Abstract
The growing demand for new, sophisticated, multifunctional materials has brought natural structural composites into focus, since they underwent a substantial optimization during long evolutionary selection pressure and adaptation processes. Marine biological materials are the most important sources of both inspiration for biomimetics and of raw materials for practical applications in technology and biomedicine. The use of marine natural products as multifunctional biomaterials is currently undergoing a renaissance in the modern materials science. The diversity of marine biomaterials, their forms and fields of application are highlighted in this review. We will discuss the challenges, solutions, and future directions of modern marine biomaterialogy using a thorough analysis of scientific sources over the past ten years.
Keywords: marine biomaterials, algal polysaccharides, chitin, spongin, collagen, gelatin, keratin, conchiolin, corals, biominerals
1. Introduction
Humanity has been using marine biomaterials since ancient times (i.e., molluscan shells, corals, bath sponges skeletons, byssus threads), but reaching an industrial level today has become real thanks to the rapid development of various kinds of processing technologies and maricultures. Often, there is a possibility of the utilization of fish, molluscs, or marine arthropod processing products in order to use them most efficiently and not only for feed production. Due to the absence of possible human pathogens in marine biomaterials, a number of them (i.e., collagen, gelatin, keratin) have become an alternative source of long and well-established biopolymers in medicine and cosmetics. Today, modern scaffolding strategies [1,2,3] for tissue engineering are based on the application of diverse already naturally pre-fabricated 3D skeletal constructs of marine invertebrates origin [4]. Sources of marine biomaterials are still plentiful [5] in spite of partial overfishing, dramatic climate changes, and the increasing pollution of the world’s oceans with industrial waste. An attempt to classify marine biomaterials by their origin is presented by us in Figure 1.
Figure 1.
Overview of the main sources of marine biomaterials used nowadays.
Due to the huge amount of scientific information available in various scientific sources, we considered carrying out its analysis to be expedient, choosing certain topics that include marine polysaccharides of invertebrates and algal origin, marine structural proteins (spongin, collagen, gelatin, keratin, conchiolin) as well as marine biominerals from corals and molluscan shells. For the first time, in order to facilitate the perception of large volumes of information and focus on especially important parameters characterizing a particular biomaterial, we took the liberty of presenting a part of information in the form of so-called “Biomaterial passports” (see Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13). This form includes scientific name, chemical formula, molecular weight, physicochemical and material properties, extraction methods, market and patent situation of corresponding biological materials discussed in this article. For brevity, some aspects will only be briefly discussed, but interested readers are referred to pertinent references.
Table 1.
Biomaterial passport: chitin.
| Scientific Name | Chitin |
|---|---|
| Chemical formula, MW, chemical structure, polymorphism |
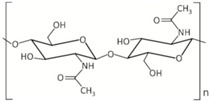
|
| (C8H13O5N)n; MW ranges from several to thousands of kDa [12]. Chitin is a linear polymer of N-acetyl-d-glucosamine units that are linked by 1,4-β-glycosidic bonds [13]. It exists in three crystalline polymorphic forms: α-, β-and γ-chitin [14,15]. Marine sources of α-chitin: crustaceans, sponges; of β-chitin: cephalopods [16]. | |
| Physicochemical properties | Due to its semicrystalline structure and hydrophobicity chitin is not soluble in usual solvents, i.e., water, the most organic solvents, though it shows solubility in hexafluoroacetone sesquihydrate, hexafluoroisopropanol, chloroalcohols (with sulfuric acid), mixture of dimethylacetamide with 5% lithium chloride [17] and diverse ionic liqiuds [18]. |
| Chitin extraction/Physical form after extraction | For commercial purposes, chitin is extracted using chemical, electrochemical and biochemical methods from the cuticles of crustaceans, mostly crabs and shripms [19,20,21,22,23,24] and corals [25]. It is isolated by chemical extraction via three stages, i.e., deproteinization by alkaline treatment, i.e., employing NaOH, Na2CO3, NaHCO3, KOH, K2CO3, demineralization using acidic (i.e., HCl, HNO3, H2SO4, CH3COOH), or EDTA-based solutions [26], and finally discoloration following the incubation in alkaline solution or by the addition of acetone or, alternatively, using KMnO4, H2O2 [27] or oxalic acid [12,28]. Currently, numerous studies aimed at developing different protocols to isolate chitin from seafood shells [29,30,31,32,33,34] as well as marine sponges [35] have been reported. Chitin is extracted in the form of flakes, powders, and scaffolds. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Elastic (Young’s) modulus ranges from 92 GPa [36] to 4 GPa [37]. Thermostability: 260–360 °C [38,39,40]. Biocompatible [1,41,42,43] and biodegradable [12]; can be hydrolyzed by chitinases [44]; non-toxic and [45] of low immunogenicity [46,47]. |
| Market situation (world market reports) | According to Global Industry Analysts, Inc. data, global chitin and chitosan market was predicted to reach US $4.2 billion by 2021 [12]. |
| Patents | Currently, about several hundreds of patents on the extraction and modification of chitin and its derivatives as well as their applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US6310188B1. Method for producing chitin or chitosan | |
| US6632941B2. Method of extracting chitin from the shells of exoskeletal animals | |
| CN106496362A. The extracting method of chitin in a kind of Carapax Eriocheir sinensis | |
| US20180186899A1. Compositions of partially deacetylated chitin derivatives | |
| JP2822174B2. Method for producing chitin chitosan fiber and structure | |
| US5623064A. Poly-β-1→-4-N-acetylglucosamine | |
| US9433698B2. High strength chitin composite material and method of making | |
| US9708634B2. Process for making chitin derivatives | |
| US7241463B2. Methods for processing crustacean material | |
| US4066735A. Process for demineralization of crustacea shells | |
| US4293098A. Recovery of active chitin and enhanced protein meal | |
| WO1986006082A1. A process for recovering chitin from materials in which chitin occurs together with or connected to proteinaceous substances | |
| US5053113A. Method of chitin production from chitin containing raw materials | |
| JPH05310804A. Production of chitin or chitosan from integument of crustacea |
Table 2.
Biomaterial passport: alginates.
| Scientific Name | Alginates |
|---|---|
| Chemical structure, MW |
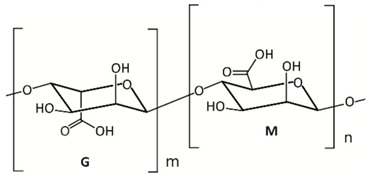
|
| Alginates are salts of alginic acid, a linear polymer composed of blocks of β-d mannuronic acid (M) and α-l guluronic acid (G) residues linked by 1-4 glycosidic bonds [90,93]. The molecular weight of alginic acid and its salts ranges from 5 to 20 kDa [94]. | |
| Physicochemical properties | Phycocolloids are known to form viscous solutions or gels [95]. Over 200 alginates with different physicochemical properties are produced [96]. Alginates can efficiently bind divalent cations, which results in hydrogel formation and crosslinked polymeric scaffolds [97]. The presence of O-acetyl groups, which was shown for algal alginates [98], increases polymer solubility affecting physicochemical parameters such as viscoelasticity and molecular mass [99]. |
| Alginate extraction/Physical form after extraction | Alginates are produced industrially from marine seaweeds, which belong to brown algae [90]. Conventional extraction of alginates consists of the following five steps: (i) acidification of seaweeds; (ii) alkaline extraction using Na2CO3; (iii) solid/liquid separation; (iv) precipitation and (v) drying [100]. In addition, seaweed tissue can be softened and bleached using formaldehyde/formalin [100]. About 25% of alginate yield can be achieved in 2 h; however, the extraction can be conducted much faster (in 15–30 min) using ultrasound treatment [101]. Usually, alginates are extracted as dry, powdered sodium alginate [95]. Alginates and their derivatives are widely used as stabilizers, thickeners, viscosifiers, additives, gel and film formers [99]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | In algae, being constituents of cell wall and inter-cellular matrix, alginates provide mechanical strength and flexibility necessary for the survival in water [100]. Due to their non-toxicity, biocompatibility, biodegradability, non-immunogenicity, and hydrophilicity alginates have a great potential for pharmaceutical and biomedical applications [99]. |
| Market situation (world market reports) | Owing to their properties such as thickeners, the ability to form gels, sodium, and calcium films alginates are widely applied in the food, printing, dyeing, textile, pharmaceutical, and cosmetic industries. According to the report of Market Data Forecast [102], the global alginates market was estimated as USD 409.2 million in 2020 and is expected to reach USD 529.2 million by 2025. Alginates market is predicted to grow mainly in Europe and Asia Pacific. |
| Patents | Currently, about several hundreds of patents on the extraction and modification of alginic acid and its derivatives as well as their applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US2653106A. Manufacture of alginates | |
| US20150289533A1. Alginate gum | |
| US8741872B2. Self-gelling alginate systems and uses thereof | |
| US2420308A. Gel-forming algin composition and method | |
| US1814981A. Process of preparing alginic acid and compounds thereof | |
| EP0345886A2. Alginate gels | |
| US5266326A. In situ modification of alginate | |
| EP0849281A1. Bioresorbable alginate derivatives | |
| US5874100A. Alginate fibres, manufacture and use | |
| WO2000009566A1. Method for producing ultra-pure alginates | |
| US6150581A. Chitosan/alginate anti-adhesion barrier | |
| US6432449B1. Biodegradable sustained-release alginate gels | |
| US10292936B2. Modified alginates for cell encapsulation and cell therapy | |
| US10426735B2. Modified alginates for anti-fibrotic materials and applications |
Table 3.
Biomaterial passport: fucoidans.
| Scientific Name | Fucoidans |
|---|---|
| Chemical Structure, MW |
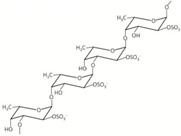
|
| Fucoidans are sulphated hetero-polysaccharides consisting of α 1-3 linked sulphated L fucose with repeating sequence of alternating α 1-3 and α 1-4 glycosidic bonds [90,144]. MW of most fucoidans was reported to vary within 200–2000 kDa [145]. | |
| Physicochemical properties | Isolated shielded opposite groups contribute to the solubility of fucoidans in solvents with higher dielectric constants, such as water, whereas solvents of lower dielectric constants, i.e., ethanol can be used for precipitation and isolation of fucoidans from other co-extracted natural compounds [146]. Fucoidan molecules, being stable in salts, i.e., NaCl and CaCl2, acid and alkaline solutions, are suitable for the use as stabilizing, thickening, and water-holding agents [147]. |
| Fucoidan extraction/Physical form after extraction | Fucoidans can be extracted from brown algae such as Undaria pinnatifida (Miyeok), a common Korean edible brown seaweed [148], by hot acidic, alkaline, enzyme-, microwave- and ultrasound-assisted aqueous methods [146]. They are extracted in the form of fluffy, hygroscopic powders, soluble in water, relatively soluble in dimethyl sulfoxide (DMSO), but insoluble in ethanol [146]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Fucoidans have specific mechanical properties. Indeed, these polysaccharides provide mechanical stability to brown seaweeds, in particular, they prevent the desiccation of the thallus tissues, especially at the lower tide levels or high summer temperatures [149]. Fucoidans were reported to be biocompatible, biodegradable and demonstrated low cytotoxicity and immunogenicity [144,150,151,152]. Some studies, however, pointed to their cytotoxicity in vitro and in vivo, which paves the way to their use as anticancer agents [153]. |
| Market situation (world market reports) |
Based on the New Research Analysis, the global fucoidan market size was USD 30 million in 2019 and is expected to reach USD 37 million in 2024 with Asia (mainly China and Japan) and the U.S.A. being the largest fucoidan consumption regions [154]. |
| Patents | Currently, about several hundreds of patents on the extraction and applications of fucoidans exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US20070087996A1. Method of extracting fucoidan | |
| US20100056473A1. Method of extracting fucoidan | |
| CN101993501A. Method for preparing fucoidan | |
| CN103665179A. Extraction device for kelp fucoidan | |
| US20080089941A1. Fucoidan compositions and methods | |
| US20050129708A1. Fucoidan-based health food | |
| CA2253573C. Fucoidan-containing foods or beverages | |
| US20150328268A1. Marine Plants Extract for Wound Healing | |
| CN101954087B. Fucoidan medicinal carrier and preparation method thereof | |
| NZ610788A. Process for isolating fucoidan and laminarin from live, harvested seaweed |
Table 4.
Biomaterial passport: carrageenans.
| Scientific Name | Carrageenans |
|---|---|
| Chemical structure, MW |
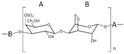
|
| Carrageenans, hydrophilic linear sulphated galactans, being composed of alternate units of d-galactose and 3,6-anhydro-galactose linked by α 1-3 and β 1-4 glycosidic bonds, are divided into groups, i.e., kappa (κ), iota (ι), lambda (λ), mu (μ), nu (υ), theta (θ) and others, which is based on their solubility in potassium chloride [90]. Their MW was reported to be within 200–800 kDa [167]. | |
| Physicochemical properties | Hydrocolloids of different solubility: κ-carrageenan is insoluble in cold water [168]; a higher hydrophilicity was shown for ι-carrageenan, while λ-carrageenan is freely soluble in water under most conditions [168] and even in cold milk [169]. λ-carrageenan is non-gelling and is used rather for its thickening properties and the ability to form creamy texture [169]. κ- and ι-carrageenans form gels [169] following heating [167,170] and cooling in the presence of K+, Ca2+, NH4+ cations [168]. ι-carrageenan is used to obtain soft gels [169] while κ-carrageenan, the main carrageenan applied in industry, forms strong, brittle gels the strength of which can be improved by locust bean gum, corn starch and wheat starch [168]. Thus, due to their specific texture properties carrageenans are widely used in food industry to improve appearance (creaminess, homogeneity), organoleptic qualities (juiciness, mouthfeel), and application (spreadability) [169]. |
| Carrageenan extraction/Physical form after extraction | Carrageenans are extracted from various red algae species [90,171,172,173] by hot alkaline treatment followed by ethanol precipitation [168] in the form of translucent plates or powders [168,174]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Carrageenans were shown to be biocompatible, biodegradable, non-immunogenic, and non-toxic compounds [19,175,176,177]. |
| Market situation (world market reports) |
The global carrageen market is predicted to reach USD 1.25 million by the end of 2024 [178]. |
| Patents | Currently, about several hundreds of patents on the extraction and applications of carrageenans exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US3956173A. Preparation of gels based on carrageenan | |
| US5502179A. Carrageenan product and a method of producing same | |
| US3094517A. Process for treating a polysaccharide of seaweeds of the gigartinaceae and solieriaceae families | |
| US3280102A. Preparation of carrageenan having improved water dispersibility | |
| US3907770A. Process of extracting carrageenan from seaweed | |
| JPS57202302A. Preparation of carrageenan | |
| US4443486A. Modified extractive of Eucheuma cottonii seaweed and composition containing same | |
| US6387354B1. Semi-refined carrageenan dentifrice binder | |
| WO2002048199A3. Production of carrageenan and carrageenan products | |
| SU756683A1. Method of obtaining jellifier from red algae | |
| CN103788225A. Production method of modified carrageenan | |
| AU2003245252A1. Carrageenan based antimicrobial compositions |
Table 5.
Biomaterial passport: ulvans.
| Scientific Name | Ulvans |
|---|---|
| Chemical Structure, MW |
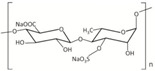
|
| Ulvans are composed of branched, complex structure without a defined backbone or a specific repeating monomer, usually consisting of rhamnose (17–45%), sulphate (14–23%), glucuronic acid (7–19%), xylose (2–12%), iduronic acid (1–9%), and glucose (1–7%) [191,192]. Their MW ranges from about 200 to 8200 kDa [193]. | |
| Physicochemical properties | Hydrocolloids, which in the presence of divalent cations, i.e., Ca2+, Cu2+, Zn2+, boric acid and slightly basic pH form gels [194]. At low and neutral pH, owing to rhamnose hydrophobicity, ulvans fold into beads-like conformation resulting in low viscosity [195,196]. At pH ~13, ulvans develop more open conformation leading to higher viscosities and gel strengths [196]. They have metal chelating ability, play the role of radical scavengers, and were shown to tolerate temperatures up to 180 °C [194]. |
| Ulvans extraction/Physical form after extraction | Ulvans are extracted from green seaweeds [191,192]. Following cold water or hot water extraction and ethanol precipitation, they are recovered as fluffy powder [196]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Ulvans were reported to be biocompatible, biodegradable, show a low toxicity, and immunogenicity [197,198]. |
| Market situation (world market reports) | There is no open access data regarding a global ulvans market. It is known that ulvan containing green algae is consumed in Asian countries and are used in Chinese medicine [199]. Due to their high vitamin and fiber content, ulvans are also consumed in Europe [199]. The main ulvan producers are represented by China and Indonesia, which account for 49% and 37% of the world production, respectively [200]. |
| Patents | Currently, about several hundreds of patents on the extraction and applications of ulvans exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US7820176B2. Ulvans as activators of plant defense and resistance reactions against biotic or abiotic stresses | |
| FR2868252B1. Use of ulvanes as elicitors of nitrogen absorption mechanisms and protein synthesis | |
| EP2582810B1. Ulvan lyase, the method for manufacturing same, and uses thereof | |
| CA2562942C. Use of ulvans as elicitors of mechanisms for nitrogen absorption and protein synthesis | |
| WO2007045795A1. Product resulting from the grafting of fatty chains to ulvans and use of a said product as a surfactant | |
| US5089481A. Polysaccharides and antiviral drugs containing the same as active ingredient | |
| US20080083160A1.Compositions of enriched seaweeds in land-based sea water ponds | |
| US20080226740A1. Marine algal extracts comprising marine algal polysaccharides of low degree polymerizaton, and the preparation processes and uses thereof | |
| CN1108310C. Algae polysaccharide and its preparation and use |
Table 6.
Biomaterial passport: agar.
| Scientific Name | Agar |
|---|---|
| Chemical structure, MW |
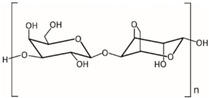
|
| Agars, (2R,3S,4S,5R)-2-(hydroxymethyl)-6-[[(4R,5S)-4-hydroxy-3-methyl-2,6-dioxabicyclo[3.2.1]octan-8-yl]oxy]-4-methoxyoxane-3,5-diol, are known as water-soluble, gel-forming polysaccharide extracts from agarophyte members of the Rhodophyta [209]. Agar is derived from the polysaccharide agarose, which forms the supporting structure in the cell walls of certain species of algae, and which is released on boiling. Average molecular weight of agar ranges between 35.7 and 144 kDa for commercial preparations [208]. | |
| Physicochemical properties | Insoluble in cold water. Main physical properties of agar include gel strength, gelling, and melting temperature [210,211]. |
| Agar extraction/Physical form after extraction | Agar can be extracted with different yields from such algae as Gelidium, Acanthopeltis, Ceramium, Gracilaria, and Gloiopeltis species by boiling in 70, 60, 50% alcohol and water [208]. Two classical extraction methods of total agar extraction with and without NaOH treatment have been described as follows: “The dried sample of 30 g of algae was boiled for 2 h with 900 mL of distilled water and used for non-alkali treatment (native agar). Another 30 g sample was incubated in 2 L of 5% NaOH solution at 80 °C for 2 h. The algae were washed in running tap water for 30 min to remove excess NaOH. The alkali-treated algae were neutralized in 2% H2SO4 solution for 1 h, then washed in running tap water overnight until complete elimination of the acid” [209]. Agar scaffolds preparation for tissue engineering was also reported: “0.02% agar was soaked in distilled water for 30 min at room temperature and then boiled to 80 °C with stirring for 2 h until it completely turned into a transparent homogeneous solution. The agar solution was poured into a mold and cooled to room temperature” [212]. The development of agar-based bioaerogels [213] and membranes [214] has also been described. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | The gel-forming ability and solubility of agar polysaccharides rely on the relative hydrophobicity of the basic repeating unit, the alternating 1,3-linked β-d-galactopyranose and 1,4-linked 3,6-anhydro-α-l-galactopyranose or agarobiose, and its substitution by hydrophobic(methoxyl) and polar (sulfate, pyruvate) groups [208]. Agar-based thermoreversible gels have a melting point at 60–97 °C [215] and can retain their structure after freeze-drying [216]. Agar biocompartibility, biodegradability, and low toxicity has been experimentally confirmed [217]. |
| Market situation (world market reports) | The global agar agar gum market size was estimated at USD 214.98 million in 2015 and USD 219 million in 2017.It is anticipated to grow at a CAGR of 4.9% from 2016 to 2025 [218]. |
| Patents | Currently, about several hundreds of patents on the extraction and modification of agar and its derivatives as well as their applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US3335127A. Fractionation of mixtures of agarose and agaropectin | |
| US2439964A. Extraction and preparation of agar | |
| US784349A. Process of manufacturing limpid solutions of agar-agar and product of same | |
| US3094517A.Process for treating a polysaccharide of seaweeds of the gigartinaceae and solieriaceae families | |
| US4780534A. Process for producing agar-agar from an algae extraction juice | |
| US20050267296A1.Cost-effective process for preparing agarose from Gracilaria spp. | |
| US3956273A. Modified agarose and agar and method of making same | |
| US3423396A. Method of making agarose | |
| US3281409A. Method for the separation of agaropectin from agarose | |
| US9045566B2. Method for the manufacture of agarose gels | |
| US3527712A. Dried agarose gel, method of preparation thereof, and production of aqueous agarose gel | |
| US3860573A. Method for cross-linking agarose or agar | |
| CN101891835A. Method for separating and preparing agarose from agar by using polyethylene glycol precipitation method | |
| US6322814B1. Manufacture of and uses for low molecular weight agars and agaroids | |
| GB1352613A. Stabilized agar product and method for its stabilization |
Table 7.
Biomaterial passport: spongin.
| Scientific Name | Spongin |
|---|---|
| Chemical structure | Spongin is a collagen derivative protein which can be referred to halogenated scleroproteins or neurokeratin-like proteins [231,232]. However, halogens (I, Br), detected within spongin structure, do not occur in collagens or keratins [232]. The biochemistry of spongin as well as its molecular weight remains to be unknown. |
| Physicochemical properties | Spongin is not soluble neither by proteases (collagenase, pepsin, trypsin, amylase, lysozyme), nor by aggressive reagents, i.e., HCl, sulfuric acid, hydrogen peroxide [233,234,235]. Treatment with alkalis dissolves spongin resulting in hydrolysates of amino acids. In the natural habitat of sponges, spongin can be destroyed by bacteria and fungi [235]. Its thermostability is species dependent and ranges between 150 °C and 360 °C [236]. Owing to spongin, the scaffolds of bath sponge Spongia officinalis are characterized by unique material properties, such as the ability to hold water, toughness, compressibility and resiliency [232]. Heating of spongin scaffolds up to 1200 °C under exclusion of oxygen leads to obtaining of turbostratic graphite [86]. |
| Spongin extraction/Physical form after extraction | Spongin skeletons can be purified using 3M HCl as was shown for Hippospongia communis [237]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Spongin was reported to be biocompatible, biodegradable, non-toxic and of low immunogenicity [4,232,238,239]. |
| Market situation (world market reports) | According to Technavio report, global commercial sponge market is predicted to reach USD 3.18 billion during 2020–2024 [240]. In addition, sponges can be cultivated and such sponge farms already exist in Japan, France, Greece, the Philippines, Micronesia, Australia, New Zealand, and East Africa [232]. |
| Patents | Currently, about several hundreds of patents on sponge cultivation, sponge scaffolds extraction, their treatments, and applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| WO2015151030A1. Method to obtain collagen/gelatin from marine sponges | |
| WO2006089660A2. Method for cleaning marine collagen and the treatment thereof to form porous sponges | |
| US20030032601A1. Method for isolating sponge collagen and producing nanoparticulate collagen, and the use thereof | |
| US20080261876A1. Method for purifying marine collagen and the processing thereof into porous sponges | |
| US20100260823A1. Preparation with marine collagen for protease inhibition | |
| JPH07100B2. Method of drying collagen sponge | |
| DE10010113A. Native sponge collagen, process for its isolation and its use, as well as native nanoparticulate sponge collagen, process for its preparation and its use |
Table 8.
Biomaterial passport: collagens.
| Scientific Name | Collagens |
|---|---|
| Chemical structure, MW |
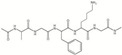
|
| Collagens belong to a superfamily of extracellular matrix structural proteins that are formed by a triple helix of three protein chains wrapped around each other [246,247]. Marine collagens resemble those of mammals, but their amino acid composition was shown to be much more diverse [230,231,248,249,250] MW of marine collagens, i.e., cod is about 300 kDa [251]. | |
| Physicochemical properties | Marine fish collagens are characterized by a high solubility in water upon heating, which was reported to be higher for warm-water fish species [230,252]. Incubation of collagen with thrombin results in the hydrolysis of peptide bonds and the formation of scaffolds in the form of hydrogel with a range of elasticity, transparency, and density parameters [251]. Upon heat denaturation collagen from fish, i.e., shark undergoes hydrolysis yielding gelatin [253]. |
| Collagen extraction/Physical form after extraction | Marine collagens, predominantly type I collagen, can be isolated from marine invertebrates (sponges, jellyfish, cephalopods, echinoderms) and marine vertebrates (fish) [176,254,255]. The raw materials for fish collagen isolation include skin, scale, fins, backbone, swimbladder, wing muscles of skate, shark placoid-scale dentine [230,254]. Marine collagen is extracted via (i) decellularization using physical methods involving freezing and disruption of cells; (ii) chemical methods based on variable reagents, i.e., acids, alkalis, chelating agents, detergents, solutions of high osmolarity; (iii) enzymatic treatments. Usually, these methods are combined [230,255]. From jellyfish, it is extracted from mesoglea via solubilization in acetic acid solution [256]. The protocols for collagen extraction from sponges were reported [255,257,258]. Upon extraction, collagen or its composites have the physical form of sheets, flakes, powder, gel, particles, fibers, film, etc. [259]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Marine collagens were shown to be biocompatible, biodegradable, non-toxic, and of weak antigenicity [255,260,261,262,263,264]. The mechanical properties of marine fish collagens can be improved by ultraviolet irradiation, gamma irradiation, dehydrothermal treatment, chemical treatment including glutaraldehyde, carbodiimide,1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide [252,262,265] as well as incorporation of other biopolymers such chitosan, alginate, and pectin [266,267]. Unique collagen mechanical properties were reported for Chondrosia reniformis demosponge. It allows the species to creep and withstand compression [231]. |
| Market situation (world market reports) | The global market for marine collagen has been steadily growing over the last years. While in 2018 it was estimated to be worth of USD 620.3 million, it is predicted to reach USD 897.5 by 2023 [268]. Primarily, marine collagen market is predicted to grow in China, India, and Brazil [268]. |
| Patents | Currently, about several thousands of patents on marine collagen extraction, purification, modification, removing odor, improving mechanical properties and applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US20060135752A1. Method of obtaining biologically active collagen from skins of the salmonidae fish | |
| DE102005041414A1. Glass sponge collagen obtained by gradually corroding glass sponge basal spicule in alkaline solution; dialyzing the obtained extract and subsequently lyophilizing, useful for the production of e.g., biological material and bullets | |
| DE102013014417A1. Sponge collagen comprehensive preparations with defined in vivo release profile especially in the colon, their production and use | |
| TWI487711B. A extraction method of collagen from tuna and product thereof | |
| KR101640801B1. Collagen extraction from aquatic animals | |
| WO2015012682A2. A method for extracting collagen from aquatic animals, collagen and products containing it | |
| JP4236850B2. Method for producing fish-derived collagen peptide, and food and drink and cosmetics containing fish-derived collagen peptide obtained by the method | |
| EP0592586B1. Use of unpigmented fish skin, particularly from flat fish, as a novel industrial source of collagen, extraction method, and collagen and biomaterial thereby obtained | |
| CN1582771B. Production of collagen peptide from fish skins | |
| US9591853B2. Jellyfish-derived polymer | |
| EP2889305A1. Method for fractionally extracting mucin and collagen | |
| WO2009090655A2. Colloidal collagen burn wound dressing produced from jellyfish | |
| US5714582A. Bioscience Consultants Invertebrate type V telopeptide collagen, methods of making, and use thereof | |
| JP2007504100A. Medical and insurance use of pufferfish type I collagen extract and method for producing the extract | |
| KR100381741B1. Collagen product containing collagen of marine origin with a low odor and with improved mechanical properties, and its use in the form of cosmetic or pharmaceutical compositions or products |
Table 9.
Biomaterial passport: gelatin.
| Scientific Name | Gelatin |
|---|---|
| Chemical structure, MW | (C102H151O39N31), the amino acid sequence of gelatin depends on its source and is similar to that of collagen, comprising of repeating sequences of Gly-X-Y triplets, where X and Y are represented by mostly proline and hydroxyproline, respectively. The average MW is in the range of 40 to 700 kDa [292,293]. |
| Physicochemical properties | Gelatin properties vary in a broad spectrum, depending on the material used, pretreatment method, extraction process parameters and its intensity. Reported pH values span from 2.98 to 4.38; isoelectric point for acid-processed gelatins is in the pH range of 6.0–9.5, while for alkali-processed gelatins it falls between the pH of 4.8 and 5.2, moisture content is in the range of 9–14% [292,293]. |
| Fish gelatin extraction/Physical form after extraction | Gelatin extraction was reported from various marine species, i.e., fish species [256,294], sponges [295], jellyfish [296] and other marine organisms such as squids [297] and snails [298]. The processing with alkaline or acidic media in elevated temperatures yields gelatin in the form of granulates or powders [292,293]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Fish gelatin is considered to be biodegradable, non-immunogenic and biocompatible [299,300,301]. It does not display toxicity or carcinogenicity and has very poor mechanical properties, dependent on the source type (cold/warm fish) or experimental conditions; e.g., tensile strength varies from 36.8 MPa for the cold-water pollock derived gelatin to 95.5 MPa for the catfish [302]. |
| Market situation (world market reports) |
Production of fish gelatin is still quite small, contributing only to ca. 1% of the global gelatin market [292,293]. |
| Patents | Currently, about several hundreds of patents on utilization of fish gelatin in food and pharmaceutical industry as components of packaging systems or drug delivery, medicine and cosmetics are available. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US20030022832A1. Method for the production of gelatin of marine origin and product thus obtained | |
| JP4738005B2. Fish skin pretreatment method | |
| JP6265350B2. Extraction method of collagen and gelatin | |
| TWI487711B. A extraction method of collagen from tuna and product thereof | |
| US6368656B1. Process for the preparation of fish gelatin | |
| WO2017216780A1. Gelatin polymer derived from natural sources of cold-adapted marine species and uses thereof | |
| WO2012160575A2. Method of producing gelatin from fish | |
| US5093474A. Process for the production of gelatin from fish skins | |
| US20050124034A1. Method for producing fish gelatin peptide | |
| CN104605006A. Freeze-drying method for swim bladder | |
| WO2019022623A1. Process for producing gelatin from fish skin by optimisation of the extraction conditions | |
| US2048728A. Process for making a clear fish glue or fish gelatin solution | |
| CN102702984A. Process for industrially producing fishskin gelatin | |
| GB2377708A. Improved alkaline process for preparing type B fish gelatin | |
| US5484888A. Gelatin production |
Table 10.
Biomaterial passport: keratin.
| Scientific Name | Keratin |
|---|---|
| Chemical structure, MW |
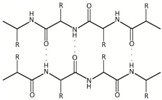
|
| Fibrous structural protein of a molecular weight ca. 66.6 kDa [311] | |
| Physicochemical properties | Keratin is a stable protein, insoluble in polar and nonpolar solvents [311]. |
| Keratin extraction/Physical form after extraction | Depending on the source, keratin extraction is quite a demanding process and its parameters influence the scope of application of the extracted keratin, available as a powder or liquid [312,313]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Keratin is biodegradable [314], biocompatible and non-toxic [315]. Reported properties of keratin differ depending on its source. Young’s modulus ranges from 10 MPa in stratum corneum to about 2.5 GPa in feathers; tensile strength varies from 2 MPa in stratum corneum to 530 MPa in dry hagfish slime threads [310]. Reported stiffness of keratin is up to 20 GPa [316]; however, it strongly depends on the level of hydration [317]: for hagfish slime threads the initial stiffness reaches 3.6 GPa in dry state and drops to 6 MPa in wet state [310]. |
| Market situation (world market reports) | There are no open access reports on the marine keratin market situation. |
| Patents | Currently, about several hundreds of patents on utilization of keratin in cosmetics, hair care products, adhesives, wound dressing or as components of antibacterial and anti-inflammatory products are available. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US7148327B2. Production of soluble keratin derivaties | |
| CN1535280A. Production of soluble keratin derivatives | |
| WO2019116357A1. Method for extracting keratin | |
| US8575313B2. Process for extracting keratin | |
| US20140228257A1. Method for Sea Floor Drilling Using Hagfish Slime as Drilling Fluid Additive | |
| US7049405B2. α-helical protein based materials and methods for making same | |
| CN106999546A. Keratin nano material and preparation method thereof | |
| WO2007095151A2. Nerve regeneration employing keratin biomaterials | |
| US20100197021A1. Keratin biomaterials for cell culture and methods of use | |
| US6110487A. Method of making porous keratin scaffolds and products of same | |
| US8920827B2. Keratin bioceramic compositions |
Table 11.
Biomaterial passport: conchiolin.
| Scientific Name | Conchiolin |
|---|---|
| Chemical structure, MW | Conchiolin is reported to be an aggregate of proteins including a significant portion of polysaccharide component [332]. When isolated from mollusk tissue and separated by PAGE, it gives three main protein bands with molecular weight of 37.8, 23.2, and 19.6 kDa. The amino acid analysis of the isolated material shows the presence of high content of glycine and alanine (30–60%) and a large number of hydrophobic residues [332]. |
| Physicochemical properties | Insoluble in water and acid [333]. |
| Conchiolin extraction/Physical form after extraction | Conchiolin can be extracted form ground mollusk shells by subsequent washing with EDTA solution, basic Tris buffer and water followed by the extraction with SDS solution at increased temperature to yield conchiolin as a powder [332]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Due to their biocompatibility, marine collagens can be applied in biomedicine, regenerative medicine, wound healing, cartilage and hard tissue engineering. Domains typical for collagen have been detected as main structural segments in other structural marine proteins including conchiolin [4]. This may suggest that conchiolin may exhibit properties similar to collagen that is highly biocompatible and applicable as a biomaterial. Conchiolin is a calcium binding protein which facilitates calcification during shell formation thus exhibiting a potential to be applied in bone engineering [334,335]. |
| Market situation (world market reports) |
Today’s market exhibits fast increase in the demand on medical devices supporting the regeneration of bone fractures and defects [336]. Due to its calcium binding properties [334], conchiolin exhibits the potential to be applied as a component of bone regeneration scaffolds. |
| Patents | Currently, several patents on conchiolin extraction, modification and application exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| US20110274792A1. Method for producing powder for supplementary food and supplementary food | |
| US5702728A. Clam extract preparation, the method of preparation and use thereof | |
| WO2010005243A2. Method for producing an extract containing water-soluble conchiolin derived from shells | |
| EP3228625A4. Preparation method for conchiolin, and water-soluble conchiolin and acid-soluble conchiolin prepared by using method | |
| FR2827478B1. Process for the preparation of a nacre-based powder, isolated protein from said powder and their uses in bone surgery and in various osteoarticular pathologies |
Table 12.
Biomaterial passport: Coral biominerals.
| Scientific Name | Coral Biominerals |
|---|---|
| Chemical structure, MW | Coral skeletons are composed mainly from CaCO3. MW: 100.1 g/mol [344]. |
| Physicochemical properties | Coral material is quite stable. It preserves highly organized porous structure after hydrothermal treatment and even sintering at 1250 °C [345]. Hydrothermal treatment of as sea received coral samples results in the transformation of crystalline aragonite (CaCO3) to hydroxyapatite [345]. |
| Coral extraction/Physical form after extraction | Coral derived materials include coral hydroxyapatite and aragonite, natural coral fragments, coral granules and coral powders [346]. |
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Coral-derived material is biocompatible, structurally similar to human bone, with Young’s modulus of 0.580 to 9.032 GN m−2 (reported for octocorals) [347], non-toxic, biodegradable and of low immunogenicity [4,348]. Mechanical properties of octocorals were shown to depend on environment, i.e., the stiffest skeletons belong to the inhabitants of deeper environments (with pressure >80 atmospheres) while the least stiff skeletons are found in the colonies from shallow environments with moderate waves [347]. |
| Market situation (world market reports) | Materials to reconstruct bone defects are in high demand. In 2021, global markets for orthopedic and dental bone graft products is predicted to reach USD 3.4 billion and USD 1.0 billion, respectively [349]. Bone allografts can be obtained from corals cultured in aquarium systems and enriched with silica and strontium increasing coral osteoconductive properties, which was patented in the U.S.A. and Israel [349]. |
| Patents | Currently, about several hundreds of patents on coral cultivation, hydrothermal treatment of coral material yielding hydroxyapatite, modification of coral material and its applications exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| WO2009066463A1. Method of producing coral powder | |
| CN-107951818-A. Reparation toothpaste containing coral powder and hydroxyapatite component and preparation method thereof | |
| WO2010078879A2. Cosmetic use of a coral powder | |
| US8936638B2. Coral bone graft substitute | |
| EP2618858B1. Coral bone graft substitute | |
| WO2009066283A2. Calcium-mediated effects of coral and methods of use thereof | |
| KR100536500B1. Mass propagation methods of Korean Corals | |
| JP2008141989A. Method for propagating coral | |
| CN101702998B. Propagation method for coral grass seedling tissue culture | |
| WO2009066283A3. Calcium-mediated effects of coral and methods of use thereof | |
| EP0952114B1. Weathered hermatypic coral material | |
| DE20311110U1. Biological dental implant consists of coral | |
| US20060147656A1. Simulated coral rock and method of manufacture | |
| RU2472516C1. Biomaterial for bone defect replacement | |
| US7608283B2. Coral purification method and coral thus obtained | |
| WO2002040398A1. Processes for treating coral and coating an object |
Table 13.
Biomaterial passport: Molluscan shell.
| Scientific Name | Molluscan Shells |
|---|---|
| Chemical structure, MW | Mostly composed of CaCO3, MW: 100.1 g/mol [377]. |
| Physicochemical properties | Molluscan shells are stable, exhibiting a high degree of morphological and crystallographic ordering [378] resulting in high values of the elastic modulus and bending strength (up to 82 GPa and 267 MPa, respectively) [379,380]. Importantly, the quality of the shell and its physical properties depend on environmental conditions [381]. High temperature treatment of shells leads to the conversion of CaCO3 to calcium oxide (CaO) [382] or it can be converted to hydroxyapatite by the hydrothermal method [383]. |
| Molluscan shell extraction/Physical form after extraction | In general, molluscan shells are collected as aquaculture industry waste byproduct and are further processed [384]. Physical forms of shells include shell fragments and powders [384]. |
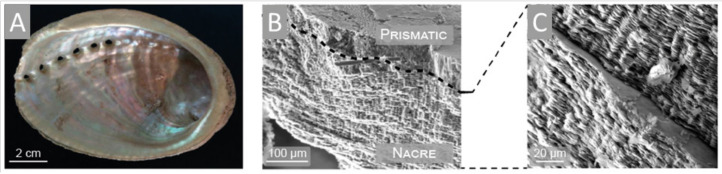
| Biomaterials properties (biocompatibility, biodegradability, toxicity, immune responses) | Molluscan shell derived materials are considered to be biocompatible [385]. The nacre was reported to be biocompatible, biodegradable and exhibit osteogenic properties [386]. Furthermore, it showed limited cytotoxicity [387] and did not elicit immune responses [388]. The nacre exhibits outstanding mechanical properties which are species dependent (Pincfada: tensile strength of 140–170 MPa, Young’s modulus of 60–70 GPa; Hydnum rufescens: tensile strength of 180 ± 20 MPa; Pinctada margaritifera: tensile strength of 220 ± 60 MPa) [386]. |
| Market situation (world market reports) | The variety of molluscan shells applications (poultry food, pet nutrition liming agents) created a market of potentially increasing demand [389]. The development of shell valorisation methods will be crucial for the market stabilization [384]. |
| Patents | Currently, about several hundreds of patents related to various application of molluscan shells (building material component, bone graft material, decontaminants) or nacre itself (composites, cosmetic ingredients) exist. |
| For search, use: https://patents.google.com/ | |
| Selected examples: | |
| CN101971982A. Oyster shell powder containing hydrogen and manufacture method thereof | |
| CN106866807A. The preparation method of pearl protein, the pearl protein prepared by the method and its application | |
| WO2008017962A8. Microcapsules with improved shells | |
| KR101357078B1. Process for seperation of cutoffs having anti-inflamentary or osteoarthritis inhibition effects using oyster shells | |
| KR101771055B1. Composition comprising water-soluble pearl powder for skin whitening, anti-inflammation and anti-aging | |
| UDS 5968772. Pearl protein (nacrein) and process for producing the same | |
| US4312099A. Process for shucking a mollusk | |
| US8067078B1. Nacre composites, methods of synthesis, and methods of use | |
| US 6251438. Method of preparing active substances from nacre, products obtained which can be used in particular as medicaments | |
| FR2777190B1. Extraction process, identification of the active ingredients contained in the internal and external shell of sea molluscs, their use in people-based thera, diagnosis and cosmetic preparations | |
| FR2799125B1. Process for the preparation of a composition by extraction of nacre, comprising the complete components of the nacre, composition obtained by this process and its use in pharmacy and cosmetics. | |
| FR2899478A1. Process for extracting nacre molecules, compositions and use | |
| US8162241B2. Apparatus and method for collecting and crushing seashells on a beach | |
| US4939814A.Cultured mussel cleaning machine | |
| WO1997015398A1.Method for producing a lime product from mussel- and/or seashells |
This review has the ambitious goal to provide a thorough and comprehensive coverage of marine biomaterials as multifaceted topic. Consequently, we strongly believe that numerous open questions raised in this review will inspire a younger generation of experts in marine biology, biochemistry, bioengineering, biomimetics, bioinspired materials science, biomineralization, marine waste processing, fishery and mariculture to research marine biomaterials as examples of renewable natural sources which stood the test of time through evolutionary development of corresponding organisms.
2. Marine Polysaccharides
Polysaccharides belong to biological materials with carbohydrate backbone-based structures. In this review, we focus attention only on structural aminopolysaccharide chitin and selected polysaccharides of algal origin. Chitosan, an artificially produced derivate of chitin, was not the goal of our analytical research exclusively due to the existence of numerous reviews related to this biopolymer (i.e., [6,7,8,9,10,11]).
2.1. Chitin
The main characteristics of chitin are summarized in Table 1.
2.2. Recent Studies in Crustacean Chitin Applications
Crustaceans (lobster, crab and krill) chitin [48,49] including chitin-based cuticles of more than 300 million tons of Antarctic krill present in the world ocean [50], remains the main industrial source of this structural biopolymer.
Importantly, crustacean shells combined with commercial chitin can be used as biosorbents to remove heavy metals from surface runoff that solves two environmental problems: the use of seafood wastes and water resources management [51]. Moreover, seafood wastes can be employed in agriculture: the use of shrimp chitin as feed additives showed a positive effect on growth and carcass characteristics of broiler chickens [52]. Another application of crustacean shells was shown in a recent research of Las Heras et al. [53] who described the generation of chitin-containing sponge like scaffolds, which were biocompatible with human mesenchymal stromal cells (hMSCs), thus representing a high potential for biomedical technologies, in particular, for tissue engineering. Likewise, novel interesting scaffolds-candidates for tissue engineering were designed from crab shells chitin and silk protein fibroin obtained from silkworm Antheraea pernyi cocoons [41]. Finally, nanomaterials from shrimp chitin (nanocrystals and nanofibers) were reported to have no cytotoxic effect, which was tested with epithelial-like and fibroblast-like cell lines [54]: this research indicates that such components can be safely used in biomedical industry.
2.3. Poriferan Chitin: Progress in the APPLICATion of Poriferan Chitinous Scaffolds
The presence of chitin in marine sponges has been revealed only recently [55,56] that was further confirmed by the detection of chitin in fossilized skeleton of 505 MYR old demosponge Vauxia gracilents [39]. Since then, chitin has been isolated from numerous species of marine [25,43,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] as well as fresh-water sponges [70].
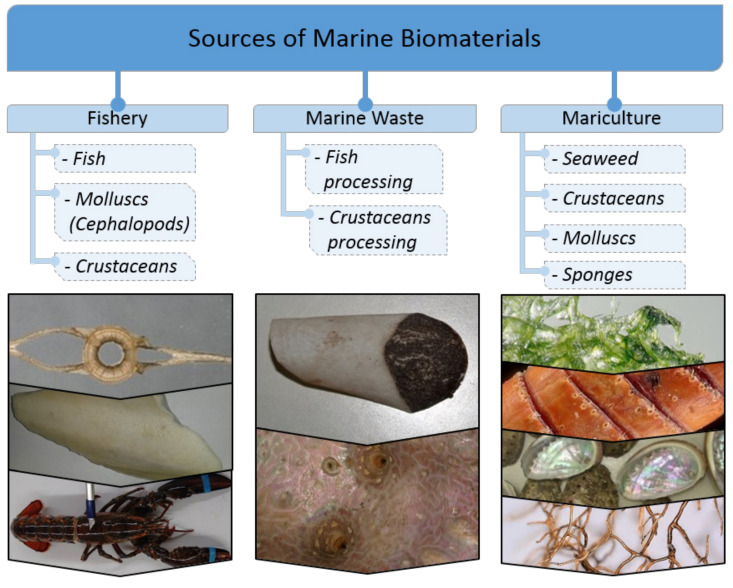
Over the last decade, 3D chitinous scaffolds of poriferan origin were reported to have a huge potential for biomedical applications due to ability of corresponding sponges to grow under marine farming conditions [71]. Indeed, being biocompatible and supporting cell adhesion, growth, and proliferation, these scaffolds serve as perfect ready-to-use 3D matrices for tissue engineering and regenerative medicine [3,4,43,72,73,74]. For example, hMSCs seeded onto Aplysina aerophoba [61], A. fulva [1], and Ianthella basta [62] chitinous scaffolds displayed good attachment, viability, proliferation, and the capability of differentiation into osteogenic (A. aerophoba, A. fulva), adipogenic (A. aerophoba, I. basta) and chondrogenic (A. aerophoba) lineages, provided that the growth media were supplemented with respective differentiation inducers. Furthermore, chitinous scaffolds from I. labyrinthus were applied for the cultivation of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs): the long-term study (20 days) demonstrated that the cells grown on investigated scaffolds formed contracting cell clusters indicating that I. labyrinthus chitin is a source of suitable matrices to conduct research on the regeneration of myocardial tissue [66]. Additionally, poriferan chitinous scaffolds can serve as templates for co-culture systems mimicking in vivo processes [1,6]. A recent study conducted by our group [75,76] explored the ability of hemocytes from Cornu aspersum snail to grow on chitinous scaffold of A. archeri, which resulted in the generation of a new calcium-layered biomimetic product.
In addition, the study of sponge chitinous scaffolds, i.e., scaffolds of Ianthella species, pointed to their elasticity and capillary effect that allows these unique matrices to assume the shape of the objects they were placed on and swell with liquids (i.e., blood), the properties, which can be used in wound treatment [35,66] (Figure 2). In addition, owing to their capillary effect, sponge chitinous scaffolds can be used as adsorbents of crude oil and synthetic dyes [35] as well as drug delivery systems, which was shown for a sponge scaffold adsorbing antimicrobial drug decamethoxine leading to the inhibition of Staphylococcus aureus pathogene [68]. Another important application of sponge chitinous scaffolds lies in waste water treatment, as was shown for the case of A. aerophoba adsorbing uranium [77].
Figure 2.
Digital microscopy images: Naturally prefabricated 3D chitinous skeletal constructs of verongiids sponges origin (A) are made of interconnected tube-like fibres with excellent ability to absorb diverse liquids including blood (B). They can be used also as biodegradable 3D scaffold-based bioreactors for cultivation of algal cultures (C,D) for further production of corresponding biologically active compounds under controlled laboratory conditions.
Intriguingly, sponge chitinous scaffolds serve as a source of inspiration for biomimetic research including the development of diverse composite materials using “extreme biomimetics route” [2,78,79]. Some sponges of Verongiida order were reported to carry unique biocomposites composed of amorphous silica, crystalline aragonite and chitin [74] that can be used as a «natural example» of chitin mineralization. Recently, a great progress in the application of sponge chitinous scaffolds as matrices for metal incorporation has been reported. For example, isolated chitinous skeleton of A. fulva demosponge was shown to undergo electrochemical deposition of copper following “sensibilization” employing silver nitrate solution [80,81]. Other examples include A. aerophoba scaffolds covered by ZrO2 [82] and Fe2O3 [83], the use of A. cauliformis as a template for the growth of GeO2 nanocrystals [84], and nanosilica depositions onto I. basta scaffolds [85]. All such treatments were conducted under hydrothermal conditions up to 185 °C. Such composites could be used for waste water treatment and photocatalytic decomposition of water to convert solar energy into chemical energy [81,86]. These findings are of a very high relevance to the current development in EV industry including the production of sensors, catalysts, electrochemical capacitors, the research focused on the latter also included I. basta chitinous scaffold to produce chitosan/sponge chitin membrane [87]. Finally, poriferan chitin-containing biocomposite materials demonstrated a potential for the adsorption of water pollutants, i.e., A. archeri scaffolds were used as a matrix for the immobilization of Trametes versicolor laccase that proved to efficiently remove tetracycline [69], while metallization of A. aerophoba scaffolds with silver nanoparticles and AgBr proved to be promising for water filtering systems with antibacterial properties [88].
Modern biocomposite-based scaffolding strategies include two key ways: to produce requested 3D constructs from corresponding precursors using technological tools or simply use naturally already pre-fabricated scaffolds if they originate from renewable sources. Such kind of 3D scaffolds remains to be one of the crucial features of skeletons of marine sponges that belong to the Verongiida order inhabiting oceans since the Precambrian [39].
Fabrication of biomimetic materials and scaffolds is usually a micro- or even nanoscale process. However, mostly all practical applications on the industrial level require larger-scale synthesis of nanoscale features. Recent development in micro-CT tomography and 3D printing not only bring us closer to the biomimicry of hierarchical 3D open cell hierarchical structures, but also clearly shows how nature is ahead of our most advanced technologies. Nevertheless, science still can benefit from the remarkable structural advancements of natural chitin-based scaffolds by simply applying them as multi-target templates in biomedicine and various modern technologies.
2.4. Polysaccharides of Algal Origin
Historically, the value of marine macroalgae (seaweeds) was greatly underestimated. Already in ancient Greece, Virgil and Horace, while referring to something completely worthless, used the term “villior alga” [89]. Seaweeds can be divided into green, red and brown algae containing a variety of polysaccharides [90,91], the properties of which were extensively studied during the last decade. Almost all representatives of brown algae are marine, mostly occurring in cold water, especially in the northern latitudes [92], and are rich in polysaccharides such as alginates (Table 2) and fucoidans (Table 3).
As represented below, alginates as biomaterials are widely applied in diverse biomedical fields. Being used as polymeric coating for therapeutic agents, so-called alginate microspheres can be applied for the delivery of different drugs [97,103,104,105,106,107,108,109,110] including tetracycline derivative minocycline [111] and vancomycin [112] antibiotics, lipopolysaccharide subunit antigen as vaccination therapy against Klebsiella pneumoniae [113], paracetamol [114], and anticancer drugs [115]. Additionally, alginates, in the form of hydrogels or composites, in particular, employing bioprinting [116,117,118,119,120,121], are widely employed in tissue engineering, such as tissue engineering of bone [122,123,124,125,126,127,128,129], cartilage [130,131], skin [132,133], muscle [134], and even neural tissue engineering [135] as well as cardiac regeneration [136]. Recently, alginates were reported to be widely researched for wound healing applications [137,138,139,140,141,142,143].
Similar to alginates, fucoidans proved to be valuable in tissue engineering [155,156,157,158,159], drug delivery [160,161,162,163], and wound healing [164,165,166].
Apart from brown seaweeds, read and green algae also produce unique polysaccharides. Brief information on carrageenans, extracted from red algae, is presented in Table 4.
Having viscosity increasing, stabilizing and gelling properties, carrageenans are widely used for controlled drug release, pharmaceuticals, food and other industries [174,179]. In addition, being biodegradable, these polysaccharides can be applied as films for food packaging: in order to increase their mechanical properties, nano-sized fillers such as melanin nanoparticles are employed as reinforcing agents [180]. Finally, carrageenans exhibit anticoagulant [181,182], antithrombotic [183], anti-HIV [151], antiviral [184,185,186], anti-cancer [187], immunomodulatory [177,188,189], and antioxidant [150,177,190] activities.
Green seaweeds, on the other hand, are abundant in ulvans (see Table 5).
Biocompatibility of ulvans, shown in in vitro cell culture assays, enables their use in wound treatment [191,201] and as substrates for cell cultivation [202]. Like other algal polysaccharides with gelling properties, ulvans can be employed for drug delivery [203,204]. Furthermore, ulvans are used for the synthesis of silver nanoparticles, the antimicrobial activity of which are essential for cosmetic and biomedical industries [205]. Iduronic acid, another rare sugar component of ulvans, is reported to have anti-thrombotic activities [206]. Finally, similar to alginates and carrageenans, ulvans are used to produce films as biodegradable material for food packaging, antioxidant, optical, thermal, and mechanical characteristics of which can be modified [207].
Selected marine algae, belonging to the phylum Rhodophyta (red algae), have been recognized as renewable sources of such polysaccharides as agar (agar-agar) (see Table 6), agarose and agaropectin. Numerous methods of agar extraction from such algae species as Gelidium, Acanthopeltis, Ceramium, Gracilaria, and Gloiopeltis have been reported [208].
Nowadays, both agar and agarose represent marine biomaterials with a high potential of their application in biomedicine and tissue engineering [213,214,217]. According to the modern view, “agarose is particularly used as a temporary scaffold for bony cells and growth factors in the field of tissue engineering, as a biocompatible substrate enriched with osteoconductive particles for bone grafting/augmentation procedures, and as a bone spacer in guided tissue regeneration“ [219]. Self-gelling properties and adjustable mechanical stability [220,221] of agarose gels are crucial for their use. For example, non-toxic [222] and biodegradable agarose gels have been effectively used in implantation surgery [219], wound healing, cartilage [223], cardiac, bone and nervous system [224], and regeneration as well as skin tissue engineering [225,226]. These directions are based on tunable features of agarose, which can result in adjustable characteristics similar to native tissues [225]. In addition, applications of this biomaterial for targeted drug delivery have been recently discussed in the review entitled “Agarose-based biomaterials for advanced drug delivery” [227]. Finally, agarose gels can be used in 3D bioprinting [228].
3. Marine Structural Proteins
According to a modern definition, “a structural protein is a protein that possesses a characteristic amino acid sequence or motif that repeats and forms a skeleton or contributes to the mechanical properties of a living organism, cell, or material” [229]. Typical examples of such proteins include actin, tubulin, collagen, elastin, sericin, fibroin, byssus, spongin, conchiolon, resilin, gorgonin, and keratin (see for overview [4,230]). A few selected structural proteins of marine origin are discussed below.
3.1. Spongin
Despite the fact that spongin (Table 7) has been studied by scientists since 1705, its true nature remains unknown and this biological material itself is attributed to one of the last mysteries of water-insoluble structural proteins that arose more than 800 million years ago, at the dawn of multicellular organisms [231,232]. The identification of spongin requires an extraordinary approach and is a challenging task that diverse research groups have failed to solve during 315 years of investigations. The low solubility of natural spongin in acids as well as after enzymatic treatments mentioned earlier [232] is a critical factor limiting its clear identification as collagen, or keratin, or a glycosylated form of one of them.
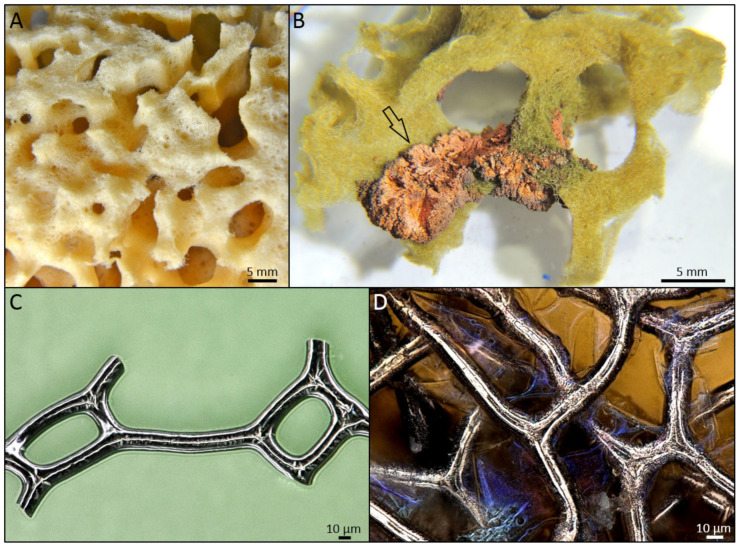
Spongin represents the biopolymers with high resistance to chemically harsh and thermally extreme conditions and is one of the main players as specialized templates for extreme biomimetics (Figure 3). Nowadays, it is very important to design a bridge between extreme biomimetics and bioinspired materials science where the basic principle is to exploit chemically and thermally stable, renewable biopolymers for the development of the next generation of biologically inspired composite materials never reported, or even suggested before, with sizes and properties which will allow their application in the extremes of modern industry including a large scale level. Recent studies have revealed that especially such renewable structural biopolymers as aminopolysaccharide chitin and proteinaceous spongin can be used as thermostable biopolymeric scaffolds with 3D architecture for the nucleation and growth of a wide range of novel nanoorganized SiO2-, GeO2- Fe2O3-, ZnO-, ZrO2-, TiO2, MnO2, and multiphased TiO2/ZrO2-based composites (see, for an overview, [2,3,79,86]).
Figure 3.
Spongin of the bath sponges origin (A) has been recently recognized as unique marine biomaterial for development of metal oxide-based composites (B, arrow) and the source for creation of mechanically stable 3D turbostratic graphite (C), which can be further functionalized with selected metals (D). For details, see [86].
In particular, using an extreme biomimetic approach, the spongin scaffold of Hippospongia communis was coated with TiO2 and such new biocomposite could efficiently remove C.I. Basic Blue 9 via adsorption and photocatalysis [237]. Secondly, the application of H. communis scaffold as a template for hydrothermal synthesis of hematite (α-Fe2O3) resulted in the generation of a composite consisting of spongin and hematite, which was shown to enhance the electrochemical properties of the capacitor electrode [241]. Thirdly, the same template was reported to undergo extreme biomimetic treatment yielding a novel MnO2-spongin composite that can be employed for the development of 3D metal oxide layered biocomposites functioning as electrodes [242]. Furthermore, due to their structure composed of 3D fibrous network and to spongin perfect sorption properties, spongin scaffolds serve as excellent matrices for enzyme immobilization. Indeed, H. communis were studied as a template for the immobilization of Candida antarctica lipase B (CALB). Astonishingly, such a biocatalytic system proved to be efficient even after 20 days of storage at 4 °C: immobilized lipase catalyzed the conversion of triglycerides to glycerol and fatty acid methyl esters that is very promising for bio-fuel industry and further research focused on spongin matrix enzyme immobilization [239]. Indeed, a follow-up study using H. communis scaffold showed a successful immobilization of laccase from Trametes versicolor mushroom, which efficiently catalyzed degradation of bisphenols, toxic compounds used in polycarbonates manufacturing [243]. The removal of contaminants, i.e., phenol, chlorophenol, fluorophenol, bisphenol A was also shown in the study that exploited the properties of another biocomposite composed of H. communis spongin and iron phthalocyanine [244]. In addition, H. communis was used to construct 3D carbonized spongin-Cu/Cu2O scaffold that was reported to catalyze the conversion of a toxic compound, 4-nitrophenol to 4-aminophenol [86]. Recently, spongin-based scaffolds isolated from Haliclona sp. marine demosponge have been successfully used for preconcentration and extraction of such substances as fenitrotion [245] and ketamine [245].
3.2. Collagens
The most important features of marine collagens are described in Table 8.
3.2.1. Marine Invertebrates Collagen
Recently, the biocompatibility and cell responses to marine invertebrate collagens have been reported in different studies. For example, employing murine fibroblast cells, biocompatibility of cryogels composed of jellyfish collagen, chitosan, and fucoidan was demonstrated [159]. Poriferan collagenous scaffolds, on the other hand, represent natural 3D scaffolds with a great potential for tissue engineering [263]. In particular, in vitro experiments using primary murine osteoblasts demonstrated a good cell attachment and proliferation when cultured on sponge collagenous scaffolds [269]. A series of experiments revealed a positive effect of sponge collagen hydrolysates on damaged or photoaged skin [270]. In addition, a recent in vivo study using rats demonstrated biocompatibility and the ability to support bone formation of biocomposites generated from collagen, isolated from A. fulva, and biosilicate [271]. Finally, powdered collagenous sponge scaffold loaded with L-cysteine hydrochlorid proved to cause a positive effect on wound healing [272].
Intriguingly, sponge collagen served as a template in several scientific projects aiming at the generation of bioinspired silica layered composite biomaterials [261] that resemble naturally occurring poriferan biocomposites [273,274]. For example, in laboratory conditions, collagen of different origin, i.e., isolated from Chondrosia reniformis marine demosponge, underwent in vitro silicification resembling the growth of siliceous spicules in glass sponges, which is promising for the generation of new collagen-silica hybrid materials on industrial scale [260,275]. Moreover, a specific amino acid motif, Gly-3Hyp-4Hyp, was discovered within the glass rope sponge Hyalonema sieboldi collagen, which presumably is predisposed for silica precipitation [276]. Thus, the modification of collagen amino acid sequence might significantly improve the construction of siliceous spicules layered biocomposites.
3.2.2. Marine Vertebrates Collagen
Both fishery and mariculture of selected fish species represent important sources of collagens (see for overview [254]). Marine fish collagen-based biomaterials (i.e., collagen gels, scaffolds, sponges, films, membranes, and composites) have a wide range of applications including drug delivery, wound healing, wound dressing, tissue engineering, i.e., bone, cartilage, dental, vascular and skin tissues, and therapeutics against skin aging, diabetes, and obesity [256,262,277,278].
The use of marine wastes including by-products of industrial plants, such as fish skin, scales and fins, as a source of fish collagen helps to fight environmental pollution and serves as a strategy to valorize marine resources [254,279]. Intriguingly, it is possible to isolate fish collagen from skin of marine Eel fish [280], codfish [281,282,283], European hake [284], smooth wolf herring [267], blue shark [285,286], small-spotted catshark [253], salmon [266,283], ocellate puffer fish, seaweed pipefish, brownstripe red snapper, brownbanded bamboo shark, carp, largefin longbarbel catfish, Japanese sea-bass, bigeye snapper, surf smelt, brown backed toadfish, Nile perch, skate, blacktip shark [255,256], bones of European hake [284], carp, Japanese sea-bass, skipjack, ayu, yellow sea bream, horse mackerel, Baltic cod [255], swim bladder of Atlantic cod [287], cartilages of brownbanded bamboo shark, blacktip shark, scales of carp, tilapia, spotted golden goatfish, grey mullet, rohu, and catla [255,256].
The application of fish collagen as biomaterial in biomedicine including tissue engineering has been thoroughly studied. Indeed, using cell culture assays, it was shown that 3D printed fish collagen/alginate scaffolds proved to be biocompatible with human MSCs [280]:
3D printed scaffolds consisting of fish collagen/alginate and phlorotannin (as a bioactive component) displayed good biocompatibility and stimulated osteogenic differentiation of osteoblast-like MG63 cells [288];
3D printed fish collagen/alginate hydrogels containing murine fibroblasts were of good biocompatible characteristics [285];
fish collagen was reported to be biocompatible with human fibroblasts [282];
3D printed scaffolds composed of fish collagen and calcium phosphates derived from two sharks, blue shark and shortfin mako shark, were biocompatible with osteoblast-like Saos-2 cells [286];
composite scaffolds from fish collagen and chitosan promoted osteogenic and chondrogenic differentiation of rat MSCs [266];
fish collagen composites cross-linked by genipin under CO2 atmosphere were biocompatible with murine chondrocytes [253].
Fish collagen is also employed in dentistry, usually as membranes and bone graft materials [257,269]. Furthermore, this structural protein is used for controlled drug release including antimicrobial agents such as tetracycline [270]. In another research, a potential of anticancer drug(s) loaded 3D printed patches from fish gelatin for anticancer treatment was demonstrated [271]. Due to its excellent absorption properties and the ability to resorb up to 56 days, fish collagen can be used to control wound blood bleeding [272]. In addition, it has a high potential for cosmetic applications: fish collagen demonstrated a moisturizing effect without irritating skin [263].
3.3. Gelatin
Gelatin (Table 9) can serve as cell carrier to repair tissue defects, i.e., gelatin extracted from marine snail Rapana venosa was reported as a biocompatible template for the growth of human keratinocytes [289]. Hence, this marine biomaterial can be used in tissue engineering, often in combination with other materials such as chitosan and silk fibroin [42]. Indeed, chitosan/gelatin and silk fibroin/gelatin composites were employed in hepatocytes research and can be applied to generate 3D hepatic microenvironments, which would shed more light on hepatic cell functions [290]. Importantly, marine gelatin can be used in the inhibition of angiotensin-converting enzyme in order to lower blood pressure and reduce the risks of myocardial infarction, congestive heart failure, stroke, and arteriosclerosis [291]. Amino acid sequences of peptides inhibiting angiotensin-converting enzyme were detected in the studies on gelatin extracts of Alaska pollack [291] and can be further applied to prevent hypertension.
Moreover, due to its gel-forming properties, marine gelatin is also applied in food industry as a stabilizer, texturizer, thickener and foaming agent in yoghurt, ice-cream, jam, cream cheese, marshmallows, etc. [303,304]. Presumably, due to the lower content of proline and hydroxyproline in comparison to beef- and pork-derived gelatins [294,305], marine gelatins form “weaker gels” [306,307]. Notably, gelatin inhibits peroxidation preventing food from deterioration and functions as an outer protective film against dehydration, oxygen, and light [304]. In addition, isinglass, a high-grade gelatin derived from fish swim bladders that can induce aggregation of yeast and other insoluble particles, can be widely applied as a commercial clarifier in beverages, i.e., wine, beer, cider [303]. Though marine gelatin may trigger allergy, i.e., 0%–8% incidence depending on local food habits and fish consumption is reported [304]. Finally, marine gelatin is widely used in capsule industry. Usually, it is applied for the encapsulation of temperature-sensitive vitamins and other nutrients [308].
3.4. Keratin
Keratin (Table 10) is a fibrous protein of a high importance in the animal kingdom. Keratin presence in horn, hoofs, hair, beaks, shells, toenails, claws, fingernails, and feathers renders it the most abundant structural protein [309]. In such marine mammals as whales, keratin is to be found as the main structural component of baleen (see, for details, [230]). In general, keratin is present in two forms, characteristic for the type of tissue it is present in: α-keratin, found in soft tissues, e.g., wool, hair or skin, and β-keratin dominating in feathers, nails, fish scales, and other hard tissues. Structurally, both keratin types show a filament-type matrix structure. However, α-keratin filaments, denoted as intermediate filaments (IF), are two times greater in diameter (7–10 nm) compared to β-keratin filaments diameter of 3–4 nm. From the mechanical point of view, keratins have high strength and stiffness; the properties typical for the tissues keratin is a component of [310].
Though keratins seem to be constituents of static matrices (i.e., baleen) [310], there are exceptions to this rule such as hagfish. Hagfish (Myxinidae) are deep water inhabiting living fossils the body of which has an eel shape with no scales present. The remarkable feature of hagfishes is their ability to, when provoked or threatened, produce and excrete large amount of slime consisting of keratin IFs. The filaments act as threads binding mucin, a protein capable of forming gels [318]. When shot out of the slime gland followed by the contact with seawater, the slime becomes extremely dilute and is capable of effectively covering or choking the hagfish predator almost instantly. Detailed mechanical analysis of hagfish threads reveals their remarkable mechanical properties, different in dry state when compared to their wet state. In particular, dry hagfish threads show high initial stiffness of 3.6 GPa and a high tensile stress of 530 MPa while wet threads exhibit stiffness of 6 MPa and tensile strength of 180 MPa [317]. The outstanding mechanical properties of dry threads combined with the ease of their synthesis have been the reason for considering hagfish slime as a substrate for engineering fibers acting as a reinforcement for various modern composite materials [319].
Due to its poor solubility and tedious extraction methods, keratin has so far found limited applications; nonetheless, attempts were made to expand its usefulness. Initial studies on potential applications were focused on cells and their behavior on keratin containing films [320] and further extended on the potential of these films to act as active molecule carriers [321] or focused on altering their mechanical and antibacterial properties [322]. Keratin films have also been proposed for ocular surface reconstruction due to their good corneal biocompatibility and transparency [323].
3.5. Conchiolin and Conchixes of Molluscan Origin
To solve the problem of conchiolin insolubility, numerous hydrolysis protocols yielding soluble peptides of better functionality and applicability were developed: hydrolyzed conchiolin protein of pearl shell origin is a common cosmetic ingredient of hair and skin conditioning agents [324] or cleansing solutions [325]. More detailed studies on the properties and possible future applications of molluscan matrix protein extracts have been performed by Latire and co-workers [326]. In the first study, the authors analyzed shell extracts (acid soluble (AS), acid insoluble (AI) and water soluble (WS)) from the marine bivalve Pecten maximus [326]. AS did increase human fibroblast metabolic activity following 24 h of incubation. Likewise, the extracts obtained from mussel Mytilus edulis (AS and WS) and oyster Crassostrea gigas (AS) led to an increase in primary human skin fibroblast metabolic activity and cell proliferation [327]. Such data indicate the potential applications of these matrix proteins or their extracts in medicine, especially in wound healing or the treatment of various skin conditions. Indeed, in vivo study employing rats with dorsal skin wounds [328] demonstrated a progressive wound reduction after the ointment containing powdered shells of Megalobulimus lopesi was applied. This effect was attributed to calcium, which, after being administered to the wound tends to enhance the healing process [329,330], though the authors do not rule out the possibility of so-called conchix proteins to be involved in the facilitation of the healing process. Conchix, a term representing the shell organic matrix, has been recently proposed by Ehrlich and co-workers [331] to underline the importance of this organic piece of mollusc shell architecture. A brief summary on conchiolin properties is provided by Table 11.
The molecular complexity of the conchix hinders its applicability, especially in highly regulated industries, e.g., pharmacy. Dealing with this issue requires an intense focus on the isolation of active matrix components [337].
The optimistic conclusions resulting from the above-mentioned studies are challenged by the vast number of conchix active components which are extremely difficult to get rid of in order to isolate a single substance exhibiting biomedical or cosmetic potential. This fact is reflected in a small variety of products utilizing shell proteins as active components.
4. Marine Biominerals
Biominerals have been recognized as the main players in skeletogenesis of diverse organisms including those inhabiting seas. Being the products of vital activity of cells and specialized tissues, they are formed as the result of the interaction of organic matrices with various mineral phases regulated at the molecular and genetic level. Biosilica, calcium carbonates, and phosphates (mostly in marine vertebrates) represent the dominant mineral phases in a broad diversity of biocomposite-based skeletal constructs. In contrast to Ca-based biominerals discussed below, we paid no attention to highly sophisticated biosilica- based constructs of sponges origin [276,338,339,340,341,342] which represent diverse biomimetic models (Figure 4); however, they are not industrially harvested, or cultivated being mostly protected.
Figure 4.
Digital microscopy images: Despite protection and the lack of industrial harvesting, glass sponges, thanks to the complex architecture of their biosilica-based skeletons (A–C)—Aphrocallistes sp., (D)–Waltheria sp.; (E)—Euplectella sp.) represent a unique source for creating 3D models for potential biomimetic functional materials.
4.1. Corals
Corals (class Anthozoa) are marine invertebrates offering great opportunities for biomedical applications. “Coralline biomaterials” [343] (see also Table 12) have been well recognized in biomaterials science community (see, for an overview, [4,42,166]).
Coral skeletons are often used as the sources for inspiration to create artificial 3D constructs “with a 3D bioprinting platform which mimics morphological features of living coral tissue and the underlying skeleton with micron resolution, including their mechanical properties” [350]. The use of bioceramics of coral origin represents an attractive alternative to metal-based constructs [351] for implantology and tissue engineering [352]. Furthermore, the coral structure can undergo chemical conversions yielding calcium phosphate particles, which could be used in tissue engineering and as drug carriers: the conversion of Tubipora musica coral at 400 °C and 800 °C resulted in plate-like calcium phosphate nanoparticles (mostly Monetite) and spherical shaped calcium phosphate nanoparticles (whitelockite and hydroxyapatite), respectively [353]. Likewise, the scleractinian corals, Porites spp., were converted to hydroxyapatite employing hydrothermal and mechanochemical treatments [353]. Hence, the properties of coral skeletons inspire a whole range of studies focused on coral bone graft substitutes [354] as well on osseointegration with human bones [344,352,353,355,356,357]. Indeed, in vivo study demonstrated efficient bone formation at critical size defects in sheep bone using MSCs-covered scaffolds from Acropora coral [358]. Another research demonstrated that murine preadipocytes cultured on coralline skeletal material obtained from Porites lutea corals differentiated into osteoblasts [359]. In addition, positive effect has been obtained with respect to activity of human osteoblast-like MG-63 cells growing on the scaffolds isolated from the coral Goniopora sp. [360]. Moreover, as recently reported by Gancz and co-workers, “the coral skeleton biomaterial may act as a strong, promotive scaffold for tissue regeneration due to its ability to reduce its rejection by inflammatory reactions such as phagocytosis” [361].
In addition to application of stony corals reported above, octocorals also possess a high biomimetic and biomedical potential [4]. Indeed, their structural architecture, the role of gorgonin-associated mineralization, and the potential of deep-sea bamboo octocoral for tissue engineering were reported [346]. The skeleton structure of black coral species, i.e., Parantipathes larix [27], or Cirrhipathes sp. [25] contains chitin that was also shown to be biocompatible and serves as a template for cell adhesion and differentiation.
Large scale production of coral-based biomaterials is limited due to the protection of coral reefs [362]. However, further investigations to use corals as model 3D porous constructs and source for bioinspiration in materials science are trending well.
4.2. Molluscan Shells
Though molluscan shells (Table 13) have been intensively studied primarily as the indicators of environmental transformations [363,364] and contamination [365,366]; over the years, this has changed with the focus on biomechanics [367,368,369,370], biomimetics, and materials science [26,371,372,373,374,375,376] of these biomineralized constructs.
With about 18 million tons of total annual production, shelled molluscs are one of the most important components of global aquaculture industry [390], being especially important for the regions of Eastern Asia (China, South Korea and Japan), and, to a less extent, North America (the USA) and South America (Chile). Due to the risks associated with freshwater shortage, energy consumption and constantly increasing human population the development of offshore mollusc farms is of an increasing interest. In order to valorize molluscan shell wastes that constitute from 59% up to over 75% of the total organism weight depending on molluscan taxa [391], several solutions were developed.
Being composed mainly from calcium carbonate (it can reach up to 99.9% of the shell mass), shell wastes can be utilized as a mortar component [392]. The authors revealed that crushed oyster shell small particles (0.074–2 mm) were more suitable than the large ones (2–4.75 mm) as sand substitutes. A similar study conducted by [393] demonstrated that mollusc based CaCO3 particles are longer and of prismatic shape in contrast to the round and shorter particles of traditionally used limestone (Figure 5), thus affecting mortar setting time and its mechanical properties.
Figure 5.
SEM micrographs showing morphology of the particles in (A) mussel and (B) quarry derived limestone. Images adapted with permission from [393].
The use of shell CaCO3 as a source of calcium in livestock feed supplements is another alternative to utilize shell wastes. Such supplements were reported to have a positive impact on animal bones and the strength of eggshells [394]. In addition, different studies indicated that both oyster and clam shells are equally effective regarding eggshell strength and egg production rate [395,396,397,398,399]. Such benefits of shell derived CaCO3, shown in earlier studies, were also confirmed in recent research [400,401].
Furthermore, molluscan shells can be applied for soil neutralization and metal decontamination. Soil neutralization, typically known as liming, is performed in order to reduce acidity, improve oxygen levels, soils fertility and structure, therefore directly affecting agricultural crop yields [402]. Indeed, the use of crushed oyster shells leads to an increase in soil pH, available phosphorus and exchangeable cations, thus, positively affecting the productivity of Chinese cabbage [403]. In addition, marine shell wastes can be applied as soil decontamination agents as was reported for soils containing copper [404], lead, cadmium [405], and arsenic (V) [406].
Another option to utilize molluscan shells lies in wastewater filtration. Several studies pointed to the capabilities of razor clam and oyster shells to accumulate Zn2+, Pb2+, and Cd2+, though with different capacities, favoring calcite rich oyster powder for Pb2+ and aragonite rich razor clam for Cd2+ [407].
Intriguingly, molluscan shells, being among the most important biominerals known to date, challenge man-made ceramics, i.e., they are characterized by a specific internal features hierarchy, structural organization, and organic-mineral phase interactions that are formed in mild conditions unlike ceramics, which require high temperature and/or high pressure [408].
The nacre, of the structural shell elements (Figure 6), has gained special attention. An increasing interest in the nacre, an acellular composite of calcium carbonate acting as an internal shell coating for bivalves, cephalopods and gastropods, stems from its structural similarity to bone and remarkable mechanical properties, i.e., Young’s modulus of 30–40 GPa for the nacre vs. about 20 GPa for human bones, resistance to failure of 185–200 MPa for the nacre vs. about 140 MPa for human bones [409,410]. Hence, nacre material has a great potential to be used as bone grafting material (Figure 7) [411].
Figure 6.
Abalone (Haliotis sp.) shell and its structure. (A) interior view of the shell and (B) scanning electron microscope micrograph showing the cross section the shell; (C) the microstructure of the nacre. Image reproduced with the permission from [412].
Figure 7.
Examples of different forms of nacre used in bone graft studies. (A) nacre powder (denoted with a ★) injected within sheep vertebrae; adapted from [413], with permission; (B) nacre in the form of a cylinder (N) implanted in sheep femoral epiphysis; adapted from [388] with permission; (C) trochlea shaped nacre in sheep femoral trochlea; adapted from [414] with permission; (D) screw shaped nacre implanted in sheep metatarsus; adapted from [415] with permission.
Indeed, osseointegrative properties of the nacre and its potential for implantology were shown in numerous studies [411,413,414,416,417,418]. Moreover, molluscan shells can be used in bone biocomposite scaffolds [385,419,420], which are characterized by porosity favouring cell seeding and adhesion (Figure 8).
Figure 8.
SEM micrographs presenting (A) porous morphology of fabricated scaffold and (B) cells attached to the surface of the scaffold matrix—figures adapted from [385] with permission.
5. Conclusions
Biological materials of marine origin represent a special scientific niche within the global biomaterialogy with a long history of their research and applications in diverse fields of human activity.
What was recently referred to as processed marine biological waste is now considered raw material for the production of biomaterials, which differ from their synthetic analogs in biocompatibility and possess excellent biodegradability. In addition, the approach to the study of marine biocomposite structures has shifted to the point of view of modern bionics and biomimetics, when these materials are thought of as models for creating new composites, which are produced according to «drawings drawn by nature» as a result of evolutionary selection. The design of such new hybrid materials is of crucial importance for fundamental science because further progress in their research and application is impossible without understanding the mechanisms of their formation as well as their structural features at the molecular and nano-level.
The progress in marine biomaterials research is mainly attributable to its strong interdisciplinary character: the exchange of expertise in marine and structural biology, bioinspired materials chemistry, biomineralogy, biomimetics, biomechanics, and solid state physics is a key action to strengthen the scientific and practical level of this modern research field. We are strongly convinced that the scientific area described herewith will include both a high degree of novelty and challenging tasks in the future. Researchers will discover the key principles of molecular structure of marine biomaterials that will finally let them realize the dream of understanding the chemistry and materials science of diverse unique marine biocomposites spanning from atomistic detail to the macrolevel.
This concept will adopt a truly multidisciplinary and multi-scale approach to study not only the structural peculiarities of marine biomaterials, but also the mechanisms of their transformation in hybrid, functionally advanced composites, hierarchically constructed during special treatments and modifications according to human goals. A holistic understanding of the creation of a new generation of bioinspired composites and its impact on large-scale biomimetics with future input in modern technologies can only be achieved by a modern multi-facetted approach, which has not been attempted before. An undoubted factor stimulating progress in marine biomaterials is a sharp move away from synthetic plastic materials due to the serious and global threat of pollution of the world’s oceans by microplastic waste. We readily believe that biomaterials of marine origin will also be actively studied because of their extreme prospects for the so-called marine bioeconomy worldwide.
Author Contributions
Conceptualization, H.E.; writing—original draft preparation, Y.K., S.L., I.P., and H.E.; writing—review and editing, H.E. and Y.K.; supervision, H.E. and I.P.; funding acquisition, H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by DFG Project HE 394/3, SMWK Project no. 02010311 (Germany), by Alexander von Humboldt Polish Honorary Research Scholarship (FNP, Poland) and OPUS19 Program (NCN, Poland). We would like also to thank the Polish National Agency for Academic Exchange for the financial support within the “Polish Returns” programme (PPN/PPO/2018/1/00071/DEC/1). Y.K. is supported by the Russian Science Foundation (Grant No. 18-13-00220).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this review, in the writing of the manuscript, or in the decision of this publication.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mutsenko V., Gryshkov O., Rogulska O., Lode A., Petrenko A., Gelinsky M., Glasmacher B., Ehrlich H. Chitinous scaffolds from marine sponges for tissue engineering. In: Choi A., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 285–309. [Google Scholar]
- 2.Tsurkan D., Wysokowski M., Petrenko I., Voronkina A., Khrunyk Y., Fursov A., Ehrlich H. Modern scaffolding strategies based on naturally pre-fabricated 3D biomaterials of poriferan origin. Appl. Phys. A Mater. Sci. Process. 2020;126:1–9. doi: 10.1007/s00339-020-03564-9. [DOI] [Google Scholar]
- 3.Petrenko I., Khrunyk Y., Voronkina A., Kovalchuk V., Fursov A., Tsurkan D., Ivanenko V. Poriferan chitin: 3D scaffolds from nano- to macroscale. A review. Lett. Appl. Nanobiosci. 2020;9:1004–1014. [Google Scholar]
- 4.Ehrlich H. Proceedings of the Biologically-Inspired Systems. Springer; Berlin/Heidelberg, Germany: 2019. Marine biological materials of Invertebrate origin. [Google Scholar]
- 5.Venkatesan J., Kim S.K. Springer Handbook of Marine Biotechnology. Springer; Berlin, Germany: 2015. Marine biomaterials. [Google Scholar]
- 6.Anitha A., Sowmya S., Kumar P.T.S., Deepthi S., Chennazhi K.P., Ehrlich H., Tsurkan M., Jayakumar R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014;39:1644–1667. doi: 10.1016/j.progpolymsci.2014.02.008. [DOI] [Google Scholar]
- 7.Patel D.P., Singh S. Chitosan: A multifacet polymer. Int. J. Curr. Pharm. Res. 2015;7:21–28. [Google Scholar]
- 8.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marpu S.B., Benton E.N. Shining light on chitosan: A review on the usage of chitosan for photonics and nanomaterials research. Int. J. Mol. Sci. 2018;19:1795. doi: 10.3390/ijms19061795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczmarek M.B., Struszczyk-Swita K., Li X., Szczęsna-Antczak M., Daroch M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019;18:577–594. doi: 10.3389/fbioe.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaneva Z., Ivanova D., Nikolova N., Tzanova M. The 21st century revival of chitosan in service to bio-organic chemistry. Biotechnol. Biotechnol. Equip. 2020 doi: 10.1080/13102818.2020.1731333. [DOI] [Google Scholar]
- 12.Oyatogun G.M., Esan T.A., Akpan E.I., Adeosun S.O., Popoola A.P.I., Imasogie B.I., Soboyejo W.O., Afonja A.A., Ibitoye S.A., Abere V.D., et al. Handbook of Chitin and Chitosan. Elsevier; Amsterdam, The Netherlands: 2020. Chitin, chitosan, marine to market; pp. 335–376. [Google Scholar]
- 13.Silva T.H., Alves A., Ferreira B.M., Oliveira J.M., Reys L.L., Ferreira R.J.F., Sousa R.A., Silva S.S., Mano J.F., Reis R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012;57:276–306. doi: 10.1179/1743280412Y.0000000002. [DOI] [Google Scholar]
- 14.Kaya M., Mujtaba M., Ehrlich H., Salaberria A.M., Baran T., Amemiya C.T., Galli R., Akyuz L., Sargin I., Labidi J. On chemistry of γ-chitin. Carbohydr. Polym. 2017;176:177–186. doi: 10.1016/j.carbpol.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 15.Tsurkan M.V., Voronkina A., Khrunyk Y., Wysokowski M., Petrenko I., Ehrlich H. Progress in chitin analytics. Carbohydr. Polym. 2020;252:117204. doi: 10.1016/j.carbpol.2020.117204. [DOI] [PubMed] [Google Scholar]
- 16.Moussian B. Advances in Experimental Medicine and Biology. Volume 1142. Springer; Cham, Switzerland: 2019. Chitin: Structure, chemistry and biology; pp. 5–18. [DOI] [PubMed] [Google Scholar]
- 17.Casadidio C., Peregrina D.V., Gigliobianco M.R., Deng S., Censi R., Di Martino P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs. 2019;17:369. doi: 10.3390/md17060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamshina J.L., Berton P. Use of ionic liquids in chitin biorefinery: A systematic review. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurienzo P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur S., Dhillon G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015;35:44–61. doi: 10.3109/07388551.2013.798256. [DOI] [PubMed] [Google Scholar]
- 21.Suryawanshi N., Jujjavarapu S.E., Ayothiraman S. Marine shell industrial wastes–an abundant source of chitin and its derivatives: Constituents, pretreatment, fermentation, and pleiotropic applications-a revisit. Int. J. Environ. Sci. Technol. 2019;16:3877–3898. doi: 10.1007/s13762-018-02204-3. [DOI] [Google Scholar]
- 22.Sanandiya N.D., Ottenheim C., Phua J.W., Caligiani A., Dritsas S., Fernandez J.G. Circular manufacturing of chitinous bio-composites via bioconversion of urban refuse. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-61664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos V.P., Marques N.S., Maia P.C., de Lima M.A., Franco L.D., Campos-Takaki G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020;21:4290. doi: 10.3390/ijms21124290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Y.N., Lee P.P., Chen W.N. Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Express. 2020;10:1–11. doi: 10.1186/s13568-020-0954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowacki K., Stępniak I., Langer E., Tsurkan M., Wysokowski M., Petrenko I., Khrunyk Y., Fursov A., Bo M., Bavestrello G., et al. Electrochemical approach for isolation of chitin from the skeleton of the black coral Cirrhipathes sp. (Antipatharia) Mar. Drugs. 2020;18:297. doi: 10.3390/md18060297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connors M.J., Ehrlich H., Hog M., Godeffroy C., Araya S., Kallai I., Gazit D., Boyce M., Ortiz C. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. 2012;277:314–328. doi: 10.1016/j.jsb.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Bo M., Bavestrello G., Kurek D., Paasch S., Brunner E., Born R., Galli R., Stelling A.L., Sivkov V.N., Petrova O.V., et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria) Int. J. Biol. Macromol. 2012;51:129–137. doi: 10.1016/j.ijbiomac.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Philibert T., Lee B.H., Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017;181:1314–1337. doi: 10.1007/s12010-016-2286-2. [DOI] [PubMed] [Google Scholar]
- 29.Maruthiah T., Palavesam A. Characterization of haloalkalophilic organic solvent tolerant protease for chitin extraction from shrimp shell waste. Int. J. Biol. Macromol. 2017;97:552–560. doi: 10.1016/j.ijbiomac.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Doan C.T., Tran T.N., Nguyen V.B., Vo T.P.K., Nguyen A.D., Wang S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019;131:706–715. doi: 10.1016/j.ijbiomac.2019.03.117. [DOI] [PubMed] [Google Scholar]
- 31.Ghorbel-Bellaaj O., Younes I., Maâlej H., Hajji S., Nasri M. Chitin extraction from shrimp shell waste using Bacillus bacteria. Int. J. Biol. Macromol. 2012;51:1196–1201. doi: 10.1016/j.ijbiomac.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Bajaj M., Freiberg A., Winter J., Xu Y., Gallert C. Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl. Microbiol. Biotechnol. 2015;99:9835–9846. doi: 10.1007/s00253-015-6841-5. [DOI] [PubMed] [Google Scholar]
- 33.Paul T., Halder S.K., Das A., Ghosh K., Mandal A., Payra P., Barman P., Das Mohapatra P.K., Pati B.R., Mondal K.C. Production of chitin and bioactive materials from Black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. Biotech. 2015;5:483–493. doi: 10.1007/s13205-014-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolesa L.D., Gupta B.S., Lee M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019;130:818–826. doi: 10.1016/j.ijbiomac.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Klinger C., Żółtowska-Aksamitowska S.Z., Wysokowski M., Tsurkan M.V., Galli R., Petrenko I., Machałowski T., Ereskovsky A., Martinović R., Muzychka L., et al. Express method for isolation of ready-to-use 3D chitin scaffolds from Aplysina archeri (Aplysineidae: Verongiida) demosponge. Mar. Drugs. 2019;17:131. doi: 10.3390/md17020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Z., Lau D. Molecular dynamics study on stiffness and ductility in chitin–protein composite. J. Mater. Sci. 2015;50:7149–7157. doi: 10.1007/s10853-015-9271-y. [DOI] [Google Scholar]
- 37.Raabe D., Sachs C., Romano P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005;53:4281–4292. doi: 10.1016/j.actamat.2005.05.027. [DOI] [Google Scholar]
- 38.Stawski D., Rabiej S., Herczyńska L., Draczyński Z. Thermogravimetric analysis of chitins of different origin. J. Therm. Anal. Calorim. 2008;93:489–494. doi: 10.1007/s10973-007-8691-6. [DOI] [Google Scholar]
- 39.Ehrlich H., Rigby J.K., Botting J.P., Tsurkan M.V., Werner C., Schwille P., Petrášek Z., Pisera A., Simon P., Sivkov V.N., et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013;3 doi: 10.1038/srep03497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stawski D. Thermogravimetric analysis of sponge chitins in thermooxidative conditions. In: Ehrlich H., editor. Extreme Biomimetics. Springer; Cham, Switzerland: 2017. pp. 191–205. [Google Scholar]
- 41.Silva S.S., Gomes J.M., Vale A.C., Lu S., Reis R.L., Kundu S.C. Green pathway for processing non-mulberry Antheraea pernyi silk fibroin/chitin-based sponges: Biophysical and biochemical characterization. Front. Mater. 2020;7 doi: 10.3389/fmats.2020.00135. [DOI] [Google Scholar]
- 42.Morganti P., Morganti G., Coltelli M.B. Chitin Nanomaterials and Nanocomposites for Tissue Repair. In: Choi A., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. [Google Scholar]
- 43.Ehrlich H., Worch H. Porifera Research: Biodiversity, Innovation and Sustainability. Museu Nacional; Rio de Janeiro, Brasil: 2007. Sponges as natural composites: From biomimetic potential to development of new biomaterials; pp. 217–223. [Google Scholar]
- 44.Wang Y.J., Jiang W.X., Zhang Y.S., Cao H.Y., Zhang Y., Chen X.L., Li C.Y., Wang P., Zhang Y.Z., Song X.Y., et al. Structural insight into chitin degradation and thermostability of a novel endochitinase from the glycoside hydrolase family 18. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niho N., Tamura T., Toyoda K., Uneyama C., Shibutani M., Hirose M. A 13-week subchronic toxicity study of chitin in F344 rats. Bull. Natl. Inst. Health Sci. 1999;117:129–134. [PubMed] [Google Scholar]
- 46.Elieh-Ali-Komi D., Hamblin M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016;4:411–427. [PMC free article] [PubMed] [Google Scholar]
- 47.Elieh Ali Komi D., Sharma L., Dela Cruz C.S. Chitin and its effects on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2018;54:213–223. doi: 10.1007/s12016-017-8600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arbia W., Arbia L., Adour L., Amrane A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013;51:12–25. [Google Scholar]
- 49.Nguyen T.T., Barber A.R., Corbin K., Zhang W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017;4:1–9. doi: 10.1186/s40643-017-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones M., Kujundzic M., John S., Bismarck A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs. 2020;18:64. doi: 10.3390/md18010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rech A.S., Rech J.C., Caprario J., Tasca F.A., Recio M.Á.L., Finotti A.R. Use of shrimp shell for adsorption of metals present in surface runoff. Water Sci. Technol. 2019;79:2221–2230. doi: 10.2166/wst.2019.213. [DOI] [PubMed] [Google Scholar]
- 52.Lokman I.H., Ibitoye E.B., Hezmee M.N.M., Goh Y.M., Zuki A.B.Z., Jimoh A.A. Effects of chitin and chitosan from cricket and shrimp on growth and carcass performance of broiler chickens. Trop. Anim. Health Prod. 2019;51:2219–2225. doi: 10.1007/s11250-019-01936-9. [DOI] [PubMed] [Google Scholar]
- 53.Las Heras K., Santos-Vizcaino E., Garrido T., Borja Gutierrez F., Aguirre J.J., de la Caba K., Guerrero P., Igartua M., Hernandez R.M. Soy protein and chitin sponge-like scaffolds: From natural by-products to cell delivery systems for biomedical applications. Green Chem. 2020;22:3445–3460. doi: 10.1039/D0GC00089B. [DOI] [Google Scholar]
- 54.Larbi F., García A., del Valle L.J., Hamou A., Puiggalí J., Belgacem N., Bras J. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin: From energy production to material cytotoxicity and Pickering emulsion properties. Carbohydr. Polym. 2018;196:385–397. doi: 10.1016/j.carbpol.2018.04.094. [DOI] [PubMed] [Google Scholar]
- 55.Ehrlich H., Krautter M., Hanke T., Simon P., Knieb C., Heinemann S., Worch H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera) J. Exp. Zool. Part B Mol. Dev. Evol. 2007;308:473–483. doi: 10.1002/jez.b.21174. [DOI] [PubMed] [Google Scholar]
- 56.Ehrlich H., Maldonado M., Spindler K.D., Eckert C., Hanke T., Born R., Goebel C., Simon P., Heinemann S., Worch H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera) J. Exp. Zool. Part B Mol. Dev. Evol. 2007;308:347–356. doi: 10.1002/jez.b.21156. [DOI] [PubMed] [Google Scholar]
- 57.Ehrlich H., Ilan M., Maldonado M., Muricy G., Bavestrello G., Kljajic Z., Carballo J.L., Schiaparelli S., Ereskovsky A., Schupp P., et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010;47:132–140. doi: 10.1016/j.ijbiomac.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Cruz-Barraza J.A., Carballo J.L., Rocha-Olivares A., Ehrlich H., Hog M. Integrative taxonomy and molecular phylogeny of genus Aplysina (Demospongiae: Verongida) from Mexican Pacific. PLoS ONE. 2012;7:e42049. doi: 10.1371/journal.pone.0042049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wysokowski M., Bazhenov V.V., Tsurkan M.V., Galli R., Stelling A.L., Stöcker H., Kaiser S., Niederschlag E., Gärtner G., Behm T., et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013;62:94–100. doi: 10.1016/j.ijbiomac.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 60.Zakrzewski A.C., Weigert A., Helm C., Adamski M., Adamska M., Bleidorn C., Raible F., Hausen H. Early divergence, broad distribution, and high diversity of animal chitin synthases. Genome Biol. Evol. 2014;6:316–325. doi: 10.1093/gbe/evu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutsenko V.V., Bazhenov V.V., Rogulska O., Tarusin D.N., Schütz K., Brüggemeier S., Gossla E., Akkineni A.R., Meißner H., Lode A., et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017;104:1966–1974. doi: 10.1016/j.ijbiomac.2017.03.116. [DOI] [PubMed] [Google Scholar]
- 62.Mutsenko V.V., Gryshkov O., Lauterboeck L., Rogulska O., Tarusin D.N., Bazhenov V.V., Schütz K., Brüggemeier S., Gossla E., Akkineni A.R., et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017;104:1955–1965. doi: 10.1016/j.ijbiomac.2017.03.161. [DOI] [PubMed] [Google Scholar]
- 63.Żółtowska-Aksamitowska S., Shaala L.A., Youssef D.T.A., Elhady S.S., Tsurkan M.V., Petrenko I., Wysokowski M., Tabachnick K., Meissner H., Ivanenko V.N., et al. First report on chitin in a non-verongiid marine demosponge: The Mycale euplectellioides case. Mar. Drugs. 2018;16:68. doi: 10.3390/md16020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Żółtowska-Aksamitowska S., Tsurkan M.V., Lim S.C., Meissner H., Tabachnick K., Shaala L.A., Youssef D.T.A., Ivanenko V.N., Petrenko I., Wysokowski M., et al. The demosponge Pseudoceratina purpurea as a new source of fibrous chitin. Int. J. Biol. Macromol. 2018;112:1021–1028. doi: 10.1016/j.ijbiomac.2018.02.071. [DOI] [PubMed] [Google Scholar]
- 65.Ehrlich H., Shaala L.A., Youssef D.T.A., Zoltowska Żółtowska-Aksamitowska S., Tsurkan M., Galli R., Meissner H., Wysokowski M., Petrenko I., Tabachnick K.R., et al. Discovery of chitin in skeletons of non-verongiid Red Sea demosponges. PLoS ONE. 2018;13:e0195803. doi: 10.1371/journal.pone.0195803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schubert M., Binnewerg B., Voronkina A., Muzychka L., Wysokowski M., Petrenko I., Kovalchuk V., Tsurkan M., Martinovic R., Bechmann N., et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (demospongiae: Verongiida) Int. J. Mol. Sci. 2019;20:5105. doi: 10.3390/ijms20205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fromont J., Żółtowska-Aksamitowska S., Galli R., Meissner H., Erpenbeck D., Vacelet J., Diaz C., Tsurkan M.V., Petrenko I., Youssef D.T.A., et al. New family and genus of a Dendrilla-like sponge with characters of Verongiida. Part II. Discovery of chitin in the skeleton of Ernstilla lacunosa. Zool. Anz. 2019;280:21–29. doi: 10.1016/j.jcz.2019.03.002. [DOI] [Google Scholar]
- 68.Kovalchuk V., Voronkina A., Binnewerg B., Schubert M., Muzychka L., Wysokowski M., Tsurkan M.V., Bechmann N., Petrenko I., Fursov A., et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs. 2019;17:574. doi: 10.3390/md17100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zdarta J., Machałowski T., Degórska O., Bachosz K., Fursov A., Ehrlich H., Ivanenko V.N., Jesionowski T. 3D Chitin scaffolds from the marine demosponge Aplysina archeri as a support for laccase immobilization and its use in the removal of pharmaceuticals. Biomolecules. 2020;10:646. doi: 10.3390/biom10040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talevski T., Talevska Leshoska A., Pejoski E., Pejin B., Machałowski T., Wysokowski M., Tsurkan M.V., Petrova O., Sivkov V., Martinovic R., et al. Identification and first insights into the structure of chitin from the endemic freshwater demosponge Ochridaspongia rotunda (Arndt, 1937) Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.06.247. [DOI] [PubMed] [Google Scholar]
- 71.Ehrlich H., Bazhenov V., Meschke S., Bürger M., Ehrlich A., Petovic S., Durovic M. Handbook of Environmental Chemistry. Springer; Cham, Switzerland: 2017. Marine invertebrates of Boka Kotorska Bay unique sources for bioinspired materials science; pp. 313–334. [Google Scholar]
- 72.Ehrlich H. Chitin of poriferan origin as a unique biological material. In: La Barre S., Bates S.S., editors. Blue Biotechnology: Production and Use of Marine Molecules. John Wiley & Sons; Chichester, UK: 2018. pp. 821–854. [Google Scholar]
- 73.Steck E., Burkhardt M., Ehrlich H., Richter W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation. 2010;17:153–159. doi: 10.1111/j.1399-3089.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 74.Ehrlich H., Steck E., Ilan M., Maldonado M., Muricy G., Bavestrello G., Kljajic Z., Carballo J.L., Schiaparelli S., Ereskovsky A., et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010;47:141–145. doi: 10.1016/j.ijbiomac.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Machałowski T., Wysokowski M., Petrenko I., Langer E., Tsurkan D., Jesionowski T., Ehrlich H. In vivo biomimetic calcification of selected organic scaffolds using snail shell regeneration: A new methodological approach. Appl. Phys. A Mater. Sci. Process. 2020;126:1–9. doi: 10.1007/s00339-020-03634-y. [DOI] [Google Scholar]
- 76.Wysokowski M., Machałowski T., Petrenko I., Schimpf C., Rafaja D., Galli R., Ziętek J., Pantović S., Voronkina A., Kovalchuk V., et al. 3D chitin scaffolds of marine demosponge origin for biomimetic mollusk hemolymph-associated biomineralization ex-vivo. Mar. Drugs. 2020;18:123. doi: 10.3390/md18020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schleuter D., Günther A., Paasch S., Ehrlich H., Kljajić Z., Hanke T., Bernhard G., Brunner E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013;92:712–718. doi: 10.1016/j.carbpol.2012.08.090. [DOI] [PubMed] [Google Scholar]
- 78.Ehrlich H. Biomimetic potential of chitin-based composite biomaterials of poriferan origin. In: Ruys A.J., editor. Biomimetic Biomaterials: Structure and Applications. Woodhead Publishing; Philadelphia, PA, USA: New Delhi, India: 2013. [Google Scholar]
- 79.Wysokowski M., Petrenko I., Stelling A.L., Stawski D., Jesionowski T., Ehrlich H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers. 2015;7:235–265. doi: 10.3390/polym7020235. [DOI] [Google Scholar]
- 80.Petrenko I., Bazhenov V.V., Galli R., Wysokowski M., Fromont J., Schupp P.J., Stelling A.L., Niederschlag E., Stöker H., Kutsova V.Z., et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017;104:1626–1663. doi: 10.1016/j.ijbiomac.2017.01.084. [DOI] [PubMed] [Google Scholar]
- 81.Petrenko I., Bazhenov V.V., Stelling A.L., Kutsova V.Z. Bioelectrometallurgy of copper on chitin. In: Ehrlich H., editor. Extreme Biomimetics. Springer; Cham, Switzerland: 2017. pp. 205–223. [Google Scholar]
- 82.Wysokowski M., Motylenko M., Bazhenov V.V., Stawski D., Petrenko I., Ehrlich A., Behm T., Kljajic Z., Stelling A.L., Jesionowski T., et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013;7:248–260. doi: 10.1007/s11706-013-0212-x. [DOI] [Google Scholar]
- 83.Wysokowski M., Motylenko M., Walter J., Lota G., Wojciechowski J., Stöcker H., Galli R., Stelling A.L., Himcinschi C., Niederschlag E., et al. Synthesis of nanostructured chitin-hematite composites under extreme biomimetic conditions. RSC Adv. 2014;4:61743–61752. doi: 10.1039/C4RA10017D. [DOI] [Google Scholar]
- 84.Wysokowski M., Motylenko M., Beyer J., Makarova A., Stöcker H., Walter J., Galli R., Kaiser S., Vyalikh D., Bazhenov V.V., et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015;8:2288–2301. doi: 10.1007/s12274-015-0739-5. [DOI] [Google Scholar]
- 85.Wysokowski M., Piasecki A., Bazhenov V.V., Paukszta D., Born R., Schupp P., Petrenko I., Jesionowski T. Poriferan chitin as the scaffold for nanosilica deposition under hydrothermal synthesis conditions. J. Chitin Chitosan Sci. 2013;1:26–38. doi: 10.1166/jcc.2013.1009. [DOI] [Google Scholar]
- 86.Petrenko I., Summers A.P., Simon P., Żółtowska-Aksamitowska S., Motylenko M., Schimpf C., Rafaja D., Roth F., Kummer K., Brendler E., et al. Extreme biomimetics: Preservation of molecular detail in centimeter-scale samples of biological meshes laid down by sponges. Sci. Adv. 2019;5:eaax2805. doi: 10.1126/sciadv.aax2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stepniak I., Galinski M., Nowacki K., Wysokowski M., Jakubowska P., Bazhenov V.V., Leisegang T., Ehrlich H., Jesionowski T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors-preparation and characterization. RSC Adv. 2016;6:4007–4013. doi: 10.1039/C5RA22047E. [DOI] [Google Scholar]
- 88.Machałowski T., Czajka M., Petrenko I., Meissner H., Schimpf C., Rafaja D., Ziętek J., Dzięgiel B., Adaszek Ł., Voronkina A., et al. Functionalization of 3D chitinous skeletal scaffolds of sponge origin using silver nanoparticles and their antibacterial properties. Mar. Drugs. 2020;18:304. doi: 10.3390/md18060304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tseng C.K. Utilization of seaweeds. Sci. Mon. 1944;59:37–46. [Google Scholar]
- 90.Joshi S., Eshwar S., Jain V. Marine polysaccharides: Biomedical and tissue engineering applications. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 443–491. [Google Scholar]
- 91.Jönsson M., Allahgholi L., Sardari R.R.R., Hreggviosson G.O., Karlsson E.N. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules. 2020;25:930. doi: 10.3390/molecules25040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mautner H.G. The chemistry of brown algae. Econ. Bot. 1954;8:174–192. doi: 10.1007/BF02984737. [DOI] [Google Scholar]
- 93.Smidsrød O., Skjåk-Bræk G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-O. [DOI] [PubMed] [Google Scholar]
- 94.Yu C., Li D. The Alginate Having Low Molecular Weight, Methods of Manufacturing It and Its Use. No. CA 2430277 A1. U.S. Patent. 2001 Jul 6;
- 95.Fertah M. Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications. Elsevier; Amsterdam, The Netherlands: 2017. Isolation and characterization of alginate from seaweed; pp. 11–26. [Google Scholar]
- 96.Tønnesen H.H., Karlsen J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002;28:621–630. doi: 10.1081/DDC-120003853. [DOI] [PubMed] [Google Scholar]
- 97.Mørch Ý.A., Donati I., Strand B.L., Skjåk-Bræk G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 98.Windhues T., Borchard W. Effect of acetylation on physico-chemical properties of bacterial and algal alginates in physiological sodium chloride solutions investigated with light scattering techniques. Carbohydr. Polym. 2003;52:47–52. doi: 10.1016/S0144-8617(02)00265-5. [DOI] [Google Scholar]
- 99.Rehm B.H.A., Fata Moradali M., editors. Alginates and Their Biomedical Applications. Springer Nature; Singapore: 2018. [Google Scholar]
- 100.Łabowska M.B., Michalak I., Detyna J. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field—A review. Open Chem. 2019;17:738–762. doi: 10.1515/chem-2019-0077. [DOI] [Google Scholar]
- 101.Youssouf L., Lallemand L., Giraud P., Soulé F., Bhaw-Luximon A., Meilhac O., D’Hellencourt C.L., Jhurry D., Couprie J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017;166:55–63. doi: 10.1016/j.carbpol.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 102.Market Data Forecast. [(accessed on 3 September 2020)]; Available online: https://www.marketdataforecast.com/market-reports/alginates-market.
- 103.Dhamecha D., Movsas R., Sano U., Menon J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019;569 doi: 10.1016/j.ijpharm.2019.118627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Somo S.I., Khanna O., Brey E.M. Alginate microbeads for cell and protein delivery. Methods Mol. Biol. 2017;1479:217–224. doi: 10.1007/978-1-4939-6364-5_17. [DOI] [PubMed] [Google Scholar]
- 105.Cong Z., Shi Y., Wang Y., Wang Y., Niu J., Chen N., Xue H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018;107:855–864. doi: 10.1016/j.ijbiomac.2017.09.065. [DOI] [PubMed] [Google Scholar]
- 106.Severino P., da Silva C.F., Andrade L.N., de Lima Oliveira D., Campos J., Souto E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019;25:1312–1334. doi: 10.2174/1381612825666190425163424. [DOI] [PubMed] [Google Scholar]
- 107.Hariyadi D.M., Hendradi E., Purwanti T., Fadil F.D.G.P., Ramadani C.N. Effect of cross linking agent and polymer on the characteristics of ovalbumin loaded alginate microspheres. Int. J. Pharm. Pharm. Sci. 2014;6:469–474. [Google Scholar]
- 108.Darrabie M.D., Kendall W.F., Opara E.C. Characteristics of Poly-L-Ornithine-coated alginate microcapsules. Biomaterials. 2005;26:6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Chandy T., Mooradian D.L., Rao G.H.R. Evaluation of modified alginate-chitosan-polyethylene glycol microcapsules for cell encapsulation. Artif. Organs. 1999;23:894–903. doi: 10.1046/j.1525-1594.1999.06244.x. [DOI] [PubMed] [Google Scholar]
- 110.Ching S.H., Bansal N., Bhandari B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017;57:1133–1152. doi: 10.1080/10408398.2014.965773. [DOI] [PubMed] [Google Scholar]
- 111.Calasans-Maia M.D., Barboza C.A.B., Soriano-Souza C.A., Novellino Alves A.T.N., De Pinheiro Uzeda M.J., Martinez-Zelaya V.R., Mavropoulos E., Rocha Leão M.H., De Santana R.B., Granjeiro J.M., et al. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019;14:4559–4571. doi: 10.2147/IJN.S201631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Unagolla J.M., Jayasuriya A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018;114:199–209. doi: 10.1016/j.ejps.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jain R.R., Mehta M.R., Bannalikar A.R., Menon M.D. Alginate microparticles loaded with lipopolysaccharide subunit antigen for mucosal vaccination against Klebsiella pneumoniae. Biologicals. 2015;43:195–201. doi: 10.1016/j.biologicals.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 114.Almurisi S.H., Doolaanea A.A., Akkawi M.E., Chatterjee B., Sarker M.Z.I. Taste masking of paracetamol encapsulated in chitosan-coated alginate beads. J. Drug Deliv. Sci. Technol. 2020;56 doi: 10.1016/j.jddst.2020.101520. [DOI] [Google Scholar]
- 115.Kim C., Kim H., Park H., Lee K.Y. Controlling the porous structure of alginate ferrogel for anticancer drug delivery under magnetic stimulation. Carbohydr. Polym. 2019;223 doi: 10.1016/j.carbpol.2019.115045. [DOI] [PubMed] [Google Scholar]
- 116.Reakasame S., Boccaccini A.R. Oxidized Alginate-based hydrogels for tissue engineering applications: A review. Biomacromolecules. 2018;19:3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 117.Rastogi P., Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11:042001. doi: 10.1088/1758-5090/ab331e. [DOI] [PubMed] [Google Scholar]
- 118.Aljohani W., Ullah M.W., Zhang X., Yang G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018;107:261–275. doi: 10.1016/j.ijbiomac.2017.08.171. [DOI] [PubMed] [Google Scholar]
- 119.Axpe E., Oyen M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016;17:1976. doi: 10.3390/ijms17121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva R., Singh R., Sarker B., Papageorgiou D.G., Juhasz-Bortuzzo J.A., Roether J.A., Cicha I., Kaschta J., Schubert D.W., Chrissafis K., et al. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018;114:614–625. doi: 10.1016/j.ijbiomac.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 121.Dodero A., Scarfi S., Pozzolini M., Vicini S., Alloisio M., Castellano M. Alginate-based electrospun membranes containing ZnO nanoparticles as potential wound healing patches: Biological, mechanical, and physicochemical characterization. ACS Appl. Mater. Interfaces. 2020;12:3371–3381. doi: 10.1021/acsami.9b17597. [DOI] [PubMed] [Google Scholar]
- 122.Chen L., Shen R., Komasa S., Xue Y., Jin B., Hou Y., Okazaki J., Gao J. Drug-loadable calcium alginate hydrogel system for use in oral bone tissue repair. Int. J. Mol. Sci. 2017;18:989. doi: 10.3390/ijms18050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharma C., Dinda A.K., Potdar P.D., Chou C.F., Mishra N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C. 2016;64:416–427. doi: 10.1016/j.msec.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 124.Kolanthai E., Sindu P.A., Khajuria D.K., Veerla S.C., Kuppuswamy D., Catalani L.H., Mahapatra D.R. Graphene oxide—A tool for the preparation of chemically crosslinking free alginate-chitosan-collagen scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces. 2018;10:12441–12452. doi: 10.1021/acsami.8b00699. [DOI] [PubMed] [Google Scholar]
- 125.Luo Z., Yang Y., Deng Y., Sun Y., Yang H., Wei S. Peptide-incorporated 3D porous alginate scaffolds with enhanced osteogenesis for bone tissue engineering. Colloids Surf. B Biointerfaces. 2016;143:243–251. doi: 10.1016/j.colsurfb.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 126.Wang P., Song Y., Weir M.D., Sun J., Zhao L., Simon C.G., Xu H.H.K. A self-setting iPSMSC-alginate-calcium phosphate paste for bone tissue engineering. Dent. Mater. 2016;32:252–263. doi: 10.1016/j.dental.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venkatesan J., Bhatnagar I., Manivasagan P., Kang K.H., Kim S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015;72:269–281. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Nabavinia M., Khoshfetrat A.B., Naderi-Meshkin H. Nano-hydroxyapatite-alginate-gelatin microcapsule as a potential osteogenic building block for modular bone tissue engineering. Mater. Sci. Eng. C. 2019;97:67–77. doi: 10.1016/j.msec.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 129.Diaz-Rodriguez P., Garcia-Triñanes P., Echezarreta López M.M., Santoveña A., Landin M. Mineralized alginate hydrogels using marine carbonates for bone tissue engineering applications. Carbohydr. Polym. 2018;195:235–242. doi: 10.1016/j.carbpol.2018.04.101. [DOI] [PubMed] [Google Scholar]
- 130.Yang X., Lu Z., Wu H., Li W., Zheng L., Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C. 2018;83:195–201. doi: 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 131.Li Y.J., Teng B.H., Zhao Y.H., Yang Q., Wang L.Y., Huang Y. Preparation and evaluation of carboxymethyl chitosan/sodium alginate hydrogel for cartilage tissue engineering. Hua Xi Kou Qiang Yi Xue Za Zhi. 2019;37:235–259. doi: 10.7518/hxkq.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu T., Jiang J., Zhao J., Chen S., Yan X. Regulating preparation of functional alginate-chitosan three-dimensional scaffold for skin tissue engineering. Int. J. Nanomed. 2019;14:8891–8903. doi: 10.2147/IJN.S210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang X., Kim G.J., Kang M.G., Lee J.K., Seo J.W., Do J.T., Hong K., Cha J.M., Shin S.R., Bae H. Marine biomaterial-based bioinks for generating 3D printed tissue constructs. Mar. Drugs. 2018;16:484. doi: 10.3390/md16120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baniasadi H., Mashayekhan S., Fadaoddini S., Haghirsharifzamini Y. Design, fabrication and characterization of oxidized alginate-gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016;31:152–161. doi: 10.1177/0885328216634057. [DOI] [PubMed] [Google Scholar]
- 135.Wu H., Liu J., Fang Q., Xiao B., Wan Y. Establishment of nerve growth factor gradients on aligned chitosan-polylactide /alginate fibers for neural tissue engineering applications. Colloids Surf. B Biointerfaces. 2017;160:598–609. doi: 10.1016/j.colsurfb.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 136.Liberski A., Latif N., Raynaud C., Bollensdorff C., Yacoub M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016;1:e201604. doi: 10.21542/gcsp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Türe H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019;123:878–888. doi: 10.1016/j.ijbiomac.2018.11.143. [DOI] [PubMed] [Google Scholar]
- 138.Rubio-Elizalde I., Bernáldez-Sarabia J., Moreno-Ulloa A., Vilanova C., Juárez P., Licea-Navarro A., Castro-Ceseña A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019;206:455–467. doi: 10.1016/j.carbpol.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 139.Varaprasad K., Jayaramudu T., Kanikireddy V., Toro C., Sadiku E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020;236 doi: 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- 140.Zhao W.Y., Fang Q.Q., Wang X.F., Wang X.W., Zhang T., Shi B.H., Zheng B., Zhang D.D., Hu Y.Y., Ma L., et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2019;28:326–337. doi: 10.1111/wrr.12789. [DOI] [PubMed] [Google Scholar]
- 141.Johnson K.A., Muzzin N., Toufanian S., Slick R.A., Lawlor M.W., Seifried B., Moquin P., Latulippe D., Hoare T. Drug-impregnated, pressurized gas expanded liquid-processed alginate hydrogel scaffolds for accelerated burn wound healing. Acta Biomater. 2020;20:30327–30335. doi: 10.1016/j.actbio.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 142.Salehi M., Ehterami A., Farzamfar S., Vaez A., Ebrahimi-Barough S. Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00731-6. [DOI] [PubMed] [Google Scholar]
- 143.Zhao X., Liu L., An T., Xian M., Luckanagul J.A., Su Z., Lin Y., Wang Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020;104:85–94. doi: 10.1016/j.actbio.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 144.Morya V.K., Kim J., Kim E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012;93:71–82. doi: 10.1007/s00253-011-3666-8. [DOI] [PubMed] [Google Scholar]
- 145.Bae J.S., Lee J.S., Kim Y.S., Sim W.J., Lee H., Chun J.H., Park K.P. Depolymerization of fucoidan by Contact Glow Discharge Electrolysis (CGDE) Korean Chem. Eng. Res. 2008;46:889–891. [Google Scholar]
- 146.Zayed A., Muffler K., Hahn T., Rupp S., Finkelmeier D., Burger-Kentischer A., Ulber R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs. 2016;14:79. doi: 10.3390/md14040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tako M. Rheological characteristics of fucoidan isolated from commercially cultured Cladosiphon okamuranus. Bot. Mar. 2003;46:461–465. doi: 10.1515/BOT.2003.047. [DOI] [Google Scholar]
- 148.Park K., Cho E., In M.J., Kim D.C., Chae H.J. Physicochemical properties and bioactivity of brown seaweed fucoidan prepared by ultra high pressure-assisted enzyme treatment. Korean J. Chem. Eng. 2012;29:221–227. doi: 10.1007/s11814-011-0165-7. [DOI] [Google Scholar]
- 149.Holtkamp A.D., Kelly S., Ulber R., Lang S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009;82:1–11. doi: 10.1007/s00253-008-1790-x. [DOI] [PubMed] [Google Scholar]
- 150.Ngo D.H., Kim S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013;62:70–75. doi: 10.1016/j.ijbiomac.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 151.Besednova N.N., Zvyagintseva T.N., Kuznetsova T.A., Makarenkova I.D., Smolina T.P., Fedyanina L.N., Kryzhanovsky S.P., Zaporozhets T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites. 2019;9:87. doi: 10.3390/metabo9050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Citkowska A., Szekalska M., Winnicka K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs. 2019;17:458. doi: 10.3390/md17080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sutapa B.M., Dhruti A., Priyanka G., Gopa R.B. Absorption, distribution, metabolism and elimination (ADME) and toxicity profile of marine sulfated polysaccharides used in bionanotechnology. Afr. J. Pharm. Pharmacol. 2018;12:1–10. doi: 10.5897/AJPP2017.4869. [DOI] [Google Scholar]
- 154.MarketWatch. [(accessed on 5 September 2020)]; Available online: https://www.marketwatch.com/press-release/global-fucoidan-market-size-2020-is-predicted-to-reach-37-million-usd-mark-by-2024-with-a-magnificent-cagr-and-covid-19-impact-analysis-with-top-countries-data-defination-industry-outlook-2020-06-18.
- 155.Tae Young A., Kang J.H., Kang D.J., Venkatesan J., Chang H.K., Bhatnagar I., Chang K.Y., Hwang J.H., Salameh Z., Kim S.K., et al. Interaction of stem cells with nano hydroxyapatite-fucoidan bionanocomposites for bone tissue regeneration. Int. J. Biol. Macromol. 2016;93:1488–1491. doi: 10.1016/j.ijbiomac.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 156.Lu H.T., Lu T.W., Chen C.H., Lu K.Y., Mi F.L. Development of nanocomposite scaffolds based on biomineralization of N,O-carboxymethyl chitosan/fucoidan conjugates for bone tissue engineering. Int. J. Biol. Macromol. 2018;120:2335–2345. doi: 10.1016/j.ijbiomac.2018.08.179. [DOI] [PubMed] [Google Scholar]
- 157.Lalzawmliana V., Mukherjee P., Kundu B., Nandi S.K. Clinical application of biomimetic marine-derived materials for tissue engineering. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 329–357. [Google Scholar]
- 158.Rohman G., Langueh C., Ramtani S., Lataillade J.J., Lutomski D., Senni K., Changotade S. The use of platelet-rich plasma to promote cell recruitment into low-molecular-weight fucoidan-functionalized poly(ester-urea-urethane) scaffolds for soft-tissue engineering. Polymers. 2019;11:1016. doi: 10.3390/polym11061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carvalho D.N., López-Cebral R., Sousa R.O., Alves A.L., Reys L.L., Silva S.S., Oliveira J.M., Reis R.L., Silva T.H. Marine collagen-chitosan-fucoidan cryogels as cell-laden biocomposites envisaging tissue engineering. Biomed. Mater. 2020;15:055030. doi: 10.1088/1748-605X/ab9f04. [DOI] [PubMed] [Google Scholar]
- 160.Tran P.H.L., Duan W., Tran T.T.D. Fucoidan-based nanostructures: A focus on its combination with chitosan and the surface functionalization of metallic nanoparticles for drug delivery. Int. J. Pharm. 2020;575 doi: 10.1016/j.ijpharm.2019.118956. [DOI] [PubMed] [Google Scholar]
- 161.Huang T.W., Ho Y.C., Tsai T.N., Tseng C.L., Lin C., Mi F.L. Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym. 2020;242 doi: 10.1016/j.carbpol.2020.116312. [DOI] [PubMed] [Google Scholar]
- 162.Cunha L., Rosa da Costa A.M., Lourenço J.P., Buttini F., Grenha A. Spray-dried fucoidan microparticles for pulmonary delivery of antitubercular drugs. J. Microencapsul. 2018;35:392–405. doi: 10.1080/02652048.2018.1513089. [DOI] [PubMed] [Google Scholar]
- 163.Chen C.H., Lin Y.S., Wu S.J., Mi F.L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018;193:163–172. doi: 10.1016/j.carbpol.2018.03.080. [DOI] [PubMed] [Google Scholar]
- 164.Park J.H., Choi S.H., Park S.J., Lee Y.J., Park J.H., Song P.H., Cho C.M., Ku S.K., Song C.H. Promoting wound healing using low molecular weight fucoidan in a full-thickness dermal excision rat model. Mar. Drugs. 2017;15:112. doi: 10.3390/md15040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murakami K., Ishihara M., Aoki H., Nakamura S., Nakamura S.I., Yanagibayashi S., Takikawa M., Kishimoto S., Yokoe H., Kiyosawa T., et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressings. Wound Repair Regen. 2010;18:478–485. doi: 10.1111/j.1524-475X.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- 166.Carson M.A., Clarke S.A. Bioactive compounds from marine organisms: Potential for bone growth and healing. Mar. Drugs. 2018;16:340. doi: 10.3390/md16090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Younes M., Aggett P., Aguilar F., Crebelli R., Filipič M., Frutos M.J., Galtier P., Gott D., Gundert-Remy U., Kuhnle G.G., et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018;16:e05238. doi: 10.2903/j.efsa.2018.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jones G.M.J. Ph.D. Thesis. University of Nottingham; Nottingham, UK: 2004. Rheological Properties of Gelatin, Carrageenan and Locust Bean Gum Mixtures. [Google Scholar]
- 169.Hotchkiss S., Brooks M., Campbell R., Philp K., Trius A. Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects. Nova Science Publishers; London, UK: 2016. The use of carrageenan in food. [Google Scholar]
- 170.Yermak I.M., Kim Y.H., Titlynov E.A., Isakov V.V., Solov’eva T.F. Chemical structure and gel properties of carrageenans from algae belonging to the Gigartinaceae and Tichocarpaceae, collected from the Russian Pacific Coast. J. Appl. Phycol. 1999:555–562. doi: 10.1023/A:1008071925884. [DOI] [Google Scholar]
- 171.Rodríguez Sánchez R.A., Canelón D.J., Cosenza V.A., Fissore E.N., Gerschenson L.N., Matulewicz M.C., Ciancia M. Gracilariopsis hommersandii, a red seaweed, source of agar and sulfated polysaccharides with unusual structures. Carbohydr. Polym. 2019;213:138–146. doi: 10.1016/j.carbpol.2019.02.071. [DOI] [PubMed] [Google Scholar]
- 172.Perez Recalde M., Canelón D.J., Compagnone R.S., Matulewicz M.C., Cerezo A.S., Ciancia M. Carrageenan and agaran structures from the red seaweed Gymnogongrus tenuis. Carbohydr. Polym. 2016;136:1370–1378. doi: 10.1016/j.carbpol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 173.Fenoradosoa T.A., Delattre C., Laroche C., Wadouachi A., Dulong V., Picton L., Andriamadio P., Michaud P. Highly sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta), a red seaweed of Madagascar marine coasts. Int. J. Biol. Macromol. 2009;45:140–145. doi: 10.1016/j.ijbiomac.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 174.Cheong K.L., Qiu H.M., Du H., Liu Y., Khan B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules. 2018;23:2451. doi: 10.3390/molecules23102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brychcy E., Malik M., Drozdzewski P., Król Z., Jarmoluk A. Physicochemical and antibacterial properties of carrageenan and gelatine hydrosols and hydrogels incorporated with acidic electrolyzedwater. Polymers. 2015;7:2638–2649. doi: 10.3390/polym7121534. [DOI] [Google Scholar]
- 176.Zhang Y., Zhou D., Chen J., Zhang X., Li X., Zhao W., Xu T. Biomaterials based on marine resources for 3D bioprinting applications. Mar. Drugs. 2019;17:555. doi: 10.3390/md17100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pangestuti R., Kim S.K. Advances in Food and Nutrition Research. Academic Press; Cambridge, MA, USA: 2014. Biological activities of Carrageenan; pp. 113–124. [DOI] [PubMed] [Google Scholar]
- 178.Market Research Future. [(accessed on 6 September 2020)]; Available online: https://www.marketresearchfuture.com/reports/carrageenan-market-704.
- 179.Zia K.M., Tabasum S., Nasif M., Sultan N., Aslam N., Noreen A., Zuber M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017;96:282–301. doi: 10.1016/j.ijbiomac.2016.11.095. [DOI] [PubMed] [Google Scholar]
- 180.Roy S., Rhim J.W. Preparation of carrageenan-based functional nanocomposite films incorporated with melanin nanoparticles. Colloids Surf. B Biointerfaces. 2019;176:317–324. doi: 10.1016/j.colsurfb.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 181.Kim S.K., Wijesekara I. Anticoagulant effect of marine algae. Adv. Food Nutr. Res. 2011;64:235–244. doi: 10.1016/B978-0-12-387669-0.00018-1. [DOI] [PubMed] [Google Scholar]
- 182.Song X., Wang K., Tang C.Q., Yang W.W., Zhao W.F., Zhao C.S. Design of carrageenan-based heparin-mimetic gel beads as self-anticoagulant hemoperfusion adsorbents. Biomacromolecules. 2018;19:1966–1978. doi: 10.1021/acs.biomac.7b01724. [DOI] [PubMed] [Google Scholar]
- 183.Sokolova E.V., Byankina A.O., Kalitnik A.A., Kim Y.H., Bogdanovich L.N., Solov’Eva T.F., Yermak I.M. Influence of red algal sulfated polysaccharides on blood coagulation and platelets activation in vitro. J. Biomed. Mater. Res. Part A. 2014;102:1431–1438. doi: 10.1002/jbm.a.34827. [DOI] [PubMed] [Google Scholar]
- 184.Diogo J.V., Novo S.G., González M.J., Ciancia M., Bratanich A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res. Vet. Sci. 2015;98:142–144. doi: 10.1016/j.rvsc.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 185.Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eccles R., Winther B., Johnston S.L., Robinson P., Trampisch M., Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial. Respir. Res. 2015;16:1–11. doi: 10.1186/s12931-015-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Z., Gao T., Yang Y., Meng F., Zhan F., Jiang Q., Sun X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules. 2019;24:4286. doi: 10.3390/molecules24234286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yao Z.A., Xu L., Wu H.G. Immunomodulatory function of κ-Carrageenan oligosaccharides acting on LPS-activated microglial cells. Neurochem. Res. 2014;39:333–343. doi: 10.1007/s11064-013-1228-4. [DOI] [PubMed] [Google Scholar]
- 189.Ai L., Chung Y.C., Lin S.Y., Jeng K.C.G., Lai P.F.H., Xiong Z.Q., Wang G. Carrageenan polysaccharides and oligosaccharides with distinct immunomodulatory activities in murine microglia BV-2 cells. Int. J. Biol. Macromol. 2018;120:633–640. doi: 10.1016/j.ijbiomac.2018.08.151. [DOI] [PubMed] [Google Scholar]
- 190.Sun Y., Yang B., Wu Y., Liu Y., Gu X., Zhang H., Wang C., Cao H., Huang L., Wang Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015;178:311–328. doi: 10.1016/j.foodchem.2015.01.105. [DOI] [PubMed] [Google Scholar]
- 191.Tziveleka L.A., Ioannou E., Roussis V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019;218:355–370. doi: 10.1016/j.carbpol.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 192.Lahaye M., Robic A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- 193.Venugopal V. Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. ECronicon Nutr. 2019;14:126–141. [Google Scholar]
- 194.Priyan Shanura Fernando I., Kim K.N., Kim D., Jeon Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019:1–15. doi: 10.1080/07388551.2018.1503995. [DOI] [PubMed] [Google Scholar]
- 195.Lahaye M. Chemistry and physico-chemistry of phycocolloids. Cah. Biol. Mar. 2001;42:137–157. doi: 10.21411/cbm.a.a7aade12. [DOI] [Google Scholar]
- 196.Kidgell J.T., Magnusson M., de Nys R., Glasson C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019;39 doi: 10.1016/j.algal.2019.101422. [DOI] [Google Scholar]
- 197.Kidgell J.T., Glasson C.R.K., Magnusson M., Vamvounis G., Sims I.M., Carnachan S.M., Hinkley S.F.R., Lopata A.L., de Nys R., Taki A.C. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int. J. Biol. Macromol. 2020;150:839–848. doi: 10.1016/j.ijbiomac.2020.02.071. [DOI] [PubMed] [Google Scholar]
- 198.Andryukov B.G., Besednova N.N., Kuznetsova T.A., Zaporozhets T.S., Ermakova S.P., Zvyagintseva T.N., Chingizova E.A., Gazha A.K., Smolina T.P. Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: Current achievements and future prospects. Biomedicines. 2020;8:301. doi: 10.3390/biomedicines8090301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Patil N.P., Le V., Sligar A.D., Mei L., Chavarria D., Yang E.Y., Baker A.B. Algal Polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 2018;5 doi: 10.3389/fcvm.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pezoa Conte R.M. Ph.D. Thesis. Abo Akademi University; Turku, Finland: 2017. Fractionation of Marine Algae to Its Constituents towards Valuable Chemicals and Energy Products. [Google Scholar]
- 201.Chen X., Yue Z., Winberg P.C., Dinoro J.N., Hayes P., Beirne S., Wallace G.G. Development of rhamnose-rich hydrogels based on sulfated xylorhamno-uronic acid toward wound healing applications. Biomater. Sci. 2019;7:3497–3509. doi: 10.1039/C9BM00480G. [DOI] [PubMed] [Google Scholar]
- 202.Toskas G., Heinemann S., Heinemann C., Cherif C., Hund R.D., Roussis V., Hanke T. Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carbohydr. Polym. 2012;89:997–1002. doi: 10.1016/j.carbpol.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 203.Cunha L., Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs. 2016;14:42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Manivasagan P., Oh J. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 2016;82:315–327. doi: 10.1016/j.ijbiomac.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 205.Massironi A., Morelli A., Grassi L., Puppi D., Braccini S., Maisetta G., Esin S., Batoni G., Della Pina C., Chiellini F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019;203:310–321. doi: 10.1016/j.carbpol.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 206.Elli S., Stancanelli E., Wang Z., Petitou M., Liu J., Guerrini M. Degeneracy of the antithrombin binding sequence in heparin: 2-O-sulfated Iduronic acid can replace the critical glucuronic acid. Chem. A Eur. J. 2020 doi: 10.1002/chem.202001346. [DOI] [PubMed] [Google Scholar]
- 207.Guidara M., Yaich H., Richel A., Blecker C., Boufi S., Attia H., Garna H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019;135:647–658. doi: 10.1016/j.ijbiomac.2019.05.196. [DOI] [PubMed] [Google Scholar]
- 208.Lahaye M., Rochas C. Chemical structure and physico-chemical properties of agar. Hydrobiologia. 1991;221:137–148. doi: 10.1007/BF00028370. [DOI] [Google Scholar]
- 209.Praiboon J., Chirapart A., Akakabe Y., Bhumibhamon O., Kajiwara T. Physical and chemical characterization of agar polysaccharides extracted from the Thai and Japanese species of Gracilaria. ScienceAsia. 2006;32:11–17. doi: 10.2306/scienceasia1513-1874.2006.32(s1).011. [DOI] [Google Scholar]
- 210.Araki C., Arai K. Studies on the Chemical Constitution of Agar-agar. XX. Isolation of a tetrasaccharide by enzymatic hydrolysis of agar-agar. Bull. Chem. Soc. Jpn. 1957;30:287–293. doi: 10.1246/bcsj.30.287. [DOI] [Google Scholar]
- 211.Murano E., Toffanin R., Zanetti F., Knutsen S.H., Paoletti S., Rizzo R. Chemical and macromolecular characterisation of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta) Carbohydr. Polym. 1992;18:171–178. doi: 10.1016/0144-8617(92)90061-T. [DOI] [Google Scholar]
- 212.Thu-Hien L., Thanh-Truc N., Van Toi V., Khon H.C., Bao B.C., Niem V.V.T., Ngoc Tuan Anh M., Hai N.D., Chuong P.D., Hiep N.T. Evaluation of the morphology and biocompatibility of natural silk fibers/agar blend scaffolds for tissue regeneration. Int. J. Polym. Sci. 2018;2018 doi: 10.1155/2018/5049728. [DOI] [Google Scholar]
- 213.Soorbaghi F.P., Isanejad M., Salatin S., Ghorbani M., Jafari S., Derakhshankhah H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019;111:964–975. doi: 10.1016/j.biopha.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 214.De Lima G.G., De Lima D.W.F., De Oliveira M.J.A., Lugaõ A.B., Alcântara M.T.S., Devine D.M., De Sá M.J.C. Synthesis and in vivo behavior of PVP/CMC/Agar hydrogel membranes impregnated with silver nanoparticles for wound healing applications. ACS Appl. Bio Mater. 2018;1:1842–1852. doi: 10.1021/acsabm.8b00369. [DOI] [PubMed] [Google Scholar]
- 215.Zuberi H.S., Bengisu M. Agar-based adaptable DIY materials. FME Trans. 2019;47:442–451. doi: 10.5937/fmet1903442H. [DOI] [Google Scholar]
- 216.Mikkonen K.S., Parikka K., Ghafar A., Tenkanen M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013;34:124–136. doi: 10.1016/j.tifs.2013.10.003. [DOI] [Google Scholar]
- 217.Martín-López E., Darder M., Ruiz-Hitzky E., Nieto Sampedro M. Agar-based bridges as biocompatible candidates to provide guide cues in spinal cord injury repair. Biomed. Mater. Eng. 2013;23:405–421. doi: 10.3233/BME-130763. [DOI] [PubMed] [Google Scholar]
- 218.Agar Agar Gum Market Size, Share and Trends Analysis Report by Product (Powder, Square, Strips), by Applicaltion (Confectioneries, Bakery and Pastry, Retail, Meat, Microbiological and Molecular), by Region and Segment Forecasts, 2018–2025. [(accessed on 13 November 2020)]; Available online: https://www.grandviewresearch.com/industry-analysis/global-agar-agar-gum-market.
- 219.Varoni E., Tschon M., Palazzo B., Nitti P., Martini L., Rimondini L. Agarose gel as biomaterial or scaffold for implantation surgery: Characterization, histological and histomorphometric study on soft tissue response. Connect. Tissue Res. 2012;53:548–554. doi: 10.3109/03008207.2012.712583. [DOI] [PubMed] [Google Scholar]
- 220.Arnott S., Fulmer A., Scott W.E., Dea I.C.M., Moorhouse R., Rees D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974;90:269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- 221.Zarrintaj P., Rezaeian I., Bakhshandeh B., Heshmatian B., Ganjali M.R. Bio—Conductive scaffold based on agarose - polyaniline for tissue engineering. J. Ski. Stem Cell. 2017;4:e67394. doi: 10.5812/jssc.67394. [DOI] [Google Scholar]
- 222.Witzler M., Ottensmeyer P.F., Gericke M., Heinze T., Tobiasch E., Schulze M. Non-cytotoxic agarose/hydroxyapatite composite scaffolds for drug release. Int. J. Mol. Sci. 2019;20:3565. doi: 10.3390/ijms20143565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Salati M.A., Khazai J., Tahmuri A.M., Samadi A., Taghizadeh A., Taghizadeh M., Zarrintaj P., Ramsey J.D., Habibzadeh S., Seidi F., et al. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers. 2020;32:1150. doi: 10.3390/polym12051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gao M., Lu P., Bednark B., Lynam D., Conner J.M., Sakamoto J., Tuszynski M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34:1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zarrintaj P., Manouchehri S., Ahmadi Z., Saeb M.R., Urbanska A.M., Kaplan D.L., Mozafari M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018;187:66–84. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 226.Campos F., Bonhome-Espinosa A.B., Chato-Astrain J., Sánchez-Porras D., García-García Ó.D., Carmona R., López-López M.T., Alaminos M., Carriel V., Rodriguez I.A. Evaluation of fibrin-agarose tissue-like hydrogels biocompatibility for tissue engineering applications. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khodadadi Yazdi M., Taghizadeh A., Taghizadeh M., Stadler F.J., Farokhi M., Mottaghitalab F., Zarrintaj P., Ramsey J.D., Seidi F., Saeb M.R., et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release. 2020;326:523–543. doi: 10.1016/j.jconrel.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 228.López-Marcial G.R., Zeng A.Y., Osuna C., Dennis J., García J.M., O’Connell G.D. Agarose-based hydrogels as suitable bioprinting materials for tissue engineering. ACS Biomater. Sci. Eng. 2018;4:3610–3616. doi: 10.1021/acsbiomaterials.8b00903. [DOI] [PubMed] [Google Scholar]
- 229.Numata K. How to define and study structural proteins as biopolymer materials. Polym. J. 2020;52:1043–1056. doi: 10.1038/s41428-020-0362-5. [DOI] [Google Scholar]
- 230.Ehrlich H. Marine collagens. In: Gorb S.N., editor. Biological Materials of Marine Origin. Vertebrates. Springer; Dordrecht, The Natherlands: Heidelberg, Germany: New York, NY, USA: London, UK: 2015. pp. 321–342. [Google Scholar]
- 231.Jesionowski T., Norman M., Żółtowska-Aksamitowska S., Petrenko I., Joseph Y., Ehrlich H. Marine spongin: Naturally prefabricated 3D scaffold-based biomaterial. Mar. Drugs. 2018;16:88. doi: 10.3390/md16030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ehrlich H., Wysokowski M., Żółtowska-Aksamitowska S., Petrenko I., Jesionowski T. Collagens of poriferan origin. Mar. Drugs. 2018;16:79. doi: 10.3390/md16030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Junqua S., Robert L., Garrone R., De Ceccatty M.P., Vacelet J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974;2:193–203. doi: 10.3109/03008207409152244. [DOI] [PubMed] [Google Scholar]
- 234.Simpson T.L. The Cell Biology of Sponges. Springer; New York, NY, USA: 1984. [Google Scholar]
- 235.Ehrlich H. Enigmatic structural protein spongin. In: Gorb S.N., editor. Marine Biological Materials of Invertebrate Origin. Springer; Cham, Switzerland: 2019. pp. 161–170. [Google Scholar]
- 236.Szatkowski T., Jesionowski T. Extreme Biomimetics. Springer; Cham, Switzerland: 2016. Hydrothermal synthesis of spongin-based materials; pp. 251–274. [Google Scholar]
- 237.Szatkowski T., Siwínska-Stefánska K., Wysokowski M., Stelling A.L., Joseph Y., Ehrlich H., Jesionowski T. Immobilization of titanium(IV) oxide onto 3D spongin scaffolds of marine sponge origin according to extreme biomimetics principles for removal of C.I. basic blue 9. Biomimetics. 2017;2:4. doi: 10.3390/biomimetics2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim M.M., Mendis E., Rajapakse N., Lee S.H., Kim S.K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009;90B:540–546. doi: 10.1002/jbm.b.31315. [DOI] [PubMed] [Google Scholar]
- 239.Zdarta J., Norman M., Smułek W., Moszyński D., Kaczorek E., Stelling A.L., Ehrlich H., Jesionowski T. Spongin-based scaffolds from Hippospongia communis demosponge as an effective support for lipase immobilization. Catalysts. 2017;7:147. doi: 10.3390/catal7050147. [DOI] [Google Scholar]
- 240.Technavio. [(accessed on 16 September 2020)]; Available online: https://www.technavio.com/
- 241.Szatkowski T., Wysokowski M., Lota G., Pęziak D., Bazhenov V.V., Nowaczyk G., Walter J., Molodtsov S.L., Stöcker H., Himcinschi C., et al. Novel nanostructured hematite-spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015;5:79031–79040. doi: 10.1039/C5RA09379A. [DOI] [Google Scholar]
- 242.Szatkowski T., Kopczyński K., Motylenko M., Borrmann H., Mania B., Graś M., Lota G., Bazhenov V.V., Rafaja D., Roth F., et al. Extreme biomimetics: A carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2018;11:4199–4214. doi: 10.1007/s12274-018-2008-x. [DOI] [Google Scholar]
- 243.Zdarta J., Antecka K., Frankowski R., Zgoła-Grześkowiak A., Ehrlich H., Jesionowski T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018;615:784–795. doi: 10.1016/j.scitotenv.2017.09.213. [DOI] [PubMed] [Google Scholar]
- 244.Norman M., Żółtowska-Aksamitowska S., Zgoła-Grześkowiak A., Ehrlich H., Jesionowski T. Iron(III) phthalocyanine supported on a spongin scaffold as an advanced photocatalyst in a highly efficient removal process of halophenols and bisphenol A. J. Hazard. Mater. 2018;347:78–88. doi: 10.1016/j.jhazmat.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 245.Ashouri V., Adib K., Rahimi-Nasrabadi M. Pre-concentration and extraction of fenitrothion using a prefabricated 3D spongin-based skeleton of marine demosponge: Optimization by experimental design. Appl. Phys. A Mater. Sci. Process. 2020;126:1–12. doi: 10.1007/s00339-020-04040-0. [DOI] [Google Scholar]
- 246.Prockop D.J., Kivirikko K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 247.Sawhney R.K., Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J. Cell Biol. 2002;157:1083. doi: 10.1083/jcb.200203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kimura S. Vertebrate skin type I collagen: Comparison of bony fishes with lamprey and calf. Comp. Biochem. Physiol. Part B Biochem. 1983;53:1315–1318. doi: 10.1016/0305-0491(83)90222-5. [DOI] [PubMed] [Google Scholar]
- 249.Nagai T., Yamashita E., Taniguchi K., Kanamori N., Suzuki N. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas) Food Chem. 2001;72:425–429. doi: 10.1016/S0308-8146(00)00249-1. [DOI] [Google Scholar]
- 250.Bae I., Osatomi K., Yoshida A., Osako K., Yamaguchi A., Hara K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008;108:49–54. doi: 10.1016/j.foodchem.2007.10.039. [DOI] [Google Scholar]
- 251.Semenycheva L.L., Egorikhina M.N., Chasova V.O., Valetova N.B., Kuznetsova Y.L., Mitin A.V. Enzymatic hydrolysis of marine collagen and fibrinogen proteins in the presence of thrombin. Mar. Drugs. 2020;18:208. doi: 10.3390/md18040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hayashi Y., Yamada S., Yanagi Guchi K., Koyama Z., Ikeda T. Advances in Food and Nutrition Research. Academic Press; Cambridge, MA, USA: 2012. Chitosan and fish collagen as biomaterials for regenerative medicine; pp. 107–120. [DOI] [PubMed] [Google Scholar]
- 253.Fernandes-Silva S., Moreira-Silva J., Silva T.H., Perez-Martin R.I., Sotelo C.G., Mano J.F., Duarte A.R.C., Reis R.L. Porous hydrogels from shark skin collagen crosslinked under dense carbon dioxide atmosphere. Macromol. Biosci. 2013;13:1621–1631. doi: 10.1002/mabi.201300228. [DOI] [PubMed] [Google Scholar]
- 254.Coppola D., Oliviero M., Vitale G.A., Lauritano C., D’Ambra I., Iannace S., de Pascale D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs. 2020;18:214. doi: 10.3390/md18040214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Moreira-Silva J., Diogo G.S., Marques A.L.P., Silva T.H., Reis R.L. Biomaterials from Nature for Advanced Devices and Therapies. Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. Marine collagen isolation and processing envisaging biomedical applications; pp. 16–36. [Google Scholar]
- 256.Silva T.H., Moreira-Silva J., Marques A.L.P., Domingues A., Bayon Y., Reis R.L. Marine origin collagens and its potential applications. Mar. Drugs. 2014;12:5881–5901. doi: 10.3390/md12125881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Swatschek D., Schatton W., Müller W.E.G., Kreuter J. Microparticles derived from marine sponge collagen (SCMPs): Preparation, characterization and suitability for dermal delivery of all-trans retinol. Eur. J. Pharm. Biopharm. 2002;54:125–133. doi: 10.1016/S0939-6411(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 258.Barros A.A., Aroso I.M., Silva T.H., Mano J.F., Duarte A.R.C., Reis R.L. Water and carbon dioxide: Green solvents for the extraction of collagen/gelatin from marine sponges. ACS Sustain. Chem. Eng. 2015;3:254–260. doi: 10.1021/sc500621z. [DOI] [Google Scholar]
- 259.Dhara S., Majumdar P., Maiti N. A Process for Extraction of Collagen from Fish Scale and Polyelectrolyte Based Bioactive Super-Absorbent Materials. No. WO 2017/122216 A1. U.S. Patent. 2017 Jul 20;
- 260.Heinemann S., Ehrlich H., Douglas T., Heinemann C., Worch H., Schatton W., Hanke T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis nardo. Biomacromolecules. 2007;8:3452–3457. doi: 10.1021/bm700574y. [DOI] [PubMed] [Google Scholar]
- 261.Ehrlich H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010;52:661–699. doi: 10.1080/00206811003679521. [DOI] [Google Scholar]
- 262.Yamada S., Yamamoto K., Ikeda T., Yanagiguchi K., Hayashi Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zheng M., Zheng J. Sponge (Porifera) collagen for bone tissue engineering. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 247–285. [Google Scholar]
- 264.Flaig I., Radenković M., Najman S., Pröhl A., Jung O., Barbeck M. In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 2020;21:4518. doi: 10.3390/ijms21124518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pina S., Ribeiro V.P., Marques C.F., Maia F.R., Silva T.H., Reis R.L., Oliveira J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12:1824. doi: 10.3390/ma12111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Raftery R.M., Woods B., Marques A.L.P., Moreira-Silva J., Silva T.H., Cryan S.A., Reis R.L., O’Brien F.J. Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 2016;43:160–169. doi: 10.1016/j.actbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 267.Seng S.K. Ph.D. Thesis. University Tunku Abdul Rahman; Perak, Malesia: 2018. Physicochemical Properties of Biomaterial Fabricated from Fish Skin Collagen and Brown Seaweed Alginate. [Google Scholar]
- 268.Markets and Markets. [(accessed on 10 September 2020)]; Available online: https://www.marketsandmarkets.com/Market-Reports/marine-collagen-market-155534506.html.
- 269.Lin Z., Solomon K.L., Zhang X., Pavlos N.J., Abel T., Willers C., Dai K., Xu J., Zheng Q., Zheng M. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011;7:968–977. doi: 10.7150/ijbs.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pozzolini M., Scarfì S., Gallus L., Castellano M., Vicini S., Cortese K., Gagliani M.C., Bertolino M., Costa G., Giovine M. Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs. 2018;16:111. doi: 10.3390/md16040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Parisi J.R., Fernandes K.R., Aparecida do Vale G.C., de França Santana A., de Almeida Cruz M., Fortulan C.A., Zanotto E.D., Peitl O., Granito R.N., Rennó A.C.M. Marine spongin incorporation into Biosilicate® for tissue engineering applications: An in vivo study. J. Biomater. Appl. 2020;35:205–214. doi: 10.1177/0885328220922161. [DOI] [PubMed] [Google Scholar]
- 272.Langasco R., Cadeddu B., Formato M., Lepedda A.J., Cossu M., Giunchedi P., Pronzato R., Rassu G., Manconi R., Gavini E. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C. 2017;70:710–720. doi: 10.1016/j.msec.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 273.Ehrlich H., Brunner E., Simon P., Bazhenov V.V., Botting J.P., Tabachnick K.R., Springer A., Kummer K., Vyalikh D.V., Molodtsov S.L., et al. Calcite reinforced silica-silica joints in the biocomposite skeleton of deep-sea glass sponges. Adv. Funct. Mater. 2011;21:3473–3481. doi: 10.1002/adfm.201100749. [DOI] [Google Scholar]
- 274.Ehrlich H., Ereskovsky A.V., Drozdov A., Krylova D., Hanke T., Meissner H., Heinemann S., Worch H. A modern approach to demineralisation of spicules in the glass sponges (Hexactinellida: Porifera) for the purpose of extraction and examination of the protein matrix. Russ. J. Mar. Biol. 2006;32:186–193. doi: 10.1134/S1063074006030060. [DOI] [Google Scholar]
- 275.Heinemann S., Heinemann C., Ehrlich H., Meyer M., Baltzer H., Worch H., Hanke T. A novel biomimetic hybrid material made of silicified collagen: Perspectives for bone replacement. Adv. Eng. Mater. 2007;9:1061–1068. doi: 10.1002/adem.200700219. [DOI] [Google Scholar]
- 276.Ehrlich H., Deutzmann R., Brunner E., Cappellini E., Koon H., Solazzo C., Yang Y., Ashford D., Thomas-Oates J., Lubeck M., et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010;2:1084–1088. doi: 10.1038/nchem.899. [DOI] [PubMed] [Google Scholar]
- 277.Lim Y.S., Ok Y.J., Hwang S.Y., Kwak J.Y., Yoon S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs. 2019;17:467. doi: 10.3390/md17080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Marques C.F., Diogo G.S., Pina S., Oliveira J.M., Silva T.H., Reis R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019;30:1–12. doi: 10.1007/s10856-019-6234-x. [DOI] [PubMed] [Google Scholar]
- 279.Shimizu J., Shimizu H., Nagashima K., Yamada K., Takamatsu M. Fish Collagen and Method of Producing Same. No. US6271350B1. U.S. Patent. 2001 Aug 7;
- 280.Govindharaj M., Roopavath U.K., Rath S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019;230:412–419. doi: 10.1016/j.jclepro.2019.05.082. [DOI] [Google Scholar]
- 281.Carvalho A.M., Marques A.P., Silva T.H., Reis R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs. 2018;16:495. doi: 10.3390/md16120495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sousa R.O., Martins E., Carvalho D.N., Alves A.L., Oliveira C., Duarte A.R.C., Silva T.H., Reis R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020;27 doi: 10.1007/s10965-020-02048-x. [DOI] [Google Scholar]
- 283.Alves A.L., Marques A.L.P., Martins E., Silva T.H., Reis R.L. Cosmetic potential of Marine fish skin collagen. Cosmetics. 2017;4:39. doi: 10.3390/cosmetics4040039. [DOI] [Google Scholar]
- 284.Blanco M., Sotelo C.G., Pérez-Martín R.I. New strategy to cope with common fishery policy landing obligation: Collagen extraction from skins and bones of undersized hake (Merluccius merluccius) Polymers. 2019;11:1485. doi: 10.3390/polym11091485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Diogo G.S., Marques C.F., Sotelo C.G., Pérez-Martín R.I., Pirraco R.P., Reis R.L., Silva T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020;6:3664–3672. doi: 10.1021/acsbiomaterials.0c00436. [DOI] [PubMed] [Google Scholar]
- 286.Diogo G.S., López-Senra E., Pirraco R.P., Canadas R.F., Fernandes E.M., Serra J., Pérez-Martín R.I., Sotelo C.G., Marques A.P., González P., et al. Marine collagen/apatite composite scaffolds envisaging hard tissue applications. Mar. Drugs. 2018;16:269. doi: 10.3390/md16080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sousa R.O., Alves A.L., Carvalho D.N., Martins E., Oliveira C., Silva T.H., Reis R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. J. Biomater. Sci. Polym. Ed. 2020:20–37. doi: 10.1080/09205063.2019.1669313. [DOI] [PubMed] [Google Scholar]
- 288.Im J.H., Choi C.H., Mun F., Lee J.H., Kim H., Jung W.K., Jang C.H., Kim G.H. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv. 2017;7:2009–2018. doi: 10.1039/C6RA25182J. [DOI] [Google Scholar]
- 289.Gaspar-Pintiliescu A., Stefan L.M., Anton E.D., Berger D., Matei C., Negreanu-Pirjol T., Moldovan L. Physicochemical and biological properties of gelatin extracted from marine snail Rapana venosa. Mar. Drugs. 2019;17:589. doi: 10.3390/md17100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Macha I.J., Ben-Nissan B., Mueller W.H. Marine-based biomaterials for tissue engineering applications. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 99–113. [Google Scholar]
- 291.Kim S.K., Ngo D.H., Vo T.S. Advances in Food and Nutrition Research. Academic Press; Cambridge, MA, USA: 2012. Marine fish-derived bioactive peptides as potential antihypertensive agents; pp. 249–260. [DOI] [PubMed] [Google Scholar]
- 292.Karim A.A., Bhat R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009;23:563–576. doi: 10.1016/j.foodhyd.2008.07.002. [DOI] [Google Scholar]
- 293.Da Trindade Alfaro A., Balbinot E., Weber C.I., Tonial I.B., Machado-Lunkes A. Fish gelatin: Characteristics, functional properties, applications and future potentials. Food Eng. Rev. 2014;7:33–44. doi: 10.1007/s12393-014-9096-5. [DOI] [Google Scholar]
- 294.Gomez-Guillen M.C., Gimenez B., Lopez-Caballero M.E., Montero M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- 295.Silva J.C., Barros A.A., Aroso I.M., Fassini D., Silva T.H., Reis R.L., Duarte A.R.C. Extraction of collagen/gelatin from the marine demosponge Chondrosia reniformis (Nardo, 1847) using water acidified with carbon dioxide—Process optimization. Ind. Eng. Chem. Res. 2016;55:6922–6930. doi: 10.1021/acs.iecr.6b00523. [DOI] [Google Scholar]
- 296.Chancharern P., Laohakunjit N., Kerdchoechuen O., Thumthanaruk B. Extraction of type A and type B gelatin from jellyfish (Lobonema smithii) Int. Food Res. J. 2016;23:419. [Google Scholar]
- 297.Uriarte-Montoya M.H., Santacruz-Ortega H., Cinco-Moroyoqui F.J., Rouzaud-Sández O., Plascencia-Jatomea M., Ezquerra-Brauer J.M. Giant squid skin gelatin: Chemical composition and biophysical characterization. Food Res. Int. 2011;44:3243–3249. doi: 10.1016/j.foodres.2011.08.018. [DOI] [Google Scholar]
- 298.Zarai Z., Balti R., Mejdoub H., Gargouri Y., Sayari A. Process for extracting gelatin from marine snail (Hexaplex trunculus): Chemical composition and functional properties. Process Biochem. 2012;47:1779–1784. doi: 10.1016/j.procbio.2012.06.007. [DOI] [Google Scholar]
- 299.Nur Hanani Z.A., Roos Y.H., Kerry J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014;71:94–102. doi: 10.1016/j.ijbiomac.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 300.Radhakrishnan N., Kanagesan S., Pandurangan A., Padmanabhan P. Nanobiomaterials in Medical Imaging: Applications of Nanobiomaterials. William Andrew; Oxford, UK: 2016. Basics to different imaging techniques, different nanobiomaterials for image enhancement; pp. 101–129. [Google Scholar]
- 301.Van Vlierberghe S., Graulus G.J., Samal S.K., Van Nieuwenhove I., Dubruel P. Biomedical Foams for Tissue Engineering Applications. Woodhead Publishing; Cambridge, UK: 2014. Porous hydrogel biomedical foam scaffolds for tissue repair; pp. 335–390. [Google Scholar]
- 302.Yang G., Xiao Z., Long H., Ma K., Zhang J., Ren X., Zhang J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rasmussen R.S., Morrissey M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007;52:237–292. doi: 10.1016/S1043-4526(06)52005-4. [DOI] [PubMed] [Google Scholar]
- 304.Salvatore L., Gallo N., Natali M.L., Campa L., Lunetti P., Madaghiele M., Blasi F.S., Corallo A., Capobianco L., Sannino A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C. 2020 doi: 10.1016/j.msec.2020.110963. [DOI] [PubMed] [Google Scholar]
- 305.Gómez-Guillén M.C., Turnay J., Fernández-Díaz M.D., Ulmo N., Lizarbe M.A., Montero P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002;16:25–34. doi: 10.1016/S0268-005X(01)00035-2. [DOI] [Google Scholar]
- 306.Choi S.S., Regenstein J.M. Physicochemical and sensory characteristics of fish gelatin. J. Food Sci. 2000;65:194–199. doi: 10.1111/j.1365-2621.2000.tb15978.x. [DOI] [Google Scholar]
- 307.Haard N.F., Simpson B.K., Sikorski Z.E. Seafood Proteins. Springer; New York, NY, USA: 1994. Biotechnological applications of seafood proteins and other nitrogenous compounds; pp. 194–216. [Google Scholar]
- 308.Abd Elgadir M., Mirghani M.E.S., Adam A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ. 2013;11:73–79. [Google Scholar]
- 309.Sharma S., Gupta A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016;59 doi: 10.1590/1678-4324-2016150684. [DOI] [Google Scholar]
- 310.Wang B., Yang W., McKittrick J., Meyers M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016;76:229–318. doi: 10.1016/j.pmatsci.2015.06.001. [DOI] [Google Scholar]
- 311.Sharma S., Kumar A. Keratin as a Protein Biopolymer: Extraction from Waste Biomass and Applications. Springer; Cham, Switzerland: 2019. [Google Scholar]
- 312.Shavandi A., Silva T.H., Bekhit A.A., Bekhit A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017;5:1699–1735. doi: 10.1039/C7BM00411G. [DOI] [PubMed] [Google Scholar]
- 313.Vineis C., Varesano A., Varchi G., Aluigi A. Keratin as a Protein Biopolymer. Springer; Cham, Switzerland: 2019. Extraction and characterization of keratin from different biomasses; pp. 35–76. [Google Scholar]
- 314.Korniłłowicz-Kowalska T., Bohacz J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011;31:1689–1701. doi: 10.1016/j.wasman.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 315.Mogosanu G., Grumezescu A., Chifiriuc M. Keratin-based biomaterials for biomedical applications. Curr. Drug Targets. 2014;15:518–530. doi: 10.2174/1389450115666140307154143. [DOI] [PubMed] [Google Scholar]
- 316.Fu J., Guerette P.A., Pavesi A., Horbelt N., Lim C.T., Harrington M.J., Miserez A. Artificial hagfish protein fibers with ultra-high and tunable stiffness. Nanoscale. 2017;9:12908–12915. doi: 10.1039/C7NR02527K. [DOI] [PubMed] [Google Scholar]
- 317.Fudge D.S., Gosline J.M. Molecular design of the α-keratin composite: Insights from a matrix-free model, hagfish slime threads. Proc. R. Soc. B Biol. Sci. 2004;271:291–299. doi: 10.1098/rspb.2003.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Böni L.J., Zurflüh R., Baumgartner M.E., Windhab E.J., Fischer P., Kuster S., Rühs P.A. Effect of ionic strength and seawater cations on hagfish slime formation. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-27975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dance A. Will hagfish yield the fibers of the future? Proc. Natl. Acad. Sci. USA. 2016;113:7005–7006. doi: 10.1073/pnas.1606279113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yamauchi K., Yamauchi A., Kusunoki T., Kohda A., Konishi Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J. Biomed. Mater. Res. 1996;31:439–444. doi: 10.1002/(SICI)1097-4636(199608)31:4<439::AID-JBM1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 321.Fujii T., Ogiwara D., Arimoto M. Convenient procedures for human hair protein films and properties of alkaline phosphatase incorporated in the film. Biol. Pharm. Bull. 2004;27:89–93. doi: 10.1248/bpb.27.89. [DOI] [PubMed] [Google Scholar]
- 322.Tanabe T., Okitsu N., Tachibana A., Yamauchi K. Preparation and characterization of keratin-chitosan composite film. Biomaterials. 2002;23:817–825. doi: 10.1016/S0142-9612(01)00187-9. [DOI] [PubMed] [Google Scholar]
- 323.Borrelli M., Joepen N., Reichl S., Finis D., Schoppe M., Geerling G., Schrader S. Keratin films for ocular surface reconstruction: Evaluation of biocompatibility in an in-vivo model. Biomaterials. 2015;42:112–120. doi: 10.1016/j.biomaterials.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 324.Heldreth B. Hydrolyzed Source Proteins as Used in Cosmetics. [(accessed on 23 November 2020)]; Available online: http://www.cir-safety.org/sites/default/files/hprtns052012slr.pdf.
- 325.Naoya Y., Masatoshi S., Kazuhiko T. Cleansing Composition. No. US20060091610A1. U.S. Patent. 2005 Jul 7;
- 326.Latire T., Legendre F., Bigot N., Carduner L., Kellouche S., Bouyoucef M., Carreiras F., Marin F., Lebel J.M., Galéra P., et al. Shell extracts from the marine bivalve Pecten maximus regulate the synthesis of extracellular matrix in primary cultured human skin fibroblasts. PLoS ONE. 2014;9:e99931. doi: 10.1371/journal.pone.0099931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Latire T., Legendre F., Bouyoucef M., Marin F., Carreiras F., Rigot-Jolivet M., Lebel J.M., Galéra P., Serpentini A. Shell extracts of the edible mussel and oyster induce an enhancement of the catabolic pathway of human skin fibroblasts, in vitro. Cytotechnology. 2017;69:815–829. doi: 10.1007/s10616-017-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Andrade P.H.M., Schmidt Rondon E., Carollo C.A., Rodrigues Macedo M.L., Viana L.H., Schiaveto De Souza A., Turatti Oliveira C., Cepa Matos M.D.F. Effect of powdered shells of the snail Megalobulimus lopesi on secondary-intention wound healing in an animal model. Evid. Based Complement. Altern. Med. 2015:120785. doi: 10.1155/2015/120785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lansdown A.B.G. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285. doi: 10.1046/j.1524-475X.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 330.Limová M. Evaluation of two calcium alginate dressings in the management of venous ulcers. Ostomy Wound Manag. 2003;49:26–33. [PubMed] [Google Scholar]
- 331.Ehrlich H., Martinović R., Joksimović D., Petrenko I., Schiaparelli S., Wysokowski M., Tsurkan D., Stelling A.L., Springer A., Gelinsky M., et al. Conchixes: Organic scaffolds which resemble the size and shapes of mollusks shells, their isolation and potential multifunctional applications. Appl. Phys. A Mater. Sci. Process. 2020;126:280. doi: 10.1007/s00339-020-03728-7. [DOI] [Google Scholar]
- 332.Bowen C.E., Tang H. Conchiolin-protein in aragonite shells of mollusks. Comp. Biochem. Physiol. A Physiol. 1996;115:269–275. doi: 10.1016/S0300-9629(96)00059-X. [DOI] [Google Scholar]
- 333.Bédouet L., Marie A., Dubost L., Péduzzi J., Duplat D., Berland S., Puisségur M., Boulzaguet H., Rousseau M., Milet C., et al. Proteomics analysis of the nacre soluble and insoluble proteins from the oyster Pinctada margaritifera. Mar. Biotechnol. 2007;9:638–649. doi: 10.1007/s10126-007-9017-1. [DOI] [PubMed] [Google Scholar]
- 334.Cariolou M.A., Morse D.E. Purification and characterization of calcium-binding conchiolin shell peptides from the mollusc, Haliotis rufescens, as a function of development. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1988;157:717–729. doi: 10.1007/BF00691002. [DOI] [Google Scholar]
- 335.Krampitz G., Drolshagen H., Hotta S. Simultaneous binding of calcium and bicarbonate by conchiolin of oyster shells. Experientia. 1983;39:1104–1105. doi: 10.1007/BF01943131. [DOI] [Google Scholar]
- 336.OECD . Health at a Glance: Europe 2016—State of Health in the EU Cycle. OECD Publishing; Paris, France: 2016. [DOI] [Google Scholar]
- 337.Kim I.W., Collino S., Morse D.E., Evans J.S. A crystal modulating protein from molluscan nacre that limits the growth of calcite in vitro. Cryst. Growth Des. 2006;6:1078–1082. doi: 10.1021/cg060056q. [DOI] [Google Scholar]
- 338.Tabachnick K.R., Menshenina L.L., Pisera A., Ehrlich H. Revision of Aspidoscopulia Reiswig, 2002 (Porifera: Hexactinellida: Farreidae) with description of two new species. Zootaxa. 2011;2883:1–22. doi: 10.11646/zootaxa.2883.1.1. [DOI] [Google Scholar]
- 339.Tabachnick K., Fromont J., Ehrlich H., Menshenina L. Hexactinellida from the Perth Canyon, Eastern Indian Ocean, with descriptions of five new species. Zootaxa. 2019;4664:047–082. doi: 10.11646/zootaxa.4664.1.2. [DOI] [PubMed] [Google Scholar]
- 340.Ehrlich H., Maldonado M., Parker A.R., Kulchin Y.N., Schilling J., Köhler B., Skrzypczak U., Simon P., Reiswig H.M., Tsurkan M.V., et al. Supercontinuum generation in naturally occurring glass sponges spicules. Adv. Opt. Mater. 2016;4:1608–1613. doi: 10.1002/adom.201600454. [DOI] [Google Scholar]
- 341.Wysokowski M., Jesionowski T., Ehrlich H. Biosilica as a source for inspiration in biological materials science. Am. Mineral. 2018;103:665–691. doi: 10.2138/am-2018-6429. [DOI] [Google Scholar]
- 342.Fernandes M.C., Aizenberg J., Weaver J.C., Bertoldi K. Mechanically robust lattices inspired by deep-sea glass sponges. Nat. Mater. 2020 doi: 10.1038/s41563-020-0798-1. [DOI] [PubMed] [Google Scholar]
- 343.Gravel M., Vago R., Tabrizian M. Use of natural coralline biomaterials as reinforcing and gas-forming agent for developing novel hybrid biomatrices: Microarchitectural and mechanical studies. Tissue Eng. 2006;12:589–600. doi: 10.1089/ten.2006.12.589. [DOI] [PubMed] [Google Scholar]
- 344.Green D.W., Ben-Nissan B., Yoon K.S., Milthorpe B., Jung H.S. Natural and synthetic coral biomineralization for human bone revitalization. Trends Biotechnol. 2017;35:43–54. doi: 10.1016/j.tibtech.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 345.Nandi S.K., Kundu B., Mukherjee J., Mahato A., Datta S., Balla V.K. Converted marine coral hydroxyapatite implants with growth factors: In vivo bone regeneration. Mater. Sci. Eng. C. 2015;49:816–823. doi: 10.1016/j.msec.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 346.Ehrlich H., Etnoyer P., Litvinov S.D., Olennikova M.M., Domaschke H., Hanke T., Born R., Meissner H., Worch H. Biomaterial structure in deep-sea bamboo coral (Anthozoa: Gorgonacea: Isididae): Perspectives for the development of bone implants and templates for tissue engineering. Mater. Werkst. 2006;37:552–557. doi: 10.1002/mawe.200600036. [DOI] [Google Scholar]
- 347.Boller M.L., Swain T.D., Lasker H.R. Skeletal morphology and material properties of a fragmenting gorgonian coral. Mar. Ecol. Prog. Ser. 2002;228:131–141. doi: 10.3354/meps228131. [DOI] [Google Scholar]
- 348.Day A.G.E., Francis W.R., Fu K., Pieper I.L., Guy O., Xia Z. Osteogenic potential of human umbilical cord mesenchymal stem cells on coralline hydroxyapatite/calcium carbonate microparticles. Stem Cells Int. 2018;2018 doi: 10.1155/2018/4258613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Israel 21C. [(accessed on 12 September 2020)]; Available online: https://www.israel21c.org/growing-new-bone-from-corals-raised-in-the-israeli-desert/
- 350.Wangpraseurt D., You S., Azam F., Jacucci G., Gaidarenko O., Hildebrand M., Kühl M., Smith A.G., Davey M.P., Smith A., et al. Bionic 3D printed corals. Nat. Commun. 2020;11:1–8. doi: 10.1038/s41467-020-15486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Khrunyk Y.Y., Belikov S.V., Tsurkan M.V., Vyalykh I.V., Markaryan A.Y., Karabanalov M.S., Popov A.A., Wysokowski M. Surface-dependent osteoblasts response to TiO2 nanotubes of different crystallinity. Nanomaterials. 2020;10:320. doi: 10.3390/nano10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Karacan I., Ben-Nissan B., Sinutok S. Marine-based calcium phosphates from hard coral and calcified algae for biomedical applications. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 137–157. [Google Scholar]
- 353.Ben-Nissan B., Choi A.H., Green D.W. Marine derived biomaterials for bone regeneration and tissue engineering: Learning from nature. In: Choi A.H., Ben-Nissan B., editors. Marine-Derived Biomaterials for Tissue Engineering Applications. Springer; Singapore: 2019. pp. 51–81. [Google Scholar]
- 354.Demers C., Reggie Hamdy C., Corsi K., Chellat F., Tabrizian M., Yahia L. Natural coral exoskeleton as a bone graft substitute: A review. Biomed. Mater. Eng. 2002;12:15–35. [PubMed] [Google Scholar]
- 355.Nabipour I. The Application of Corals in Bone Tissue Engineering. Iran. South Med. J. 2017;20:217–244. doi: 10.29252/ismj.20.2.217. [DOI] [Google Scholar]
- 356.Chapman-Sheath P., Cain S., Debes J., Svehla M., Bruce W., Yu Y., Walsh W.R. In vivo response of coral biomaterials. Orthop. Proc. 2018 doi: 10.1302/0301-620X.85BSUPP_I.0850001b. [DOI] [Google Scholar]
- 357.Sheehy E.J., Lemoine M., Clarke D., Vazquez A.G., O’Brien F.J. The incorporation of marine coral microparticles into collagen-based scaffolds promotes osteogenesis of human mesenchymal stromal cells via calcium ion signalling. Mar. Drugs. 2020;18:74. doi: 10.3390/md18020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Manassero M., Viateau V., Deschepper M., Oudina K., Logeart-Avramoglou D., Petite H., Bensidhoum M. Bone regeneration in sheep using acropora coral, a natural resorbable scaffold, and autologous mesenchymal stem cells. Tissue Eng. Part A. 2013;19:1554–1563. doi: 10.1089/ten.tea.2012.0008. [DOI] [PubMed] [Google Scholar]
- 359.Birk R.Z., Abramovitch-Gottlib L., Margalit I., Aviv M., Forti E., Geresh S., Vago R. Conversion of adipogenic to osteogenic phenotype using crystalline porous biomatrices of marine origin. Tissue Eng. 2006;12:21–31. doi: 10.1089/ten.2006.12.21. [DOI] [PubMed] [Google Scholar]
- 360.Julia V., Abbas B., Bachtiar E.W., Latief B.S., Kuijpers-Jagtman A.M. Effect of coral Goniopora Sp scaffold application on human osteoblast-like MG-63 cell activity in vitro. Makara J. Health Res. 2019:23. doi: 10.7454/msk.v23i2.10752. [DOI] [Google Scholar]
- 361.Gancz A., Zueva Y., Weiss O.E., Hendler R.M., Minnes R., Baranes D. Coralline skeleton biomaterial reduces phagocytosis in mouse blood in vitro. Isr. J. Chem. 2020;60:586–592. doi: 10.1002/ijch.201900151. [DOI] [Google Scholar]
- 362.Pohl T., Al-Muqdadi S.W., Ali M.H., Fawzi N.A.M., Ehrlich H., Merkel B. Discovery of a living coral reef in the coastal waters of Iraq. Sci. Rep. 2014;4 doi: 10.1038/srep04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Pfister L., Grave C., Beisel J.N., McDonnell J.J. A global assessment of freshwater mollusk shell oxygen isotope signatures and their relation to precipitation and stream water. Sci. Rep. 2019;9:1–6. doi: 10.1038/s41598-019-40369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Chandra Rajan K., Vengatesen T. Molecular adaptation of molluscan biomineralisation to high-CO2 oceans—The known and the unknown. Mar. Environ. Res. 2020;155 doi: 10.1016/j.marenvres.2020.104883. [DOI] [PubMed] [Google Scholar]
- 365.Zuykov M., Pelletier E., Harper D.A.T. Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere. 2013;93:201–208. doi: 10.1016/j.chemosphere.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 366.Bruggmann S., Klaebe R.M., Paulukat C., Frei R. Heterogeneity and incorporation of chromium isotopes in recent marine molluscs (Mytilus) Geobiology. 2019;17:417–435. doi: 10.1111/gbi.12336. [DOI] [Google Scholar]
- 367.Kamat S., Su X., Ballarini R., Heuer A.H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature. 2000;405:1036–1040. doi: 10.1038/35016535. [DOI] [PubMed] [Google Scholar]
- 368.Yang W., Kashani N., Li X.W., Zhang G.P., Meyers M.A. Structural characterization and mechanical behavior of a bivalve shell (Saxidomus purpuratus) Mater. Sci. Eng. C. 2011;31:724–729. doi: 10.1016/j.msec.2010.10.003. [DOI] [Google Scholar]
- 369.Hrabánková I., Frýda J., Šepitka J., Sasaki T., Frýdová D., Lukeš J. Mechanical properties of deep-sea molluscan shell. Comput. Methods Biomech. Biomed. Eng. 2013;16:287–289. doi: 10.1080/10255842.2013.815873. [DOI] [PubMed] [Google Scholar]
- 370.Li X.W., Ji H.M., Yang W., Zhang G.P., Chen D.L. Mechanical properties of crossed-lamellar structures in biological shells: A review. J. Mech. Behav. Biomed. Mater. 2017;74:54–71. doi: 10.1016/j.jmbbm.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 371.Checa A.G., Esteban-Delgado F.J., Rodríguez-Navarro A.B. Crystallographic structure of the foliated calcite of bivalves. J. Struct. Biol. 2007;157:393–402. doi: 10.1016/j.jsb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 372.Meyers M.A., Chen P.Y., Lin A.Y.M., Seki Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008;53:1–206. doi: 10.1016/j.pmatsci.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 373.De Paula S.M., Silveira M. Studies on molluscan shells: Contributions from microscopic and analytical methods. Micron. 2009;40:669–690. doi: 10.1016/j.micron.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 374.Yadav R., Goud R., Dutta A., Wang X., Naebe M., Kandasubramanian B. Biomimicking of hierarchal molluscan shell structure via layer by layer 3D printing. Ind. Eng. Chem. Res. 2018;57:10832–10840. doi: 10.1021/acs.iecr.8b01738. [DOI] [Google Scholar]
- 375.Schoeppler V., Lemanis R., Reich E., Pusztai T., Gránásy L., Zlotnikov I. Crystal growth kinetics as an architectural constraint on the evolution of molluscan shells. Proc. Natl. Acad. Sci. USA. 2019;116:20388–20397. doi: 10.1073/pnas.1907229116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Strąg M., Maj Ł., Bieda M., Petrzak P., Jarzębska A., Gluch J., Topal E., Kutukova K., Clausner A., Heyn W., et al. Anisotropy of mechanical properties of Pinctada margaritifera mollusk shell. Nanomaterials. 2020;10:634. doi: 10.3390/nano10040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Currey J.D. The design of mineralised hard tissues for their mechanical functions. J. Exp. Biol. 1999;202:3285–3294. doi: 10.1242/jeb.202.23.3285. [DOI] [PubMed] [Google Scholar]
- 378.Chateigner D., Hedegaard C., Wenk H.R. Mollusc shell microstructures and crystallographic textures. J. Struct. Geol. 2000;22:1723–1735. doi: 10.1016/S0191-8141(00)00088-2. [DOI] [Google Scholar]
- 379.Currey J.D. Further studies on the mechanical properties of mollusc shell material. J. Zool. 1976;180:445–453. doi: 10.1111/j.1469-7998.1976.tb04690.x. [DOI] [Google Scholar]
- 380.Kuhn-Spearing L.T., Kessler H., Chateau E., Ballarini R., Heuer A.H., Spearing S.M. Fracture mechanisms of the Strombus gigas conch shell: Implications for the design of brittle laminates. J. Mater. Sci. 1996;31:6583–6594. doi: 10.1007/BF00356266. [DOI] [Google Scholar]
- 381.Parker L.M., Ross P.M., O’Connor W.A., Pörtner H.O., Scanes E., Wright J.M. Predicting the response of molluscs to the impact of ocean acidification. Biology. 2013;2:651–692. doi: 10.3390/biology2020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Hu S., Wang Y., Han H. Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production. Biomass Bioenerg. 2011;35:3627–3635. doi: 10.1016/j.biombioe.2011.05.009. [DOI] [Google Scholar]
- 383.Vecchio K.S., Zhang X., Massie J.B., Wang M., Kim C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007;3:910–918. doi: 10.1016/j.actbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 384.Morris J.P., Backeljau T., Chapelle G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019;11:42–57. doi: 10.1111/raq.12225. [DOI] [Google Scholar]
- 385.George P., Hamid Z.A., Zakaria M.Z.A.B., Perimal E.K., Bharatham H. A short review on cockle shells as biomaterials in the context of bone scaffold fabrication. Sains Malays. 2019;48:1539–1545. doi: 10.17576/jsm-2019-4807-23. [DOI] [Google Scholar]
- 386.Gerhard E.M., Wang W., Li C., Guo J., Ozbolat I.T., Rahn K.M., Armstrong A.D., Xia J., Qian G., Yang J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017;54:21–34. doi: 10.1016/j.actbio.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 387.Agarwal V., Tjandra E.S., Iyer K.S., Humfrey B., Fear M., Wood F.M., Dunlop S., Raston C.L. Evaluating the effects of nacre on human skin and scar cells in culture. Toxicol. Res. 2014;3:223–227. doi: 10.1039/C4TX00004H. [DOI] [Google Scholar]
- 388.Atlan G., Delattre O., Berland S., LeFaou A., Nabias G., Cot D., Lopez E. Interface between bone and nacre implants in sheep. Biomaterials. 1999;20:1017–1022. doi: 10.1016/S0142-9612(98)90212-5. [DOI] [PubMed] [Google Scholar]
- 389.Food and Agriculture Organization of the United Nations (FAO) The State of World Fisheries and Aquaculture. FAO; Rome, Italy: 2020. Sustainability in Action. [DOI] [Google Scholar]
- 390.Food and Agriculture Organization of the United Nations. [(accessed on 25 October 2020)]; Available online: http://www.fao.org/documents/card/en/c/ca1213en/
- 391.Tokeshi M., Ota N., Kawai T. A comparative study of morphometry in shell-bearing molluscs. J. Zool. 2000;251:31–38. doi: 10.1111/j.1469-7998.2000.tb00590.x. [DOI] [Google Scholar]
- 392.Yoon H., Park S., Lee K., Park J. Oyster shell as substitute for aggregate in mortar. Waste Manag. Res. 2004;22:158–170. doi: 10.1177/0734242X04042456. [DOI] [PubMed] [Google Scholar]
- 393.Ballester P., Mármol I., Morales J., Sánchez L. Use of limestone obtained from waste of the mussel cannery industry for the production of mortars. Cem. Concr. Res. 2007;37:559–564. doi: 10.1016/j.cemconres.2007.01.004. [DOI] [Google Scholar]
- 394.Suttle N.F. Mineral Nutrition of Livestock. 4th ed. CABI Publishing; Cambridge, MA, USA: 2010. [Google Scholar]
- 395.Scott M.L., Hull S.J., Mullenhoff P.A. The calcium requirements of laying hens and effects of dietary oyster shell upon egg shell quality. Poult. Sci. 1971;50:1055–1063. doi: 10.3382/ps.0501055. [DOI] [Google Scholar]
- 396.Aletor V.A., Aturamu O.A. Use of oyster shell as calcium supplement. Part 2. An assessment of the responses of hepatic and serum enzymes, relative organ weights, and bone mineralization in the broiler chicken fed gossypol-containing cottonseed cake supplemented with oyster shell. Food/Nahrung. 1990;34:319–324. doi: 10.1002/food.19900340404. [DOI] [PubMed] [Google Scholar]
- 397.Aletor V.A., Onibi O.E. Use of oyster shell as calcium supplement. Part 1. Effect on the utilization of gossypol-containing cotton seed cake by the chicken. Food/Nahrung. 1990;34:311–318. doi: 10.1002/food.19900340403. [DOI] [PubMed] [Google Scholar]
- 398.Ajakaiye A., Atteh J.O., Leeson S. Biological availability of calcium in broiler chicks from different calcium sources found in Nigeria. Anim. Feed Sci. Technol. 2003;104:209–214. doi: 10.1016/S0377-8401(02)00332-2. [DOI] [Google Scholar]
- 399.Guinotte F., Nys Y., de Monredon F. The effects of particle size and origin of calcium carbonate on performance and ossification characteristics in broiler chicks. Poult. Sci. 1991;70:1908–1920. doi: 10.3382/ps.0701908. [DOI] [PubMed] [Google Scholar]
- 400.Çatli A.U., Bozkurt M., Küçükyilmaz K., Çinar M., Bintas E., Çöven F., Atik H. Performance and egg quality of aged laying hens fed diets supplemented with meat and bone meal or oyster shell meal. S. Afr. J. Anim. Sci. 2012;41 doi: 10.4314/sajas.v42i1.9. [DOI] [Google Scholar]
- 401.McLaughlan C., Rose P., Aldridge D.C. Making the Best of a Pest: The potential for using invasive Zebra mussel (Dreissena polymorpha) biomass as a supplement to commercial chicken feed. Environ. Manag. 2014;54:1102–1109. doi: 10.1007/s00267-014-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Haynes R.J., Naidu R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998;51:123–137. doi: 10.1023/A:1009738307837. [DOI] [Google Scholar]
- 403.Lee C.H., Lee D.K., Ali M.A., Kim P.J. Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Manag. 2008;28:2702–2708. doi: 10.1016/j.wasman.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 404.Garrido-Rodríguez B., Fernández-Calviño D., Nóvoa Muñoz J.C., Arias-Estévez M., Díaz-Raviña M., Álvarez-Rodríguez E., Fernández-Sanjurjo M.J., Núñez-Delgado A. pH-dependent copper release in acid soils treated with crushed mussel shell. Int. J. Environ. Sci. Technol. 2013;10:983–994. doi: 10.1007/s13762-013-0201-8. [DOI] [Google Scholar]
- 405.Ok Y.S., Lim J.E., Moon D.H. Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ. Geochem. Health. 2011;33:83–91. doi: 10.1007/s10653-010-9329-3. [DOI] [PubMed] [Google Scholar]
- 406.Osorio-López C., Seco-Reigosa N., Garrido-Rodríguez B., Cutillas-Barreiro L., Arias-Estévez M., Fernández-Sanjurjo M.J., Álvarez-Rodríguez E., Núñez-Delgado A. As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: Kinetics and fractionation. J. Taiwan Inst. Chem. Eng. 2014;45:1007–1014. doi: 10.1016/j.jtice.2013.10.001. [DOI] [Google Scholar]
- 407.Du Y., Lian F., Zhu L. Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environ. Pollut. 2011;159:1763–1768. doi: 10.1016/j.envpol.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 408.Tam C.H., Lee S.C., Chang S.H., Tang T.P., Ho H.H., Bor H.Y. Effects of the temperature of hot isostatic pressing treatment on Cr-Si targets. Ceram. Int. 2009;35:565–570. doi: 10.1016/j.ceramint.2008.01.009. [DOI] [Google Scholar]
- 409.Song F., Soh A.K., Bai Y.L. Structural and mechanical properties of the organic matrix layers of nacre. Biomaterials. 2003;24:3623–3631. doi: 10.1016/S0142-9612(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 410.Currey J.D., Zioupos P., Davies P., Casinos A. Mechanical properties of nacre and highly mineralized bone. Proc. R. Soc. B Biol. Sci. 2001;268:107–111. doi: 10.1098/rspb.2000.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhang G., Brion A., Willemin A.S., Piet M.H., Moby V., Bianchi A., Mainard D., Galois L., Gillet P., Rousseau M. Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. Part A. 2017;105:662–671. doi: 10.1002/jbm.a.35939. [DOI] [PubMed] [Google Scholar]
- 412.Morris J.P., Wang Y., Backeljau T., Chapelle G. Biomimetic and bio-inspired uses of mollusc shells. Mar. Genomics. 2016;27:85–90. doi: 10.1016/j.margen.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 413.Lamghari M., Berland S., Laurent A., Huet H., Lopez E. Bone reactions to nacre injected percutaneously into the vertebrae of sheep. Biomaterials. 2001;22:555–562. doi: 10.1016/S0142-9612(00)00213-1. [DOI] [PubMed] [Google Scholar]
- 414.Rousseau M., Delattre O., Gillet P., Lopez E. Subchondral nacre implant in the articular zone of the sheep’s knee: A pilot study. Biomed. Mater. Eng. 2012;22:227–234. doi: 10.3233/BME-2012-0712. [DOI] [PubMed] [Google Scholar]
- 415.Pascaretti-Grizon F., Libouban H., Camprasse G., Camprasse S., Mallet R., Chappard D. The interface between nacre and bone after implantation in the sheep: A nanotomographic and Raman study. J. Raman Spectrosc. 2014;45:558–564. doi: 10.1002/jrs.4506. [DOI] [Google Scholar]
- 416.Asvanund P., Chunhabundit P. Alveolar bone regeneration by implantation of nacre and B-tricalcium phosphate in guinea pig. Implant Dent. 2012;21:248–253. doi: 10.1097/ID.0b013e3182563ae0. [DOI] [PubMed] [Google Scholar]
- 417.Green D.W., Kwon H.J., Jung H.S. Osteogenic potency of nacre on human mesenchymal stem cells. Mol. Cells. 2015;38:267–272. doi: 10.14348/molcells.2015.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Brion A., Zhang G., Dossot M., Moby V., Dumas D., Hupont S., Piet M.H., Bianchi A., Mainard D., Galois L., et al. Nacre extract restores the mineralization capacity of subchondral osteoarthritis osteoblasts. J. Struct. Biol. 2015;192:500–509. doi: 10.1016/j.jsb.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 419.Mahmood S.K., Zakaria M.Z.A.B., Razak I.S.B.A., Yusof L.M., Jaji A.Z., Tijani I., Hammadi N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017;10:237–251. doi: 10.1016/j.bbrep.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Bharatham H., Zakaria M.Z.A.B., Perimal E.K., Yusof L.M., Hamid M. Mineral and physiochemical evaluation of Cockle shell (Anadara granosa) and other selected Molluscan shell as potential biomaterials. Sains Malays. 2014;43:1023–1029. [Google Scholar]