534. doi: 10.1183/09059180.00006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Provencher S., Archer S.L., Ramirez F.D., Hibbert B., Paulin R., Boucherat O., Lacasse Y., Bonnet S. Standards and Methodological Rigor in Pulmonary Arterial Hypertension Preclinical and Translational Research. Circ. Res. 2018;122:1021–1032. doi: 10.1161/CIRCRESAHA.117.312579. [DOI] [PubMed] [Google Scholar]
- 249.Rol N., Kurakula K.B., Happé C., Bogaard H.J., Goumans M.-J. TGF-β and BMPR2 Signaling in PAH: Two Black Sheep in One Family. Int. J. Mol. Sci. 2018;19:2585. doi: 10.3390/ijms19092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ten Dijke P., Goumans M.-J., Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 251.Upton P.D., Davies R.J., Trembath R.C., Morrell N.W. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem. 2009;284:15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Barnes J.W., Kucera E.T., Tian L., Mellor N.E., Dvorina N., Baldwin W.W., Aldred M.A., Farver C.F., Comhair S.A.A., Aytekin M., et al. Bone Morphogenic Protein Type 2 Receptor Mutation-Independent Mechanisms of Disrupted Bone Morphogenetic Protein Signaling in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2016;55:564–575. doi: 10.1165/rcmb.2015-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Good R.B., Gilbane A.J., Trinder S.L., Denton C.P., Coghlan G., Abraham D.J., Holmes A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015;185:1850–1858. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 254.Liu T., Zou X.-Z., Huang N., Ge X.-Y., Yao M.-Z., Liu H., Zhang Z., Hu C.-P. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci. 2019;227:64–73. doi: 10.1016/j.lfs.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 255.Vanderpool R.R., El-Bizri N., Rabinovitch M., Chesler N.C. Patchy deletion of Bmpr1a potentiates proximal pulmonary artery remodeling in mice exposed to chronic hypoxia. Biomech. Model. Mechanobiol. 2013;12:33–42. doi: 10.1007/s10237-012-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tie L., Wang D., Shi Y., Li X. Aquaporins in Cardiovascular System. Adv. Exp. Med. Biol. 2017;969:105–113. doi: 10.1007/978-94-024-1057-0_6. [DOI] [PubMed] [Google Scholar]
- 257.Clapp C., Martínez de la Escalera G. Aquaporin-1: A novel promoter of tumor angiogenesis. Trends Endocrinol. Metab. 2006;17:1–2. doi: 10.1016/j.tem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 258.Schuoler C., Haider T.J., Leuenberger C., Vogel J., Ostergaard L., Kwapiszewska G., Kohler M., Gassmann M., Huber L.C., Brock M. Aquaporin 1 controls the functional phenotype of pulmonary smooth muscle cells in hypoxia-induced pulmonary hypertension. Basic Res. Cardiol. 2017;112:30. doi: 10.1007/s00395-017-0620-7. [DOI] [PubMed] [Google Scholar]
- 259.Kunichika N., Landsberg J.W., Yu Y., Kunichika H., Thistlethwaite P.A., Rubin L.J., Yuan J.X.-J. Bosentan inhibits transient receptor potential channel expression in pulmonary vascular myocytes. Am. J. Respir. Crit. Care Med. 2004;170:1101–1107. doi: 10.1164/rccm.200312-1668OC. [DOI] [PubMed] [Google Scholar]
- 260.Pozeg Z.I., Michelakis E.D., McMurtry M.S., Thébaud B., Wu X.-C., Dyck J.R.B., Hashimoto K., Wang S., Moudgil R., Harry G., et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 261.Sheeba C.J., Logan M.P.O. The Roles of T-Box Genes in Vertebrate Limb Development. Curr. Top. Dev. Biol. 2017;122:355–381. doi: 10.1016/bs.ctdb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 262.Zhang W., Menke D.B., Jiang M., Chen H., Warburton D., Turcatel G., Lu C.-H., Xu W., Luo Y., Shi W. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol. 2013;11:111. doi: 10.1186/1741-7007-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rieder M.J., Reiner A.P., Rettie A.E. Gamma-glutamyl carboxylase (GGCX) tagSNPs have limited utility for predicting warfarin maintenance dose. J. Thromb. Haemost. 2007;5:2227–2234. doi: 10.1111/j.1538-7836.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- 264.Napolitano M., Mariani G., Lapecorella M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet J. Rare Dis. 2010;5:21. doi: 10.1186/1750-1172-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Li Q., Schurgers L.J., Smith A.C.M., Tsokos M., Uitto J., Cowen E.W. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: Compound heterozygosity for mutations in the GGCX gene. Am. J. Pathol. 2009;174:534–540. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Moreau M.E., Garbacki N., Molinaro G., Brown N.J., Marceau F., Adam A. The Kallikrein-Kinin System: Current and Future Pharmacological Targets. J. Pharmacol. Sci. 2005;99:6–38. doi: 10.1254/jphs.SRJ05001X. [DOI] [PubMed] [Google Scholar]
- 267.Carretero O.A., Scicli A.G. The renal kallikrein-kinin system in human and in experimental hypertension. Klin. Wochenschr. 1978;56(Suppl. 1):113–125. doi: 10.1007/BF01477462. [DOI] [PubMed] [Google Scholar]
- 268.Madeddu P., Emanueli C., El-Dahr S. Mechanisms of disease: The tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat. Clin. Pract. Nephrol. 2007;3:208–221. doi: 10.1038/ncpneph0444. [DOI] [PubMed] [Google Scholar]
- 269.Woodley-Miller C., Chao J., Chao L. Restriction fragment length polymorphisms mapped in spontaneously hypertensive rats using kallikrein probes. J. Hypertens. 1989;7:865–871. doi: 10.1097/00004872-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 270.Madeddu P., Varoni M.V., Demontis M.P., Chao J., Simson J.A., Glorioso N., Anania V. Kallikrein-kinin system and blood pressure sensitivity to salt. Hypertension. 1997;29:471–477. doi: 10.1161/01.HYP.29.1.471. [DOI] [PubMed] [Google Scholar]
- 271.Hua H., Zhou S., Liu Y., Wang Z., Wan C., Li H., Chen C., Li G., Zeng C., Chen L., et al. Relationship between the regulatory region polymorphism of human tissue kallikrein gene and essential hypertension. J. Hum. Hypertens. 2005;19:715–721. doi: 10.1038/sj.jhh.1001875. [DOI] [PubMed] [Google Scholar]
- 272.Alexander-Curtis M., Pauls R., Chao J., Volpi J.J., Bath P.M., Verdoorn T.A. Human tissue kallikrein in the treatment of acute ischemic stroke. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286418821918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Anthony T.G., McDaniel B.J., Byerley R.L., McGrath B.C., Cavener D.R., McNurlan M.A., Wek R.C. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 274.Waypa G.B., Osborne S.W., Marks J.D., Berkelhamer S.K., Kondapalli J., Schumacker P.T. Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2013;49:885–891. doi: 10.1165/rcmb.2013-0191OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kim K., Kim I.-K., Yang J.M., Lee E., Koh B.I., Song S., Park J., Lee S., Choi C., Kim J.W., et al. SoxF Transcription Factors Are Positive Feedback Regulators of VEGF Signaling. Circ. Res. 2016;119:839–852. doi: 10.1161/CIRCRESAHA.116.308483. [DOI] [PubMed] [Google Scholar]
- 276.Varshney G.K., Carrington B., Pei W., Bishop K., Chen Z., Fan C., Xu L., Jones M., LaFave M.C., Ledin J., et al. A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat. Protoc. 2016;11:2357–2375. doi: 10.1038/nprot.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vickovic S., Eraslan G., Salmén F., Klughammer J., Stenbeck L., Schapiro D., Äijö T., Bonneau R., Bergenstråhle L., Navarro J.F., et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods. 2019;16:987–990. doi: 10.1038/s41592-019-0548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Grunewald J., Eklund A. Sex-Specific Manifestations of Löfgren’s Syndrome. Am. J. Respir. Crit. Care Med. 2007;175:40–44. doi: 10.1164/rccm.200608-1197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Landini S., Mazzinghi B., Becherucci F., Allinovi M., Provenzano A., Palazzo V., Ravaglia F., Artuso R., Bosi E., Stagi S., et al. Reverse Phenotyping after Whole-Exome Sequencing in Steroid-Resistant Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2020;15:89–100. doi: 10.2215/CJN.06060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nelson M.R., Tipney H., Painter J.L., Shen J., Nicoletti P., Shen Y., Floratos A., Sham P.C., Li M.J., Wang J., et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 2015;47:856–860. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 281.Whicher D., Philbin S., Aronson N. An overview of the impact of rare disease characteristics on research methodology. Orphanet J. Rare Dis. 2018;13:14. doi: 10.1186/s13023-017-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.UK Biobank. [(accessed on 17 October 2020)]; Available online: https://www.ukbiobank.ac.uk.
- 283.Welcome to eMerge > Collaborate. [(accessed on 17 October 2020)]; Available online: https://emerge-network.org.
- 284.National Institutes of Health (NIH)—All of Us. [(accessed on 17 October 2020)]; Available online: https://www.nih.gov/precision-medicine-initiative-cohort-program.
- 285.Polubriaginof F.C.G., Vanguri R., Quinnies K., Belbin G.M., Yahi A., Salmasian H., Lorberbaum T., Nwankwo V., Li L., Shervey M.M., et al. Disease Heritability Inferred from Familial Relationships Reported in Medical Records. Cell. 2018;173:1692–1704. doi: 10.1016/j.cell.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Firth H.V., Richards S.M., Bevan A.P., Clayton S., Corpas M., Rajan D., Van Vooren S., Moreau Y., Pettett R.M., Carter N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Philippakis A.A., Azzariti D.R., Beltran S., Brookes A.J., Brownstein C.A., Brudno M., Brunner H.G., Buske O.J., Carey K., Doll C., et al. The Matchmaker Exchange: A platform for rare disease gene discovery. Hum. Mutat. 2015;36:915–921. doi: 10.1002/humu.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Boycott K.M., Rath A., Chong J.X., Hartley T., Alkuraya F.S., Baynam G., Brookes A.J., Brudno M., Carracedo A., den Dunnen J.T., et al. International Cooperation to Enable the Diagnosis of All Rare Genetic Diseases. Am. J. Hum. Genet. 2017;100:695–705. doi: 10.1016/j.ajhg.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kopanos C., Tsiolkas V., Kouris A., Chapple C.E., Albarca Aguilera M., Meyer R., Massouras A. VarSome: The human genomic variant search engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Freeman P.J., Hart R.K., Gretton L.J., Brookes A.J., Dalgleish R. VariantValidator: Accurate validation, mapping, and formatting of sequence variation descriptions. Hum. Mutat. 2018;39:61–68. doi: 10.1002/humu.23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Corbin L.J., Tan V.Y., Hughes D.A., Wade K.H., Paul D.S., Tansey K.E., Butcher F., Dudbridge F., Howson J.M., Jallow M.W., et al. Formalising recall by genotype as an efficient approach to detailed phenotyping and causal inference. Nat. Commun. 2018;9:711. doi: 10.1038/s41467-018-03109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Koolen D.A., Kramer J.M., Neveling K., Nillesen W.M., Moore-Barton H.L., Elmslie F.V., Toutain A., Amiel J., Malan V., Tsai A.C.-H., et al. Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat. Genet. 2012;44:639–641. doi: 10.1038/ng.2262. [DOI] [PubMed] [Google Scholar]
- 293.Potocki L., Chen K.-S., Park S.-S., Osterholm D.E., Withers M.A., Kimonis V., Summers A.M., Meschino W.S., Anyane-Yeboa K., Kashork C.D., et al. Molecular mechanism for duplication 17p11.2—The homologous recombination reciprocal of the Smith-Magenis microdeletion. Nat. Genet. 2000;24:84–87. doi: 10.1038/71743. [DOI] [PubMed] [Google Scholar]
- 294.Potocki L., Bi W., Treadwell-Deering D., Carvalho C.M.B., Eifert A., Friedman E.M., Glaze D., Krull K., Lee J.A., Lewis R.A., et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am. J. Hum. Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ghigna M.-R., Guignabert C., Montani D., Girerd B., Jaïs X., Savale L., Hervé P., Thomas de Montpréville V., Mercier O., Sitbon O., et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur. Respir. J. 2016;48:1668–1681. doi: 10.1183/13993003.00464-2016. [DOI] [PubMed] [Google Scholar]
- 296.Austin E.D., Phillips J.A., Cogan J.D., Hamid R., Yu C., Stanton K.C., Phillips C.A., Wheeler L.A., Robbins I.M., Newman J.H., et al. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir. Res. 2009;10 doi: 10.1186/1465-9921-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Girerd B., Coulet F., Jaïs X., Eyries M., Van Der Bruggen C., De Man F., Houweling A., Dorfmüller P., Savale L., Sitbon O., et al. Characteristics of pulmonary arterial hypertension in affected carriers of a mutation located in the cytoplasmic tail of bone morphogenetic protein receptor type 2. Chest. 2015;147:1385–1394. doi: 10.1378/chest.14-0880. [DOI] [PubMed] [Google Scholar]
- 298.Ballif B.C., Theisen A., Rosenfeld J.A., Traylor R.N., Gastier-Foster J., Thrush D.L., Astbury C., Bartholomew D., McBride K.L., Pyatt R.E., et al. Identification of a recurrent microdeletion at 17q23.1q23.2 flanked by segmental duplications associated with heart defects and limb abnormalities. Am. J. Hum. Genet. 2010;86:454–461. doi: 10.1016/j.ajhg.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nimmakayalu M., Major H., Sheffield V., Solomon D.H., Smith R.J., Patil S.R., Shchelochkov O.A. Microdeletion of 17q22q23.2 encompassing TBX2 and TBX4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am. J. Med Genet. Part A. 2011;155:418–423. doi: 10.1002/ajmg.a.33827. [DOI] [PubMed] [Google Scholar]
- 300.German K., Deutsch G.H., Freed A.S., Dipple K.M., Chabra S., Bennett J.T. Identification of a deletion containing TBX4 in a neonate with acinar dysplasia by rapid exome sequencing. Am. J. Med Genet. Part A. 2019;179:842–845. doi: 10.1002/ajmg.a.61096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Suhrie K., Pajor N.M., Ahlfeld S.K., Dawson D.B., Dufendach K.R., Kitzmiller J.A., Leino D., Lombardo R.C., Smolarek T.A., Rathbun P.A., et al. Neonatal Lung Disease Associated with TBX4 Mutations. J. Pediatr. 2019;206:286–292.e1. doi: 10.1016/j.jpeds.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Iannuzzi M.C., Baughman R.P. Reverse phenotyping in sarcoidosis. Am. J. Respir. Crit. Care Med. 2007;175:4–5. doi: 10.1164/rccm.200610-1459ED. [DOI] [PubMed] [Google Scholar]
- 303.Stram M., Gigliotti T., Hartman D., Pitkus A., Huff S.M., Riben M., Henricks W.H., Farahani N., Pantanowitz L. Logical Observation Identifiers Names and Codes for Laboratorians. Arch. Pathol. Lab. Med. 2020;144:229–239. doi: 10.5858/arpa.2018-0477-RA. [DOI] [PubMed] [Google Scholar]
- 304.Grubert F., Zaugg J.B., Kasowski M., Ursu O., Spacek D.V., Martin A.R., Greenside P., Srivas R., Phanstiel D.H., Pekowska A., et al. Genetic Control of Chromatin States in Humans Involves Local and Distal Chromosomal Interactions. Cell. 2015;162:1051–1065. doi: 10.1016/j.cell.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Perenthaler E., Yousefi S., Niggl E., Barakat T.S. Beyond the Exome: The Non-coding Genome and Enhancers in Neurodevelopmental Disorders and Malformations of Cortical Development. Front. Cell. Neurosci. 2019;13:352. doi: 10.3389/fncel.2019.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Reyes-Palomares A., Gu M., Grubert F., Berest I., Sa S., Kasowski M., Arnold C., Shuai M., Srivas R., Miao S., et al. Remodeling of active endothelial enhancers is associated with aberrant gene-regulatory networks in pulmonary arterial hypertension. Nat. Commun. 2020;11:1673. doi: 10.1038/s41467-020-15463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Machado R.D., James V., Southwood M., Harrison R.E., Atkinson C., Stewart S., Morrell N.W., Trembath R.C., Aldred M.A. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation. 2005;111:607–613. doi: 10.1161/01.CIR.0000154543.07679.08. [DOI] [PubMed] [Google Scholar]
- 308.Aldred M.A., Comhair S.A., Varella-Garcia M., Asosingh K., Xu W., Noon G.P., Thistlethwaite P.A., Tuder R.M., Erzurum S.C., Geraci M.W., et al. Somatic Chromosome Abnormalities in the Lungs of Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2010;182:1153–1160. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Drake K.M., Comhair S.A., Erzurum S.C., Tuder R.M., Aldred M.A. Endothelial chromosome 13 deletion in congenital heart disease-associated pulmonary arterial hypertension dysregulates SMAD9 signaling. Am. J. Respir. Crit. Care Med. 2015;191:850–854. doi: 10.1164/rccm.201411-1985LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Best D.H., Vaughn C., McDonald J., Damjanovich K., Runo J.R., Chibuk J.M., Bayrak-Toydemir P. Mosaic ACVRL1 and ENG mutations in hereditary haemorrhagic telangiectasia patients. J. Med Genet. 2011;48:358–360. doi: 10.1136/jmg.2010.088286. [DOI] [PubMed] [Google Scholar]
- 311.Eyries M., Coulet F., Girerd B., Montani D., Humbert M., Lacombe P., Chinet T., Gouya L., Roume J., Axford M.M., et al. ACVRL1 germinal mosaic with two mutant alleles in hereditary hemorrhagic telangiectasia associated with pulmonary arterial hypertension. Clin. Genet. 2012;82:173–179. doi: 10.1111/j.1399-0004.2011.01727.x. [DOI] [PubMed] [Google Scholar]
- 312.McDonald J., Gedge F., Burdette A., Carlisle J., Bukjiok C.J., Fox M., Bayrak-Toydemir P. Multiple sequence variants in hereditary hemorrhagic telangiectasia cases: Illustration of complexity in molecular diagnostic interpretation. J. Mol. Diagn. 2009;11:569–575. doi: 10.2353/jmoldx.2009.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Culley M.K., Chan S.Y. Mitochondrial metabolism in pulmonary hypertension: Beyond mountains there are mountains. J. Clin. Investig. 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rhodes C.J., Ghataorhe P., Wharton J., Rue-Albrecht K.C., Hadinnapola C., Watson G., Bleda M., Haimel M., Coghlan G., Corris P.A., et al. Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension. Circulation. 2017;135:460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Farha S., Hu B., Comhair S., Zein J., Dweik R., Erzurum S.C., Aldred M.A. Mitochondrial Haplogroups and Risk of Pulmonary Arterial Hypertension. PLoS ONE. 2016;11:e0156042. doi: 10.1371/journal.pone.0156042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bris C., Goudenege D., Desquiret-Dumas V., Charif M., Colin E., Bonneau D., Amati-Bonneau P., Lenaers G., Reynier P., Procaccio V. Bioinformatics Tools and Databases to Assess the Pathogenicity of Mitochondrial DNA Variants in the Field of Next Generation Sequencing. Front. Genet. 2018;9:632. doi: 10.3389/fgene.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Bell J.T., Pai A.A., Pickrell J.K., Gaffney D.J., Pique-Regi R., Degner J.F., Gilad Y., Pritchard J.K. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 2011;12:R10. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cheung W.A., Shao X., Morin A., Siroux V., Kwan T., Ge B., Aïssi D., Chen L., Vasquez L., Allum F., et al. Correction to: Functional variation in allelic methylomes underscores a strong genetic contribution and reveals novel epigenetic alterations in the human epigenome. Genome Biol. 2019;20 doi: 10.1186/s13059-019-1702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Archer S.L., Marsboom G., Kim G.H., Zhang H.J., Toth P.T., Svensson E.C., Dyck J.R.B., Gomberg-Maitland M., Thébaud B., Husain A.N., et al. Epigenetic Attenuation of Mitochondrial Superoxide Dismutase 2 in Pulmonary Arterial Hypertension. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bonnet S., Michelakis E.D., Porter C.J., Andrade-Navarro M.A., Thébaud B., Bonnet S., Haromy A., Harry G., Moudgil R., Sean McMurtry M., et al. An Abnormal Mitochondrial–Hypoxia Inducible Factor-1α–Kv Channel Pathway Disrupts Oxygen Sensing and Triggers Pulmonary Arterial Hypertension in Fawn Hooded Rats. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 321.Hyun K., Jeon J., Park K., Kim J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017;49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhao L., Chen C.-N., Hajji N., Oliver E., Cotroneo E., Wharton J., Wang D., Li M., McKinsey T.A., Stenmark K.R., et al. Histone deacetylation inhibition in pulmonary hypertension: Therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126:455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hon C.-C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J.L., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J., et al. An atlas of human long non-coding RNAs with accurate 5’ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.St Laurent G., Wahlestedt C., Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Su H., Xu X., Yan C., Shi Y., Hu Y., Dong L., Ying S., Ying K., Zhang R. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir. Res. 2018;19 doi: 10.1186/s12931-018-0956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Jandl K., Thekkekara Puthenparampil H., Marsh L.M., Hoffmann J., Wilhelm J., Veith C., Sinn K., Klepetko W., Olschewski H., Olschewski A., et al. Long non-coding RNAs influence the transcriptome in pulmonary arterial hypertension: The role of PAXIP1-AS1. J. Pathol. 2019;247:357–370. doi: 10.1002/path.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang D., Xu H., Wu B., Jiang S., Pan H., Wang R., Chen J. Long non‑coding RNA MALAT1 sponges miR‑124‑3p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int. J. Mol. Med. 2019;44:871–884. doi: 10.3892/ijmm.2019.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Leung A., Trac C., Jin W., Lanting L., Akbany A., Sætrom P., Schones D.E., Natarajan R. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ. Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yang L., Liang H., Shen L., Guan Z., Meng X. LncRNA Tug1 involves in the pulmonary vascular remodeling in mice with hypoxic pulmonary hypertension via the microRNA-374c-mediated Foxc1. Life Sci. 2019;237:116769. doi: 10.1016/j.lfs.2019.116769. [DOI] [PubMed] [Google Scholar]
- 330.Zhang H., Liu Y., Yan L., Wang S., Zhang M., Ma C., Zheng X., Chen H., Zhu D. Long noncoding RNA Hoxaas3 contributes to hypoxia-induced pulmonary artery smooth muscle cell proliferation. Cardiovasc. Res. 2019;115:647–657. doi: 10.1093/cvr/cvy250. [DOI] [PubMed] [Google Scholar]
- 331.Xing Y., Zheng X., Fu Y., Qi J., Li M., Ma M., Wang S., Li S., Zhu D. Long Noncoding RNA-Maternally Expressed Gene 3 Contributes to Hypoxic Pulmonary Hypertension. Mol. Ther. 2019;27:2166–2181. doi: 10.1016/j.ymthe.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhu T.-T., Sun R.-L., Yin Y.-L., Quan J.-P., Song P., Xu J., Zhang M.-X., Li P. Long noncoding RNA UCA1 promotes the proliferation of hypoxic human pulmonary artery smooth muscle cells. Pflugers Arch. 2019;471:347–355. doi: 10.1007/s00424-018-2219-8. [DOI] [PubMed] [Google Scholar]
- 333.Sun Z., Nie X., Sun S., Dong S., Yuan C., Li Y., Xiao B., Jie D., Liu Y. Long Non-Coding RNA MEG3 Downregulation Triggers Human Pulmonary Artery Smooth Muscle Cell Proliferation and Migration via the p53 Signaling Pathway. Cell. Physiol. Biochem. 2017;42:2569–2581. doi: 10.1159/000480218. [DOI] [PubMed] [Google Scholar]
- 334.Zhu B., Gong Y., Yan G., Wang D., Qiao Y., Wang Q., Liu B., Hou J., Li R., Tang C. Down-regulation of lncRNA MEG3 promotes hypoxia-induced human pulmonary artery smooth muscle cell proliferation and migration via repressing PTEN by sponging miR-21. Biochem. Biophys. Res. Commun. 2018;495:2125–2132. doi: 10.1016/j.bbrc.2017.11.185. [DOI] [PubMed] [Google Scholar]
- 335.Gong J., Chen Z., Chen Y., Lv H., Lu H., Yan F., Li L., Zhang W., Shi J. Long non-coding RNA CASC2 suppresses pulmonary artery smooth muscle cell proliferation and phenotypic switch in hypoxia-induced pulmonary hypertension. Respir. Res. 2019;20:53. doi: 10.1186/s12931-019-1018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chen J., Guo J., Cui X., Dai Y., Tang Z., Qu J., Usha Raj J., Hu Q., Gou D. The Long Noncoding RNA LnRPT Is Regulated by PDGF-BB and Modulates the Proliferation of Pulmonary Artery Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2018;58:181–193. doi: 10.1165/rcmb.2017-0111OC. [DOI] [PubMed] [Google Scholar]
- 337.Xiang Y., Zhang Y., Tang Y., Li Q. MALAT1 Modulates TGF-β1-Induced Endothelial-to-Mesenchymal Transition through Downregulation of miR-145. Cell. Physiol. Biochem. 2017;42:357–372. doi: 10.1159/000477479. [DOI] [PubMed] [Google Scholar]
- 338.Neumann P., Jaé N., Knau A., Glaser S.F., Fouani Y., Rossbach O., Krüger M., John D., Bindereif A., Grote P., et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat. Commun. 2018;9:237. doi: 10.1038/s41467-017-02431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kang H., Louie J., Weisman A., Sheu-Gruttadauria J., Davis-Dusenbery B.N., Lagna G., Hata A. Inhibition of microRNA-302 (miR-302) by bone morphogenetic protein 4 (BMP4) facilitates the BMP signaling pathway. J. Biol. Chem. 2012;287:38656–38664. doi: 10.1074/jbc.M112.390898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pullamsetti S.S., Doebele C., Fischer A., Savai R., Kojonazarov B., Dahal B.K., Ghofrani H.A., Weissmann N., Grimminger F., Bonauer A., et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 341.Brock M., Samillan V.J., Trenkmann M., Schwarzwald C., Ulrich S., Gay R.E., Gassmann M., Ostergaard L., Gay S., Speich R., et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur. Heart J. 2014;35:3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 342.Caruso P., MacLean M.R., Khanin R., McClure J., Soon E., Southgate M., MacDonald R.A., Greig J.A., Robertson K.E., Masson R., et al. Dynamic Changes in Lung MicroRNA Profiles During the Development of Pulmonary Hypertension due to Chronic Hypoxia and Monocrotaline. Arterioscler. Thromb. Vasc. Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 343.Uchida S., Dimmeler S. Long Noncoding RNAs in Cardiovascular Diseases. Circ. Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 344.Thum T., Condorelli G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ. Res. 2015;116:751–762. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 345.Chinnappan M., Mohan A., Agarwal S., Dalvi P., Dhillon N.K. Network of MicroRNAs Mediate Translational Repression of Bone Morphogenetic Protein Receptor-2: Involvement in HIV-Associated Pulmonary Vascular Remodeling. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Durrington H.J., Upton P.D., Hoer S., Boname J., Dunmore B.J., Yang J., Crilley T.K., Butler L.M., Blackbourn D.J., Nash G.B., et al. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J. Biol. Chem. 2010;285:37641–37649. doi: 10.1074/jbc.M110.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Dalvi P., O’Brien-Ladner A., Dhillon N.K. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: Implications for HIV-related pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2013;33:2585–2595. doi: 10.1161/ATVBAHA.113.302054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mansoor J.K., Schelegle E.S., Davis C.E., Walby W.F., Zhao W., Aksenov A.A., Pasamontes A., Figueroa J., Allen R. Analysis of volatile compounds in exhaled breath condensate in patients with severe pulmonary arterial hypertension. PLoS ONE. 2014;9:e95331. doi: 10.1371/journal.pone.0095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hakim M., Broza Y.Y., Barash O., Peled N., Phillips M., Amann A., Haick H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012;112:5949–5966. doi: 10.1021/cr300174a. [DOI] [PubMed] [Google Scholar]
- 350.Montani D., Lau E.M., Descatha A., Jaïs X., Savale L., Andujar P., Bensefa-Colas L., Girerd B., Zendah I., Le Pavec J., et al. Occupational exposure to organic solvents: A risk factor for pulmonary veno-occlusive disease. Eur. Respir. J. 2015;46:1721–1731. doi: 10.1183/13993003.00814-2015. [DOI] [PubMed] [Google Scholar]
- 351.Barker D. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet. 1986;327:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 352.Barker D.J.P., Osmond C., Winter P.D., Margetts B., Simmonds S.J. Weight in Infancy and Death from Ischaemic Heart Disease. Lancet. 1989;334:577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 353.Hales C.N., Barker D.J., Clark P.M., Cox L.J., Fall C., Osmond C., Winter P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Stern D.A., Morgan W.J., Wright A.L., Guerra S., Martinez F.D. Poor airway function in early infancy and lung function by age 22 years: A non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Berry C.E., Billheimer D., Jenkins I.C., Lu Z.J., Stern D.A., Gerald L.B., Carr T.F., Guerra S., Morgan W.J., Wright A.L., et al. A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am. J. Respir. Crit. Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Mann S.L., Wadsworth M.E., Colley J.R. Accumulation of factors influencing respiratory illness in members of a national birth cohort and their offspring. J. Epidemiol. Community Health. 1992;46:286–292. doi: 10.1136/jech.46.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Grigoriadis S., Vonderporten E.H., Mamisashvili L., Tomlinson G., Dennis C.-L., Koren G., Steiner M., Mousmanis P., Cheung A., Ross L.E. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: Systematic review and meta-analysis. BMJ. 2014;348:f6932. doi: 10.1136/bmj.f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Marques F.Z. Missing Heritability of Hypertension and Our Microbiome. Circulation. 2018;138:1381–1383. doi: 10.1161/CIRCULATIONAHA.118.036224. [DOI] [PubMed] [Google Scholar]
- 359.Ranchoux B., Bigorgne A., Hautefort A., Girerd B., Sitbon O., Montani D., Humbert M., Tcherakian C., Perros F. Gut-Lung Connection in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2017;56:402–405. doi: 10.1165/rcmb.2015-0404LE. [DOI] [PubMed] [Google Scholar]
- 360.Sofianopoulou E., Kaptoge S., Gräf S., Hadinnapola C., Treacy C.M., Church C., Coghlan G., Gibbs J.S.R., Haimel M., Howard L.S., et al. Traffic exposures, air pollution and outcomes in pulmonary arterial hypertension: A UK cohort study analysis. Eur. Respir. J. 2019;53 doi: 10.1183/13993003.01429-2018. [DOI] [PubMed] [Google Scholar]
- 361.Gazzo A., Raimondi D., Daneels D., Moreau Y., Smits G., Van Dooren S., Lenaerts T. Understanding mutational effects in digenic diseases. Nucleic Acids Res. 2017;45:e140. doi: 10.1093/nar/gkx557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Eichstaedt C.A., Song J., Benjamin N., Harutyunova S., Fischer C., Grünig E., Hinderhofer K. EIF2AK4 mutation as “second hit” in hereditary pulmonary arterial hypertension. Respir. Res. 2016;17:141. doi: 10.1186/s12931-016-0457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Pousada G., Baloira A., Valverde D. Pulmonary arterial hypertension associated with several mutations. Eur. Respir. J. 2016;48:PA2478. [Google Scholar]