Abstract
In the present study, four new compounds including a pair of 2-benzoyl tetrahydrofuran enantiomers, namely, (−)-1S-myrothecol (1a) and (+)-1R-myrothecol (1b), a methoxy-myrothecol racemate (2), and an azaphilone derivative, myrothin (3), were isolated along with four known compounds (4–7) from cultures of the deep-sea fungus Myrothecium sp. BZO-L062. Enantiomeric compounds 1a and 1b were separated through normal-phase chiral high-performance liquid chromatography. The absolute configurations of 1a, 1b, and 3 were assigned by ECD spectra. Among them, the new compound 1a and its enantiomer 1b exhibited anti-inflammatory activity, inhibited nitric oxide formation in lipopolysaccharide-treated RAW264.7 cells, and exhibited antioxidant activity in the 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and oxygen radical absorbance capacity assays.
Keywords: deep sea marine-derived fungus, Myrothecium sp., myrothecol, nitric oxide (NO), antioxidant activity
1. Introduction
Natural products are a rich source of new drugs and they are frequently used for the discovery and development of new drugs [1]. Natural marine products with unique architectures and distinct biological activities are treasure troves for natural product chemists [2,3]. Among marine organisms, fungi produce a diverse range of biologically active metabolites [3], including polyketides [4,5,6], terpenoids [7,8,9], polypeptides [10], and alkaloids [11,12,13].
Microorganisms of the deep-sea are an attractive source of candidate drugs. While screening inhibitors of lipopolysaccharide (LPS)-induced nitric oxide (NO) production, we recently isolated cyclopenol and cyclopenin from the extract of the fungal strain Aspergillus sp. SCSIOW2 collected from a depth of approximately 2000 m in the sea [14]. At non-toxic concentrations, these compounds inhibited LPS-induced NO production and IL-6 secretion in RAW264.7 cells. This inhibitory effect of cyclopenol and cyclopenin was attributed to the suppression of the upstream signal of NF-B activation. These compounds also suppressed the expression of IL-1β, IL-6, and iNOS in microglia cells (macrophages in the mouse brain) [14]. In Alzheimer’s disease, amyloid β-peptide induces inflammation in the brain. Between the two compounds, cyclopenin showed ameliorative effects in an in vivo Alzheimer’s model using flies [14].
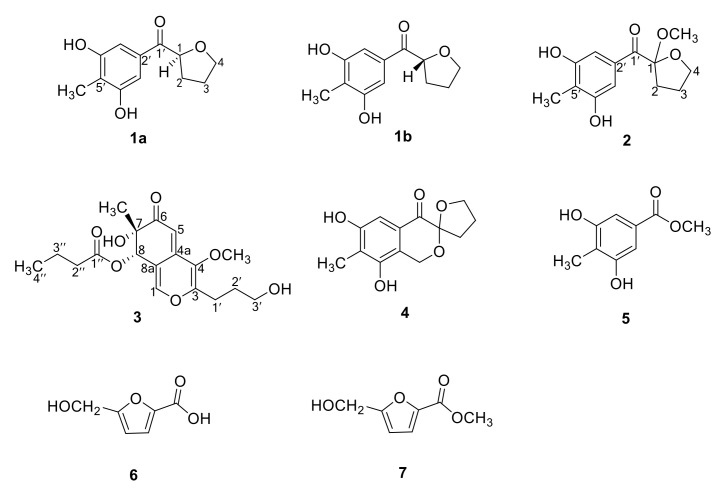
To explore new bioactive secondary metabolites from deep marine-derived fungi [15,16,17], a fungal strain, Myrothecium sp. BZO-L062, isolated from sediment samples collected from the sea bottom near Yongxing Island, was used for chemical investigation. Seven pure components, including four new compounds (1a, 1b, 2, and 3), were isolated and identified from the ethyl acetate extract of the fungus (Figure 1). The absolute configurations of the new compounds (1a, 1b, and 3) were assigned by comparison of their experimental CD spectra with the theoretically calculated spectra. The NO production inhibitory activity and antioxidant activity of the new compounds were also evaluated. Known compounds 4–7 were identified as terreinol (4) [18], 3,5-dihydroxy-4-methylbenzoic acid methyl ester (5), 5-hydroxymethyl-2-furoic acid (6) [19], and 5-hydroxymethyl-2-furancarboxylic acid methyl ester (7) [20] by comparing their spectroscopic data with those previously reported.
Figure 1.
Compounds 1–7 isolated from Myrothecium sp. BZO-L062, including (−)-(1S)-myrotheciol (1a), (+)-(1R)-myrotheciol (1b), 1-methoxy-myrotheciol (2), myrothin (3), terreinol (4), 3,5-dihydroxy-4-methylbenzoic acid methyl ester (5), 5-hydroxymethyl-2-furoic acid (6), and 5-hydroxymethyl-2-furancarboxylic acid methyl ester (7).
2. Results and Discussion
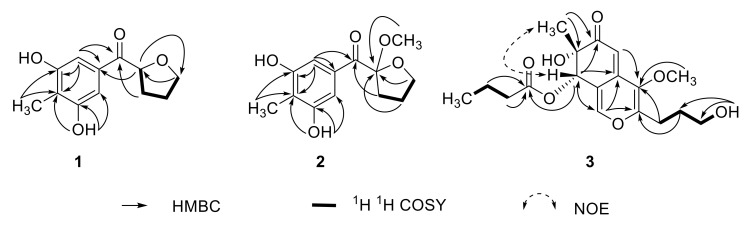
The molecular formula of 1 was determined as C12H14O4 by high-resolution electrospray ionization mass spectrometry (HRESIMS) at m/z 223.0958 [M + H]+ and 245.0780 [M + Na]+ (calculated for C12H15O4+, 223.0965; C12H14O4Na+, 245.0784) (Figure S1). 1H NMR, 13C NMR, and 2D-NMR data of 1 (Table 1, Figures S2–S8) revealed the presence of 12 resonance signals, including those for one sp3 methyl, one sp3 oxygenated methine, three sp3 methylenes, two symmetric sp2 methines, two symmetric sp2 oxygenated quaternary carbons, two sp2 quaternary carbons, and one ketone carbonyl carbon. The 1H-1H correlation spectroscopy (COSY) data from H-1 to H2-4 and the ¹H-¹³C heteronuclear multiple bond correlations (HMBC) from H-1 to oxygenated C-4 and from H2-4 to C-1 suggested the presence of a tetrahydro-2-furanyl moiety (Table 1 and Figure 2). The four aromatic carbon signals indicated the presence of one symmetrically substituted benzene ring. The HMBC experiment correlations confirmed the presence of a 3,5-dihydroxy-4-methyl benzoyl moiety (Table 1 and Figure 2). Finally, the key HMBC correlations from H2-2 to C-1 and from H-1 to C-2 allowed the linkage of the 3,5-dihydroxy-4-methyl benzoyl and tetrahydro-2-furanyl groups (Table 1 and Figure 2). Accordingly, 1 was established as (3,5-dihydroxy-4-methylphenyl)-(tetrahydro-2-furanyl)methanone and denoted as a myrotheciol.
Table 1.
1H NMR (600 MHz) and 13C NMR (150 MHz) spectral data of 1.
| No. | δ C | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 79.1 | 5.09, dd (8.4, 5.6) | 2 | C-2,3,4 |
| 2 | 29.0 | 2.17, m; 1.92, m | 1,3 | C-1,3,4,1′ |
| 3 | 25.2 | 1.84, m | 2,4 | C-1,2,4 |
| 4 | 68.4 | 3.81, t (6.7) | 3 | C-1,2,3 |
| 1′ | 198.0 | - | ||
| 2′ | 132.7 | - | ||
| 3′,7′ | 106.1 | 6.92, s | C-1′,2′,4′(6′),5′ | |
| 4′,6′ | 156.1 | - | ||
| 5′ | 116.7 | - | ||
| 4′−OH/6′−OH | - | 9.51, s | C-3′,4′,5′/C-5′,6′,7′ | |
| 5′−CH3 | 8.9 | 1.99, s | C-4′,5′,6′ |
Figure 2.
Key 2D NMR correlations of 1–3.
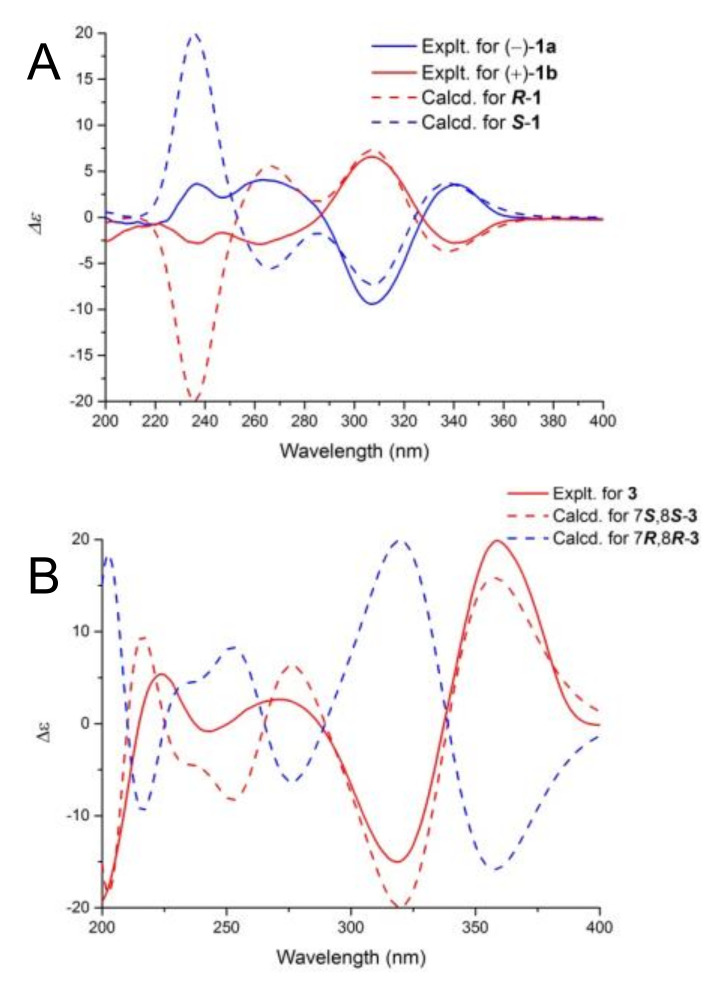
The absence of the Cotton effect in the CD spectrum and zero specific rotation indicated that 1 was a racemate. Generally, enantiomers are more advantageous than racemates for drug development. To detect the enantiomers of 1, chiral HPLC was performed using a Chiralpak IC column; the HPLC results showed two separate peaks (Figure S9). The two enantiomers, (−)-1a and (+)-1b, were obtained in a ratio of 1:1. (−)-1a and (+)-1b showed mirror image-like CD curves (Figure 3) and opposite specific rotations (1a: [α] − 25.3; 1b: [α] + 26.3). The experimental CD spectra of 1a were consistent with the theoretically calculated ECD spectrum of the 1-S enantiomer with four Cotton effects observed at 237 nm (positive), 270 nm (positive), 310 nm (negative), and 348 nm (positive) (Figure 3). In contrast, the CD spectrum of 1b was consistent with the ECD spectrum of the 1-R enantiomer but different from that of 1-S with three negative Cotton effects at 237 nm, 270 nm, and 348 nm, and one positive Cotton effect at 310 nm. Thus, the absolute configurations of 1a and 1b were assigned as (−)-(1S)-myrotheciol and (+)-(1R)-myrotheciol, respectively (Figure 1).
Figure 3.
ECD spectra of compounds 1a and 1b (A), and 3(B).
The molecular formula of 2 was determined as C13H16O5 through HRESIMS at m/z 275.0896 [M + Na]+ (calculated for C13H16O5Na+, 275.0890), which was 30 mass units larger than 1 (Figure S10). The 1H and 13C NMR data of 2 (Table 2, Figures S11–S16) closely resembled those of 1, except for three major differences: the presence of an additional methoxy group (δH 3.09, δC 50.2), the absence of a methine proton (δH 5.09), and the chemical shift of C-1 (from δC 79.1 to 109.5); these differences indicated the substitution of the methine proton at C-1 by a methoxy group. The position of the new methoxy group was confirmed by HMBC correlation from 1-OMe to C-1 (Table 2 and Figure 2). Thus, 2 was established as 1-methoxy-myrotheciol. The structure of 2 was validated through a detailed analysis of 2D NMR data (Table 2 and Figure 2).
Table 2.
1H NMR (600 MHz) and 13C NMR (150 MHz) spectral data of 2.
| No. | δ C | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 109.5 | - | ||
| 2 | 34.6 | 2.13, m | 3 | C-1,3,4,1′ |
| 3 | 24.0 | 1.88, m; 2.01, m | 2,4 | C-1,2,4 |
| 4 | 68.1 | 3.96, m | 3 | C-1,2,3 |
| 1′ | 194.8 | - | ||
| 2′ | 131.7 | - | ||
| 3′,7′ | 107.3 | 7.09, s | C-1′,2′,4′(6′),5′ | |
| 4′,6′ | 155.9 | - | ||
| 5′ | 116.7 | - | ||
| 1−OCH3 | 50.2 | 3.09, s | C-1 | |
| 4′−OH/6′−OH | - | 9.47, s | C-3′,4′,5′/ C-5′,6′,7′ | |
| 5′−CH3 | 8.9 | 1.98, s | C-4′,5′,6′ |
Compound 2 was also considered as a racemic mixture based on the zero specific rotation and absence of the Cotton effect in its CD spectrum. The chiral HPLC performed using the same condition as that used for 1 revealed two peaks, attributable to 2a and 2b, at a ratio of approximately 1:1 (Figure S17). However, due to the limited sample size, further isolation was not carried out.
(+)-HRESIMS at m/z 353.1599 [M + H]+ and 375.1418 [M + Na]+ (calculated for C18H25O7+, 353.1595; C18H24O7Na+, 375.1414) revealed the molecular formula of 3 as C18H24O7 (Figure S18). The 1D- and 2DNMR results revealed the presence of one sp3 oxygenated quaternary carbon, one sp3 oxygenated methine, two sp2 aromatic methines, four sp2 quaternary carbons, one ketone carbonyl carbon, one methoxy group, and one angular methyl group (Table 3 and Figures S19–25). Other than these signals, the 1H-1H COSY correlations from H-2″ to H-4″, along with the HMBC correlations from H-2″ and 3″ to C-1″ (Table 3 and Figure 2) indicated the presence of the butyl ester fragment. The 1H-1H COSY correlations from H-1 to 3-OH corresponded to the hydroxypropyl fragment (Table 3, Figure 2). The NMR data of the core structure of 3 closely resembled those of C-8 dihydro-azaphilone [21,22]. Careful HMBC analysis confirmed this structure (Table 3 and Figure 2). Finally, the key HMBC correlation from H-8 to C-1″connected the butyl ester side chain to C-8, that from H-1 and H-2 to C-3 connected the hydroxypropyl group moiety to C-3, and that from 4-OCH3 to C-4 connected the methoxy group to C-4 (Table 3 and Figure 2). Accordingly, 3 was established as myrothin (Figure 1).
Table 3.
1H NMR (600 MHz) and 13C NMR (150 MHz) data of 3.
| No. | δ C | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 146.9 | 7.66, d (1.2) | C-3,4a,8,8a | |
| 3 | 154.7 | - | ||
| 4 | 138.3 | - | ||
| 4a | 139.6 | - | ||
| 5 | 99.7 | 5.28, d (1.2) | C-4,7,8a | |
| 6 | 196.5 | - | ||
| 7 | 73.29 | - | ||
| 8 | 73.30 | 5.54, s | C-1,4a,6,7,8a,1″ | |
| 8a | 116.5 | - | ||
| 1′ | 24.2 | 2.58, m | 2′ | C-3,4,2′,3′ |
| 2′ | 29.6 | 1.68, m | 1′,3′ | C-3,1′,3′ |
| 3′ | 59.88 | 3.44, m | 2′,3′-OH | C-1′,2′ |
| 3′-OH | - | 4.58, t (5.1) | 3′ | C-2′,3′ |
| 4-OCH3 | 59.94 | 3.62, s | C-4 | |
| 7-CH3 | 23.4 | 1.16, s | C-6,7 | |
| 7-OH | - | 5.07, s | C-6,7,7-CH3 | |
| 1″ | 172.2 | - | ||
| 2″ | 35.4 | 2.26, t (7.2) | 3″ | C-1″,3″,4″ |
| 3″ | 17.9 | 1.49, m | 2″, 4″ | C-1″,2″,4″ |
| 4″ | 13.2 | 0.82, t (7.4) | 3″ | C-2″,3″ |
The relative configuration of 3 at C-7 and C-8 was assigned by nuclear overhauser effect spectroscopy (NOESY) correlations. The strong NOESY correlation between 7-CH3 and H-8 indicated that 7-CH3 and H-8 occupied the same side of the ring (Figure 2 and Figure S25). Thus, the stereo-configurations of C-7 and C-8 are either S,S or R,R. The experimental ECD curve of 3 was consistent with that of the 7S, 8S epimer (Figure 3). The chiral carbons C-7 and C-8 were thus determined as 7S and 8S.
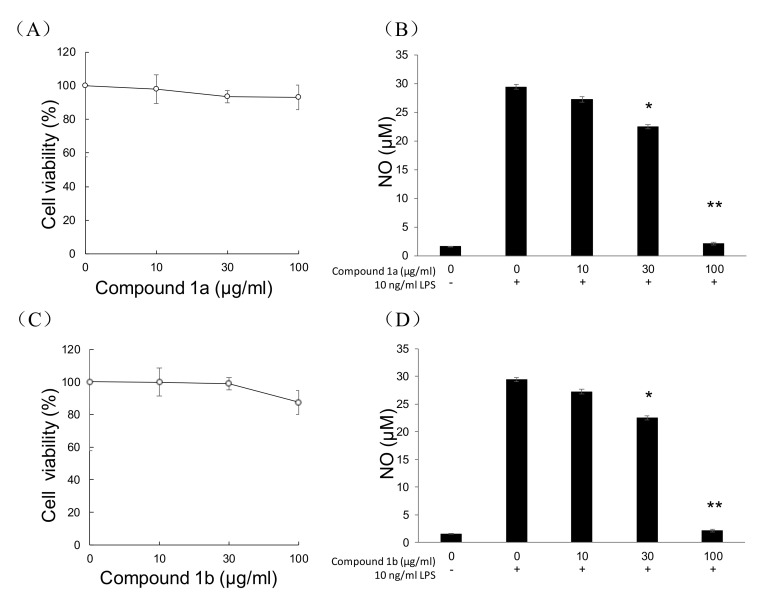
LPS-induced NO production in RAW264.7 cells was used to evaluate the anti-inflammatory activity of different compounds [14]. NO is produced by NF-κB-dependent inducible NO synthase. All the isolated compounds were evaluated for cytotoxicity and for their effects on LPS-induced NO production. Among all the tested compounds, only two new compounds (1a and 1b) significantly inhibited LPS-induced NO production at non-toxic concentrations (Figure 4).
Figure 4.
NO production inhibitory activity of 1a and 1b in RAW264.7 cells. Effect of 1a (A) or 1b (C) on the viability of RAW264.7 cells. Inhibition of LPS-induced NO production by 1a (B) or 1b (D). Values represent the means ± SEM of three independent experiments. *, p < 0.05; **, p < 0.001 vs. control.
Antioxidant activities were measured through 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) scavenging activity, 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging capacity, and the oxygen radical absorbance capacity (ORAC) assay. As shown in Table 4, new compounds 1a and 1b exhibited antioxidant activity in the ABTS assay with EC50 of 1.20 and 1.41 µgmL−1, respectively, which were comparable with EC50 values of the positive controls L-ascorbic acid (1.55 µgmL−1) and trolox (1.61 µgmL−1). In the ORAC assay, the antioxidant ability was expressed as μmol trolox equivalents per μmol of sample solution. Compounds 1a and 1b showed high antioxidant activity (1.41 μM trolox/μM for 1a and 1.19 μM trolox/μM for 1b). Generally, the scavenging activities of ABTS are significantly higher than the scavenging activities of DPPH in phenolic compounds [23]. Compounds 1a and 1b did not show antioxidant activity in the DPPH assay, even at the highest concentration of 10 µgmL−1.
Table 4.
Antioxidant activities of 1a and 1b.
| Compounds | ABTS | ORAC |
|---|---|---|
| EC50, μg/mL | μM Trolox Equivalent/μM | |
| 1a | 1.20 ± 0.18 | 1.41 ± 0.27 |
| 1b | 1.41 ± 0.19 | 1.19 ± 0.19 |
| L-Ascorbic acid | 1.55 ± 0.15 | 0.35 ± 0.14 |
| Trolox | 1.61 ± 0.09 | NA |
In the present research, we isolated several compounds including new structures from a deep-sea fungus. We found cellular anti-inflammatory activity in 1a and 1b. Microorganisms often produce useful compounds for therapy. However, the role of these compounds on producing organisms is not clear. At the beginning of antibiotic research, antibiotics are considered to protect the producing organisms by killing their enemy microorganisms. But later, many enzyme inhibitors such as pepstatin and leupeptin were discovered from the secondary metabolites of Streptomyces, and they showed no antibiotic activity. Therefore, it is unlikely that these secondary metabolites are useful for the producers. From this point of view, new compounds, 1a and 1b, may be remnants of microorganisms in their evolution.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded on an Anton Paar MCP-100 polarimeter (Anton Paar GmbH, Graz, Austria). ECD spectra were measured on a JASCO-810 spectropolarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were obtained on a UV-1800 spectrophotometer (Shimadzu Corporation, Tokyo, Japan). IR spectra were recorded on a Nicolet Avatar 330 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) using KBr disks. NMR spectra were acquired on a Bruker ASCEND 500 MHz or 600 MHz NMR magnet system (Bruker, Ettlingen, Germany) using tetramethylsilane (TMS) as the internal standard. HRESIMS was performed using a Triple TOF 6600 (AB SCIEX LLC, Framingham, MA, USA). Column chromatography (CC) was conducted using silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Thin-layer chromatography (TLC) was performed on Merck TLC plates silica gel 60 F254 and silica gel 60 RP-18 F254S (Merck Millipore Corporation, Darmstadt, Germany). HPLC was carried out on a Shimadzu LC-16P HPLC system (Shimadzu Corporation, Tokyo, Japan) using YMC-pack Pro C18 Column (4.6 × 250 mm, 5 µm; 10 × 250 mm, 5 µm; YMC Co., Ltd., Kyoto, Japan) for analysis and semi-preparation. Optical pure compounds were prepared using a DAICEL Chiralpak IC column (250 mm × 4.6 mm, 5 µm; YMC Co., Ltd., Kyoto, Japan). All the chemical reagents for isolation were either of analytical (Damao Chemical Factory, Tianjin, China) or HPLC grade (Kermel Chemical Co., Ltd., Tianjin, China).
3.2. Fungal Material
The fungus Myrothecium sp. BZO-L062 used in this study was isolated from a deep-sea (2130 m depth) sediment sample collected from an area close to Yongxing Island, China. The strain was identified as Myrothecium sp. based on the morphological features and internal transcribed spacer sequence analysis. This strain was deposited at the Marine Natural Products Laboratory, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen, China.
3.3. Fermentation and Extraction
The fungus Myrothecium sp. BZO-L062 was activated on petri dishes containing potato dextrose agar supplemented with 3% sea salt at 28 °C for three days [24]. Agar plugs were inoculated in a 500 mL Erlenmeyer flask containing 150 mL of liquid potato dextrose culture medium [24] supplemented with 3% sea salt as seed cultures and were incubated at 28 °C on a rotary shaker at 180 rpm for three days. Large-scale fermentation (70 L) was conducted using the same medium as that for seed cultures at 28 °C and 180 rpm for seven days. After seven days, the fermentation broth was filtered through cheesecloth to separate the supernatant from the mycelia. The supernatant was then concentrated to 8 L and successively extracted three times with EtOAc (3 × 8 L), yielding a crude extract (40.0 g).
3.4. Isolation and Purification
The crude extract was separated using silica gel CC through CH2Cl2/MeOH gradient elution (100:0, 100:1, 100:5, 100:10, 100:20, 100:50, and 0:100; 600 mL each) and was grouped into nine fractions (Fr.) based on the TLC analysis (Fr.1 to Fr.9). Fr.3 was purified by semi-preparative HPLC (28% MeCN/H2O, flow rate 3 mLmin⁻1) to yield 4 (tR 16.2 min, 10.1 mg). Fr.4 was subjected to HPLC using a medium-pressure octadecyl-silica (ODS) column and separated with MeOH/H2O (20–100%) into five fractions (Fr.4.1–Fr.4.5). Fr.4.1 was further fractionated by HPLC (5% MeOH/H2O, a flow rate of 3 mLmin⁻1) to obtain 6 (tR 15.0 min, 5.0 mg) and 7 (tR 24.0 min, 5.0 mg). Fr.4.2 was purified by HPLC (25% MeOH/H2O, a flow rate of 3 mLmin⁻1) to obtain 3 (tR 21.0 min, 1.0 mg). Fr.4.3 was refined by HPLC (25% MeCN/H2O, a flow rate of 3 mLmin⁻1) to obtain 2 (tR 20.0 min, 1.4 mg). Finally, Fr.5 was subjected to HPLC (17% MeCN/H2O, flow rate 3 mLmin⁻1) to obtain 1 (tR 20.0 min, 14.2 mg) and 5 (tR 21.2 min, 10.2 mg).
The racemic compound 1 was resolved into enantiomers (−)-1a (3.0 mg, tR 10.2 min) and (+)-1b (3.6 mg, tR 18.1 min) using a chiral HPLC equipped with a DAICEL® Cellulose Chiralpak IC column (5 µm, 4.6 × 250 mm) using n-hexane-ethanol (89:11) as mobile phase at a flow rate of 1 mLmin−1.
3.5. Spectral Data of the Compounds
3.5.1. (±)-Myrothecol (1)
Myrothecol (1) is a colorless oil; [α] 0°(c 0.1, MeOH); UV (MeOH) λmax (log ε) 280 nm (7.18) and 218 nm (7.46); IR (KBr) νmax 3325, 2956, 1678, 1591, 1423, 1325, 1198, 1088, 1040, 934, and 851; HRESIMS m/z 223.0958 [M+H]+, 245.0780 [M+Na]+ (calculated for C12H15O4, 223.0965; C12H14O4Na, 245.0784); for 1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) spectral data, see Table 1. (−)-1a: [α]—25.3° (c 0.3, MeOH); ECD (2.3 mM, MeOH) λmax (Δε) 237 nm (+0.49), 262 nm (+0.54), 307 nm (−1.27), 341 nm (+0.48). (+)-1b: [α] + 26.3° (c 0.27, MeOH); ECD (2.3 mM, MeOH) λmax (Δε) 237 nm (−0.38), 262 nm (−0.39), 307 nm (+0.88), and 341 nm (−0.37).
3.5.2. Methoxy-myrothecol (2)
Methoxy-myrothecol (2) is a colorless oil; [α] 0° (c 0.1, MeCN); UV (MeOH) λmax(log ε) 285 nm (6.99) and 218 nm (7.24); HRESIMS m/z 275.0896 [M+Na]+ (calculated for C13H16O5Na, 275.0890); HRESIMS m/z 353.1599 [M+H]+, 375.1418 [M+Na]+ (calculated for C18H25O7, 353.1595; C18H24O7Na, 375.1414); for ¬1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) spectral data, see Table 2.
3.5.3. Myrothin (3)
Myrothin (3) is a light-yellow colored oil; UV (MeOH) λmax (log ε) 246 nm (3.13) and 350 nm (3.56); IR (KBr) νmax 3405, 2925, 2376, 2316, 1621, 1385, 1036, 910, 790, 731, and 635 cm⁻1; HRESIMS m/z 353.1599 [M+H]+, 375.1418 [M+Na]+, 727.2948 [2M+Na]+ (calculated for C18H25O7, 353.1595; C18H24O7Na, 375.1414; C36H48O14Na, 727.2936); for ¬1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) spectral data, see Table 3. [α] + 15.7°; ECD (2.8 mM, MeOH) λmax (Δε) 224 nm (+0.7), 243 nm (−0.11), 271 nm (+0.34), 319 nm (+1.96), and 359 nm (+2.59).
3.6. ECD Calculation
The conformational distribution search was conducted with the MMFF94 molecular mechanics force field in Spartan 12 software (Wavefunction Inc., Irvine, CA, USA). The lowest energy conformers within the 5-kcalmol−1 energy window were optimized using the Gaussian 09 program [25]. TDDFT calculations for all optimized conformers were performed at the B3LYP/6-31G (d, p) level. The ECD spectra were generated using the software SpecDis [26].
3.7. MTT and NO Production Assay
MTT and NO production inhibitory activities of the isolated compounds in RAW264.7 cells were determined as reported previously [14].
3.8. Antioxidant Activity
The ABTS and DPPH scavenging assays were carried out as reported earlier [23]. L-ascorbic acid and trolox were used as positive controls. The ORAC assay was conducted according to a previously reported protocol [27]. The results were expressed as μmol Trolox equivalents per μmol of sample solution.
4. Conclusions
In this study, four new components, (−)-1S-myrothecol (1a), (+)-1R-myrothecol (1b), methoxy-myrothecol (2), and myrothin (3), along with four known compounds (4–7), were isolated from the deep-sea fungus Myrothecium sp. BZO-L062. The enantiomers 1a and 1b were purified by chiral HPLC. The absolute configurations of 1a, 1b, and 3 were determined by the calculated ECD.
Among these compounds, new compounds 1a and 1b showed anti-inflammatory and antioxidant activities at non-toxic concentrations. Derivatives of these compounds could be potent and safe and may be useful for the development of new anti-inflammatory agents.
Acknowledgments
The authors thank the Instrumentation Analysis Center of Shenzhen University for help in acquiring NMR and MS data.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/12/597/s1, Figure S1: HR-ESI MS spectrum of compound 1, Figure S2–S8: 1D and 2D NMR spectra of compound 1, Figure S9: Chiral separation of racemic 1, Figure S10: HR-ESI MS spectrum of compound 2, Figure S11–16: 1D and 2D NMR spectra of compound 2, Figure S17: Chiral separation of racemic 2, Figure S18: HR-ESI MS spectrum of compound 3, Figure S19–S25: 1D and 2D NMR spectra of compound 3.
Author Contributions
The contributions of the authors are as follows: X.L. (Xiaojie Lu) was involved in performing fermentation, extraction, structure elucidation, and manuscript preparation; J.H. and N.D. were involved in compound isolation and data acquisition; Y.W. contributed to the evaluation of bioactivities; X.L. (Xiaofan Li), J.J., and Z.H. were involved in manuscript revision; K.U. was involved in the evaluation of biological data and manuscript preparation. L.W. was involved in experimental design, manuscript preparation, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Project 2019YFC0312501 and 2018YFA0902504; the Science and Technology Project of Shenzhen City, Shenzhen Bureau of Science, Technology, and Information under Grant JCYJ20180305123659726; and by the Interdisciplinary Innovation Team Project of Shenzhen University. This research was also supported by AMED under Grant No. JP18fk0310118 of Japan.
Conflicts of Interest
K.U. and Y.W. belong to the laboratory supported by Shenzhen Wanhe Pharmaceutical Co., Ltd, Shenzhen, China, Meiji Seika Pharma Co., Ltd, Tokyo, Japan, Fukuyu Medical Corporation, Nisshin, Japan, and Brunaise Co., Ltd, Nagoya, Japan. The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 2.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2018;35:8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 3.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2020;37:175–223. doi: 10.1039/C9NP00069K. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P.P., Deng Y.L., Lin X.J., Chen B., Li J., Liu H.J., Chen S.H., Liu L. Anti-inflammatory mono- and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019;82:947–957. doi: 10.1021/acs.jnatprod.8b01029. [DOI] [PubMed] [Google Scholar]
- 5.El-Kashef D.H., Daletos G., Plenker M., Hartmann R., Proksch P. Polyketides and a dihydroquinolone alkaloid from a marine-derived strain of the fungus Metarhizium marquandii. J. Nat. Prod. 2019;82:2460–2469. doi: 10.1021/acs.jnatprod.9b00125. [DOI] [PubMed] [Google Scholar]
- 6.Yang B.Y., He Y., Lin S., Zhang J.W., Li H.Q., Wang J.P., Hu Z.X., Zhong Y.H. Antimicrobial dolabellanes and atranones from a marine-derived strain of the toxigenic fungus Stachybotrys chartarum. J. Nat. Prod. 2019;82:1923–1929. doi: 10.1021/acs.jnatprod.9b00305. [DOI] [PubMed] [Google Scholar]
- 7.Fang W., Wang J.J., Wang J.F., Shi L.Q., Li K.L., Lin X.P., Min Y., Yang B., Tang L., Liu Y.H., et al. Cytotoxic and antibacterial eremophilane sesquiterpenes from the marine-derived fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018;81:1405–1410. doi: 10.1021/acs.jnatprod.8b00015. [DOI] [PubMed] [Google Scholar]
- 8.Wen H.L., Yang X.L., Liu Q., Li S.J., Li Q., Zang Y., Chen C.M., Wang J.W., Zhu H.C., Zhang Y.H. Structurally diverse meroterpenoids from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 2019;83:99–104. doi: 10.1021/acs.jnatprod.9b00878. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y.H., Geng C., Zhang X.W., Zhu H.J., Shao C.L., Cao F., Wang C.Y. Discovery of bioactive indole-diketopiperazines from the marine-derived fungus Penicillium brasilianum aided by genomic information. Mar. Drugs. 2019;17:514. doi: 10.3390/md17090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo X.W., Lin Y., Lu Y.J., Zhou X.F., Liu Y.H. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin. J. Nat. Med. 2019;17:149–154. doi: 10.1016/S1875-5364(19)30017-2. [DOI] [PubMed] [Google Scholar]
- 11.Chamni S., Sirimangkalakitti N., Chanvorachote P., Saito N., Suwanborirux K. Chemistry of renieramycins. 17. A new generation of renieramycins: Hydroquinone 5-O-monoester analogues of renieramycin M as potential cytotoxic agents against non-small-cell lung cancer cells. J. Nat. Prod. 2017;80:1541–1547. doi: 10.1021/acs.jnatprod.7b00068. [DOI] [PubMed] [Google Scholar]
- 12.Li C.J., Chen P.N., Li H.J., Mahmud T., Wu D.L., Xu J., Lan W.J. Potential antidiabetic fumiquinazoline alkaloids from the marine-derived fungus Scedosporium apiospermum F41-1. J. Nat. Prod. 2020;83:1082–1091. doi: 10.1021/acs.jnatprod.9b01096. [DOI] [PubMed] [Google Scholar]
- 13.Wang J.J., Chen F.M., Liu Y.C., Liu Y.X., Li K.L., Yang X.L., Liu S.W., Zhou X.F., Wang J. Spirostaphylotrichin X from a marine-derived fungus as an anti-influenza agent targeting RNA polymerase PB2. J. Nat. Prod. 2018;81:2722–2730. doi: 10.1021/acs.jnatprod.8b00656. [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Li M., Lin Y., Du S., Liu Z., Ju J., Suzuki H., Sawada M., Umezawa K. Inhibition of cellular inflammatory mediator production and amelioration of learning deficit in flies by deep sea Aspergillus-derived cyclopenin. J. Antibiot. 2020;73:622–629. doi: 10.1038/s41429-020-0302-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X., Fang P., Tang J., Wu Z., Li X., Li S., Wang Y., Liu G., He Z., Gou D., et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016;30:52–57. doi: 10.1080/14786419.2015.1033623. [DOI] [PubMed] [Google Scholar]
- 16.Li X.F., Xia Z.Y., Tang J.Q., Wu J.H., Tong J., Li M.J., Ju J.H., Chen H.R., Wang L.Y. Identification and biological evaluation of secondary metabolites from marine derived fungi-Aspergillus sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents. Molecules. 2017;22:1302. doi: 10.3390/molecules22081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L.Y., Li M.J., Tang J.Q., Li X.F. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules. 2016;21:473. doi: 10.3390/molecules21040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macedo F.C., Porto A.L.M., Marsaioli A.J. Terreinol—A novel metabolite from Aspergillus terreus: Structure and 13C labeling. Tetrahedron Lett. 2004;45:53–55. doi: 10.1016/j.tetlet.2003.10.128. [DOI] [Google Scholar]
- 19.Matsui T., Kudo A., Tokuda S., Matsumoto K., Hosoyama H. Identification of a new natural vasorelaxatant compound, (+)-osbeckic acid, from rutin-free tartary buckwheat extract. J. Agric. Food. Chem. 2010;58:10876–10879. doi: 10.1021/jf1028416. [DOI] [PubMed] [Google Scholar]
- 20.Schmuck C., Machon U. 2-(Guanidiniocarbonyl)furans as a new class of potential anion hosts: Synthesis and first binding studies. Eur. J. Org. Chem. 2006;19:4385–4392. doi: 10.1002/ejoc.200600324. [DOI] [Google Scholar]
- 21.Steyn P.S., Vleggaar R. The structure of dihydrodeoxy-8-epi-austdiol and the absolute configuration of the azaphilones. J. Chem. Soc. Perkin Trans. 1. 1976:204–206. doi: 10.1039/p19760000204. [DOI] [PubMed] [Google Scholar]
- 22.Nukina M., Marumo S. Lunatoic acid A and B, aversion factor and its related metabolite of Cochliobolus lunata. Tetrahedron Lett. 1977;18:2603–2606. doi: 10.1016/S0040-4039(01)83831-4. [DOI] [Google Scholar]
- 23.Bai Y., Chang J., Xu Y., Cheng D., Liu H., Zhao Y., Yu Z. Antioxidant and myocardial preservation activities of natural phytochemicals from mung bean (Vigna radiata L.) seeds. J. Agric. Food. Chem. 2016;64:4648–4655. doi: 10.1021/acs.jafc.6b01538. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Lee J., Kim K.-J., Sung Y., Park K.-H., Oh E., Park C., Son Y.-J., Kang H. Austalides, osteoclast differentiation inhibitors from a marine-derived strain of the fungus Penicillium rudallense. J. Nat. Prod. 2019;82:3083–3088. doi: 10.1021/acs.jnatprod.9b00690. [DOI] [PubMed] [Google Scholar]
- 25.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian 09 Revision D. 01. Gaussian Inc.; Wallingford, CT, USA: 2009. [Google Scholar]
- 26.Bruhn T., Schaumloeffel A., Hemberger Y., Bringmann G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality. 2013;25:243–249. doi: 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
- 27.Wakamatsu J., Stark T.D., Hofmann T. Antioxidative maillard reaction products generated in processed aged garlic extract. J. Agric. Food. Chem. 2019;67:2190–2200. doi: 10.1021/acs.jafc.8b06907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.