Abstract
Simple Summary
Ensiling total mixed ration allows preservation and saves labor for small farms. This study evaluated the substitution relationship between lactic acid bacteria (Lactobacillus plantarum) and silage components, and verified the practicality of preservative (potassium sorbate) in total mixed ration silage. The results showed that potassium sorbate greatly improved the preservation efficiency of total mixed ration silages. The alfalfa silage could directly produce an acidic environment for fresh total mixed ration before ensiling and showed comparable function to inoculant in the improvement of fermentation quality. Therefore, the application of the inoculant is not necessary when the total mixed ration contains a certain percentage of silage. These findings could provide guidance for farmers to avoid the blind use of inoculants and the spoilage of total mixed ration silage, which could directly improve economic efficiency.
Abstract
This study aimed to evaluate the effect of the application of an inoculant and a preservative on the fermentation quality, in vitro digestibility, and aerobic stability of alfalfa silage-based fermented total mixed ration (TMR). The TMR was ensiled with (1) no additives (control), (2) Lactobacillus plantarum (LP), or (3) potassium sorbate (PS). The V-scores of all silages were higher than 80 points during the 30 days of ensiling. The addition of LP and PS had no effects on the in vitro parameters, such as in vitro digestibility and in vitro gas production (p > 0.05). LP-treated silage showed similar fermentation quality and comparable aerobic stability to the control (110 h). The LP only decreased the ammonia nitrogen (NH3-N) content (p < 0.05) during ensiling. The PS significantly increased the pH of TMR silages (p < 0.05). Meanwhile, the addition of PS improved the aerobic stability (>162 h) of TMR silage, indicated by the higher water-soluble carbohydrate content and lower NH3-N content in comparison with those in the control after aerobic exposure (p < 0.05). The improvement in fermentation quality is extremely small in terms of applying LP in TMR silage based on a large percentage of other silage ingredients. The PS is effective in conserving unpacked TMR silage and showed the potential to reduce the risk of ruminal acidosis in livestock.
Keywords: TMR silage, potassium sorbate, Lactobacillus plantarum, fermentation quality, in vitro digestibility, aerobic stability
1. Introduction
Silage is a product based on fermentation, whereby lactic acid bacteria (LAB) convert water-soluble carbohydrates (WSC) to organic acids under anaerobic conditions. Additives such as LAB, chemicals, and enzymes are often applied in silage to enhance its preservation [1]. Well-preserved silage has dominant lactic acid content, low NH3-N content, and negligible butyric acid content [2]. The appropriate adjustment in density, moisture content, chopping length, and the application of additives can significantly improve the fermentation quality, digestibility, and aerobic stability of silage.
Total mixed ration (TMR) is a form of complete formula feed consisting of roughage, concentrate, minerals, vitamins, and other additives in certain proportions. It is widely used to provide ruminants with adequate and balanced nutrition, which can stabilize microbial function and enhance energy and protein utilization in the rumen [3]. However, the processing of TMR requires professional equipment or sufficient labor. Fresh TMR is also a highly deteriorative feedstuff that cannot be preserved for long periods. Ensiling can prevent the spoilage of TMR and improve its palatability by anaerobic fermentation [4]. Baled TMR silages can be transported to provide year-round nutritional balance feed for small-scale farms that lack labor.
Silage is also a common roughage in TMR, which has low pH and a large number of lactic acid bacteria attached to it. Fermented feedstuffs have been successfully used as raw materials for TMR silage in industry [5]. Sometimes a part of protein feed may be replaced by relatively inexpensive alfalfa (Medicago sativa) silage with poor quality in TMR production to reduce feeding costs. Alfalfa silage is favorable for ruminal fermentation and can also improve the dry matter intake of cows [6]. Nishino et al. [7] found that the LAB species in TMR silages were selected during the ensiling process, and the bacterial community was unrelated to the ingredient crop silages. It was not conclusive as to whether the silage could directly stabilize the fermentation of TMR silage when the single silage composition accounted for more than half of the dry matter of the ingredients. Lactobacillus plantarum (LP) has been added to TMR silage and has proven to be effective in altering fermentation characteristics [8,9]. However, few studies have investigated whether it is necessary to reinoculate LP for TMR silage based on a large percentage of other silage ingredients, as well-fermented silage might already play the role of inoculant. Furthermore, the main factor limiting the use of TMR silage by ruminants is the digestibility of the fiber. In an early study, LP improved the in vitro dry matter digestibility (DMD) of TMR silages [9]. Thus, an inoculant of LP was applied in alfalfa silage-based TMR silage to estimate the improvement of digestibility and to verify the necessity of inoculation.
TMR silages are inherently unstable after re-exposure to air during the feed-out phase [10]. It is a common scenario that unpacked TMR silage bales cannot be completely fed in time. It may take more than 5 days to consume an 800–1000 kg TMR silage bale for some small-scale family farms. Potassium sorbate (PS) is a typical inexpensive preservative. The efficiency of PS in silage preservation has also been demonstrated for a variety of crops, such as fescue, Leymus chinensis, and corn [11,12,13]. Therefore, PS can be a potential antiseptic factor for the TMR silage bale. However, to the best of our knowledge, no research has investigated the effect of PS on TMR silage preservation. This study aimed to estimate the performance of PS in the preservation of TMR silages.
Therefore, this article reports on the effects of applying LP inoculant and PS on the fermentation quality, in vitro digestibility, and aerobic stability of TMR silage based on alfalfa silage.
2. Materials and Methods
2.1. Total Mixed Ration (TMR) Silage Preparation
The chemical composition of the ingredients and the composition of the TMR are given in Table 1 and Table 2, respectively. First-cutting alfalfa (Medicago sativa L) was harvested at the full-bloom stage in Guyuan, Ningxia, China (106°17′ E, 36°28′ N, elevation 1529 m), on 19 June 2019. The wilted alfalfa was chopped to a length of 2–5 cm and baled without any additives by a round baler (Comprima, Krone, Germany). Alfalfa silage bales were unpacked after 55 days of ensiling. Crushed corn cob and corn grain were obtained from a private medium-scale cattle farm in Guyuan, Ningxia, China. The mixed concentrate was purchased from Botai Company (Guyuan, China) and was composed of soybean meal, rapeseed meal, cotton meal, corn gluten meal, distillers’ dried grains, corn peel, and vitamin–mineral mix. TMR mixtures (750 g, fresh matter) were mixed well and then tightly packed into plastic silos (1 L). The TMR was treated with (1) distilled water (control), (2) LP, and (3) PS. The application rate of LP and the concentration of PS applied to each TMR were 106 colony-forming units (cfu) g−1 and 2 g kg−1 on a fresh matter (FM) basis, respectively. Additives were dissolved in water, and the control treatment was sprayed with an equal amount of water. There were 15 silos for each treatment, among which triplicate silos were opened on days 7 and 14 for TMR silage fermentation quality determination, while the other 9 silos were opened on day 30 for both TMR silage quality determination and the aerobic stability test. Anaerobic fermentation was conducted at an ambient temperature of 22–28 °C. At the time of ensiling, triplicate silos of well-mixed TMR were taken as 0-day TMR silages for initial characterization analysis.
Table 1.
Chemical composition and fermentation characteristics of ingredients used for the total mixed ration.
| Item 1 | Alfalfa Silage | Corn Cob | Corn Grain | Mixed 2 Concentrate |
|---|---|---|---|---|
| Dry matter (g kg−1 FM) | 446.82 | 940.04 | 887.88 | 921.03 |
| Crude protein (g kg−1 DM) | 145.83 | 35.87 | 83.01 | 325.29 |
| Neutral detergent fiber (g kg−1 DM) | 423.30 | 810.26 | 98.50 | 263.31 |
| Acid detergent fiber (g kg−1 DM) | 296.02 | 417.88 | 38.09 | 110.84 |
| Ether extract (g kg−1 DM) | 41.69 | 38.21 | 48.84 | 58.09 |
| Lactic acid (g kg−1 DM) | 42.47 | - | - | - |
| Acetic acid (g kg−1 DM) | 4.56 | - | - | - |
| Propionic acid (g kg−1 DM) | 10.00 | - | - | - |
| Butyric acid (g kg−1 DM) | ND | - | - | - |
| Ammonia nitrogen (g kg−1 TN) | 31.06 | - | - | - |
ND, not detected. 1 FM, fresh matter; DM, dry matter; TN, total nitrogen. 2 The mixed concentrate was purchased from Botai Company (Guyuan, China) and was composed of soybean meal, rapeseed meal, cotton meal, corn gluten meal, distillers’ dried grains, corn peel, and vitamin–mineral mix.
Table 2.
Ingredient composition and chemical composition of the total mixed ration.
| Item 1 | TMR |
|---|---|
| Ingredient composition (g kg−1 DM) | |
| Alfalfa silage | 600 |
| Corn cob | 60 |
| Corn grain | 240 |
| Mixed concentrate 2 | 100 |
| Total | 1000 |
| Chemical composition (g kg−1 FM) | |
| Dry matter | 487.51 |
| Crude protein | 146.18 |
| Water soluble carbohydrate | 15.33 |
| Neutral detergent fiber | 349.61 |
| Acid detergent fiber | 231.16 |
1 DM, dry matter; FM, fresh matter. 2 The mixed concentrate was purchased from Botai Company (Guyuan, China) and was composed of soybean meal, rapeseed meal, cotton meal, corn gluten meal, distillers’ dried grains, corn peel, and vitamin–mineral mix.
2.2. Chemical and Microbiological Analyses
The contents of each silo were removed and blended thoroughly after opening. The dry matter (DM) content was determined by a forced-draft oven at 65 °C for 48 h. The dried samples were ground to allow them to pass through a 1.0-mm screen and were used for chemical analysis. The crude protein (CP) content was determined according to the Kjeldahl procedure [14] and calculated as total N × 6.25. Examination of the ether extract (EE) was conducted by the method of the Association of Official Analytical Chemists (AOAC) [15]. The WSC content was analyzed by the anthrone–sulfuric acid method [16]. Neutral detergent fiber (aNDF) and acid detergent fiber (ADF) analyses were performed following the procedure described by Van Soest et al. [17]. Sodium sulfite and α-amylase were applied for aNDF determination, and both the aNDF and ADF content reported include residual ash.
TMR silage samples (20 g) from each silo were mixed with 180 mL of sterilized distilled water and then homogenized for 2 min in a blender jar. The mixtures were then filtered through 4 layers of cheesecloth and filter paper. The filtrate was centrifuged at 10,000× g at 4 °C for 10 min to analyze the pH and ammonia nitrogen (NH3-N) content. The pH was measured using an electrode (PHS-3C, INESA Scientific Instrument, Shanghai, China). The NH3-N content was determined according to the sodium hypochlorite and phenol method [18]. The supernatant was further processed with a 0.22-μm dialyzer to analyze organic acids. Lactic, acetic, propionic, and butyric acids were analyzed by high-performance liquid chromatography according to Tian et al. [19]. The V-score was calculated to evaluate the silage quality according to the volatile fatty acid and NH3-N contents [20]. Enumerations of LAB, yeasts, molds, and coliform bacteria were performed from fresh TMR silages using the method of Wang et al. [21].
2.3. In Vitro Incubation and Degradability Measurement
In vitro fermentation was carried out in Ankom RFS bottles using the pressure transducer technique (Ankom Technologies, Macedon, NY, USA) as described by Yuan et al. [4]. Rumen fluid was collected from 4 dry Holstein cows and kept at 39 °C. These cows were fed 3.5 kg (FM) of wheat straw, 2.8 kg of oat hay, 11 kg of whole-plant corn silage, 1 kg of rapeseed meal, 1 kg of soybean meal, 0.5 kg of distiller’s dried grains with solubles, 1.3 kg of corn peel, 0.05 kg of urea, and 0.2 kg of 5% premix. Filtered rumen fluid was mixed with buffer at a ratio of 1:4. The buffer consisted of 1330 mL of buffer A (KH2PO4, 10.0 g/L; MgSO4·7H2O, 0.5 g/L; NaCl, 0.5 g/L; CaCl2·2H2O, 0.1 g/L; and urea, 0.5 g/L) and 266 mL of buffer B (Na2CO3, 15.0 g/L and Na2S·9H2O, 1.0 g/L). Approximately 1 g of ground samples from 30-day TMR silage were added to rumen fluid–buffer mixtures in Ankom RFS bottles under CO2. The mixtures were incubated at 39 °C, and gas production in the bottles was measured every hour for 48 h. The blank was 3 RFS bottles with the only inoculum added. Cumulative gas production data were fitted to a gas production model modified from the Gompertz growth equation [22]:
| V(t) = V(∞) exp [−exp (ke (λ − t)/V(∞) + 1)] | (1) |
where V(t) is cumulative gas production (mL), V(∞) is maximal cumulative gas production (mL), k is maximum gas production rate (mL·h−1), λ is lag time (h), t is time elapsed (h), and e is exponential of 1 (2.718).
In vitro DMD and in vitro neutral detergent fiber digestibility (NDFD) were determined with an Ankom DaisyII incubator (Ankom Technologies, Macedon, NY, USA). Approximately 0.5 g of ground samples was put into the same fluid–buffer mixtures and incubated under CO2 for 48 h. The reduced weight of TMR samples was used to calculate the in vitro DMD. The aNDF content of the residue after incubation was also determined to calculate the in vitro NDFD.
2.4. Aerobic Stability Test
After 30 days of ensiling, 9 silos of each treatment were opened, and every third silo was mixed thoroughly and taken as the 30-day TMR silage sample (also as the 0-day aerobic exposure sample) for fermentation quality analysis. The remaining TMR silage of the 3 silos was placed into 4 new 1 L plastic silos without compaction (marked with days 1, 3, 5, and 7). The new silos were covered with 4 layers of cheesecloth and stored at ambient temperature (25 °C). A total of 12 silos marked with different exposure times for each treatment were exposed to air for the aerobic stability test.
A multichannel data logger (MDL-1048A, Tianhe, Shanghai, China) was used to record the temperature of the exposure samples every half hour. Aerobic stability was defined as the time before TMR silage temperatures increased by 2 °C above ambient temperature. Triplicate silos of aerobic exposure samples were collected as marked after 1, 3, 5, and 7 days to determine the pH and NH3-N, organic acid, DM, CP, and WSC levels, as well as microbial counts.
2.5. Statistical Analyses
Microbial data were log10-transformed and presented on a wet-weight basis. The data were subjected to one-way or two-way analysis of variance with fixed effects of ensiling time (or exposure time) and additives, analyzed by SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range method was used to judge the differences among the means of the treatments and ensiling days (or exposure days). Means were considered significantly different at p < 0.05.
3. Results
3.1. Fermentation Quality of TMR Silages
The changes in fermentative characteristics of TMR silages during ensiling are presented in Table 3. The interaction between additives and ensiling period significantly affected (p < 0.05) the pH; lactic acid, acetic acid, propionic acid, and NH3-N levels; and V-score. The effect of additives on the pH; lactic acid, propionic acid, and NH3-N levels; and V-score of the TMR silages during ensiling was evident (p < 0.05). The days of ensiling also had a significant effect (p < 0.05) on all fermentative characteristics except butyric acid.
Table 3.
Changes in fermentative characteristics during ensiling of total mixed ration (TMR) silages.
| Item 1 | Treatment 2 | Days of Ensiling | SEM | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 30 | D | T | D × T | |||
| Fermentative Characteristics | |||||||||
| pH | Control | 4.65 A | 4.32 bB | 4.3b B | 4.26 bB | 0.01 | <0.001 | <0.001 | <0.001 |
| LP | 4.70 A | 4.26 bB | 4.24 bB | 4.25 bB | |||||
| PS | 4.67 A | 4.70 aA | 4.72 aA | 4.59 aB | |||||
| LA | Control | 36.12 D | 53.27 aC | 64.43 aB | 75.44 aA | 0.77 | <0.001 | <0.001 | <0.001 |
| LP | 30.05 C | 39.47 bB | 71.91 aA | 69.39 aA | |||||
| PS | 33.47 C | 18.07 cD | 51.05 bB | 56.23 bA | |||||
| AA | Control | 3.56 B | 3.16 aB | 4.09 bB | 12.06 A | 0.10 | <0.001 | 0.230 | 0.009 |
| LP | 3.27 C | 2.39 aC | 5.15 aB | 12.25 A | |||||
| PS | 3.44 B | 1.09 bC | 4.61 abB | 12.39 A | |||||
| PA | Control | 9.83 AB | 8.31 aB | 9.89 bAB | 12.51 A | 0.20 | <0.001 | 0.008 | <0.001 |
| LP | 9.77 B | 5.84 abC | 5.84 cC | 12.40 A | |||||
| PS | 9.51 B | 3.83 bC | 12.86 aA | 12.32 A | |||||
| BA | Control | ND | ND | ND | ND | - | - | - | - |
| LP | ND | ND | ND | ND | |||||
| PS | ND | ND | ND | ND | |||||
| NH3-N | Control | 22.09 C | 5.60 D | 57.44 aA | 42.53 aB | 0.64 | <0.001 | <0.001 | 0.004 |
| LP | 21.80 C | 3.53 D | 41.17 bA | 31.40 bB | |||||
| PS | 19.52 C | 5.08 C | 56.44 aA | 41.14 aB | |||||
| V-score | Control | 91.31 | 92.71 b | 89.51 b | 90.00 | 0.22 | <0.001 | 0.019 | 0.001 |
| LP | 91.50 C | 95.21 abA | 93.09 aB | 90.00 D | |||||
| PS | 91.58 B | 97.76 aA | 88.71 bC | 90.00 BC | |||||
Means within the same row (A–D) or within the same column (a–c) with different superscripts differ significantly from each other (p < 0.05). SEM, standard error of the mean; ND, not detected. 1 LA, lactic acid (g kg−1 DM); AA, acetic acid (g kg−1 DM); PA, propionic acid (g kg−1 DM); BA, butyric acid (g kg−1 DM); NH3-N, ammonia nitrogen (g kg−1 TN); V-score, score used to evaluate the silage quality according to the volatile fatty acid and NH3-N contents; TN, total nitrogen; DM, dry matter. 2 LP, Lactobacillus plantarum inoculant; PS, potassium sorbate. 3 D, effect of ensiling days; T, effect of treatment; D × T, interaction between ensiling days and treatment.
During the ensiling procedure (30 days), the organic acid contents in all silages gradually increased (p < 0.05). LP silages showed lower NH3-N content than other silages (p < 0.05). The NH3-N content decreased during the first 7 days, but increased during the next 7 days of ensiling (p < 0.05). The pH of the PS silages remained at 4.72 in the first 14 days, while the pH of the control and LP silages were both lower than 4.32 after 7 days of ensiling. The content of acetic acid in all silages increased greatly during days 14–30 (p < 0.05). The PS silages showed significantly higher (p < 0.05) WSC content than the other silages (Table 4). No butyric acid was detected in TMR silages during the 30 days of ensiling.
Table 4.
Chemical compositions and in vitro digestibility of the total mixed ration silages after 30 days of ensiling.
| Item 1 | Treatment 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | LP | PS | |||
| Chemical compositions | |||||
| Dry matter (g kg−1 FM) | 487.57 | 480.56 | 481.02 | 3.54 | 0.732 |
| Crude protein (g kg−1 DM) | 150.40 | 154.20 | 153.60 | 1.06 | 0.331 |
| Water soluble carbohydrate (g kg−1 DM) | 9.03 B | 7.03 B | 29.01 A | 3.59 | <0.001 |
| Neutral detergent fiber (g/kg (g kg−1 DM) | 366.29 | 347.68 | 342.78 | 8.40 | 0.548 |
| Acid detergent fiber (g kg−1 DM) | 231.43 | 222.87 | 218.45 | 6.91 | 0.789 |
| In vitro degradability | |||||
| DMD (g kg−1) | 579.90 | 606.71 | 608.87 | 6.37 | 0.113 |
| NDFD (g kg−1) | 355.29 | 354.55 | 330.79 | 21.84 | 0.902 |
| In vitro gas production parameters | |||||
| V24h (mL) | 57.15 | 57.01 | 56.57 | 0.72 | 0.957 |
| V48h (mL) | 65.68 | 65.10 | 65.04 | 0.80 | 0.951 |
| V(∞) (mL) | 64.10 | 63.74 | 63.43 | 0.79 | 0.955 |
| k (mL·h−1) | 3.60 | 3.69 | 3.58 | 0.08 | 0.861 |
Means within the same row (A,B) with different superscripts differ significantly from each other (p < 0.05). SEM, standard error of the mean. 1 FM, fresh matter; DM, dry matter; DMD, dry matter digestibility; NDFD, neutral detergent fiber digestibility; V24h, 24-h cumulative gas production; V48h, 48-h cumulative gas production; V(∞), maximal cumulative gas production; k, maximum gas production rate. 2 LP, Lactobacillus plantarum inoculant; PS, potassium sorbate.
3.2. In Vitro Degradability of TMR Silages
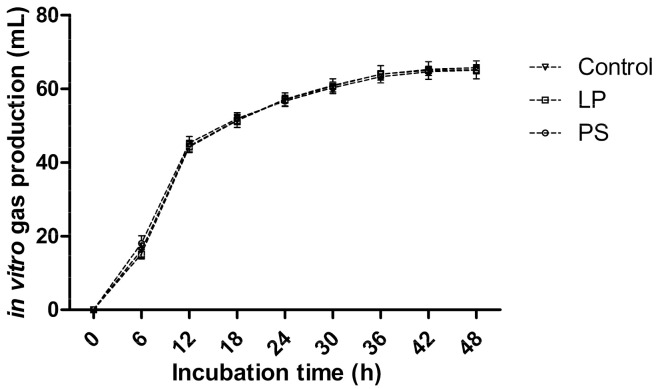
The in vitro gas production profiles and in vitro parameters of 30-day TMR silages are presented in Figure 1 and Table 4. The curves for gas production of different treatments almost coincided. The additives showed no effects on in vitro DMD, in vitro NDFD, in vitro gas productions, and in vitro maximum gas production rate (p > 0.05).
Figure 1.
Gas production profiles from in vitro fermentation of the TMR silage for 48 h (bars indicate standard errors of the means). LP, Lactobacillus plantarum inoculant; PS, potassium sorbate.
3.3. Aerobic Stability of TMR Silages
The changes in the fermentative characteristics and chemical compositions of TMR silages during the aerobic exposure period are presented in Table 5. Significant interactions between additives and days of aerobic exposure were observed for the pH, CP, and NH3-N levels and V-score (p < 0.05). The additives significantly affected the pH; CP, WSC, lactic acid, and NH3-N levels; and V-score (p < 0.05). The days of aerobic exposure had a significant effect on the CP and WSC content and fermentative characteristics (p < 0.05), except for propionic acid and butyric acid.
Table 5.
Changes in fermentative characteristics and chemical compositions of TMR silages after exposure to air.
| Item 1 | Treatment 2 | Days of Air Exposure | SEM | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | D | T | D × T | |||
| Fermentative characteristics | ||||||||||
| pH | Control | 4.26 bC | 4.28 bC | 4.28 bC | 4.53 B | 7.52 aA | 0.01 | <0.001 | <0.001 | <0.001 |
| LP | 4.25 bC | 4.28 bC | 4.31 bC | 4.56 B | 7.51 aA | |||||
| PS | 4.59 a | 4.63 a | 4.65 a | 4.68 | 4.70 b | |||||
| LA | Control | 75.44 aA | 71.51 A | 71.55 abA | 71.55 A | 42.46 B | 1.17 | 0.006 | 0.001 | 0.178 |
| LP | 69.39 aAB | 67.69 AB | 80.73 aA | 72.89 A | 48.67 B | |||||
| PS | 56.23 b | 56.16 | 44.22 b | 51.16 | 50.84 | |||||
| AA | Control | 12.06 A | 11.20 A | 9.41 A | 4.80 bB | 2.61 B | 0.35 | <0.001 | 0.172 | 0.224 |
| LP | 12.25 A | 10.93 AB | 11.46 A | 5.64 abC | 6.02 BC | |||||
| PS | 12.39 A | 11.45 AB | 7.85 C | 8.73 aBC | 7.47 C | |||||
| PA | Control | 12.51 | 11.91 | 10.82 | 10.75 | 9.48 b | 0.32 | 0.224 | 0.178 | 0.907 |
| LP | 12.41 | 11.20 | 12.63 | 12.30 | 11.19 a | |||||
| PS | 12.32 | 11.37 | 9.47 | 9.74 | 9.28 b | |||||
| BA | Control | ND | ND | ND | ND | ND | - | - | - | - |
| LP | ND | ND | ND | ND | ND | |||||
| PS | ND | ND | ND | ND | ND | |||||
| NH3-N | Control | 42.53 aB | 47.69 abB | 41.71 B | 44.30 B | 135.85 aA | 2.20 | <0.001 | 0.001 | <0.001 |
| LP | 31.40 bB | 39.74 bB | 32.88 B | 53.18 B | 164.96 aA | |||||
| PS | 41.14 a | 52.15 a | 44.24 | 41.51 | 44.04 b | |||||
| V-score | Control | 90.00 A | 89.84 A | 90.65 A | 89.89 A | 67.90 abB | 0.75 | <0.001 | 0.002 | <0.001 |
| LP | 90.00 A | 90.00 A | 90.00 A | 89.37 A | 54.97 bB | |||||
| PS | 90.00 | 89.45 | 90.80 | 90.05 | 89.60 a | |||||
| Chemical compositions | ||||||||||
| DM | Control | 487.57 | 492.51 | 494.73 | 500.26 | 498.86 | 2.33 | 0.466 | 0.058 | 0.918 |
| LP | 480.56 | 483.04 | 489.44 | 482.92 | 476.77 | |||||
| PS | 481.02 | 489.89 | 499.81 | 499.66 | 504.83 | |||||
| CP | Control | 150.40 A | 150.88 A | 152.19 bA | 153.71 A | 144.00 bB | 0.39 | 0.003 | 0.015 | <0.001 |
| LP | 154.20 AB | 151.60 B | 157.18 aA | 155.84 AB | 146.07 bC | |||||
| PS | 153.60 AB | 154.03 AB | 149.20 bC | 151.04 BC | 155.46 aA | |||||
| WSC | Control | 9.03 b | 11.71 b | 8.76 b | 7.92 b | 8.78 c | 0.35 | 0.037 | <0.001 | 0.202 |
| LP | 7.03 bC | 8.11 bBC | 6.91 bC | 10.22 bAB | 11.83 bA | |||||
| PS | 29.01 a | 32.86 a | 27.18 a | 29.34 a | 30.20 a | |||||
Means within the same row (A–C) or within the same column (a–c) with different superscripts differ significantly from each other (p < 0.05). SEM, standard error of the mean. 1 LA, lactic acid (g kg−1 DM); AA, acetic acid (g kg−1 DM); PA, propionic acid (g kg−1 DM); BA, butyric acid (g kg−1 DM); NH3-N, ammonia nitrogen (g kg−1 TN); V-score, score used to evaluate the silage quality according to the volatile fatty acid and NH3-N contents; TN, total nitrogen; FM, fresh matter; DM, dry matter (g kg−1 FM); CP, crude protein (g kg−1 DM); WSC, water-soluble carbohydrate (g kg−1 DM). 2 LP, Lactobacillus plantarum inoculant; PS, potassium sorbate. 3 D, effect of aerobic exposure day; T, effect of treatment; D × T, interaction between aerobic exposure day and treatment.
The pH of the control and LP silages significantly increased (p < 0.05) after 5 days of aerobic exposure, and it even increased above 7.50 with a substantial decrease in lactic acid content after 7 days of aerobic exposure. At the same time, the CP levels of the control and LP silages also decreased with a sharp increase in NH3-N content, and the V-scores of the control and LP silages were much lower than the PS silages (p < 0.05). Aerobic exposure had a relatively weak effect on the chemical compositions or fermentative characteristics of PS silages. TMR silages treated with PS also preserved greater amounts of WSC than other silages from treatments (p < 0.05). Additives showed no significant effect on DM content (p > 0.05). The levels of acetic acid and propionic acid in all silages steadily decreased during aerobic deterioration. No butyric acid was detected in TMR silages during the 7 days of air exposure.
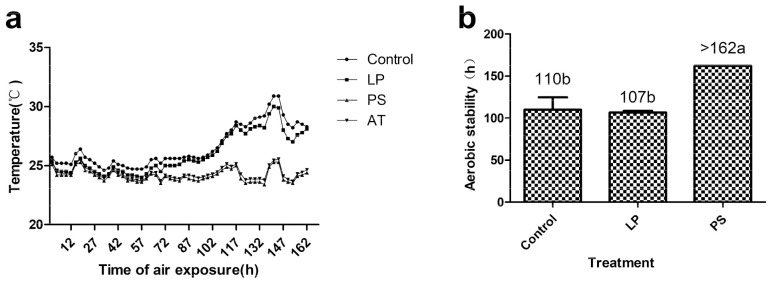
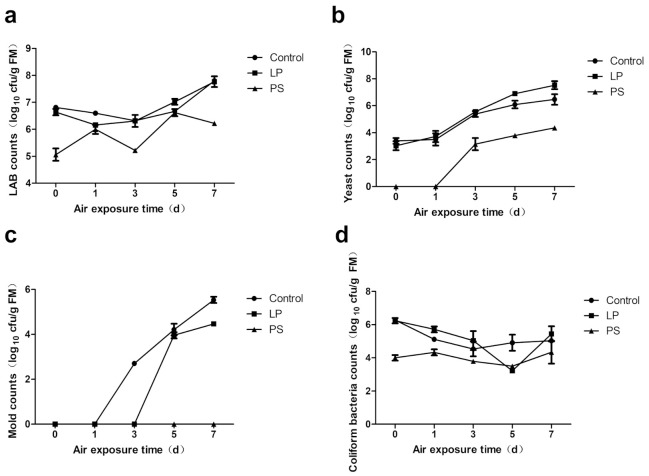
The dynamic changes in temperature during aerobic exposure are presented in Figure 2a. The periods during which TMR silages remained aerobically stable ranged from 107 to >162 h (Figure 2b). LP silages showed almost the same temperature trend and aerobic stability duration as control silages. The temperature of PS silages did not increase in the present experiment. Dynamic changes in viable microbial counts during aerobic exposure are shown in Figure 3. All the microbial counts of PS silages were lower than those of other groups (p < 0.05). The molds in the control silage were detected after 3 days, while those in the LP silage were detected after 5 days.
Figure 2.
Dynamic changes in temperatures (a) and hours of aerobic stability (b) of TMR silages during air exposure (bars indicate standard error of the means). Values with different letters show significant differences among the treatments (p < 0.05). LP, Lactobacillus plantarum inoculant; PS, potassium sorbate; AT, ambient temperature.
Figure 3.
Dynamic changes in lactic acid bacteria (LAB) counts (a), yeast counts (b), mold counts (c), and coliform bacteria counts (d) of TMR silages during the processing of aerobic deterioration (bars indicate standard error of the means). cfu, colony-forming units; FM, fresh matter; LP, Lactobacillus plantarum inoculant; PS, potassium sorbate.
4. Discussion
4.1. Effects of Additives on the Fermentation Quality of TMR Silages
All TMR silages were well preserved during fermentation. Due to the low NH3-N content and lack of butyric acid detected in all silage samples, the V-scores of all silages were higher than 80 points, indicating good silage quality [2]. The significant interaction between the additives and ensiling days for V-score, organic acids, and NH3-N content might have been due to the different fermentation levels of the TMR silage at the seventh day. Due to the reductions in acetic acid and propionic acid content, all silages exhibited the highest V-score after 7 days of ensiling.
Generally, ensiling is performed to inhibit the growth of detrimental anaerobes by decreasing the pH, and inoculants such as LP could be used to accelerate this procedure. However, TMR silages could cause ruminal acidosis if the pH is too low, especially when the TMR silage contains high grain content, which is a cause for concern. Some buffer salts, such as ammonium formate, propionate, and sodium bicarbonate, were applied in silages in early studies, but none of these salts increased the pH of silage [23,24]. However, the PS showed an inhibitory effect on the growth of LAB in this study. The addition of PS decreased the contents of lactic acid and increased the pH of TMR silage during ensiling, which may reduce the risk of ruminal acidosis in livestock. The weaker fermentation in PS group preserved more WSC and also explained the significant interaction between the additives and ensiling days for pH. Ethanol has been proven to have a similar effect on the pH of TMR silage, but the cost may be higher than that of PS due to the addition of 25 mL kg−1 [4]. Further animal studies are required to judge the specific influence of PS-treated TMR silage in the rumen environment.
The NH3-N content of LP silages was lower than that of the control, suggesting that the inoculant outgrew the epiphytic LAB from alfalfa silage and dominated the fermentation, which reduced ammonia production from proteolysis [10,25]. All NH3-N levels decreased after 7 days of fermentation. These results may be due to some aerobic microorganisms from alfalfa silage or other ingredients that degrade NH3-N remaining active or becoming active again, such as Bacillus sp. strains [26]. Before the residual oxygen was completely consumed, the aerobic microorganisms and lactic acid bacteria may have degraded or utilized a portion of the remaining NH3-N, which was mainly derived from the unpacked alfalfa silage. The NH3-N content increased again after the aerobic microorganisms were inhibited. A similar result was reported by Anjos et al. [27], who found relatively low NH3-N content in re-ensiled sorghum silage. This finding may provide a strategy to reduce NH3-N content in silage-based TMR silages. However, the pH of the 0-day TMR silage was low, and no significant difference was found in the organic acid or WSC content between LP silages and control silages after 30 days of ensiling. The inoculation rates of the LAB strains are usually 105–106 cfu g−1 on an FM basis, which is often sufficient for the inoculants to become the predominant population in the TMR silage [25]. In contrast, the LAB counts in well-fermented alfalfa silage were proven to be much higher than 106 cfu g−1 on an FM basis [28,29]. Lactobacillus, which plays a critical role in fermentation, usually dominates the bacterial community in alfalfa silage [30]. The results suggested that the alfalfa silage could directly produce an acidic environment for fresh TMR before ensiling and had a comparable function to LAB inoculant in the improvement or stabilization of fermentation, except for NH3-N content. In future experiments, we will investigate the specific percentage of silage that can play the role of inoculant in the TMR silage.
4.2. Effects of Additives on the In Vitro Degradability of TMR Silages
Digestibility is commonly accepted as a measure of feed nutritional value and intake [2]. Some studies showed that LP has a promoting effect on the digestibility of TMR silages [8,31,32], whereas other studies showed that LP did not have such an effect [4,33,34]. The contradictions and inconsistencies may be caused by the other different characteristics of the LP inoculants or the different composition of TMR silage. Neither PS nor LP improved the in vitro digestibility of TMR silages after ensiling in this study. Filya et al. [35] found that DMD was correlated with various fiber constituents. Both aNDF and ADF contents of the treated groups were similar to those of the control group. Similar results were reported by Zhao et al. [2]. In vitro gas production can be used to estimate the rate and extent of ruminal DM degradation [36]. Meanwhile, this parameter is also an indicator that can be used for the prediction of DM intake [37]. However, there was no significant difference in any of these in vitro parameters, which could also be due to the similar fiber constituents among the ensiled TMR silages. In fact, gas production and DMD are also positively correlated [38]. The results indicated that the addition of PS or LP had no adverse or beneficial effects on rumen utilization of the TMR silage.
4.3. Effects of Additives on the Aerobic Stability of TMR Silages
Many experiments have proven that TMR silage with sufficient fermentation will have higher aerobic stability than fresh TMR [33,39,40]. In the present study, the aerobic stability of untreated TMR silage was less than 5 days, which may have been due to the relatively simple composition of the ingredients [40]. The short aerobic stabilization time further proved the necessity of applying preservatives in some TMR silages. PS is a well-known additive in the conservation of a variety of feeds because of its antifungal properties [12]. Yeast is the key factor in the aerobic deterioration of silage, and the silage more easily deteriorated when the number of yeasts reached 105 cfu g−1 (FM) [41]. Many yeast species could oxidatively metabolize lactic acid via the tricarboxylic acid cycle, which decreased the acidity of TMR silage and allowed for the growth of spoilage bacteria [42]. The PS inhibited the growth of yeasts and stabilized the acidic environment of silage. Therefore, TMR silages with PS showed low counts (<105 cfu g−1 FM) in all detrimental microorganisms and high aerobic stability during this aerobic stability test. Meanwhile, PS preserved more WSC and lactic acid, reduced silage protein breakdown, and kept the NH3-N content stable during the whole process of aerobic exposure, which directly contributed to high V-scores until the end of the test. Therefore, the significant interaction between additives and aerobic exposure day for pH, V-score, NH3-N, and CP content was due to the inhibition of yeasts in PS silage. Specifically, the PS silage showed better fermentation quality at the seventh day of exposure.
Numerous studies have demonstrated that inoculation with homofermentative LAB reduces the aerobic stability of silages [8,11,43]. However, the LP group showed aerobic stability similar to that of the control group in this study. Lactic acid and WSC were potential sources of available substrate for the growth of undesirable microorganisms, and their levels were negatively correlated with aerobic stability [44]. The comparable lactic acid, WSC, and protective volatile fatty acid contents of LP and control silages before exposure may partly account for the similar aerobic stability. The LP strain did not inhibit the growth of yeast, which was the main cause of aerobic spoilage [44], and this result is in agreement with the results of Cai et al. [45]. In previous research by Filya et al. [43], both corn and sorghum silages inoculated with LP showed higher mold counts than control silages after 5 days of aerobic exposure. However, it is hard to explain why the addition of LP delayed and inhibited the growth of mold in comparison with the control in this study. We interpret that the LP silages produced some substances with antioxidant and antifungal properties during ensiling. Out of curiosity, we tested the phenolic acid contents of the 30-day TMR silages. We found that 11.0% and 18.8% more ferulic acid was extracted from the LP silage than from the control and PS silages, respectively, which may partially explain the inhibition of mold growth of LP silage. Additional research is needed for further verification of the antifungal properties of the silages treated with LP.
5. Conclusions
The addition of LP and PS did not improve the digestibility of TMR silage in this study. Although LP slightly decreased the NH3-N content and inhibited the growth of molds during aerobic exposure, the alfalfa silage showed comparable function to LAB inoculant in the improvement of fermentation quality. When TMR silage contains enough single silage ingredient to stabilize fermentation, there is no need to apply LP. The PS greatly improved the aerobic stability of TMR silages and showed the potential to reduce the risk of ruminal acidosis in livestock by increasing the pH of the silages.
Author Contributions
Conceptualization, Y.X. and Z.Y.; methodology, Y.X. and Z.Y.; formal analysis, Y.X. and Z.Y.; data curation, Y.X. and Z.Y.; writing—original draft preparation, Y.X.; writing—review and editing, Y.X., S.X., W.L., M.W., Z.W., J.B., T.J., and Z.Y.; supervision, Z.Y.; project administration, Z.Y.; funding acquisition, Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Modern Agro-Industry Technology Research System (CARS-34), the Key Technologies Research and Demonstration for Efficient Utilization of Modern Artificial Grassland (2017BY082), the Demonstration Project of Exploitation and Utilization of High Quality Green and Rough Feed Resources (16190051), and the National Key Research and Development Program of China (2017YFD0502102).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muck R.E., Nadeau E.M.G., McAllister T.A., Contreras-Govea F.E., Santos M.C., Kung L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018;101:3980–4000. doi: 10.3168/jds.2017-13839. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J., Dong Z., Chen L., Wang S., Shao T. The replacement of whole-plant corn with bamboo shoot shell on the fermentation quality, chemical composition, aerobic stability and in vitro digestibility of total mixed ration silage. Anim. Feed Sci. Technol. 2020;259:114348. doi: 10.1016/j.anifeedsci.2019.114348. [DOI] [Google Scholar]
- 3.Nishino N., Harada H., Sakaguchi E. Evaluation of fermentation and aerobic stability of wet brewers’ grains ensiled alone or in combination with various feeds as a total mixed ration. J. Sci. Food Agric. 2003;83:557–563. doi: 10.1002/jsfa.1395. [DOI] [Google Scholar]
- 4.Yuan X., Guo G., Wen A., Desta S.T., Wang J., Wang Y., Shao T. The effect of different additives on the fermentation quality, in vitro digestibility and aerobic stability of a total mixed ration silage. Anim. Feed Sci. Technol. 2015;207:41–50. doi: 10.1016/j.anifeedsci.2015.06.001. [DOI] [Google Scholar]
- 5.Bueno A.V.I., Lazzari G., Jobim C.C., Daniel J.L.P. Ensiling total mixed ration for ruminants: A review. Agronomy. 2020;10:879. doi: 10.3390/agronomy10060879. [DOI] [Google Scholar]
- 6.Ruppert L.D., Drackley J.K., Bremmer D.R., Clark J.H. Effects of tallow in diets based on corn silage or alfalfa silage on digestion and nutrient use by lactating dairy cows. J. Dairy Sci. 2003;86:593–609. doi: 10.3168/jds.S0022-0302(03)73638-8. [DOI] [PubMed] [Google Scholar]
- 7.Nishino N., Ogata Y., Han H., Yamamoto Y. Identification of bacteria in total mixed ration silage produced with and without crop silage as an ingredient. Anim. Sci. J. 2015;86:45–50. doi: 10.1111/asj.12234. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Guo G., Yuan X., Zhang J., Li J., Shao T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016;96:1678–1685. doi: 10.1002/jsfa.7271. [DOI] [PubMed] [Google Scholar]
- 9.Yuan X., Wen A., Wang J., Guo G., Desta S.T., Shao T. Effects of ethanol, molasses and Lactobacillus plantarum on the fermentation quality, in vitro digestibility and aerobic stability of total mixed ration silages in the Tibetan plateau of China. Anim. Sci. J. 2016;87:681–689. doi: 10.1111/asj.12477. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson J.M., Davies D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013;68:1–19. doi: 10.1111/j.1365-2494.2012.00891.x. [DOI] [Google Scholar]
- 11.Seppälä A., Heikkilä T., Mäki M., Rinne M. Effects of additives on the fermentation and aerobic stability of grass silages and total mixed rations. Grass Forage Sci. 2016;71:458–471. doi: 10.1111/gfs.12221. [DOI] [Google Scholar]
- 12.Zhang Q., Yu Z., Na R.S. Effects of different additives on fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 2018;73:413–419. doi: 10.1111/gfs.12301. [DOI] [Google Scholar]
- 13.Kung L., Smith M.L., Benjamim da Silva E., Windle M.C., da Silva T.C., Polukis S.A. An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2018;101:5949–5960. doi: 10.3168/jds.2017-14006. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamoorthy U., Muscato T.V., Sniffen C.J., Van Soest P.J. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 1982;65:217–225. doi: 10.3168/jds.S0022-0302(82)82180-2. [DOI] [Google Scholar]
- 15.AOAC . Official Methods of Analysis of AOAC International. 17th ed. Association of Official Analytical Chemists; Gaithersburg, MD, USA: 2005. [Google Scholar]
- 16.McDonald P., Henderson A.R. Determination of water-soluble carbohydrates in grass. J. Sci. Food Agric. 1964;15:395–398. doi: 10.1002/jsfa.2740150609. [DOI] [Google Scholar]
- 17.Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 18.Broderick G.A., Kang J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980;63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8. [DOI] [PubMed] [Google Scholar]
- 19.Tian J., Li Z., Yu Z., Zhang Q., Li X. Interactive effect of inoculant and dried jujube powder on the fermentation quality and nitrogen fraction of alfalfa silage. Anim. Sci. J. 2017;88:633–642. doi: 10.1111/asj.12689. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T., Horiguchi K.I., Goto M. Effect of crushing unhulled rice and the addition of fermented juice of epiphytic lactic acid bacteria on the fermentation quality of whole crop rice silage, and its digestibility and rumen fermentation status in sheep. Anim. Sci. J. 2005;76:353–358. doi: 10.1111/j.1740-0929.2005.00275.x. [DOI] [Google Scholar]
- 21.Wang M., Xu S., Wang T., Jia T., Xu Z., Wang X., Yu Z. Effect of inoculants and storage temperature on the microbial, chemical and mycotoxin composition of corn silage. Asian-Australas. J. Anim. Sci. 2018;31:1903–1912. doi: 10.5713/ajas.17.0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benbelkacem H., Bayard R., Abdelhay A., Zhang Y., Gourdon R. Effect of leachate injection modes on municipal solid waste degradation in anaerobic bioreactor. Bioresour. Technol. 2010;101:5206–5212. doi: 10.1016/j.biortech.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Adesogan A.T., Salawu M.B., Ross A.B., Davies D.R., Brooks A.E. Effect of Lactobacillus buchneri, Lactobacillus fermentum, Leuconostoc mesenteroides inoculants, or a chemical additive on the fermentation, aerobic stability, and nutritive value of crimped wheat grains. J. Dairy Sci. 2010;86:1789–1796. doi: 10.3168/jds.S0022-0302(03)73764-3. [DOI] [PubMed] [Google Scholar]
- 24.Bal E.B.B., Bal M.A. Effects of chemical additives and ensiling time on whole plant wheat silage microbial profiles inferred by phenotypic and 16S ribosomal DNA analyses. World J. Microbiol. Biotechnol. 2012;28:767–776. doi: 10.1007/s11274-011-0864-6. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg Z.G., Muck R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996;19:53–68. doi: 10.1111/j.1574-6976.1996.tb00253.x. [DOI] [Google Scholar]
- 26.Su Z., Li Y., Pan L., Xue F. An investigation on the immunoassays of an ammonia nitrogen-degrading bacterial strain in aquatic water. Aquaculture. 2016;450:17–22. doi: 10.1016/j.aquaculture.2015.07.001. [DOI] [Google Scholar]
- 27.dos Anjos G.V.S., Gonçalves L.C., Rodrigues J.A.S., Keller K.M., Coelho M.M., Michel P.H.F., Ottoni D., Jayme D.G. Effect of re-ensiling on the quality of sorghum silage. J. Dairy Sci. 2018;101:6047–6054. doi: 10.3168/jds.2017-13687. [DOI] [PubMed] [Google Scholar]
- 28.Yuan X., Wen A., Dong Z., Desta S.T., Shao T. Effects of formic acid and potassium diformate on the fermentation quality, chemical composition and aerobic stability of alfalfa silage. Grass Forage Sci. 2017;72:833–839. doi: 10.1111/gfs.12296. [DOI] [Google Scholar]
- 29.Wen A., Yuan X., Wang J., Desta S.T., Shao T. Effects of four short-chain fatty acids or salts on dynamics of fermentation and microbial characteristics of alfalfa silage. Anim. Feed Sci. Technol. 2017;223:141–148. doi: 10.1016/j.anifeedsci.2016.11.017. [DOI] [Google Scholar]
- 30.Guo L., Yao D., Li D., Lin Y., Bureenok S., Ni K., Yang F. Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front. Microbiol. 2020;10:2998. doi: 10.3389/fmicb.2019.02998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Yuan X.J., Li J.F., Dong Z.H., Wang S.R., Guo G., Shao T. Effects of applying lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of forage-based total mixed ration silage in Tibet. Anim. Prod. Sci. 2019;59:376–383. doi: 10.1071/AN16062. [DOI] [Google Scholar]
- 32.Chen L., Yuan X., Li J., Wang S., Dong Z., Shao T. Effect of lactic acid bacteria and propionic acid on conservation characteristics, aerobic stability and in vitro gas production kinetics and digestibility of whole-crop corn based total mixed ration silage. J. Integr. Agric. 2017;16:1592–1600. doi: 10.1016/S2095-3119(16)61482-X. [DOI] [Google Scholar]
- 33.Restelatto R., Novinski C.O., Pereira L.M., Silva E.P.A., Volpi D., Zopollatto M., Schmidt P., Faciola A.P. Chemical composition, fermentative losses, and microbial counts of total mixed ration silages inoculated with different Lactobacillus species. J. Anim. Sci. 2019;97:1634–1644. doi: 10.1093/jas/skz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y., Takahashi T., Horiguchi K., Yoshida N. Effect of adding lactic acid bacteria and molasses on fermentation quality and in vitro ruminal digestion of total mixed ration silage prepared with whole crop rice. Grassl. Sci. 2010;56:19–25. doi: 10.1111/j.1744-697X.2009.00168.x. [DOI] [Google Scholar]
- 35.Filya I., Muck R.E., Contreras-Govea F.E. Inoculant effects on alfalfa silage: Fermentation products and nutritive value. J. Dairy Sci. 2007;90:5108–5114. doi: 10.3168/jds.2006-877. [DOI] [PubMed] [Google Scholar]
- 36.Contreras-Govea F.E., Muck R.E., Mertens D.R., Weimer P.J. Microbial inoculant effects on silage and in vitro ruminal fermentation, and microbial biomass estimation for alfalfa, bmr corn, and corn silages. Anim. Feed Sci. Technol. 2011;163:2–10. doi: 10.1016/j.anifeedsci.2010.09.015. [DOI] [Google Scholar]
- 37.Blümmel M., Becker K. The degradability characteristics of fifty-four roughages and roughage neutral-detergent fibres as described by in vitro gas production and their relationship to voluntary feed intake. Br. J. Nutr. 1997;77:757–768. doi: 10.1079/BJN19970073. [DOI] [PubMed] [Google Scholar]
- 38.Blümmel M., Makkar H.P.S., Becker K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997;77:24–34. doi: 10.1111/j.1439-0396.1997.tb00734.x. [DOI] [Google Scholar]
- 39.Wang H., Sun Q., Yang F., Xu C. Evaluation of fermentation and aerobic stability of total mixed ration silage containing wet brewers’ grains and corn straw. Adv. Mater. Res. 2012;347–353:189–192. doi: 10.4028/www.scientific.net/AMR.590.189. [DOI] [Google Scholar]
- 40.Wang F., Nishino N. Resistance to aerobic deterioration of total mixed ration silage: Effect of ration formulation, air infiltration and storage period on fermentation characteristics and aerobic stability. J. Sci. Food Agric. 2008;88:133–140. doi: 10.1002/jsfa.3057. [DOI] [Google Scholar]
- 41.Jiang D., Niu D., Zuo S., Tian P., Zheng M., Xu C. Yeast population dynamics on air exposure in total mixed ration silage with sweet potato residue. Anim. Sci. J. 2020;91:e13397. doi: 10.1111/asj.13397. [DOI] [PubMed] [Google Scholar]
- 42.Fleet G. Spoilage yeasts. Crit. Rev. Biotechnol. 1992;12:1–44. doi: 10.3109/07388559209069186. [DOI] [PubMed] [Google Scholar]
- 43.Filya I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J. Dairy Sci. 2003;86:3575–3581. doi: 10.3168/jds.S0022-0302(03)73963-0. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg Z.G., Ashbell G., Hen Y., Azrieli A. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J. Appl. Bacteriol. 1993;75:512–518. doi: 10.1111/j.1365-2672.1993.tb01588.x. [DOI] [Google Scholar]
- 45.Cai Y., Benno Y., Ogawa M., Kumai S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999;82:520–526. doi: 10.3168/jds.S0022-0302(99)75263-X. [DOI] [PubMed] [Google Scholar]