Abstract
Docosahexaenoic acid (DHA) is an essential human nutrient that may promote neural health and development. DHA occurs naturally in milk in concentrations that are influenced by many factors, including the dietary intake of the cow and the rumen microbiome. We reviewed the literature of milk DHA content and the biohydrogenation pathway in rumen of dairy cows aim to enhance the DHA content. DHA in milk is mainly derived from two sources: α-linolenic acid (ALA) occurring in the liver and consumed as part of the diet, and overall dietary intake. Rumen biohydrogenation, the lymphatic system, and blood circulation influence the movement of dietary intake of DHA into the milk supply. Rumen biohydrogenation reduces DHA in ruminal environmental and limits DHA incorporation into milk. The fat-1 gene may increase DHA uptake into the body but this lacks experimental confirmation. Additional studies are needed to define the mechanisms by which different dietary sources of DHA are associated with variations of DHA in milk, the pathway of DHA biohydrogenation in the rumen, and the function of the fat-1 gene on DHA supply in dairy cows.
Keywords: α-linolenic acid, Biohydrogenation, Dairy cows, Docosahexaenoic acid, fat-1 gene, Ruminal microorganism
Introduction
Some researchers reported that milk fat could increase triglycerides in blood. However, many researches showed the milk fat had no adverse effects on the concentrations of fasting blood lipids, glucose, and insulin (Benatar, Sidhu & Stewart, 2013; Engel, Elhauge & Tholstrup, 2017). Some studies even showed the benefit for milk fat as a blood pressure supplement (Rietsema et al., 2019). Substances found in enriched milk, including medium and odd chain SFA (saturated fatty acid), globular phospholipids, unsaturated fatty acids, branched-chain fatty acids, natural trans fatty acids, vitamins K1 and K2, and calcium, have been found to have positive health effects (Mozaffarian & Wu, 2018). Among them, conjugated linoleic acid (CLA), which is peculiarly originated from the rumen (Jaglan et al., 2019), and omega-3 polyunsaturated fatty acids (n-3 PUFA) have been found to show health benefits to humans (Swanson, Block & Mousa, 2012).
Docosahexaenoic acid (DHA, C22:6n-3) is an n-3 PUFA found in the mammalian central nervous system (Gázquez, 2017), making up 10% to 15% of the total cerebral fatty acids (about 10 µmol/g brain). Some bacteria and lower eukaryotes can produce DHA de novo via a polyketide synthase pathway (Kabeya et al., 2018) but humans lack the key fat desaturase enzyme for synthesizing DHA (especially Δ12 and Δ13/n-3 desaturase). The FAO/WHO (2008) recommended a daily intake of DHA+EPA of 300 mg for lactating women, recent studies shown a daily intake of 100 mg DHA for infants and 250 mg/day for adolescents DHA+ Eicosapentaenoic acid (EPA, C20:5n-3) (Saini & Keum, 2018). The American Heart Association recommends an intake of 2–4 g/day of DHA+EPA for hypertriglyceridemia patients (Miller et al., 2011). Gebauer et al. (2006) recommended an intake of approximately 500 mg/d of EPA+DHA to reduce the risk of cardiovascular disease. However, most populations only get approximately 100 mg of DHA+EPA per day, which is much lower than the recommendations (Afshin et al., 2019).
The human body can synthesize DHA in extremely limited amounts using α-linolenic acid (ALA, C18:3n-3) (Plourde & Cunnane, 2007), and only approximately 0–4% of dietary ALA may be converted to DHA (Burdge & Wootton, 2002), so DHA needs to be supplemented (Hashimoto et al., 2017).
Milk is a possible dietary source of DHA, dietary source of DHA, but its concentration is particularly low (Bai et al., 2018; Ishaq & Nawaz, 2018; Shingfield, Bonnet & Scollan, 2013). The DHA content of milk is influenced by the rumen microbiota, endogenous synthesis, and dietary intakes of DHA by dairy cows. The rumen biohydrogen content limits the efficient dietary incorporation of DHA into milk and the pathway of DHA hydrogen in the rumen is still unclear. Fat-1 gene was also used to increase DHA in milk (Wu et al., 2012), but researches is limited. We mainly analyzed the literature and defined factors affecting the conversion of dietary DHA into milk and explored strategies to increase the DHA content in milk.
Methodology
The scholarly articles in this review were obtained from web of knowledge, google scholar Baidu scholar and subject-specific professional websites, the date from 1999-2019. The keywords “dairy cow”, “dairy cattle”, “rumen”, “bacteria”, “biohydrogenation” “DHA”, “microalgae” and “fish oil” were used in the search. All of the articles included in this review were peer-reviewed. The article chose in this paper should show the relation between DHA with dairy cow bacteria or rumen biohydrogenation. The qualitative and quantitative articles were reviewed in this paper. The qualitative articles provide insights into problems by helping to understand the reason and opinions. The quantitative articles use measurable data to express facts and discover research patterns.
Sources of DHA in Milk
DHA in milk comes from three major sources: those synthesized from endogenous ALA, those synthesized by the microorganisms in the rumen and intestines of cows, and those converted from the diet.
Metabolic conversion of ALA to DHA
DHA can be synthesized from ALA through metabolic pathways in the liver (Fig. 1) (Kabeya et al., 2018; Kim et al., 2014). The process occurs in the endoplasmic reticulum and peroxisome, which are organelles. ALA is desaturated to stearidonic acid in the endoplasmic reticulum (C18:4n-3) catalyzed in a rate-limiting reaction by Δ6 desaturase and then converted to tetracosahexaenoic acid (C24:6n-3). Tetracosahexaenoic acid is then transferred into peroxisome where it undergoes β-oxidation to form DHA. The reaction sequence for converting ALA to eicosatetraenoic acid (C20:4n-3) is as follows: ALA → eicosatrienoic acid (C20:3n-3) → C20:4n-3 (Kabeya et al., 2018). Δ6 and Δ5 desaturases are the key enzymes in the metabolic pathways (Missotten et al., 2009) and the activity of these two desaturate enzymes can determine the amount of DHA synthesized. For example, the expressions of Δ6 and Δ5 desaturase in human subjects are positively correlated with SFA and PUFA but negatively correlated with linoleic acid (LA) and ALA in foods (Xiang et al., 2006). Omega-6 polyunsaturated fatty acids (n-6 PUFA) are essential in human and animal diets (Saini & Keum, 2018). Changing the ratio of n-3:n-6 PUFA in the diet can influence the expression of Δ6 desaturase enzyme in rats (Missotten et al., 2009; Neuringer, Anderson & Connor, 1988). Missotten et al. (2009) added fish oil and linseed oil to pig diets and determined the expressions of Δ6 and Δ5 desaturase in the liver, subcutaneous fat, and the longissimus dorsi muscle. The addition of fish oil increased the expression of Δ5 desaturase only in the longissimus dorsi muscle, but not in the liver or subcutaneous fat; the addition of linseed oil had no effect on the expression of Δ5 desaturase in all three tissues; the expression of Δ6 desaturase in all three tissues was not affected by either type of oil (Missotten et al., 2009). Studies in rats have shown that protein (Narce et al., 1988) and micromineral (Johnson et al., 1989) depletion in the diet reduced the activity of the Δ6 desaturase enzyme. There have been no reports on the effect of dietary fat on the expression of Δ6 and Δ5 desaturases in cows.
Figure 1. Biosynthetic conversion pathway of ALA to DHA.
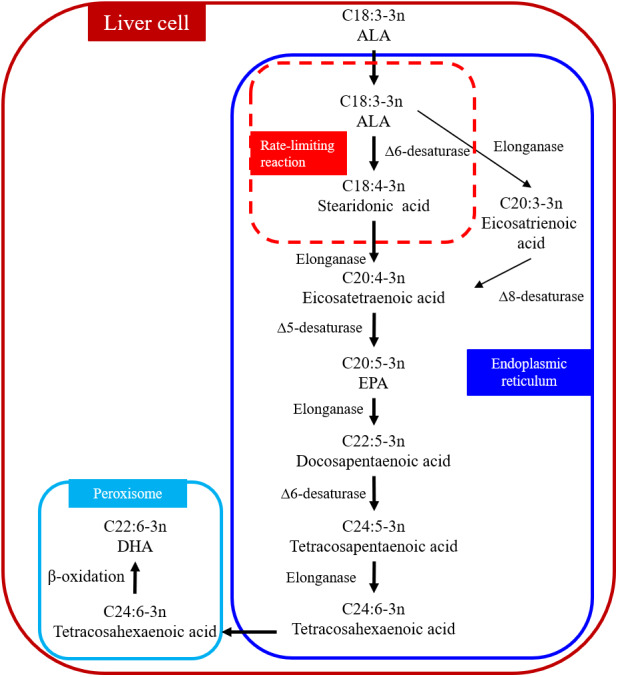
Abbreviations: ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. The first step that ALA is converted to stearidonic acid (C18:4-3n) is a rate-limiting reaction. Data is taken from Kabeya et al. (2018) and Kim et al. (2014).
Synthesis of DHA by rumen microorganisms
Microorganisms, including microbes in the ocean and environment, are able to synthesize DHA de novo via a polyketide synthase pathway (Kabeya et al., 2018; Dongming, Jackson & Quinn, 2015; Xue et al., 2013). Diverse and interdependent populations of bacteria, protozoa, and fungi inhabit the rumen of dairy cattle (Russell & Rychlik, 2001) but no studies have been conducted to date that show the relationship between rumen microbes and DHA metabolism. Some articles have indicated that microbes in the rumen may synthesize DHA independently. For example, Bianchi et al. (2014) found that Cellulophaga can produce DHA. Cellulophaga belongs to phylum Bacteroidetes which is the most abundant phylum in the rumen of older dairy cows (Jami et al., 2013), and infers that there may be a kind of microbe (belong to phylum Bacteroidetes) in the rumen that can produce DHA. Yarrowia lipolytica is a yeast widely distributed in the natural environment that can also produce DHA (Dongming, Jackson & Quinn, 2015; Damude et al., 2006; Gong et al., 2014), however, it is not clear if yeast living in the rumen can produce DHA (Prakasan et al., 2013). In an in vivo experiment with dairy cows, the animals were fed diets containing linseeds or no linseeds, and no DHA at all, but DHA was subsequently found in the duodenal chyme (0.07 g/d and 0.08 g/d, respectively) (Shingfield et al., 2011; Kairenius, Toivonen & Shingfield, 2011). The DHA in the chyme came from the rumen and originated from synthesis by rumen microorganisms or from the blood circulation into salivary secretions.
Additional research may need to focus on the identification of DHA-producing bacteria in the rumen. Increasing the content of DHA-producing bacteria present may increase DHA synthesis in the rumen, which results in more DHA in milk. In addition, there may exist interrelationships in DHA synthesis between microbial species with a functional network (Moraïs & Mizrahi, 2019), which should be explored.
Dietary DHA
An increase in dietary DHA can significantly increase the bodily content of DHA and the subsequent milk content of DHA (Scollan et al., 2001a; Vahmani, Fredeen & Glover, 2013). Currently, the major dietary DHA sources for cows are fish oil and microalgae products.
Fish oil
Fish oils contain a variety of n-3 PUFA, of which EPA and DHA are the most abundant (Mahla et al., 2017). Studies have been conducted on supplementing the diets of dairy cattle with fish oil (Table 1) and most studies indicate that this practice could reduce milk fat. The study conducted by Pirondini et al. (2015) showed no negative effects of fish oil (0.8% dry matter) on milk fat when cattle were provided a low starch diet. The type of diet fed, including the percentage of forage (Shingfield et al., 2003) and type of forage (Chilliard, Ferlay & Doreau, 2001), plays an important role in milk fat concentrations.
Table 1. The effects of dietary supplementation of fish oil on milk fat content.
| Treatment | Fish oil supplement | Diet DHA intake (g/d) | Milk fatty acid content (%) | DHA content in milk (g/d) | Increase of DHA content in milk (compared with control group) (g/d) | Reference |
|---|---|---|---|---|---|---|
| C=basal diet | – | – | 3.52 | 0.26 | – | Vahmani, Fredeen & Glover (2013) |
| T=basal diet + RUFO | 200 g/d | 24.7 | 3.37 | 1.68 | 1.42 | |
| Ca=basal diet | – | 0.00 | 4.30 | 0.00 | – | Pirondini et al. (2015) |
| Ta=basal diet + RUFO | 0.80% | 17.6 | 4.51 | 0.40 | 0.40 | |
| C=basal diet | – | 0.21 | 3.46 | 0.40 | – | AbuGhazaleh et al. (2002) |
| T1=basal diet +RUFO | 2.00% | 21.3 | 3.22 | 2.49 | 2.09 | |
| T2=basal diet +RUFO | 1.00% | 12.2 | 3.45 | 1.46 | 1.06 | |
| C = basal diet | – | ND | 3.28 | ND | – | Ramaswamy et al. (2001) |
| T = basal diet + RUFO | 2.00% | ND | 2.56 | ND | – | |
| C=basal diet | – | 0.35 | 3.40 | 0.47 | – | Vafa et al. (2012) |
| T1=basal diet + RUFO | 2.00% | 31.7 | 2.30 | 2.18 | 1.71 | |
| T2=basal diet + RUFO | 1.00% | 12.9 | 2.45 | 0.84 | 0.37 | |
| Ca=basal diet | – | – | 3.48 | 0.56 | – | Whitlock et al. (2002) |
| Ta1=basal diet +RUFO | 2.00% | 11.1 | 2.87 | 1.59 | 1.03 | |
| Ta2=basal diet +RUFO | 1.00% | 9.07 | 3.11 | 0.97 | 0.41 | |
| Cb = basal diet | – | – | 4.36 | 0.12 | – | |
| Tb = basal diet + RUFO | 0.80% | 18.5 | 3.87 | 0.34 | 0.22 | |
| C = basal diet | – | ND | 3.27 | 0.30 | – | Kairenius et al. (2015) |
| T1 = basal diet + RUFO | 75 g/d | ND | 3.23 | 0.28 | −0.02 | |
| T2 = basal diet + RUFO | 150 g/d | ND | 3.14 | 0.42 | 0.12 | |
| T3 = basal diet + RUFO | 300 g/d | ND | 3.33 | 0.59 | 0.29 | |
| C = basal diet | – | ND | ND | 0.30 | – | Lacasse et al. (2002) |
| T1 = basal diet + RUFO | 3.70% | ND | ND | 1.03 | 0.73 | |
| T2 = basal diet + RPFO | 1.80% | ND | ND | 0.89 | 0.59 | |
| T3 = basal diet + RPFO | 3.70% | ND | ND | 1.04 | 0.74 | |
| C=basal diet | – | – | 2.94 | 0.19 | – | Donovan et al. (2000) |
| T1=basal diet +RUFO | 1.00% | 14.30 | 2.77 | 0.57 | 0.38 | |
| T2=basal diet +RUFO | 2.00% | 50.66 | 2.35 | 1.97 | 1.78 | |
| T3=basal diet +RUFO | 3.00% | 92.63 | 2.28 | 1.25 | 1.06 | |
| C = basal diet | – | ND | 3.34 | ND | – | Baer et al. (2001) |
| T = basal diet + RUFO | 2.00% | ND | 2.27 | ND | – | |
| C = basal diet | – | ND | 4.56 | 1 | – | Shingfield et al. (2006) |
| T = basal diet + RUFO | 1.50% | ND | 2.87 | 0.54 | 0.54 |
Notes.
Fatty acid ≈ triacylglycerols + diacylglycerols + monoacylglycerols + free fatty acids.
Milk fatty acid = milk fat content × 99.13% (MacGibbon & Taylor, 2006).
C, control; Ca,b, controls in article; Ta,b, treatments in article; T1, 2, 3, treatments.
- RPFO
- rumen protected fish oil
- RUFO
- rumen unprotected fish oil
- ND
- Not detected
The supplementation of fish oil alone or fish oil combined with other oils (such as extruded soybean, canola oil) all resulted in improved DHA concentrations in milk (Vahmani, Fredeen & Glover, 2013; AbuGhazaleh et al., 2002; Ramaswamy et al., 2001; Vafa et al., 2012; Whitlock et al., 2002). Kairenius et al. (2015), supplemented with fish oil at doses of 75, 150 and 300g/day (around 0.4, 0.8 and 1.88% diet) which increased the DHA concentration in milk (0.03, 0.05 and 0.10 g/100g total milk fatty acid or 0.22, 0.39 and 0.67 g/day in milk). Other studies have shown a positive correlation for DHA content between dietary intake and milk concentrations (Lacasse et al., 2002). However, increased supplementation had no constant linear relationship between dietary DHA intakes and DHA concentrations in milk. Donovan et al. (2000) showed that supplementation with fish oil at 0, 1 and 2% of total diet increased DHA concentrations in milk, but that the concentration decreased with 3% of total diet fish oil supplementation. Kairenius et al. (2015) reported no difference between the control group and 75 g/day supplementation group for DHA concentrations (0.03 to 0.03 g/100g total milk fatty acid or 0.22 to 0.22 g/day in milk). There may be a liner relationship between fish oil supplementation and milk DHA within certain range, which may be between 0.4% to 3%.
The DHA content of milk is also affected by the host’s metabolism and the biohydrogenation pathway in the rumen. In theory, minimizing the effects of rumen biohydrogenation in the rumen could increase the DHA content of milk (Casta Eda-Gutiérrez et al., 2007). However, Lacasse et al. (2002) reported that supplementation with fish oil or rumen-protected fish oil at the same doses in the diet made no difference in the DHA concentration of milk. This effect may be due to the reduced digestibility of DHA in rumen-protected fish oil. Dietary supplementation of fish oil can increase the DHA content of milk, but the effect of DHA intake is affected by many factors that need to be quantitatively defined.
Microalgae
Microalgae are microscopic photosynthetic organisms found in marine and fresh waters that are used as an animal feed (Priyadarshani & Rath, 2012). Microalgae are a good source of protein, carbohydrates, and long chain PUFA, some of which are rich in DHA (Ryckebosch et al., 2014). Microalgae have been shown to improve the DHA content in milk when used as an additive to dairy cattle feed (Altomonte et al., 2018). The effects of microalgae supplementation on the fatty acid profile of milk are summarized in Table 2.
Table 2. The effects of dietary supplementation of microalgae on milk fat content.
| Treatment | Microalgae supplement (g/d) | Diet DHA intake (g/d) | Milk fatty acid (%) | DHA content in milk g/d | Increase of DHA content in milk (compared with control group) (g/d) | Reference |
|---|---|---|---|---|---|---|
| C=basal diet | – | – | 4.75 | 0.13 | – | Boeckaert et al. (2008) |
| T=basal diet + RPA | 899 | 43.7 | 2.18 | 1.45 | 1.32 | |
| C=basal diet | – | 0.05 | 3.36 | 0.70 | – | Fougère, Delavaud & Bernard (2018) |
| T=basal diet + RUA | 310 | 115 | 2.62 | 13.6 | 12.9 | |
| C=basal diet | – | ND | 4.75 | 0.10 | – | Póti et al. (2015) |
| T=basal diet + RUA | 150 | ND | 3.46 | 0.14 | 0.04 | |
| C=basal diet | – | – | 3.67 | 0.00 | – | Franklin et al. (1999) |
| T1=basal diet + RPA | 910 | 29.2 | 2.92 | 5.15 | 5.15 | |
| T2=basal diet + RUA | 910 | 35.9 | 2.92 | 3.23 | 3.23 | |
| C=basal diet | – | – | 3.47 | 0.10 | – | Stamey et al. (2012) |
| T1= basal diet + 0.5 × RUA | 150 | 21.6 | 3.97 | 0.50 | 0.40 | |
| T2= basal diet + 1 × RUA | 300 | 43.2 | 3.27 | 0.59 | 0.49 | |
| T3= basal diet + 1 × RUA oil | 194 | 27.4 | 3.27 | 0.30 | 0.20 | |
| C=basal diet | – | – | 4.93 | 0.44 | – | Moate et al. (2013) |
| T1=basal diet + RUA | 125 | 25.0 | 3.75 | 3.51 | 3.07 | |
| T2=basal diet + RUA | 250 | 50.0 | 3.67 | 5.09 | 4.65 | |
| T3=basal diet + RUA | 375 | 75.0 | 3.80 | 7.70 | 7.26 | |
| C=basal (diet + Hydrogenated palm oil fat | – | – | 3.90 | – | – | Moran et al. (2018) |
| T=basal diet + RUA | 100 | 17.8 | 3.81 | 1.32 | 1.32 |
Notes.
Fatty acid ≈ triacylglycerols + diacylglycerols + monoacylglycerols + free fatty acids.
Milk fatty acid = milk fat content × 99.13% (MacGibbon & Taylor, 2006).
C, control; T1, 2, 3, treatments.
- RPA
- rumen protected algae
- RUA
- rumen unprotected algae
- ND
- Not detected
Supplementation with microalgae has been shown to improve the DHA concentration of milk with a negative effect on the overall fat content of milk. Microalgae supplementation has a liner relationship with the DHA content of milk (Altomonte et al., 2018; Boeckaert et al., 2008; Fougère, Delavaud & Bernard, 2018; Póti et al., 2015) and fish oil has been shown to have the same effect. Three microalgae feeding styles were utilized (microalgae, rumen protect microalgae, and microalgae oil) and each produced unique results. The feeding of rumen-protected microalgae can improve the concentration of milk DHA markedly, compared to feeding microalgae alone (Franklin et al., 1999). Rumen-protected microalgae can reduce the biohydrogenation of DHA in the rumen. Stamey et al. (2012) supplemented with 150 g/day of microalgae and 194 g/day of microalgae oil, respectively, and found that the microalgae oil supplementation produced a lower milk fat content, DHA concentration and efficient transport of dietary DHA into milk compared with supplementation of microalgae. The DHA in microalgae oil is able to be biohydrogenated in the rumen more easily than microalgae, although DHA is chosen as a source of dietary DHA more often. Moate et al. (2013) reported that there is an exact linear relationship between microalgae intake and the DHA content of milk. However, no experiments have revealed the range in which microalgae supplementation has a linear relationship with the DHA concentration of milk.
An increased concentration of DHA in milk depends on the DHA content in dietary microalgae and is dependent on the species of microalgae and its processing methods. There are many kinds of microalgae that can be used in animal diets with substantially different levels of DHA (Madeira et al., 2017) that can be influenced by the way they are processed. Protecting microalgae from rumen degradation can preserve approximately 45% of the DHA content versus un-protected microalgae (Stamey et al., 2012).
Transport ratio of DHA in milk
The efficiency of DHA incorporation from the feed into milk was low, as showed in Table 3. The incorporation efficiency of DHA can be calculated as the ratio of milk DHA content to dietary DHA intake. Fish oil supplementation increased the DHA content in milk by approximately 6.86% (range from 1.35% to 14.4%), while microalgae supplementation increased it approximately 7.08% (range from 1.09% to 14.0%). The efficiency can be influenced by many factors.
Table 3. Efficiency of dietary incorporation of DHA into milk.
| DHA source | DHA in take (g/d) | Milk DHA yield (g/d) | Efficiency (%) | Reference |
|---|---|---|---|---|
| Fish oil | 14.30 | 0.57 | 3.99 | Donovan et al. (2000) |
| Fish oil | 50.66 | 1.97 | 3.89 | Donovan et al. (2000) |
| Fish oil | 92.63 | 1.25 | 1.35 | Donovan et al. (2000) |
| Fish oil | 21.27 | 2.49 | 11.7 | AbuGhazaleh et al. (2002) |
| Fish oil | 12.20 | 1.46 | 12.0 | AbuGhazaleh et al. (2002) |
| Fish oil | 11.07 | 1.59 | 14.4 | Whitlock et al. (2002) |
| Fish oil | 9.07 | 0.97 | 10.7 | Whitlock et al. (2002) |
| Fish oil | 31.71 | 2.18 | 6.87 | Vafa et al. (2012) |
| Fish oil | 12.87 | 0.84 | 6.53 | Vafa et al. (2012) |
| Fish oil | 24.66 | 1.68 | 6.81 | Vahmani, Fredeen & Glover (2013) |
| Fish oil | 17.55 | 0.40 | 2.28 | Pirondini et al. (2015) |
| Fish oil | 18.53 | 0.34 | 1.83 | Pirondini et al. (2015) |
| Microalgae | 35.94 | 3.23 | 8.99 | Franklin et al. (1999) |
| Microalgae | 43.68 | 1.45 | 3.32 | Boeckaert et al. (2008) |
| Microalgae | 25.00 | 3.51 | 14.0 | Moate et al. (2013) |
| Microalgae | 50.00 | 5.09 | 10.2 | Moate et al. (2013) |
| Microalgae | 75.00 | 7.70 | 10.3 | Moate et al. (2013) |
| Microalgae | 21.61 | 0.50 | 2.31 | Stamey et al. (2012) |
| Microalgae | 43.23 | 0.59 | 1.36 | Stamey et al. (2012) |
| Microalgae oil | 27.41 | 0.30 | 1.09 | Stamey et al. (2012) |
| Microalgae | 17.82 | 1.32 | 7.41 | Moran et al. (2018) |
| Microalgae | 115.5 | 13.6 | 11.8 | Fougère, Delavaud & Bernard (2018) |
Notes.
DHA intake: reported in the article or calculation: dry matter intake × fatty content of dietDHA content.
Fatty acid ≈ triacylglycerols + diacylglycerols + monoacylglycerols + free fatty acids.
Milk fatty acid = milk fat content × 99.13% (MacGibbon & Taylor, 2006).
Milk DHA yield: reported in the article or calculation: milk fatty yieldDHA content.
Efficiency: milk DHA/diet DHA.
Transportation of Dietary DHA into Milk
Figure 2 shows how dietary DHA is moved through the body into the milk. The majority of dietary DHA is hydrogenated in the rumen, with 60–98% of DHA transformed in the rumen to the corresponding geometric isomers via cis-trans isomerization of double bonds in DHA (NRC, 2001; Scollan et al., 2001a; Scollan et al., 2001b; Kim et al., 2008; Kim et al., 2008; Shingfield et al., 2011; Shingfield et al., 2012; Kairenius et al., 2018). Intact DHA (or by-pass) flows into the small intestine where approximately 70–100% is absorbed (Doreau & Ferlay, 1994; Wachira et al., 2000; NRC, 2001; Scollan et al., 2001a; Scollan et al., 2001b; Mattos et al., 2004). It is then absorbed via the lymphatic system into the blood circulation system (Doreau & Ferlay, 1994; Scollan et al., 2001b; NRC, 2001; Wachira et al., 2000) and is transported via the blood into various tissues and organs of the body, including the brain (Al-Ghannami, Al-Adawi & Ghebremeskel, 2019), bones (Saini & Keum, 2018), and the reproductive system (Gholami et al., 2010), where it is used for tissue repair or energy supply via the β-oxidative pathway. Only 13–25% of DHA absorbed from the small intestine is transported into milk through the mammary gland cells (Shingfield, Bonnet & Scollan, 2013).
Figure 2. The pathway of DHA transportation into milk.
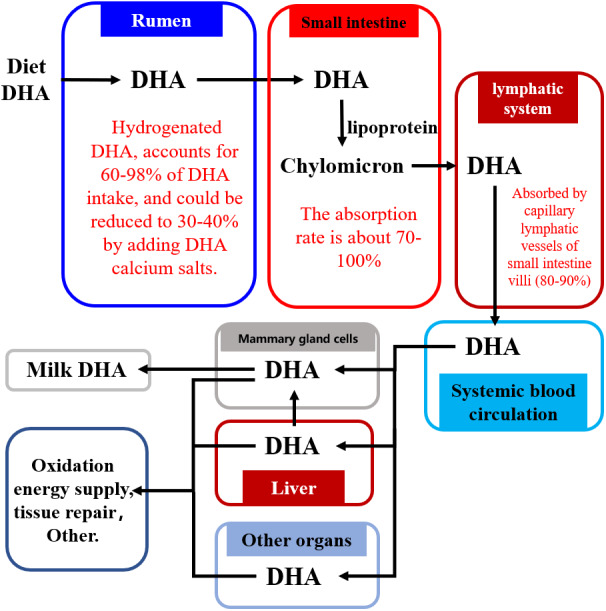
Abbreviations: DHA, docosahexaenoic acid. Data is taken from Shingfield et al. (2003), NRC (2001), Wachira et al. (2000), and Mattos et al. (2004).
The biohydrogenation of DHA in the rumen increases the amount of DHA lost by the body (Fig. 2) (Kairenius et al., 2018; Kim et al., 2008; Mattos et al., 2004; Scollan et al., 2001a; Scollan et al., 2001b; Shingfield et al., 2003; Shingfield et al., 2012; Shingfield et al., 2011; NRC, 2001; Wachira et al., 2000). Therefore, reducing the biohydrogenation of DHA in the rumen is important for improving the DHA concentration of milk.
Ruminal Biohydrogenation
Ruminal biohydrogenation limits the transportation of dietary DHA into milk and is influenced by rumen microbes. Rumen microorganisms include bacteria, protozoa, and fungi. Bacteria play an important role in the biohydrogenation process (Louren, Ramos-Morales & Wallace, 2010). DHA has two dietary forms: free fatty acids and triacylglycerols. Triacylglycerols must be converted into free fatty acids and then must undergo biohydrogenation by the rumen microbes. Thus, two kinds of microorganisms exist with lipolytic effects and biohydrogenation properties.
The lipolytic effect of triacylglycerols mainly depended on the lipase, Anaerovibrio lipolyticus, which is a prominent ruminal lipase-producing bacterium (Hungate, 1966). Three putative lipase genes were identified from the draft genome of Anaerovibrio lipolyticus (alipA, alipB, alipC) (Privé et al., 2013) and had greater hydrolytic activity against caprylate (C8:0), laurate (C12:0), and myristate (C14:0). Butyrivibrio fibrisolvens, Propionibacterium (Edwards et al., 2012) Clostridium, Propionibacterium, Staphylococcus (Edwards et al., 2013), and Pseudomonas aeruginosa (Priji et al., 2017) are among the bacteria that have the ability to decompose triacylglycerols. Sargolzehi et al. (2015) showed that pyridostigmine bromide could decrease the lipase activity and the immunization against lipase may also inhibit the decomposition of triacylglycerols, just like the immunization against rumen urease inhibits ureolysis in the rumen (Zhao et al., 2015).
Butyrivibrio sp. is a genus of an important microbe that hydrogenates PUFA in the rumen; it includes Butyrivibrio fibrisolvens (B. fibrisolvens), and Butyrivibrio proteoclasticus (B. proteoclasticus). B. fibrisolvens can produce isomerase and change the PUFA structure (for example, converting LA into CLA) (Kepler et al., 1966). Trans-11 vaccenic acid (C18:1), converted from LA, can be hydrogenated to stearic acid (C18:0) by B. proteoclasticus (Jenkins et al., 2007). However, some studies show that B. fibrisolvens failed to successfully induce DHA hydrogenation in the rumen (Jeyanathan et al., 2016; Maia et al., 2007). B. proteoclasticus could hydrogenate DHA (Jeyanathan et al., 2016) in vitro in a growth medium containing autoclaved ruminal fluid. Bacterial species, such as Acetobacter (Bainbridge et al., 2016) and Bacillus (Petri et al., 2014), but not Butyrivibrio sp., can affect DHA biohydrogenation. However, an experiment by Sakurama et al. (2014) reported that no bacteria (100 strains of anaerobic bacteria were used, Acetobacter was included) metabolized DHA. Dietary PUFA has been shown to strongly influence microbial profiles in the rumen. Many studies have shown that DHA intake in a reduction of B. fibrisolvens in the rumen in a dose-dependent manner (Shingfield et al., 2012; Maia et al., 2010; Shinji et al., 2009). Abughazaleh & Ishlak (2014) reported that supplement with DHA could reduce the abundance of B. proteoclasticus, but other experiments have shown no effect. Shingfield et al. (2012) proposed that DHA and other unsaturated fatty acids could lengthen the bacteria’s lag phase. It is well known that rumen bacteria release hydrogens and secrete isomerases, which may hydrogenate the double bonds in unsaturated fatty acids. The biohydrogenase in the rumen is a major factor regulating the biohydrogenation of PUFA. Further studies should focus on the relationship between rumen microbes, DHA, and biohydrogenase.
The formation of DHA can also be influenced by the intake of LA. LA can be converted into highly unsaturated fatty acids (HUFA) in vivo. LA and ALA share the same family of enzymes in the formation of HUFA (Fleming & Kris-Etherton, 2014), and compete with one another for enzyme uptake (Gibson, Muhlhausler & Makrides, 2015). Increasing the intake of LA may reduce the formation of DHA from ALA. A study showed that high LA diet could reduce the content of DHA in milk (Aprianita et al., 2014). However, DHA synthesis by ALA in tissues is very low and yet not reported in dairy cows. Ruminal biohydrogenation process is well-studied and understood. The content of unsaturated fatty acids in the diet can influence the biohydrogenation of DHA in the rumen, to various effects. In an in vitro study, Chow et al. (2004) found that adding LA and ALA could reduce the biohydrogenation of DHA, which was confirmed in an in vitro experiment (Wasowska et al., 2006). Shingfield et al. (2011) found that dietary supplementation of both fish oil and linseed oil at a ratio of 1:1 reduced the hydrogenation of DHA, but increased the hydrogenation of ALA in the rumen. However, Kairenius et al. (2018) reported that dietary addition of linseed oil or sunflower seed oil could promote the biohydrogenation of DHA, EPA, and ALA compared with fish oil alone. We determined that biohydrogenase is not fatty-acid specific and competition exists among unsaturated fatty acids. Short-chain unsaturated fatty acids may tend to be biohydrogenated more readily than long-chain PUFA. Therefore, understanding the mechanisms of biohydrogenation for unsaturated fatty acids and the interactions among these fatty acids in the rumen will help develop dietary strategies to reduce DHA biohydrogenation.
Biohydrogenation pathways of DHA in rumen
Studies on the DHA biohydrogenation pathway in the rumen are limited. In 2007, Jenkins et al. (2007) speculated that the first step in the process of DHA biohydrogenation is to convert DHA to a C22:6 isomer that is then hydrogenated to C22:5 fatty acid. However, Kairenius, Toivonen & Shingfield (2011) showed that the C22:6 isomer was not detectable in the DHA hydrogenation process, likely due to its short lifetime or the limitation of the analytic method (Escobar et al., 2016). Aldai et al. (2018) investigated the biohydrogenation process of DHA in in vitro fermentation using sheep rumen fluid as the inoculator, and determined the metabolites of DHA at 0, 1-, 2-, 3-, and 6- hours after fermentation. They found that DHA was initially transformed into mono trans methylene interrupted DHA and monoconjugated DHA. Nevertheless, the DHA hydrogenation process started from the isomer formation.
Jeyanathan et al. (2016) used in vitro anaerobic fermentation with a single strain of Butyrivibrio proteoclasticus P18 to explore the biohydrogenation process of DHA during fermentation and showed the product in the DHA biohydrogenation pathway. This experiment showed that 12 kinds of DHA intermediates (C22:5, C22:4, C22:3 and C22:2 isomers) were transformed in 48 h. Toral et al. (2018) found that Docosapentaenoic acid may be a major DHA intermediate product. DHA intermediates and the hydrogenation pathway in the rumen are illustrated in Fig. 3.
Figure 3. The putative biohydrogenation pathway of DHA in the rumen.
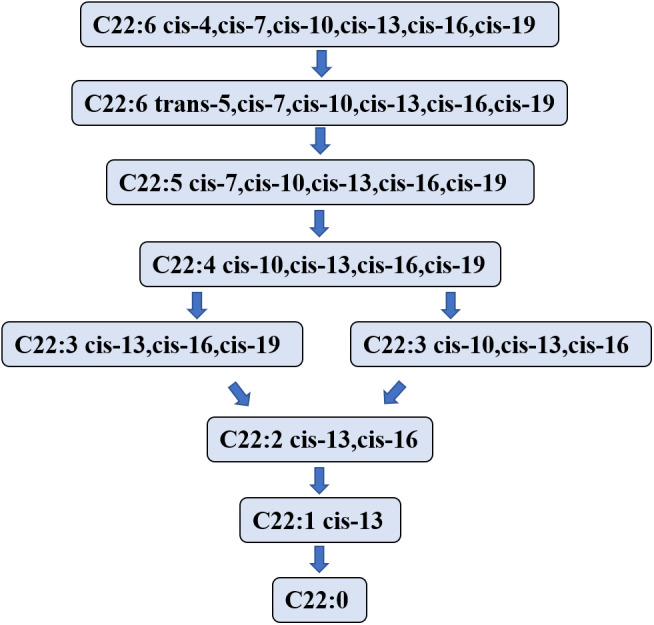
Abbreviations: DHA, docosahexaenoic acid, C22:6 cis-4,cis-7,cis-10,cis-13,cis-16,cis-19. The arrows represent possible major pathways. Neither all putative fatty acids nor the numerous interconversions among C22:6 isomers are presented. Data is taken from Kairenius et al. (2018), Shingfield et al. (2012), Jenkins et al. (2007), Aldai et al. (2018), and Jeyanathan et al. (2016).
The literature is lacking for enzymes that may regulate the DHA hydrogenation pathway in the rumen. According to a report by Toral et al. (2018), some enzymes may exist relating to hydrogenation, isomerization, and migration in the EPA hydrogenation pathway. However, the specific enzymes have not been identified yet, and further studies should focus on the enzymes that regulate the DHA hydrogenation pathway in the rumen.
Other Factor
Fat-1 gene modification
The Fat-1 gene is present in Caenorhabditis elegans, a free-living nematode. Spychalla, Kinney & Browse (2007) first reported the fat-1 gene in Caenorhabditis elegans, and specifically expressed the gene in Arabidopsis, confirming the cDNA (complementary DNA) sequence of the fat-1 gene. The translation product of the fat-1 gene is n-3 PUFA dehydrogenase, which can catalyze the formation of the corresponding n-3 PUFA using 18-20 carbon n-6 PUFA as the substrate (Kang, 2005). The expression of the fat-1 gene can promote the synthesis of n-3 PUFA in nematodes. Liu et al. (2017) constructed the eukaryotic expression vector pef-gfp-fat-1, then transfected pef-gfp-fat-1 into cow fetal fibroblast cells and determined the fatty acid profile. They found that the expression of fat-1 gene could increase the DHA concentration in the cells. The birth of transgenic cows that carried and expressed the mammalianized fat-1 gene (mfat-1) (Wu et al., 2012) and a transgenic cow showed increased n-3 PUFA profiles and reduced n-6 PUFA in their tissues and milk (Liu et al., 2017; Wu et al., 2012). The effect of the fat-1 gene on the conversion of n-6 to n-3 PUFA was also confirmed in a transgenic-pig model (Kang et al., 2004; Li et al., 2018). These findings need to be validated in a large cohort of transgenic animals to support these conclusions.
Conclusions
The literature is limited regarding the conversion of ALA to DHA in tissues and its effect on DHA content in milk. Many studies have focused on increasing the DHA concentration in milk by manipulation of the DHA supply in the diet. Many dietary factors can influence DHA’s passage into milk and their effects need to be quantitated. The majority of dietary DHA is biohydrogenated in the rumen. It is extremely important to reduce our reliance on rumen biohydrogenation and find alternative means for synthesizing DHA.
The fat-1 gene from nematodes is highly effective in converting n-6 PUFA to n-3 PUFA. Since the gene does not exist in mammals, transgenic techniques have been applied, which have been successful in cows, pigs and mice. Thus, it may be worthwhile to examine enlarging the transgenic population.
Acknowledgments
We thank Professor Shimin Liu from the University of Western Australia for their advice.
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant number: 31601963), The Agricultural Science and Technology Innovation Program (ASTIP-IAS12) and Modern Agro-Industry Technology Research System of the PR China (CARS-36), The Scientific Research Project for Major Achievements of The Agricultural Science and Technology Innovation Program (CAAS-ZDXT2019004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper. Xueyin Qu is employed by Tianjin Mengde Groups Co., Ltd. Our lab collaborates with Tianjin Mengde Groups Co., Ltd.
Author Contributions
Guoxin Huang and Yangdong Zhang conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Qingbiao Xu conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Nan Zheng, Shengguo Zhao, Kaizhen Liu, Xueyin Qu and Jing Yu performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jiaqi Wang conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a review article; there is no raw data or code.
References
- Abughazaleh & Ishlak (2014).Abughazaleh AA, Ishlak A. Effects of incremental amounts of fish oil on trans fatty acids and b utyrivibrio bacteria in continuous culture fermenters. Journal of Animal Physiology and Animal Nutrition. 2014;98(2):271–278. doi: 10.1194/jlr.M045450. [DOI] [PubMed] [Google Scholar]
- AbuGhazaleh et al. (2002).AbuGhazaleh AA, Schingoethe DJ, Hippen AR, Kalscheur KF, Whitlock LA. Fatty acid profiles of milk and rumen digesta from cows fed fish oil, extruded soybeans or their blend. Journal of Dairy Science. 2002;85(9):2266–2276. doi: 10.3168/jds.S0022-0302(02)74306-3. [DOI] [PubMed] [Google Scholar]
- Afshin et al. (2019).Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama J, Mullany EC, Abate KH, Abbafati C, Abebe Z, Afarideh M, Aggarwal A, Agrawal S, Akinyemiju T, Alahdab F, Bacha U, Bachman VF, Badali H, Badawi A, Bensenor IM, Bernabe E, Biadgilign S, Biryukov S, Cahill LE, Carrero JJ, Cercy K, Dandona L, Dandona R, Dang AK, Degefa MG, Zaki MES, Esteghamati A, Esteghamati S, Fanzo J, Farinha CSES, Farvid MS, Farzadfar F, Feigin VL, Fernandes JC, Flor LS, Foigt N, Forouzanfar MH, Ganji M, Geleijnse JM, Gillum RF, Goulart AC, Grosso G, Guessous I, Hamidi S, Hankey GJ, Harikrishnan S, Hassen HY, Hay SI, Hoang CL, Horino M, Islami F, Jackson MD, James SL, Johansson L, Jonas JB, Kasaeian A, Khader Y, Khalil IA, Khang Y, Kimokoti RW, Kokubo Y, Kumar GA, Lallukka T, Lopez A, Lorkowski S, Lotufo PA, Lozano R, Malekzadeh R, Marz W, Meier T, Melaku YA, Mendoza W, Mensink GBM, Micha R, Miller TR, Mirarefin M, Mohan V, Mokdad AH, Mozaffarian D, Nagel G, Naghavi M, Nguyen CT, Nixon MR, Ong KL, Pereira DM, Poustchi H, Qorbani M, Rai RK, Razogarcia C, Rehm CD, Rivera JA, Rodriguezramirez S, Roshandel G, Roth GA, Sanabria JR, Sanchezpimienta TG, Sartorius B, Schmidhuber J, Schutte AE, Sepanlou SG, Shin M, Sorensen RJD, Springmann M, Szponar L, Thornelyman AL, Thrift AG, Touvier M, Tran BX, Tyrovolas S, Ukwaja KN, Ullah I, Uthman OA, Vaezghasemi M, Vasankari T, Vollset SE, Vos T, Vu GT, Vu LG, Weiderpass EV, Werdecker A, Wijeratne T, Willett WC, Wu JHY, Xu G, Yonemoto N, Yu C, Murray CJL. Health effects of dietary risks in 195 countries, 1990–2017 a systematic analysis for the global burden of disease study 2017. The Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldai et al. (2018).Aldai N, Delmonte P, Alves S, Bessa RJB, Kramer J. Evidence for the initial steps of DHA biohydrogenation by mixed ruminal microorganisms from sheep involves formation of conjugated fatty acids. Journal of Agricultural and Food Chemistry. 2018;66(4):842–855. doi: 10.1021/acs.jafc.7b04563. [DOI] [PubMed] [Google Scholar]
- Al-Ghannami, Al-Adawi & Ghebremeskel (2019).Al-Ghannami SS, Al-Adawi S, Ghebremeskel K. Randomised open-label trial of docosahexaenoic acid -enriched fish oil and fish meal on cognitive and behavioural functioning in omani children. Nutrition. 2019;57:167–172. doi: 10.1016/j.nut.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Altomonte et al. (2018).Altomonte I, Salari F, Licitra R, Martini M. Use of microalgae in ruminant nutrition and implications on milk quality—a review. Livestock Science. 2018;214:25–35. doi: 10.1016/j.livsci.2018.05.006. [DOI] [Google Scholar]
- Aprianita et al. (2014).Aprianita A, Donkor ON, Moate PJ, Williams SR, Auldist MJ, Greenwood JS, Hannah MC, Wales WJ, Vasiljevic T. Effects of dietary cottonseed oil and tannin supplements on protein and fatty acid composition of bovine milk. Journal of Dairy Research. 2014;81(2):183–192. doi: 10.1017/s0022029914000065. [DOI] [PubMed] [Google Scholar]
- Baer et al. (2001).Baer RJ, Ryali J, Schingoethe DJ, Kasperson KM, Donovan DC, Hippen AR, Franklin ST. Composition and properties of milk and butter from cows fed fish oil 1. Journal of Dairy Science. 2001;84(2):345–353. doi: 10.3168/jds.S0022-0302(01)74483-9. [DOI] [PubMed] [Google Scholar]
- Bai et al. (2018).Bai Z, Lee MRF, Ma L, Ledgard S, Oenema O, Velthof GL, Ma W, Guo M, Zhao Z, Wei S, Li S, Liu X, Havlík P, Luo J, Hu C, Zhang F. Global environmental costs of china’s thirst for milk. Global Change Biology. 2018;24(5):2198–2211. doi: 10.1111/gcb.14047. [DOI] [PubMed] [Google Scholar]
- Bainbridge et al. (2016).Bainbridge ML, Cersosimo LM, Wright AG, Kraft J. Rumen bacterial communities shift across a lactation in holstein, jersey and holstein × jersey dairy cows and correlate to rumen function, bacterial fatty acid composition and production parameters. Fems Microbiology Ecology. 2016;92(5):fiw059. doi: 10.1093/femsec/fiw059. [DOI] [PubMed] [Google Scholar]
- Benatar, Sidhu & Stewart (2013).Benatar JR, Sidhu K, Stewart RAH. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLOS ONE. 2013;8(10):e76480. doi: 10.1371/journal.pone.0076480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi et al. (2014).Bianchi AC, Olazábal L, Torre A, Loperena L. Antarctic microorganisms as source of the omega-3 polyunsaturated fatty acids. World Journal of Microbiology & Biotechnology. 2014;30(6):1869–1878. doi: 10.1007/s11274-014-1607-2. [DOI] [PubMed] [Google Scholar]
- Boeckaert et al. (2008).Boeckaert C, Vlaeminck B, Dijkstra J, Issa-Zacharia A, Van Nespen T, Van Straalen W, Fievez V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. Journal of Dairy Science. 2008;91(12):4714–4727. doi: 10.3168/jds.2008-1178. [DOI] [PubMed] [Google Scholar]
- Burdge & Wootton (2002).Burdge GC, Wootton SA. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. British Journal of Nutrition. 2002;88(4):411–420. doi: 10.1079/bjn2002689. [DOI] [PubMed] [Google Scholar]
- Casta Eda-Gutiérrez et al. (2007).Casta Eda-Gutiérrez E, Veth MJD, Lock AL, Dwyer DA, Murphy KD, Bauman DE. Effect of supplementation with calcium salts of fish oil on n-3 fatty acids in milk fat. Journal of Dairy Science. 2007;90(9):4149–4156. doi: 10.3168/jds.2006-856. [DOI] [PubMed] [Google Scholar]
- Chilliard, Ferlay & Doreau (2001).Chilliard Y, Ferlay A, Doreau M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (cla) and polyunsaturated fatty acids. Livestock Production Science. 2001;70(1):31–48. doi: 10.1016/s0301-6226(01)00196-8. [DOI] [Google Scholar]
- Chow et al. (2004).Chow TT, Fievez V, Moloney AP, Raes K, Demeyer D, Smet SD. Effect of fish oil on in vitro rumen lipolysis, apparent biohydrogenation of linoleic and linolenic acid and accumulation of biohydrogenation intermediates. Animal Feed Science & Technology. 2004;117(1):1–12. doi: 10.1016/j.anifeedsci.2004.08.008. [DOI] [Google Scholar]
- Damude et al. (2006).Damude HG, Zhang H, Farrall L, Ripp KG, Tomb JF, Hollerbach D, Yadav NS. Identification of bifunctional delta-12/omega-3 fatty acid desaturases for improving the ratio of omega-3 to omega-6 fatty acids in microbes and plants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9446–9451. doi: 10.1073/pnas.0511079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongming, Jackson & Quinn (2015).Dongming X, Jackson EN, Quinn Z. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered yarrowia lipolytica: from fundamental research to commercial production. Applied Microbiology and Biotechnology. 2015;99(4):1599–1610. doi: 10.1007/s00253-014-6318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan et al. (2000).Donovan DC, Schingoethe DJ, Baer RJ, Ryali J, Hippen AR, Franklin ST. Influence of dietary fish oil on conjugated linoleic acid and other fatty acids in milk fat from lactating dairy cows. Journal of Dairy Science. 2000;83(11):2620–2628. doi: 10.3168/jds.s0022-0302(00)75155-1. [DOI] [PubMed] [Google Scholar]
- Doreau & Ferlay (1994).Doreau M, Ferlay A. Digestion and utilisation of fatty acids by ruminants. Animal Feed Science and Technology. 1994;45(3-4):379–396. doi: 10.1016/0377-8401(94)90039-6. [DOI] [Google Scholar]
- Edwards et al. (2012).Edwards HD, Anderson RC, Miller RK, Taylor TM, Hardin MD, Smith SB, Krueger NA, Nisbet DJ. Glycerol inhibition of ruminal lipolysis in vitro. Journal of Dairy Science. 2012;95(9):5176–5181. doi: 10.3168/jds.2011-5236. [DOI] [PubMed] [Google Scholar]
- Edwards et al. (2013).Edwards HD, Anderson RC, Taylor TM, Miller RK, Hardin MD, Nisbet DJ, Krueger NA, Smith SB. Interactions between oil substrates and glucose on pure cultures of ruminal lipase-producing bacteria. Lipids. 2013;48(7):749–755. doi: 10.1007/s11745-013-3793-3. [DOI] [PubMed] [Google Scholar]
- Engel, Elhauge & Tholstrup (2017).Engel S, Elhauge M, Tholstrup T. Effect of whole milk compared with skimmed milk on fasting blood lipids in healthy adults: a 3-week randomized crossover study. European Journal of Clinical Nutrition. 2017;72(2):249–254. doi: 10.1038/s41430-017-0042-5. [DOI] [PubMed] [Google Scholar]
- Escobar et al. (2016).Escobar M, Vlaeminck B, Jeyanathan J, Thanh LP, Shingfield KJ, Wallace RJ, Fievez V. Effect of adsorbants on in vitro biohydrogenation of 22:6n-3 by mixed cultures of rumen microorganisms. Animal. 2016;10(9):1439–1447. doi: 10.1017/s1751731116000367. [DOI] [PubMed] [Google Scholar]
- Fleming & Kris-Etherton (2014).Fleming JA, Kris-Etherton PM. The evidence for α-linolenic acid and cardiovascular disease benefits: comparisons with eicosapentaenoic acid and docosahexaenoic acid. Advances in Nutrition. 2014;5(6):863S–876S. doi: 10.3945/an.114.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougère, Delavaud & Bernard (2018).Fougère H, Delavaud C, Bernard L. Diets supplemented with starch and corn oil, marine algae, or hydrogenated palm oil differentially modulate milk fat secretion and composition in cows and goats: a comparative study. Journal of Dairy Science. 2018;101(9):8429–8445. doi: 10.3168/jds.2018-14483. [DOI] [PubMed] [Google Scholar]
- Franklin et al. (1999).Franklin ST, Martin KR, Baer RJ, Schingoethe DJ, Hippen AR. Dietary marine algae (schizochytrium sp.) Increases concentrations of conjugated linoleic, docosahexaenoic and transvaccenic acids in milk of dairy cows. Journal of Nutrition. 1999;129(11):2048–2054. doi: 10.1093/jn/129.11.2048. [DOI] [PubMed] [Google Scholar]
- Gázquez (2017).Gázquez AHIL. Docosahexaenoic acid supplementation during pregnancy as phospholipids did not improve the incorporation of this fatty acid into rat fetal brain compared with the triglyceride form. Nutrition Research. 2017;37:78–86. doi: 10.1016/j.nutres.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Gebauer et al. (2006).Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. N-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. American Journal of Clinical Nutrition. 2006;83(6 Suppl):1526S–1535S. doi: 10.1186/1476-511X-5-14. [DOI] [PubMed] [Google Scholar]
- Gholami et al. (2010).Gholami H, Chamani M, Towhidi A, Fazeli MH. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in holstein bulls. Theriogenology. 2010;74(9):1548–1558. doi: 10.1016/j.theriogenology.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Gibson, Muhlhausler & Makrides (2015).Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (lcpufas), with a focus on pregnancy, lactation and the first 2 years of life. Maternal and Child Nutrition. 2015;7(s2):17–26. doi: 10.1111/j.1740-8709.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong et al. (2014).Gong Y, Wan X, Jiang M, Hu C, Hu H, Huang F. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Progress in Lipid Research. 2014;56:19–35. doi: 10.1016/j.plipres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Hashimoto et al. (2017).Hashimoto M, Hossain S, Mamun AA, Matsuzaki K, Arai H. Docosahexaenoic acid: one molecule diverse functions. Critical Reviews in Biotechnology. 2017;37(5):579–597. doi: 10.1080/07388551.2016.1207153. [DOI] [PubMed] [Google Scholar]
- Hungate (1966).Hungate RE. The rumen and its microbes. Blackie Academic and Professional Press; London: 1966. [DOI] [Google Scholar]
- Ishaq & Nawaz (2018).Ishaq Z, Nawaz MA. Analysis of contaminated milk with organochlorine pesticide residues using gas chromatography. International Journal of Food Properties l. 2018;21:879–891. doi: 10.1080/10942912.2018.1460607. [DOI] [Google Scholar]
- Jaglan et al. (2019).Jaglan N, Kumar S, Choudhury PK, Tyagi B, Tyagi AK. Isolation, characterization and CLA production potential of bifidobacterial isolates from ruminal fluid samples of Murrah buffaloes. Anaerobe. 2019;56:40–45. doi: 10.1016/j.anaerobe.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Jami et al. (2013).Jami E, Israel A, Kotser A, Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. Isme Journal. 2013;7(6):1069–1079. doi: 10.1038/ismej.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins et al. (2007).Jenkins TC, Wallace RJ, Moate PJ, Mosley EE. Board-invited review: recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. Journal of Animal Science. 2007;86(2):397–412. doi: 10.2527/jas.2007-0588. [DOI] [PubMed] [Google Scholar]
- Jeyanathan et al. (2016).Jeyanathan J, Escobar M, Wallace RJ, Fievez V, Vlaeminck B. Biohydrogenation of 22:6n-3 by butyrivibrio proteoclasticus p18. BMC Microbiology. 2016;16(1):104. doi: 10.1186/s12866-016-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al. (1989).Johnson SB, Kramer TR, Briske-Anderson M, Holman RT. Fatty acid pattern of tissue phospholipids in copper and iron deficiencies. Lipids. 1989;24(2):141–145. doi: 10.1007/BF02535252. [DOI] [PubMed] [Google Scholar]
- Kabeya et al. (2018).Kabeya N, Fonseca MM, Ferrier DEK, Navarro JC, Bay LK, Francis DS, Tocher DR, Castro LFC, Monroig Ó. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Science Advances. 2018;4(5):r6849. doi: 10.1126/sciadv.aar6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairenius et al. (2015).Kairenius P, Ärölä A, Leskinen H, Toivonen V, Ahvenjärvi S, Vanhatalo A, Huhtanen P, Hurme T, Griinari JM, Shingfield KJ. Dietary fish oil supplements depress milk fat yield and alter milk fatty acid composition in lactating cows fed grass silage-based diets. Journal of Dairy Science. 2015;98(8):5653–5671. doi: 10.3168/jds.2015-9548. [DOI] [PubMed] [Google Scholar]
- Kairenius et al. (2018).Kairenius P, Leskinen H, Toivonen V, Muetzel S, Ahvenjärvi S, Vanhatalo A, Huhtanen P, Wallace RJ, Shingfield KJ. Effect of dietary fish oil supplements alone or in combination with sunflower and linseed oil on ruminal lipid metabolism and bacterial populations in lactating cows. Journal of Dairy Science. 2018;101(4):S1331039752. doi: 10.3168/jds.2017-13776. [DOI] [PubMed] [Google Scholar]
- Kairenius, Toivonen & Shingfield (2011).Kairenius P, Toivonen V, Shingfield KJ. Identification and ruminal outflow of long-chain fatty acid biohydrogenation intermediates in cows fed diets containing fish oil. Lipids. 2011;46(7):587–606. doi: 10.1007/s11745-011-3561-1. [DOI] [PubMed] [Google Scholar]
- Kang (2005).Kang JX. From fat to fat-1: a tale of omega-3 fatty acids. Journal of Membrane Biology. 2005;206(2):165–172. doi: 10.1007/s00232-005-0790-3. [DOI] [PubMed] [Google Scholar]
- Kang et al. (2004).Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- Kepler et al. (1966).Kepler CR, Hirons KP, Mcneill JJ, Tove SB. Intermediates and products of the biohydrogenation of linoleic acid by butyrivibrio fibrisolvens. Journal of Biological Chemistry. 1966;241(6):1350–1354. [PubMed] [Google Scholar]
- Kim et al. (2008).Kim EJ, Huws SA, Lee MR, Wood JD, Muetzel SM, Wallace RJ, Scollan ND. Fish oil increases the duodenal flow of long chain polyunsaturated fatty acids and trans-11 18:1 and decreases 18:0 in steers via changes in the rumen bacterial community. Journal of Nutrition. 2008;138(5):889–896. doi: 10.1093/jn/138.5.889. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2014).Kim KB, Nam YA, Kim HS, Hayes AW, Lee BM. α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food & Chemical Toxicology. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Lacasse et al. (2002).Lacasse P, Kennelly JJ, Delbecchi L, Ahmadi CE. Addition of protected and unprotected fish oil to diets for dairy cows. I. Effects on the yield, composition and taste of milk. Journal of Dairy Research. 2002;69(4):511–520. doi: 10.1017/s0022029902005770. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018).Li M, Ouyang H, Yuan H, Li J, Xie Z, Wang K, Yu T, Liu M, Chen X, Tang X, Jiao H, Pang D. Site-specific fat-1 knock-in enables significant decrease of n-6pufas/n-3pufas ratio in pigs. G3 (Bethesda, Md.) 2018;8(5):1747–1754. doi: 10.1534/g3.118.200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu XF, Wei ZY, Bai CL, Ding XB, Li X, Su GH, Cheng L, Zhang L, Guo H, Li GP. Insights into the function of n-3 pufas in fat-1 transgenic cattle. Journal of Lipid Research. 2017;58(8):1524–1535. doi: 10.1194/jlr.M072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louren, Ramos-Morales & Wallace (2010).Louren OM, Ramos-Morales E, Wallace RJ. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal An International Journal of Animal Bioscience. 2010;4(7):1008–1023. doi: 10.1017/S175173111000042X. [DOI] [PubMed] [Google Scholar]
- Madeira et al. (2017).Madeira MS, Carlos C, Lopes PA, DiogoCoelho C, Bandarra NM, Prates JAM. Microalgae as feed ingredients for livestock production and meat quality: a review. Livestock Science. 2017;205:111–121. doi: 10.1016/j.livsci.2017.09.020. [DOI] [Google Scholar]
- MacGibbon & Taylor (2006).MacGibbon AKH, Taylor MW. Composition and structure of bovine milk lipids. In: Fox PF, McSweeney PLH, editors. Advanced Dairy Chemistry. Springer; New York: 2006. 3rd ed. Vol. 2: Lipids. [DOI] [Google Scholar]
- Mahla et al. (2017).Mahla AS, Chaudhari RK, Verma AK, Singh AK, Singh SK, Singh G, Sarkar M, Dutta N, Kumar H, Krishnaswamy N. Effect of dietary supplementation of omega-3 polyunsaturated fatty acid (pufa) rich fish oil on reproductive performance of the goat (capra hircus) Theriogenology. 2017;99:79–89. doi: 10.1016/j.theriogenology.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Maia et al. (2010).Maia MR, Chaudhary LC, Bestwick CS, Richardson AJ, McKain N, Larson TR, Graham IA, Wallace RJ. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, butyrivibrio fibrisolvens. BMC Microbiology. 2010;10(1):52. doi: 10.1186/1471-2180-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia et al. (2007).Maia MRG, Chaudhary LC, Figueres L, Wallace RJ. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie van Leeuwenhoek. 2007;91(4):303–314. doi: 10.1007/s10482-006-9118-2. [DOI] [PubMed] [Google Scholar]
- Mattos et al. (2004).Mattos R, Staples CR, Arteche A, Wiltbank MC, Diaz FJ, Jenkins TC, Thatcher WW. The effects of feeding fish oil on uterine secretion of pgf2alpha, milk composition, and metabolic status of periparturient holstein cows. Journal of Dairy Science. 2004;87(4):921–932. doi: 10.3168/jds.s0022-0302(04)73236-1. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2011).Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: a scientific statement from the american heart association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Missotten et al. (2009).Missotten J, Smet SD, Raes K, Doran O. Effect of supplementation of the maternal diet with fish oil or linseed oil on fatty-acid composition and expression of Δ5- and Δ6-desaturase in tissues of female piglets. Animal An International Journal of Animal Bioscience. 2009;3(8):1196–1204. doi: 10.1017/S1751731109004455. [DOI] [PubMed] [Google Scholar]
- Moate et al. (2013).Moate PJ, Williams SR, Hannah MC, Eckard RJ, Auldist MJ, Ribaux BE, Jacobs JL, Wales WJ. Effects of feeding algal meal high in docosahexaenoic acid on feed intake, milk production, and methane emissions in dairy cows. Journal of Dairy Science. 2013;96(5):3177–3188. doi: 10.3168/jds.2012-6168. [DOI] [PubMed] [Google Scholar]
- Moraïs & Mizrahi (2019).Moraïs S, Mizrahi I. The road not taken: the rumen microbiome, functional groups, and community states. Trends in Microbiology. 2019;27(6):538–549. doi: 10.1016/j.tim.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Moran et al. (2018).Moran CA, Morlacchini M, Keegan JD, Fusconi G. The effect of dietary supplementation with aurantiochytrium limacinum on lactating dairy cows in terms of animal health, productivity and milk composition. Journal of Animal Physiology & Animal Nutrition. 2018;102(2):1–15. doi: 10.1111/jpn.12827. [DOI] [PubMed] [Google Scholar]
- Mozaffarian & Wu (2018).Mozaffarian D, Wu J. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circulation Research. 2018;122(2):369–384. doi: 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narce et al. (1988).Narce M, Poisson JP, Belleville J, Chanussot B. Time-course effects of protein malnutrition on hepatic fatty acids Δ6 and Δ5 desaturation in the growing rat. British Journal of Nutrition. 1988;60:389–402. doi: 10.1079/bjn19880108. [DOI] [PubMed] [Google Scholar]
- Neuringer, Anderson & Connor (1988).Neuringer M, Anderson GJ, Connor WE. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annual Review of Nutrition. 1988;8(1):517–541. doi: 10.1146/annurev.nu.08.070188.002505. [DOI] [PubMed] [Google Scholar]
- NRC (2001). National Research Council . 7th rev. ed. Washington, D.C.: National Academies Press; 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- WHO (2008).Organization WHO Interim summary of conclusions and dietary recommendations on total fat & fatty acids. From the joint FAO/WHO expert consultation on fats and fatty acids in human nutrition.2008. pp. 10–14. [Google Scholar]
- Petri et al. (2014).Petri RM, Mapiye C, Dugan ME, Mcallister TA. Subcutaneous adipose fatty acid profiles and related rumen bacterial populations of steers fed red clover or grass hay diets containing flax or sunflower-seed. PLOS ONE. 2014;9(8):e104167. doi: 10.1371/journal.pone.0104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirondini et al. (2015).Pirondini M, Colombini S, Mele M, Malagutti L, Rapetti L, Galassi G, Crovetto GM. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. Journal of Dairy Science. 2015;98(1):357–372. doi: 10.3168/jds.2014-8092. [DOI] [PubMed] [Google Scholar]
- Plourde & Cunnane (2007).Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Applied Physiology, Nutrition, and Metabolism. 2007;32(4):619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- Póti et al. (2015).Póti P, Pajor F, Bodnár Á, Penksza KK. Effect of micro-alga supplementation on goat and cow milk fatty acid composition. Chilean Journal of Agricultural Research. 2015;75(2):259–263. doi: 10.4067/S0718-58392015000200017. [DOI] [Google Scholar]
- Prakasan et al. (2013).Prakasan P, Unni KN, Sajith S, Sailas B. Candida tropicalis bpu1, a novel isolate from the rumen of the malabari goat, is a dual producer of biosurfactant and polyhydroxybutyrate. Yeast. 2013;30(3):103–110. doi: 10.1002/yea.2944. [DOI] [PubMed] [Google Scholar]
- Priji et al. (2017).Priji P, Sajith S, Unni KN, Anderson RC, Benjamin S. Pseudomonas sp. Bup6, a novel isolate from malabari goat produces an efficient rhamnolipid type biosurfactant. Journal of Basic Microbiology. 2017;57(1):21–33. doi: 10.1002/jobm.201600158. [DOI] [PubMed] [Google Scholar]
- Privé et al. (2013).Privé F, Kaderbhai NN, Girdwood S, Worgan HJ, Pinloche E, Scollan ND, Huws SA, Newbold CJ. Identification and characterization of three novel lipases belonging to families ii and v from anaerovibrio lipolyticus 5st. PLOS ONE. 2013;8(8):69076. doi: 10.1371/journal.pone.0069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshani & Rath (2012).Priyadarshani I, Rath B. Commercial and industrial applications of micro algae–a review. Journal of Algal Biomass Utilization. 2012;3(4):89–100. [Google Scholar]
- Ramaswamy et al. (2001).Ramaswamy N, Baer RJ, Schingoethe DJ, Hippen AR, Kasperson KM, Whitlock LA. Composition and flavor of milk and butter from cows fed fish oil, extruded soybeans, or their combination1. Journal of Dairy Science. 2001;84(10):2144–2151. doi: 10.3168/jds.S0022-0302(01)74659-0. [DOI] [PubMed] [Google Scholar]
- Rietsema et al. (2019).Rietsema S, Eelderink C, Joustra ML, Van V, Iris MY, Van Londen M, Corpeleijn E, Singh-Povel CM, Geurts JMW, Kootstra-Ros JE, Westerhuis R, Navis G, Bakker SJL. Effect of high compared with low dairy intake on blood pressure in overweight middle-aged adults: results of a randomized crossover intervention study. The American Journal of Clinical Nutrition. 2019;110(2):340–348. doi: 10.1093/ajcn/nqz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell & Rychlik (2001).Russell JB, Rychlik JL. Factors that alter rumen microbial ecology. Science. 2001;292(5519):1119–1122. doi: 10.1126/science.1058830. [DOI] [PubMed] [Google Scholar]
- Ryckebosch et al. (2014).Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chemistry. 2014;160(1):393–400. doi: 10.1016/j.foodchem.2014.03.087. [DOI] [PubMed] [Google Scholar]
- Saini & Keum (2018).Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance—a review. Life Sciences. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- Sakurama et al. (2014).Sakurama H, Kishino S, Mihara K, Ando A, Ogawa J. Biohydrogenation of c-20 polyunsaturated fatty acids by anaerobic bacteria. Journal of Lipid Research. 2014;55(9):1855–1863. doi: 10.1194/jlr.M045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolzehi et al. (2015).Sargolzehi MM, Naserian A, Asoodeh A, Roknabadi MR, Shin JS, Ghassemi NJ. Short communication application of esterase inhibitors: a possible new approach to protect unsaturated fatty acids from ruminal biohydrogenation. European Journal of Lipid Science and Technology. 2015;117(10):1667–1672. doi: 10.1002/ejlt.201400567. [DOI] [Google Scholar]
- Scollan et al. (2001a).Scollan ND, Choi NJ, Kurt E, Fisher AV, Enser M, Wood JD. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. British Journal of Nutrition. 2001a;85(1):115–124. doi: 10.1079/bjn2000223. [DOI] [PubMed] [Google Scholar]
- Scollan et al. (2001b).Scollan ND, Dhanoa MS, Choi NJ, Maeng WJ, Enser M, Wood JD. Biohydrogenation and digestion of long chain fatty acids in steers fed on different sources of lipid. Journal of Agricultural Science. 2001b;136(5):345–355. doi: 10.1017/S0021859601008796. [DOI] [Google Scholar]
- Shingfield et al. (2003).Shingfield KJ, Ahvenjarvi S, Toivonen V, Arola A, Nurmela KVV, Huhtanen P, Griinari JM. Effect of dietary fish oil on biohydrogenation of fatty acids and milk fatty acid content in cows. Animal Science. 2003;77(4):165–179. doi: 10.1017/S1357729800053765. [DOI] [Google Scholar]
- Shingfield, Bonnet & Scollan (2013).Shingfield KJ, Bonnet M, Scollan ND. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal. 2013;7(s1):132–162. doi: 10.1017/s1751731112001681. [DOI] [PubMed] [Google Scholar]
- Shingfield et al. (2012).Shingfield KJ, Kairenius P, Arölä A, Paillard D, Muetzel S, Ahvenjärvi S, Vanhatalo A, Huhtanen P, Toivonen V, Griinari JM, Wallace RJ. Dietary fish oil supplements modify ruminal biohydrogenation, alter the flow of fatty acids at the omasum, and induce changes in the ruminal butyrivibrio population in lactating cows. Journal of Nutrition. 2012;142(8):1437–1448. doi: 10.3945/jn.112.158576. [DOI] [PubMed] [Google Scholar]
- Shingfield et al. (2011).Shingfield KJ, Lee MRF, Humphries DJ, Scollan ND, Toivonen V, Beever DE, Reynolds CK. Effect of linseed oil and fish oil alone or as an equal mixture on ruminal fatty acid metabolism in growing steers fed maize silage-based diets. Journal of Animal Science. 2011;89(11):3728–3741. doi: 10.2527/jas.2011-4047. [DOI] [PubMed] [Google Scholar]
- Shingfield et al. (2006).Shingfield KJ, Reynolds CK, Hervás G, Griinari JM, Grandison AS, Beever DE. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. Journal of Dairy Science. 2006;89(2):714–732. doi: 10.3168/jds.s0022-0302(06)72134-8. [DOI] [PubMed] [Google Scholar]
- Shinji et al. (2009).Shinji F, Yumiko N, Eisuke C, Hiroshi O, Tsuneo H, Jun K. Evaluation and characterization of bacterial metabolic dynamics with a novel profiling technique, real-time metabolotyping. PLOS ONE. 2009;4(3):e4893. doi: 10.1371/journal.pone.0004893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychalla, Kinney & Browse (2007).Spychalla JP, Kinney AJ, Browse J. Identification of an animal v-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2007;94:1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamey et al. (2012).Stamey JA, Shepherd DM, Veth MJD, Corl BA. Use of algae or algal oil rich in n-3 fatty acids as a feed supplement for dairy cattle. Journal of Dairy Science. 2012;95(9):5269–5275. doi: 10.3168/jds.2012-5412. [DOI] [PubMed] [Google Scholar]
- Swanson, Block & Mousa (2012).Swanson D, Block R, Mousa SA. Omega-3 fatty acids epa and dha: health benefits throughout life. Advances in Nutrition. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toral et al. (2018).Toral PG, Hervás G, Leskinen H, Shingfield KJ, Frutos P. In vitro ruminal biohydrogenation of eicosapentaenoic (EPA), docosapentaenoic (DPA), and docosahexaenoic acid (DHA) in cows and ewes: intermediate metabolites and pathways. Journal of Dairy Science. 2018;101(7):6109–6121. doi: 10.3168/jds.2017-14183. [DOI] [PubMed] [Google Scholar]
- Vafa et al. (2012).Vafa TS, Naserian AA, Moussavi ARH, Reza V, Mohsen DM. Effect of supplementation of fish and canola oil in the diet on milk fatty acid composition in early lactating holstein cows. Asian-Australasian Journal of Animal Sciences. 2012;25(3):311–319. doi: 10.5713/ajas.2010.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahmani, Fredeen & Glover (2013).Vahmani P, Fredeen AH, Glover KE. Effect of supplementation with fish oil or microalgae on fatty acid composition of milk from cows managed in confinement or pasture systems. Journal of Dairy Science. 2013;96(10):6660–6670. doi: 10.3168/jds.2013-6914. [DOI] [PubMed] [Google Scholar]
- Wachira et al. (2000).Wachira AM, Sinclair LA, Wilkinson RG, Hallett K, Enser M, Wood JD. Rumen biohydrogenation of n-3 polyunsaturated fatty acids and their effects on microbial efficiency and nutrient digestibility in sheep. Journal of Agricultural Science. 2000;135(4):419–428. doi: 10.1017/s0021859699008370. [DOI] [Google Scholar]
- Wasowska et al. (2006).Wasowska I, Maia MRG, Nied Wiedzka KM, Czauderna M, Ribeiro JMCR, Devillard E, Shingfield KJ, Wallace RJ. Influence of fish oil on ruminal biohydrogenation of c18 unsaturated fatty acids. British Journal of Nutrition. 2006;95(6):1199–1211. doi: 10.1079/bjn20061783. [DOI] [PubMed] [Google Scholar]
- Whitlock et al. (2002).Whitlock LA, Schingoethe DJ, Hippen AR, Kalscheur KF, Baer RJ, Ramaswamy N, Kasperson KM. Fish oil and extruded soybeans fed in combination increase conjugated linoleic acids in milk of dairy cows more than when fed separately. Journal of Dairy Science. 2002;85(1):234–243. doi: 10.3168/jds.S0022-0302(02)74072-1. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2012).Wu X, Ouyang H, Duan B, Pang D, Zhang L, Yuan T, Xue L, Ni D, Cheng L, Dong S, Wei Z, Li L, Yu M, Sun QY, Chen DY, Lai L, Dai Y, Li GP. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Research. 2012;21(3):537–543. doi: 10.1007/s11248-011-9554-2. [DOI] [PubMed] [Google Scholar]
- Xiang et al. (2006).Xiang M, Rahman MA, Ai H, Li X, Harbige LS. Diet and gene expression- delta-5 and delta-6 desaturases in healthy chinese and european subjects. Annals of Nutrition and Metabolism. 2006;50(6):492–498. doi: 10.1159/000095829. [DOI] [PubMed] [Google Scholar]
- Xue et al. (2013).Xue ZX, Sharpe PL, Hong SP, Yadav NS, Xie DM, Short DR, Damude HG, Rupert RA, Seip JE, Wang J, Pollak DW, Bostick MW, Bosak MD, Macool DJ, Hollerbach DH, Zhang HX, Arcilla DM, Bledsoe SA, Croker K, McCord EF, Tyreus BD, Jackson EN, Zhu Q. Production of omega-3 eicosapentaenoic acid by metabolic engineering of yarrowia lipolytica. Nature Biotechnology. 2013;31(8):734–740. doi: 10.1038/nbt.2622. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2015).Zhao S, Wang J, Zheng N, Bu D, Sun P, Yu Z. Reducing microbial ureolytic activity in the rumen by immunization against urease therein. BMC Veterinary Research. 2015;11(1):94. doi: 10.1186/s12917-015-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a review article; there is no raw data or code.


