Abstract
Simple Summary
Sweetpotato whitefly, Bemisia tabaci Gennadius, is a serious pest of many agricultural crops worldwide. Numerous studies have examined the genetic structure of whitefly populations separated by geographical barriers; however, very few have assessed the population structure of B. tabaci at a farmscape level. A farmscape in this study is defined as heterogenous habitat with crop and non-crop areas spanning approximately 8 square kilometers. To assess the roles of farmscapes as drivers of B. tabaci genetic variation, thirty-five populations of the sweetpotato whitefly were collected from crop and non-crop plant species from fifteen farmscapes. Using mitochondrial COI gene sequences (mtCOI) and six nuclear microsatellite markers, the genetic diversity and genetic differentiation among collected B. tabaci MEAM1 populations were examined. Haplotype analysis using mtCOI sequences revealed the presence of a single B. tabaci MEAM1 haplotype across farmscapes of Georgia. Results from microsatellite markers further showed no significant genetic structuring among populations that corresponded to plant species or farmscapes from which they were collected. Annual whitefly population explosions and subsequent dispersal might have facilitated the persistence of a single panmictic B. tabaci population over all sampled farmscapes in this region.
Abstract
Bemisia tabaci is a whitefly species complex comprising important phloem feeding insect pests and plant virus vectors of many agricultural crops. Middle East–Asia Minor 1 (MEAM1) and Mediterranean (MED) are the two most invasive members of the B. tabaci species complex worldwide. The diversity of agroecosystems invaded by B. tabaci could potentially influence their population structure, but this has not been assessed at a farmscape level. A farmscape in this study is defined as heterogenous habitat with crop and non-crop areas spanning ~8 square kilometers. In this study, mitochondrial COI gene (mtCOI) sequences and six microsatellite markers were used to examine the population structure of B. tabaci MEAM1 colonizing different plant species at a farmscape level in Georgia, United States. Thirty-five populations of adult whiteflies on row and vegetable crops and weeds across major agricultural regions of Georgia were collected from fifteen farmscapes. Based on morphological features and mtCOI sequences, five species/cryptic species of whiteflies (B. tabaci MEAM1, B. tabaci MED, Dialeurodes citri, Trialeurodes abutiloneus, T. vaporariorum) were found. Analysis of 102 mtCOI sequences revealed the presence of a single B. tabaci MEAM1 haplotype across farmscapes in Georgia. Population genetics analyses (AMOVA, PCA and STRUCTURE) of B. tabaci MEAM1 (microsatellite data) revealed only minimal genetic differences among collected populations within and among farmscapes. Overall, our results suggest that there is a high level of gene flow among B. tabaci MEAM1 populations among farmscapes in Georgia. Frequent whitefly population explosions driven by a single or a few major whitefly-suitable hosts planted on a wide spatial scale may be the key factor behind the persistence of a single panmictic population over Georgia’s farmscapes. These population structuring effects are useful for delineating the spatial scale at which whiteflies must be managed and predicting the speed at which alleles associated with insecticide resistance might spread.
Keywords: Bemisia tabaci, farmscape, genetic diversity, microsatellite markers, population genetics
1. Introduction
Insect herbivores rely on living plants for food and habitat. Accordingly, host plants are among the most important ecological factors that drive genetic diversity within and among insect herbivore populations [1]. Insect herbivores are also exposed to selection pressure from several factors such as agricultural practices including spatial and temporal cropping patterns and insecticide usage that can influence insect population genetics [2,3,4,5,6,7,8]. Together, these selective forces may act at the level of a “farmscape”, which is a heterogenous habitat with crop and non-crop areas that herbivores can relatively easily move between [2]. For instance, farmscapes defined by a single ephemeral crop and managed with similar agricultural practices may favor selection for a narrow range of herbivore traits that closely match those relatively homogeneous conditions. On the other hand, very diverse farmscapes that include many crop and non-crop plant species managed using different practices can encourage the maintenance of genetically diverse herbivore populations with the broad variety of traits needed to exploit different habitats; this may be particularly true for polyphagous herbivores capable of exploiting many different host plant species. In either case, selective pressures at the farmscape level, coupled with reproductive isolation, can result in the development of host- or farmscape-associated genetic differentiation [3,4] and landscape/farmscape-associated populations [5,6,7].
Sweetpotato whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) is a serious pest of open-field crop production systems throughout the world. Nymphs and adults of B. tabaci are phloem feeders and are typically found on the abaxial leaf surfaces of their hosts [8,9]. Their direct feeding causes phytotoxic effects to crops such as silvering in leaves of squash (Cucurbita pepo L.) and irregular fruit ripening in tomato (Solanum lycopersicum L.) [10,11,12]. Aside from causing direct feeding damage, B. tabaci transmits multiple plant-pathogenic viruses to important crops [13,14,15]. Bemisia tabaci is a species complex that encompasses more than 40 cryptic species [16,17,18]. Middle East–Asia Minor 1 (MEAM1, formerly known as the B biotype) and Mediterranean (MED, formerly known as the Q biotype) are the two most invasive members of B. tabaci worldwide [19,20]. MEAM1 cryptic species of B. tabaci was first reported in the United States in the mid-1980s, and has since become the predominant cryptic species in the country [21,22,23]. It readily colonizes squash, watermelon (Citrullus lanatus L.), cantaloupe (Cucumis melo L.), tomato, snap bean (Phaseolus vulgaris L.), and other vegetable crops, while transmitting a wide range of plant viruses in the southwestern and southeastern United States [24]. In 2004, B. tabaci MED was first documented on poinsettia (Euphorbia pulcherrima Willd. ex Klotsch) in Arizona [25]. Since then, B. tabaci MED has rapidly spread in the United states, but is restricted primarily to greenhouse-grown ornamentals [26].
Numerous studies have examined the population genetics of B. tabaci at broad spatial scales [27,28,29,30,31,32], but less is known about how the genetic differentiation and diversity of B. tabaci populations vary at a farmscape level, i.e., between spatially adjacent crop and non-crop habitats. Variability in host-plant resistance, cropping patterns, landscape composition and configuration, and insecticide application patterns all might alter B. tabaci genetic population structure [4,33,34,35]. In turn, documenting low or high rates of gene flow can allow crop managers to determine if whiteflies fall into local or regional populations, so that pest control efforts can be organized at the appropriate scale. The mitochondrial cytochrome oxidase subunit I (mtCOI) gene, which mutates at a rapid rate compared with nuclear genes, has typically been used for studying B. tabaci evolutionary patterns and phylogenetic relationships [36,37,38,39]. The partial sequence of mtCOI gene is most effective as a molecular marker for the taxonomy and identification of species within the genus Bemisia because of the similarities in the morphology of whitefly adults and pupae of several members within this species complex [19,40]. However, the usefulness of mtCOI as a molecular marker to exclusively identify differences at a population level may be limited by its lack of resolution. Insect herbivores’ population structure could be more effectively examined by microsatellite markers due to their ease of use, high polymorphism, co-dominant inheritance, and even distribution throughout the genome [41]. Many microsatellite markers have been developed for whitefly population genetic analysis, identification of hybrids between cryptic species, determination of insecticide resistance levels among populations, and population structure of B. tabaci at a broad scale [26,28,29,30,31,32,42].
To assess the roles of host plants and farmscapes as drivers of the population structure of B. tabaci in Georgia, we collected whiteflies from 35 populations, 14 plant species, and 15 farmscapes (defined here as an area ~8 square kilometers and at least 16 km apart). Whiteflies were then identified to the species level using a combination of morphology and mtCOI sequencing, and B. tabaci populations were grouped into MED and MEAM1 cryptic species [43]. We then used six polymorphic microsatellite markers to determine the population structure of the B. tabaci MEAM1 populations in order to determine whether B. tabaci MEAM1 populations exhibited genetic differentiation by (1) host plant and/or (2) farmscape.
2. Materials and Methods
2.1. Whitefly Collections
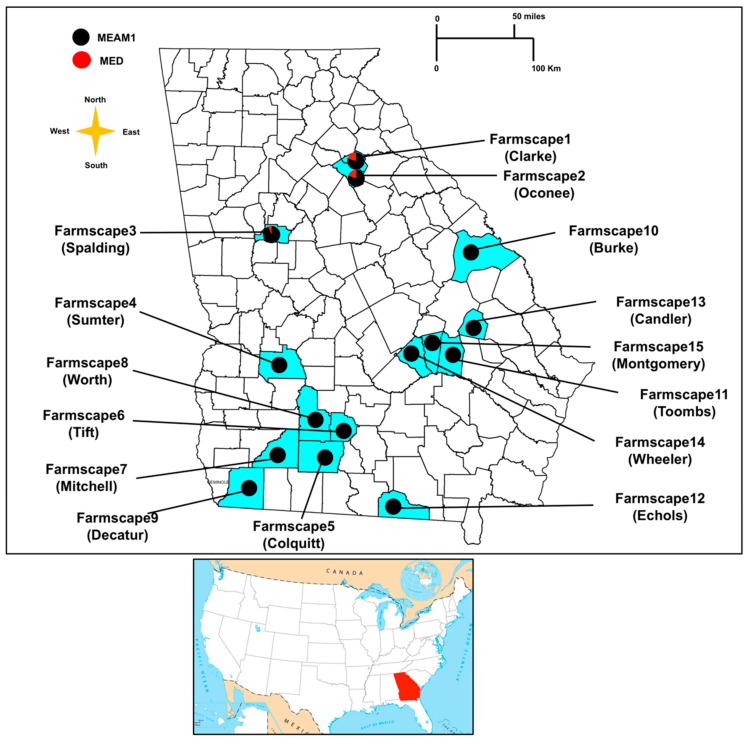
A total of 35 different populations of whiteflies were collected from 15 farmscapes located in 15 different counties of Georgia, USA (Table 1). Here, “population” refers to whiteflies collected from a single plant species, while “farmscape” is a broader area as defined above [44,45]. For every population, approximately 100 whitefly adults were collected from 5 to 10 different plants of the same species that were at least 1 m apart using an aspirator. Samples were stored in 95% ethanol at −80 °C until DNA extraction. For population structure analyses, populations were either grouped by host plants or farmscapes.
Table 1.
Collection data for whiteflies analyzed in this study.
| Population Number | Farmscape | County | Host Plant | Collection Date | GPS Coordinates (DMS) a |
|---|---|---|---|---|---|
| 1 | Farmscape 1 | Oconee | Snap bean (Phaseolus vulgaris L.) | 08/23/2019 | 33°43’26.5″ N 83°19’41.5″ W |
| 2 | Farmscape 2 | Clarke | Lantana (Lantana camara L.) | 08/23/2019 | 33°54’02.9″ N 83°22’56.6″ W |
| 3 | Farmscape 3 | Spalding | Okra (Abelmoschus esculentus (L.) | 07/19/2019 | 33°15’48.0″ N 84°18’25.5″ W |
| 4 | Farmscape 3 | Spalding | Dandelion (Taraxacum officinale Weber) | 07/20/2019 | 33°15’57.1″ N 84°18’22.4″ W |
| 5 | Farmscape 3 | Spalding | Eggplant (Solanum melongena L.) | 07/20/2019 | 33°15’46.7″ N 84°17’30.6″ W |
| 6 | Farmscape 3 | Spalding | Squash (Cucurbita pepo L.) | 07/21/2019 | 33°15’45.2″ N 84°17’06.8″ W |
| 7 | Farmscape 4 | Sumter | Cotton (Gossypium hirsutum L.) | 08/19/2019 | 32°02’35.2″ N 84°22’13.4″ W |
| 8 | Farmscape 5 | Colquitt | Cotton (Gossypium hirsutum L.) | 08/01/2019 | 31°11’32.0″ N 83°40’18.6″ W |
| 9 | Farmscape 5 | Colquitt | Squash (Cucurbita pepo L.) | 08/01/2019 | 31°12’07.6″ N 83°40’10.8″ W |
| 10 | Farmscape 5 | Colquitt | Eggplant (Solanum melongena L.) | 08/01/2019 | 31°11’23.5″ N 83°43’41.2″ W |
| 11 | Farmscape 6 | Tift | Snap bean (Phaseolus vulgaris L.) | 07/23/2019 | 31°28’17.7″ N 83°31’47.7″ W |
| 12 | Farmscape 6 | Tift | Squash (Cucurbita pepo L.) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 13 | Farmscape 6 | Tift | Tomato (Solanum lycopersicum Mill) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 14 | Farmscape 6 | Tift | Soybean (Glycine max Merrill) | 07/23/2019 | 31°29’01.3″ N 83°31’18.3″ W |
| 15 | Farmscape 6 | Tift | Tobacco (Nicotiana tabacum L.) | 07/23/2019 | 31°28’13.0″ N 83°31’54.1″ W |
| 16 | Farmscape 6 | Tift | Cotton (Gossypium hirsutum L.) | 08/13/2019 | 31°30’07.5″ N 83°32’43.0″ W |
| 17 | Farmscape 7 | Mitchell | Cotton (Gossypium hirsutum L.) | 08/16/2019 | 31°16’49.0″ N 84°17’38.1″ W |
| 18 | Farmscape 7 | Mitchell | Horseweed (Conyza canadensis L.) | 08/16/2019 | 31°16’39.8″ N 84°17’54.5″ W |
| 19 | Farmscape 8 | Worth | Cotton (Gossypium hirsutum L.) | 08/06/2019 | 31°35’19.6″ N 83°49’38.4″ W |
| 20 | Farmscape 8 | Worth | Redroot pigweed (Amaranthus retroflexus L.) | 08/06/2019 | 31°35’08.4″ N 83°50’03.3″ W |
| 21 | Farmscape 9 | Decatur | Cotton (Gossypium hirsutum L.) | 08/16/2019 | 30°45’49.7″ N 84°29’09.7″ W |
| 22 | Farmscape 10 | Burke | Cotton (Gossypium hirsutum L.) | 08/15/2019 | 32°52’35.1″ N 82°13’05.2″ W |
| 23 | Farmscape 11 | Toombs | Soybean (Glycine max Merrill) | 08/15/2019 | 32°01’03.2″ N 82°13’15.5″ W |
| 24 | Farmscape 11 | Toombs | Cotton (Gossypium hirsutum L.) | 08/15/2019 | 32°00’55.2″ N 82°13’19.8″ W |
| 25 | Farmscape 12 | Echols | Cotton (Gossypium hirsutum L.) | 08/01/2019 | 30°38’47.1″ N 83°01’42.8″ W |
| 26 | Farmscape 12 | Echols | Squash (Cucurbita pepo L.) | 08/01/2019 | 30°37’43.1″ N 83°02’20.0″ W |
| 27 | Farmscape 12 | Echols | Eggplant (Solanum melongena L.) | 08/01/2019 | 30°39’56.9″ N 83°01’54.9″ W |
| 28 | Farmscape 12 | Echols | Lantana (Lantana camara L.) | 08/01/2019 | 30°39’56.9″ N 83°01’54.9″ W |
| 29 | Farmscape 13 | Candler | Cotton (Gossypium hirsutum L.) | 09/02/2019 | 32°25’44.0″ N 82°04’50.7″ W |
| 30 | Farmscape 13 | Candler | Snap bean (Phaseolus vulgaris L.) | 09/02/2019 | 32°25’52.5″ N 82°04’27.5″ W |
| 31 | Farmscape 13 | Candler | Squash (Cucurbita pepo L.) | 09/02/2019 | 32°26’03.6″ N 82°03’59.1″ W |
| 32 | Farmscape13 | Candler | Redroot pigweed (Amaranthus retroflexus L.) | 09/02/2019 | 32°26’03.6″ N 82°03’59.1″ W |
| 33 | Farmscape 13 | Candler | Purple morning glory (Ipomoea purpurea L.) | 09/02/2019 | 32°26’15.7″ N 82°03’52.4″ W |
| 34 | Farmscape 14 | Wheeler | Cotton (Gossypium hirsutum L.) | 08/21/2019 | 32°06’12.3″ N 82°48’21.6″ W |
| 35 | Farmscape 15 | Montgomery | Cotton (Gossypium hirsutum L.) | 08/21/2019 | 32°11’59.7″ N 82°30’12.6″ W |
a Coordinates are in the degrees, minutes, seconds format (DMS).
2.2. DNA Extraction
Total DNA was extracted from individual whiteflies using InstaGene Matrix containing six percent Chelex resin (Bio-Rad, Hercules, CA, USA). Individual whiteflies were homogenized in 1 mL of autoclaved distilled water in a 1.5 mL microcentrifuge tube and centrifuged for 1 min at 12,000 rpm. The supernatants were discarded and 50 μL of InstaGene matrix was added to the pellet. Microcentrifuge tubes were then incubated at 56 °C for 20 min and vortexed for 10 s. The tubes were again incubated for 8 min at 100 °C, vortexed for 10 s, and centrifuged at 12,000 rpm for 3 min. Extracted DNA was stored at −20 °C until used.
2.3. Determination of Whitefly Species
Collected whitefly populations were placed in individual 9 cm Petri dishes, and under a dissecting microscope at 20× magnification, individuals were grouped into species using a whitefly identification guide [46]. Species identity was further confirmed by amplifying and sequencing the 5′ end of the mitochondrial DNA barcode region of three representative whiteflies from each group using universal primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) [43]. Polymerase chain reaction (PCR) was conducted using 2X GoTaq® Green Master Mix (Promega, Madison, WI, USA) in an Eppendorf Mastercycler® pro thermocycler (Eppendorf, Hamburg, Germany). The 50 μL PCR mixture contained 25 μL of Master Mix, 0.5μM of forward and reverse primers, 20 ng DNA, and nuclease-free water. PCR conditions were 5 min of initial denaturation followed by five cycles of 40 s at 94 °C, 40 s at 45 °C, and 60 s at 72 °C; and then 35 cycles of 40 s at 94 °C, 40 s at 51 °C, and 60 s at 72 °C, and a final extension period of 72 °C for 10 min [47]. Successful amplification was confirmed by running 10 μL of PCR products on 1% agarose gels stained with GelRed (Biotium, Fremont, CA, USA). Remaining PCR products were purified using the GeneJET PCR Purification Kit as per the manufacturer’s instructions (ThermoFisher Scientific, Waltham, MA, USA). Purified PCR products were sequenced using the SimpleSeq Kit (Eurofins Genomics, Louisville, KY, USA), and whitefly species identity was confirmed using Basic Local Alignment Search Tool (BLAST) available at the National Center for Biotechnology Information (NCBI) webpage.
The mtCOI region amplified by primer pairs LCO1490 and HCO2198 was not effective in differentiating B. tabaci cryptic species. Therefore, B. tabaci individuals (three per population: 35 × 3 = 105) were further identified to the cryptic species level by amplifying and sequencing 867 bp of the 3′end of mtCOI gene using the primers and conditions described by Mugerwa et al. 2018 [18]. Briefly, 0.5 μM of the primers 2195Bt (5′-TGRTTTTTTGGTCATCCRGAAGT-3′) and C012/Bt-sh2 (5′-TTTACTGCACTTTCTGCC-3′) were combined with 20 ng DNA, 2× GoTaq Green Master Mix, and nuclease-free water to a final reaction volume of 50 μL. PCR was performed in an Eppendorf Mastercycler® pro thermocycler with an initial denaturation at 94 °C for 5 min followed by 40 cycles of 40 s at 94 °C, 40 s at 52 °C, and 60 s at 72 °C, and a final extension period of 72 °C for 10 min. PCR products were purified and sequenced as described above. Bemisia tabaci cryptic species determinations were based on direct sequence comparisons using the web based NCBI BLAST sequence comparison application. Whitefly species delimitation was based on 3.50% partial mtCOI gene sequence divergence [19]. Bemisia tabaci individuals were designated as MEAM1 or MED based on ≥96.50% mtCOI sequence similarity with the MEAM1 (GenBank accession number KR559508) and MED (GenBank accession numbers MH205753) mtCOI reference sequences.
2.4. Haplotype Analysis
Haplotype analysis was carried out using 105 B. tabaci (102 MEAM1 and three MED) sequences obtained through 2195Bt and C012/Bt-sh2 primers (GenBank accession numbers MW024919–MW024949, MW025170–MW025179, MW025184–MW025197, MW031122–MW031131, MW046877–MW046891, MW160137–MW160161). Sequences were aligned using MUSCLE in MEGA X [48], and the number of haplotypes were determined based on aligned sequence fragments using DnaSP version 4.10.0 [49]. Minimum spanning haplotype network between B. tabaci MEAM1 and B. tabaci MED haplotypes was constructed using PopART software [50].
2.5. Microsatellite Genotyping
For each population, twelve females of B. tabaci were genotyped at six loci using the following primers: BEM6, BEM11, BEM15, BEM23, BEM25, and BEM31 [51]. PCR amplification with microsatellite primers was conducted in 12.5 μL reactions composed of 6.25 μL of 2× Type-it Multiplex PCR Master Mix (QIAGEN, Germantown, MD, USA), 1 μL of sterile water, 1.25 μL of forward and reverse primer mix (10 pmol), and 20 ng of DNA template. The forward primers were labeled with the fluorescein derivative 5-carboxyfluorescein (FAM) for microsatellite scoring. The PCR conditions included 94 °C for 7 min, followed by 35 cycles of 1 min at 94 °C, 1 min at 47 °C, 1 min at 73 °C, and a final extension of 72 °C for 1 h. Ready-to-run genotyping reaction solution was generated by mixing 1 μL of PCR products with 9 μL formamide and 1 μL ROX 500 size standard. In 96 well polypropylene micro-titer plates, 1 μL genotyping reaction solution was sent to the Georgia Genomics and Bioinformatics Core (UGA, Athens, GA, USA) for genotyping and amplicon size analysis. In order to estimate the genotyping error rates, duplicate genotypes were generated for each sample across all loci. Alleles were treated as missing data in cases of amplification failure, presence of three or more peaks in the electropherogram, peaks having insufficient height, or mismatches between duplicates. Based on the size of PCR products, two microsatellite markers BEM6 and BEM23 were used for the identification of cryptic species of B. tabaci [52]. All genotyped females (n: 12 × 35 = 420) were identified to cryptic species as MEAM1 or MED using these loci. Since only nine out of 420 genotyped females were identified as MED, further population genetic analyses were limited to the 411 MEAM1 genotypes from 35 populations representing 13 host plants and 15 farmscapes (Microsatellite Dataset: https://doi:10.5061/dryad.xgxd254f7).
2.6. Genetic Diversity of B. tabaci MEAM1
Genetic diversity across loci was estimated with several descriptive statistics: number of alleles, evenness of allele frequency, observed heterozygosity, expected heterozygosity, inbreeding coefficient (FIS), and fixation index (FST), using the ‘poppr’ package in R version 3.6.0 [53,54]. Genetic diversity across populations was estimated according to: number of alleles, number of effective alleles, observed heterozygosity, expected heterozygosity, Shannon’s information index and FST using GenAlEx6.5 [55]. Whitefly genotypes from each population and locus were assessed for the presence of null alleles, departure from the Hardy–Weinberg equilibrium (HWE), and for linkage disequilibrium between locus pairs using the ‘poppr’ R package.
Mutation-drift equilibrium among populations was tested using BOTTLENECK v.1.2.02 [56]. The probability of a bottleneck (indicated by significant heterozygote excess) in each population was estimated using a one-tailed Wilcoxon sign-rank test (p < 0.05) according to three models: infinite alleles model (IAM), two-phase model (TPM), and stepwise mutation model (SMM) (parameters for TPM: variance = 30.0%, probability = 70.0%, 1000 replications). The probability of a bottleneck was estimated using the Wilcoxon sign-rank test [56]. Signatures of bottlenecks in populations were visually confirmed by examining mode shifts in populations’ allele frequency distributions, where a relative underrepresentation of low-frequency alleles was considered a recent population bottleneck [57].
2.7. Genetic Differentiation among B. tabaci MEAM1 Populations
Pairwise FST, analysis of molecular variance (AMOVA), genetic isolation by distance (Mantel test), STRUCTURE, and principal components analysis (PCA) were used to characterize the population structure and genetic differentiation among collected B. tabaci MEAM1 populations. First, pairwise FST was calculated using 10,000 bootstrap pseudoreplicates over loci, while accounting for null alleles, using the ‘poppr’ R package. Significance of pairwise FST values was estimated with the ‘hierfstat’ R package with 999 permutations [58]. Values of pairwise FST < 0.05 were taken as evidence of low differentiation among the populations, while values of FST > 0.15 were taken as evidence of high genetic differentiation [29]. Second, AMOVA was performed in R using the ‘poppr’ R package to partition the genetic variance among farmscapes, among host plants within a farmscape, among populations and within populations [53], and significance was tested with 1000 permutations using the randtest function in the ‘ade4’ R package [59]. Third, correlation between pairwise genetic distance (FST/(1 − FST)) and pairwise geographic distance (Ln km) between all pairs of populations was analyzed by Mantel test (9999 permutations) using GenAlEx6.5 [55]. Fourth, Bayesian clustering was implemented in STRUCTURE v2.3.4 [60]. The admixture ancestry model was run with the correlated allele frequency model to calculate the number of distinct genetic clusters (K). STRUCTURE was used to identify the distinct genetic clusters (K) within the dataset by detecting allele frequency differences and assigning the individuals to those clusters based on analysis of likelihoods. The range of possible distinct genetic clusters (K) was set from 1 to 10 with 20 runs for each genetic cluster. Clustering was analyzed with a burn-in period of 50,000 iterations and 1,000,000 Markov Chain Monte Carlo (MCMC) replicates. The most likely number of genetic clusters in the B. tabaci MEAM1 populations collected over the farmscape was estimated using log-likelihood values of each K and ΔK in STRUCTURE HARVESTER [57]. Fifth, PCA was implemented in R using ‘adegenet’ and ‘ade4′ R packages [59,61]. PCA was run using the dudi.pca function and the first three principal components (PC) encompassing the majority of genetic variability among host plants (PC1: 48.62%, PC2: 24.00%, and PC3: 14.76%) and farmscapes (PC1: 54.34%, PC2: 22.12%, and PC3: 11.06%) were retained.
3. Results
3.1. Determination of Whitefly Species
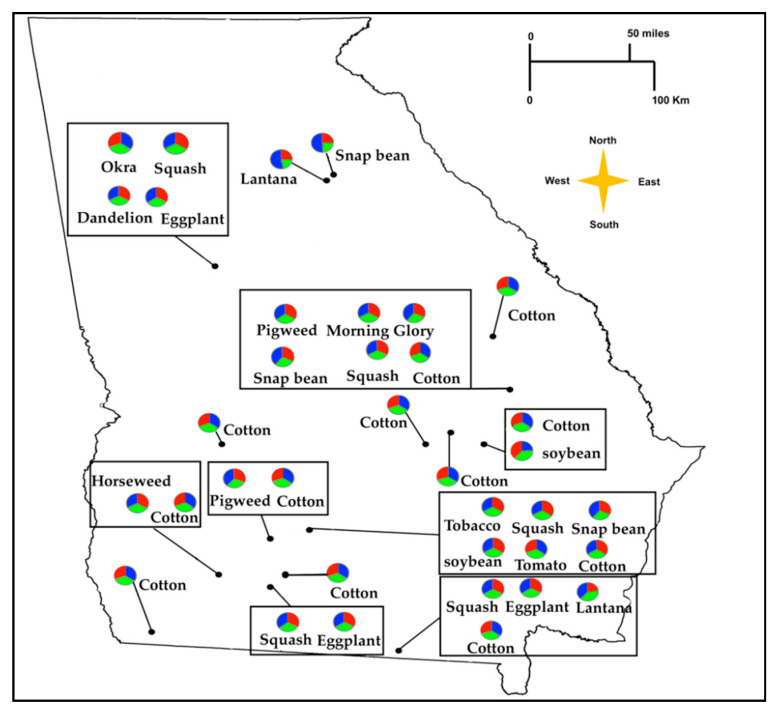
A total of three different whitefly species and two B. tabaci cryptic species were observed among the collected populations. The bandedwinged whitefly, Trialeurodes abutilonea Haldeman (GenBank accession number MT976143 −99.83% nucleotide similarity with GenBank reference sequence MG817067) and B. tabaci MEAM1 (GenBank accession number KR559508 −98.78% nucleotide similarity with GenBank reference sequence LN614546) were present at all collection sites. The greenhouse whitefly, Trialeurodes vaporariorum Westwood (GenBank accession number MT976141 −100.00% nucleotide similarity with GenBank reference sequence MK490855) was found on field squash in Spalding county. The citrus whitefly, Dialeurodes citri Ashmead (GenBank accession number MT976142 −96.81% nucleotide similarity with GenBank reference sequence JQ340192) was found on horseweed growing in the non-crop vegetation located next to cotton in Mitchell and Sumter counties. Bemisia tabaci MED was detected from snap bean, lantana, and eggplant in Clarke, Oconee, and Spalding counties, respectively (GenBank accession number MT976144 −99.75% nucleotide similarity with GenBank reference sequence MH205753). In farms from all three above-stated counties/farmscapes, mixed populations of B. tabaci MEAM1 and B. tabaci MED were present in the same field (Figure 1).
Figure 1.
Distribution of B. tabaci cryptic species (MEAM1 and MED) in Georgia in 2019 based on mtCOI sequences and microsatellite markers analysis. Pie charts represent the proportion of B. tabaci MEAM1 (black) and MED (red) individuals in collected populations.
3.2. Haplotype Analysis
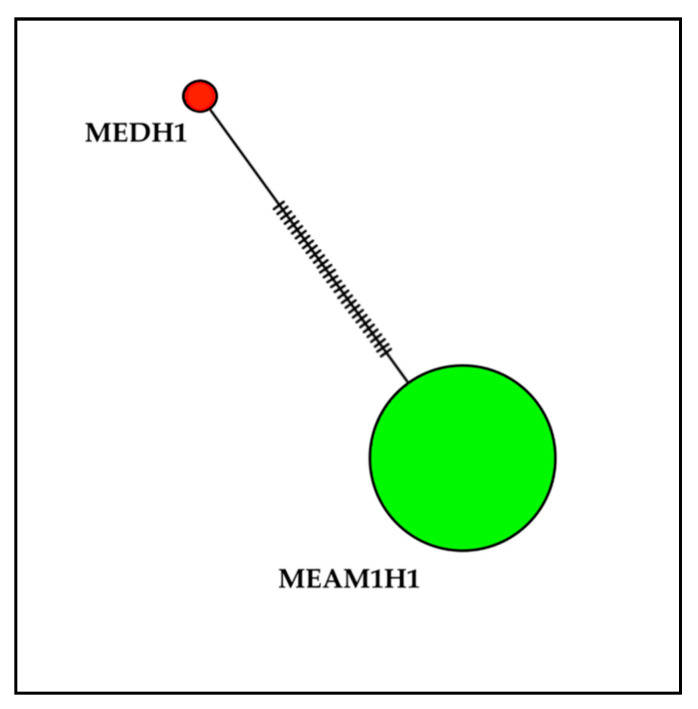
Analyses of B. tabaci mtCOI sequences revealed that out of 105 sequences, 102 were identified as B. tabaci MEAM1 and three were identified as B. tabaci MED. Bemisia tabaci MEAM1 mtCOI sequences had a 98.50 to 100.00% nucleotide identity to the reference sequence (GenBank accession number KR559508). Bemisia tabaci MED mtCOI sequences had a 99.50% to 99.75% nucleotide identity to the reference sequence (GenBank accession number MH205753). The mtCOI amino acid sequences of B. tabaci MEAM1 and MED were 100.00% identical to their respective reference sequences (MEAM 1: GenBank accession numbers KR559508; MED: GenBank accession number MH205753). Examination of aligned B. tabaci mtCOI sequences (690 bp) revealed the presence of one MEAM1 (MEAM1H1) and one MED (MEDH1) haplotype (Figure 2).
Figure 2.
Minimum spanning network of B. tabaci haplotypes based on mtCOI sequences. Size of the circles are proportional to the number of individuals in each haplotype. MEDH1 and MEAM1H1 are haplotypes of MED and MEAM1, respectively. Dashed lines between circles represent mutational steps.
3.3. Genetic Diversity of B. tabaci MEAM1
All loci exhibited variation among populations in evenness of allele frequencies (0.43–0.83) and expected heterozygosity (Hexp) (0.12–0.73), confirming the utility of these microsatellite markers for detecting variability among populations (Table 2). The average frequency of null alleles ranged from 0.021 to 0.12. The number of alleles per locus ranged from 4 to 7 (Table 2). The expected (Hexp) heterozygosity ranged from 0.12 (BEM23) to 0.73 (BEM15) and observed heterozygosity (Ho) ranged from 0.05 (BEM23) to 0.63 (BEM25) (Table 2). FIS, which describes the difference between observed and expected heterozygosity (FIS > 0 implies a heterozygote deficit and FIS < 0 implies heterozygote excess), ranged from −0.16 to 0.51 across the loci. Loci BEM6 and BEM23 had a significantly positive FIS value, suggesting heterozygote deficits at these loci. FST across loci ranged from 0.04 to 0.19 (Table 2). The highest FST was observed for the BEM6 marker. No population exhibited evidence of linkage disequilibrium between any loci.
Table 2.
Genetic diversity of thirty-five B. tabaci populations across six microsatellite markers.
| Locus | Number of Alleles | Evenness | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) | Fixation Index (FST) |
|---|---|---|---|---|---|---|
| BEM6 | 5.00 | 0.43 | 0.14 | 0.08 | 0.36 | 0.19 |
| BEM11 | 7.00 | 0.74 | 0.55 | 0.61 | −0.16 | 0.08 |
| BEM15 | 7.00 | 0.83 | 0.73 | 0.59 | 0.14 | 0.05 |
| BEM23 | 5.00 | 0.44 | 0.12 | 0.05 | 0.51 | 0.10 |
| BEM25 | 7.00 | 0.72 | 0.60 | 0.63 | 0.03 | 0.04 |
| BEM31 | 4.00 | 0.43 | 0.15 | 0.10 | 0.33 | 0.18 |
| Mean | 5.80 | 0.60 | 0.38 | 0.34 | 0.12 | 0.11 |
Genetic diversity observed in B. tabaci populations collected from different host plants and farmscapes are shown in Table 3 and Table 4. The mean number of alleles ranged from 2.00 to 4.83 and 2.33 to 4.00 for populations collected from different host plants and farmscapes, respectively (Table 3 and Table 4). The expected (Hexp) and observed heterozygosity (Ho) for populations from different host plants ranged from 0.30 to 0.42 and 0.29 to 0.44, respectively. For populations from different farmscapes, expected (Hexp) and observed heterozygosity (Ho) ranged from 0.32 to 0.49 and 0.14 to 0.47, respectively (Table 4). FIS for populations collected from different host plants ranged from −0.17 to 0.29 and FIS for populations collected from different farmscapes ranged from −0.29 to 0.55 (Table 3 and Table 4). Among collections by host plant, significant heterozygote excess was found among whiteflies collected from horseweed and tobacco, and whiteflies collected from eggplant exhibited a significant heterozygote deficit (Table 3). Among collections by farmscape, significant heterozygote excess was found in farmscape 14 and 15 (Wheeler and Montgomery counties, respectively), and significant heterozygote deficits were found in farmscape 3 and 4 (Spalding and Sumter counties, respectively) (Table 4).
Table 3.
Genetic diversity of B. tabaci MEAM1 populations collected from different plant species based on six microsatellites markers.
| Population | Sample Size | Mean number of Alleles | Shannon’s Information Index (I) | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) 1 |
|---|---|---|---|---|---|---|
| Cotton | 144 | 4.83 | 0.80 | 0.41 | 0.36 | 0.23 |
| Soybean | 24 | 3.17 | 0.72 | 0.35 | 0.37 | −0.08 |
| Squash | 60 | 4.33 | 0.72 | 0.37 | 0.38 | −0.04 |
| Tomato | 12 | 2.83 | 0.66 | 0.33 | 0.29 | 0.08 |
| Snap bean | 36 | 3.50 | 0.66 | 0.35 | 0.41 | 0.10 |
| Lantana | 24 | 3.33 | 0.74 | 0.38 | 0.35 | 0.25 |
| Horseweed | 10 | 2.83 | 0.70 | 0.35 | 0.40 | −0.15 |
| Pigweed | 24 | 3.17 | 0.71 | 0.40 | 0.31 | 0.22 |
| Okra | 12 | 2.00 | 0.53 | 0.39 | 0.32 | 0.21 |
| Dandelion | 12 | 2.50 | 0.61 | 0.38 | 0.44 | 0.01 |
| Eggplant | 36 | 4.17 | 0.86 | 0.42 | 0.32 | 0.29 |
| Tobacco | 12 | 2.67 | 0.57 | 0.31 | 0.37 | −0.17 |
| Morning glory | 12 | 2.50 | 0.55 | 0.30 | 0.29 | 0.01 |
| Mean | 32.15 | 3.22 | 0.68 | 0.37 | 0.30 | 0.10 |
1 Numbers indicated in bold font and underlined are significantly different from zero. Significant FIS indicates that populations are not mating randomly.
Table 4.
Genetic diversity of B. tabaci MEAM1 populations collected from different farmscapes based on six microsatellites markers.
| Population | Sample Size | Mean No. of Alleles | Shannon’s Information Index (I) | Expected Heterozygosity (Hexp) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FIS) 1 |
|---|---|---|---|---|---|---|
| Farmscape1 | 12 | 2.83 | 0.70 | 0.39 | 0.36 | 0.23 |
| Farmscape2 | 12 | 2.83 | 0.72 | 0.39 | 0.38 | 0.09 |
| Farmscape3 | 48 | 3.33 | 0.80 | 0.41 | 0.32 | 0.33 |
| Farmscape4 | 12 | 2.67 | 0.70 | 0.34 | 0.14 | 0.55 |
| Farmscape5 | 36 | 4.00 | 0.88 | 0.49 | 0.47 | 0.07 |
| Farmscape6 | 72 | 3.83 | 0.82 | 0.37 | 0.36 | −0.02 |
| Farmscape7 | 22 | 3.17 | 0.79 | 0.34 | 0.36 | −0.06 |
| Farmscape8 | 24 | 2.83 | 0.64 | 0.37 | 0.37 | 0.24 |
| Farmscape9 | 12 | 2.83 | 0.74 | 0.35 | 0.39 | 0.08 |
| Farmscape10 | 12 | 2.83 | 0.61 | 0.32 | 0.33 | −0.08 |
| Farmscape11 | 24 | 2.83 | 0.57 | 0.35 | 0.35 | 0.13 |
| Farmscape12 | 48 | 3.33 | 0.65 | 0.34 | 0.35 | 0.03 |
| Farmscape13 | 60 | 3.83 | 0.68 | 0.35 | 0.35 | 0.11 |
| Farmscape14 | 12 | 2.33 | 0.54 | 0.33 | 0.43 | −0.29 |
| Farmscape15 | 12 | 2.33 | 0.61 | 0.33 | 0.42 | −0.26 |
| Mean | 27.87 | 3.05 | 0.69 | 0.39 | 0.36 | 0.23 |
1 Numbers indicated in bold font and underlined are significantly different from zero. Significant FIS indicates that populations are not mating randomly.
Under all three population genetics models (IAM, TPM, and SMM) significant heterozygote excess was observed among whiteflies collected from okra (Farmscape 3, Spalding) and horseweed (Farmscape 7, Mitchell) (Table 5). However, a mode shift in the allele frequency distribution was only observed for whiteflies collected from horseweed (Table 5). Significant heterozygote excess was additionally detected among populations collected from farmscape 5, but only according to the IAM model. Among farmscapes, mode shifts—indicative of population bottlenecks—were apparent in populations collected from farmscapes 1, 2, 14, and 15 (Table 6). Overall, there was no evidence of widespread recent bottlenecks among B. tabaci MEAM1 populations.
Table 5.
Wilcoxon signed-rank test for mutation-drift equilibrium for 35 B. tabaci MEAM1 populations collected from different host plants, based on six microsatellite loci.
| Wilcoxon Test p-Values 1 | ||||
|---|---|---|---|---|
| Infinite Alleles Model IAM | Two-Phase Model TPM | Stepwise Mutation Model SMM | ||
| Host Plants | Heterozygosity Excess | Heterozygosity Excess | Heterozygosity Excess | Mode Shift |
| Cotton | 0.50 | 0.78 | 0.99 | L |
| Soybean | 0.31 | 0.50 | 0.68 | L |
| Squash | 0.71 | 0.98 | 1.00 | L |
| Tomato | 0.56 | 0.93 | 0.96 | L |
| Snap bean | 0.68 | 0.68 | 0.96 | L |
| Lantana | 0.31 | 0.50 | 0.89 | L |
| Horseweed | 0.06 | 0.06 | 0.06 | S |
| Pigweed | 0.31 | 0.31 | 0.40 | L |
| Okra | 0.03 | 0.03 | 0.03 | L |
| Dandelion | 0.31 | 0.68 | 1.00 | L |
| Eggplant | 0.40 | 0.68 | 1.00 | L |
| Tobacco | 0.06 | 0.12 | 0.81 | L |
| Morning glory | 0.13 | 0.13 | 0.81 | L |
1 Numbers indicated in bold font and underlined are significant at p < 0.05; L: normal L-shaped distribution; S: shifted mode distribution.
Table 6.
Wilcoxon signed-rank test for mutation-drift equilibrium for 35 B. tabaci MEAM1 populations collected from different farmscapes, based on six microsatellite loci.
| Wilcoxon Test p-Values 1 | ||||
|---|---|---|---|---|
| Infinite Alleles Model IAM | Two-Phase Model TPM | Stepwise Mutation Model SMM | ||
| Farmscapes | Heterozygosity Excess | Heterozygosity Excess | Heterozygosity Excess | Mode Shift |
| Farmscape1 | 0.92 | 0.40 | 0.89 | S |
| Farmscape2 | 0.31 | 0.41 | 0.41 | S |
| Farmscape3 | 0.05 | 0.31 | 0.41 | L |
| Farmscape4 | 0.56 | 0.84 | 0.94 | L |
| Farmscape5 | 0.04 | 0.42 | 0.96 | L |
| Farmscape6 | 0.78 | 0.57 | 0.98 | L |
| Farmscape7 | 0.63 | 0.63 | 0.63 | L |
| Farmscape8 | 0.05 | 0.40 | 0.59 | L |
| Farmscape9 | 0.84 | 0.84 | 1.00 | L |
| Farmscape10 | 0.16 | 0.16 | 0.56 | L |
| Farmscape11 | 0.41 | 0.69 | 0.92 | L |
| Farmscape12 | 0.58 | 0.92 | 0.98 | L |
| Farmscape13 | 0.59 | 0.92 | 0.98 | L |
| Farmscape14 | 0.06 | 0.06 | 0.06 | S |
| Farmscape15 | 0.06 | 0.06 | 0.06 | S |
1 Numbers indicated in bold font and underlined are significant at p < 0.05; L: normal L-shaped distribution; S: shifted mode distribution.
3.4. Genetic Differentiation among B. tabaci MEAM1 Populations
Pairwise FST values among B. tabaci MEAM1 populations collected from different host plants ranged from 0.01 to 0.05 and pairwise FST values among populations collected from different farmscapes ranged from 0.01 to 0.07 (Table 7 and Table 8). Overall, B. tabaci MEAM1 populations collected from different host plants and farmscapes exhibited low genetic differentiation. Pairwise FST values between populations collected from different host plants or farmscapes were low but were significant between numerous populations (Table 7 and Table 8). The highest significant pairwise FST value (0.05) for different hosts was found between tobacco and okra (Table 7) and highest pairwise significant FST (0.07) was observed between farmscape 4 and farmscape 15 (Table 8).
Table 7.
Pairwise FST values among B. tabaci MEAM1 populations collected from different host plants based on six microsatellites markers.
| Cotton | Soybean | Squash | Tomato | Snapbean | Lantana | Horseweed | Pigweed | Okra | Dandelion | Eggplant | Tobacco | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean | 0.01 | |||||||||||
| Squash | 0.01 | 0.02 | ||||||||||
| Tomato | 0.01 | 0.03 | 0.02 | |||||||||
| Snapbean | 0.01 | 0.02 | 0.02 | 0.01 | ||||||||
| Lantana | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | |||||||
| Horseweed | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | ||||||
| Pigweed | 0.01 | 0.03 | 0.02 | 0.03 | 0.01 | 0.02 | 0.03 | |||||
| Okra | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.04 | 0.01 | ||||
| Dandelion | 0.00 | 0.02 | 0.01 | 0.04 | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | |||
| Eggplant | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | ||
| Tobacco | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | 0.05 | 0.04 | 0.02 | |
| Morning Glory | 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.04 | 0.02 | 0.04 | 0.03 | 0.01 | 0.01 |
Pairwise FST values in bold font and underlined are significant at p < 0.05.
Table 8.
Pairwise FST values among B. tabaci MEAM1 populations collected from different farmscapes based on six microsatellites markers.
| Farmscape 1 |
Farmscape 2 |
Farmscape 3 |
Farmscape 4 |
Farmscape 5 |
Farmscape 6 |
Farmscape 7 |
Farmscape 8 |
Farmscape 9 |
Farmscape 10 |
Farmscape 11 |
Farmscape 12 |
Farmscape 13 |
Farmscape 14 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farmscape 2 | 0.04 | |||||||||||||
| Farmscape 3 | 0.02 | 0.01 | ||||||||||||
| Farmscape 4 | 0.04 | 0.04 | 0.02 | |||||||||||
| Farmscape 5 | 0.02 | 0.03 | 0.02 | 0.03 | ||||||||||
| Farmscape 6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | |||||||||
| Farmscape 7 | 0.03 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | ||||||||
| Farmscape 8 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |||||||
| Farmscape 9 | 0.03 | 0.04 | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | 0.01 | ||||||
| Farmscape 10 | 0.03 | 0.05 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | |||||
| Farmscape 11 | 0.03 | 0.03 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | ||||
| Farmscape 12 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| Farmscape 13 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Farmscape 14 | 0.05 | 0.05 | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | |
| Farmscape 15 | 0.06 | 0.06 | 0.03 | 0.07 | 0.02 | 0.01 | 0.02 | 0.04 | 0.04 | 0.06 | 0.05 | 0.03 | 0.02 | 0.02 |
Pairwise FST values in bold font and underlined are significant at p < 0.05
The analysis of molecular variance (AMOVA) revealed that most of the genetic variance was partitioned within populations (among and within individuals of a population) (Table 9). The variance partitioned among B. tabaci MEAM1 populations from different farmscapes and host plants was 2.00% (Table 9). Overall, AMOVA results suggested that there were no significant genetic differences among populations. Mantel test results revealed no correlation between genetic and geographic distances among populations (r2 = 0.0008, p = 0.429).
Table 9.
Hierarchical analysis of molecular variance (AMOVA) for the 35 B. tabaci MEAM1 populations collected from Georgia, USA, based on six microsatellite markers. (A) Among populations collected from different host plants. (B) Among populations collected from different farmscapes.
| Source of Variation | Degrees of Freedom | Sums of Squares | Mean Sums of Squares | % Variation | p-Value |
|---|---|---|---|---|---|
| A, Host Plants | |||||
| Among host plants | 12 | 34.35 | 2.86 | 2.00 | 0.39 |
| Among populations within a host plant | 22 | 36.51 | 1.66 | 1.00 | 0.32 |
| Among individuals within a population | 409 | 708.27 | 1.64 | 23.00 | <0.001 |
| Within individuals | 444 | 442.00 | 1.00 | 74.00 | <0.001 |
| Total | 887 | 1184.63 | 1.34 | 100.00 | |
| B, Farmscapes | |||||
| Among farmscapes | 14 | 45.88 | 3.28 | 2.00 | 0.43 |
| Among populations within a farmscape | 20 | 40.42 | 2.01 | 0.00 | 0.63 |
| Among individuals within a population | 409 | 680.32 | 1.59 | 22.00 | <0.001 |
| Within individuals | 444 | 446.50 | 1.01 | 76.00 | <0.001 |
| Total | 887 | 1172.69 | 1.32 | 100.00 | |
Significance at p < 0.01 based on 999 permutation.
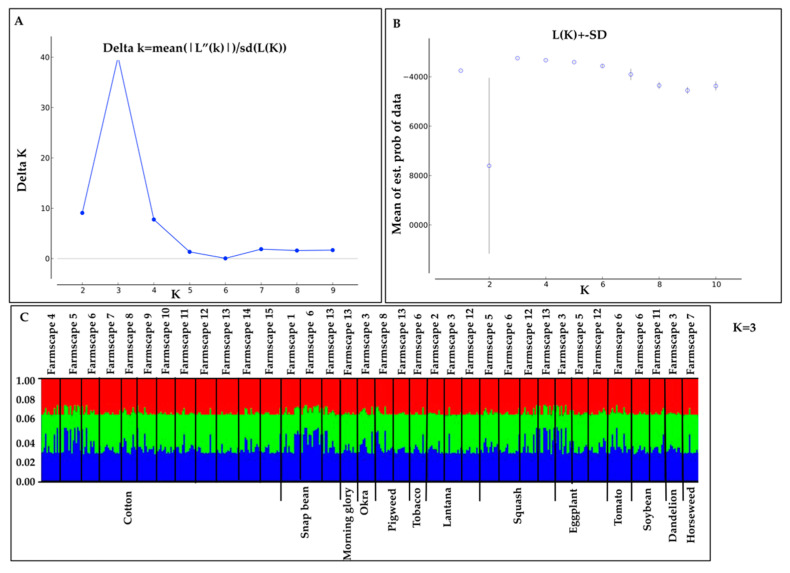
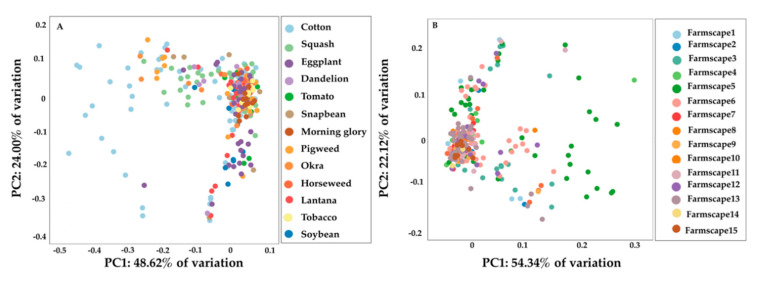
Bayesian cluster analysis performed using STRUCTURE identified K = 3 as the optimal number of genetic clusters according to log-likehood values of each K and ΔK (Figure 3A,B, Supplementary Figure S1). However, all populations exhibited a relatively even distribution of ancestry proportions from each genetic cluster, and separation by host plant or farmscape was not observed (Figure 3C, Figure 4). PCA results showed broad overlap among B. tabaci individuals along PC1 and PC2, which together accounted for 72.62% and 76.46% of the variation in microsatellite genotypes among whiteflies collected from host plants and farmscapes, respectively. (Figure 5).
Figure 3.
Bayesian clustering analysis results for 35 B. tabaci MEAM1 populations based on six microsatellite markers using STRUCTURE v.2.3.2. (A) Optimal number of genetic clusters (K = 3) following methods described by Evanno et al. 2005. (B) Plot of average likelihood L(K) and variance per K. (C) Scatter plots at K = 3. The length of each line in the bars represents the proportion of the genome in different clusters. Whitefly populations collected from different host plants or farmscapes are separated by a continuous vertical black line.
Figure 4.
Pie charts showing the proportion of alleles that were inherited from a postulated ancestral population to individuals of 35 B. tabaci MEAM1 populations collected from Georgia, USA, using Bayesian clustering implemented in STRUCTURE 2.3.4 at K = 3.
Figure 5.
Population structure based on principal component analysis for B. tabaci MEAM1 populations collected from different host plants (A), and farmscapes (B). Dots represent single individuals within the populations. All individuals are clustered together indicating minimum genetic isolation between individuals.
4. Discussion
Several factors, such as host plants and local agricultural practices, can influence the genetic diversity and population structure of insect pests inhabiting farmscapes [1,29,62]. The influence of such factors in shaping the population structure and genetics of insect pests such as whiteflies at the farmscape level has been sparsely explored. This study examined the genetic differentiation and structure of B. tabaci MEAM1 populations occurring in heterogeneous farmscapes of Georgia, USA. Partial mtCOI gene sequences and six nuclear microsatellite markers were utilized to examine patterns of genetic diversity and differentiation among populations of B. tabaci MEAM1. Comparison of partial mtCOI sequences led to the identification of a single predominant B. tabaci MEAM1 haplotype occurring throughout the farmscapes of Georgia. Analyses of microsatellite markers further revealed low levels of genetic diversity and differentiation among MEAM1 populations and found no evidence of host-or farmscape-associated differentiation. Overall, results show that a single panmictic population of B. tabaci MEAM1 dominates weeds and all crops across the farmscapes that we sampled. In addition to B. tabaci MEAM1 and MED cryptic species, three other whitefly species viz., D. citri, T. abutiloneus, and T. vaporariorum were also identified in the collected populations.
The citrus whitefly, D. citri is a serious citrus pest in Florida [63]. However, it is seldom considered a pest in vegetables and row crops in Georgia. Bandedwinged whiteflies, T. abutiloneus, and B. tabaci MEAM1 were present in all farmscapes, but B. tabaci MEAM1 was far more abundant than T. abutiloneus. Trialeurodes abutiloneus is native and widely distributed throughout United States. Although distributed throughout the farmscapes of Georgia, T. abutiloneus rarely reaches numbers that justify treatment with insecticides. The greenhouse whitefly, T. vaporariorum, was found on field-grown squash in Spalding county. There is growing evidence that T. vaporariorum may not necessarily be limited to greenhouse environments [64,65]. However, in the current study, T. vaporariorum was found in just one squash field located near urban landscapes. Therefore, the T. vaporariorum individuals that were collected might have dispersed into the squash field from a nearby greenhouse. Trialeurodes is the only whitefly genus other than Bemisia that has been documented as a plant virus vector [66]. Both T. abutiloneus and T. vaporariorum are reported vectors of plant viruses in the family Closteroviridae [65,66]. Bemisia tabaci MED cryptic species was found in snap bean and eggplant fields in Clarke and Spalding counties located in North Georgia, respectively. At both locations, MED individuals were present in the same field as MEAM1 and were limited in number (<15% of the individuals examined for each county). Both locations were in close proximity to urban landscapes; therefore, there is a high likelihood that these isolated MED individuals may have dispersed into these crops from nearby greenhouses or ornamentals. Bemisia tabaci MED has a high propensity to develop resistance to insecticides, and its presence in field-grown vegetables can have profound impacts on whitefly management programs [67]. Bemisia tabaci MED has replaced B. tabaci MEAM1 as the dominant whitefly in certain regions of China [68,69,70]. In the United States, since its documentation ~15 years ago, B. tabaci MED has been primarily restricted to ornamentals in greenhouses [67]. Recently, it has also been detected in residential landscapes in Florida [67]. Predicting what will trigger a B. tabaci MED outbreak in field crops and vegetables in the United States as in other places is not obvious. As of now, B. tabaci MEAM1 seems to be better adapted to the farmscapes in the southeastern United States than B. tabaci MED. In a recent study, McKenzie et al., also documented the reoccurrence of New World cryptic species (NW, biotype A) of B. tabaci in the United States following its disappearance in the late 1980s [26,71]. Our results provide no evidence for reoccurrence of the indigenous biotype-A within the farmscapes of Georgia; however, we acknowledge that its presence may have gone undetected due to the limited number of samples tested in this study.
Genetic differentiation (pairwise FST) between B. tabaci MEAM1 populations collected from different host plants or farmscapes was very low. Likewise, results from population structure analysis (AMOVA, STRUCTURE, and PCA) did not suggest evidence of host- or farmscape-specific genetic clustering. Furthermore, a test of isolation by distance (Mantel test) indicated no correlation between genetic differentiation and geographic distance among all populations. Taken together, results suggest that there could be extensive gene flow among whitefly populations inhabiting various crops and farmscapes aided by frequent wind-aided dispersal and high spatial synchrony among B. tabaci populations across farmscapes [72,73]. Low genetic diversity among B. tabaci MEAM1 populations observed in the current study could also be influenced by population bottlenecks, founder effects, or high mortality caused by insecticides [34,74,75]. Invasive insects such as B. tabaci MEAM1 often experience genetic bottlenecks that can lead to low genetic diversity [74,76]. In the current study, only one out of 13 populations collected from different host plants and one out of 15 populations collected from different farmscapes exhibited evidence of a genetic bottleneck. Overall, there was no substantial evidence for bottleneck effects driving the low genetic differentiation observed among B. tabaci MEAM1 populations in Georgia. Nevertheless, the heterozygote excess associated with population bottlenecks is not expected to last more than few generations [77]. This signature could rapidly erode in insects such as B. tabaci, which have high reproductive potential and can complete multiple generations (up to 12 in Georgia, United States) within a single calendar year [30]. Thus, signatures associated with earlier population bottleneck effects influencing B. tabaci MEAM1 populations since its introduction to the United States in the 1980s might not have been captured in this study.
Bemisia tabaci population genetic analyses carried out at fine spatial scales with no major geographical barriers tend to show no or minimal genetic differences among populations [32,78,79]. However, studies carried out over large geographical areas have reported substantial population structure [31,80]. These studies suggest that B. tabaci populations tend to cluster between regions isolated by geographical barriers. Results in this study are in agreement with earlier reports; the low level of genetic diversity observed in the current study might be influenced by lack of geographical barriers between populations. Furthermore, cropping patterns in Georgia might have also contributed to the low genetic differentiation in B. tabaci MEAM1 populations. In Georgia, summer cotton is planted within a rotation of spring, fall, and winter vegetable crops [81,82], essentially providing suitable host plants for B. tabaci MEAM1 year-around. Cotton is one of the most widely grown crops in Georgia; approximately 1.4 million acres of cotton were planted in 2019 [83]. Widespread availability of susceptible hosts and higher temperatures during the summer allow whiteflies to reproduce extensively, and cotton defoliation could trigger mass dispersal of whiteflies from cotton into fall-planted vegetable crops and also weeds. Over the years, this annual dispersal of whiteflies from cotton to nearby vegetation might have resulted in the genetic uniformity among B. tabaci MEAM1 populations across farmscapes.
Dispersal is a vital component of B. tabaci ecology, which not only enables host finding and colonization in constantly changing land cover, but also assists in distribution of favorable genetic traits such as insecticide resistance among populations [73]. In the current study, we did not find genetic differences between whiteflies collected from vegetables (squash, okra, tomato, eggplant, snap beans), row crops (cotton, soybean, tobacco), and weeds (horseweed, lantana), suggesting that whiteflies on vegetables and field crops might regularly disperse from weeds in the vicinity and vice-versa. Cases of insecticide resistance in B. tabaci have been well-documented in many parts of the world including in the southeastern United States [84,85,86,87]. Insect growth regulators, diamides, and neonicotinoids are vital classes of insecticides for integrated whitefly management programs in the southeastern United States [67,88,89,90]. A resistance gene arising against these insecticides can quickly disperse into interbreeding populations of B. tabaci MEAM1. Bemisia tabaci is haplodiploid, wherein the females are diploid, and the males are haploid. Because recessive alleles are always expressed in haploid males, recessive resistance traits can quickly become fixed in populations, especially those with a high ratio of males to females. Occurrence of such rapid fixation of insecticide resistance conferring alleles could essentially jeopardize management programs that rely on applications of insecticides in multiple crops. Knowledge about the genetic uniformity of B. tabaci populations over the farmscapes offers an intuitive avenue for slowing the evolution of insecticide resistance and enhancing sustainability in whitefly management. All above mentioned insecticide classes act differently on whiteflies (different modes of action) [91]. Therefore, rotation of these insecticides, along with robust insecticide resistance management programs, would not only slow the resistance evolution but also give a leeway to alter the insecticide application patterns if resistance arises anywhere in the farmscapes. For instance, if high levels of insecticide resistance can be ascribed to a single widespread genetic cluster, then insecticide applications can be adjusted across farmscapes accordingly.
5. Conclusions
Whitefly population genetics at broad spatial scales and with respect to invasion routes have been well studied [28,32]. However, less is known about levels of population structure among whitefly populations at finer scales such as farmscapes. Here, we find evidence that whitefly populations occurring in heterogeneous farmscapes comprise a single panmictic population. Such homogeneity among populations could arise from extensive gene flow, though the importance of a recent founder effect cannot be precluded. Extensive gene flow could facilitate the rapid spread of any new trait arising in a local population and warrants further investigation with higher-resolution genetic markers. Results from the current study provide clear evidence for the presence of a single panmictic population over the summer and early fall in Georgia and identify avenues where this information can be used in whitefly management programs. With such low genetic variation within summer and fall populations, one would expect the same B. tabaci MEAM1 genetic cluster to prevail and circulate with changing cropping patterns in cooler seasons. However, it remains to be empirically examined.
Acknowledgments
We thank Habibu Mugerwa for assistance rendered during sample collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/12/834/s1, Figure S1: Bayesian clustering analysis results for 35 B. tabaci MEAM1 populations based on six microsatellite markers using STRUCTURE v.2.3.2. Structure bar plots at K =1–10.
Author Contributions
Conceptualization, S.G. and R.S.; methodology, S.G.; software, S.G.; formal analysis, S.G. and M.S.C.; investigation, S.G. and M.S.C.; resources, R.S.; data curation, S.G.; Writing—Original draft preparation, S.G.; Writing—Review and editing, R.S., B.D., T.C., W.E.S., M.S.C., A.d.S., and A.M.S.; supervision, R.S., B.D., and T.C.; project administration, R.S.; funding acquisition, A.M.S., and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the USDA Non-Assistance Cooperative Agreement #58-6080-9-006 “Managing Whiteflies and Whitefly-transmitted Viruses in Vegetable Crops in the Southeastern U.S.” to the University of Georgia.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ehrlich P.R., Raven P.H. Butterflies and Plants: A Study in Coevolution. Evolution (N. Y.) 1964;18:586. [Google Scholar]
- 2.Philips C.R., Rogers M.A., Kuhar T.P. Understanding Farmscapes and Their Potential for Improving IPM Programs. J. Integr. Pest Manag. 2014;5:1–9. doi: 10.1603/IPM13018. [DOI] [Google Scholar]
- 3.Morales-Hojas R., Sun J., Iraizoz F.A., Tan X., Chen J. Contrasting population structure and demographic history of cereal aphids in different environmental and agricultural landscapes. Ecol. Evol. 2020;10:9647–9662. doi: 10.1002/ece3.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crossley M.S., Rondon S.I., Schoville S.D. Effects of contemporary agricultural land cover on Colorado potato beetle genetic differentiation in the Columbia Basin and Central Sands. Ecol. Evol. 2019;9:9385–9394. doi: 10.1002/ece3.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantz A., Plantegenest M., Mieuzet L., Simon J.-C. Ecological specialization correlates with genotypic differentiation in sympatric host-populations of the pea aphid. J. Evol. Biol. 2006;19:392–401. doi: 10.1111/j.1420-9101.2005.01025.x. [DOI] [PubMed] [Google Scholar]
- 6.Peccoud J., Ollivier A., Plantegenest M., Simon J.-C. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. USA. 2009;106:7495–7500. doi: 10.1073/pnas.0811117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Z., Li Y., Zhang Z. Genetic diversity of melon aphids Aphis gossypii associated with landscape features. Ecol. Evol. 2018;8:6308–6316. doi: 10.1002/ece3.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J.K., Frohlich D.R., Rosell R.C. The Sweetpotato or Silverleaf Whiteflies: Biotypes of Bemisia tabaci or a Species Complex? Annu. Rev. Entomol. 1995;40:511–534. doi: 10.1146/annurev.en.40.010195.002455. [DOI] [Google Scholar]
- 9.Schuster D.J., Stansly P.A., Polston J.E. In: Expressionsofplant Damage by Bemisia. Gerling D., Mayer R., editors. Intercept Ltd.; Andover, UK: 1996. [Google Scholar]
- 10.McCollum T., Stoffella P., Powell C., Cantliffe D., Hanif-Khan S. Effects of silverleaf whitefly feeding on tomato fruit ripening. Postharvest Biol. Technol. 2004;31:183–190. doi: 10.1016/j.postharvbio.2003.09.001. [DOI] [Google Scholar]
- 11.Yokomi R.K. Relationships Between the Sweetpotato Whitefly and the Squash Silverleaf Disorder. Phytopathology. 1990;80:895. doi: 10.1094/Phyto-80-895. [DOI] [Google Scholar]
- 12.Summers C.G. Chlorotic Streak of Bell Pepper: A New Toxicogenic Disorder Induced by Feeding of the Silverleaf Whitefly, Bemisia argentifolii. Plant Dis. 1996;80:822. doi: 10.1094/PD-80-0822A. [DOI] [Google Scholar]
- 13.Jones D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003;109:195–219. doi: 10.1023/A:1022846630513. [DOI] [Google Scholar]
- 14.Navas-Castillo J., Fiallo-Olivé E., Sánchez-Campos S. Emerging Virus Diseases Transmitted by Whiteflies. Annu. Rev. Phytopathol. 2011;49:219–248. doi: 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- 15.Gautam S., Gadhave K.R., Buck J.W., Dutta B., Coolong T., Adkins S., Srinivasan R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020;286:198069. doi: 10.1016/j.virusres.2020.198069. [DOI] [PubMed] [Google Scholar]
- 16.Boykin L.M., Shatters R.G., Jr., Rosell R.C., McKenzie C.L., Bagnall R.A., De Barro P., Frohlich D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007;44:1306–1319. doi: 10.1016/j.ympev.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Dinsdale A., Cook L., Riginos C., Buckley Y.M., De Barro P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010;103:196–208. doi: 10.1603/AN09061. [DOI] [Google Scholar]
- 18.Mugerwa H., Seal S., Wang H.L., Patel M.V., Kabaalu R., Omongo C.A., Alicai T., Tairo F., Ndunguru J., Sseruwagi P., et al. African ancestry of New World, Bemisia tabaci-whitefly species. Sci. Rep. 2018;8:2734. doi: 10.1038/s41598-018-20956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Barro P.J., Liu S.-S., Boykin L.M., Dinsdale A.B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 20.Wan F., Zhang G., Liu S., Luo C., Chu D., Zhang Y., Zang L., Jiu M., Lü Z., Cui X., et al. Invasive mechanism and management strategy of Bemisia tabaci (Gennadius) biotype B: Progress report of 973 Program on invasive alien species in China. Sci. China Ser. C Life Sci. 2009;52:88–95. doi: 10.1007/s11427-008-0135-4. [DOI] [PubMed] [Google Scholar]
- 21.Hamon A., Salguero V. Bemisia tabaci, sweetpotato whitefly, in Florida (Homoptera: Aleyrodidae: Aleyrodinae) Fla. Dep. Agric. Consum. Serv. Div. Plant Ind. 1987. Entomology circular No. 292.
- 22.Schuster D., Price J., King J., Everett P. Integrated management of the sweetpotato whiteßy on commercial tomato. Univ. Fla. IFAS Bradent. GCREC Res. Rep. 1989. BRA1989-12.
- 23.Hoelmer K.A., Osborne L.S., Yokomi R.K. Foliage Disorders in Florida Associated with Feeding by Sweetpotato Whitefly, Bemisia tabaci. Fla. Entomol. 1991;74:162. doi: 10.2307/3495258. [DOI] [Google Scholar]
- 24.Adkins S., Webster C.G., Kousik C.S., Webb S.E., Roberts P.D., Stansly P.A., Turechek W.W. Ecology and management of whitefly-transmitted viruses of vegetable crops in Florida. Virus Res. 2011;159:110–114. doi: 10.1016/j.virusres.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Dennehy T., Degain B., Harpold V., Brown J., Morin S., Fabrick J., Byrne F., Nichols R.L. New challenges to management of whitefly resistance to insecticides in Arizona. Univ. Ariz. Coop. Ext. Veg. Rep. 2005;T Series:144. [Google Scholar]
- 26.McKenzie C.L., Bethke J.A., Byrne F.J., Chamberlin J.R., Dennehy T.J., Dickey A.M., Gilrein D., Hall P.M., Ludwig S., Oetting R.D., et al. Distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) Biotypes in North America After the Q Invasion. J. Econ. Entomol. 2012;105:753–766. doi: 10.1603/EC11337. [DOI] [PubMed] [Google Scholar]
- 27.Dickey A.M., Osborne L.S., Shatters R.G., Hall P.M., Mckenzie C.L. Population Genetics of Invasive Bemisia tabaci (Hemiptera: Aleyrodidae) Cryptic Species in the United States Based on Microsatellite Markers. J. Econ. Entomol. 2013;106:1355–1364. doi: 10.1603/EC12512. [DOI] [PubMed] [Google Scholar]
- 28.Ben Abdelkrim A., Hattab T., Fakhfakh H., Belkadhi M.S., Gorsane F. A landscape genetic analysis of important agricultural pest species in Tunisia: The whitefly Bemisia tabaci. PLoS ONE. 2017;12:e0185724. doi: 10.1371/journal.pone.0185724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park Y., Nam H.Y., Baek S., Lee S.H., Lee J.-H. Population genetic structure of Bemisia tabaci MED (Hemiptera: Aleyrodidae) in Korea. PLoS ONE. 2019;14:e0220327. doi: 10.1371/journal.pone.0220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H.-R., Pan H.-P., Tao Y.-L., Zhang Y.-J., Chu D. Population genetics of an alien whitefly in China: Implications for its dispersal and invasion success. Sci. Rep. 2017;7:2228. doi: 10.1038/s41598-017-02433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz F., Endersby N.M., Hoffmann A.A. Genetic structure of the whitefly Bemisia tabaci populations in Colombia following a recent invasion. Insect Sci. 2015;22:483–494. doi: 10.1111/1744-7917.12129. [DOI] [PubMed] [Google Scholar]
- 32.Delatte H., David P., Granier M., Lett J.M., Goldbach R., Peterschmitt M., Reynaud B. Microsatellites reveal extensive geographical, ecological and genetic contacts between invasive and indigenous whitefly biotypes in an insular environment. Genet. Res. 2006;87:109–124. doi: 10.1017/S0016672306008135. [DOI] [PubMed] [Google Scholar]
- 33.Thomas S., Vanlerberghe-Masutti F., Mistral P., Loiseau A., Boissot N. Insight into the durability of plant resistance to aphids from a demo-genetic study of Aphis gossypii in melon crops. Evol. Appl. 2016;9:756–768. doi: 10.1111/eva.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brévault T., Carletto J., Tribot J., Vanlerberghe-Masutti F. Insecticide use and competition shape the genetic diversity of the aphid Aphis gossypii in a cotton-growing landscape. Bull. Entomol. Res. 2011;101:407–413. doi: 10.1017/S0007485310000635. [DOI] [PubMed] [Google Scholar]
- 35.Pannell J.R., Charlesworth B. Effects of metapopulation processes on measures of genetic diversity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000;355:1851–1864. doi: 10.1098/rstb.2000.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manani D., Ateka E., Nyanjom S., Boykin L. Phylogenetic Relationships among Whiteflies in the Bemisia tabaci (Gennadius) Species Complex from Major Cassava Growing Areas in Kenya. Insects. 2017;8:25. doi: 10.3390/insects8010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajaei Shoorcheh H., Kazemi B., Manzari S., Brown J.K., Sarafrazi A. Genetic variation and mtCOI phylogeny for Bemisia tabaci (Hemiptera, Aleyrodidae) indicate that the ‘B’ biotype predominates in Iran. J. Pest Sci. 2008;81:199–206. doi: 10.1007/s10340-008-0206-0. [DOI] [Google Scholar]
- 38.Baoli Q., Coats S.A., Shunxiang R., Idris A.M., Caixia X., Brown J.K. Phylogenetic relationship of native and introduced Bemisia tabaci (Homoptera: Aleyrodidae) from China and India based on mtCOI DNA sequencing and host plant comparisons. Prog. Nat. Sci. 2007;17:645–654. doi: 10.1080/10002007088537453. [DOI] [Google Scholar]
- 39.Brown W.M., George M., Wilson A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frohlich D.R., Torres-Jerez I., Bedford I.D., Markham P.G., Brown J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999;8:1593–1602. doi: 10.1046/j.1365-294x.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 41.Schlötterer C. Genealogical inference of closely related species based on microsatellites. Genet. Res. 2001;78:209–212. doi: 10.1017/S0016672301005444. [DOI] [PubMed] [Google Scholar]
- 42.Chu D., Guo D., Tao Y., Jiang D., Li J., Zhang Y. Evidence For Rapid Spatiotemporal Changes in Genetic Structure of an Alien Whitefly During Initial Invasion. Sci. Rep. 2015;4:4396. doi: 10.1038/srep04396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 44.Bayhan E., Ulusoy M.R., Brown J.K. Host range, distribution, and natural enemies of Bemisia tabaci ‘B biotype’ (Hemiptera: Aleyrodidae) in Turkey. J. Pest Sci. 2006;79:233–240. doi: 10.1007/s10340-006-0139-4. [DOI] [Google Scholar]
- 45.Athar H.-R., Bhatti A.R., Bashir N., Zafar Z.U., Abida, Farooq A. Modulating infestation rate of white fly (Bemicia tabaci) on okra (Hibiscus esculentus L.) by nitrogen application. Acta Physiol. Plant. 2011;33:843–850. doi: 10.1007/s11738-010-0609-4. [DOI] [Google Scholar]
- 46.Hodges G.S., Gregory A.E. An identification guide to the whiteflies (Hemiptera: Aleyrodidae) of the southeastern united states. Fla. Entomol. 2005;88:518–534. doi: 10.1653/0015-4040(2005)88[518:AIGTTW]2.0.CO;2. [DOI] [Google Scholar]
- 47.Ovalle T.M., Parsa S., Hernández M.P., Becerra Lopez-Lavalle L.A. Reliable molecular identification of nine tropical whitefly species. Ecol. Evol. 2014;4:3778–3787. doi: 10.1002/ece3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozas J., Sanchez-DelBarrio J.C., Messeguer X., Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 50.Leigh J.W., Bryant D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 51.De Barro P.J., Scott K.D., Graham G.C., Lange C.L., Schutze M.K. Isolation and characterization of microsatellite loci in Bemisia tabaci. Mol. Ecol. Notes. 2002;3:40–43. doi: 10.1046/j.1471-8286.2003.00344.x. [DOI] [Google Scholar]
- 52.Mckenzie C.L., Hodges G., Osborne L.S., Byrne F.J., Shatters R.G. Distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) Biotypes in Florida–Investigating the Q Invasion. J. Econ. Entomol. 2009;102:670–676. doi: 10.1603/029.102.0227. [DOI] [PubMed] [Google Scholar]
- 53.Kamvar Z.N., Tabima J.F., Grünwald N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ. 2014;2:e281. doi: 10.7717/peerj.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 55.Peakall R., Smouse P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piry S., Luikart G., Cornuet J.-M. Computer note. BOTTLENECK: A computer program for detecting recent reductions in the effective size using allele frequency data. J. Hered. 1999;90:502–503. doi: 10.1093/jhered/90.4.502. [DOI] [Google Scholar]
- 57.Luikart G. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998;89:238–247. doi: 10.1093/jhered/89.3.238. [DOI] [PubMed] [Google Scholar]
- 58.Goudet J. HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes. 2005;5:184–186. doi: 10.1111/j.1471-8286.2004.00828.x. [DOI] [Google Scholar]
- 59.Dray S., Dufour A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007;22 doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 60.Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jombart T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 62.Bagley R.K., Sousa V.C., Niemiller M.L., Linnen C.R. History, geography and host use shape genomewide patterns of genetic variation in the redheaded pine sawfly (Neodiprion lecontei) Mol. Ecol. 2017;26:1022–1044. doi: 10.1111/mec.13972. [DOI] [PubMed] [Google Scholar]
- 63.Fasulo T.R., Weems H.V. Citrus Whitefly, Dialeurodes citri (Ashmead) (Insecta: Hemiptera: Aleyrodidae) IFAS Ext. 1996;128:1–6. doi: 10.32473/edis-in241-2002. [DOI] [Google Scholar]
- 64.BI J.L., Toscano N.C., Ballmer G.R. Seasonal Population Dynamics of the Greenhouse Whitefly Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on Strawberries in Southern California. J. Econ. Entomol. 2002;95:1179–1184. doi: 10.1603/0022-0493-95.6.1179. [DOI] [PubMed] [Google Scholar]
- 65.Wintermantel W.M. Emergence of Greenhouse Whitefly (Trialeurodes vaporariorum) Transmitted Criniviruses as Threats to Vegetable and Fruit Production in North America. APSnet Featur. Artic. 2004 doi: 10.1094/APSnetFeature-2004-0604. [DOI] [Google Scholar]
- 66.Navas-Castillo J., López-Moya J.J., Aranda M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. Appl. Biol. 2014;165:155–171. doi: 10.1111/aab.12147. [DOI] [Google Scholar]
- 67.McKenzie C.L., Osborne L.S. Bemisia tabaci MED (Q biotype) (Hemiptera: Aleyrodidae) in Florida is on the Move to Residential Landscapes and May Impact Open-Field Agriculture. Fla. Entomol. 2017;100:481–484. doi: 10.1653/024.100.0213. [DOI] [Google Scholar]
- 68.Chu D., Wan F.H., Zhang Y.J., Brown J.K. Change in the Biotype Composition of Bemisia tabaci in Shandong Province of China From 2005 to 2008. Environ. Entomol. 2010;39:1028–1036. doi: 10.1603/EN09161. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z., Yan H., Yang Y., Wu Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010;66:1360–1366. doi: 10.1002/ps.2023. [DOI] [PubMed] [Google Scholar]
- 70.Yuan L., Wang S., Zhou J., Du Y., Zhang Y., Wang J. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 2012;31:67–71. doi: 10.1016/j.cropro.2011.09.017. [DOI] [Google Scholar]
- 71.Perring T.M. Biological differences of two species of Bemisia that contribute to adaptive advantage. In: Gerling D., Mayer R.T., editors. Bemisia 1995: Tax- Onomy, Biology, Damage Control and Management. Intercept Scientific, Medical and Technical Publications; Andover, UK: 1996. pp. 3–16. [Google Scholar]
- 72.Brewster C., Allen J., Schuster D., Stansly P.A. Simulating the dynamics of Bemisia argentifolii (Homoptera: Aleyrodidae) in an organic cropping system with a spatiotemporal model. Environ. Entomol. 1997;26:603–616. doi: 10.1093/ee/26.3.603. [DOI] [Google Scholar]
- 73.Naranjo S.E., Castle S.J., De Barro P.J., Liu S.-S. Bemisia: Bionomics and Management of a Global Pest. Springer; Dordrecht, The Netherlands: 2009. Population Dynamics, Demography, Dispersal and Spread of Bemisia tabaci; pp. 185–226. [Google Scholar]
- 74.Puillandre N., Dupas S., Dangles O., Zeddam J.-L., Capdevielle-Dulac C., Barbin K., Torres-Leguizamon M., Silvain J.-F. Genetic bottleneck in invasive species: The potato tuber moth adds to the list. Biol. Invasions. 2008;10:319–333. doi: 10.1007/s10530-007-9132-y. [DOI] [Google Scholar]
- 75.Orantes L.C., Zhang W., Mian M.A.R., Michel A.P. Maintaining genetic diversity and population panmixia through dispersal and not gene flow in a holocyclic heteroecious aphid species. Heredity. 2012;109:127–134. doi: 10.1038/hdy.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsutsui N.D., Suarez A.V., Holway D.A., Case T.J. Reduced genetic variation and the success of an invasive species. Proc. Natl. Acad. Sci. USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luikart G., Cornuet J.-M. Empirical Evaluation of a Test for Identifying Recently Bottlenecked Populations from Allele Frequency Data. Conserv. Biol. 2008;12:228–237. doi: 10.1111/j.1523-1739.1998.96388.x. [DOI] [Google Scholar]
- 78.Dalmon A., Halkett F., Granier M., Delatte H., Peterschmitt M. Genetic structure of the invasive pest Bemisia tabaci: Evidence of limited but persistent genetic differentiation in glasshouse populations. Heredity. 2008;100:316–325. doi: 10.1038/sj.hdy.6801080. [DOI] [PubMed] [Google Scholar]
- 79.Tahiri A., Halkett F., Granier M., Gueguen G., Peterschmitt M. Evidence of gene flow between sympatric populations of the Middle East-Asia Minor 1 and Mediterranean putative species of Bemisia tabaci. Ecol. Evol. 2013;3:2619–2633. doi: 10.1002/ece3.655. [DOI] [Google Scholar]
- 80.Simón B., Cenis J.L., De La Rúa P. Distribution patterns of the Q and B biotypes of Bemisia tabaci in the Mediterranean Basin based on microsatellite variation. Entomol. Exp. Appl. 2007;124:327–336. doi: 10.1111/j.1570-7458.2007.00586.x. [DOI] [Google Scholar]
- 81.Westerfield R. Vegetable Planting Chart. UGA Ext. 2019. Circular 963.
- 82.Lee R.D., Johnson J.T. Planting Guide for Row Crops in Georgia. UGA Ext. 2014. Circular 813.
- 83.Georgia Cotton Commisison. [(accessed on 10 August 2020)]; Available online: http://www.georgiacottoncommission.org/index.cfm?show=10&mid=5.
- 84.Nauen R., Stumpf N., Elbert A. Toxicological and mechanistic studies on neonicotinoid cross resistance in Q-type Bemisia tabaci (Hemiptera: Aleyrodidae) Pest Manag. Sci. 2002;58:868–875. doi: 10.1002/ps.557. [DOI] [PubMed] [Google Scholar]
- 85.Roditakis E., Roditakis N.E., Tsagkarakou A. Insecticide resistance in Bemisia tabaci (Homoptera: Aleyrodidae) populations from Crete. Pest Manag. Sci. 2005;61:577–582. doi: 10.1002/ps.1029. [DOI] [PubMed] [Google Scholar]
- 86.Cahill M., Byrne F.J., Gorman K., Denholm I., Devonshire A.L. Pyrethroid and organophosphate resistance in the tobacco whitefly Bemisia tabaci (Homoptera: Aleyrodidae) Bull. Entomol. Res. 1995;85:181–187. doi: 10.1017/S0007485300034258. [DOI] [Google Scholar]
- 87.Naveen N.C., Chaubey R., Kumar D., Rebijith K.B., Rajagopal R., Subrahmanyam B., Subramanian S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017;7:40634. doi: 10.1038/srep40634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellsworth P.C., Martinez-Carrillo J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001;20:853–869. doi: 10.1016/S0261-2194(01)00116-8. [DOI] [Google Scholar]
- 89.Palumbo J., Horowitz A., Prabhaker N. Insecticidal control and resistance management for Bemisia tabaci. Crop Prot. 2001;20:739–765. doi: 10.1016/S0261-2194(01)00117-X. [DOI] [Google Scholar]
- 90.Caballero R., Cyman S., Schuster D.J., Portillo H.E., Slater R. Baseline susceptibility of Bemisia tabaci (Genn.) biotype B in southern Florida to cyantraniliprole. Crop Prot. 2013;44:104–108. doi: 10.1016/j.cropro.2012.10.013. [DOI] [Google Scholar]
- 91.Sparks T.C., Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.