Abstract
Objective
The sympathetic nervous system has a recognized role in transmission of pain, and the lumbar sympathetic blockade is intended to provide analgesia. We share our experiences of lumbar sympathetic blockade in the treatment of cancer-related pain.
Methods
We performed a retrospective analysis of patients with cancer-related pain in the back, abdomen, pelvis, or legs treated at Memorial Sloan Kettering Cancer Center between 2000 and 2018 undergoing lumbar sympathetic blockade at L2 or L3. Blocks were accomplished by injection of local anesthetic or local anesthetic with steroid under fluoroscopy. We measured numerical rating scale scores, percent relief, and relief time. The primary end point was defined as “effective” or “ineffective” pain relief. Effective pain relief was defined as ≥30% relief for at least one day.
Results
We identified 124 data points of lumbar sympathetic blockade at L2 or L3, of which 57 were with complete data and used for analysis. Peri-injection, 42 data points had active disease whereas 15 were in remission. Lumbar sympathetic blockade was 67% effective in the back pain cohort, 82% effective in the abdominopelvic pain cohort, and 75% effective in the leg pain cohort. Seventeen data points went on to neurolysis, two to neuromodulation, and eight to intrathecal pump implantation.
Conclusions
Lumbar sympathetic blockade is effective for back, abdominopelvic, and leg pain related to cancer and its treatments. Future research should be aimed at refining its role within multimodal pain management.
Keywords: Lumbar Sympathetic Block, Cancer Pain
Introduction
The role of the sympathetic nervous system in the transmission of pain makes it an appealing target for interventional pain management. Visceral afferent pain fibers, including thoracic and lumbar splanchnic nerves, travel with sympathetic and parasympathetic nerves, whose cell bodies are in the dorsal root ganglion [1]. These fibers then ascend within the spinothalamic and spinohypothalamic tracts to the cerebral cortex, where pain is processed [2].
The lumbar sympathetic block (LSB) is a proposed mechanism to treat visceral pain in diverse patient populations. It was first described by Jaboulay in 1899 and first performed via blind technique by Felix Mandl in 1924, but since 1944 it has been done primarily under fluoroscopy [3–5]. Blockade of the sympathetic chains between L1 and L4 is possible due to their predictable locations within the retroperitoneum, posterior to the great vessels and anterior to the psoas muscles and vertebral bodies [6]. Alcohol, local anesthetics, or steroids may be injected to produce a physiological block and accompanying analgesia. This technique has been used in conditions such as limb ischemia, complex regional pain syndromes, postherpetic neuralgia, and phantom limb pain [4,7].
De Oliveira and colleagues described benefits of LSB in addition to medical management for visceral cancer pain over medical management alone, but there is little in the literature in the last decade regarding this technique [5,8]. The current evidence suggests that opioids are able reduce moderate to severe cancer pain to mild to none within 14 days of treatment in the majority of patients who are able to tolerate their side effects [9]. Intrathecal pump implantation has been used with success, but not every patient will be a candidate or will accept pump placement. Accordingly, steroid injection, radiofrequency ablation, and chemical neurolysis of the sympathetic nerves and accompanying visceral afferent fibers become attractive options for pain control in these patients.
We share the experience of lumbar sympathetic blockade and its role in the treatment of cancer-related pain at an academic cancer center. Although lumbar sympathetic blocks have anecdotally been performed for oncologic pain management, a formal paradigm including the optimal technique and patient population has not been established. We evaluate the efficacy and safety of lumbar sympathetic blockade for patients with cancer-related pain.
Methods
This was an institutional review board (IRB)–approved single-center retrospective analysis of patients at Memorial Sloan Kettering Cancer Center (MSKCC) between 2000 and 2018 undergoing lumbar sympathetic blockade. This retrospective review was approved via a waiver for informed consent by the MSKCC IRB and supported by an MSKCC support grant (P30 core grant).
Inclusion Criteria
This review focused on patients who were being treated for cancer-related pain in the back, abdomen, pelvis, or legs in the inpatient and outpatient settings and had had a lumbar sympathetic block performed at L2 or L3. Only data points with complete data were included. Abdominal pain and pelvic pain were grouped together in the current analysis for ease of interpretation. Patients were included whether they had one block performed or a series of blocks performed, such that each data point represents a single injection but not necessarily a unique patient.
Exclusion Criteria
Patients were excluded if they presented for celiac plexus blocks or superior hypogastric plexus blocks performed at L4 or lower. Patients with incomplete follow-up, missing data, or poor documentation were excluded from the analysis.
Lumbar Sympathetic Block Technique
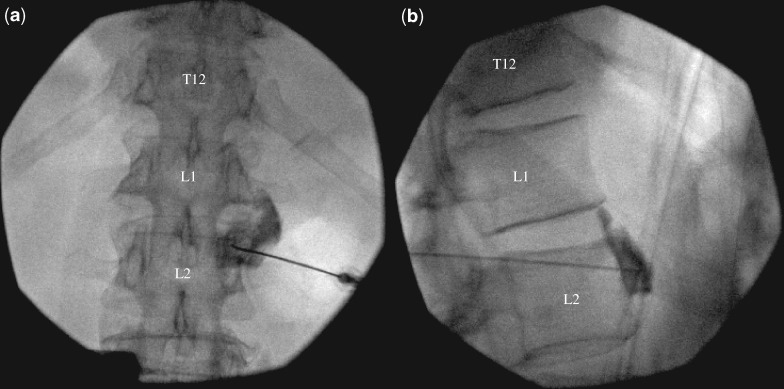
Lumbar sympathetic blocks were performed in the prone position with fluoroscopic guidance between levels L2 and L3 with the occasional neurolysis done under computed tomography (CT) guidance. Patients did not receive sedation during the block, although inpatients were able to use their patient-controlled analgesia if available for pain associated with positioning. Preprocedure planning for both angle of entry and decision for lumbar level was made with the patient’s most recent abdominopelvic CT scan. Once the patient was positioned on the procedure table, fluoroscopic images were obtained in an oblique view with the lateral edge of the transverse process eclipsing the vertebral body. The type of needle used was decided by the operator. The needle was advanced under anteroposterior and lateral views until the tip lay anterior to and within the upper one-third of the vertebral body and at its anterior border in lateral view (Figure 1). After aspiration was negative for air, blood, or cerebrospinal fluid, 1 mL of iohexol (either 180 mg/mL or 300 mg/mL) was injected. In cases where patients had documented allergies to contrast dye, 1 mL of gadopentetate dimeglumine was used instead. After adequate rostral and caudal spread (avoiding intravascular, psoas, and crural spread) along the anterior surface of the vertebral body with contrast, 10 mL of 0.25% bupivacaine or 9 mL of 0.25% bupivacaine with 1 mL of 40 mg/mL of triamcinolone was injected.
Figure 1.
Anterior–posterior (left) and lateral (right) fluoroscopy films of contrast injection before lumbar sympathetic block.
Data Collection
Data points were sorted using Pain Management payment codes and electronic medical record procedure notes identifying patients who underwent lumbar sympathetic blockade for back, abdominopelvic, or leg cancer–related pain. Data points were refined by using only those procedures performed at spinal levels L2 or L3. This was determined by attending review of the saved radiographic images.
Collected baseline data points included sex, oncologic diagnosis, pain complaint and location, spinal level of injection, and injectate medicine. Pre- and postinjection pain medications were gathered and converted to 24-hour oral morphine milligram equivalents (mmeq) for comparison using the Centers for Disease Control and Prevention’s mobile application “CDC Opioid Guideline.” The overwhelming majority of our cohort’s pain was oncologic in origin, that is, from the mass itself, metastases, postresection changes, or radiation treatment. We collected outcome data regarding patients’ pre- and postinjection statuses, such as numerical rating scale (NRS) scores 0–10, percent relief, and total relief time (measured in weeks). (Of note, NRS scores were generally taken from the Events Since Last Visit section of medical notes, as this was found to be the most accurate and updated description of patients’ clinical statuses.)
All data points were categorized into cohorts based on their primary anatomical pain site, be it the back, abdominopelvis, or legs. Subcohorts were delineated by injectate medicine: either local anesthetic or local anesthetic with steroid.
Analysis
Our primary end points were “effective” vs “ineffective” blockade. Effective pain relief was defined as ≥30% relief for at least one day. Ineffective pain relief was defined as not meeting the mentioned effective criteria. Secondary end points included the mean pre- and postinjection NRS scores, percent relief, and relief time. Mathematical analysis was completed using SPSS, version 25 (IBM Corp., Armonk, NY, USA).
Results
A total of 124 data points underwent LSB at L2 (N = 37) or L3 (N = 87): 12 with primarily back pain, 17 with abdominopelvic pain, and 95 with leg pain. Of those, LSB was effective 80% of the time in the back pain cohort, 79% in the abdominopelvic pain cohort, and 72% in the leg pain cohort (Table 1).
Table 1.
Demographics for patients in the LSB cohort
| Back Pain |
Abdominopelvic Pain |
Leg Pain |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | 12 | 17 | 95 | |||
| Female | 2 | 17 | 10 | 59 | 52 | 55 |
| Male | 10 | 83 | 7 | 41 | 43 | 45 |
| Level | 12 | 17 | 95 | |||
| L2 | 4 | 33 | 7 | 41 | 26 | 27 |
| L3 | 8 | 67 | 10 | 59 | 69 | 73 |
| Overall Outcome | 10 | 14 | 71 | |||
| Effective | 8 | 80 | 11 | 79 | 51 | 72 |
| Ineffective | 2 | 20 | 3 | 21 | 20 | 28 |
All data points with lumbar sympathetic blockade at L2 or L3.
There were four data points of the 124 that underwent bilateral blockade (one patient underwent three bilateral blocks, and one patient underwent one bilateral block). These were done similarly to the unilateral blocks with 10 mL of injectate volume to each side.
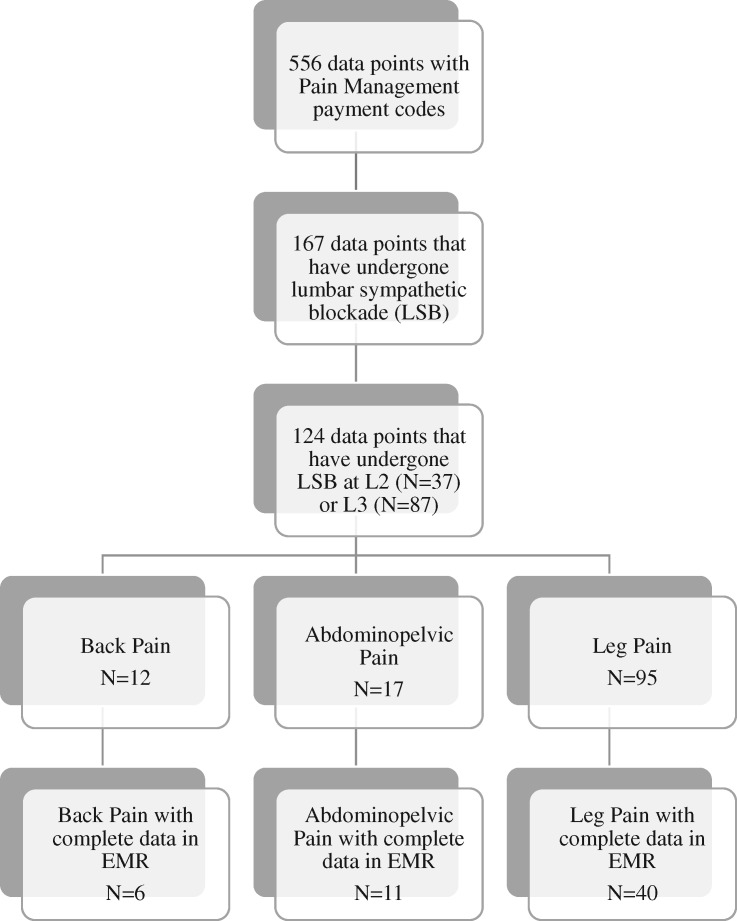
Of the 124 initial data points, 57 (41 unique patients) were used for primary analysis: six back pain, 11 abdominopelvic pain, and 40 leg pain (Figure 2). The remainder were excluded from further analysis due to partial missing data (secondary end points) despite explicit documentation of effective or ineffective LSB.
Figure 2.
Recorded data metrics included pre- and postinjection numeric rating scale scores, percent relief, and relief time in weeks.
Back Pain
In the back pain cohort (N = 6), 67% of LSBs were effective. Mean pre- and post-NRS scores were 8.0±1.8 and 3.7±1.6, respectively, whereas mean percent relief was 55% and mean time of relief was 9.2 weeks (Table 2).
Table 2.
Back pain cohort
| No. | % | Results | No. | Mean | SD | No. | Mean | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 6 | Local anesthetic | 2 | |||||||
| Female | 2 | 33 | All | 6 | Effective | 2 | ||||
| Male | 4 | 67 | Prescore (NRS) | 8.0 | 1.8 | Precore (NRS) | 8.0 | 1.4 | ||
| Postscore (NRS) | 3.7 | 1.6 | Postscore (NRS) | 2.5 | 0.7 | |||||
| Cancer | 6 | Relief, % | 55 | 18 | Relief, % | 69 | 16 | |||
| GI | 1 | 17 | Relief, wk | 9.2 | 12.3 | Relief, wk | 21.0 | 15.6 | ||
| GYN | 1 | 17 | Ineffective | 0 | ||||||
| Neurologic | 1 | 17 | Local anesthetic | 2 | Prescore (NRS) | – | – | |||
| Osteogenic | 1 | 17 | Prescore (NRS) | 8.0 | 1.4 | Postscore (NRS) | – | – | ||
| Soft tissue | 1 | 17 | Postscore (NRS) | 2.5 | 0.7 | Relief, % | – | – | ||
| Urologic | 1 | 17 | Relief, % | 69 | 16 | Relief, wk | – | – | ||
| Relief, wk | 21.0 | 15.6 | ||||||||
| Level | 6 | Local anesthetic + steroid | 4 | |||||||
| L2 | 2 | 33 | Local anesthetic + steroid | 4 | Effective | 2 | ||||
| L3 | 4 | 67 | Prescore (NRS) | 8.0 | 2.2 | Prescore (NRS) | 9.5 | 0.7 | ||
| Postscore (NRS) | 4.3 | 1.7 | Postscore (NRS) | 4.5 | 0.7 | |||||
| Outcome | 6 | Relief, % | 48 | 16 | Relief, % | 53 | 4 | |||
| Effective | 4 | 67 | Relief, wk | 3.3 | 5.8 | Relief, wk | 6.1 | 8.3 | ||
| Ineffective | 2 | 33 | Ineffective | 2 | ||||||
| Prescore (NRS) | 6.5 | 2.1 | ||||||||
| Postscore (NRS) | 4.0 | 2.8 | ||||||||
| Relief, % | 43 | 25 | ||||||||
| Relief, wk | 0.5 | 0.7 |
Effective relief was defined as ≥30% relief for one or more days.
GI = gastrointestinal; GYN = gynecological; NRS = numerical rating scale.
Back Pain with Local Anesthetic
Among patients with back pain, the local anesthetic cohort (N = 2) had 100% effective blockade. Mean pre- and post-NRS scores for these data points were 8.0±1.4 and 2.5±0.7, respectively, whereas the mean percent relief was 69% and mean time of relief was 21 weeks.
Back Pain with Local Anesthetic and Steroid
In the back pain with local anesthetic and steroid cohort (N = 4), 50% had effective blockade. Mean pre- and post-NRS scores for the cohort were 8.0±2.2 and 4.3±1.7, respectively, whereas mean percent relief was 48% and mean time of relief was 3.3 weeks.
Abdominopelvic Pain
In the abdominopelvic pain cohort (N = 11), 82% of LSBs were effective. Mean pre- and post-NRS scores were 7.4±1.9 and 2.3±2.2, respectively, whereas mean percent relief was 66% and mean time of relief was 2.5 weeks (Table 3).
Table 3.
Abdominopelvic pain cohort
| No. | % | Results | No. | Mean | SD | No. | Mean | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 11 | Local anesthetic | 6 | |||||||
| Female | 8 | 73 | All | 11 | Effective | 5 | ||||
| Male | 3 | 27 | Prescore (NRS) | 7.4 | 1.9 | Prescore (NRS) | 8.3 | 1.0 | ||
| Postscore (NRS) | 2.3 | 2.2 | Postscore (NRS) | 1.5 | 1.7 | |||||
| Cancer | 11 | Relief, % | 66 | 38 | Relief, % | 83 | 20 | |||
| Endocrine | 1 | 9 | Relief, wk | 2.5 | 3.1 | Relief, wk | 2.9 | 3.8 | ||
| GI | 3 | 27 | Ineffective | 1 | ||||||
| GYN | 6 | 55 | Local anesthetic | 6 | Prescore (NRS) | 4.0 | – | |||
| Urologic | 1 | 9 | Prescore (NRS) | 7.6 | 2.0 | Postscore (NRS) | 4.0 | – | ||
| Postscore (NRS) | 1.9 | 1.8 | Relief, % | 0 | – | |||||
| Level | 11 | Relief, % | 69 | 38 | Relief, wk | 0.7 | – | |||
| L2 | 2 | 18 | Relief, wk | 2.6 | 3.5 | |||||
| L3 | 9 | 82 | Local anesthetic + steroid | 5 | ||||||
| Local anesthetic + steroid | 5 | Effective | 4 | |||||||
| Outcome | 11 | Prescore (NRS) | 7.2 | 1.9 | Prescore (NRS) | 7.5 | 2.1 | |||
| Effective | 9 | 82 | Postscore (NRS) | 2.8 | 2.8 | Postscore (NRS) | 2.0 | 2.4 | ||
| Ineffective | 2 | 18 | Relief, % | 63 | 41 | Relief, % | 78 | 26 | ||
| Relief, wk | 2.4 | 3.1 | Relief, wk | 3.0 | 3.2 | |||||
| Ineffective | 1 | |||||||||
| Prescore (NRS) | 6.0 | – | ||||||||
| Postscore (NRS) | 6.0 | – | ||||||||
| Relief, % | 0 | – | ||||||||
| Relief, wk | 0.0 | – |
Effective relief was defined as ≥30% relief for one or more days.
GI = gastrointestinal; GYN = gynecological; NRS = numerical rating scale.
Abdominopelvic Pain with Local Anesthetic
Among patients with abdominopelvic pain, the local anesthetic cohort (N = 6) had 83% effective blockade. Mean pre- and post-NRS scores for these data points were 7.6±2.0 and 1.9±1.8, respectively, whereas mean percent relief was 69% and mean time of relief was 2.6 weeks.
Abdominopelvic Pain with Local Anesthetic and Steroid
In the abdominopelvic pain with local anesthetic and steroid cohort (N = 5), 80% had effective blockade. Mean pre- and post-NRS scores for the cohort were 7.2±1.9 and 2.8±2.8, respectively, whereas mean percent relief was 63% and mean time of relief was 2.4 weeks.
Leg Pain
In the leg pain cohort (N = 40), 75% of LSBs were effective. Mean pre- and post-NRS scores were 7.8±1.7 and 3.7±2.5, respectively, whereas mean percent relief was 53% and mean time of relief was 4.8 weeks (Table 4).
Table 4.
Leg pain cohort
| No. | % | Results | No. | Mean | SD | No. | Mean | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 40 | Local anesthetic | 22 | |||||||
| Female | 23 | 58 | All | 40 | Effective | 15 | ||||
| Male | 17 | 43 | Prescore (NRS) | 7.8 | 1.7 | Prescore (NRS) | 7.7 | 1.6 | ||
| Postscore (NRS) | 3.7 | 2.5 | Postscore (NRS) | 2.7 | 1.8 | |||||
| Cancer | 40 | Relief, % | 53 | 31 | Relief, % | 68 | 23 | |||
| Endocrine | 4 | 10 | Relief, wk | 4.8 | 7.3 | Relief, wk | 4.2 | 6.5 | ||
| GYN | 8 | 20 | Ineffective | 7 | ||||||
| Lung | 1 | 3 | Local anesthetic | 22 | Prescore (NRS) | 8.0 | 1.4 | |||
| Neurologic | 5 | 13 | Prescore (NRS) | 7.8 | 1.5 | Postscore (NRS) | 7.1 | 1.3 | ||
| Other | 2 | 5 | Postscore (NRS) | 4.1 | 2.7 | Relief, % | 11 | 9 | ||
| Soft tissue | 12 | 30 | Relief, % | 50 | 33 | Relief, wk | 2.5 | 6.0 | ||
| Urologic | 8 | 20 | Relief, wk | 3.7 | 6.2 | |||||
| Local anesthetic + steroid | 18 | |||||||||
| Level | 40 | Local anesthetic + steroid | 18 | Effective | 15 | |||||
| L2 | 13 | 33 | Prescore (NRS) | 7.7 | 1.9 | Prescore (NRS) | 7.7 | 2.0 | ||
| L3 | 27 | 68 | Postscore (NRS) | 3.3 | 2.3 | Postscore (NRS) | 2.6 | 1.6 | ||
| Relief, % | 58 | 29 | Relief, % | 67 | 21 | |||||
| Outcome | 40 | Relief, wk | 6.3 | 8.4 | Relief, wk | 7.4 | 8.8 | |||
| Effective | 30 | 75 | Ineffective | 3 | ||||||
| Ineffective | 10 | 25 | Prescore (NRS) | 8.0 | 2.0 | |||||
| Postscore (NRS) | 6.7 | 2.1 | ||||||||
| Relief, % | 12 | 13 | ||||||||
| Relief, wk | 0.7 | 1.2 |
Effective relief was defined as ≥30% relief for one or more days.
GI = gastrointestinal; GYN = gynecological; NRS = numerical rating scale.
Leg Pain with Local Anesthetic
Among patients with leg pain, the local anesthetic cohort (N = 22) had 68% effective blockade. Mean pre- and post-NRS scores for these data points were 7.8±1.5 and 4.1±2.7, respectively, whereas mean percent relief was 50% and mean time of relief was 3.7 weeks.
Leg Pain with Local Anesthetic and Steroid
In the leg pain with local anesthetic and steroid cohort (N = 18), 83% had effective blockade. Mean pre- and post-NRS scores for the cohort were 7.7±1.9 and 3.3±2.3, respectively, whereas mean percent relief was 58% and mean time of relief was 6.3 weeks.
Morphine Milligram Equivalents
For all data points, mean pre-injection morphine milligram equivalents was 220.5, whereas mean postinjection morphine milligram equivalents was 211.9 mmeq (Table 5). In the back pain cohort, average pre-injection narcotic consumption decreased from 245.1 mmeq to 218.3 mmeq postinjection. In the abdominopelvic pain cohort, the average peri-injection mmeq lessened from 299.9 mmeq to 294.2 mmeq. Lastly, in the leg pain cohort, average pre-injection consumption was reduced from 195.0 mmeq to 188.2 mmeq.
Table 5.
Peri-injection opioids
| Mean Morphine, mmeq |
||
|---|---|---|
| Pre-injection | Postinjection | |
| All | 220.5 | 211.9 |
| Back pain | 245.1 | 218.3 |
| Local anesthetic | 429.0 | 382.5 |
| Local anesthetic + steroid | 153.1 | 136.3 |
| Abdominopelvic pain | 299.9 | 294.2 |
| Local anesthetic | 170.0 | 167.9 |
| Local anesthetic + steroid | 455.8 | 445.8 |
| Leg pain | 195.0 | 188.2 |
| Local anesthetic | 303.9 | 281.4 |
| Local anesthetic + steroid | 61.9 | 74.4 |
mmeq = morphine milligram equivalents.
Discussion
In this study, we presented 57 data points (41 patients) of lumbar sympathetic blockade in patients who had cancer-related pain. At the time of injection, 42 data points (30 patients) had active disease and 15 data points (11 patients) were in remission. Of the entire cohort, 75% of LSBs provided effective relief.
Oncologic disease commonly induces an inflammatory response and may even cause states of immunosuppression. In this setting, systemic steroids are a common oncologic intervention for exacerbation of tumor-related pain. The injection of particulate steroid via LSB may have a local effect near the tumor or a low-dose systemic effect similar to intravenous (IV) or oral steroids. These interactions are complex, and our injection of steroid was based on hundreds of injections performed at MSKCC, which we have empirically observed over years of practice, to lead to improved pain control over local anesthetic. Accordingly, our data demonstrate more relief with steroid injection vs no steroid (78% vs 73%). Nonetheless, local anesthetic alone often demonstrated nearly equivalent effective relief. It is possible that local anesthetic without steroid was often selected as the injectate because the patient population had less severe disease and pain to begin with or perhaps a higher baseline functional status. Alternatively, perhaps the local anesthetic with steroid cohorts had higher expectations of pain relief that weren’t met or well managed and thereby influenced their NRS scores.
We did not anticipate finding patients who received prolonged benefit from local anesthetics without steroids. A neurolytic effect of local anesthetics in the retroperitoneum is unlikely [10]. It is possible that a “reset” of central sensitization occurred or that a break in patients’ pain symptoms led to more sustained relief through improved coping. Another explanation may be that LSB averts the wind-up phenomenon. It is conceivable that simply injecting a volume of fluid dilutes inflammatory mediators. If this holds true, there may be a dose response curve with increased injectate volumes. It is plausible that patients’ pain was on the trajectory toward recovery and the local anesthetic injection merely helped improve the patient's pain during their recovery. We also acknowledge that changes to peri-injection pharmacotherapy may skew outcomes.
It is unclear why cancer-related back pain may improve with lumbar sympathetic blockade. It is possible that back pain from tumors may be more anatomically related to retroperitoneal structures that transmit pain via the sympathetic axis. Additionally, it is acknowledged that the anterior two-thirds of vertebral bodies are at least partially sympathetically innervated, which would explain relief in patients with vertebral body–related back pain (e.g., multiple myeloma or similar spinal metastases) [11].
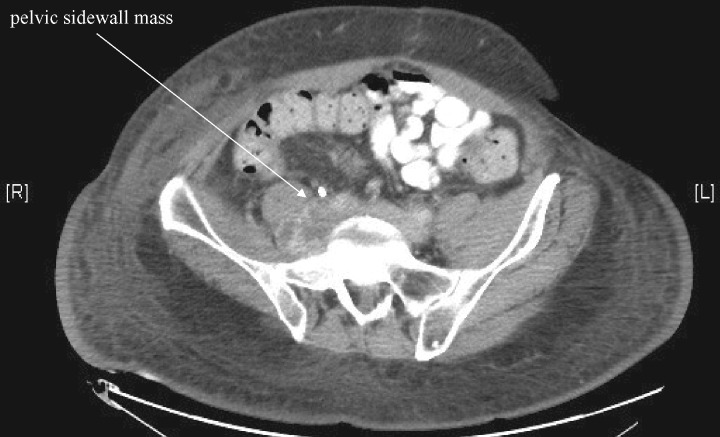
It is possible that abdominal and pelvic tumors may cause regional pain from bowel obstruction or stretching (e.g., patients with colon cancer), which may transmit impulses to the central nervous system via general visceral afferent fibers that pass through the sympathetic chain. Our current understanding is that the sympathetic chain travels around the aorta and inferior vena cava to become part of the superior hypogastric plexus (SHP), and we chose to interrupt this at L2 or L3 because of pelvic masses that are at the level of the lower lumbar spine (yet higher than the bladder, etc.). Along with the descending sympathetic chain are the lumbar splanchnic nerves, which may be involved in pain transmission of the lumbar nerve roots and mid and hind gut organs [12]. We used LSB over SHP block if the mass or tumor encompassed the retroperitoneal space at the level of the aortic bifurcation and below, making SHP block difficult to perform anatomically. Other anatomical considerations include pelvic sidewall masses, such as that of one of our patients (Figure 3), which have a femoral or sciatic neuropathic component of pain—an anatomical reason to choose LSB. Similarly, abdominopelvic tumor excisions or radiation may lead to unintended adjacent nerve injury, for which LSBs may provide relief [13]. All this has led to our routine use of LSB as described, and hence the present article to encourage its use based on our shared experiences.
Figure 3.
Patient with abdominopelvic pain secondary to a sidewall mass.
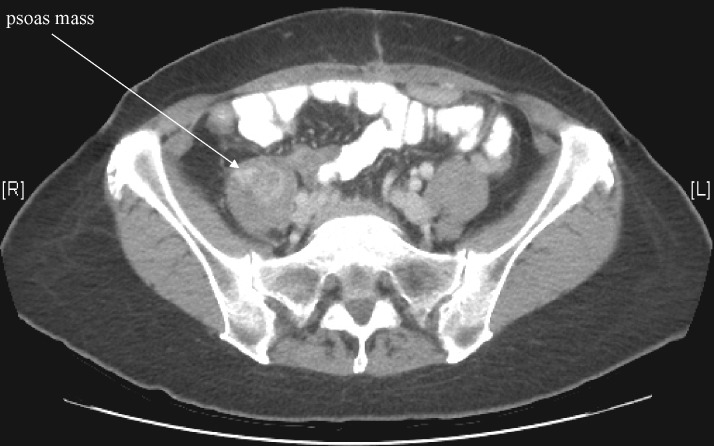
Leg pain may improve after an LSB, since many times the pain symptoms are similar to patients suffering from Complex Regional pain Syndrome (CRPS). CRPS or CRPS-like symptoms often result in nerve injury secondary to oncologic surgical resections or tumor invasions, where these syndromes are frequently treated interventionally by first choice sympathetic blockade [14]. Alternatively, tumor invasion into the psoas (Figure 4) may result in direct damage to the lumbar plexus with referred pain to the legs that may be alleviated by LSB [15]. It is also feasible to consider that LSB injectate medication may spread toward the psoas muscles and lumbosacral plexus, thereby blocking somatic afferent fibers as well [14].
Figure 4.
Patient with referred leg pain secondary to a right-sided psoas mass.
Opioid Consumption
On the whole, we appreciated a generalized trend toward decreased opioid consumption, as calculated in mean morphine milligram equivalents. Perhaps the most notable exception was in the leg pain local anesthetic with steroid cohort, where opioid consumption increased from 61.9 mmeq to 74.4 mmeq. On further review, this was skewed by a single data point with renal cell malignancy; the patient was started on methadone after his lumbar sympathetic block.
Neurolysis
LSB with local anesthetic is often intended for diagnostic purposes to consider neurolytic blockade. However, steroids can act to prolong the action of local anesthetics, decrease inflammation, and have a systemic effect on pain [16–18]. Accordingly, an effective initial block that includes steroids may be therapeutic as well. Yet, there may be contraindications to injection with steroids, such as poorly controlled diabetes or immunotherapy. In our study, there were 17 data points (two with back pain, seven with abdominopelvic pain, and eight with leg pain) that underwent neurolytic blockade (either radiofrequency ablation or with alcohol). Of the data points in this cohort, the mean preneurolysis NRS score was 7.6, whereas the mean postneurolysis NRS score was 5.2. Similarly, narcotic usage decreased perineurolysis from an average of 372.3 mmeq to 248.6 mmeq. Average relief time was 2.7 weeks. We acknowledge that this relief time is not particularly prolonged compared with our local anesthetic alone or local anesthetic with steroid cohorts. We speculate as to whether these neurolytic data points ultimately had more refractory pain to begin with. Notably, there was one neurolysis that was complicated by a self-limited psoas hematoma.
Adverse Effects
The safety of lumbar sympathetic blockade has been well established, but risks exist. Concerns include bleeding, infection, and nerve damage as with any procedure. Lumbar sympathetic blockade may result in loss of sympathetic tone yielding hypotension and unopposed parasympathetic tone (with symptoms such as diarrhea). Hematuria from kidney puncture is a rare complication and usually self-limited. Local anesthetic toxicity is unlikely at the doses used for proper lumbar sympathetic blockade; however, it may be a more real concern with inadvertent intravenous injection. As such, preprocedural IV placement is a reasonable consideration with an understanding of the need for access to resuscitative equipment. Neurolysis may be associated with anterior thigh pain, presumably from damage to the genitofemoral nerve, the rate of which has been quoted to be as high as 10% of patients [19]. Injection into radicular arteries can result in anterior spinal artery syndrome with devastating consequences of paralysis, which necessitates careful injection with contrast dye to detect vascular uptake. This complication is more likely at higher spinal levels, as only ≤25% of people have an artery of Adamkiewicz arising below T12 [20]. In our local and steroid cohorts, there was one complication of unilateral facial and upper extremity erythema. This may have been a steroid reaction, or a possible cephalad spread and a Horner Syndrome.
The major adverse effect of lumbar sympathetic blockade is lack of efficacy. In the patient population that we studied, nerve anatomy is often distorted by tumors, resections, or radiation, thus making the spread of medication unpredictable. The use of CT guidance may improve the efficacy and safety profile of this procedure. Furthermore, large or multiple tumors may cause pain transmission rostrally through a variety of pathways, and thus a single injection to the sympathetic nerves may be insufficient to block complete transmission of nociception.
Limitations
Our goal was to clarify where in the paradigm of cancer pain treatments lumbar sympathetic blockade may fit, but we acknowledge limitations with the present study. Some medical notes incompletely documented the amount of relief achieved, and several patients were lost to follow-up. When possible, NRS scores were taken from the Events Since Last Visit section of the medical record where the physician was usually able to tease out the true score as related to the pain site at hand; nonetheless, it is conceivable that some patients took NRS scores to imply a more global pain or discomfort. It is possible that some patients received 100% relief, became accustomed to the pain, transferred care elsewhere, or progressed to a critical care/comatose state where pain was a lesser concern for the intensive care team. We acknowledge that each data point was a single injection, not a unique patient, which may skew the data. We did not search for statistical significance in our data, as we felt the thrust of our manuscript here was to share our years of experience providing LSB in patients with cancer-related pain, not to prove, for example, steroidal injection superiority over local anesthetic alone. There were a handful of patients’ pain complaints that were difficult to categorize into our back, abdominopelvic, or leg cohorts. This was often due to radiation of pain from one location to another or pain present at two sites. This is a retrospective cohort without a control group, so it is unknown how many of these patients might have improved with time or more conservative treatments. Propensity score matching or other retrospective attempts to control the data were not feasible due to the sample size and heterogeneity of the cohorts. Cancer pain often changes with treatment and although an LSB may be successful, it may not seem to last long due to progression of disease. Conversely, a block may seem to have a prolonged effect in the setting of successful oncologic treatment. We did not control for analgesic regimen, and inpatients often received higher-strength intravenous opioids while outpatients were usually managed with multimodal topical and oral pain medications. Along those lines, peri-injection adjustments of pain medication regimen may blur the accuracy of NRS scoring as related to the injection alone. In regard to our mmeq calculations, prn (as needed) dosing, when able, was accurately teased out of the Events Since Last Visit section of the medical record. However, when not mentioned, it was standardized and assumed that patients took half of their daily prn regimen. Although we saw a trend toward decreased opioid consumption, this does not account for patients’ multimodal regimens, which included nonsteroidal drugs, acetaminophen, muscle relaxants, neuropathic agents, and more. Lastly, we defined an effective LSB as relief for at least one day, and although placebo effect is always possible, when we proceeded to neurolytic consideration, we had exhausted many treatment options already. We expect local anesthetic to have only hours’ duration of relief, but can only contact the patient the following day, hence the one-day effect. In our population, we appreciate a lower placebo effect typically due to progressing oncologic disease, leading to organic pain.
Conclusions
Patients may have differing ideas about what duration of pain relief from lumbar sympathetic blockade is acceptable. In patients who have short survival time frames, implantation of an intrathecal pump may be contraindicated, and temporary pain relief from an LSB may be an attractive and safe option. As more patients with cancer survive, short-term pain relief from local anesthetics or steroids may be less desirable, in which case neurolysis may be superior.
Lumbar sympathetic blockade is not completely effective as a treatment for back, abdominopelvic, or leg cancer–related pain, and thus we do not recommend it in isolation. We present it as a step in a paradigm in the treatment of cancer-related pain where opioids and adjuvants are inadequate. Accordingly, our algorithm uses LSB with local anesthetic +/- steroid as an initial treatment for our oncologic patient population. Should pain relief be significant but short-lived, then consideration is for neurolysis. Alternatively, if there is significant disease progression or changes in pain character, then neuraxial relief with intrathecal drug delivery or neuromodulation may be considered [21,22]. Percutaneous neurosurgical techniques may be warranted when other treatments have failed [23].
In the future, aggressive pain control with well-established data-driven protocols will shorten the path that patients take to adequate relief. Establishing lumbar sympathetic blockade as effective for back pain, abdominopelvic pain, and leg pain in the cancer population will help inform the conversation that patients have with their care team. Future research should refine the role of lumbar sympathetic blockade in multimodal cancer-related pain treatment.
Acknowledgments
Technical help was used for initial gathering of Pain Management payment codes.
Funding sources: Funded by MSKCC Core grant P 30.
Conflicts of interest: Matthew A. Spiegel: none; Lee Hingula: none; Grant H. Chen: none; Aron Legler: none; Vinay Puttanniah: none; Amitabh Gulati: consultant for Medtronic.
References
- 1. Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T.. Spinal afferent nerve endings in visceral organs: Recent advances. Am J Physiol Gastrointest Liver Physiol 2016;311(6):G1056–63. [DOI] [PubMed] [Google Scholar]
- 2. Willis WD, Al-Chaer ED, Quast MJ, Westlund KN.. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci U S A 1999;96(14):7675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang J. The History of Chemical Lumbar Sympathectomy. Cleveland; The internet jounral of Anesthesia 2002;7(1).
- 4. Zechlinski JJ, Hieb RA. Lumbar sympathetic neurolysis: How to and when to use? Tech Vasc Interv Radiol 2016;19(2):163–8. [DOI] [PubMed]
- 5. Baig S, Moon JY, Shankar H.. Review of sympathetic blocks: Anatomy, sonoanatomy, evidence, and techniques. Reg Anesth Pain Med 2017;42(3):377–91. [DOI] [PubMed] [Google Scholar]
- 6. Furman MB, Lee TS, Berkwits L.. Atlas of Image-Guided Spinal Procedures. Philadelphia: Elsevier Saunders; 2013. [Google Scholar]
- 7. Day M. Sympathetic blocks: The evidence. Pain Pract 2008;8(2):98–109. [DOI] [PubMed] [Google Scholar]
- 8. De Oliveira R, Dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain 2004;110(1-2):400–8. [DOI] [PubMed]
- 9. Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA.. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev 2017;7:CD012592.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Memari E, Hosseinian M-A, Mirkheshti A, et al. Comparison of histopathological effects of perineural administration of bupivacaine and bupivacaine-dexmedetomidine in rat sciatic nerve. Exp Toxicol Pathol 2016;68(10):559–64. [DOI] [PubMed] [Google Scholar]
- 11. Bogduk N, Tynan W, Wilson AS.. The nerve supply to the human lumbar intervertebral discs. J Anat 1981;132(Pt 1):39–56. [PMC free article] [PubMed] [Google Scholar]
- 12. Cramer GD, Darby SA.. Basic and Clinical Anatomy of the Spine, Spinal Cord and ANS. 3rd ed. St. Louis, MO: Elsevier;2013. [Google Scholar]
- 13. Wilson M, Nowicki M.. Persistent abdominal pain. Clin Pediatr (Phila) 2005;44(6):553–5. [DOI] [PubMed] [Google Scholar]
- 14. van Eijs F, Stanton-Hicks M, Van Zundert J, et al. Evidence-based interventional pain medicine. 16. Complex regional pain syndrome. Pain Pract 2011;11(1):70–87. [DOI] [PubMed] [Google Scholar]
- 15. Kirchmair L, Lirk P, Colvin J, Mitterschiffthaler G, Moriggl B.. Lumbar plexus and psoas major muscle: Not always as expected. Reg Anesth Pain Med 2008;33(2):109–14. [DOI] [PubMed] [Google Scholar]
- 16. Naghipour B, Aghamohamadi D, Azarfarin R, et al. Dexamethasone added to bupivacaine prolongs duration of epidural analgesia. Middle East J Anaesthesiol 2013;22(1):53–7. [PubMed] [Google Scholar]
- 17. Haywood A, Good P, Khan S, et al. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database Syst Rev 2015;4:CD010756. [DOI] [PMC free article] [PubMed]
- 18. Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane database Syst Rev 2017;11:CD011770. [DOI] [PMC free article] [PubMed]
- 19. Rathmell JP. Atlas of Image Guided Intervention in Regional Anesthesia and Pain Medicine. 2nd ed. philadelphia PA USA: Wolters Kluwer: | Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 20. Hurst RW. Spinal vascular disorders. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. 2nd ed. Philadelphia: Lippincott; 1996:1387–412. [Google Scholar]
- 21. Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20(19):4040–9. [DOI] [PubMed] [Google Scholar]
- 22. Legler A, Laufer I, Yamada J, Gulati A.. Case report considerations for the use of dorsal column stimulation for lum-bosacral radiation neuritis of the nerve root in the spine tumor patient (a case series). J Clin Oncol. 2017;1(5):163–72. [Google Scholar]
- 23. Kanpolat Y. Percutaneous destructive pain procedures on the upper spinal cord and brain stem in cancer pain: CT-guided techniques, indications and results. Adv Tech Stand Neurosurg 2007;32:147–73. [DOI] [PubMed] [Google Scholar]