Abstract
Despite the high regenerative capacity of bone tissue, there are some cases where bone repair is insufficient for a complete functional and structural recovery after damage. Current surgical techniques utilize natural and synthetic bone grafts for bone healing, as well as collagen sponges loaded with drugs. However, there are certain disadvantages associated with these techniques in clinical usage. To improve the therapeutic efficacy of bone tissue regeneration, a number of drug delivery systems based on biodegradable natural and synthetic polymers were developed and examined in in vitro and in vivo studies. Recent studies have demonstrated that biodegradable polymers play a key role in the development of innovative drug delivery systems and tissue engineered constructs, which improve the treatment and regeneration of damaged bone tissue. In this review, we discuss the most recent advances in the field of polymer-based drug delivery systems for the promotion of bone tissue regeneration and the physical-chemical modifications of polymers for controlled and sustained release of one or more drugs. In addition, special attention is given to recent developments on polymer nano- and microparticle-based drug delivery systems for bone regeneration.
Keywords: polymer, nanoparticle, microparticle, drug delivery system, bone tissue regeneration
1. Introduction
Bone tissue has a high regenerative ability, however, large bone defects produced by cancer, tumor resection, severe mechanical trauma, infectious diseases and congenital disorders do not recover spontaneously and require considerable amounts of bone grafting [1,2]. Insufficient regeneration of damaged bone tissue remains a common problem in orthopedics and traumatology. The standard surgical treatment for such defects is an autogeneic bone graft. Autografts possess major components for achieving osteoinduction, osteogenesis and osteoconduction after transplantation [3]. However, autologous bone grafting has a number of drawbacks related to a high risk of morbidity at the donor site, limited availability, prolonged wound drainage, large hematomas and reoperation [4]. In addition, the quality of the autograft can be highly variable depending on age and metabolic abnormalities of the patient [5]. To avoid these drawbacks and limitations, various bone substitutes such as allografts and xenografts are used in clinical practice [6]. Relative availability and absence of donor site morbidity make these bone substitutes a good alternative to the autograft. However, they have some disadvantages related to high risk of immunorejection and transmission of infections after transplantation, and possessing low osteogenic and osteoinductive properties [4]. Other commonly used bone repair techniques may involve distraction osteogenesis, bone cement fillers, pharmaceutical agents and soluble growth factors [5,6].
In repair and regeneration of damaged bone tissue, osteoinductive and angiogenic growth factors, such as bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) play an essential role [7]. BMPs are key factors in the reconstruction and restoration of damaged bone tissue [8]. BMPs have been shown to have powerful osteoinductive effects and the ability to stimulate the formation of new bone tissue due to the differentiation of mesenchymal stem cells (MSCs) into osteoblasts [9]. Some recombinant BMPs, such as BMP-2 and BMP-7, have been approved by the U.S. FDA and are currently being used in clinical practice to repair non-union fractures [10,11]. However, despite the high efficiency of recombinant BMPs, there are still some problems associated with their clinical use. This is primarily due to the short life of BMPs. Proteins loaded into the lesion site lose their biological activity in a short period of time, and therefore, large doses of recombinant BMPs are used in clinical practice in order to achieve complete bone regeneration [10]. For example, for BMP-2, an effective dose for bone regeneration is 1.5 mg/mL defect, which is 4–5 times higher than the endogenous dose. Such high doses of recombinant BMPs can diffuse from the lesion site and cause side effects, including abnormal bone overgrowth, osteolysis and an immune response [12].
In order to avoid these problems, there is a need to develop delivery systems that can help drugs safely mediate their biological activity for an extended period of time, protect from degradation, and control their slow release to the targeted site. To date, a number of natural and synthetic polymer-based systems have been designed and evaluated for the delivery of drugs for bone repair and regeneration [13,14]. Biodegradable synthetic polymers such as polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), poly(propylene fumarate) (PPF), polycaprolactone (PCL) can easily and readily be fabricated into desired shapes with relatively high mechanical strength, but they are not fully appropriate for the generation of bioactive and biocompatible tissues [15,16,17]. Natural polymers, such as collagen, gelatin, hyaluronic acid, fibrin, alginate and chitosan, are excellent scaffolds for cell growth and adhesion, but their weak mechanical properties render them susceptible to compression when transplanted into the defective area [18]. In this regard, approaches including extensive purification, crosslinking, or blending with synthetic polymers, have been used to improve the properties of natural polymers [19,20]. In this review, we discuss polymeric drug delivery systems of natural and synthetic origins for bone tissue regeneration. We also discuss the types of delivery systems and controlled release of growth factors and the potential use of polymer nanoparticle- and microparticle-based drug delivery systems in bone regeneration.
2. Types of Drug Delivery Systems
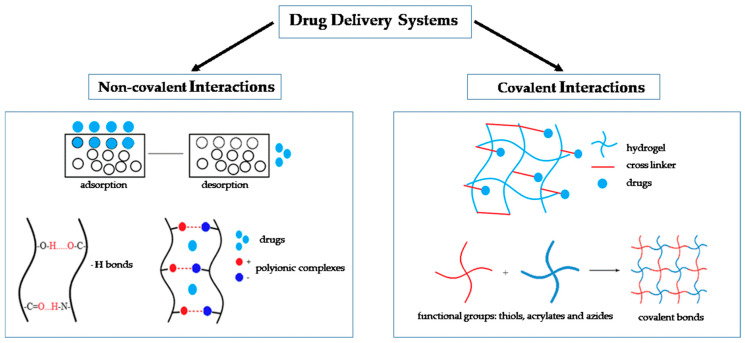
There are several approaches to classifying drug delivery systems (DDS). One approach involves dividing DDS into two main groups: uncontrolled and controlled. The first is characterized by the fact that the release rate of substances depends on the physical and chemical properties of the materials of the carrier [21]. For example, the release of substances from conventional polymer particles is usually controlled by a diffuse mechanism and degradation of the matrix. In controlled systems, the release of the substance can be regulated by interactions between the material of the carrier and its environment (pH, temperature, light intensity, etc.) Moreover, when changing the parameters of the structure of the carrier, it is possible to create a controlled delivery system of substances from an uncontrolled system [22,23,24,25]. The most common classification of DDS is their division based on the method of interaction of a substance with a carrier-scaffold (Figure 1). There are two main types of binding: non-covalent and covalent [26].
Figure 1.
Types of drug delivery systems based on the method of interaction of a substance with a carrier-scaffold. Non-covalent interactions can be created through adsorption, H-bonds or polyionic complexes. Covalent interactions are the result of the formation of covalent bonds (between functional groups).
2.1. Non-Covalent Interactions
Non-covalent interactions of substances with a scaffold are often used to place biologically active substances and drugs on the carrier. Non-covalent interactions can be realized through passive adsorption of the substance on the surface of the scaffold, physical capture of the molecules of the substance in the scaffold-carrier through the formation of hydrogen bonds or ionic and hydrophobic interactions due to Van der Waals forces [27]. Adsorption of substances on the surface of the scaffolds is considered one of the most available methods for fabrication of DDS. For example, INFUSE® and InductOS® bone grafts are a simple mixture of recombinant human BMP-2 (rhBMP-2) and bovine Type I collagen, where rhBMP-2 is adsorbed onto the collagen matrix. A release of the substance loaded with adsorption method is mediated by desorption and biodegradation of the scaffold. The release kinetics of a drug has a high degree of dependence on properties of the carrier material and drug substance [28]. The main disadvantages of these DDS are insufficient control of the release kinetics and a low level of efficiency of their loading. Such delivery systems are characterized by explosive release, followed by the release of drugs, mediated by the process of biodegradation of the scaffold. The method for physical encapsulation or immobilization of drug substances in a scaffold is to mix biologically active substances and drugs with polymers prior to their gelation or hardening [29,30,31]. To create such scaffolds, natural and synthetic polymers such as collagen, fibrin, chitosan and polylactide are used. The main advantage of physical immobilization is that during the incorporation of the substance, no harsh chemicals are used in the scaffold, which reduces the risk of loss of activity of growth factors or drugs.
Ionic complexation is one option for non-covalent immobilization of substances in DDS. It is based on the creation of polyionic complexes from molecules of substances and functionalizing scaffolds, with different isoelectric points of the charged structure-forming molecules of the scaffold material. One example of this type of transport is the use of polyelectrolyte multilayer films, for example, as a carrier for recombinant BMP-2. The use of polyelectrolyte multilayer films with BMP-2 significantly increases the rate of bone healing [32]. However, in some cases, the formation of ionic complexes may be accompanied by a decrease in biological activity of the immobilized substance. A special case of non-covalent binding is affinity binding [27]. A number of polymers, such as fibrin, have specific biological sites with a high degree of affinity for certain regions of growth factors. Typically, DDS based on affinity binding have a more stable release profile compared to DDS with non-covalent binding. This activation method is physiological and today is positioned as one of the most suitable method for delivery of biologically active substances. For example, BMP-2 binds to heparin-conjugated fibrin and controlled delivery from fibrin hydrogels enhances their effect on formation and vascularization of a new bone [33].
2.2. Covalent Interactions
Covalent immobilization is a chemical interaction of biologically active substances with a matrix with the formation of covalent bonds (Figure 1). As a rule, for the implementation of this approach for the delivery of biologically active substances and drugs, it is important that the molecules contain reactive functional groups such as thiols, acrylates and azides [34,35]. Covalent binding of the substance with scaffold is not widely used, however this method of immobilization is required if the molecules of the loaded drug are not capable of non-covalent interaction with the material scaffold or non-covalent loading efficiency is extremely low [36]. It is known that this method can provide sustained drug release. Drug release kinetics in this case will be mediated by hydrolysis or cell-mediated enzymatic cleavage [37]. One positive property of covalent immobilization is control over the amount and the distribution of substances in the composition of the scaffold [14]. However, this approach has disadvantages associated with the complexity and the high cost of its preparation. Moreover, covalent immobilization can lead to conformational disturbances of drugs, which can significantly affect their biological activity [35]. Thus, a variety of DDS gives researchers a wide choice of approaches and methods for loading drug substances into scaffolds. At the same time, each method has its advantages and disadvantages. The development of improved approaches for drug delivery is a leading direction in the research and development of DDS. One possible option for optimizations is combining methods for loading substances into the carrier.
3. Biodegradable Polymers for Drug Delivery
To date, there are various biodegradable polymers being studied as DDS for bone tissue regeneration [14]. Biodegradable polymers are mostly applied where the transient existence of materials is required and they find applications as scaffolds for tissue regeneration, tissue adhesives and transient barriers for tissue adhesion, as well as DDS. Each of these applications demands materials with unique physical, chemical, biological and biomechanical properties to achieve efficient therapy. The polymers used for bone regeneration should be biodegradable, biocompatible, non-immunogenic and non-toxic to normal bone healing events. Biodegradable polymer materials fall into three general types. These are (1) natural polymers, of which collagen is the most widely used, (2) synthetic polymers, of which the poly(α-hydroxy acids) are most commonly used, and 3) a combination of these. Each type of polymer material has its advantages and drawbacks, which are summarized in Table 1.
Table 1.
Advantages and limitations of polymer-based drug delivery systems for bone tissue regeneration based on a polymer type.
| Source | Polymer | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Natural | Collagen | The most abundant protein, capable of altering its mechanical properties by crosslinking, safety, biocompatibility | Strong burst release, causing inflammation, swelling, ectopic bone formation and osteolysis | Busra and Lokanathan, 2019 Sheikh et al., 2015 Zara et al., 2011 |
| Chitosan | Biocompatibility, biodegradability, antibacterial and wound healing activity, bioadhesion, presence of functional groups on the surface, enabling physical and chemical functionalization | Requirement for additional modifications for controlled and sustained release of osteopromotive drugs | Levengood and Zhang, 2014 Saravanan et al., 2016 Venkatesan et al., 2017 |
|
| Hyaluronic acid | Good biocompatibility, biodegradability, high viscoelasticity, hydrophilicity, non-immunogenicity, capability to modification, low burst release, sustained drug release | Low mechanical and physical properties, fast degradation rate, complicated crosslinking method | Hemshekhar et al., 2016 Zhao et al., 2016, Kim et al., 2019 |
|
| Alginate | Good biodegradability, biocompatibility, non-immunogenicity, capability of modification, significant promotion of the proliferation and osteogenic differentiation | Poor cell attachment ability, limited availability of cellular adhesion sites | Ray et al., 2020 Su et al., 2013, Purohit et al., 2020 |
|
| Fibrin | Adhesion, hemostasis, sealant capability, presence of multiple binding sites | Requirement for additional modification | Bujoli et al., 2019 Noori et al., 2017 Khodakaram-Tafti et al., 2017 Martino and Hubbell, 2010 Censi et al., 2012 |
|
| Synthetic | Poly (ε-caprolactone) | Elasticity, biocompatibility, bioabsorbtion and biodegradability | Limited polymer-cell interaction due to its hydrophobicity and mechanical properties, used as one of the scaffold components | Narayanan et al., 2016 Ghassemi et al., 2018 Miroshnichenko et al., 2019 Aldemir Dikici et al., 2019 Pazarçeviren et al., 2020 Wang et al., 2017 |
| Poly(lactic-co-glycolic acid) | Biocompatibility, biodegradability, increased adhesion and cell viability and proliferation, sustained release of GF, induced osteogenic differentiation, quick integration with surrounding tissues | Rapid biodegradation | Turnbull et al., 2018 Bharadwaz and Jayasuriya, 2020 Cao et al., 2020 Zhang et al., 2019 Deng et al., 2019 Das et al., 2016 Kim et al., 2019 Jakus et al., 2016 Generali et al., 2017 |
|
| Polyethylene glycol | Hydrophilicity, biocompatibility, non-immunogenicity, biodegradability | Absence of functional groups on the surface, used as one of the composite components | Saroia et al., 2018 Wang et al., 2019 Kutikov and Song, 2015 Eğri and Eczacıoğlu, 2017 |
3.1. Natural Polymers for Drug Delivery
Natural polymers have good biocompatibility and osteoinductive properties. In addition, they are safe, used in low concentrations and are capable of chemical modification and hydrogel formation. When used as grafts, these polymers are readily rearranged by the cells of the recipient. Moreover, the fibrous properties of polymers allow one to regulate the structure and porosity during the manufacture of scaffolds [18].
3.1.1. Collagen
Collagen is the most abundant protein in various tissues, including bone. Collagen scaffolds are very attractive for the creation of bioengineering structures since its mechanical properties can be changed by crosslinking with various chemical agents (glutaraldehyde, formaldehyde, etc.), or by their physical treatment (UV irradiation, heating, etc.) [38]. Collagen-based scaffold for drug delivery has frequently been used as a template to induce bone regeneration due to its safety and good biocompatibility. For the first time, bone regeneration was induced in femoral defects of rat and sheep and mandibular defects in dogs by BMPs in a collagen carrier in the early 1990s [39]. Several studies showed that recombinant human BMP-2, -4, -7 and -9 delivered with absorbable collagen sponge (ACS) effectively induced bone formation in a critically sized rat calvarial defect [40,41]. Currently, rhBMP-2 and rhBMP-7 absorbed on a collagen sponge are approved by the FDA for spinal fusion, tibial non-unions and oral-maxillofacial reconstructions [42]. However, clinical studies revealed a number of adverse side effects after transplantation of ACS loaded with high supraphysiological doses of rhBMP-2 or rhBMP-7 [43]. The strong burst release of high doses of BMPs from the collagen sponge in the first two days caused inflammation, swelling, ectopic bone formation and osteolysis in patients. In order to reduce these adverse events, differently modified collagen-based polymers for long-term delivery of rhBMP-2 were developed and evaluated, including an injectable collagen matrix, vitrified type I collagen membrane, apatite-coated collagen sponge and surface-treated collagen sponge [44]. For example, to enhance BMP-2 binding to collagen scaffolds, a porous collagen scaffold was chemically modified using Traut’s reagent and Sulfo-SMCC [45]. The modified collagen scaffold demonstrated a slower release of BMP-2 and preserved its biological activity. In addition, an in vivo study showed that subcutaneous implants of cross-linked collagen with immobilized BMP-2 led to more ectopic bone formation in experimental animals.
Another study demonstrated that the controlled and sustained release of rhBMP-2 from collagen hydrogel can be regulated by blood plasma fibronectin [46]. Collagen hydrogel with fibronectin slowly released rhBMP-2 in the first 3 days which avoided adverse effects in clinics caused by the rapid release of large concentrations of rhBMP-2 from ACS. Recently, Lui and colleagues designed a mineralized collagen delivery system for BMP-2 and VEGF for promotion of bone formation and vascularization in mandibular defects. A mineralized collagen scaffold was prepared through self-assembling hydroxyapatite crystals that grew within the collagen fibrils to form triple collagen self-assembled helices. They showed that BMP-2 released from the mineralized collagen scaffold enhanced new mandibular bone formation. However, the combination of BMP-2 and VEGF exerted a synergistic effect and thereby significantly increased mandibular bone regeneration. It was suggested that the use of mineralized collagen delivery system for growth factors could provide a new therapeutic strategy to generate large-size vascularized bone grafts [47]. Despite the widespread use of ACS loaded with rhBMP-2 in clinical applications, recently it was found that rhBMP-9 is a more osteopromotive growth factor among the 14 BMPs [48]. It was revealed that ACS loaded with rhBMP-9, even at low concentrations of 10 ng/mL, was able to significantly induce over a two-fold increase in osteoblast differentiation and mineralization potential when compared to rhBMP-2 at a high concentration of 100 ng/mL. An in vivo study demonstrated that as little as 1 μg of rhBMP-9 loaded on ACS could promote new bone formation in a rat critically sized bone defect [41].
3.1.2. Chitosan
Chitosan is a hydrophilic and positively charged linear polysaccharide prepared by alkaline deacetylation of crustacean chitin. The surface of chitosan is rich in amino functional groups that favor chemical or physical interaction with the nanoparticles, drugs, polymers and cells [49,50]. Despite a weak mechanical feature and a fast degradation rate, chitosan has many beneficial properties, such as its biocompatible, biodegradable, antibacterial, wound healing and bioadhesive properties that make it an excellent carrier for the safe and effective delivery of drugs and osteoprogenitor cells to sites for bone tissue regeneration [51,52].
Multiple studies demonstrated that chitosan scaffolds have a high potential as DDS to improve regeneration of damaged bone tissue [53,54]. However, to provide controlled and sustained release of osteopromotive drugs, certain chemical modifications in chitosan were proposed [51]. It was shown that photo-cross-linkable chitosan-lactide hydrogel with immobilized BMP-2 significantly enhanced osteoblast differentiation and mineralization of preosteoblast mouse bone marrow stromal cells and mouse myoblast cells due to the sustained release of BMP-2 from hydrogel [55]. In addition, the sustained release of BMP-2 from photo-cross-linkable chitosan-lactide hydrogel significantly enhanced mineralization in the cells. In another study, Kim and colleagues showed that photo-cross-linkable chitosan-lactide-fibrinogen hydrogels containing BMP-2 induced neo-osteogenesis and accelerated healing of the bone defects in a dose-dependent manner [56]. Several research groups developed a thiolated chitosan scaffold for effective BMP-2 delivery and bone formation [57,58]. For example, Bae and colleagues showed that thiolated chitosan was able to sustain delivery of BMP-2 and enhance osteoblastic differentiation of preosteoblast cells. Moreover, transplantation of thiolated chitosan for local delivery of BMP-2 induced more ectopic bone formation to a greater extent (1.8 fold) in comparison to collagen gel [57]. Heparinized chitosan scaffolds were also examined as potential carriers for BMP-2 delivery. It was shown that a heparinized chitosan-BMP-2 scaffold increased alkaline phosphatase (ALP) activity, calcium deposition and gene expression of osteocalcin and osteopontin in osteoblast cells compared to heparinized chitosan alone [59]. In another study, Kim and colleagues developed a novel heparinized methacrylated glycol chitosan (Hep-MeGC) carrier for demineralized bone matrix that can stabilize and enhance BMP-2 activity for increased osteoinduction [60]. Moreover, they demonstrated that Hep-MeGC hydrogels can effectively deliver bone marrow stromal cells, enhance endogenous activity of BMP-2 by sequestering and localizing the cell-produced BMPs and exert protective effects on bioactivity of BMP-2 against physiological stressors related to bone fracture healing.
3.1.3. Hyaluronic Acid
Hyaluronic acid (HA) is a simple linear mucopolysaccharide containing repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid [61]. HA has good biocompatibility, biodegradability, high viscoelasticity, hydrophilicity, non-immunogenicity, and can be easily modified and synthesized for bone tissue engineering applications [62,63]. HA is currently being used as a vehicle for drug delivery. Usually for controlled and sustained delivery of drugs or osteoinductive growth factors, chemically modified HA scaffolds are used. For example, Bhakta and colleagues demonstrated that in comparison to a collagen sponge, thiol-modified HA (Glycosil™) hydrogel exhibits a low burst followed by a sustained release of BMP-2 and leads to ectopic bone formation in experimental animals [64]. In another study, Kisiel and colleagues determined that incorporation of fibronectin (FN) fragment containing the major integrin-binding domain of full-length FN into HA hydrogel can improve attachment of MSCs and enhance the osteogenic potential of rhBMP-2 [65]. They also showed that delivery of rhBMP-2 with HA hydrogel containing FN resulted in significant ectopic bone formation with more homogeneous and organized collagen fibers compared to non-functionalized HA hydrogel with loaded rhBMP-2. Furthermore, 2-aminoethyl methacrylate conjugated HA hydrogel containing the growth and differentiation factor 5 and simvastatin was used for the stimulation of osteogenesis and repair of damaged parietal bone in rabbits [66,67]. An in vitro study demonstrated that hydrogel promoted osteogenic differentiation of adipose-derived stromal cells and increased expression levels of osteocalcin and osteopontin. However, an in vivo study showed that bone formation after implantation of methacrylate conjugated HA hydrogel was only slightly higher in comparison to control HA hydrogel.
3.1.4. Alginate
Alginate is a linear polysaccharide extracted from red and brown seaweeds and consists of 1,4-linked β-D mannuronic acid (M) and α-L-glucoronic acid (G) units. It is similar to chitin and chitosans in its chemical structure. In the presence of bivalent cations such as Mg2+, Ca2+, Ba2+ and Sr2+, these polysaccharides are exposed to ionotropic gel formation Alginate hydrogels have a good biodegradability, biocompatibility, non-immunogenicity and susceptibility for chemical modifications that make them suitable biopolymers for many biomedical as well as pharmaceutical applications [68]. Alginate is easily chemically modified due to a number of free hydroxyl (OH) and carboxyl (COOH) groups along its polysaccharidic structural backbone. To improve its properties, alginate hydrogel can be chemically modified by acetylation, phosphorylation, sulfation and esterification [69,70,71]. Nano-composite polymeric scaffolds (PLGA-PCL-nanoHA) containing sodium alginate hydrogel as the carrier for dual delivery of BMP-2 and basic FGF showed good sustained release ability, leading to significant promotion of the proliferation and osteogenic differentiation of MSCs [72]. In addition, an in vivo study revealed that the delivery of both growth factors can markedly enhance new bone formation and vascularization in large bone defect regeneration in rabbit mandibles compared with delivery of BMP-2 or basic FGF alone.
In another study, Choi and colleagues fabricated core-shell microcapsules using poly(L-lactide-co-glycolide) (PLGA) and sodium alginate for dual delivery BMP-2 and dexamethasone (Dex) [73]. They observed an initial burst of Dex over 5 days, followed by a sustained release during 30 days, while the release kinetics of BMP-2 was more sustained. Moreover, it was found that released BMP-2 and Dex enhanced gene expression of ALP, osteocalcin and osteopontin in rat bone marrow stromal cells encapsulated with core-shell microcapsules into alginate hydrogels. The authors of the study suggested that dual controlled delivery of BMP-2 and Dex using core-shell microcapsules in alginate hydrogel can maintain the osteogenic potential of stromal stem cells. Kanczler and colleagues also demonstrated that alginate scaffold containing human bone marrow-derived MSCs, VEGF and BMP-2 was effective for regeneration of critically-sized femur defects in mice through the controlled and sustained release of growth factors [74]. Alginate hydrogel has also been applied as beads for multiple delivery of growth factors, for example for simultaneous delivery of BMP-2 and platelet rich plasma (PRP), which contains a number of growth factors such as FGF-2, platelet derived growth factor, tumor growth factor-β (TGF-β), IGF and VEGF. Fernandes and colleagues demonstrated that dual controlled release of PRP and BMP-2 from alginate beads significantly increased in vitro proliferation, osteogenic differentiation and mineralization activity of MSCs [75].
3.1.5. Fibrin
Fibrin is a natural biopolymer endowed with adhesive, sealant and hemostatic properties, and is suitable for cell adhesion and tissue stability [76]. Fibrin is widely used as scaffolds and DDS, enabling the precise application of biomaterials, cells, growth factors and nanoparticles [77]. Traditional commercial fibrin hydrogels have been produced from thrombin and fibrinogen of human plasma in the presence of calcium ions [78]. Fibrin has multiple binding sites for cells, growth factors and extracellular matrix (ECM) components. Thus, fibrin can provide main growth factors required for bone regeneration including BMP-2, TGF-β, FGF, VEGF, PDGF and IGF [79]. However, in other cases, the covalent binding of these growth factors to fibrinogen is considered. It generally requires certain chemical modifications with reactive functional groups allowing a stable association [37]. For example, to fabricate an injectable fibrin hydrogel for sustained delivery of BMP-2, Yang and colleagues synthesized heparin-conjugated fibrinogen with a carbodiimide chemistry method to keep BMP-2 for a long duration of time [33]. They showed that BMP-2-loaded heparin-conjugated fibrin (HCF) hydrogel controlled and sustained release of BMP-2 for 13 days in comparison to fibrin hydrogel. BMP-2 released from HCF significantly increased ALP activity in cultured osteoblasts and significantly enhanced ectopic bone formation in rats. Moreover, a comparative study demonstrated that BMP-2-loaded HCF hydrogel is more effective for stimulation of bone regeneration than BMP-2-loaded collagen sponge [80].
Recently, Sanchez-Casanova and colleagues developed a novel technological platform for spatiotemporal control of BMP-2 production using a heat-activated and dimerizer-dependent transgene expression system that was incorporated into MSCs [81]. They encapsulated genetically engineered MSCs in hydrogels based on fibrin and plasmonic gold nanoparticles that transduced incident energy of an NIR laser into heat. In the presence of a dimerizer, photoinduced mild hyperthermia induced the release of bioactive BMP-2 from NIR responsive cell constructs. An in vivo study showed that induction of NIR-responsive cell constructs conditionally expressing BMP-2 in a critically sized bone defect bone defect resulted in the formation of new mineralized tissue in mice.
3.2. Synthetic Polymers for Drug Delivery
In tissue engineering and drug delivery, synthetic polymers are used as carriers for peptides, drugs, genes, proteins, etc. Synthetic polymers are biocompatible and decompose into non-toxic products [82]. The most commonly used synthetic polymers in tissue engineering are poly (lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), poly (ε-caprolactone) (PCL) and polyethylene glycol (PEG) [83].
3.2.1. Poly (ε-caprolactone)
Poly (ε-caprolactone) (PCL)—elastic, biocompatible, bio absorbable and biodegradable linear aliphatic polyester, has good environmental stability, is easy to process and is used in the development of biomaterials. PCL is a semicrystalline polyester, with a melting temperature around 55–60 °C and a Tg of 60 °C, synthesized by the ring-opening polymerization of ε-caprolactone. PCL is soluble in a wide range of organic solvents and is easily processed into different structures [84,85]. However, as known, pure PCL polymer is not suitable for use in bone regeneration due to its mechanical properties and degradation. In addition, PCL, like many synthetic polymers, is hydrophobic, which limits its polymer–cell interaction [86,87]. Generally, PCL is used as one of the material components [88,89], so minerals such as hydroxyapatite (HA), tetracalcium phosphate (TCP), etc. [90,91,92] are added to the composites to improve the mechanical strength of PCL scaffolds. Xiaogang Bao and colleagues obtained a “functional artificial bone” based on a 3D printing scaffold from HA and PCL. Implanted scaffolds in rabbit tibia defect (1.2 cm) showed better defect repair results in 12 weeks, similar to autogenous bone graft, compared to the original gel/scaffold. The cytokine-loaded hydrogel has good biodegradability and can stably release VEGF-165/BMP-2 [93]. Yao Q.Q. and colleagues succeeded in synthesizing microspheres-aggregated 3D PCL scaffolds of various structures (macro/micro/nano). The scaffold provided a significantly slow release of the low molecular weight drug (phenamil) and BMP-2 [94]. By obtaining bilayer scaffolds by sequential electrospinning of a PCL solution containing a decellularized (dECM), Junka R. and colleagues demonstrated that the incorporation of dECM into polymer fibers limits the access of phenotypes that disrupt bone formation during the healing process [95]. In another study, Yao Q. and colleagues fabricated 3D electrospun PCL/PLA blend (mass ratio: 4/1) nanofibrous scaffolds with high porosity (~96%). PCL/PLA scaffolds improved osteogenic differentiation of human MSCs, showed homogeneous scaffold cellularity and increased cell viability, osteogenic gene expression and apatite-like deposition in vitro. The results of new bone formation in a model of a skull bone defect in mice of critical size in vivo showed that with the same amount of rhBMP2 (0.75 μg) added per scaffold group, the greatest bone formation was observed in the PCL/PLA-rhBMP2 group (4.56%) than in the PCL-rhBMP2 group (0.99%) [96].
3.2.2. Poly(lactic-co-glycolic acid)
Poly(lactic-co-glycolic acid) (PLGA) is also a biocompatible synthetic copolymer of poly-L-lactic acid (PLLA) and polyglycolic acid (PGA), which is used in medical applications, therapeutic tools and DDS. PLGA is synthesized by ring-opening copolymerization of two different monomers of glycolic acid and lactic acid [97,98,99]. Cao S.S. and colleagues used two relatively distinct 3D printing (3DP) technologies to produce 3D poly(lactic-co-glycolic acid)/β-tricalcium phosphate (3D-PLGA/TCP) and 3D beta-tricalcium phosphate (3D-TCP) scaffolds. Administration of TCP increased the adhesion and proliferation of human dental pulp stem cells (hDPSC) [100]. Another group of scientists used a cryogenic 3D printing method to develop a bioactive scaffold of PLGA/β-TCP, in which graphene oxide (GO) and BMP-2 were loaded in situ (PTG/P). The pore size averaged 400 ± 50 μm. Rat MSCs were cultured directly on a scaffold. In an in vivo study, the authors successfully corrected the rat cranial bone defect [101]. Deng and colleagues using a synthesized scaffold based on 3D-printed PLGA/HA/chitosan/rhBMP-2, were able to successfully repair a bone defect in vivo in the mandible of a rabbit model. In an in vitro experiment, a gradual release of rhBMP-2 from scaffold was observed [102]. There are also known studies using bone morphogenetic proteins BMP-6 and BMP-7, which, when combined with scaffold, can promote bone regeneration in vivo [103,104]. Jakus, A.E. and others were able to 3D print hyperelastic “bone” based on 90 wt% hydroxyapatite and 10 wt% polycaprolactone or poly(lactic-co-glycolic acid). In in vitro studies with human MSCs derived from bone marrow, the scaffolds showed good cell viability and proliferation and induced osteogenic differentiation after four weeks without any osteo-inducing factors in the medium. The authors conducted in vivo studies on a mouse subcutaneous implant model for biocompatibility (7 and 35 days), on a posterolateral fusion in rats for new bone formation (8 weeks) and in a non-human primate calvarial defect case study (4 weeks). Thus, they demonstrated the efficacy of hyperelastic “bone”, which did not induce a negative immune response, became vascularized, quickly integrated with surrounding tissues, quickly ossified and supported the growth of new bone without using the biological factors [105].
3.2.3. Poly(lactic acid)
Poly (lactic acid) (PLA) is a bioresorbable material and exists in three forms—D-PLA, PDLA and L-PLA (PLLA). The polymerization of these monomers leads to the formation of semicrystalline polymers and the degree of crystallinity depends on the molecular weight and polymer processing parameters. This polymer can be degraded into nontoxic components with a controllable in-vivo degradation rate and has a long history of use in DDS and tissue engineering scaffolds [106,107]. PLA-based biopolymers are often synthesized in various modifications [98,108,109,110]. Zhang H. and colleagues obtained a PLA/HA 3D printing scaffold that was cultured with bone marrow stromal cells embedded in the vascular bundle and surrounded by a periosteal capsule and examined after four and eight weeks. PLA/HA scaffold had better osteogenic capacity and osteoinductive activity, and created an environment for bone development [111]. Mohammadi M. and colleagues synthesized electrospun PLA nanofibers to stimulate osteogenic differentiation of MSCs. The resulting scaffolds provided a sustained release profile of BMP-2 peptide up to day 21, while maintaining cell attachment and proliferation without cytotoxicity [112]. D. Ma and colleagues used PLA/PLGA polymeric particles as vehicles for rhBMP-2 transport. Particles of PLA/PLGA suppressed the pulsed release characteristics of BMP-2 [113]. Other researchers have synthesized hierarchical macroporous biocompatible (HmPB) scaffolds based on PLLA and PCL, which are readily fabricated by solvent evaporation of 3D printed Pickering HIPEs stabilized by h-SiO2 nanoparticles. In vitro studies show that this HmPB is a suitable drug carrier. Loaded 1.2 wt.% enrofloxacin released almost 80% in the first 2.5 h, and the release rate of all samples reached more than 98% after 10 h. The resulting scaffold is structurally similar to the natural ECM, thereby promoting good biocompatibility and cell adhesion of mouse bone marrow-derived MSCs [114]. In another study, Ren and colleagues synthesized nano-fibrous meshes of poly (L-lactide) (PLLA) and gelatin (1:1 in weight ratio). The study showed that the use of nano-fibrous meshes promoted osteogenic differentiation of bone marrow-derived MSCs compared to the control. The resulting composites were implanted into rat skull defects. As shown by the results in comparison with the control group, 12 weeks after surgery in the groups filled with composite, there was a significant formation of new calcified bone [115].
3.2.4. Polyethylene Glycol
Polyethylene glycol (PEG) is a hydrophilic non-ionic polymer, non-toxic and non-immunogenic, soluble in aqueous solutions and organic solvents [116,117,118]. PEG is suitable for making composites with other polymers. So to improve its biocompatibility and degradation rate, Sinan Eğri and colleagues fabricated scaffold PLA-PEG-PLA terpolymers. The release of BMP-2 from the synthesized scaffold was much slower (by several months) than that of VEGF (2 days after insertion). The addition of BMP-2 and VEGF to scaffold PLA-PEG-PLA led to an increase in cell adhesion and proliferation [119]. Lin Dan and colleagues performed work on the reconstruction of bone-cartilage defects using PEGylated poly (glycerol sebacate) (PEGS) with a macro-/microporous surface. PEGS served as one of the layers of a two-layer scaffold PEGS/mesoporous bioactive glass (MBG). As shown by in vivo results for 12 weeks, the two-layer scaffold viscoelastic PEGS/MBG successfully reconstructed cartilage and subchondral bone, demonstrating exceptional regenerative efficiency [120]. In another study, Yang Y. and colleagues fabricated a novel class of bone-targeting anabolic compound based on PTH-PEG-BP (parathyroid hormone- polyethylene glycol-bisphosphonate) for the treatment of osteoporosis and related bone disorders. According to the authors, in vitro and in vivo studies showed that PTH-PEG-BP conjugates had significant enhanced affinity for bone mineral and improved the trabecular bone volume and structural strength [121].
4. Polymer Nanoparticle- and Microparticle-Based Drug Delivery Systems for Bone Regeneration
There are four key aspects that the bone tissue engineering field is aiming for in providing a successful bone regeneration at the defect site with minimal complications: (1) osteogenic cells that generate bone tissue matrix; (2) a biocompatible framework (carrier) that mimics the natural ECM of the bone; (3) vascularization; (4) morphogenetic signals that direct the cells [122]. The general idea behind bone tissue engineering is that eventually the bioactive framework should be resorbed and replaced by the newly regenerated tissue of the body. Therefore, the biomaterial should be both osteoinductive and osteoconductive, as well as capable of integrating into surrounding bone [98]. Additionally, the biomaterial should be feasible in preparation and sterilization and possess stable mechanical and chemical properties in the host environment and non-thrombogenic activity [122]. Polymer nanoparticles are a promising class of biomaterials that meet these criteria. Nanoparticle-based DDS have been attracting considerable attention in biomedical and pharmaceutical sciences over the last two decades due to their unique physicochemical properties, including their ultra small size, high reactivity and large surface area to mass ratio, which can offer significant benefits compared to traditional therapeutic and diagnostic agents [123]. They have been successfully applied as drug carriers [124,125], diagnostic tools [126,127], labelling and tracking agents [128,129]. Among various types of nanoparticles, polymer nanoparticles are of particular interest for drug delivery as they exhibit certain advantages over their counterparts, which include controllable size and size distribution, longer clearance time, lower toxicity, higher drug loading capacity and good biocompatibility [130].
Chitosan is one of the most abundant natural amino polysaccharides. Chitosan-based nanoparticle DDS are very promising in biomedical fields and tissue engineering, due to their non-toxic, biocompatible, biodegradable, non-carcinogenic and antibacterial properties [131,132]. However, chitosan exhibits low mechanical characteristics and non-osteogenic inductivity, hence, has limited functions in bone tissue engineering [131,133]. To overcome this limitation, hybridized nanocomposite formulations of chitosan and other natural or inorganic components are often used for adequate application in tissue engineering. Nanocomposite formulations in tissue engineering are aiming to combine the mechanical properties of inorganic moiety and improved degradation and compatibility profiles of a polymer component [134]. Incorporation of TiO2 nanoparticles into chitosan sponge resulted in the formation of novel scaffold for bone tissue engineering with improved bone regenerating capability [135]. Even distribution of TiO2 nanoparticles on the surface of chitosan sponges provided stronger mechanical properties of the composite, while biological activity also demonstrated promising results. Nano chitosan–TiO2 (50%) scaffolds were capable of osteogenesis, showing the highest expression on Dentin Matrix Protein 1 and osteocalcin gene. Ikono and colleagues demonstrated that chitosan-TiO2 nanocomposites exhibited improved robustness, biomineralization, osteogenic induction and biocompatibility, which makes them attractive in the bone tissue engineering field, particularly in cases with complex defects and cavities [135]. Another chitosan-based nanocomposite modified with TiO2 was later reported by another group to mimic the human bone ECM. A polymer film-construct of Chitosan/poly (vinyl alcohol)/Nanohydroxyapatite (CPHT I-III) doped with TiO2 nanoparticles demonstrated enhanced mechanical and biological properties, highly cytocompatible nature for osteoblast-like MG-63 cell attachment and proliferation [136]. Various inorganic components could be applied to the formation of chitosan-hybrid nanocomposites, such as silica [137], POSS (the smallest silica particle) [138], bioglass [139], as well as polymers [140,141], resulting in improved mechanical properties, antimicrobial activity, cytocompatibility, osteoblast adhesion, osteocalcin secretion and biomineralization of cells, thus providing a promising biomaterial for bone tissue engineering.
Other biological polymers, such as hyaluronic acid, gelatin, alginate and collagen, can also be used for nanocomposite fabrication for bone tissue regeneration [142,143,144,145,146]. Purohit et al. reported the successful formation of gelatin-alginate nanocomposite scaffold with incorporation of cerium oxide nanoparticles (nanoceria). The effect of nanoceria concentration on mechanical and biological properties of nanocomposite was evaluated, demonstrating improved bio-mineralization properties and decreased in vitro weight loss of a scaffold. Gelatin-alginate-nanoceria composites exhibited a high potential to accelerate differentiation of MSCs to osteoblast and reduce free radicals [147]. Hyaluronic acid was shown to optimize a nanoparticle formulation for delivery of alendronate (a bone anti-resorptive agent) and curcumin (a bone density enhancing drug) inducing an increase in proliferation, differentiation and mineralization of MC3T3-E1 cells leading to improved bone formation [148].
Along with natural polymers, synthetic polymers also demonstrated promise as candidates in the bone tissue engineering field owing to reproducible physical and mechanical properties, biocompatibility and adjustable biodegradation kinetics [149]. The most often used synthetic polymers for bone regeneration include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic acid-co-glycolic acid) (PLGA) and poly(-caprolactone) (PCL). The table below summarizes the most recent and relevant publications on both natural and synthetic polymer-based nano- and micro-formulations for bone tissue engineering (Table 2). Synthetic polymers have found their wide application in the development of 3D-printed materials for bone regeneration [150,151,152]. Polycaprolactone-based nanocomposite 3D matrix with inorganic nanoparticles incorporated were shown to significantly enhance osteogenic differentiation in vitro and to exhibit tissue mineralization and early bone defect repair [153]. Polycaprolactone nanocomposites also demonstrated better cell attachment, proliferation and enhanced calcium phosphate deposition, suggesting a high potential as bone tissue engineering material [154]. Similarly to natural polymers, synthetic polymers significantly improve proliferation, cell attachment and anchorage, however, they are low in osteopromotive properties, hence cannot provide complete bone tissue regeneration alone [155]. A mineral-based component is crucial in tissue restoration, therefore minerals are used for nanoscaffold fabrication for bone regeneration, for example hydroxyapatite (HA) [156]. Often, natural and synthetic polymers are employed simultaneously in the development of nanocomposites for bone repair. A nanocomposite of PLGA-HA-chitosan loaded with BMP2 provided a sustained release of the growth factor, induced osteogenic effects and demonstrated a successful bone defect repair in vivo [102]. Another three-layered composite membrane based on PLGA and collagen was reported to be a promising platform for guided tissue regeneration under the concept of functional graded material [157].
Table 2.
A summary of the most recent and relevant publications on nano- and micro-particle polymer formulations for bone regeneration.
| Source | Polymer | Formulation | Function | Reference |
|---|---|---|---|---|
| Natural | Chitosan | Nanocomposites | Improved osteoblast adhesion and proliferation, osteocalcin secretion and biomineralization of cells | Tamburaci et al., 2020 |
| Sponge | Improved biomineralization and osteogenic induction | Ikono et al., 2019 | ||
| Nanocomposite film | Accelerated tissue ingrowth, enhanced capability to mimic human bone extracellular matrix, vascularization, antibacterial efficacy, compatibility with human erythrocytes, advanced cell attachment and high proliferation with human osteoblasts | Khan et al., 2019 | ||
| Nanoparticle-containing electrospun fibers | Increased bioavailability and osteogenic capability | Balagangadharan et al., 2019 | ||
| Coated nanoparticles | Improved antimicrobial activity | Ignjatović et al., 2016 | ||
| Hyaluronic acid | Nanocomposite | Improved antibacterial activity, high bone differentiation of mesenchymal stem cells | Makvandi et al., 2020 | |
| Nanoparticle | Sustained delivery of alendronate and curcumin, increased proliferation, differentiation and mineralization of MC3T3-E1 cells | Dong et al., 2018 | ||
| Gelatin | Composite scaffold | Improved cell proliferation and bone healing, cytocompatibility, osteoinductivity | Hashemi et al., 2020 | |
| Nanocomposite scaffold | Accelerated differentiation of mesenchymal stem cells to osteoblast and reduce free radicals | Purohit et al., 2020 | ||
| Nanotubes/hydrogel | Enhanced osteogenic differentiation, osteoimmunomodulatory and antibacterial activities | Ou et al., 2020 | ||
| Alginate | Nanocomposite scaffold | Improved bio-mineralization | Purohit et al., 2020 | |
| Microbeads | Enhanced bone formation, higher injectability and washout resistance | Amirian et al., 2020 | ||
| Collagen | Nanocomposite | Enhanced bone regeneration, improved healing and tissue remodeling | Patel et al., 2020 | |
| Three-layered composite membrane | Guided tissue regeneration | Liao et al., 2005 | ||
| Synthetic | Poly(-caprolactone) (PCL) | Composite nanofibers | Improved fiber morphology, cell attachment, proliferation, differentiation, biomineralization, calcium-phosphate deposition | Awasthi et al., 2020 |
| Nanocomposite 3D matrix | Enhanced osteogenic differentiation, early bone defect repair, tissue mineralization | Shen et al., 2019 | ||
| PLGA | Nanocomposite | Induced osteogenic effects and successful bone defect repair in vivo, BMP2 delivery | Deng et al., 2019 | |
| Microspheres | Controlled delivery of magnesium ions, enhanced cell attachment, proliferation, osteogenic differentiation, cell migration of bone marrow mesenchymal stromal cells, promotion of mineral depositions | Yuan et al., 2019 | ||
| Scaffolds | Improved cell attachment, proliferation, induction of cartilage formation | Lin et al., 2018 | ||
| Microspheres | Sustained Simvastatin delivery, induced proliferation of MC3T3-E1 cells, increased differentiation and bone mineralization | Terukina et al., 2016 | ||
| Microspheres | Sustained drug release, good biocompatibility | Nath et al., 2013 |
Although nanosized materials are currently recognized to be more bioactive than their microsized counterparts, there is a series of recent studies demonstrating the high potential of PLGA microparticles in bone regeneration. The performance profiles of PLGA micro- and nanoparticles loaded with simvastatin, a cholesterol lowering drug, were compared and their effects on bone regeneration were studied in vitro and in vivo [158]. PLGA microspheres exhibited a sustained release of simvastatin for one month, as opposed to one week for PLGA nanospheres. Simvastatin-loaded PLGA microspheres were also reported to induce the proliferation of MC3T3-E1 cells and increased ALP activity. PLGA nanospheres, on the other hand, did not show such properties [159]. These data suggested that PLGA microparticles exhibit far superior properties, compared to PLGA nanoparticles, in bone tissue regeneration. An earlier study also reported that PLGA microparticles loaded with simvastatin demonstrated their high potential for bone regeneration [160]. PLGA microspheres could serve as excellent platforms for the formation of DDS with a high promise for bone tissue engineering. PLGA-alginate core-shell microspheres provided a controlled release of magnesium ions that exhibited osteogenic tendency in vitro. This PLGA-based delivery system stimulated in situ bone regeneration in vivo, enhanced bone mineral density [161]. Injectable PLGA microspheres with tunable magnesium ion release promoted attachment, proliferation, osteogenic differentiation and, particularly, cell migration of bone marrow-derived mesenchymal stromal cells [162]. PLGA microparticles were reported to be effective in treating infections and bone defects caused by Staphylococcus aureus. PLGA microparticles were assembled with human demineralized bone matrix in the development of antimicrobial bone paste with a controlled release of vancomycin hydrochloride. This DDS provided a sustained release of antibiotic for over two weeks, effectively inhibiting growth of the bacteria, making the PLGA paste promising for the controlled delivery of bioactive molecules for bone repair.
The future of polymer nano- and microparticle formulations for bone tissue regeneration is undoubtedly highly promising and merits further investigation. Over the past few decades, tremendous progress has been made in the development of nanocomposites and micro-formulations employing both natural and synthetic polymers, studying their characteristics and eventually contributing to the field of tissue engineering. The novel nano- and micro-formulated biomaterials are designed for the delivery of drugs, cells and growth factors, promoting antimicrobial activity, biomineralization, improving mechanical and biological properties of the platform, and hence, bone tissue repair and formation. However, further understanding of the interaction at the cell/surface interface, as well as a closer look at the toxicity, hemotoxicity and long-term biocompatibility of the material and the host tissue are required for clinical applications. This overall highlights the importance of close interaction and inter-disciplinary collaboration of medical, biological, chemical and material communities to move from the bench to clinical applications.
5. Clinical Trials of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration
Despite a number of developed polymer-based DDS to treat bone defects, only a few have reached clinical application. Currently, there are several commercially available carriers for delivery of osteoinductive growth factors such as OP-1, INFUSE®, InductOS® and AUGMENT® [8]. OP-1 was the first FDA approved osteoinductive biomaterial containing rhBMP-7, type I bovine collagen matrix and the putty additive carboxymethyl cellulose sodium [163,164]. A number of prospective, randomized, multicenter studies have demonstrated that OP-1 is safe and very effective for the treatment of open tibial fractures, distal tibial fractures, tibial nonunions, scaphoid nonunions, atrophic long bone nonunions and the posterolateral spine [165,166]. For example, Ristiniemi and colleagues investigated the efficacy of OP-1 in the treatment of distal tibial fractures in a case-control study in 40 patients [166]. They divided the patients into two groups: OP-1 (20) and without OP-1 (20). The results of this clinical study showed that implantation of OP-1 significantly accelerated bone regeneration in distal tibial fractures compared to the control group (6.3 months vs. 9.0 months). In clinical practice, INFUSE® Bone Graft is widely used for the treatment of bone fractures, nonunions, interbody spinal fusions and in oral maxillofacial surgery [167]. It is the most studied osteoinductive biomaterial and is one of the most significant advances in traumatology and orthopaedics. INFUSE® Bone Graft consists of rhBMP-2 (1.5 mg/mL) and ACS as a carrier. Multiple clinical trials conducted from 2002 to 2017 to evaluate the safety, efficacy and dose-dependent effects of INFUSE® implantation in anterior cervical discectomy and fusion (ACDF) demonstrated that patients treated with rhBMP-2/ACS had significantly higher fusion rates compared to non-rhBMP-2 patients, not only in total value but also in 3 tiers of rhBMP-2 doses [168]. It should be noted that ACS loaded with a low dose of rhBMP-2 (<0.7 mg/level) showed the highest fusion rate among all rhBMP-2 doses. However, patients treated with rhBMP-2/ACS had a higher complication rate, dysphagia and wound infections than that in the non-rhBMP-2 group. In 2-level ACDF, the fusion rate was significantly higher in rhBMP-2 treated patients than non-rhBMP-2, but not in the complication rate.
Furthermore, a randomized clinical trial (RCT) with 160 patients demonstrated the efficacy of rhBMP-2/ACS in comparison with commonly used autogenous bone graft for maxillary sinus augmentation [169]. The bone density was higher in the rhBMP-2/ACS group and the success rate of implant integration reached 79% (exceeding the targeted success rate of the protocol by 6%). Additionally, application of rhBMP-2/ACS helped avoid the long-term presence of complications such as prolonged paresthesia, gait disturbance and pain, which are commonly associated with the graft harvesting procedure. Moreover, rhBMP-2/ACS can be effective in bone synthesis for the placement of dental implants. RCT on 80 patients demonstrated that delivery of 2 doses (1.5 mg/mL) of rhBMP-2/ACS after tooth extraction increased bone augmentation in the alveolar ridge, and that the adequacy of bone formation was at least twice greater in the rhBMP-2/ACS group than in the placebo or no treatment groups [170]. However, another RCT with 177 patients with open tibial fractions demonstrated no significant acceleration of bone synthesis in comparison with routine soft-tissue management [171]. Proportion of fracture healing was higher by 12% in the rhBMP-2/ACS group at week 13, however, it demonstrated almost the same results at week 20 (68% for the rhBMP-2/ACS group and 67% for the control group). Furthermore, a recent systematic review of polymer-based delivery of BMP-2 in 17 RCTs for the treatment of craniofacial bone defects did not find evidence of the efficacy rhBMP-2/ACS and rhBMP/hydrogels with HA in the maxillary sinus augmentation and the reconstruction of cranial vault defects. At the same time, the authors state that the treatment with rhBMP-2/ACS can be superior for alveolar ridge augmentation, alveolar cleft reconstruction and in spine surgery. More clinical trials are needed to evaluate the efficacy of rhBMP-2/ACS and rhBMP/hydrogels treatments for the reconstruction of mandibular bone and osteodistraction [10].
More recently, Min and colleagues reported on the administration of rhBMP-2/ACS for medication-related osteonecrosis of the jaw (MRONJ) treatment [172]. They demonstrated that rhBMP-2/ACS (0.5 mg/mL) could promote bone healing by enhancing bone remodeling in MRONJ patients more than 6 months after surgery. Another research group used a higher dose of 1.5 mg/mL of rhBMP-2 to treat MRONJ patients [173]. They reported that large critical-sized defects of the mandible in MRONJ patients were completely repaired in all 3 patients in 3 months after implantation of rhBMP-2/ACS.
Like BMPs, platelet-derived growth factor (PDGF) also plays an important role in bone regeneration [174]. Several recent clinical studies reported that β-TCP-collagen loaded with rhPDGF-BB (Augment® Injectable Bone Graft, Wright Medical Technologies) is effective in ankle and hindfoot fusions [175,176]. Prospective RCT showed that rhPDGF-BB/β-TCP-collagen significantly reduced the time needed for complete fusion (14.3 ± 8.9 weeks) in comparison to autograft-treated patients (19.7 ± 11.5 weeks). Moreover, clinical success rate was achieved in 57 of 63 (91%) rhPDGF-BB/β-TCP-collagen-treated patients and 120 of 154 (78%) autograft-treated patients at 52 weeks [175]. The results of another RCT of rhPDGF-BB/β-TCP-collagen with autograft in ankle or hindfoot arthrodesis using a propensity score (PS) subclassification cohort study design showed that rhPDGF-BB/β-TCP-collagen rhPDGF-BB/β-TCP-collagen was as effective as an autograft for ankle and hindfoot fusions, with less pain and morbidity than treatment with autograft [176]. Thus, commercially available polymer-based DDS for bone regeneration demonstrated good therapeutic efficacy in various clinical applications.
6. Conclusions
Multiple studies showed that biodegradable natural and synthetic polymers play a key role in the development of innovative drug delivery systems and tissue engineered constructs, which improve the treatment and regeneration of damaged bone tissue. Several types of biodegradable and biocompatible polymers have been studied as potential drug delivery systems, including nano- and microparticles. Polymers after chemical or physical modifications acquire improved properties for controlled and sustained release of various drugs, including osteoinductive growth factors for bone regeneration. A special place among the various approaches for drug delivery is occupied by scaffolds with incorporated nano- and microparticles loaded with drugs or biological active substances. These innovative drug delivery systems have a number of advantages that distinguish them from other systems. The small size and high specific surface of nano- and micro-particles provide high efficiency for loading several drugs simultaneously. Their composition and physico-chemical properties allow for the protection of the loaded drugs from enzymatic digestion and other aggressive agents in the wound environment. Moreover, use of nano- and microparticles allows for more effective and controlled drug release from scaffold over time in relevant therapeutic concentrations. These controlled drug delivery systems are able to effectively stimulate osteogenesis and accelerate bone regeneration without significant side effects. Despite the promising results in preclinical studies, implementation of additional clinical trials is required to enhance the translation of developed innovative drug delivery systems for effective bone healing.
Author Contributions
Conceptualization, V.O., E.A.M. and A.S.; writing—original draft preparation, V.O., E.A.M., G.K., M.B., K.K. and Z.Z.; writing—review and editing V.O. and A.S.; supervision, V.O. and A.S.; project administration, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Education and Science of the Republic of Kazakhstan (AP05135207).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghiasi M.S., Chen J., Vaziri A., Rodriguez E.K., Nazarian A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017;6:87–100. doi: 10.1016/j.bonr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marongiu G., Dolci A., Verona M., Capone A. The biology and treatment of acute long-bones diaphyseal fractures: Overview of the current options for bone healing enhancement. Bone Rep. 2020;12:100249. doi: 10.1016/j.bonr.2020.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen M.K., Miclau T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl. 1):S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Yeung K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017;2:224–247. doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcei J.G., Rodeo S.A. Orthobiologics for Bone Healing. Clin. Sports Med. 2019;38:79–95. doi: 10.1016/j.csm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn T.A., Gerstenfeld L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda Y., Kawai T., Goto K., Matsuda S. Clinical application of injectable growth factor for bone regeneration: A systematic review. Inflamm. Regen. 2019;39:20. doi: 10.1186/s41232-019-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Bialy I., Jiskoot W., Reza Nejadnik M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017;34:1152–1170. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Guo J., Zhou Y., Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng. Part B Rev. 2014;20:84–92. doi: 10.1089/ten.TEB.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramly E.P., Alfonso A.R., Kantar R.S., Wang M.M., Siso J.R.D., Ibrahim A., Coelho P.G., Flores R.L. Safety and efficacy of recombinant human bone morphogenetic protein-2 (rhBMP-2) in craniofacial surgery. Plast. Reconstr. Surg. Glob. Open. 2019;7:e2347. doi: 10.1097/gox.0000000000002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreira A.C., Lojudice F.H., Halcsik E., Navarro R.D., Sogayar M.C., Granjeiro J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014;93:335–345. doi: 10.1177/0022034513518561. [DOI] [PubMed] [Google Scholar]
- 12.James A.W., LaChaud G., Shen J., Asatrian G., Nguyen V., Zhang X., Ting K., Soo C. A Review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016;22:284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Y., Hoque J., Varghese S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019;93:152–168. doi: 10.1016/j.actbio.2019.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki K., Saito N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics. 2020;12:95. doi: 10.3390/pharmaceutics12020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor D.N., Bhatia A., Kaur R., Sharma R., Kaur G., Dhawan S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 16.Yan Q., Xiao L.Q., Tan L., Sun W., Wu T., Chen L.W., Mei Y., Shi B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. Part A. 2015;103:3580–3589. doi: 10.1002/jbm.a.35499. [DOI] [PubMed] [Google Scholar]
- 17.Olthof M.G.L., Kempen D.H.R., Herrick J.L., Yaszemski M.J., Dhert W.J.A., Lu L. Effect of different sustained bone morphogenetic protein-2 release kinetics on bone formation in poly(propylene fumarate) scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018;106:477–487. doi: 10.1002/jbm.b.33866. [DOI] [PubMed] [Google Scholar]
- 18.Filippi M., Born G., Chaaban M., Scherberich A. Natural polymeric scaffolds in bone regeneration. Front Bioeng. Biotechnol. 2020;8:474. doi: 10.3389/fbioe.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.X., Zhu R., Xu Z.L., Ke Q.F., Zhang C.Q., Guo Y.P. Self-assembly of pifithrin-α-loaded layered double hydroxide/chitosan nanohybrid composites as a drug delivery system for bone repair materials. J. Mater. Chem. B. 2017;5:2245–2253. doi: 10.1039/C6TB02730J. [DOI] [PubMed] [Google Scholar]
- 20.Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011;36:1254–1276. doi: 10.1016/j.progpolymsci.2011.05.003. [DOI] [Google Scholar]
- 21.Brudno Y., Mooney D.J. On-demand drug delivery from local depots. J. Control. Release. 2015;219:8–17. doi: 10.1016/j.jconrel.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Kim S., Chen Y., Ho E.A., Liu S. Reversibly pH-responsive polyurethane membranes for on-demand intravaginal drug delivery. Acta Biomater. 2017;47:100–112. doi: 10.1016/j.actbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Wei L., Chen J., Zhao S., Ding J., Chen X. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 2017;58:44–53. doi: 10.1016/j.actbio.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Sultankulov B., Berillo D., Sultankulova K., Tokay T., Saparov A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9:470. doi: 10.3390/biom9090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansurov N., Chen W.C., Awada H., Huard J., Wang Y., Saparov A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018;12:e1164–e1172. doi: 10.1002/term.2451. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell A.C., Briquez P.S., Hubbell J.A., Cochran J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atienza-Roca P., Cui X., Hooper G.J., Woodfield T.B., Lim K.S. Cutting-Edge Enabling Technologies for Regenerative Medicine. Springer; New York, NY, USA: 2018. Growth factor delivery systems for tissue engineering and regenerative medicine; pp. 245–269. [DOI] [PubMed] [Google Scholar]
- 28.Draenert F.G., Nonnenmacher A.L., Kämmerer P.W., Goldschmitt J., Wagner W. BMP-2 and bFGF release and in vitro effect on human osteoblasts after adsorption to bone grafts and biomaterials. Clin. Oral Implant. Res. 2013;24:750–757. doi: 10.1111/j.1600-0501.2012.02481.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Wang Z., Lu W.W., Zhen W., Yang D., Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017;9:e435. doi: 10.1038/am.2017.171. [DOI] [Google Scholar]
- 30.Nurkesh A., Jaguparov A., Jimi S., Saparov A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 2020;8:638. doi: 10.3389/fcell.2020.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimi S., Jaguparov A., Nurkesh A., Sultankulov B., Saparov A. Sequential delivery of cryogel released growth factors and cytokines accelerates wound healing and improves tissue regeneration. Front. Bioeng. Biotechnol. 2020;8:345. doi: 10.3389/fbioe.2020.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouyer M., Guillot R., Lavaud J., Plettinx C., Olivier C., Curry V., Boutonnat J., Coll J.-L., Peyrin F., Josserand V. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016;104:168–181. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H.S., La W.-G., Bhang S.H., Jeon J.-Y., Lee J.H., Kim B.-S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. Part A. 2010;16:1225–1233. doi: 10.1089/ten.tea.2009.0390. [DOI] [PubMed] [Google Scholar]
- 34.De Witte T.M., Fratila-Apachitei L.E., Zadpoor A.A., Peppas N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018;5:197–211. doi: 10.1093/rb/rby013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lienemann P.S., Lutolf M.P., Ehrbar M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Deliv. Rev. 2012;64:1078–1089. doi: 10.1016/j.addr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Lee K., Silva E.A., Mooney D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Censi R., Di Martino P., Vermonden T., Hennink W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release Off. J. Control. Release Soc. 2012;161:680–692. doi: 10.1016/j.jconrel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Busra M.F.M., Lokanathan Y. Recent development in the fabrication of collagen scaffolds for tissue engineering applications: A review. Curr. Pharm. Biotechnol. 2019;20:992–1003. doi: 10.2174/1389201020666190731121016. [DOI] [PubMed] [Google Scholar]
- 39.Sheikh Z., Javaid M.A., Hamdan N., Hashmi R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials. 2015;8:1778–1816. doi: 10.3390/ma8041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyun S.J., Han D.K., Choi S.H., Chai J.K., Cho K.S., Kim C.K., Kim C.S. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J. Periodontol. 2005;76:1667–1674. doi: 10.1902/jop.2005.76.10.1667. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T., Shirakata Y., Shinohara Y., Miron R.J., Furue K., Noguchi K. Osteogenic potential of recombinant human bone morphogenetic protein-9/absorbable collagen sponge (rhBMP-9/ACS) in rat critical size calvarial defects. Clin. Oral Investig. 2017;21:1659–1665. doi: 10.1007/s00784-016-1963-4. [DOI] [PubMed] [Google Scholar]
- 42.Lo K.W.-H., Ulery B.D., Ashe K.M., Laurencin C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012;64:1277–1291. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zara J.N., Siu R.K., Zhang X., Shen J., Ngo R., Lee M., Li W., Chiang M., Chung J., Kwak J. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal V., Sinha M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017;105:904–925. doi: 10.1002/jbm.b.33599. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., He Q.-F., Zhang T.-H., Yu X.-L., Liu Q., Deng F.-I. Improvement in the delivery system of bone morphogenetic protein-2: A new approach to promote bone formation. Biomed. Mater. 2012;7:045002. doi: 10.1088/1748-6041/7/4/045002. [DOI] [PubMed] [Google Scholar]
- 46.Osidak E., Osidak M., Sivogrivov D., Portnaya T., Grunina T., Soboleva L., Lunin V., Karyagina A., Domogatskii S. Regulation of the binding of the BMP-2 growth factor with collagen by blood plasma fibronectin. Appl. Biochem. Microbiol. 2014;50:200–205. doi: 10.1134/S0003683814020148. [DOI] [PubMed] [Google Scholar]
- 47.Liu K., Meng C.X., Lv Z.Y., Zhang Y.J., Li J., Li K.Y., Liu F.Z., Zhang B., Cui F.Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020;7:435–440. doi: 10.1093/rb/rbaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujioka-Kobayashi M., Schaller B., Saulacic N., Pippenger B.E., Zhang Y., Miron R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017;3:32–40. doi: 10.1002/cre2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levengood S.K.L., Zhang M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B. 2014;2:3161–3184. doi: 10.1039/c4tb00027g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saravanan S., Leena R.S., Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016;93:1354–1365. doi: 10.1016/j.ijbiomac.2016.01.112. [DOI] [PubMed] [Google Scholar]
- 51.Venkatesan J., Anil S., Kim S.K., Shim M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017;104:1383–1397. doi: 10.1016/j.ijbiomac.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 52.Sultankulov B., Berillo D., Kauanova S., Mikhalovsky S., Mikhalovska L., Saparov A. Composite cryogel with polyelectrolyte complexes for growth factor delivery. Pharmaceutics. 2019;11:650. doi: 10.3390/pharmaceutics11120650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernkop-Schnürch A., Dünnhaupt S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Aguilar A., Zein N., Harmouch E., Hafdi B., Bornert F., Offner D., Clauss F., Fioretti F., Huck O., Benkirane-Jessel N., et al. Application of chitosan in bone and dental engineering. Molecules. 2019;24:3009. doi: 10.3390/molecules24163009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S., Kang Y., Mercado-Pagán Á.E., Maloney W.J., Yang Y. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;102:1393–1406. doi: 10.1002/jbm.b.33118. [DOI] [PubMed] [Google Scholar]
- 56.Kim S., Bedigrew K., Guda T., Maloney W.J., Park S., Wenke J.C., Yang Y.P. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 2014;10:5021–5033. doi: 10.1016/j.actbio.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae I.-H., Jeong B.-C., Kook M.-S., Kim S.-H., Koh J.-T. Evaluation of a thiolated chitosan scaffold for local delivery of BMP-2 for osteogenic differentiation and ectopic bone formation. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/878930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Yu B., Huang Q., Liu R., Feng Q., Cai Q., Mi S. In vitro BMP-2 peptide release from thiolated chitosan based hydrogel. Int. J. Biol. Macromol. 2016;93:314–321. doi: 10.1016/j.ijbiomac.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 59.Yun Y.-P., Kim S.E., Kang E.Y., Kim H.-J., Park K., Song H.-R. The effect of bone morphogenic protein-2 (BMP-2)-immobilizing heparinized-chitosan scaffolds for enhanced osteoblast activity. Tissue Eng. Regen. Med. 2013;10:122–130. doi: 10.1007/s13770-013-0386-4. [DOI] [Google Scholar]
- 60.Kim S., Fan J., Lee C.-S., Chen C., Bubukina K., Lee M. Heparinized chitosan stabilizes the bioactivity of BMP-2 and potentiates the osteogenic efficacy of demineralized bone matrix. J. Biol. Eng. 2020;14:1–12. doi: 10.1186/s13036-020-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemshekhar M., Thushara R.M., Chandranayaka S., Sherman L.S., Kemparaju K., Girish K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016;86:917–928. doi: 10.1016/j.ijbiomac.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 62.Zhao N., Wang X., Qin L., Zhai M., Yuan J., Chen J., Li D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. Part A. 2016;104:1560–1569. doi: 10.1002/jbm.a.35681. [DOI] [PubMed] [Google Scholar]
- 63.Kim W.K., Choi J.H., Shin M.E., Kim J.W., Kim P.Y., Kim N., Song J.E., Khang G. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int. J. Biol. Macromol. 2019;141:51–59. doi: 10.1016/j.ijbiomac.2019.08.176. [DOI] [PubMed] [Google Scholar]
- 64.Bhakta G., Lim Z.X., Rai B., Lin T., Hui J.H., Prestwich G.D., van Wijnen A.J., Nurcombe V., Cool S.M. The influence of collagen and hyaluronan matrices on the delivery and bioactivity of bone morphogenetic protein-2 and ectopic bone formation. Acta Biomater. 2013;9:9098–9106. doi: 10.1016/j.actbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Kisiel M., Martino M.M., Ventura M., Hubbell J.A., Hilborn J., Ossipov D.A. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials. 2013;34:704–712. doi: 10.1016/j.biomaterials.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Bae M.S., Ohe J.Y., Lee J.B., Heo D.N., Byun W., Bae H., Kwon Y.D., Kwon I.K. Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration. Bone. 2014;59:189–198. doi: 10.1016/j.bone.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Bae M.S., Yang D.H., Lee J.B., Heo D.N., Kwon Y.D., Youn I.C., Choi K., Hong J.H., Kim G.T., Choi Y.S., et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials. 2011;32:8161–8171. doi: 10.1016/j.biomaterials.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 68.Ray P., Maity M., Barik H., Sahoo G.S., Hasnain M.S., Hoda M.N., Nayak A.K. Alginates in Drug Delivery. Elsevier; New York, NY, USA: 2020. Alginate-based hydrogels for drug delivery applications; pp. 41–70. [Google Scholar]
- 69.Pawar S.N., Edgar K.J. Chemical modification of alginates in organic solvent systems. Biomacromolecules. 2011;12:4095–4103. doi: 10.1021/bm201152a. [DOI] [PubMed] [Google Scholar]
- 70.Pawar S.N., Edgar K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Pawar S.N., Edgar K.J. Alginate esters via chemoselective carboxyl group modification. Carbohydr. Polym. 2013;98:1288–1296. doi: 10.1016/j.carbpol.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Su J., Xu H., Sun J., Gong X., Zhao H. Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. Int. J. Mol. Sci. 2013;14:12714–12728. doi: 10.3390/ijms140612714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi D.H., Park C.H., Kim I.H., Chun H.J., Park K., Han D.K. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release Off. J. Control. Release Soc. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 74.Kanczler J.M., Ginty P.J., White L., Clarke N.M., Howdle S.M., Shakesheff K.M., Oreffo R.O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 75.Fernandes G., Wang C., Yuan X., Liu Z., Dziak R., Yang S. Combination of controlled release platelet-rich plasma alginate beads and bone morphogenetic protein-2 genetically modified mesenchymal stem cells for bone regeneration. J. Periodontol. 2016;87:470–480. doi: 10.1902/jop.2016.150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bujoli B., Scimeca J.C., Verron E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics. 2019;11:556. doi: 10.3390/pharmaceutics11110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noori A., Ashrafi S.J., Vaez-Ghaemi R., Hatamian-Zaremi A., Webster T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017;12:4937. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khodakaram-Tafti A., Mehrabani D., Shaterzadeh-Yazdi H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent. Res. J. 2017;14:79. [PMC free article] [PubMed] [Google Scholar]
- 79.Martino M.M., Hubbell J.A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 80.Yang H.S., La W.G., Cho Y.M., Shin W., Yeo G.D., Kim B.S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp. Mol. Med. 2012;44:350–355. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez-Casanova S., Martin-Saavedra F.M., Escudero-Duch C., Falguera Uceda M.I., Prieto M., Arruebo M., Acebo P., Fabiilli M.L., Franceschi R.T., Vilaboa N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials. 2020;241:119909. doi: 10.1016/j.biomaterials.2020.119909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao S.H., Harini B., Shadamarshan R.P.K., Balagangadharan K., Selvamurugan N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018;110:88–96. doi: 10.1016/j.ijbiomac.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 83.Iqbal N., Khan A.S., Asif A., Yar M., Haycock J.W., Rehman I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019;64:91–126. doi: 10.1080/09506608.2018.1460943. [DOI] [Google Scholar]
- 84.Narayanan G., Vernekar V.N., Kuyinu E.L., Laurencin C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016;107:247–276. doi: 10.1016/j.addr.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghassemi T., Shahroodi A., Ebrahimzadeh M.H., Mousavian A., Movaffagh J., Moradi A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018;6:90–99. [PMC free article] [PubMed] [Google Scholar]
- 86.Miroshnichenko S., Timofeeva V., Permykova E., Ershov S., Kiryukhantsev-Korneev P., Dvořaková E., Shtansky D.V., Zajíčková L., Solovieva A., Manakhov A. Plasma-coated polycaprolactone nanofibers with covalently bonded platelet-rich plasma enhance adhesion and growth of human fibroblasts. Nanomaterials. 2019;9:637. doi: 10.3390/nano9040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aldemir Dikici B., Dikici S., Reilly G.C., MacNeil S., Claeyssens F. A novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials. 2019;12:2643. doi: 10.3390/ma12162643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pazarçeviren A.E., Dikmen T., Altunbaş K., Yaprakçı V., Erdemli Ö., Keskin D., Tezcaner A. Composite clinoptilolite/PCL-PEG-PCL scaffolds for bone regeneration: In vitro and in vivo evaluation. J. Tissue Eng. Regen. Med. 2020;14:3–15. doi: 10.1002/term.2938. [DOI] [PubMed] [Google Scholar]
- 89.Wang H., Tong D., Wang L., Chen L., Yu N., Li Z. A facile strategy for fabricating PCL/PEG block copolymer with excellent enzymatic degradation. Polym. Degrad. Stab. 2017;140:64–73. doi: 10.1016/j.polymdegradstab.2017.04.015. [DOI] [Google Scholar]
- 90.Huang B., Caetano G., Vyas C., Blaker J.J., Diver C., Bártolo P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tri-calcium phosphate. Materials. 2018;11:129. doi: 10.3390/ma11010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bittner S.M., Smith B.T., Diaz-Gomez L., Hudgins C.D., Melchiorri A.J., Scott D.W., Fisher J.P., Mikos A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019;90:37–48. doi: 10.1016/j.actbio.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park S.H., Park S.A., Kang Y.G., Shin J.W., Park Y.S., Gu S.R., Wu Y.R., Wei J., Shin J.W. PCL/β-TCP Composite scaffolds exhibit positive osteogenic differentiation with mechanical stimulation. Tissue Eng. Regen. Med. 2017;14:349–358. doi: 10.1007/s13770-017-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bao X., Zhu L., Huang X., Tang D., He D., Shi J., Xu G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017;7:7814. doi: 10.1038/s41598-017-08412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao Q., Liu Y., Pan Y., Miszuk J.M., Sun H. One-pot porogen free method fabricated porous microsphere-aggregated 3D PCL scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:2699–2710. doi: 10.1002/jbm.b.34601. [DOI] [PubMed] [Google Scholar]
- 95.Junka R., Yu X. Polymeric nanofibrous scaffolds laden with cell-derived extracellular matrix for bone regeneration. Mater. Sci. Eng. C. 2020;113:110981. doi: 10.1016/j.msec.2020.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Q., Cosme J.G., Xu T., Miszuk J.M., Picciani P.H., Fong H., Sun H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turnbull G., Clarke J., Picard F., Riches P., Jia L., Han F., Li B., Shu W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018;3:278–314. doi: 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bharadwaz A., Jayasuriya A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;110:110698. doi: 10.1016/j.msec.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Generali M., Kehl D., Capulli A.K., Parker K.K., Hoerstrup S.P., Weber B. Comparative analysis of poly-glycolic acid-based hybrid polymer starter matrices for in vitro tissue engineering. Colloids Surf. B Biointerfaces. 2017;158:203–212. doi: 10.1016/j.colsurfb.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 100.Cao S., Han J., Sharma N., Msallem B., Jeong W., Son J., Kunz C., Kang H.W., Thieringer F.M. In vitro mechanical and biological properties of 3D printed polymer composite and β-tricalcium phosphate scaffold on human dental pulp stem cells. Materials. 2020;13:3057. doi: 10.3390/ma13143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y., Wang C., Fu L., Ye S., Wang M., Zhou Y. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3d printing for repairing critical-sized bone defect. Molecules. 2019;24:1669. doi: 10.3390/molecules24091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng N., Sun J., Li Y., Chen L., Chen C., Wu Y., Wang Z., Li L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral Biol. 2019;102:16–25. doi: 10.1016/j.archoralbio.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 103.Das A., Fishero B.A., Christophel J.J., Li C.J., Kohli N., Lin Y., Dighe A.S., Cui Q. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell Tissue Res. 2016;364:125–135. doi: 10.1007/s00441-015-2301-x. [DOI] [PubMed] [Google Scholar]
- 104.Kim H.J., Han M.A., Shin J.Y., Jeon J.H., Lee S.J., Yoon M.Y., Kim H.J., Choi E.J., Do S.H., Yang V.C., et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control. Release Off. J. Control. Release Soc. 2019;302:169–180. doi: 10.1016/j.jconrel.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Jakus A.E., Rutz A.L., Jordan S.W., Kannan A., Mitchell S.M., Yun C., Koube K.D., Yoo S.C., Whiteley H.E., Richter C.P., et al. Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 2016;8:358ra127. doi: 10.1126/scitranslmed.aaf7704. [DOI] [PubMed] [Google Scholar]
- 106.Hajebi S., Mohammadi Nasr S., Rabiee N., Bagherzadeh M., Ahmadi S., Rabiee M., Tahriri M., Tayebi L., Hamblin M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2020:1–15. doi: 10.1080/00914037.2020.1765365. [DOI] [Google Scholar]
- 107.Botlhoko O.J., Ramontja J., Ray S.S. A new insight into morphological, thermal, and mechanical properties of melt-processed polylactide/poly (ε-caprolactone) blends. Polym. Degrad. Stab. 2018;154:84–95. doi: 10.1016/j.polymdegradstab.2018.05.025. [DOI] [Google Scholar]
- 108.Li H., Qiao T., Song P., Guo H., Song X., Zhang B., Chen X. Star-shaped PCL/PLLA blended fiber membrane via electrospinning. J. Biomater. Sci. Polym. Ed. 2015;26:420–432. doi: 10.1080/09205063.2015.1015865. [DOI] [PubMed] [Google Scholar]
- 109.Qiao T., Song P., Guo H., Song X., Zhang B., Chen X. Reinforced electrospun PLLA fiber membrane via chemical crosslinking. Eur. Polym. J. 2016;74:101–108. doi: 10.1016/j.eurpolymj.2015.11.012. [DOI] [Google Scholar]
- 110.Scaffaro R., Lopresti F., Botta L., Rigogliuso S., Ghersi G. Preparation of three-layered porous PLA/PEG scaffold: Relationship between morphology, mechanical behavior and cell permeability. J. Mech. Behav. Biomed. Mater. 2016;54:8–20. doi: 10.1016/j.jmbbm.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H., Mao X., Zhao D., Jiang W., Du Z., Li Q., Jiang C., Han D. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: An in vivo bioreactor model. Sci. Rep. 2017;7:15255. doi: 10.1038/s41598-017-14923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohammadi M., Alibolandi M., Abnous K., Salmasi Z., Jaafari M.R., Ramezani M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomedicine. 2018;14:1987–1997. doi: 10.1016/j.nano.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Ma D., An G., Liang M., Liu Y., Zhang B., Wang Y. A composited PEG-silk hydrogel combining with polymeric particles delivering rhBMP-2 for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;65:221–231. doi: 10.1016/j.msec.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 114.Yang T., Hu Y., Wang C., Binks B.P. Fabrication of hierarchical macroporous biocompatible scaffolds by combining pickering high internal phase emulsion templates with three-dimensional printing. ACS Appl. Mater. Interfaces. 2017;9:22950–22958. doi: 10.1021/acsami.7b05012. [DOI] [PubMed] [Google Scholar]
- 115.Ren Z., Ma S., Jin L., Liu Z., Liu D., Zhang X., Cai Q., Yang X. Repairing a bone defect with a three-dimensional cellular construct composed of a multi-layered cell sheet on electrospun mesh. Biofabrication. 2017;9:025036. doi: 10.1088/1758-5090/aa747f. [DOI] [PubMed] [Google Scholar]
- 116.Saroia J., Yanen W., Wei Q., Zhang K., Lu T., Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio Des. Manuf. 2018;1:265–279. doi: 10.1007/s42242-018-0029-7. [DOI] [Google Scholar]
- 117.Wang J.Z., You M.L., Ding Z.Q., Ye W.B. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;97:1021–1035. doi: 10.1016/j.msec.2019.01.057. [DOI] [PubMed] [Google Scholar]
- 118.Kutikov A.B., Song J. Biodegradable peg-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater. Sci. Eng. 2015;1:463–480. doi: 10.1021/acsbiomaterials.5b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eğri S., Eczacıoğlu N. Sequential VEGF and BMP-2 releasing PLA-PEG-PLA scaffolds for bone tissue engineering: I. Design and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2017;45:321–329. doi: 10.3109/21691401.2016.1147454. [DOI] [PubMed] [Google Scholar]
- 120.Lin D., Cai B., Wang L., Cai L., Wang Z., Xie J., Lv Q.X., Yuan Y., Liu C., Shen S.G. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials. 2020;253:120095. doi: 10.1016/j.biomaterials.2020.120095. [DOI] [PubMed] [Google Scholar]
- 121.Yang Y., Aghazadeh-Habashi A., Panahifar A., Wu Y., Bhandari K.H., Doschak M.R. Bone-targeting parathyroid hormone conjugates outperform unmodified PTH in the anabolic treatment of osteoporosis in rats. Drug Deliv. Transl. Res. 2017;7:482–496. doi: 10.1007/s13346-017-0407-2. [DOI] [PubMed] [Google Scholar]
- 122.Venkanna A., Kwon O.W., Afzal S., Jang C., Cho K.H., Yadav D.K., Kim K., Park H.G., Chun K.H., Kim S.Y., et al. Pharmacological use of a novel scaffold, anomeric N,N-diarylamino tetrahydropyran: Molecular similarity search, chemocentric target profiling, and experimental evidence. Sci. Rep. 2017;7:12535. doi: 10.1038/s41598-017-12082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11:18–25. doi: 10.1016/S1369-7021(08)70086-5. [DOI] [Google Scholar]
- 124.Zhang L., Gu F.X., Chan J.M., Wang A.Z., Langer R.S., Farokhzad O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 125.Jalali N., Moztarzadeh F., Mozafari M., Asgari S., Motevalian M., Alhosseini S.N. Surface modification of poly (lactide-co-glycolide) nanoparticles by d-α-tocopheryl polyethylene glycol 1000 succinate as potential carrier for the delivery of drugs to the brain. Colloids Surf. A Physicochem. Eng. Asp. 2011;392:335–342. doi: 10.1016/j.colsurfa.2011.10.012. [DOI] [Google Scholar]
- 126.Venkatpurwar V., Shiras A., Pokharkar V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm. 2011;409:314–320. doi: 10.1016/j.ijpharm.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 127.Canfarotta F., Whitcombe M.J., Piletsky S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013;31:1585–1599. doi: 10.1016/j.biotechadv.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 128.Cheng Y., Morshed R.A., Auffinger B., Tobias A.L., Lesniak M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jo J., Aoki I., Tabata Y. Design of iron oxide nanoparticles with different sizes and surface charges for simple and efficient labeling of mesenchymal stem cells. J. Control. Release Off. J. Control. Release Soc. 2010;142:465–473. doi: 10.1016/j.jconrel.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 130.Zhou C.-J., Wang S.-H., Yu Z., Rong P.-F., Chen Z.-Z., Liu J.-Y., Zhou J.-D. Folate-conjugated Fe3O4 nanoparticles for in vivo tumor labeling. Trans. Nonferrous Met. Soc. China. 2013;23:2079–2084. doi: 10.1016/S1003-6326(13)62699-0. [DOI] [Google Scholar]
- 131.Sur S., Rathore A., Dave V., Reddy K.R., Chouhan R.S., Sadhu V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Objects. 2019;20:100397. doi: 10.1016/j.nanoso.2019.100397. [DOI] [Google Scholar]
- 132.Calori I.R., Braga G., de Jesus P.D.C.C., Bi H., Tedesco A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020;129:109621. doi: 10.1016/j.eurpolymj.2020.109621. [DOI] [Google Scholar]
- 133.George A., Shah P.A., Shrivastav P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019;561:244–264. doi: 10.1016/j.ijpharm.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 134.Ikono R., Li N., Pratama N.H., Vibriani A., Yuniarni D.R., Luthfansyah M., Bachtiar B.M., Bachtiar E.W., Mulia K., Nasikin M., et al. Enhanced bone regeneration capability of chitosan sponge coated with TiO(2) nanoparticles. Biotechnol. Rep. 2019;24:e00350. doi: 10.1016/j.btre.2019.e00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iannazzo D., Pistone A., Salamò M., Galvagno S. Hybrid Polymer Composite Materials. Elsevier; New York, NY, USA: 2017. Hybrid ceramic/polymer composites for bone tissue regeneration; pp. 125–155. [Google Scholar]
- 136.Khan S., Garg M., Chockalingam S., Gopinath P., Kundu P.P. TiO(2) doped chitosan/poly (vinyl alcohol) nanocomposite film with enhanced mechanical properties for application in bone tissue regeneration. Int. J. Biol. Macromol. 2020;143:285–296. doi: 10.1016/j.ijbiomac.2019.11.246. [DOI] [PubMed] [Google Scholar]
- 137.Li Y., Guo Y., Ge J., Ma P.X., Lei B. In situ silica nanoparticles-reinforced biodegradable poly (citrate-siloxane) hybrid elastomers with multifunctional properties for simultaneous bioimaging and bone tissue regeneration. Appl. Mater. Today. 2018;10:153–163. doi: 10.1016/j.apmt.2017.11.007. [DOI] [Google Scholar]
- 138.Tamburaci S., Tihminlioglu F. Chitosan-hybrid poss nanocomposites for bone regeneration: The effect of poss nanocage on surface, morphology, structure and in vitro bioactivity. Int. J. Biol. Macromol. 2020;142:643–657. doi: 10.1016/j.ijbiomac.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 139.Vukajlovic D., Parker J., Bretcanu O., Novakovic K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;96:955–967. doi: 10.1016/j.msec.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 140.Ignjatović N., Wu V., Ajduković Z., Mihajilov-Krstev T., Uskoković V., Uskoković D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;60:357–364. doi: 10.1016/j.msec.2015.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Balagangadharan K., Trivedi R., Vairamani M., Selvamurugan N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr. Polym. 2019;216:1–16. doi: 10.1016/j.carbpol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 142.Makvandi P., Ali G.W., Della Sala F., Abdel-Fattah W.I., Borzacchiello A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;107:110195. doi: 10.1016/j.msec.2019.110195. [DOI] [PubMed] [Google Scholar]
- 143.Hashemi S.F., Mehrabi M., Ehterami A., Gharravi A.M., Bitaraf F.S., Salehi M. In-vitro and in-vivo studies of PLA/PCL/gelatin composite scaffold containing ascorbic acid for bone regeneration. J. Drug Deliv. Sci. Technol. 2020:102077. doi: 10.1016/j.jddst.2020.102077. [DOI] [Google Scholar]
- 144.Ou Q., Huang K., Fu C., Huang C., Fang Y., Gu Z., Wu J., Wang Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2020;382:123019. doi: 10.1016/j.cej.2019.123019. [DOI] [Google Scholar]
- 145.Amirian J., Makkar P., Lee G.H., Paul K., Lee B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020;272:127830. doi: 10.1016/j.matlet.2020.127830. [DOI] [Google Scholar]
- 146.Patel A., Zaky S.H., Schoedel K., Li H., Sant V., Beniash E., Sfeir C., Stolz D.B., Sant S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020;112:262–273. doi: 10.1016/j.actbio.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Purohit S.D., Singh H., Bhaskar R., Yadav I., Chou C.F., Gupta M.K., Mishra N.C. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;116:111111. doi: 10.1016/j.msec.2020.111111. [DOI] [PubMed] [Google Scholar]
- 148.Dong J., Tao L., Abourehab M.A.S., Hussain Z. Design and development of novel hyaluronate-modified nanoparticles for combo-delivery of curcumin and alendronate: Fabrication, characterization, and cellular and molecular evidences of enhanced bone regeneration. Int. J. Biol. Macromol. 2018;116:1268–1281. doi: 10.1016/j.ijbiomac.2018.05.116. [DOI] [PubMed] [Google Scholar]
- 149.Hasnain M.S., Ahmad S.A., Chaudhary N., Hoda M.N., Nayak A.K. Applications of Nanocomposite Materials in Orthopedics. Elsevier; New York, NY, USA: 2019. Biodegradable polymer matrix nanocomposites for bone tissue engineering; pp. 1–37. [Google Scholar]
- 150.Okamoto M., John B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013;38:1487–1503. doi: 10.1016/j.progpolymsci.2013.06.001. [DOI] [Google Scholar]
- 151.Neufurth M., Wang X., Wang S., Steffen R., Ackermann M., Haep N.D., Schröder H.C., Müller W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017;64:377–388. doi: 10.1016/j.actbio.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 152.Shen J., Wang W., Zhai X., Chen B., Qiao W., Li W., Li P., Zhao Y., Meng Y., Qian S. 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration. Appl. Mater. Today. 2019;16:493–507. doi: 10.1016/j.apmt.2019.07.012. [DOI] [Google Scholar]
- 153.Babilotte J., Martin B., Guduric V., Bareille R., Agniel R., Roques S., Héroguez V., Dussauze M., Gaudon M., Le Nihouannen D. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C. 1920;118:111334. doi: 10.1016/j.msec.2020.111334. [DOI] [PubMed] [Google Scholar]
- 154.Awasthi G.P., Kaliannagounder V.K., Maharjan B., Lee J.Y., Park C.H., Kim C.S. Albumin-induced exfoliation of molybdenum disulfide nanosheets incorporated polycaprolactone/zein composite nanofibers for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;116:111162. doi: 10.1016/j.msec.2020.111162. [DOI] [PubMed] [Google Scholar]
- 155.Wang C., Huang W., Zhou Y., He L., He Z., Chen Z., He X., Tian S., Liao J., Lu B., et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020;5:82–91. doi: 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin Y.X., Ding Z.Y., Zhou X.B., Li S.T., Xie de M., Li Z.Z., Sun G.D. In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed. Environ. Sci. BES. 2015;28:1–12. doi: 10.3967/bes2015.001. [DOI] [PubMed] [Google Scholar]
- 157.Liao S., Wang W., Uo M., Ohkawa S., Akasaka T., Tamura K., Cui F., Watari F. A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration. Biomaterials. 2005;26:7564–7571. doi: 10.1016/j.biomaterials.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 158.Terukina T., Naito Y., Tagami T., Morikawa Y., Henmi Y., Prananingrum W., Ichikawa T., Ozeki T. Research paper. J. Drug Deliv. Sci. Technol. 2016;33:136–142. doi: 10.1016/j.jddst.2016.03.005. [DOI] [Google Scholar]
- 159.Nath S.D., Son S., Sadiasa A., Min Y.K., Lee B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013;443:87–94. doi: 10.1016/j.ijpharm.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 160.Lin Z., Wu J., Qiao W., Zhao Y., Wong K.H.M., Chu P.K., Bian L., Wu S., Zheng Y., Cheung K.M.C., et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 2018;174:1–16. doi: 10.1016/j.biomaterials.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 161.Yuan Z., Wei P., Huang Y., Zhang W., Chen F., Zhang X., Mao J., Chen D., Cai Q., Yang X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85:294–309. doi: 10.1016/j.actbio.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 162.Govoni M., Lamparelli E.P., Ciardulli M.C., Santoro A., Oliviero A., Palazzo I., Reverchon E., Vivarelli L., Maso A., Storni E., et al. Demineralized bone matrix paste formulated with biomimetic PLGA microcarriers for the vancomycin hydrochloride controlled delivery: Release profile, citotoxicity and efficacy against S. aureus. Int. J. Pharm. 2020;582:119322. doi: 10.1016/j.ijpharm.2020.119322. [DOI] [PubMed] [Google Scholar]
- 163.Vaccaro A.R., Patel T., Fischgrund J., Anderson D.G., Truumees E., Herkowitz H., Phillips F., Hilibrand A., Albert T.J. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2003;12:495–500. doi: 10.1007/s00586-003-0561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vaccaro A.R., Patel T., Fischgrund J., Anderson D.G., Truumees E., Herkowitz H., Phillips F., Hilibrand A., Albert T.J. A 2-year follow-up pilot study evaluating the safety and efficacy of op-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2005;14:623–629. doi: 10.1007/s00586-004-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.White A.P., Vaccaro A.R., Hall J.A., Whang P.G., Friel B.C., McKee M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ristiniemi J., Flinkkilä T., Hyvönen P., Lakovaara M., Pakarinen H., Jalovaara P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J. Bone Jt. Surg. Br. Vol. 2007;89:265–272. doi: 10.1302/0301-620X.89B2.18230. [DOI] [PubMed] [Google Scholar]
- 167.McKay W.F., Peckham S.M., Badura J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int. Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wen Y.D., Jiang W.M., Yang H.L., Shi J.H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J. Orthop. Transl. 2020;24:166–174. doi: 10.1016/j.jot.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Triplett R.G., Nevins M., Marx R.E., Spagnoli D.B., Oates T.W., Moy P.K., Boyne P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2009;67:1947–1960. doi: 10.1016/j.joms.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 170.Fiorellini J.P., Howell T.H., Cochran D., Malmquist J., Lilly L.C., Spagnoli D., Toljanic J., Jones A., Nevins M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005;76:605–613. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- 171.Aro H.T., Govender S., Patel A.D., Hernigou P., Perera de Gregorio A., Popescu G.I., Golden J.D., Christensen J., Valentin A. Recombinant human bone morphogenetic protein-2: A randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Jt. Surg. Am. Vol. 2011;93:801–808. doi: 10.2106/JBJS.I.01763. [DOI] [PubMed] [Google Scholar]
- 172.Daniels T.R., Younger A.S., Penner M.J., Wing K.J., Le I.L., Russell I.S., Lalonde K.A., Evangelista P.T., Quiton J.D., Glazebrook M., et al. Prospective Randomized Controlled Trial of Hindfoot and Ankle Fusions Treated With rhPDGF-BB in Combination With a β-TCP-Collagen Matrix. Foot Ankle Int. 2015;36:739–748. doi: 10.1177/1071100715576370. [DOI] [PubMed] [Google Scholar]
- 173.Daniels T.R., Anderson J., Swords M.P., Maislin G., Donahue R., Pinsker E., Quiton J.D. Recombinant Human Platelet-Derived Growth Factor BB in Combination with a Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019;40:1068–1078. doi: 10.1177/1071100719851468. [DOI] [PubMed] [Google Scholar]
- 174.Hollinger J.O., Hart C.E., Hirsch S.N., Lynch S., Friedlaender G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. Vol. 2008;90(Suppl. 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 175.Min S.H., Kang N.E., Song S.I., Lee J.K. Regenerative effect of recombinant human bone morphogenetic protein-2/absorbable collagen sponge (rhBMP-2/ACS) after sequestrectomy of medication-related osteonecrosis of the jaw (MRONJ) J. Korean Assoc.Oral Maxillofac. Surg. 2020;46:191–196. doi: 10.5125/jkaoms.2020.46.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim M.S., Kim K.J., Kim B.J., Kim C.H., Kim J.H. Immediate reconstruction of mandibular defect after treatment of medication-related osteonecrosis of the jaw (MRONJ) with rhBMP-2/ACS and miniplate: Review of 3 cases. Int. J. Surg. Case Rep. 2020;66:25–29. doi: 10.1016/j.ijscr.2019.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]