Abstract
Simple Summary
Antimicrobial resistance issues and growing consumer demand promote the need for antibiotic-free meat production. Fostering animal productivity without antibiotic growth promoters accelerates the use of non-antibiotic feed additives and encourages researchers to gain a deeper understanding of diet-gut microbiota interactions. Little information is available about the effects of single strain probiotic bacteria Clostridium butyricum and wheat bran on the gut microbiota of chickens using next-generation sequencing. Therefore, these components were evaluated in the present study on gut microbiota composition and other gut health characteristics of broiler chickens. Results showed that probiotic supplementation decreased cecal Akkermansia spp. abundance, whereas wheat bran supplementation increased the relative abundance of Akkermansia spp. compared to the control and symbiotic groups, respectively. Dietary treatment also altered cecal crypt depth and had a trend to modify cecal fermentation profiles. Besides, the combination of probiotic and wheat bran supplementation did not have further effects on any investigated parameters. Members of the Akkermansia genus have several beneficial health effects in mammals, but less is known about its role in chicken health. The results of the present study expand our understanding of diet-gut microbiota interaction in chickens, which helps to approximate antibiotic-free meat production.
Abstract
Feed additives that can improve intestinal health and maintain a diverse and resilient intestinal microbiota of poultry are of great importance. Thus, the current study investigated the effects of a single strain butyric acid-producing Clostridium (C. butyricum) with (symbiotic) or without wheat bran supplementation on cecal microbiota composition and gut health characteristics of broiler chickens. In total, 384 male Ross 308 day-old chickens were divided into four dietary treatment groups and fed ad libitum until day 37 of life. Cecal samples were taken for Illumina sequencing and pH and short-chain fatty acid analyses, as well as for histological analysis at the end of the experimental period. Neither of the supplemented diets improved chicken growth performance. Caecum was dominated by the members of Bacteroidetes phyla followed by Firmicutes in each dietary group. At the genus level, Bacteroides, Oscillospira, Akkermansia, Faecalibacterium, Ruminococcus and Streptococcus genera exceeded 1% relative abundance. Dietary treatment influenced the relative abundance of the Akkermansia genus, which had a lower relative abundance in the C. butyricum group than in the other groups and in the symbiotic group compared to the wheat bran supplemented group. Dietary treatment also altered cecal crypt depth and had a trend to modify the cecal fermentation profile. Additive effects of wheat bran and C. butyricum supplementation were not detected. Our results suggest that Akkermansia muciniphila colonization in chicken can be influenced by diet composition.
Keywords: chicken, wheat bran, microbiota, Akkermansia muciniphila, Clostridium butyricum, gut health
1. Introduction
There is growing interest in the development of alternatives to antibiotics in the poultry industry in order to substitute their beneficial effects, such as improved performance [1]. Intestinal immunity, integrity and functionality are three main components in the characterization of intestinal health status, which reflect gut health [2]. Microorganisms residing within the gastrointestinal tract, their metabolites, such as short-chain fatty acids (SCFA), characteristics of the intestinal wall and their interactions, substantially determine gut health [2,3]. Dietary manipulation of the microbiota could be a feasible way to optimize gut health and avoid performance losses due to impaired gut functions [4]. Over the past decade, next-generation sequencing (NGS) has become a tool to discover novelties with regard to relationships within the gut ecosystem [1]. Although numerous studies have been conducted on chicken gut microbiota with the help of NGS technologies, there is still a knowledge gap and inconsistency in results concerning the effects of pre- and probiotics on chicken gut health.
Wheat bran is a byproduct of the milling industry and contains high amounts of insoluble and soluble non-starch polysaccharides (NSPs), which cannot be digested directly by the host, and instead are degraded by hindgut microbiota. [5]. NSPs occur in the form of arabinoxylans (70%), cellulose (24%) and beta-glucans (6%) in wheat bran [6]. Studies performed in chickens mostly use high dietary inclusion rates (14–50%) of wheat bran, which has adverse effects by increasing intestinal viscosity [7]. In spite of negative effects, hosts may benefit from low amounts of wheat bran supplementation as it provides a dietary substrate for specific groups of intestinal bacteria [1]. The effects of moderate wheat bran supplementation and the combination of wheat bran with probiotics on the gut health and gut microbiota of broiler chickens at slaughter age has rarely been addressed previously [8,9].
Clostridium butyricum (C. butyricum) is a strict anaerobe, spore-forming and butyric acid-producing bacillus, which can be found in soil and in the intestines of healthy animals [10]. It can survive low pH and high bile concentrations. Clostridium butyricum seems to be a promising probiotic to provide protection against intestinal infections [11], and some reports show beneficial effects of C. butyricum strains on growth performance, lipid metabolism, immune function and culturable microbiota [12,13]. Although wheat bran and C. butyricum supplementations have been investigated from various aspects in broilers at slaughter age, little information is available on the effects of wheat bran using culture-independent methods [5,14,15,16]. The information concerning C. butyricum is limited only to culturable microbiota in broiler chickens. Because of the probiotic potential of C. butyricum mentioned above, and since it is commercially available for poultry, its effect on chicken gut microbiota is of great interest. We hypothesize that wheat bran and C. butyricum supplementation have beneficial effects on chicken growth performance and on gut physicochemical and histological characteristics, which may be correlated to certain gut microbiota changes. Therefore, the current study aims to reveal the effect of wheat bran and C. butyricum supplementation (either alone or in combination) on growth performance, pH and short-chain fatty acid composition of the cecal content, and the histology of the cecal gut wall in correlation with cecal microbiota composition.
2. Materials and Methods
2.1. Animals and Treatments
All husbandry and euthanasia procedures were performed in accordance with the Hungarian Government Decree 40/2013 and in full consideration of animal welfare ethics. The animal experiment was approved by the Institutional Ethics Committee (Animal Welfare Committee, Georgikon Campus, Szent István University) under the license number MÁB-9/2019. A total of 384 Ross 308 broiler hybrids were used in the experiment. Day-old broiler cockerels were purchased from a commercial hatchery and sorted randomly into 4 dietary treatment groups. Chickens were arranged in 4 replicate pens with 24 chicken per pen. Dietary treatment groups included: control group (C), wheat bran supplemented group, Clostridium butyricum (C. butyricum) supplemented group, and a combination of these (symbiotic). The control diet was based on corn and soybean. The composition and nutrient content analysis of the control and wheat bran diets are shown in Table 1 and Table 2. The chickens received starter (day 1–10), grower (day 11–24), and finisher (day 25–37) diets. Feed and water were provided ad libitum. Experimental diets were formulated according to recommendations for Ross 308 hybrids [17]. Probiotic supplementation consisted of spores of a single strain butyric acid-producing bacteria, C. butyricum CBM 588 (Miya-Gold®, Huvepharma, Sofia, Bulgaria). The wheat bran and symbiotic diets contained 3, 6 and 6% wheat bran in the starter, grower and finisher diets, respectively. Total arabinoxylan content was 90.3 mg/g, and water-extractable arabinoxylan content was 10.8 mg/g in the wheat bran diet. The C. butyricum and symbiotic diets contained 2.5 × 109 cfu/kg C. butyricum CBM 588 spores in each phase. Chickens were kept on chopped straw bedding in floor pens at a stocking density of 10 chickens/m2, which was in accordance with the European Union Council Directive 2007/43/CE, and the computer-controlled environmental conditions matched breeder recommendations [18].
Table 1.
Composition of experimental diets (g/kg as fed).
| Ingredient | Starter (Day 1 to 10 of Life) |
Grower (Day 11 to 24 of Life) |
Finisher (Day 25 to 37 of Life) |
|||
|---|---|---|---|---|---|---|
| C | Wheat Bran | C | Wheat Bran | C | Wheat Bran | |
| Maize | 466 | 434 | 534 | 469 | 589 | 524 |
| Wheat bran | 0 | 30 | 0 | 60 | 0 | 60 |
| ESM | 338 | 333 | 361 | 352 | 310 | 300 |
| Sunflower oil | 63 | 70 | 62 | 76 | 60 | 74 |
| Limestone | 19 | 19 | 15 | 15 | 15 | 15 |
| Sunflower meal | 80 | 80 | 0 | 0 | 0 | 0 |
| MCP | 15 | 15 | 14 | 14 | 13 | 13 |
| L-LYS HCL | 5 | 5 | 2 | 2 | 2 | 2 |
| DL-MET | 4 | 4 | 3 | 3 | 3 | 3 |
| L-THR | 1 | 1 | 1 | 1 | 0 | 1 |
| Val | 1 | 1 | 0 | 0 | 0 | 0 |
| NaCl | 3 | 3 | 3 | 3 | 3 | 3 |
| NaHCO3 | 1 | 1 | 1 | 1 | 1 | 1 |
| Premix 1 | 4 | 4 | 4 | 4 | 3.5 | 3.5 |
| Phytase 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| NSP enzyme 3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Abbreviations: C—control; ESM—extracted soybean meal; MCP—monocalcium phosphate; LYS—lysine; MET—methionine; THR—threonine; VAL—valine. 1 Premix was supplied by UBM Ltd. (Pilisvörösvár, Hungary). The active ingredients contained in the premix were as follows (per kg of diet): Starter and grower premixes—retinyl acetate—5.0 mg, cholecalciferol—130 µg, dl-alpha-tocopherol-acetate—91 mg, menadione—2.2 mg, thiamin—4.5 mg, riboflavin—10.5 mg, pyridoxin HCL—7.5 mg, cyanocobalamin—80 µg, niacin—41.5 mg, pantothenic acid—15 mg, folic acid—1.3 mg, biotin—150 µg, betaine—670 mg, monensin-Na—110 mg (only grower), narasin—50 mg (only starter), nicarbazin—50 mg (only starter), antioxidant—25 mg, Zn (as ZnSO4·H2O)—125 mg, Cu (as CuSO4·5H2O)—20 mg, Fe (as FeSO4·H2O)—75 mg, Mn (as MnO)—125 mg, I (as KI)—1.35 mg, Se (as Na2SeO3)—270 µg; Finisher premix—retinyl acetate—3.4 mg, cholecalciferol—97 µg, dl-alpha-tocopherol-acetate—45.5 mg, menadione—2.7 mg, thiamin—1.9 mg, riboflavin—5.0 mg, pyridoxin HCL—3.2 mg, cyanocobalamin—19 µg, niacin—28.5 mg, pantothenic acid—10 mg, folic acid—1.3 mg, biotin—140 µg, L-ascorbic acid—40 mg, betaine—193 mg, antioxidant—25 mg, Zn (as ZnSO4·H2O)—96 mg, Cu—9.6 mg, Fe (as FeSO4·H2O)—29 mg, Mn (as MnO)—29 mg, I (as KI)—1.2 mg, Se (as Na2SeO3)—350 µg. 2 Phytase was Quantum Blue® (AB Vista, Marlborough, UK). 3 NSP enzyme was Econase XT® (AB Vista, Marlborough, UK).
Table 2.
Analyzed nutrient content of experimental diets (g/kg as fed) 1.
| Ingredient | Starter (Day 1 to 10 of Life) |
Grower (Day 11 to 24 of Life) |
Finisher (Day 25 to 37 of Life) |
|||
|---|---|---|---|---|---|---|
| C | Wheat Bran | C | Wheat Bran | C | Wheat Bran | |
| AMEn (MJ/kg) 2 | 12.1 | 12.2 | 13.1 | 13.0 | 13.0 | 13.1 |
| Dry matter | 888 | 890 | 885 | 888 | 882 | 888 |
| Crude protein | 229 | 230 | 207 | 212 | 188 | 191 |
| Crude fat | 83 | 92 | 91 | 101 | 89 | 100 |
| Crude fiber | 40.2 | 45.8 | 37.7 | 41.8 | 36.3 | 43.3 |
| Crude ash | 66.9 | 68.3 | 56.1 | 59.6 | 54.3 | 56.9 |
| Ca | 10.7 | 10.8 | 9.4 | 9.4 | 8.9 | 8.9 |
| P | 8.0 | 8.1 | 6.7 | 7.1 | 6.6 | 7.0 |
| Starch | 305 | 294 | 369 | 336 | 387 | 364 |
1 C—control corn-soybean-based diet; wheat bran—corn-soybean-based diet supplemented with 30, 60 and 60 g/kg wheat bran in the starter, grower and finisher diets, respectively. 2 Calculated value.
2.2. Sampling
Growth rate, feed intake and feed conversion data were collected over the 37 days of the experimental period. Feed intake and feed conversion rates were calculated for each pen (4 dietary groups, 4 replicate pens per group with 24 chickens in each). Bodyweight was measured individually on day 37. On day 37 of life, 2 chickens (8 per dietary treatment) were randomly selected from each pen and euthanized by bleeding out the jugular vein under general carbon dioxide anesthesia induction. Immediately after the opening of the abdominal cavity, tissue and chymus samples were taken from the cecum. Fresh chymus samples were used for the determination of pH values. Chymus samples collected from the cecum were stored at −20 °C for bacterial cultivation and at −80 °C in a deep freezer until laboratory analyses of SCFA content and 16S rRNA were performed. Tissue samples for the histomorphology analyses were fixed and stored in 5% phosphate-buffered formalin.
2.3. DNA Extraction, PCR Amplification of the 16S rRNA Genes, and Illumina MiSeq Sequencing
Bacterial DNA was extracted from 15 mg samples with an AquaGenomic Kit (MoBiTec GmbH, Göttingen, Germany), and further purified with KAPA Pure Beads (Roche, Basel, Switzerland) according to manufacturer protocols. The concentration of genomic DNA was measured with a Qubit 3.0 Fluorometer and Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bacterial DNA was amplified with tagged primers (forward 5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and reverse 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) covering the V3–V4 region of the bacterial 16S rRNA gene [19]. Polymerase chain reactions (PCRs) and DNA purifications were performed according to the Illumina Demonstrated Protocol [20]. PCR product libraries were quantified and qualified using the High Sensitivity D1000 ScreenTape system on a TapeStation 2200 instrument (Agilent Technologies, Santa Clara, USA). Equimolar concentrations of libraries were pooled and sequenced on an Illumina MiSeq platform using the MiSeq Reagent Kit v3 (600-cycle, Illumina Inc., San Diego, CA, USA) 300 bp read length paired-end protocol.
Sequences were analyzed by Quantitative Insights Into Microbial Ecology (QIIME 2, version 2020.2.) software [21]. Operational taxonomic units (OTUs) were clustered by an open-reference OTU picking strategy based on 97% similarity level. Greengenes Database (version 13.8) and UCLUST algorithm [22] were applied for OTU clustering. Taxonomic identification was assigned by RDP naive Bayesian classifier [23] with a confidence threshold of 0.8.
2.4. Chemical Analyses
Fresh cecal contents were diluted with distilled water (1:5) immediately after collection and shaken manually for 1 min. pH measurements were carried out with a SNEX electrode (pH200A Portable pH meter equipped with CS1068 SNEX pH Sensor (CLEAN Instruments, Shanghai, China). Gas chromatography (TRACE 2000, Thermo Scientific, Waltham, MA, USA) method was applied for SCFA analysis as described by [24]. Briefly, frozen samples were melted and thoroughly mixed. Thereafter, 250 μL digesta were taken and mixed with 600 μL of 1.11 M HCl. Gas chromatograph was equipped with a 30 m (0.25 mm i.d.) fused silica column (Nukol column, Supelco Inc., Bellefonte, PA, USA). Flame Ionization detector (FID) was used with a split injector (1:50), the injection volume was set as 1 μL at 220 °C, and the detection was performed at 250 °C. The carrier gas was helium with a pressure of 83 kPa. Standard mixtures of SCFAs (1, 4, 8 and 20 mM), consisting of acetate, propionate, n-butyrate and n-valerate as external standards, were used for calibration.
2.5. Histomorphological Analysis
Tissue samples were taken from the left cecum close to the apex. Samples were fixed in 5% phosphate-buffered formalin. Processing consisted of serial dehydration, clearing and wax impregnation. Tissue sections were cut in 5 µm thicknesses (3 cross sections) from each of the 8 chickens per treatment. The sections were cut by a microtome and fixed on slides. A routine staining procedure was carried out with hematoxylin and eosin. The slides were examined under a Leica DMi8 Microscope (Leica Microsystems CMS GmbH, Wetzlar Germany) fitted with a digital video camera. Images were analyzed with ImageJ software (version 1.47) developed by the National Institutes of Health (Bethesda, MD, USA). A total of 10 intact, well-oriented villus-crypt units were selected in triplicate from each intestinal cross section.
2.6. Feed Analyses
Experimental diets were analyzed for dry matter (ISO 6496), crude protein (ISO 5983-1:2005), crude fat (ISO 6492), crude fiber (ISO 6865:2001), total P (ISO 6491:2001) and Ca (ISO 6869:2001) content. A polarimetric method was used for starch content measurement in line with the European Directive 152/2009. The water-extractable arabinoxylan content of wheat bran was analyzed using a colorimetric method described by [25]. Five different samples from each experimental diet were taken for feed analyses, and results showed satisfactory homogeneity.
2.7. Statistical Analyses
Growth characteristics, SCFA, pH and histomorphology data were analyzed with two-way ANOVA using SPSS 24.0 software. Differences were considered significant at a level of p < 0.05, and trends were observed for 0.1 > p ≥ 0.05. Diversity indices and principal coordinate analyses were estimated and visualized with MicrobiomeAnalyst [26]. For the identification of over- or underrepresented OTUs among dietary treatments, the edgeR algorithm was applied in MicrobiomeAnalyst to perform the differential abundance analysis method. Samples analyzed with MicrobiomeAnalyst were filtered for low abundance sequences (<4) based on the mean abundance of OTUs, and for low variability (<10%) using interquantile range assessment. After being filtered, OTU abundances were transformed by relative log expression. The false discovery rate (FDR) was calculated using the Benjamini and Hochberg method, and q-values less than 0.05 were considered statistically significant. Abundances of microbial taxa were expressed as percentages of total 16S rRNA gene sequences.
3. Results
3.1. Growth Characteristics
No differences were observed in growth parameters among the treatment groups in the starter, grower or finisher phases of the experiment (Table 3).
Table 3.
Effect of wheat bran and Clostridium butyricum (C. butyricum) supplementation on growth parameters in broiler chickens (from day 0 to day 37 of age) 1.
| Dietary Treatments | Daily Gain (g) | Feed Intake (g) | Feed Conversion Ratio (FCR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | ||
| Control | 206 | 764 | 1457 | 2427 | 242 | 1410 | 2385 | 4036 | 1.171 | 1.848 | 1.639 | 1.665 | |
| C. butyricum | 208 | 781 | 1486 | 2474 | 242 | 1402 | 2498 | 4142 | 1.163 | 1.796 | 1.684 | 1.675 | |
| Wheat bran | 210 | 773 | 1458 | 2440 | 241 | 1368 | 2374 | 3982 | 1.150 | 1.771 | 1.630 | 1.633 | |
| SYN | 207 | 754 | 1515 | 2475 | 235 | 1436 | 2399 | 4070 | 1.140 | 1.904 | 1.588 | 1.646 | |
| Wheat bran | |||||||||||||
| No | 207 | 772 | 1471 | 2450 | 242 | 1406 | 2441 | 4089 | 1.167 | 1.822 | 1.662 | 1.670 | |
| Yes | 208 | 763 | 1486 | 2458 | 238 | 1402 | 2386 | 4026 | 1.145 | 1.838 | 1.609 | 1.639 | |
| C. butyricum | |||||||||||||
| No | 208 | 768 | 1458 | 2434 | 241 | 1389 | 2379 | 4009 | 1.161 | 1.810 | 1.635 | 1.649 | |
| Yes | 207 | 768 | 1500 | 2475 | 239 | 1419 | 2448 | 4105 | 1.152 | 1.850 | 1.636 | 1.660 | |
| Pooled SEM | 3.0 | 6.9 | 15.3 | 18.5 | 4.2 | 10.9 | 29.3 | 30.6 | 0.012 | 0.019 | 0.028 | 0.018 | |
| Wheat bran | 0.870 | 0.540 | 0.638 | 0.856 | 0.700 | 0.841 | 0.371 | 0.317 | 0.426 | 0.608 | 0.395 | 0.441 | |
| C. butyricum | 0.900 | 0.977 | 0.205 | 0.322 | 0.783 | 0.154 | 0.265 | 0.133 | 0.747 | 0.190 | 0.985 | 0.769 | |
| Wheat bran x C. butyricum | 0.753 | 0.234 | 0.672 | 0.875 | 0.783 | 0.082 | 0.472 | 0.887 | 0.974 | 0.008 | 0.483 | 0.976 | |
1 SYN—C. butyricum and wheat bran supplemented group. Starter: day 0 to day 10; Grower: day 11 to day 24; Finisher: day 25 to day 37.
3.2. Cecal Histology, pH and SCFA Composition
The wheat bran supplemented diet resulted in increased cecal crypt depth (p = 0.001), whereas C. butyricum supplementation had no effect on cecal crypt depth (Table 4). Neither wheat bran nor C. butyricum supplementation had a significant effect on cecal pH, acetate, butyrate, valerate, total SCFA concentration and acetate/butyrate ratio. Clostridium butyricum supplementation had a tendency (p = 0.063) to decrease cecal propionate concentration.
Table 4.
Effect of wheat bran and Clostridium butyricum (C. butyricum) supplementation on cecal histological and physicochemical characteristics in broiler chickens at 37 days of age 1.
| Dietary Treatments | Cecal Crypt Depth 2 | Cecal pH | Acetate 3 | Propionate 3 | Butyrate 3 | Valerate 3 | Total SCFA 3 | Acetate/Butyrate Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 65.5 | 6.58 | 38.9 | 8.90 | 11.6 | 1.03 | 61.5 | 3.71 | |
| C. butyricum | 74.4 | 6.57 | 41.1 | 8.07 | 12.8 | 1.06 | 64.0 | 3.37 | |
| Wheat bran | 93.7 | 6.50 | 45.1 | 9.93 | 10.3 | 1.03 | 67.4 | 4.23 | |
| SYN | 95.5 | 6.52 | 37.5 | 6.58 | 11.1 | 0.95 | 57.0 | 3.65 | |
| Wheat bran | |||||||||
| No | 69.7 b | 6.58 | 40.0 | 8.49 | 12.2 | 1.04 | 62.8 | 3.54 | |
| Yes | 94.6 a | 6.51 | 41.9 | 8.49 | 10.6 | 0.99 | 62.9 | 3.96 | |
| C. butyricum | |||||||||
| No | 79.7 | 6.54 | 42.2 | 9.45 | 10.9 | 1.03 | 64.7 | 3.97 | |
| Yes | 85.7 | 6.55 | 39.5 | 7.39 | 12.0 | 1.01 | 60.8 | 3.50 | |
| Pooled SEM | 3.9 | 0.05 | 2.2 | 0.55 | 1.0 | 0.07 | 3.3 | 0.19 | |
| p-Values | |||||||||
| Wheat bran | 0.001 | 0.535 | 0.778 | 0.830 | 0.464 | 0.701 | 0.306 | 0.325 | |
| C. butyricum | 0.428 | 0.960 | 0.566 | 0.063 | 0.616 | 0.872 | 0.856 | 0.255 | |
| Wheat bran x C. butyricum | 0.280 | 0.883 | 0.294 | 0.251 | 0.915 | 0.720 | 0.353 | 0.768 | |
1 SYN—C. butyricum and wheat bran supplemented group. 2 µm. 3 µmol/g. a,b means that those in the same row with no common superscripts are significantly different.
3.3. Microbiota Composition
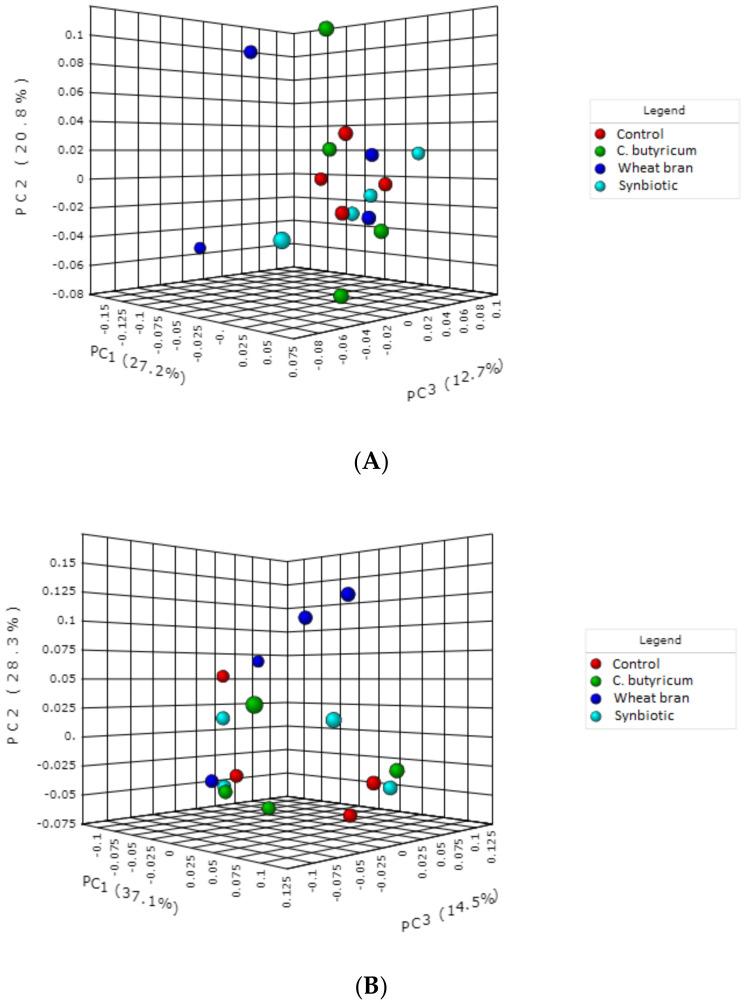
Sequencing 16 samples yielded 667,737 quality-controlled sequences with an average count of 41,733 per sample. The sequences were clustered into 655 operational taxonomic units (OTUs, 0.03 similarity). Average sequence numbers were 43,192 for the C, 40,636 for C. butyricum, 38,512 for wheat bran and 44,594 for the symbiotic groups. After filtering, we observed 343 remaining OTUs, which were assigned into 7 phyla, 11 classes, 15 orders, 21 families and 19 genera. Using ACE and Shannon and Simpson indices, we found similar species richness for the four dietary treatment groups (Table 5). Differences between dietary treatments (p ≥ 0.172) could not be revealed. At the OTU level, microbial community composition of cecal contents were not found to differ when dietary treatments were compared by using unweighted (Figure 1A, p < 0.975) or weighted (Figure 1B, p < 0.378) UniFrac distances.
Table 5.
Operational taxonomic units (OTUs) and diversity indices from cecal contents of broiler chickens (day 37 of life) 1.
| Dietary Treatments | Observed | ACE | Shannon | Simpson |
|---|---|---|---|---|
| Control | 296 | 297 | 3.13 | 0.82 |
| C. butyricum | 300 | 300 | 3.12 | 0.82 |
| Wheat bran | 274 | 275 | 3.05 | 0.84 |
| SYN | 297 | 299 | 3.14 | 0.84 |
| Pooled SEM | 4.66 | 4.62 | 0.05 | 0.01 |
1C. butyricum—Control group supplemented with 2.5 × 109 cfu/kg Clostridium butyricum CBM 588 spores; Wheat bran—corn-soybean-based diet supplemented with 60 g/kg wheat bran; SYN—C. butyricum and wheat bran supplemented group.; ACE – Abundance-based Coverage Estimator.
Figure 1.
Beta diversity plots based on unweighted (A) and weighted (B) UniFrac from cecal bacteriota of chickens that received control, Clostridium butyricum supplemented, wheat bran supplemented and Clostridium butyricum + wheat bran (synbiotic) supplemented diets.
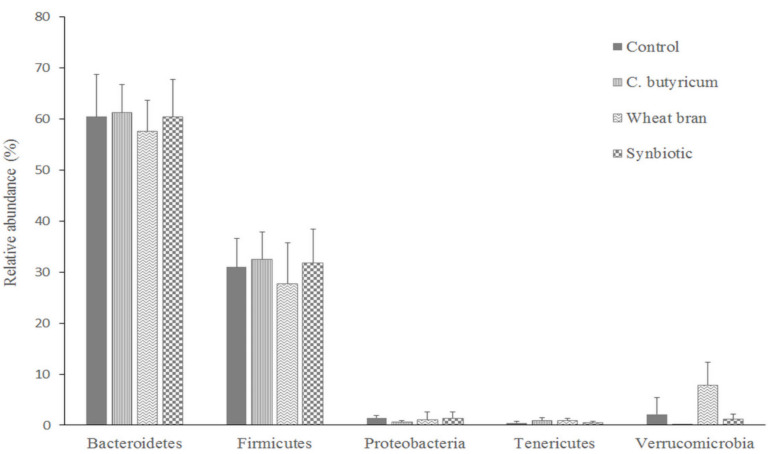
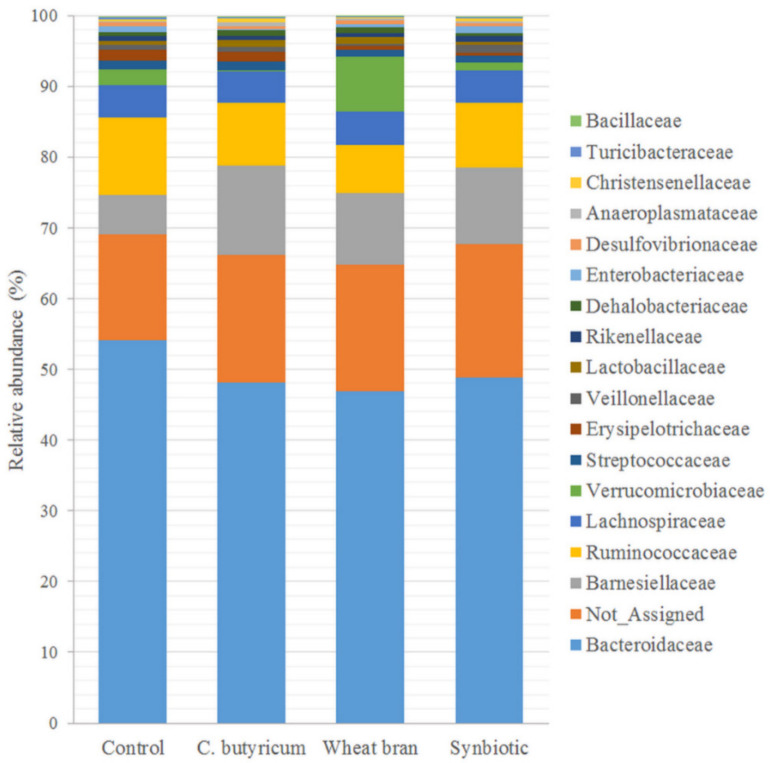
For all four dietary groups, seven bacterial phyla were identified, of which Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia and Tenericutes were found to be the most abundant (Figure 2). The five phyla represented more than 95.1% of the examined bacterial population. The Firmicutes to Bacteroidetes ratio ranged between 0.37 and 0.69 in the samples; however, it was unchanged when the four dietary groups were compared. On the other hand, diet-driven shifts in phylum composition could be observed for Verrucomicrobia phylum. The relative abundance of the Verrucomicrobia phylum was lower in the C. butyricum group, compared with all other groups (p ≤ 0.001). Similarly, bacterial composition at the family level did not differ among dietary treatments, except for Verrucomicrobiaceae (p < 0.001). At the family level, Bacteroidaceae (49.5%), Barnesiellaceae (9.8%) and Ruminococcaceae (8.9%) represented the three most abundant families (Figure 3). Families with more than 1% of relative abundance also included Lachnospiraceae (4.6%) and Verrucomicrobiaceae (2.8%). At the genus level, the 16 samples consisted of 19 genera, of which six had a relative abundance of more than 1% in one of the groups. These six genera represented more than 64.0% of the total bacterial population in the control group and 56.7% in the supplemented groups. At the genus level, Bacteroides, Oscillospira, Akkermansia, Faecalibacterium, Ruminococcus and Streptococcus were found most abundant in the control, wheat bran and symbiotic groups, whereas Akkermansia genus was almost missing in the C. butyricum group (Table 6). Akkermansia genus had a lower relative abundance in the C. butyricum group than in the other groups (p ≤ 0.003) and the relative abundance of Akkermansia decreased in the symbiotic group compared to the wheat bran supplemented group (p = 0.043). No significant differences were found between other genera (p ≥ 0.05). Dietary treatment had a tendency to influence the relative abundance of the Anaerotruncus genus, and the control group showed the highest abundance and C. butyricum group had the lowest abundance. During taxonomic classification of 16S rRNA sequences within the Akkermansia genus, Akkermansia muciniphila could be identified solely (95% identity).
Figure 2.
Relative abundances (%) of the most abundant phyla in the cecum of broiler chickens (at day 37 of life) fed control diet, and Clostridium butyricum or diets supplemented by wheat bran respectively or combined the two. Data are presented as the mean values and SEM. Abbreviations: C. butyricum—control group supplemented with 2.5 × 109 cfu/kg Clostridium butyricum CBM 588 spores; Wheat bran—wheat bran supplemented group; SYN—Clostridium butyricum and wheat bran supplemented group.
Figure 3.
Relative abundances of bacteria at the family level in the cecal content of chickens fed control (C), Clostridium butyricum supplemented (C. butyricum), wheat bran supplemented and Clostridium butyricum + wheat bran supplemented (SYN) diets (day 37 of life).
Table 6.
Relative abundances (%) of bacterial genera (>0.1%) in the cecal contents of broiler chickens (at day 37 of life) 1.
| Genus 2 | C | C. butyricum | Wheat Bran | SYN | Pooled SEM | p-Value | q-Value 2 |
|---|---|---|---|---|---|---|---|
| Bacteroides | 54.1 | 48.1 | 46.8 | 48.9 | 2.37 | 0.865 | 0.956 |
| Oscillospira | 2.57 | 1.91 | 1.43 | 2.12 | 0.201 | 0.521 | 0.956 |
| Akkermansia | 2.17 a,b | 0.02 c | 7.77 a | 1.17 b | 1.071 | <0.001 | 0.004 |
| Faecalibacterium | 2.09 | 1.88 | 0.77 | 1.72 | 0.250 | 0.936 | 0.956 |
| Ruminococcus | 1.83 | 1.50 | 1.33 | 1.78 | 0.143 | 0.667 | 0.956 |
| Streptococcus | 1.24 | 1.37 | 0.90 | 0.91 | 0.181 | 0.813 | 0.956 |
| Lactobacillus | 0.55 | 0.89 | 0.94 | 0.42 | 0.142 | 0.469 | 0.956 |
| Dehalobacterium | 0.52 | 0.71 | 0.77 | 0.51 | 0.100 | 0.606 | 0.956 |
| Anaeroplasma | 0.11 | 0.60 | 0.24 | 0.31 | 0.086 | 0.016 | 0.106 |
| Clostridium | 0.20 | 0.17 | 0.31 | 0.32 | 0.040 | 0.895 | 0.956 |
| Coprococcus | 0.24 | 0.16 | 0.22 | 0.22 | 0.022 | 0.430 | 0.956 |
| Butyricicoccus | 0.31 | 0.16 | 0.18 | 0.19 | 0.027 | 0.133 | 0.663 |
| Turicibacter | 0.27 | 0.23 | 0.14 | 0.17 | 0.028 | 0.856 | 0.956 |
| Anaerotruncus | 0.41 | 0.06 | 0.20 | 0.09 | 0.065 | 0.007 | 0.072 |
| Blautia | 0.20 | 0.19 | 0.12 | 0.15 | 0.019 | 0.956 | 0.956 |
1 C—control corn-soybean-based diet; C. butyricum—C group supplemented with 2.5 × 109 cfu/kg Clostridium butyricum spores; Wheat bran—corn-soybean based diet supplemented with 60 g/kg wheat bran; SYN—C. butyricum and wheat bran supplemented group. 2 q-value: the false discovery rate (FDR) is used to adjust p-value using Benjamini and Hochberg method. Statistically significant values are formatted in bold. a,b,c means that those in the same row with no common superscripts are significantly different.
4. Discussion
The present study indicated that dietary wheat bran or C. butyricum supplementation did not influence growth parameters of broiler chickens at 37 days of life. This result is in line with previous chicken trials conducted to study dietary supplementation of wheat bran, wheat bran-derived arabinoxylans [7,27] or C. butyricum [28,29]. In our experiment, the results of the C. butyricum diet did not provide any effect either on cecal SCFA concentration or on growth performance. Earlier studies demonstrated that the growth-promoting effect of C. butyricum supplementation can be at least partly attributed to an elevated cecal SCFA production [30]. This finding may explain the fact that the growth-promoting effect of the C. butyricum diet failed to occur in our trial. Furthermore, the wheat bran diet resulted in deeper cecal crypts in the present study, which indicates an extended absorption area of the cecum. Cecum, harboring the highest densities of bacteria, is the main site for bacterial fermentation in the chicken intestine and plays an important role in water and electrolyte absorption [31]. The main end-products of bacterial fermentation in the hindgut are SCFAs, which influence gut health in several ways. These compounds, in particular, have selective antimicrobial and anti-inflammatory properties and promote epithelial cell proliferation [3]. Only a few studies have investigated the effect of the C. butyricum diet on cecal SCFA concentration in chicken. Han et al. [12] described elevated cecal acetate concentration when chickens received the C. butyricum diet. Increased cecal acetate, butyrate and total SCFA concentration were reported by Zhang et al. [13]. In these two trials, chickens were kept on wire mesh floor, which was substantially different from our experiment, where wheat straw litter was used as bedding. According to the sequencing analysis applied in this study, a very high Bacteroidetes (50–62%) dominance was found in chicken cecal content at the phylum level. A large number of existing studies on chicken cecal microbiota described Firmicutes dominance (49.0–96.0%) [32,33,34,35,36,37], whereas only Xiao et al. [38] had outcomes similar to the results of this paper. The results of Xiao et al. [38] and those of the present study both show a dominance of the Bacteroides genus (40–50% relative abundance, phylum: Bacteroidetes) in chicken cecal samples. As compared with other reports [32,33,34,35], this dominance appears to be overwhelming in light of the high microbiota diversity of chicken cecal samples at slaughter age. Housing conditions may partly explain the differing results, as the experiments referred to above were performed in pens with wire floor, wood shavings or unknown bedding material. Bacteroides are normally found in the gut, upper respiratory and genital tract of healthy animals, and their immunostimulatory effect has been described. Some strains of Bacteroides are novel probiotic candidates [39].
The partial O2 pressure and redox potential of the intestinal lumen may also serve as potential explanations for a high Bacteroidetes/Bacteroides abundance. Wei et al. [40] concluded that factors resulting in lower partial O2 pressure and redox potential contributed to higher colonization rates of strict anaerobe bacteria, such as Bacteroides and Faecalibacterium. Interestingly, the Oscillospira genus was not reported among the most dominant genera in chicken cecum [33,40,41]. However, its colonization can be associated with a slow passage rate as Oscillospira species are slow-growing bacteria [42]. It is possible that a relatively slow passage rate or low partial O2 pressure occurred in the cecum of chickens in our experiment, which contributed to the results. Among other dominant genera observed in the current study, Ruminococcus is also known to participate in polysaccharide degradation and utilization [38], whereas Faecalibacterium is a well-known butyrate producer and also shows anti-inflammatory effects [43]. Ruminococcus and Faecalibacterium have been found to be among the dominant genera of chicken ceca [40].
It is known from previous in vitro studies [44] and in vivo [14] chicken experiments that wheat bran has a bifidogenic effect. In contrast to our trial, both of these studies were based on control microbiota dominated by Firmicutes phylum rather than Bacteroidetes. In our experiment, the Bacteroides genus was the main representative of Bacteroidetes. This phylum has powerful nutrient utilization capabilities, especially with regard to degrading complex polysaccharides. Furthermore, patterns of competition, such as secretion of antimicrobial peptides, also support the improved ecological fitness of Bacteroidetes over Firmicutes [39]. The composition of intestinal microbiota is well-known to affect the bioavailability or efficacy of various dietary substances [45]. Thus, a dominance of Bacteroidetes is likely to have limited the potential influence of the wheat bran or C. butyricum diet on cecal microbiota.
Besides, the outcomes of the present study showed a decrease in Verrucomicrobia and Akkermansia muciniphila (A. muciniphila) abundance in the chicken cecal content when chickens received the C. butyricum diet. At the same time, the addition of wheat bran eliminated the effect of C. butyricum on A. muciniphila abundance. In our trial, A. muciniphila was the sole delegate of Verrucomicrobia phylum in the chicken cecum. This bacterium is a recently identified, common resident of the intestinal microbiota in mammals, showing beneficial health effects [46,47]. Studies in mice show that the abundance of A. muciniphila is inversely correlated with several disease statuses and can be enhanced by dietary intervention [46,48]. A. muciniphila colonizes the mucus layer in the intestine and plays an important role in the maintenance of mucus layer integrity. The main fermentation products of A. muciniphila are acetate and propionate [48]. In our experiment, the cecal propionate concentration was the highest in the wheat bran group, and a trend for lower cecal propionate was observed in the C. butyricum group, which corresponded to the fermentation activity of A. muciniphila. Studies in mice with C. butyricum supplementation have shown positive [49] or no [50] correlation with Akkermansia abundance in stool samples. A few reports carry data about the presence of A. muciniphila in chicken ceca; however, little is known about its relation to gut health. Relations between A. muciniphila colonization in chicken ceca and body weight [51] or feed efficiency of chickens [52] have been described in two chicken studies; however, their outcomes are contradictory. To our knowledge, only one chicken study has shown alteration of cecal A. muciniphila abundance [53]. This study included Bacillus licheniformis supplementation during a Clostridium perfringens challenge. Abundance of A. muciniphila was below 1% in the above-mentioned chicken studies, whereas a higher A. muciniphila abundance was observed in our experiment (2.8% on average), which was more similar to findings in mammals [47,54]. Furthermore, the wheat bran group had the highest A. muciniphila abundance in our study, however, the difference was not significant. The beneficial effect of wheat bran supplementation on A. muciniphila abundance has been described in mice [55], but not in chickens.
5. Conclusions
The cecal microbiota of broiler chickens were highly dominated by strict anaerobe bacteria, including members of the Bacteroides, Oscillospira, Faecalibacterium and Akkermansia genera, and the relative abundances of some dominant genera were considerably different from the results of previous studies. This difference might have partly resulted from different housing conditions. Dietary effect on cecal microbiota was detected only for Akkermansia spp. abundance. The overwhelming Bacteroides dominance might have constituted a relatively stable and adaptive microbiota during the dietary intervention trial with wheat bran or C. butyricum diets. In addition, our results suggested that the dietary manipulation of A. muciniphila colonization may have relevance not only in mammals but also for the chicken hindgut.
Acknowledgments
Special thanks should be addressed to Angéla Vörös-Mezei for her indispensable help in management and administration. It is a pleasure to acknowledge the help of Mariann Rakszegi for her help in arabinoxylan measurement. The authors are also grateful to Michael Hess, Claudia Hess, Dieter Liebhart, and Patricia Wernsdorf at the Poultry Clinic of the Veterinary University of Vienna for their kind help and assistance in histology work. The authors are grateful for the project EFOP-3.6.3-VEKOP-16-2017-00008, which is financed by the European Union and the European Social Fund.
Author Contributions
Conceptualization: K.D., F.H. and N.S.; animal trial: N.S. and L.P.; lab analyses: N.S. and L.W.; data acquisition: N.S., V.F. and A.M.; statistical analysis: V.F., L.M. and A.M.; manuscript draft preparation: A.M.; review and editing: F.H., K.D. and L.P. Funding acquisition: K.D. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This material was supported by the Hungarian Government and the European Union with the cofunding of the European Regional Development Fund in the frames of Interreg V-A Austria-Hungary 2014–2020 cooperation program, and Széchenyi 2020 program GINOP-2.3.2-15-2016-00054.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ricke S.C., Lee S.I., Kim S.A., Park S.H., Shi Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020;99:670–677. doi: 10.1016/j.psj.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeurissen S.H.M., Lewis F., van der Klis J.D., Mroz Z., Rebel J.M.J., ter Huurne A.A.H.M. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002;3:1–14. [PubMed] [Google Scholar]
- 3.Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance. J. Appl. Poult Res. 2013;22:647–658. doi: 10.3382/japr.2013-00742. [DOI] [Google Scholar]
- 4.Dhama K., Tiwari R., Khan R.U., Chakraborty S., Gopi M., Karthik K., Saminathan M., Desingu P.A., Sunkara L.T. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trends and advances-a review. Int. J. Pharmacol. 2014;10:129–159. doi: 10.3923/ijp.2014.129.159. [DOI] [Google Scholar]
- 5.Vermeulen K., Verspreet J., Courtin C.M., Haesebrouck F., Baeyen S., Haegeman A., Ducatelle R., Van Immerseel F. Reduced-Particle-Size Wheat Bran Is Efficiently Colonized by a Lactic Acid-Producing Community and Reduces Levels of Enterobacteriaceae in the Cecal Microbiota of Broilers. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.01343-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes C., Delcour J.A. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002;35:315–326. doi: 10.1006/jcrs.2001.0439. [DOI] [Google Scholar]
- 7.Courtin C.M., Swennen K., Broekaert W.F., Swennen Q., Buyse J., Decuypere E., Michiels C.W., De Ketelaere B., Delcour J.A. Effects of dietary inclusion of xylooligosaccharides, arabinoxylooligosaccharides and soluble arabinoxylan on the microbial composition of caecal contents of chickens. J. Sci. Food Agric. 2008;88:2517–2522. doi: 10.1002/jsfa.3373. [DOI] [Google Scholar]
- 8.Such N., Molnár A., Farkas V., Pál L., Husvéth F., Koltay I.A., Rawash M.A., Mezőlaki Á., Dublecz K. Feeding two single strain probiotic bacteria and wheat bran failed to modify the production traits but altered some gut characteristics in broiler chickens. J. Cent. Eur. Agric. 2020;21:499–507. doi: 10.5513/JCEA01/21.3.2745. [DOI] [Google Scholar]
- 9.Chen Y., Wen C., Zhou Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J. Sci. Food Agric. 2018;98:3343–3350. doi: 10.1002/jsfa.8838. [DOI] [PubMed] [Google Scholar]
- 10.Cassir N., Benamar S., La Scola B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016;22:37–45. doi: 10.1016/j.cmi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Latif M.A., El-Hack M.E.A., Swelum A.A., Saadeldin I.M., Elbestawy A.R., Shewita R.S., Ba-Awadh H.A., Alowaimer A.N., El-Hamid H.S.A. Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals. 2018;8:184. doi: 10.3390/ani8100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J., Wang Y., Song D., Lu Z., Dong Z., Miao H., Wang W., He J., Li A. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric. Immunol. 2018;29:797–807. doi: 10.1080/09540105.2018.1457013. [DOI] [Google Scholar]
- 13.Zhang B., Yang X., Guo Y., Long F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 2011;65:329–339. doi: 10.1080/1745039X.2011.568274. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y., Wang L., Khan A., Zhao R., Wei S., Jing X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 2020;99:263–271. doi: 10.3382/ps/pez482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang Q.H., Liu S.J., He T.F., Liu H.S., Mahfuz S., Ma X.K., Piao X.S. Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. Poult. Sci. 2020;99:4929–4938. doi: 10.1016/j.psj.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W.C., Lee T.T. Effects of Laetiporus sulphureus-fermented wheat bran on growth performance, intestinal microbiota and digesta characteristics in broiler chickens. Animals. 2020;10:1457. doi: 10.3390/ani10091457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aviagen Group . Aviagen Ross 308 Broiler Nutrition Specifications. Aviagen Group; Huntsville, AL, USA: 2014. [Google Scholar]
- 18.Aviagen Group . Aviagen Ross 308 Broiler Management Handbook. Aviagen Group; Huntsville, AL, USA: 2014. [Google Scholar]
- 19.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illumina Inc. 16S Metagenomic Sequencing Library Preparation-Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System. Illumina Inc.; San Diego, CA, USA: 2013. [Google Scholar]
- 21.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pẽa A.G., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnár A., Dublecz F., Pál L., Wágner L., Hess C., Hess M., Husvéth F., Dublecz K. Soluble nondigestible carbohydrates improve intestinal function and increase caecal coliform load in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018;102:1615–1624. doi: 10.1111/jpn.12985. [DOI] [PubMed] [Google Scholar]
- 25.Rakszegi M., Molnár I., Lovegrove A., Darkó É., Farkas A., Láng L., Bedő Z., Doležel J., Molnár-Láng M., Shewry P. Addition of Aegilops U and M Chromosomes Affects Protein and Dietary Fiber Content of Wholemeal Wheat Flour. Front. Plant Sci. 2017;8:1529. doi: 10.3389/fpls.2017.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Leblois J., Taminiau B., Schroyen M., Beckers Y., Bindelle J., Everaert N. The effect of inulin and wheat bran on intestinal health and microbiota in the early life of broiler chickens. Poult. Sci. 2018;97:3156–3165. doi: 10.3382/ps/pey195. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M., McCartney E., Knox A., Francesch M., Oka K., Wada K., Ideno M., Uno K., Kozłowski K., Jankowski J., et al. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim. Sci. J. 2018;89:895–905. doi: 10.1111/asj.13006. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X., Shen Y., Shao D., Shi S.R., Ding X., Yang Z.B. Effects of Clostridium butyricum on growth performance, lipid metabolism and the caecal microecological environment of broilers. Eur. Poult. Sci. 2017;81 doi: 10.1399/eps.2017.187. [DOI] [Google Scholar]
- 30.Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Zhou L., Zhang L., Xiao Y.P., Chen A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012;91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- 31.Svihus B., Choct M., Classen H.L. Function and nutritional roles of the avian caeca: A review. Worlds. Poult. Sci. J. 2013;69:249–264. doi: 10.1017/S0043933913000287. [DOI] [Google Scholar]
- 32.Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohd Shaufi M.A., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015;7:4. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakley B.B., Buhr R.J., Ritz C.W., Kiepper B.H., Berrang M.E., Seal B.S., Cox N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014;10:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegerstetter S.-C., Schmitz-Esser S., Magowan E., Wetzels S.U., Zebeli Q., Lawlor P.G., O’Connell N.E., Metzler-Zebeli B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS ONE. 2017;12:e0187766. doi: 10.1371/journal.pone.0187766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017;101:4547–4559. doi: 10.1007/s00253-017-8193-9. [DOI] [PubMed] [Google Scholar]
- 37.Waite D.W., Taylor M.W. Exploring the avian gut microbiota: Current trends and future directions. Front. Microbiol. 2015;6:673. doi: 10.3389/fmicb.2015.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- 39.Tan H., Zhai Q., Chen W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019;116:637–644. doi: 10.1016/j.foodres.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 40.Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- 41.Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gophna U., Konikoff T., Nielsen H.B. Oscillospira and related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- 43.Miquel S., Martín R., Rossi O., Bermúdez-Humarán L.G., Chatel J.M., Sokol H., Thomas M., Wells J.M., Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 44.D’hoe K., Conterno L., Fava F., Falony G., Vieira-Silva S., Vermeiren J., Tuohy K., Raes J. Prebiotic Wheat Bran Fractions Induce Specific Microbiota Changes. Front. Microbiol. 2018;9:31. doi: 10.3389/fmicb.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vázquez-Baeza Y., Callewaert C., Debelius J., Hyde E., Marotz C., Morton J.T., Swafford A., Vrbanac A., Dorrestein P.C., Knight R. Impacts of the Human Gut Microbiome on Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2018;58:253–270. doi: 10.1146/annurev-pharmtox-042017-031849. [DOI] [PubMed] [Google Scholar]
- 46.Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods. 2017;33:194–201. doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pruss K.M., Marcobal A., Southwick A.M., Dahan D., Smits S.A., Ferreyra J.A., Higginbottom S.K., Sonnenburg E.D., Kashyap P.C., Choudhury B., et al. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. bioRxiv. 2020 doi: 10.1038/s41396-020-00798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geerlings S., Kostopoulos I., de Vos W., Belzer C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms. 2018;6:75. doi: 10.3390/microorganisms6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia L., Li D., Feng N., Shamoon M., Sun Z., Ding L., Zhang H., Chen W., Sun J., Chen Y.Q. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-07335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H., Ma X., Liu Y., Ma L., Chen Z., Lin X., Si L., Ma X., Chen X. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front. Immunol. 2019;10:1662. doi: 10.3389/fimmu.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han G.G., Kim E.B., Lee J., Lee J.Y., Jin G., Park J., Huh C.S., Kwon I.K., Kil D.Y., Choi Y.J., et al. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus. 2016;5 doi: 10.1186/s40064-016-2604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan W., Sun C., Yuan J., Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017;7:45308. doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., et al. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Huang Y., Song L., Xiao Y., Lu S., Xu J., Li J., Ren Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods. 2020;73:104103. doi: 10.1016/j.jff.2020.104103. [DOI] [Google Scholar]
- 55.Suriano F., Bindels L.B., Verspreet J., Courtin C.M., Verbeke K., Cani P.D., Neyrinck A.M., Delzenne N.M. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci. Rep. 2017;7:5621. doi: 10.1038/s41598-017-05698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]